Abstract
Rationale: Electronic cigarette (e-cig) use has been widely adopted under the perception of safety. However, possibly adverse effects of e-cig vapor in never-smokers are not well understood.
Objectives: To test the effects of nicotine-containing e-cig vapors on airway mucociliary function in differentiated human bronchial epithelial cells isolated from never-smokers and in the airways of a novel, ovine large animal model.
Methods: Mucociliary parameters were measured in human bronchial epithelial cells and in sheep. Systemic nicotine delivery to sheep was quantified using plasma cotinine levels, measured by ELISA.
Measurements and Main Results: In vitro, exposure to e-cig vapor reduced airway surface liquid hydration and increased mucus viscosity of human bronchial epithelial cells in a nicotine-dependent manner. Acute nicotine exposure increased intracellular calcium levels, an effect primarily dependent on TRPA1 (transient receptor potential ankyrin 1). TRPA1 inhibition with A967079 restored nicotine-mediated impairment of mucociliary parameters including mucus transport in vitro. Sheep tracheal mucus velocity, an in vivo measure of mucociliary clearance, was also reduced by e-cig vapor. Nebulized e-cig liquid containing nicotine also reduced tracheal mucus velocity in a dose-dependent manner and elevated plasma cotinine levels. Importantly, nebulized A967079 reversed the effects of e-cig liquid on sheep tracheal mucus velocity.
Conclusions: Our findings show that inhalation of e-cig vapor causes airway mucociliary dysfunction in vitro and in vivo. Furthermore, they suggest that the main nicotine effect on mucociliary function is mediated by TRPA1 and not nicotinic acetylcholine receptors.
Keywords: electronic nicotine delivery systems, mucus, TRPA1, mucociliary transport, airway epithelium
At a Glance Commentary
Scientific Knowledge on the Subject
E-cigarettes are marketed as safer alternatives to conventional cigarettes because of their defined composition and noncombustible nature. However, it is unclear how exposure to e-cigarette vapor, colloquially referred to as “vape,” affects naive airway epithelia. It is largely unknown to what extent individual constituents of vape, such as nicotine and flavoring agents, influence pulmonary function, if at all. The TRPA1 (transient receptor potential ankyrin 1) is a molecular target for vape effects because of its expression in airway epithelia and its reported gating by nicotine, reactive oxidants, and flavors, especially cinnamaldehyde.
What This Study Adds to the Field
This study implicates nicotine as a key vape constituent that acutely impairs airway mucociliary functions in vitro and in vivo (sheep). A functional, nicotine-sensitive TRPA1 receptor is natively expressed in human and sheep bronchial epithelial cells and mediates the effects of nicotine and e-cigarette vapors. Importantly, its inhibition prevents mucociliary dysfunction in vitro and in vivo. These findings implicate TRPA1 as a driver of mucociliary dysfunction induced by nicotine-containing e-cigarette vapor.
Since their introduction, electronic cigarettes (e-cigs) have continuously evolved in design, in part to compensate for their relatively poor ability to deliver nicotine compared with tobacco cigarettes (1). E-cigs originally rose in popularity as a potentially “safe” and “cleaner” method for inhaling nicotine, leading to their consideration as a cessation tool (2). However, unsubstantiated safety claims and availability of countless palatable flavors have led to an upsurge in e-cig use among never-smoking adolescents (3) and young adults (4). Despite this emerging public health issue, especially the recent report of chronic bronchitis in young e-cig users (5), informative e-cig studies on pulmonary physiology remain sparse. Ongoing debates for public policy, such as whether e-cigs are helpful or harmful or the ethics of e-cig dissemination (6), require more scientifically based investigations.
Vapor generated from e-cig liquids, colloquially known as “vape,” consists of droplets, 5–500 nm in diameter (Gaussian distributed particle size) (7) of e-cig liquid vehicle components propylene glycol (PG) and/or vegetable glycerin (VG), which contain chemical additives, such as nicotine and various flavoring agents. Between factors, such as wattage settings for the heating element, PG/VG composition, and a user’s experience with a device, a single session of inhaled vape can deliver nicotine doses that exceed those in smoke from one cigarette (8). Furthermore, recent efforts have partially demonstrated how PG/VG and various e-cig flavorings cause negative outcomes in vitro and in vivo, such as cell death, airway inflammation, and impaired airway hydration (9–14).
Early nicotine-related pulmonary research focused on airway smooth muscle because nicotine was discovered to elicit extracellular calcium (Ca2+) influx via nAChRs (nicotinic acetylcholine receptors). In the lung, nicotine induces both bronchoconstriction and bronchodilation in a dose-dependent manner. However, formative studies were often confounded by the effects of other cigarette smoke constituents (e.g., acrolein). Many e-cigs were designed for nicotine delivery and manufacturers have rapidly improved this function. However, it is currently unknown how inhaling e-cig vapor containing nicotine impacts functions of the ciliated airway epithelium in the bronchi, which is subjected to a large surface dose (15).
In a previous study, we found that e-cig vapor containing nicotine impairs mucociliary parameters, such as airway surface liquid (ASL) volume, ciliary beat frequency, and transepithelial ion transport in differentiated primary human bronchial epithelial cells (HBECs) (14). To address the mechanism of how e-cig vapor induces mucociliary dysfunction, we again used the primary HBEC culture system and a novel, large ovine animal model of e-cig vapor exposure. Sheep are particularly suited to probe effects of inhaled aerosols because of their airway responses to stimuli being similar to humans, such as in asthmatic and cystic fibrosis models (16–18). Indeed, nicotine deposited by e-cig vapor impaired various parameters of airway mucociliary function in HBECs and in sheep. However, we found that nicotine preferentially stimulates TRPA1 (transient receptor potential ankyrin 1) channels. Importantly, TRPA1 inhibition prevented nicotine’s deleterious effects on mucociliary clearance (MCC) in vitro and in sheep airways in vivo. Some of these results have been previously reported in abstracts (19, 20).
Methods
Methods used are described in greater detail in the online supplement.
Tissue Culture
Lungs were provided by organ procurement organizations with institutional review board approval. Primary HBECs were isolated with informed consent from deidentified donor lungs of never-smoking individuals rejected for transplant; 18 males and 13 females, ages 26 ± 2.3 and 25.5 ± 3.7 years, respectively (see Table E1 in the online supplement). Donors did not suffer from any documented airway disease. The study was not powered to dissect sex differences. Primary HBECs were cultured and differentiated at the air–liquid interface (ALI) for 4 weeks as described (14). Viability of cultures was assessed by the presence of active ciliary beating before and after any measurements.
In Vitro Aerosol and E-Cig Exposures of HBECs
E-cig vapor was generated using a Joyetech eVic supreme e-cig coupled to a Joyetech Delta atomizer and connected to a Vitrocell VC-1 smoking robot (14). E-cig exposure parameters were modified from the International Organization for Standardization (ISO) standards for cigarette-smoking machines (21) and described in the online supplement. Cultures were nebulized with defined solutions supplemented with NaCl using the Vitrocell Cloud device (14, 22). Final DMSO concentrations did not exceed 0.1%.
ASL Volume
Meniscus scanning of cultures was used to estimate ASL volume as described (23).
In Vitro Mucociliary Transport
HBECs were cultured on modified Transwell inserts to encourage mucociliary transport (MCT) development for 6–8 weeks (24). Movement of fluorescent microbeads (ThermoFisher Scientific) was measured to estimate MCT speed (μm/s) using the Manual Tracking ImageJ plugin.
mRNA and Protein Expression
mRNA and protein expression were measured by quantitative (q)PCR and Western blotting, respectively, according to standard techniques.
Calcium Imaging Using GCaMP6s Sensor
HBEC cultures were infected in an undifferentiated state with lentiviruses to deliver pEF1 (elongation factor1 promoter)-Puromycin–expressing GCaMP6s cDNA (25). This construct was designed using pGP-CMV-GCaMP6s (Addgene plasmid #40753) gifted by Dr. Douglas Kim (26). GCaMP6s-expressing cultures were perfused at room temperature with N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid–buffered Hanks’ balanced salt solution, pH 7.4, at 250 μl/min (27). GCaMP6s emissions were recorded every 3 seconds using MetaFluor (Molecular Devices) and recorded data reported as relative calcium levels (Fx/F0). Approximate Ca2+ sensitivity was confirmed with uridine-5′-triphosphate (10 μM) after a 10-minute recovery period. Data were analyzed using IGOR software (WaveMetrics).
Fluorescence Recovery after Photobleaching
Relative mucus viscosity was indirectly assessed by measuring diffusion rate of fluorescein isothiocyanate–dextran using fluorescence recovery after photobleaching (FRAP). Greater FRAP half-life (t1/2) correlates with an increased mucus viscosity. Cultures were layered overnight with fluorescein isothiocyanate–dextran (70 kD; Sigma-Aldrich). After treatments, emission from fluorescein isothiocyanate–labeled mucus layer was recorded as previously described (28, 29). Photobleaching was done at 10% of full laser power for two iterations (∼500 ms). FRAP was recorded at least three times per filter and averaged. A one-phase association nonlinear regression was then fit to estimate FRAP t1/2.
Mucus Concentration (Percent Solids)
“Initial weight” of absorptive paper was measured before use. After placement on cultures, it was gently lifted and measured immediately for “wet weight.” “Dry weight” was assessed after overnight exposure to 60°C. Mucus concentration in sheep tracheal secretions were measured using a fixed volume rather than absorptive paper. Percent mucus solids was calculated as previously described (30).
Animal Study
Conscious, adult female sheep (ewes) were nasally intubated (17). Male sheep (rams) were not used because they are naturally aggressive and are not amenable for experimentation without the use of general anesthesia. All procedures were approved by Mount Sinai Medical Center Animal Research Committee. Exposures are described in greater detail in the online supplement.
Tracheal mucus velocity (TMV) was measured by tracking radiopaque Teflon trioxide insufflated into the trachea using videotaped fluoroscopy (17). Sheep plasma cotinine was determined in plasma, diluted 10-fold in 1% bovine serum albumin, 0.05% Tween-20 phosphate-buffered saline, using Cotinine ELISA Kit (Abnova) following the manufacturer’s protocol.
Statistical Analyses
Data are presented as mean ± SEM and were analyzed by PRISM software (GraphPad). Each sample (n) represents a single biologic replicate. Normal distribution of data was confirmed using the Shapiro-Wilk normality test. Data were considered significant if P less than 0.05 for compared means with both parametric and nonparametric tests. Statistical differences were tested using paired Student’s t tests and one-way and two-way ANOVA with post hoc tests as deemed appropriate for collected data. Additional information on statistical analyses is described in the figure legends.
Results
Nicotine in E-Cig Vapor Impairs Parameters of Airway Mucociliary Function In Vitro
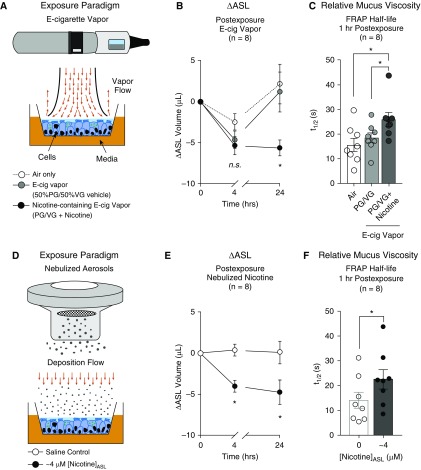
E-cig vapor containing 36 mg/ml nicotine (PG/VG + nicotine), delivered according to the methods in the online supplement, deposits 12.5 ± 1.1 μM (n = 6) nicotine in our exposure system (see Figure E1A), which is about half the nicotine deposited in the ASL after smoke exposure from one cigarette (31). Cultures exposed to nicotine-containing e-cig vapor exhibited larger decreases in ASL (Figures 1A and 1B) after 24 hours. ASL volume after exposure to e-cig vapor containing no nicotine (PG/VG) recovered after 24 hours like the air control. Cultures exposed to PG/VG + nicotine had greater FRAP t1/2 than either PG/VG or air-exposed cultures, indicative of increased mucus viscosity (Figure 1C). These outcomes revealed that airway hydration and mucus viscosity were impaired after nicotine deposition during e-cig vapor exposure.
Figure 1.
E-cigarette (e-cig) vapor containing nicotine impairs various parameters of mucociliary function in primary human air–liquid interface (ALI) cultures. (A) General schema illustrating exposure of ALI cultures to e-cig vapor. (B) Treatment effects were assessed by comparing absolute changes in airway surface liquid (ASL) volume from preexposure (ΔASL) at each time-point. Cultures exposed to e-cig vapor containing nicotine (propylene glycol [PG]/vegetable glycerin [VG] + nicotine) exhibited reduced ASL after 24 hours compared with e-cig vapor generated from vehicle (PG/VG) or air controls (n = 8). (C) Mucus viscosity estimated by fluorescence recovery after photobleaching (FRAP): cultures exposed to e-cig vapor containing nicotine (PG/VG + nicotine) had greater FRAP half-life (t1/2), indicating higher mucus viscosity, compared with PG/VG or air-only controls (n = 8). (D) General schema illustrating deposition of nebulized nicotine (or drugs) onto ALI cultures. (E) Cultures nebulized with nicotine had lower ASL compared with control-exposed cultures at 4 and 24 hours after exposure (n = 8). (F) Cultures nebulized with nicotine also exhibited greater FRAP t1/2 compared with saline control (0.1% NaCl) exposed cultures (n = 8). All data sets passed normality (Shapiro-Wilk). Each n represents a unique lung donor. *P < 0.05 between treatments, determined by Sidak post hoc test after two-way ANOVA (B and E), by Sidak post hoc test after one-way ANOVA (C), or by two-tailed paired Student’s t test (F). n.s. = not significant.
To investigate whether most of the vape-induced mucociliary dysfunction could be attributed to the effects of nicotine, solutions were deposited onto the apical surface of cultures by a mesh nebulizer in the CLOUD exposure system (Figure 1D). Mass deposition of a single dose of various nicotine tartrate salt solutions (100, 300, 600, or 900 μM) yielded 41.3 ± 9.4, 79.4 ± 14.4, 124.7 ± 26.5, and 176.5 ± 29.7 ng/cm2, respectively (see Figure E1). The lowest deposition (∼40 ng/cm2) equates to approximately 1 μM nicotine in the apical surface liquid.
Nicotine deposition affects ASL volume in a dose-dependent manner reaching a plateau at concentrations ≥4 μM (see Figure E1). Specifically, 4-μM deposited nicotine (by nebulizing 600 μM nicotine solution) lowered ASL volume more than saline control (0.1% NaCl in distilled water) at 4 and 24 hours after exposure (Figure 1E). Nicotine also increased mucus viscosity (Figure 1F). These data indicate nicotine as an active ingredient of e-cig vapor that impairs parameters of mucociliary function in ciliated airway epithelial cultures.
TRPA1 Is Expressed in Airway Epithelial Cells and Functions as a Nicotine Receptor
Nicotinic signaling in the airway is theoretically possible via several receptors expressed in various lung tissues (32, 33). TRPA1 is a Ca2+-selective ion channel that was first reported to be nicotine-sensitive in chemosensory neurons (34). TRPA1’s relevance to airway epithelial cell responses to agonists is poorly understood, however, possibly because of its low abundance in these cells.
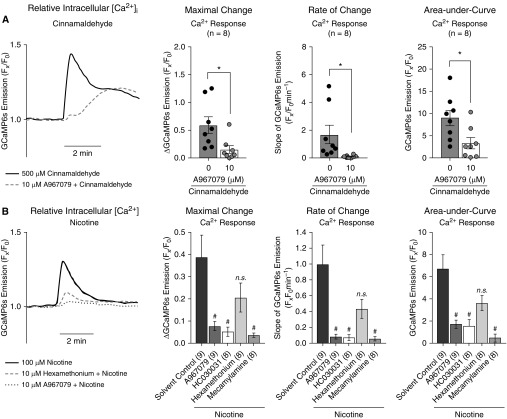
Consistent with previous studies (35, 36), we found that TRPA1 is expressed in primary HBECs (see Figure E2). We therefore assessed TRPA1 function in cultures by probing effects of cinnamaldehyde, a TRPA1 agonist and common e-cig flavor, on intracellular calcium levels ([Ca2+]i) using the fluorescent Ca2+ sensor GCaMP6s (26). These experiments were done in the presence or absence of the TRPA1 antagonists A967079 (10 μM) and HC030031 (10 μM) at doses previously shown to selectively inhibit TRPA1 under submerged conditions (35–37). A967079 (10 μM) had no effect on activation of TRPV4, another TRP channel, in our cultures (see Figure E3). Cinnamaldehyde perfusion rapidly increased [Ca2+]i, a response significantly reduced by A967079 (Figure 2A). This [Ca2+]i response occurred on the apical membrane of the cells (see Video E1). Nicotine perfusion (100 μM), approximate to the experimentally determined EC50 in our cultures, also increased [Ca2+]i (Figure 2B). This response was again significantly reduced by A967079 and HC030031, and mecamylamine, a dual inhibitor for TRPA1 and nAChRs (34, 38). The nAChR-selective antagonist hexamethonium did not significantly reduce the nicotine-induced Ca2+ response, suggesting nicotine elicited Ca2+ responses preferentially via TRPA1. Experiments with the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor thapsigargin suggested that nicotine triggered an extracellular influx of Ca2+ in a TRPA1-dependent manner (see Figure E4), consistent with apically located increases in [Ca2+]i caused by purinergic stimulation (see Video E2). These data suggest that TRPA1 is a major contributor to nicotine-induced [Ca2+]i changes in airway epithelial cell cultures.
Figure 2.
Primary human air–liquid interface cultures exhibit TRPA1 function. (A) Cinnamaldehyde (500 μM), a TRPA1 (transient receptor potential ankyrin 1) agonist, increased intracellular Ca2+ (GCaMP6s fluorescence). This response was partially blocked by the TRPA1 antagonist A967079 (10 μM), as measured by maximum, rate, and cumulative Ca2+ responses (n = 8). (B) Nicotine (100 μM) also increased intracellular Ca2+ and this response was again prevented by TRPA1 antagonists, including A967079 or HC030031 (10 μM), or the dual TRPA1/nAChRs (nicotinic acetylcholine receptors) antagonist mecamylamine (10 μM). Inhibition by the nAChR antagonist hexamethonium (10 μM) was not statistically significant. All n ≥ 8 for each treatment. “Maximal Change” and “Rate of Change” data for nicotine (B) did not pass the Shapiro-Wilk normality test and nonparametric tests were used. *P < 0.05 between treatments, determined by two-tailed paired Student’s t tests. #P < 0.05 between treatments with n > 2 treatments groups, determined by Sidak (parametric) or Kruskal-Wallis (nonparametric) post hoc test after one-way ANOVA. n.s. = not significant.
Inhibiting TRPA1 Prevents ASL Volume Loss and Increased Mucus Density Caused by Nicotine-Containing E-Cig Vapor
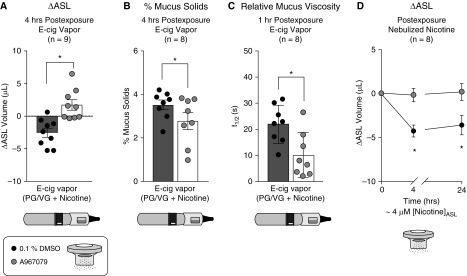
Exposure of HBECs to a selective TRPA1 agonist (JT010; ∼1 μM) mimicked the effects of nicotine on ASL volume (see Figure E5). We therefore tested the ability of TRPA1 inhibition to prevent e-cig vapor– and nicotine-induced mucociliary dysfunction. Cultures were nebulized to apically deposit approximately 2 μM A967079 and then exposed to e-cig vapor–containing nicotine or nebulized nicotine. The significant decrease in ASL in cultures exposed to PG/VG + nicotine was prevented by nebulized A967079 (Figure 3A). Cultures exposed to PG/VG + nicotine also exhibited mucus dehydration, measured by percent mucus solids (3.53 ± 0.2%), which was close to the reported value in mucus samples of patients with chronic bronchitis (30). A967079 reduced percent mucus solids under these conditions (2.78 ± 0.4%) (Figure 3B), suggesting TRPA1 inhibition improved mucus hydration during nicotine exposure and predicting a significant improvement in MCC (30). Indeed, A967079 protected against nicotine’s adverse effect on mucus viscosity (Figure 3C). Similar findings were observed when 10 μM A967079 was added to the basolateral media (see Figure E5). Nebulized nicotine also reduced ASL at 4 and 24 hours, and A967079 partially prevented this decrease (Figure 3D). Lastly, cultures infected with TRPA1 shRNA were protected from nicotine-induced ASL volume loss, providing further evidence that these effects are specific for TRPA1 (see Figure E5).
Figure 3.
TRPA1 (transient receptor potential ankyrin 1) inhibition prevents airway surface liquid (ASL) volume loss and increased mucus viscosity after nicotine delivery by e-cigarette (e-cig) vapor or nebulization. (A) A967079 was deposited onto air–liquid interface cultures by nebulization 1 hour before exposure to e-cig vapor containing nicotine (propylene glycol [PG]/vegetable glycerin [VG] + nicotine). A967079 improved ASL volume from preexposure (ΔASL) compared with 0.1% DMSO-exposed control subjects (n = 9). (B and C) Nebulized A967079 also reduced the percent mucus solids (n = 8) (B) and lowered fluorescence recovery after photobleaching half-time (t1/2) (n = 8) (C) after PG/VG + nicotine exposure. Note that the percentage of mucus solids with PG/VG + nicotine exposure is close to the values of patients with chronic bronchitis. (D) ASL volume of cultures pretreated with A967079 were protected from nebulized nicotine at 4 and 24 hours after exposure (n = 8). All data sets passed normality (Shapiro-Wilk). *P < 0.05 between treatments, determined by two-tailed paired Student’s t tests, or Sidak post hoc test after two-way ANOVA (ASL change over time).
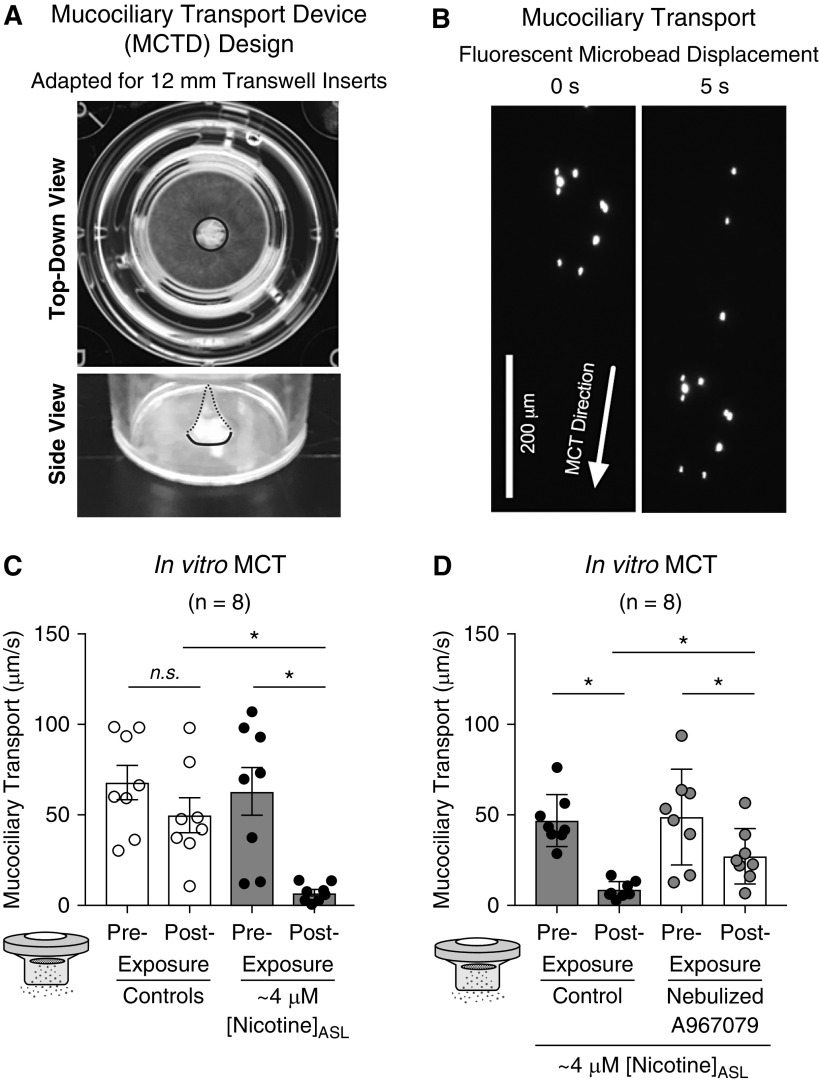
Next, pairs of cultures from the same donor were differentiated at the ALI on Transwell inserts (Figure 4A) modified to encourage development of directed MCT (Figure 4B). Paired cultures with similar baseline MCT were chosen for experiments. Consistent with ΔASL and mucus viscosity, nicotine significantly reduced MCT 1 hour after nebulization (Figure 4C). However, preexposure to nebulized A967079 prevented nicotine’s deleterious effect on MCT (Figure 4D). Technical restrictions did not allow us to repeat these in vitro experiments with e-cig vapor. Nevertheless, these results suggest that TRPA1 inhibition prevents nicotine-induced mucociliary dysfunction in vitro.
Figure 4.
Nebulized nicotine impairs mucociliary transport (MCT) in vitro. (A) Adaptation of the original “mucociliary transport device” design by Sears and colleagues (24) to 12-mm Transwell inserts. Marine-safe silicone sealant was applied to the center of 12-mm Transwell inserts to form a cone (outlined in blue) with an approximate 1- to 1.5-mm diameter at its base. (B) Primary human bronchial epithelial cell cultures were differentiated at the air–liquid interface for 6–8 weeks until continuous MCT developed. MCT was estimated by recording the movement of 1-μm-diameter fluorescent beads placed on the apical surface of cultures the prior evening. MCT velocity was calculated from distance traveled by each bead (i.e., displacement) over time (arrow). (C) Baseline MCT was similar between cultures exposed to nicotine (67.9 ± 9.5 μm/s; n = 8) and control subjects (63.0 ± 13.3 μm/s; n = 8). Nebulized nicotine significantly reduced MCT after 1 hour compared with nebulized saline control. (D) Baseline MCT was similar between cultures exposed to DMSO (46.8 ± 5.1 μm/s; n = 8) and A967079 (48.7 ± 9.4 μm/s; n = 8). A967079-exposed cultures had significantly improved MCT compared with DMSO-treated cultures after nicotine exposure. Baseline MCT differences in C and D are possibly caused by difference in donor lungs with no overlap. All data sets passed normality (Shapiro-Wilk). *P < 0.05 between treatments, determined by Sidak post hoc test after one-way ANOVA. ASL = airway surface liquid; n.s. = not significant.
Nicotine in E-Cig Vapor Impairs Sheep Tracheal MCC in a Dose-Dependent Manner and Is Prevented by TRPA1 Inhibition
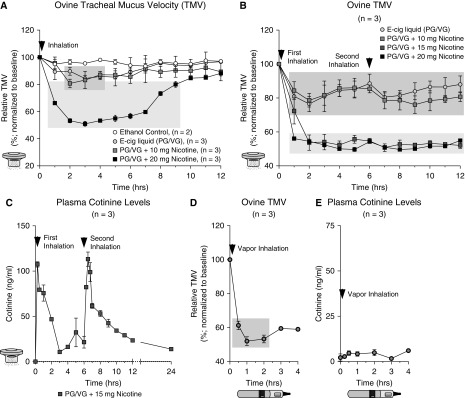
To confirm the effects of e-cig vapor on mucociliary function in vivo, a large animal exposure model was developed using sheep. Ewes have been previously used by us to measure TMV, a surrogate for MCC, because of their airway similarities to humans including the predictability of pharmacologic testing for human airway diseases, such as asthma and cystic fibrosis (16, 18). Average baseline sheep TMV was 10.2 ± 0.169 mm/min (n = 24).
First, vapor fluid (PG/VG) with different concentrations of nicotine was aerosolized into the airways in a controlled fashion. After recording baseline TMV, TMV values were recorded for a total of 12 hours. PG/VG alone decreased TMV to approximately 80% of baseline TMV for about 6 hours (Figure 5A). Nicotine-containing (10 mg) e-cig liquid had a similar effect as PG/VG alone but 20-mg nicotine caused TMV to reduce to approximately 50%. To see whether repeated exposures caused similar reductions in TMV, aerosols were readministered 6 hours after the initial application. The repeated exposures showed similar reductions in TMV regarding decrease and duration (Figure 5B). Plasma cotinine levels after 15-mg nicotine exposures were elevated to levels estimated in human beings after smoking two cigarettes (Figure 5C). No obvious toxic nicotine effects were observed in the sheep. These data show that aerosolized e-cig liquid reduces TMV and that nicotine adds to the effect in a concentration-dependent manner. Next, sheep were directly exposed to e-cig vapor generated from PG/VG + nicotine (36 mg/ml; 40 inhalations). Sheep TMV was halved after exposure (Figure 5D) despite a low but detectable level of plasma cotinine (Figure 5E).
Figure 5.
Sheep tracheal mucus clearance (tracheal mucus velocity [TMV]) is inhibited by e-cigarette (e-cig) vapor mainly in a nicotine-dependent manner (propylene glycol [PG]/vegetable glycerin [VG] had a small but initially significant effect). Sheep TMV was measured by recording movement of small radiopaque Teflon trioxide disks before and after exposures. E-cig liquids were dissolved in 100% ethanol because the e-cig liquid is otherwise too viscous, then administered by nebulization based on total delivered mass of dissolved nicotine. E-cig vapor was generated using a Joyetech e-cig device at settings used for in vitro experiments as described in the online supplement. Arrowheads indicate exposure times. (A) TMV was reduced after PG/VG ± nicotine inhalation by nebulized e-cig liquid (n = 3 per treatment) in a nicotine dose-dependent manner. Ethanol inhalation alone had no influence on TMV (n = 2). (B) Reduction of TMV was again seen after a second exposure at 6 hours after the initial dose (n = 3). (C) Plasma levels of cotinine, the primary metabolite of nicotine, showing nicotine uptake after inhalation of nebulized e-cig liquid containing 15-mg nicotine. (D) TMV was also reduced after vaping sheep with PG/VG + nicotine (36 mg/ml; 40 inhalations). (E) As expected from our published human study in veterans (53), e-cig vapor is a poor nicotine delivery medium showing low but detectable plasma cotinine levels. Dark gray box is P < 0.05 compared with baseline TMV using two-way ANOVA of repeated measures followed by Sidak’s multiple comparison test; light gray box is P < 0.05 compared with baseline, PG/VG, and 10-mg nicotine using the same analysis.
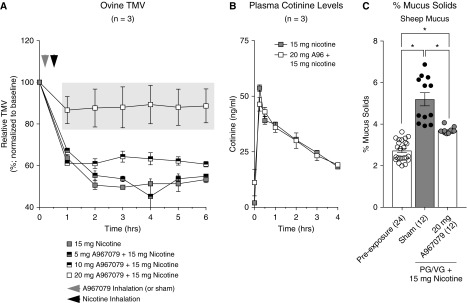
The ability of the TRPA1 inhibitor A967079 to prevent nicotine-dependent TMV decreases was tested next. First, TRPA1 expression was confirmed in freshly isolated ovine airway epithelial cells (see Figure E2). Sheep were pretreated with nebulized A967079 (0, 5, 10, or 20 mg) or vehicle control (diluted ethanol) before exposure to 15-mg nicotine-containing e-liquid (in PG/VG). Inhalation of 0, 5, or 10 mg A967079 did not prevent the TMV decrease on exposure to 15-mg nicotine (Figure 6A). However, inhalation of 20-mg A967079 prevented most of the TMV reduction caused by 15-mg nicotine. Plasma cotinine levels were not affected by A967079 (Figure 6B), confirming that nicotine uptake and metabolism were unaltered.
Figure 6.
Inhaled A967079 prevents impaired sheep tracheal mucociliary clearance caused by nicotine inhalation. (A) Sheep were nebulized with varying doses of A967079 before propylene glycol (PG)/vegetable glycerin (VG) + 15 mg of nicotine (n = 3 per treatment). Tracheal mucus velocity (TMV) for 5- and 10-mg A967079-exposed sheep were not significantly different from PG/VG + 15 mg of nicotine only. However, 20-mg A967079-exposed sheep exhibited TMV values that were significantly improved compared with PG/VG + 15 mg of nicotine. (B) Plasma cotinine levels were not significantly different between sheep exposed to PG/VG + 15 mg of nicotine with or without 20-mg A967079 pretreatment, confirming nicotine delivery. (C) Percent mucus solids were assessed in sheep mucus collected before and at the end of TMV measurements from two sheep per treatment (DMSO or A967079). Mucus solids were measured in triplicates for two separate mucus collections per sheep (n = 12). Nicotine-exposed sheep had significantly increased percentage of mucus solids that was reduced with 20-mg A967079 pretreatment. Light gray box is P < 0.05 compared with lower doses of A967079 (0, 5, and 10 mg) using two-way ANOVA of repeated measures followed by Sidak’s multiple comparison test. *P < 0.05 between treatments, determined by Sidak post hoc test after one-way ANOVA.
Furthermore, mucus hyperconcentration was found in collected sheep tracheal secretions after inhalation of aerosolized 15-mg nicotine-containing e-cig liquid, consistent with the decrease in TMV. In addition, sheep pretreated with aerosolized A967079 (20 mg; Figure 6C) revealed a lower mucus concentration on inhalation of 15-mg nicotine, consistent with the in vitro observations.
Discussion
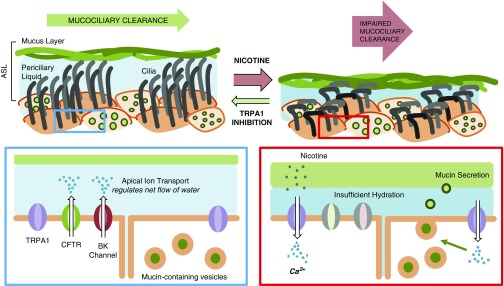
In this study, we investigated effects of e-cig vapor on airway mucociliary function. In vitro, ASL hydration and mucus viscosity were negatively impacted by nicotine-containing e-cig vapor. The TRPA1 agonists cinnamaldehyde and nicotine elicited Ca2+ responses in HBEC cultures but not in the presence of TRPA1 antagonists. The TRPA1 antagonist A967079 protected against both ASL volume loss and increased mucus viscosity during nicotine delivery by e-cig vapor. Sheep TMV was impaired with PG/VG but more with nicotine in a dose-dependent manner. This was again prevented by A967079 pretreatment. Our working theory is that nicotine delivered by e-cig vapor mediates its effects mostly via TRPA1 to affect proper ASL volume and mucus viscosity, leading to a decrease in MCC (Figure 7).
Figure 7.
Working model for impaired airway mucociliary function by nicotine delivery from e-cigarette vapor. Generalized schema outlining components of the ciliated airway epithelium. At the apical surface of ciliated airway epithelial tissue, TRPA1 (transient receptor potential ankyrin 1) is expressed and functional. On exposure to inhaled nicotine, TRPA1 is activated, leading to Ca2+ influx with subsequent signaling that, over time, results in net loss of airway surface liquid hydration and increased mucus solids and viscosity, leading to impaired mucociliary clearance. ASL = airway surface liquid; BK = voltage-dependent K+; CFTR = cystic fibrosis transmembrane conductance regulator.
To test whether nicotine had effects independent of other e-cig vapor constituents, we used the Vitrocell CLOUD exposure system to nebulize fixed nicotine doses onto the apical surface of ALI cultures (22, 39). Notably, the nicotine used differed between the e-cig vapor and nebulized exposures. Nicotine suspended in e-cig liquid is shifted toward the free-base (uncharged) form because of the basic pH as previously reported (40). E-cig liquids used in this study had pH ranging between 8.8 and 9.3. However, we measured that approximately 0.02-μl e-cig liquid is deposited into the ASL of cultures after vape exposures (not shown), suggesting that pH of the ASL itself is likely unaffected by vape deposition. Even so, ciliary beating is not significantly influenced by changes in pH between 7.5 and 10.5 in isolated human bronchial cells, suggesting a degree of buffering in the ASL (41). Nicotine tartrate salt, which was used for nebulized exposures, is negatively charged and is more acidic in solution (pH < 7). However, it induces receptor-mediated Ca2+ influx in our cultures (Figure 2) and both nicotine in e-cig vapor and in salt form induced similar mucociliary dysfunction outcomes.
Nicotine itself reduced ASL hydration and MCT in vitro, mirroring our previous findings (14). In that previous study, e-cig vapor downregulated apical ion transport through cystic fibrosis transmembrane conductance regulator (CFTR) and large conductance, and Ca2+-activated and voltage-dependent K+ (BK) channels. Impaired CFTR-driven Cl− efflux has also been reported in rats chronically ingesting nicotine (42). Because dynamic ion transport by CFTR and BK channels helps to maintain an approximately 7-μm ASL height necessary for adequate ciliary beating (43), sustained downregulation of apical ion flux can reduce ASL height and lead to impaired ciliary movement and reduced MCC (44).
Nicotine stimulation of TRPA1 channels caused an apical influx of Ca2+. In the short-term, this would be expected to increase CFTR conductance and ciliary beating (27) and activate Ca2+-sensitive BK channels. However, we previously observed reduced CFTR and BK channel conductance after e-cig vapor exposure. We speculate that sustained TRPA1 activation will disrupt short-term Ca2+ signaling possibly by diminishing ER Ca2+ stores. The initial [Ca2+]i elevation can also induce mucus secretion (45). This together with reduced ion transport will lead to ASL volume loss and MCC slowing.
Previous research on nicotine effects on the airway focused on the role of nAChR subunits (46). Most airway nAChR studies implicated a role for the α7 subunit because its expression is elevated in smokers (47, 48) and it plays a role in smoking-related lung cancer pathogenesis (32, 49). α7-nAChR is a pentameric Ca2+ channel. Its function is limited by desensitization (46). In contrast, TRPA1 is a tetrameric Ca2+ channel that is activated by reactive electrophiles (e.g., cinnamaldehyde and acrolein), prostaglandins, bradykinin, hypoxia, and as more recently shown, also by nicotine (50). Sensory neurons in trachea of TRPA1 knockout mice had a significant reduction in CGRP (calcitonin gene-related peptide) release in response to nicotine-containing particulate matter (38). Consistent with this notion of nicotine activating TRPA1 channels, we observed that TRPA1 antagonists reduced nicotine-induced Ca2+ response to a larger extent than hexamethonium in airway epithelial cell cultures, even though only low TRPA1 expression was seen in airway epithelia (35). However, it has been shown that smoke exposure upregulates TRPA1 expression in A549 cells (51), suggesting that airway tissue of chronic smokers or e-cig users might have greater TRPA1 expression. Although it is possible that our observations were confounded by off-target A967079 effects, A967079 is known to be weak (ED50 > 5 μM) or not active against various G-protein coupled receptors, enzymes, transporters, and ion channels, including other TRP channels (52). Indeed, we observed no effect of A967079 (10 μM) on TRPV4-induced calcium influx (see Figure E3). Furthermore, concentrations of TRPA1 antagonists used in this study were consistent with other in vitro studies (35–37).
To better validate our in vitro findings, we adapted inhalation of e-cig vapor to a large animal model with sheep to measure whole animal TMV as a marker of MCC. Sheep are particularly suited to probe effects of inhaled aerosols because their airway responses to stimuli are similar to humans (16, 17). Although e-cig vapor containing nicotine drastically reduced TMV when vaped into sheep, it resulted in low amounts of systemic nicotine uptake, indicated by plasma cotinine levels. This mirrored our recent clinical observations for smoking cessation in veterans using e-cigs: the major reason for failing to switch from tobacco smoking to e-cig vaping was insufficient systemic nicotine uptake if vaping did not follow a certain inhalation topography (53).
Nebulization of e-cig liquid into sheep had an effect similar to vapor on TMV, but also a much better systemic nicotine delivery. This indicated that nebulized liquid is a more effective nicotine delivery method than vapor. TMV reductions after a single dose lasted for approximately 6 hours, upon which a repeat dose decreased TMV again. Similar findings of vapor containing nicotine on MCC were reported in a murine model (11). In contrast, that study did not find that e-cig liquid had an immediate effect on murine MCC, suggesting that the large animal is more suited to predict human responses. Reductions in ovine TMV on inhalation of nicotine-containing liquid were markedly protected by pretreatments with nebulized A967079. Notably, sheep required higher concentrations of A967079 because of the low deposition efficiency (∼10%) of nebulized treatments (54). Furthermore, previous studies showed that doses approximately 20-fold higher than effective in vitro doses are optimal for nebulized treatments in sheep (55, 56). A small remaining TMV decrease may be related to direct effects of PG/VG because of its physiochemical properties that can hinder ciliary beating as recently reported (57, 58).
Lastly, TRPA1 may not only be stimulated by nicotine, but also by free radicals generated during the vaporization process of e-liquid (59), either directly or by downstream lipid peroxidation products through its redox-sensitive cysteine residues. The latter is the primary mechanism of activation for the TRPA1 agonists cinnamaldehyde and allyl isothiocyanate (60). Coincidentally, cinnamaldehyde is the main e-cig liquid additive for cinnamon flavoring and several widely used e-cig flavoring additives, such as vanillin and menthol, are known effectors of other TRP Ca2+ channels (61). It is therefore plausible that many e-cig flavors influence airway MCC via TRP family members.
Conclusions
This study demonstrates that nebulized and vaporized e-cig liquids with nicotine have significant and deleterious effects on airway mucociliary function of naive airway epithelial cells from never-smoking individuals and of the sheep trachea. This effect was mainly mediated via TRPA1 receptors. How other e-cig vapor constituents, such as certain types of flavoring agents, affect these processes are areas of future investigation.
Acknowledgments
Acknowledgment
The authors thank the Florida International University Advanced Mass Spectrometry Facility for their assistance with nicotine quantifications, Dr. Douglas Kim (Janelia) and Addgene for providing the GCaMP6s construct, Dr. David Julius (University of California, San Francisco) for providing the construct encoding the human TRPA1 cDNA, Dr. Lawrence Ostrowski (University of North Carolina at Chapel Hill) for providing mucociliary transport device samples and guidance, Dr. Michael Myerburg (University of Pittsburgh) for providing the airway surface liquid volume analysis software, Dr. Jianghua He (University of Kansas Medical Center) for biostatistical advice, Dr. Brian Button (University of North Carolina) for providing the mucus solids measurement technique, Dr. Siddarth Rawal and Dr. Ashutosh Agarwal (University of Miami Miller School of Medicine) for their assistance in generating laser cut absorbent mesh for mucus solids measurements, and the LifeCenter Northwest (WA) and Life Alliance Organ Recovery Agency (FL) for their assistance with organ procurement.
Footnotes
Supported by the Flight Attendant Medical Research Institute, CIA #130033 (M.S.); James and Esther King Florida Biomedical Research Program, grant #5JK02 (M.S.); and NIH F32-HL140729 (S.C.) and R01 HL139365 (M.S.).
Author Contributions: S.C., N.B., W.M.A., M.D.K., and M.S. contributed to the concept and/or design of the study. All authors contributed to the acquisition, critical analysis, and interpretation of the data. S.C. drafted the manuscript. All authors (except for the late W.M.A.) revised it for intellectual content and approved the final version before submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201811-2087OC on June 7, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2017;1394:5–30. doi: 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks AS, Sando K, McBane S. Do electronic cigarettes have a role in tobacco cessation? Pharmacotherapy. 2018;38:555–568. doi: 10.1002/phar.2103. [DOI] [PubMed] [Google Scholar]
- 3.Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, et al. Tobacco use among middle and high school students—United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2017;66:597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195–1202. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 5.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, et al. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195:1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlowski LT, Sweanor DT. ‘Not harmless’ messages without comparisons disserve consumers, potential consumers, and public health approaches to tobacco/nicotine products. Addict Behav. 2018;76:390–391. doi: 10.1016/j.addbeh.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Pyrgiotakis G, Demokritou P. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal Toxicol. 2016;28:658–669. doi: 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prévôt N, de Oliveira F, Perinel-Ragey S, Basset T, Vergnon JM, Pourchez J. Nicotine delivery from the refill liquid to the aerosol via high-power e-cigarette device. Sci Rep. 2017;7:2592. doi: 10.1038/s41598-017-03008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313:L52–L66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17:57. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laube BL, Afshar-Mohajer N, Koehler K, Chen G, Lazarus P, Collaco JM, et al. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol. 2017;29:197–205. doi: 10.1080/08958378.2017.1336585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, et al. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16:e2003904. doi: 10.1371/journal.pbio.2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197:492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71:1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manigrasso M, Buonanno G, Stabile L, Morawska L, Avino P. Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environ Pollut. 2015;202:24–31. doi: 10.1016/j.envpol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Sabater JR, Lee TA, Abraham WM. Comparative effects of salmeterol, albuterol, and ipratropium on normal and impaired mucociliary function in sheep. Chest. 2005;128:3743–3749. doi: 10.1378/chest.128.5.3743. [DOI] [PubMed] [Google Scholar]
- 17.Terryah ST, Fellner RC, Ahmad S, Moore PJ, Reidel B, Sesma JI, et al. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2018;314:L192–L205. doi: 10.1152/ajplung.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham WM. Modeling of asthma, COPD and cystic fibrosis in sheep. Pulm Pharmacol Ther. 2008;21:743–754. doi: 10.1016/j.pupt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Chung S, Sailland-Tschudi J, Baumlin-Schmid N, Salathe M. Nicotine-induced airway liquid volume loss depends on apical nicotinic acetylcholine receptors and possibly TRPA1 [abstract] Am J Respir Crit Care Med. 2017;195:A2432. [Google Scholar]
- 20.Chung S, Moore R, Dennis JS, Baumlin N, Kim MD, Salathe M. Nicotine activates epithelial TRPA1 receptors to cause mucociliary dysfunction [abstract] Am J Respir Crit Care Med. 2018;197:A3812. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Organisation for Standardisation. ISO 3308: routine analytical cigarette-smoking machine: definitions and standard conditions. Geneva, Switzerland: International Organisation for Standardisation; 2012. [Google Scholar]
- 22.Lenz AG, Stoeger T, Cei D, Schmidmeir M, Semren N, Burgstaller G, et al. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am J Respir Cell Mol Biol. 2014;51:526–535. doi: 10.1165/rcmb.2013-0479OC. [DOI] [PubMed] [Google Scholar]
- 23.Harvey PR, Tarran R, Garoff S, Myerburg MM. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol. 2011;45:592–599. doi: 10.1165/rcmb.2010-0484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2015;309:L99–L108. doi: 10.1152/ajplung.00024.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumlin-Schmid N, Salathe M, Fregien NL.Optimal lentivirus production and cell culture conditions necessary to successfully transduce primary human bronchial epithelial cells J Vis Exp [online ahead of print] 22 Jul201610.3791/54176. [DOI] [PubMed] [Google Scholar]
- 26.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salathe M, Bookman RJ. Coupling of [Ca2+]i and ciliary beating in cultured tracheal epithelial cells. J Cell Sci. 1995;108:431–440. doi: 10.1242/jcs.108.2.431. [DOI] [PubMed] [Google Scholar]
- 28.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 2011;25:2325–2332. doi: 10.1096/fj.10-179549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lennox AT, Coburn SL, Leech JA, Heidrich EM, Kleyman TR, Wenzel SE, et al. ATP12A promotes mucus dysfunction during type 2 airway inflammation. Sci Rep. 2018;8:2109. doi: 10.1038/s41598-018-20444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med. 2015;192:182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol. 2008;32:201–207. doi: 10.1093/jat/32.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nastrucci C, Russo P. α7 nAChR in airway respiratory epithelial cells. Curr Drug Targets. 2012;13:666–670. doi: 10.2174/138945012800398865. [DOI] [PubMed] [Google Scholar]
- 33.Lam DC, Luo SY, Fu KH, Lui MM, Chan KH, Wistuba II, et al. Nicotinic acetylcholine receptor expression in human airway correlates with lung function. Am J Physiol Lung Cell Mol Physiol. 2016;310:L232–L239. doi: 10.1152/ajplung.00101.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 35.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prandini P, De Logu F, Fusi C, Provezza L, Nassini R, Montagner G, et al. Transient receptor potential ankyrin 1 channels modulate inflammatory response in respiratory cells from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 2016;55:645–656. doi: 10.1165/rcmb.2016-0089OC. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Zhang Y, Xu M, Zhang H, Chen Y, Chung KF, et al. Roles of TRPA1 and TRPV1 in cigarette smoke-induced airway epithelial cell injury model. Free Radic Biol Med. 2019;134:229–238. doi: 10.1016/j.freeradbiomed.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Kichko TI, Kobal G, Reeh PW. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am J Physiol Lung Cell Mol Physiol. 2015;309:L812–L820. doi: 10.1152/ajplung.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagalla RB, Smaldone GC. Capturing the efficiency of vibrating mesh nebulizers: minimizing upper airway deposition. J Aerosol Med Pulm Drug Deliv. 2014;27:341–348. doi: 10.1089/jamp.2014.1152. [DOI] [PubMed] [Google Scholar]
- 40.El-Hellani A, El-Hage R, Baalbaki R, Salman R, Talih S, Shihadeh A, et al. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem Res Toxicol. 2015;28:1532–1537. doi: 10.1021/acs.chemrestox.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11:330–333. doi: 10.1183/09031936.98.11020330. [DOI] [PubMed] [Google Scholar]
- 42.Roomans GM, Vanthanouvong V, Dragomir A, Kozlova I, Wróblewski R. Effects of nicotine on intestinal and respiratory epithelium. J Submicrosc Cytol Pathol. 2002;34:381–388. [PubMed] [Google Scholar]
- 43.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011;286:19830–19839. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Button B, Anderson WH, Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc. 2016;13:S156–S162. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorrieri G, Scudieri P, Caci E, Schiavon M, Tomati V, Sirci F, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016;6:36016. doi: 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouzat C, Sine SM. Nicotinic acetylcholine receptors at the single-channel level. Br J Pharmacol. 2018;175:1789–1804. doi: 10.1111/bph.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bordas A, Cedillo JL, Arnalich F, Esteban-Rodriguez I, Guerra-Pastrián L, de Castro J, et al. Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget. 2017;8:67878–67890. doi: 10.18632/oncotarget.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu XW, Lindstrom J, Spindel ER. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;41:93–99. doi: 10.1165/rcmb.2008-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo P, Cardinale A, Margaritora S, Cesario A. Nicotinic receptor and tobacco-related cancer. Life Sci. 2012;91:1087–1092. doi: 10.1016/j.lfs.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich A, Steinritz D, Gudermann T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium. 2017;67:123–137. doi: 10.1016/j.ceca.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Nie Y, Huang C, Zhong S, Wortley MA, Luo Y, Luo W, et al. Cigarette smoke extract (CSE) induces transient receptor potential ankyrin 1(TRPA1) expression via activation of HIF1αin A549 cells. Free Radic Biol Med. 2016;99:498–507. doi: 10.1016/j.freeradbiomed.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 52.McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerrero-Cignarella A, Luna Diaz LV, Balestrini K, Holt G, Mirsaeidi M, Calderon-Candelario R, et al. Differences in vaping topography in relation to adherence to exclusive electronic cigarette use in veterans. PLoS One. 2018;13:e0195896. doi: 10.1371/journal.pone.0195896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ari A, de Andrade AD, Sheard M, AlHamad B, Fink JB. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J Aerosol Med Pulm Drug Deliv. 2015;28:281–289. doi: 10.1089/jamp.2014.1149. [DOI] [PubMed] [Google Scholar]
- 55.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, et al. Pharmacology of INS37217 [P(1)-(uridine 5′)-P(4)- (2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 56.Scott DW, Walker MP, Sesma J, Wu B, Stuhlmiller TJ, Sabater JR, et al. SPX-101 is a novel epithelial sodium channel-targeted therapeutic for cystic fibrosis that restores mucus transport. Am J Respir Crit Care Med. 2017;196:734–744. doi: 10.1164/rccm.201612-2445OC. [DOI] [PubMed] [Google Scholar]
- 57.Carson JL, Zhou L, Brighton L, Mills KH, Zhou H, Jaspers I, et al. Temporal structure/function variation in cultured differentiated human nasal epithelium associated with acute single exposure to tobacco smoke or E-cigarette vapor. Inhal Toxicol. 2017;29:137–144. doi: 10.1080/08958378.2017.1318985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis ES, Sassano MF, Goodell H, Tarran R. E-liquid autofluorescence can be used as a marker of vaping deposition and third-hand vape exposure. Sci Rep. 2017;7:7459. doi: 10.1038/s41598-017-07862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, et al. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem Res Toxicol. 2018;31:4–12. doi: 10.1021/acs.chemrestox.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 61.Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6:79–96. doi: 10.2174/157015908783769644. [DOI] [PMC free article] [PubMed] [Google Scholar]