Abstract
Rationale: Despite effective treatments, a large proportion of patients with asthma do not achieve sustained asthma control. The “preventable” burden associated with lack of proper control is likely taking a high toll at the personal and population level.
Objectives: We predicted the future excess health and economic burden associated with uncontrolled asthma among American adolescents and adults for the next 20 years.
Methods: We built a probabilistic model that linked state-specific estimates of population growth, aging, asthma prevalence, and asthma control levels. We conducted several meta-analyses to estimate the adjusted differences in healthcare resource use, quality-adjusted life years (QALYs), and productivity loss across control levels. We projected, nationally and at the state level, total direct and indirect (due to productivity loss) costs (in 2018 dollars) and QALYs lost because of uncontrolled asthma from 2019 to 2038.
Measurements and Main Results: Total 20-year direct costs associated with uncontrolled asthma are estimated to be $300.6 billion (95% confidence interval [CI], $190.1 billion–411.1 billion). When indirect costs are added, total economic burden will be $963.5 billion (95% CI, $664.1 billion–1,262.9 billion). American adolescents and adults will lose an estimated 15.46 million (95% CI, 12.77 million–18.14 million) QALYs over this period because of uncontrolled asthma. Across states, the average 20-year per capita costs due to uncontrolled asthma ranged from $2,209 (Arkansas) to $6,132 (Connecticut).
Conclusions: The burden of uncontrolled asthma is substantial and will continue to grow. Given that a substantial fraction of this burden is preventable, better adherence to evidence-informed asthma management strategies by care providers and patients has the potential to substantially reduce costs and improve quality of life.
Keywords: asthma, asthma control, costs, quality-adjusted life years, forecasting
At a Glance Commentary
Scientific Knowledge on the Subject
With proper care, the majority of asthma patients should achieve asthma control. However, studies have over and again demonstrated that, owing to suboptimal disease management, a substantial fraction of patients with asthma remain poorly controlled. While previous studies have evaluated the economic burden of asthma, to the best of our knowledge, no study previously evaluated the extra burden of uncontrolled versus controlled asthma at the population level.
What This Study Adds to the Field
By using a computer model that combined multiple sources of evidence, this study estimated and projected the economic and humanistic burden of asthma among U.S. adults. Uncontrolled asthma will cost the U.S. economy an estimated $300 billion (in 2018 dollar values) in the next 20 years in direct medical costs alone. This value increases to an estimated $963 billion if costs due to loss of work productivity are included. The impact on quality of life is projected to be equal to loss of 15.5 million years with full health. These results demonstrate the extent to which better adherence to evidence-informed asthma management strategies by care providers and patients can reduce costs and improve quality of life.
Asthma is a very common chronic disease globally. The prevalence of asthma has increased over the last decade in many regions of the world (1). Despite the fact that asthma imposes a substantial burden on patients and healthcare systems, it has not yet been identified as a healthcare priority in many countries (1). In the United States, there are approximately 26 million patients with physician-diagnosed asthma (2). Asthma cost the U.S. economy an estimated $81.9 billion in 2013 alone (3).
Conventional wisdom suggests that asthma is not a curable disease. Indeed, evidence indicates that airway hyperresponsiveness and inflammation persist in individuals whose asthma has been dormant for many years (4). Therefore, the contemporary asthma management paradigm is based on achieving symptom control and reducing the risk of exacerbations (5). It is widely accepted that through avoidance of triggers and use of inhaled antiinflammatory agents (namely inhaled corticosteroids), asthma can be controlled and exacerbation risk can be significantly reduced in the majority of patients (6). Achieving asthma control is associated with improvement in quality of life, reduction in medical costs, and better work performance (7, 8). Unfortunately, the reality of asthma care is highlighted by poor adherence to treatments and other disease management modalities (e.g., avoidance of triggers), resulting in a significant proportion of patients with asthma experiencing suboptimal asthma control (9).
A good understanding of the future burden of diseases can support the search for efficiency and equity in health care. Many studies have estimated the total burden of asthma in the United States (1, 3, 10). However, given the focus of contemporary asthma management is on achieving asthma control, the relevant figure of merit for policymaking and prioritizing future research is the added burden due to uncontrolled (vs. controlled) asthma, rather than the burden of asthma itself. In a small minority of patients, achieving symptom control can be difficult or out of reach (11). However, given the availability and efficacy of inexpensive management strategies to control asthma in the majority of patients, the excess burden between uncontrolled and controlled asthma is largely “preventable.” Such burden can be considered as the maximum space available for contemporary asthma management strategies to reduce asthma burden at the population level. The resources required for interventions and programs aimed at improving asthma control can be juxtaposed against their estimated impact on the burden of suboptimal asthma control to evaluate whether such programs are worth implementing.
The purpose of the present study was to document the current, and project the future, preventable economic and health burden associated with suboptimal asthma control among the U.S. adolescent and adult population for the next 20 years. We answered the question “How much cost could be saved, and quality of life could be improved, if all adolescent and adult patients with asthma in the United States achieve symptom control in the next 20 years?”
Some of the results of this study have been previously reported in the form of a preprint (https://www.biorxiv.org/content/10.1101/516740v1).
Methods
To enable projections, we reconciled evidence from multiple sources into a time-in-state computer model of asthma. The projection period was from 2019 to 2038 (20 yr). We adopted a societal perspective in the primary analysis; thus, costs were included no matter who had incurred them. The analyses were performed for the entire U.S. population ≥15 years of age, as well as at the state level. We defined asthma control according to the score on the Asthma Control Test (ACT) (12). ACT was used because major sources of evidence for this study used this instrument (13–19). This test classifies a patient’s asthma status into poorly controlled (score ≤ 15), not well controlled (score 16–19), and well controlled (score 20–25). As the focus was on achieving (well) controlled asthma, for the main analysis, the outcomes of very poorly controlled and not well controlled were combined into one category (henceforth referred to as “uncontrolled”). The supplementary material provides details of the methodology. Table 1 provides point estimates and probability distribution assigned to each model parameter.
Table 1.
Input Parameters for the Model
| Parameter | Point Estimate | Probability Distribution* | Source |
|---|---|---|---|
| Forecast of U.S. population growth and aging | — | — | U.S. National Population Projection (20, 21) (see online supplement) |
| Prevalence of asthma in United States across age, sex, and state | — | — | Global Burden of Disease studies (22, 23) (see online supplement) |
| Association between asthma control and age/sex† | Based on model calibration (see online supplement) | ||
| OR for well controlled (vs. very poorly controlled) | |||
| Age | 1.09 | Lognormal (0.08, 1.23) | — |
| Sex | 0.74 | Lognormal (−0.30, 1.32) | — |
| OR for not well controlled (vs. very poorly controlled) | |||
| Age | 0.99 | Lognormal (−0.01, 1.28) | — |
| Sex | 1.10 | Lognormal (0.09, 1.47) | — |
| Pooled OR of outpatient visit, uncontrolled (vs. well controlled) | 1.86 | Lognormal (0.62, 0.53) | Meta-analysis (see test and online supplement) |
| Pooled OR of emergency visit, uncontrolled (vs. well controlled) | 1.44 | Lognormal (0.36, 0.05) | Meta-analysis (see test and online supplement) |
| Pooled OR of hospitalization, uncontrolled (vs. well controlled) | 1.54 | Lognormal (0.43, 0.29) | Meta-analysis (see test and online supplement) |
| Pooled OR of medication use, uncontrolled (vs. well controlled) | 1.58 | Lognormal (0.45, 0.32) | Meta-analysis (see test and online supplement) |
| Average excess direct medical costs (per person-year),‡ uncontrolled (vs. well controlled) | 1,349 | Normal (1,349, 480) | Estimated through calibration, combining distribution of asthma controls and resource use ratios (above rows) with estimate of overall costs of asthma (3) |
| Pooled mean difference of average percentage of work time lost due to asthma, uncontrolled (vs. well controlled) | 12.70% | Lognormal (−2.06, 3.3) | Meta-analysis (see test and online supplement) |
| Average excess indirect medical costs (productivity loss, per person-year), uncontrolled (vs. well controlled) | 3,350 | Normal (3,350, 886) | Estimated through calibration, combining distribution of asthma controls and overall work impairment (above row) with estimate of average income (24) |
| Pooled mean difference of average QALYs lost due to asthma, uncontrolled (vs. well controlled) | 0.07 | Lognormal (−2.65, 0.01) | Meta-analysis (see test and online supplement) |
Definition of abbreviations: OR = odds ratio; QALY = quality-adjusted life year.
Normal (x, y): normal distribution with mean x and SD y; lognormal (x, y): lognormal distribution with mean x and SD y for the log-transformed values.
We modeled probability of three levels of control in the model but pooled “very poorly controlled” and “not well controlled” into “uncontrolled group” in order to report outcomes.
All costs are in 2018 U.S. dollars.
Data Sources
We used the following six major sources of evidence to populate the model. Details of the estimated parameters and methodologies are provided in online supplement (Section 1).
-
1.
Forecasts of population growth and aging during the projection period, nationally and for each state, were derived from the National Population Projections conducted by the Census Bureau–Population Division (20, 21).We used the midpoint of two sets of national population projections based on the 2010 Census for base case estimates.
-
2.
Estimates of the prevalence of asthma, stratified by age and sex for each state, were obtained from the Global Burden of Disease studies in 2016 (22, 23). These studies used the systematic analysis of published literature and other sources to estimate, using a consistent methodology, the burden of several health conditions, including asthma (24).
-
3.
Distribution of control levels in the asthma population, stratified by sex and age groups, were derived using calibration techniques from a recent study based on the U.S. National Health and Wellness Survey between 2011 and 2013 (13). We estimated the sex- and age-specific distribution of control levels by solving the coefficients of a multinomial logit equation (with prevalence of asthma control levels as the outcome, and sex and age as independent variables), such that the prevalence of asthma control levels matched U.S. National Health and Wellness Survey estimates within sex and age groups (Table 1). Details of calibration techniques are provided in the online supplement (Section 1.3).
-
4.
Healthcare resource use and quality-adjusted life year (QALY) differences across control levels were based on dedicated literature reviews and meta-analyses (13–19, 25–27) (online supplement, Section 1.4.1). We retrieved all relevant U.S.-based studies that assessed the adjusted association between asthma control and healthcare resource use (separately for healthcare provider visits, emergency visits, hospitalizations, and medication use), time lost from work due to health, and health-related quality of life. Studies were included if they controlled for potential confounding variables. There was statistically significant heterogeneity (at P = 0.10) for healthcare provider visits; therefore, a random-effects model was used to estimate the pooled adjusted odd ratios (ORs) of the association between asthma control and rate of healthcare provider visits. For all other outcomes, fixed-effects models were used. Details of the meta-analyses are provided in the online supplement (Section 1.4).
-
5.
We performed model calibration to convert estimates of resource use to direct costs. This method combines the distribution of asthma control levels in the population, ratio of resource use between uncontrolled and controlled asthma, and total direct costs of asthma in the United States, to solve for the costs of uncontrolled versus controlled asthma. The first two components were obtained as described above. For total costs of asthma in the United States, we relied on a recent large study (n = 214,000) by the CDC (3). This study used population-based sampling to estimate the overall costs of asthma. We solved for the costs of uncontrolled and controlled asthma that produced the desired ratio between the two that matched the results of our meta-analyses of resource use and summed up to the total costs of asthma. Details of this methodology are provided in the online supplement (Section 1.5).
-
6.
To estimate the difference in productivity loss (also referred to as indirect costs: the monetary value of time lost from work due to the health condition) between uncontrolled and controlled asthma, we obtained the sex- and age-specific wages as reported by the Bureau of Labor Statistics (28). For the youngest age group (15–19 yr), we used the reported value for the 16- to 19-year-old age groups. These estimates were combined with the pooled estimates of differences in work time lost because of asthma across control levels to calculate the monetary value of productivity loss due to uncontrolled asthma.
Analysis
All projections were made for the 2019 to 2038 period. The primary projections were made for the entire U.S. adolescent and adult population. State-level projections were provided as secondary results. In the main analysis, we estimated undiscounted total direct costs, indirect costs, and QALYs lost attributable to uncontrolled asthma. In a sensitivity analysis, we calculated outcomes after applying a 3% annual discount rate (29). All costs were adjusted to 2018 U.S. dollars using historical inflation rates (30). In another sensitivity analysis, we further separated uncontrolled asthma into very poorly controlled and not–well-controlled asthma. For each of direct costs, indirect costs, and QALYs lost, only two studies reported on the adjusted differences across such control levels; thus, this sensitivity analysis was not based on pooled estimates from the previously mentioned meta-analysis.
Uncertainty was modeled by assigning probability distributions to all input parameters (e.g., on the basis of the reported 95% confidence interval [CI]) and was propagated to the uncertainty in the projections using Monte Carlo simulation: within each simulation loop, we randomly drew from the distribution of all model parameters, performed model calibrations as described above, and projected the outcomes. Uncertainty was presented in terms of 95% CIs around point estimates of projections. A Web application was developed as an accompanying tool for this paper to enable fuller exploration of results. It can be accessed at http://resp.core.ubc.ca/ipress/burdenofasthmainus.
Results
Systematic Review and Meta-analysis of Resource Use across Control Levels
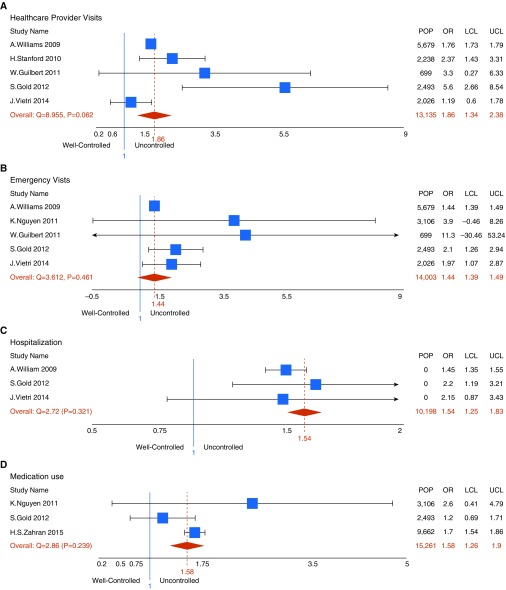
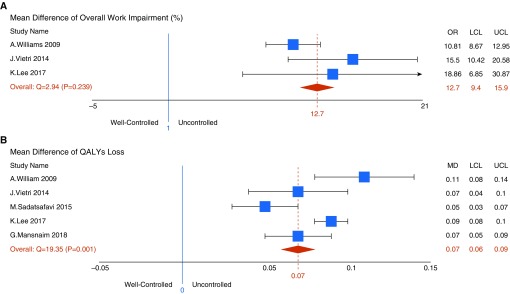
We identified 10 studies that reported on the adjusted differences in direct costs, indirect costs, or QALYs lost across control levels in the United States. Forest plots for adjusted ORs and mean differences are shown in Figures 1 and 2, respectively. For healthcare resource use, the pooled adjusted ORs in the uncontrolled versus the controlled group were as follows: 1.86 (95% CI, 1.34–2.38; five studies) for healthcare provider visits, 1.44 (95% CI, 1.39–1.49; five studies) for emergency room visits, 1.54 (95% CI, 1.25–1.80; three studies) for asthma-related hospitalizations, and 1.58 (95% CI, 1.26–1.90; three studies) for medication use (all P < 0.001). Combining these ratios with the distribution of control levels and overall costs of asthma (as described in the online supplement, Section 1.5), the estimated excess direct costs associated with uncontrolled versus controlled asthma were $1,349 (95% CI, $868–1,829) per patient-year. On average, a person with uncontrolled asthma lost an extra 12.7% of their work time compared with a person with controlled asthma (95% CI, 9.4–16.0%; P < 0.001; three studies). Assuming 52 work weeks in a year, this translates to a loss of 6.6 extra weeks of productivity loss per year. Excess indirect costs between the two groups were estimated to be $3,350 (95% CI, $2,464–4,236) per patient-year. Finally, the pooled estimate of the mean reduction in QALYs due to uncontrolled (vs. controlled) asthma was 0.07 (95% CI, 0.06–0.09; P < 0.001; five studies). Further details on these results are provided in the Tables E4–E7 and Figure E1 in the online supplement.
Figure 1.
Forest plots of adjusted odds ratio (OR) of (A) healthcare provider visits, (B) emergency department visits, (C) hospitalization, and (D) medication use associated with uncontrolled versus controlled asthma. LCL = lower confidence level; POP = population; UCL = upper confidence level.
Figure 2.
Forest plots of adjusted mean difference of (A) overall productivity loss and (B) quality-adjusted life years (QALYs) lost between uncontrolled and controlled asthma. LCL = lower confidence level; MD = mean difference; OR = odds ratio; UCL = upper confidence level.
Projection of Burden of Suboptimal Asthma Control
The size of the U.S. adolescent and adult population was projected to increase by 11%, from 266.87 million in 2019 to 298.22 million in 2038. In 2019, there will be 15.88 million adolescents and adults with asthma in the country, which is expected to increase to 17.65 million by 2038, representing 10% growth; 62% of patients with asthma will be women. During the 20-year projection window, in 175.32 million patient-years (52% of total patient-years of asthma), asthma will be uncontrolled. Total undiscounted direct costs of asthma across all control levels combined over 20 years are estimated to be $1,537 billion. The total undiscounted 20-year direct costs, indirect costs, and QALYs lost associated with uncontrolled asthma within sex and major age groups are provided in Tables 2 and E8.
Table 2.
The Undiscounted Projected 20-Year Direct Costs, Indirect Costs, and Quality-Adjusted Life Years Lost Associated with Suboptimal Control of Asthma within Sex Group
| Sex | Excess Direct Costs (95% CI) (Million $)* | Excess Indirect Costs (95% CI) (Million $)* | Excess QALYs Lost (95% CI) (Thousands) |
|---|---|---|---|
| F | |||
| 15–30 yr | 43.94 (27.74–60.14) | 188.46 (77.47–139.49) | 2,260 (1,865–2,655) |
| 30–65 yr | 91.12 (59.73–124.57) | 227.87 (161.06–288.68) | 4,686 (3,878–5,493) |
| >65 yr | 29.67 (18.68–40.66) | 24.62 (17.60–31.63) | 1,526 (1,253–1,798) |
| M | |||
| 15–30 yr | 40.06 (25.15–54.96) | 98.89 (70.12–127.66) | 2,060 (1,685–2,436) |
| 30–65 yr | 75.96 (47.72–104.20) | 187.50 (133.04–241.97) | 3,907 (3,195–4,618) |
| >65 yr | 19.88 (12.36–27.39) | 18.54 (13.08–24.00) | 1,022 (824–1,220) |
| Total | 300.65 (190.14–411.16) | 662.91 (474.02–851.8) | 15,462 (12,778–18,146) |
Definition of Abbreviations: CI = confidence interval; QALY = quality-adjusted life year.
All costs are in 2018 U.S. dollars.
Direct costs
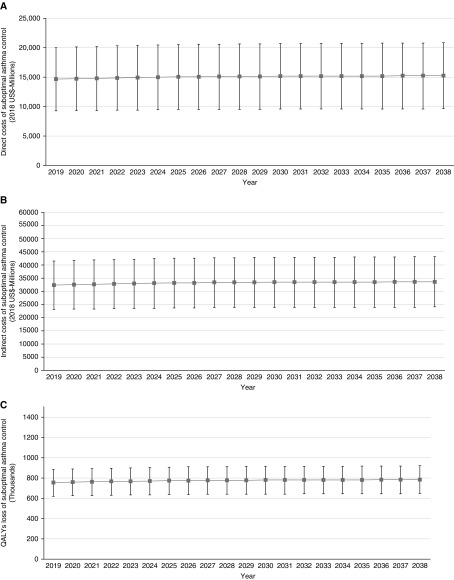
Trends of undiscounted excess direct costs due to uncontrolled asthma are shown in Figure 3A. In 2019, these costs are estimated to be $14.62 billion; this value is estimated to increase to $15.08 billion in 2028 and to $15.23 billion in 2038, representing a 4.2% growth during 20 years. Over this period, total direct costs associated with uncontrolled asthma are estimated to be $300.65 billion (95% CI, $190.1 billion–411.1 billion).
Figure 3.
Trends of (A) undiscounted direct costs, (B) undiscounted indirect costs, and (C) quality-adjusted life years (QALYs) lost because of uncontrolled (vs. controlled) asthma in the United States. Squares are point estimates and lines are 95% confidence intervals.
Indirect costs
Figure 3B depicts the projections for indirect costs associated with uncontrolled asthma. These costs are estimated to be $32.2 billion in 2019; this value is estimated to increase to $33.2 billion in 2028 and to $33.5 billion in 2038, corresponding to a 4.0% increase over 20 years. Total excess indirect costs associated with uncontrolled asthma control are estimated to be $662.9 billion (95% CI, $474 billion–851 billion) over the 20 years.
QALYs lost
The total undiscounted QALYs lost because of uncontrolled asthma are estimated to be 752,230 in 2019, increasing to 775,791 in 2028 and to 783,474 in 2038; this represents a 4.2% increase during the next 20 years. Over the 20 years, patients with asthma will lose an estimated 15.46 million (95% CI, 12.77 million–18.14 million) QALYs because of uncontrolled asthma. Trends of QALYs lost associated with uncontrolled asthma are shown in Figure 3C.
Sensitivity Analyses
When future values were discounted at the rate of 3%, projections of the burden of uncontrolled asthma changed as follows: total costs decreased to $210.4 billion (95% CI, $133 billion–287.7 billion), total indirect costs decreased to $463.9 billion (95% CI, $331.7 billion–596.1 billion), and total QALYs lost decreased to 10.82 million (95% CI, 8.94 million–12.70 million). An additional analysis that separated uncontrolled asthma into not well controlled and very poorly controlled indicated that the latter is responsible for an estimated 76% of direct costs, 83% of indirect costs, and 77% of the QALYs lost.
State-Level Analysis
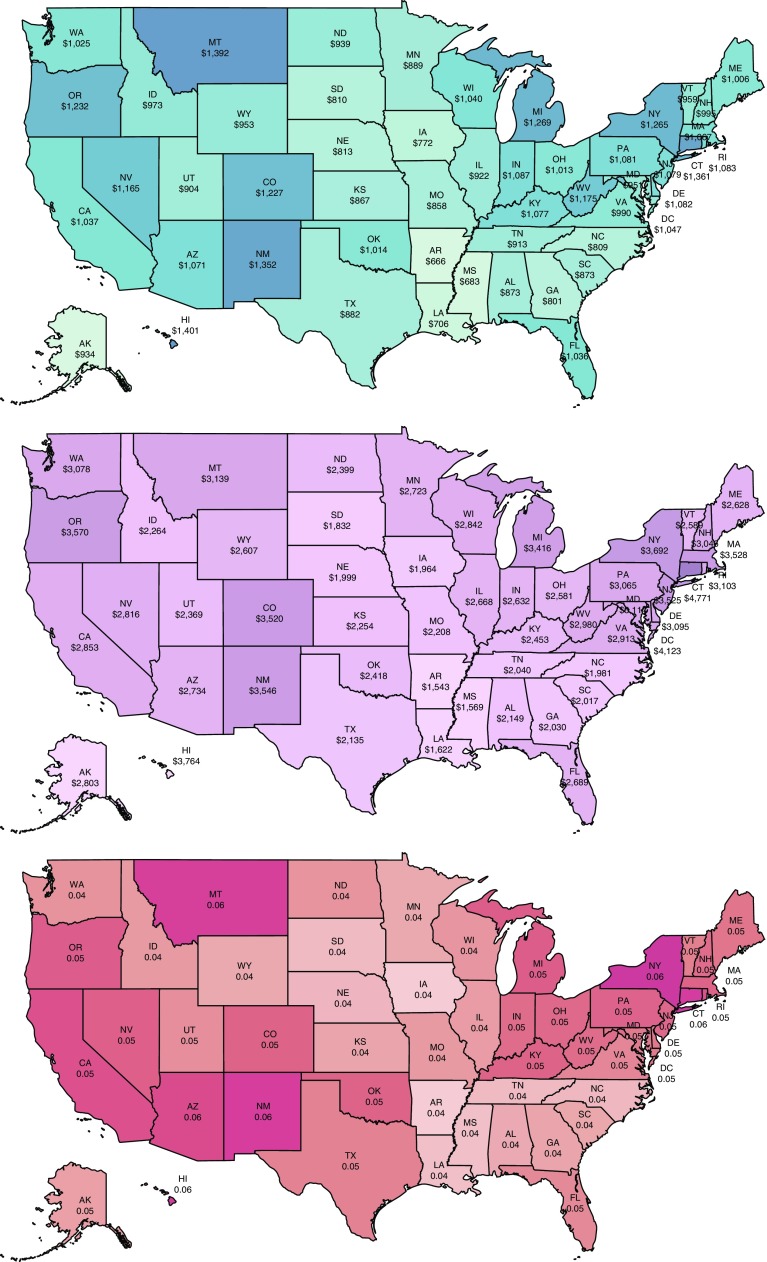
Results of state-level analyses are provided in Figure 4 and Table E9. To facilitate comparisons, we divided the total burden over the projected population size for each state to estimate the average projected “per capita” burden of asthma over 20 years. On this metric, Hawaii ranked the first in terms of the direct per capita costs of suboptimal asthma control ($1,401 over 20 yr), and Connecticut ranked the first in terms of indirect per capita costs ($4,771). Arkansas had the lowest direct and indirect per capita costs ($666 and $1,543, respectively). In terms of combined direct and indirect costs, Arkansas and Connecticut had the lowest and highest values, respectively ($2,209 and $6,132). Iowa had the lowest per capita QALYs lost (0.036 QALYs), and New York had the highest values (0.061). South Dakota and District of Columbia showed the largest and smallest increase in per capita total direct and indirect costs of uncontrolled asthma, at 8.74% and 6.94%, respectively, over 20 years.
Figure 4.
Average 20-year per capita estimates of (top) direct costs, (middle) indirect costs, and (bottom) quality-adjusted life years lost associated with uncontrolled (vs. controlled) asthma for each state (in 2018 U.S. dollars).
Discussion
We predicted the overall burden of uncontrolled asthma over the 2019 to 2038 period in the U.S. adolescent and adult population, in total and across states, if no paradigm shift occurs in contemporary asthma management. Of the 175.3 million patient-years with asthma in the next 20 years, 52% will be associated with uncontrolled asthma. Total estimated undiscounted direct costs of asthma, across all levels of control, will be $1,537 billion during this period; however, if all patients achieve asthma control during the next 20 years, $300.6 billion in direct costs can be saved. Our results therefore indicate that around 20% of direct costs of asthma can potentially be prevented by achieving asthma control in this population. When indirect costs are added, the potentially preventable burden of asthma was estimated to be $963.5 billion. In addition, there will be $15.46 million QALYs lost because of uncontrolled asthma over this period.
Indeed, strategies and interventions toward better asthma control are likely to be associated with costs and are unlikely to result in complete asthma control in all patients. As such, these values can be seen as population-based estimates of the maximum potential return on investment from strategies that are aimed at improving asthma control. Previous research from the United States has repeatedly showed that the prevalence of uncontrolled asthma is remarkably higher than the proportion of patients who fail to achieve asthma control in clinical trials (13–15). Multiple factors are considered as potential culprits for such discrepancy. Such factors include failure to avoid asthma triggers, unacceptably low adherence to controlled medication in patients with asthma (9), inefficient uptake of inhaled medications due to poor inhalation techniques (31), and overreliance on reliever versus controller use by both care providers and patients (32), to name a few.
To the best of our knowledge, ours is the first study that provides projections of asthma burden due to suboptimal asthma control. A 2005 study calculated burden of uncontrolled asthma in a managed care setting in United States and reported an incremental 2-year mean total cost of $7,760 (in 2015 U.S. dollars, equal to incremental 1-yr costs of $4,873 in 2018 U.S. dollars) between uncontrolled and controlled asthma (33). This estimate is higher than our baseline estimate ($4,699), but nonetheless within the 95% CI of our results. We have previously conducted a similar analysis in the Canadian context using a similar methodology (34). The undiscounted direct and indirect costs (in 2014 Canadian dollars [CAD$]) and QALYs lost attributable to suboptimal asthma control from 2014 to 2033 were, respectively, CAD$24.40 billion, CAD$256.09 billion, and 1.82 million (34). Adjusting for difference in the population sizes and currency exchange rates, the corresponding per capita estimates of these values are $523 (2018 U.S. dollars), $5,489.9 (2018 U.S. dollars), and 0.048. The loss of QALY was very similar between the U.S. and the Canadian study (0.04% different). On the other hand, higher per capita estimate of direct costs for the United States is likely due to the differences in healthcare resource use and the unit costs of medical services. As for the indirect costs, the overall extent of productivity loss is comparable between the two countries (5.07 h/wk in the United States vs. 4.10 h/wk in Canada). However, the average weekly income differs between the countries, resulting in different estimates of costs due to loss of productivity.
Nurmagambetov and colleagues (35) projected the economic burden of asthma in United States at the state level from 2015 to 2020. Total 5-year costs associated with asthma ranged from $336.7 million in District of Columbia to $26.3 billion in California (2014 U.S. dollars) (35). The corresponding 5-year per capita values in 2018 U.S. dollars range from $521 in District of Columbia to $1,106 in Connecticut. In contrast to the above-mentioned study which reported overall burden of asthma, we reported the excess burden due to suboptimal asthma control. Nevertheless, state-level per capita estimates of total asthma costs are also obtainable from our model and are very close to the estimates by Nurmagambetov and colleagues (35). In the above-mentioned study, Connecticut had the highest per capita economic burden of asthma over 5 years (35); our study also highlights this state as having the highest per capita costs of suboptimal asthma control over 20 years. Similarly, District of Columbia had the slowest increase rate in the indirect costs in both studies. Outside North America, our findings about the distribution of asthma control are similar to the trends observed in Europe. For example, we reported that asthma was uncontrolled in 52.13% of patients in the United States, and a similar value (56.5%) was recently reported by a European study (36).
A major strength of our study is the use of diverse sources of evidence (projection of population growth and aging, prevalence of asthma, distribution of asthma control levels, estimates of resource use and direct and indirect costs, and loss of quality of life across control levels). Furthermore, the choice of the analytical framework allowed us to translate such evidence and associated uncertainties into estimates of burden. We conducted multiple systematic reviews and meta-analyses to ensure our estimates of resource use differences across control levels reflect the best available evidence. The use of model calibration techniques allowed us to estimate parameters that were not obtainable directly but were estimated such that they remained compatible with evidence. For example, we solved for cost values associated with control levels that reflected the differences in healthcare resource use of various types between uncontrolled and controlled asthma, in the meantime adding up to the reported overall costs of asthma from a recent large and representative study (3). The limitations of this study should also be mentioned. Our study assumes the overall prevalence of asthma across sex and age groups will stay the same during the projection period. Although such a “default” assumption makes sense for estimating baseline projections for future burden of a disease, it is likely that the contribution of many risk factors (e.g., environmental and occupational pollutions) will change over this time, and the reported ranges (i.e., 95% CIs) in our projections do not reflect this source of uncertainty. Similarly to risk factors, novel therapies will arrive and guidelines and best-practice recommendations will change, adding further uncertainty to predictions that are not captured in our results. In addition, the quality of the projections cannot be higher than the quality of the underlying evidence. For example, the observed difference between the burden of uncontrolled and controlled asthma is likely to be confounded by many factors, namely the severity of underlying disease. As such, the estimate of the reduction in burden once asthma control is achieved relies on the extent original studies successfully controlled confounding factors. Furthermore, different studies have used different definitions of asthma control (e.g., on the basis of cutoffs on the ACT test or symptom control as defined by the Global Initiative for Asthma). Although we attempted to use a consistent definition of control, the availability of evidence forced us to relax this assumption at times. For example, in estimating the association between outcomes and controlled levels, we considered other definitions of asthma control, such as those put forth by the Global Initiative for Asthma and the National Asthma Education and Prevention Program (8, 26, 27), to be exchangeable with the definition on the basis of ACT.
Last but not least, our study did not include the pediatric population. Important differences in the definition of asthma and asthma control between the pediatric and adult populations, challenges in estimating quality of life in children, and nuances of evaluating healthcare resource and productivity loss by caregivers demand a significantly different methodology for such a study.
Although estimating the overall burden of a disease indeed has its own merits and should be rigorously pursued, evaluating the aspect of the burden that can realistically be prevented can more directly inform priorities in research, policy, and clinical care. Our findings highlight the sizeable potential for cost saving and improvement in quality of life associated with better asthma control. Research into improving adherence to existing medications should be put on an equal footing with investments in novel asthma therapies. Many of the effective asthma therapies are now off patent, and research and development in the private sector are understandably shifted toward developing novel therapies. However, healthcare management organizations, patient groups, governments, and society at large will benefit from investing in areas with proven capacities for improving patient outcomes and reducing costs.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Zafar Zafari, Zahra Sadat-Fatemi, Bryan Ng, and Ainsleigh Hill for their help in data collection and web application development.
Footnotes
Supported by Genome Canada, Genome British Columbia, and The Canadian Respiratory Research Network. M.S. receives salary support from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. The funders had no role in the study design, data collection, analysis, or preparation of the manuscript.
Author Contributions: J.M.F. and M.S. conceived the study question. M.Y. developed the analytic plan, performed the literature review, and conducted the analyses. M.S. supervised the study progress and provided regular feedback. A.A. and A.S. contributed to the study design and performed some of the statistical analyses. A.S. supervised statistical analyses. M.Y. wrote the first draft of the manuscript. All authors revised the manuscript and approved the final copy.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201901-0016OC on June 5, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Canadian Respiratory Research Network
References
- 1.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Data, statistics, and surveillance: asthma surveillance data. 2018 [updated 2018 Oct 3; accessed 2018 Aug 2]. Available from: https://www.cdc.gov/asthma/asthmadata.htm.
- 3.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 4.Aalbers R, Vogelmeier C, Kuna P. Achieving asthma control with ICS/LABA: a review of strategies for asthma management and prevention. Respir Med. 2016;111:1–7. doi: 10.1016/j.rmed.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma. 2018 GINA report: global strategy for asthma management and prevention. 2018 [accessed 2018 Oct 3]. Available from: www.ginasthma.org.
- 6.Bateman ED, Bousquet J, Braunstein GL. Is overall asthma control being achieved? A hypothesis-generating study. Eur Respir J. 2001;17:589–595. doi: 10.1183/09031936.01.17405890. [DOI] [PubMed] [Google Scholar]
- 7.Doz M, Chouaid C, Com-Ruelle L, Calvo E, Brosa M, Robert J, et al. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med. 2013;13:15. doi: 10.1186/1471-2466-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen HV, Nadkarni NV, Sankari U, Mital S, Lye WK, Tan NC. Association between asthma control and asthma cost: results from a longitudinal study in a primary care setting. Respirology. 2017;22:454–459. doi: 10.1111/resp.12930. [DOI] [PubMed] [Google Scholar]
- 9.Braido F.Failure in asthma control: reasons and consequences Scientifica 20132013549252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozano P, Sullivan SD, Smith DH, Weiss KB. The economic burden of asthma in US children: estimates from the National Medical Expenditure Survey. J Allergy Clin Immunol. 1999;104:957–963. doi: 10.1016/s0091-6749(99)70075-8. [DOI] [PubMed] [Google Scholar]
- 11. doi: 10.1183/09031936.00202013. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343–73. [Published erratum appears in Eur Respir J 2018;52:1352020.] [DOI] [PubMed] [Google Scholar]
- 12.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55:208–219. doi: 10.1080/02770903.2017.1316394. [DOI] [PubMed] [Google Scholar]
- 14.Williams SA, Wagner S, Kannan H, Bolge SC. The association between asthma control and health care utilization, work productivity loss and health-related quality of life. J Occup Environ Med. 2009;51:780–785. doi: 10.1097/JOM.0b013e3181abb019. [DOI] [PubMed] [Google Scholar]
- 15.Vietri J, Burslem K, Su J. Poor asthma control among US workers: health-related quality of life, work impairment, and health care use. J Occup Environ Med. 2014;56:425–430. doi: 10.1097/JOM.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 16.Stanford RH, Gilsenan AW, Ziemiecki R, Zhou X, Lincourt WR, Ortega H. Predictors of uncontrolled asthma in adult and pediatric patients: analysis of the Asthma Control Characteristics and Prevalence Survey Studies (ACCESS) J Asthma. 2010;47:257–262. doi: 10.3109/02770900903584019. [DOI] [PubMed] [Google Scholar]
- 17.Guilbert TW, Garris C, Jhingran P, Bonafede M, Tomaszewski KJ, Bonus T, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48:126–132. doi: 10.3109/02770903.2010.535879. [DOI] [PubMed] [Google Scholar]
- 18.Zahran HS, Bailey CM, Qin X, Moorman JE. Assessing asthma control and associated risk factors among persons with current asthma: findings from the child and adult Asthma Call-back Survey. J Asthma. 2015;52:318–326. doi: 10.3109/02770903.2014.956894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosnaim G, Lee LK, Carpinella C, Ariely R, Gabriel S, Lugogo NL. The impact of uncontrolled asthma on quality of life among treated, adherent patients with persistent asthma [abstract] J Allergy Clin Immunol. 2018;141:AB222. [Google Scholar]
- 20.U.S. Census Bureau. Population projections [updated 2018 Oct 27; accessed 2018 Sept 10] Available from: https://www.census.gov/programs-surveys/popproj.html.
- 21.Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. Population estimates and projections. 2017. [accessed 2018 Sept 15]. Available from: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf.
- 22.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Health Metrics and Evaluation (IHME). GBD compare. Seattle, WA: IHME, University of Washington. 2015 [accessed 2018 Sept 15]. Available from: http://vizhub.healthdata.org/gbd-compare.
- 24.Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, et al. US Burden of Disease Collaborators. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–1472. doi: 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold LS, Smith N, Allen-Ramey FC, Nathan RA, Sullivan SD. Associations of patient outcomes with level of asthma control. Ann Allergy Asthma Immunol. 2012;109:260–265, e2. doi: 10.1016/j.anai.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Sadatsafavi M, McTaggart-Cowan H, Chen W, Mark FitzGerald J Economic Burden of Asthma (EBA) Study Group. Quality of life and asthma symptom control: room for improvement in care and measurement. Value Health. 2015;18:1043–1049. doi: 10.1016/j.jval.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen K, Zahran H, Iqbal S, Peng J, Boulay E. Factors associated with asthma control among adults in five New England states, 2006-2007. J Asthma. 2011;48:581–588. doi: 10.3109/02770903.2011.576744. [DOI] [PubMed] [Google Scholar]
- 28.US Bureau Of Labor Statistics. Highlights of Women’s Earnings in 2012. Washington, D.C.; 2013 [accessed 2018 Sept 20]. Available from: https://www.bls.gov/opub/reports/womens-earnings/archive/womensearnings_2012.pdf.
- 29.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 30.US Inflation Calculator [updated 2018 Dec 11; accessed 2018 Dec 15] Available from: https://www.usinflationcalculator.com/
- 31.Lavorini F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy. 2013;2013:102418. doi: 10.1155/2013/102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahidi N, Fitzgerald JM. Current recommendations for the treatment of mild asthma. J Asthma Allergy. 2010;3:169–176. doi: 10.2147/JAA.S14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan SD. The burden of uncontrolled asthma on the U.S. health care system. Manag Care. 2005;14:4–7. [Discussion, pp. 25–27.] [PubMed] [Google Scholar]
- 34.Zafari Z, Sadatsafavi M, Chen W, FitzGerald JM. The projected economic and health burden of sub-optimal asthma control in Canada. Respir Med. 2018;138:7–12. doi: 10.1016/j.rmed.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Nurmagambetov T, Khavjou O, Murphy L, Orenstein D. State-level medical and absenteeism cost of asthma in the United States. J Asthma. 2017;54:357–370. doi: 10.1080/02770903.2016.1218013. [DOI] [PubMed] [Google Scholar]
- 36.Braido F, Brusselle G, Guastalla D, Ingrassia E, Nicolini G, Price D, et al. LIAISON Study Group. Determinants and impact of suboptimal asthma control in Europe: the International Cross-Sectional and Longitudinal Assessment on Asthma Control (LIAISON) study. Respir Res. 2016;17:51. doi: 10.1186/s12931-016-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.