Abstract
Rationale: The loss of pulmonary endothelial cells in emphysema is associated with increased lung ceramide. Ceramide perturbations may cause adaptive alterations in other bioactive sphingolipids, with pathogenic implications. We previously reported a negative correlation between emphysema and circulating glycosphingolipids (GSLs). Glucosylceramide (GlcCer), the initial GSL synthesized from ceramide by GCS (GlcCer synthase), is required for embryonic survival, but its role in the lung is unknown.
Objectives: To determine if cigarette smoke (CS) alters lung GlcCer and to elucidate the role of GCS in lung endothelial cell fate.
Methods: GlcCer was measured by tandem mass spectrometry in BAL fluid of CS- or elastase-exposed mice, and GCS was detected by Western blotting in chronic obstructive pulmonary disease lungs and CS extract–exposed primary human lung microvascular endothelial cells (HLMVECs). The role of GlcCer and GCS on mTOR (mammalian target of rapamycin) signaling, autophagy, lysosomal function, and cell death were studied in HLMVECs with or without CS exposure.
Measurements and Main Results: Mice exposed to chronic CS or to elastase, and patients with chronic obstructive pulmonary disease, exhibited significantly decreased lung GlcCer and GCS. In mice, lung GlcCer levels were negatively correlated with airspace size. GCS inhibition in HLMVEC increased lysosomal pH, suppressed mTOR signaling, and triggered autophagy with impaired lysosomal degradation and apoptosis, recapitulating CS effects. In turn, increasing GlcCer by GCS overexpression in HLMVEC improved autophagic flux and attenuated CS-induced apoptosis.
Conclusions: Decreased GSL production in response to CS may be involved in emphysema pathogenesis, associated with autophagy with impaired lysosomal degradation and lung endothelial cell apoptosis.
Keywords: sphingolipids, apoptosis, chronic obstructive pulmonary disease, autophagy, endothelium
At a Glance Commentary
Scientific Knowledge on the Subject
Excessive ceramide triggers pulmonary endothelial cell apoptosis, which is implicated in emphysema pathogenesis. Ceramide is actively metabolized to other sphingolipids with distinct structural or signaling functions. We previously reported an inverse correlation between plasma glycosphingolipid (GSL) levels and emphysema. However, the role of GSLs in lung structural cells is unknown.
What This Study Adds to the Field
We discovered that the initial step in the GSL synthesis from ceramide, catalyzed by GCS (glucosylceramide [GlcCer] synthase), is inhibited by cigarette smoke exposure and in chronic obstructive pulmonary disease. We show that inhibition of GCS was sufficient to trigger autophagy with impaired lysosomal degradation and suppressed mTOR (mammalian target of rapamycin) signaling that culminates in cell death. Increasing levels of GlcCers markedly improved autophagic flux and survival during cigarette smoke exposure, which suggests that this metabolic pathway may be of pathogenic and therapeutic importance in emphysema.
The mechanisms driving the development and progression of chronic obstructive pulmonary disease (COPD), in particular pulmonary emphysema, remain incompletely understood. The role of sphingolipids in COPD pathogenesis has been supported by studies implicating excessive ceramide accumulation that, when unopposed by compensatory increases in S1P (sphingosine 1-phosphate), triggers lung structural cell death that explains the alveolar cell loss characteristic of emphysema (1–4). More recently, metabolomic studies have identified a significant inverse association between glycosphingolipids (GSLs)—glycosylated metabolites of ceramide—and the presence of emphysema in smokers (5). However, with the exception of the report by Vij and colleagues (6), which implicated GSL in lung inflammation in cigarette smoke (CS)-exposed C57BL/6 mice, their roles in human lung cell function during homeostasis or stress, such as CS exposure, remain unknown.
Given that GSLs are mediators of proangiogenic effects of VEGF (vascular endothelial growth factor) in endothelial cells (7), and that VEGF is required for the maintenance of lung alveolar structures (8), it is conceivable that GSLs are required for lung vascular endothelial cell survival. Indeed, deficiency of GCS (glucosylceramide [GlcCer] synthase), which mediates the first enzymatic step in GSL synthesis, producing GlcCer from ceramide, is associated with embryonic lethality (9). GCS promotes cellular survival during stress, an effect that may be linked to autophagy. For example, GCS expression correlates with resistance of cancer cells to treatment, which may be explained by the ability of GCS to inhibit autophagy-associated apoptosis of cells treated with chemotherapeutics (10). However, in neurons, GCS inhibition alone enhanced autophagy with increased lysosomal degradation by inhibiting AKT (Ak strain–transforming) and mTOR (mammalian target of rapamycin) signaling (11). These effects may be at play in COPD, because autophagy markers are increased in COPD lungs and autophagy-related cell death is important in emphysema pathogenesis (12–14). CS exposure both triggers autophagy and impairs its appropriate completion via lysosomal degradation of autophagolysosomes, also known as autophagic flux, in lung epithelial and endothelial cells and macrophages that comprise the alveolar structures (15, 16). We therefore hypothesized that GCS and GlcCer are essential for lung endothelial cell survival, during both homeostasis and stress linked to CS exposure.
Using COPD lung tissue and primary human lung microvascular endothelial cells (HLMVECs), we report here that COPD lungs have decreased GCS levels, and that increasing GlcCer levels is sufficient to improve cell survival during CS exposure. Some of the results of these studies have been previously reported in the form of abstracts (17, 18).
Methods
Detailed methods are provided in the online supplement.
Reagents
All chemical reagents were from MilliporeSigma, unless otherwise stated.
Animal Studies
Animal studies were approved by the Animal Care and Use Committee of National Jewish Health. C57BL/6 mice and BALB/c mice were obtained from the Jackson Laboratory. C57BL/6 mice were exposed to CS or ambient air control (AC) for 6 months (19). BALB/c mice were injected intratracheally with porcine pancreatic elastase (Elastin Products Co.) or saline. After 6 months of CS exposure or 28 days of elastase administration, mouse lungs were obtained and morphometric analysis was performed (1).
Cells
Primary HLMVECs isolated from nonsmokers were obtained from Lonza and maintained in the culture media (CC3202, 5% fetal bovine serum; Lonza) at 37°C with 5% CO2. At least three independent experiments using distinct donors (biological replicates) were performed. Culture media was changed to media containing 2% fetal bovine serum for 2 hours before treatment. Transfection methods are described in the online supplement.
CS Extract Preparation
CS extract (CSE; 100%) was prepared by bubbling smoke from two cigarettes or AC into 20 ml of phosphate-buffered saline, followed by pH adjustment to 7.4 and 0.2-μm filtration (20).
Sphingolipid Quantification and GCS Activity Assay
Sphingolipids in either BAL fluid (BALF), plasma of mice, or whole-cell lysates from HLMVECs were measured using liquid chromatography with tandem mass spectrometry (LC-MS/MS), as described in the online supplement. The assay for GCS enzyme activity was performed according to a previously published method (21) with modifications detailed in the online supplement.
Cell Viability, Lactate Dehydrogenase Release, Cathepsin B Enzymatic Activity, and Endolysosomal pH Assay
Cell viability was assessed by Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) or trypan blue exclusion assay (Thermo Fisher Scientific). LDH (lactate dehydrogenase) release was measured by the LDH detection kit (Roche Diagnostics). Cathepsin B enzymatic activity was measured using the Magic Red Cathepsin B detection kit (ImmunoChemistry Technologies). Endolysosomal pH was determined using Lysosensor yellow/blue (Thermo Fisher Scientific) (22), as described in the online supplement.
Human Lungs
COPD lungs (n = 7) were obtained from the Lung Tissue Research Consortium. Lung tissues from never-smokers (n = 6) and nondiseased smokers (n = 7) were obtained from donors whose lungs were donated for medical research through the National Disease Research Interchange and the International Institute for the Advancement of Medicine, as described in the online supplement. All protocols were approved by the Institutional Review Board at National Jewish Health.
Western Blot
Western blotting was performed (23) using antibodies described in the online supplement.
Immunofluorescence
After treatments, cells were incubated with DAPGreen (Dojindo Molecular Technologies) and LysoTracker deep red (Thermo Fisher Scientific) (24), as described in the online supplement.
Statistical Analysis
All data were expressed as mean (±SEM). Statistical analyses were performed with SPSS version 16.0 software (SPSS Inc.) and Prism 6 (GraphPad Software Inc.) using the Mann-Whitney U test or one-way ANOVA with Tukey’s multiple comparisons test. A P value less than 0.05 was considered statistically significant.
Results
Decreased GlcCer in Experimental Emphysema Induced by CS or Elastase in Mice
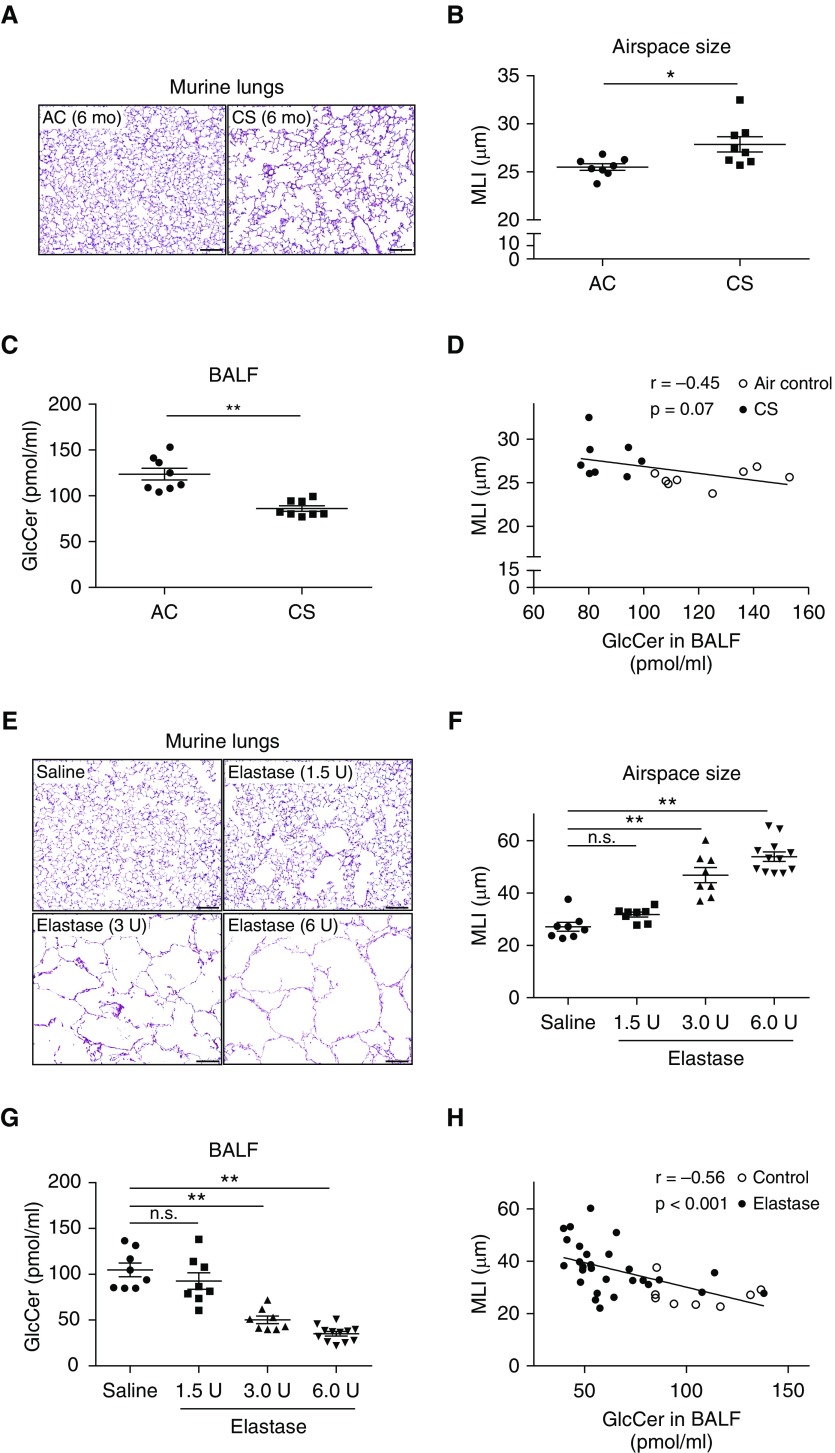
We previously identified an inverse association between emphysema and plasma GSL levels, measured by untargeted metabolomic assays (5). To determine whether a similar relationship exists between emphysema and lung GSL, we used two experimental models of emphysema. Using targeted quantitative mass spectrometry, GlcCer levels were measured in BALF and plasma from animal models of CS- and elastase-induced emphysema. C57BL/6 mice (2-mo-old male and female) were exposed to CS or AC for 6 months. As expected, when compared with the AC group, chronic CS exposure significantly increased airspace size, measured by mean linear intercept (MLI; Figures 1A and 1B). Chronic CS exposure significantly decreased GCS levels in lung tissues (Figure E1A in the online supplement), and GlcCer levels in BALF (Figure 1C) and plasma (Figure E1B). There was a negative correlation between MLI and BALF GlcCer levels in C57BL/6 mice (Figure 1D). We then measured GlcCer levels in BALB/c mice (2-mo-old male) that received intratracheal instillations of elastase (1.5, 3, or 6 U/mouse) or saline control. At 28 days after administration, elastase markedly increased airspace size (Figures 1E and 1F) and significantly decreased, in a dose-dependent manner, GlcCer levels in the BALF (Figure 1G) and in plasma of mice (Figure E1C) compared with control. There was a significant negative correlation between MLI and BALF GlcCer levels in BALB/c mice (Figure 1H).
Figure 1.
Effect of cigarette smoke (CS) or elastase administration on lung glucosylceramide (GlcCer) levels in mice. (A–D) Morphology and GlcCer levels in C57BL/6 mice exposed to CS or ambient air control (AC) for 6 months. (A) Representative hematoxylin and eosin–stained lung sections of lung parenchyma, showing airspace enlargement and alveolar wall destruction in CS-exposed mice. Scale bars, 100 μm. (B) Air space size measured by mean linear intercept (MLI; mean ± SEM; *P < 0.05 by Mann-Whitney U test; n = 8). (C) GlcCer levels measured by liquid chromatography with tandem mass spectrometry in BAL fluid (BALF) (mean ± SEM; **P < 0.01 by Mann-Whitney U test; n = 8). (D) Correlation between air space size, measured by MLI, and GlcCer levels in BALF of CS-exposed C57BL/6 mice (n = 8). Pearson correlation analysis; each dot represents an individual mouse. (E–H) Morphology and GlcCer levels in BALB/c mice at 28 days after intratracheal instillations of elastase (1.5 or 3 or 6 U/mouse) or saline control. (E) Representative hematoxylin and eosin–stained lung sections, showing airspace enlargement and alveolar wall destruction in the elastase group. Scale bars, 100 μm. (F and G) MLI (F) and GlcCer levels in BALF (G) (mean ± SEM; **P < 0.01, one-way ANOVA with Tukey’s multiple comparisons test; n = 8–12). (H) Negative correlation between MLI and GlcCer levels in BALF of elastase-treated BALB/c mice (n = 8–12). Pearson correlation analysis; each dot represents an individual mouse. n.s. = not significant.
GCS Inhibition Decreases GlcCer Levels and Causes Apoptosis in Primary HLMVECs
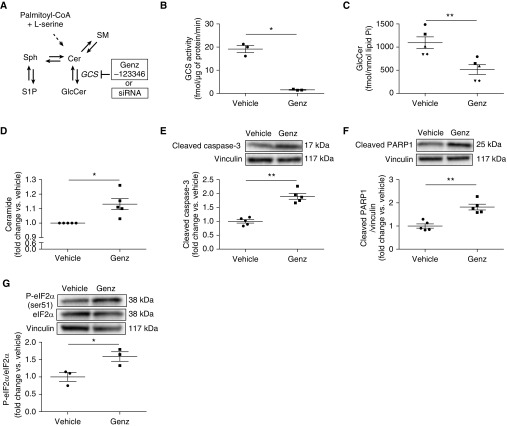
Given the involvement of lung endothelial cell in emphysema pathogenesis, we determined if GCS is important in maintaining primary HLMVEC homeostasis. Genz-123346 (Genz; GCS inhibitor) has been demonstrated to selectively inhibit GCS in several cell types (25–27). To determine the optimal concentration of Genz for use in HLMVEC experiments, we assessed the effect of Genz on cell survival and GCS activity. HLMVECs were treated with Genz (1, 2, 5, or 10 μM) or vehicle (DMSO) for 6 hours. Genz had a dose-dependent effect on cell viability and LDH release (Figures E2A and E2B). To confirm that Genz inhibits GCS activity in HLMVECs, we studied the lowest Genz concentration (2 μM) that significantly decreased cell viability. After Genz treatment, LC-MS/MS was used to measure cellular GCS activity and levels of several key sphingolipids (Figure 2A). Genz markedly suppressed GCS activity (Figure 2B) and decreased GlcCer levels (Figure 2C). Genz increased ceramide levels (Figures 2D and E2C) but did not significantly alter S1P, sphingomyelin, or sphingosine levels (Figures E2D–E2F). These results demonstrate that Genz selectively inhibited GCS in HLMVECs. We next assessed whether Genz induces lung endothelial cell apoptosis using Western blotting for several apoptosis markers. Genz significantly increased Bax/Bcl-2 ratio (Figure E2G), cleaved caspase-3, and cleaved PARP1 (poly [ADP-ribose] polymerase 1) (Figures 2E and 2F). CS exposure or ceramide accumulation can induce apoptosis in human endothelial cells via endoplasmic reticulum (ER) stress (28, 29). Genz increased the phosphorylation of eIF2α (eukaryotic translation initiation factor 2α) and IRE1α (inositol-requiring enzyme 1α) levels, (Figure 2G and Figure E2H). These results indicate that GCS inhibition was sufficient to increase apoptosis and ER stress in HLMVECs.
Figure 2.
Effect of GCS (glucosylceramide [GlcCer] synthase) inhibition on GlcCer levels and apoptosis in primary human lung microvascular endothelial cells. (A) Schematic of sphingolipid metabolism; GCS inhibition was achieved with Genz-123346 (Genz) or siRNA against GCS. (B–D) GCS activity (B), GlcCer levels (C), and ceramide levels (D), measured by liquid chromatography with tandem mass spectrometry in primary human lung microvascular endothelial cells treated with vehicle (DMSO) or Genz (2 μM; 6 h) (n = 3–5). (E–G) Representative Western blots and densitometry of cleaved caspase-3 (E), cleaved PARP1 (F), and phospho (p)- and total eIF2α (G) (n = 3–5). Vinculin was used as loading control. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by Mann-Whitney U test. SM = sphingomyelin; Sph = sphingosine.
GCS Inhibition Suppresses mTOR Signaling in HLMVECs
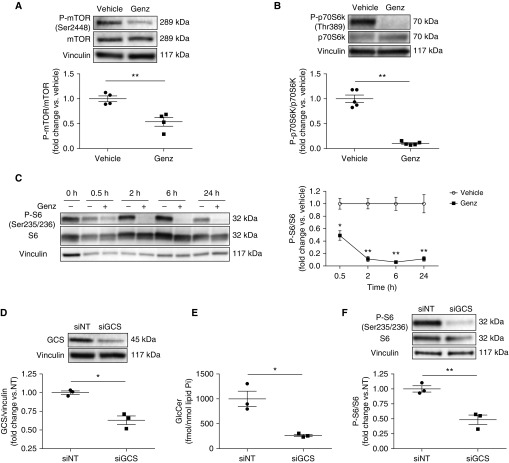
One of the first cellular responses to ER stress includes mTOR inhibition and autophagy induction (30). To further understand how GCS controls HLMVEC fate, we determined the effect of GCS inhibition on mTOR signaling. HLMVECs were treated with vehicle or Genz for up to 24 hours, and the phosphorylation of mTOR and its downstream targets, p70S6K (p70 ribosomal protein S6 kinase) and S6 (S6 ribosomal protein), were assessed by Western blotting (Figure E3A). Genz markedly decreased the phosphorylation of mTOR and p70S6K (Figures 3A and 3B) resulting in marked inhibition of S6 phosphorylation that began as early as 30 minutes and persisted for up to 24 hours (Figure 3C). Because pharmacological approaches might exhibit off-target effects, we complemented these studies with siRNA-mediated GCS knockdown. HLMVECs were transfected with 5 nM of control siRNA (siNT) or siRNA against GCS (siGCS) for more than 24 hours. After treatments, GCS mRNA and protein expression were both decreased, as measured by real-time qRT-PCR (Figure E3B) and Western blotting (Figure 3D), respectively. Similar to Genz, siGCS significantly decreased GlcCer (Figure 3E) and increased ceramide levels (Figures E2C–E2F). Furthermore, siGCS decreased S6 phosphorylation and cell viability and increased LDH release (Figure 3F and Figures E3G and E3H). These results corroborate our results with Genz, and indicate that GCS is required for HLMVEC survival. To assess if GCS inhibition impacted upstream targets of mTOR signaling (Figure E3A), we measured phosphorylation of Akt and AMPK (adenosine monophosphate–activated protein kinase). GCS inhibition did not change the phosphorylation of Akt or AMPK (Figures E3I and E3J), indicating that it may affect mTOR signaling at the level of mTOR activation.
Figure 3.
Effect of GCS inhibition on mTOR signaling in human lung microvascular endothelial cells (HLMVECs). (A–C) Representative Western blots and densitometric analysis of p-mTOR and total mTOR (A) and p-p70S6K and total p70S6K (B) after treatment with vehicle (DMSO) or Genz (2 μM; 6 h) (n = 4–5); and p-S6 and total S6 (C) after treatment with vehicle or Genz (2 μM) for up to 24 hours (n = 3–5). (D) Representative Western blots and densitometric analysis of GCS in HLMVECs transfected with 5 nM of control siRNA (siNT) or siRNA against GCS (siGCS) for 24 hours (n = 3). (E) GlcCer levels measured by liquid chromatography with tandem mass spectrometry in HLMVECs transfected with 5 nM of siNT or siGCS for 72 hours (n = 3). (F) Representative Western blots and densitometric analysis of p-S6 and total S6 in HLMVECs transfected with 5 nM of siNT or siGCS for 24 hours (n = 3). Western blots shown are representative of at least three independent experiments. Vinculin was used as loading control. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by Mann-Whitney U test. GCS = glucosylceramide synthase; Genz = Genz-123346; GlcCer = glucosylceramide; mTOR = mammalian target of rapamycin.
GCS Inhibition Impairs Lysosomal Function and Autophagic Flux in HLMVECs
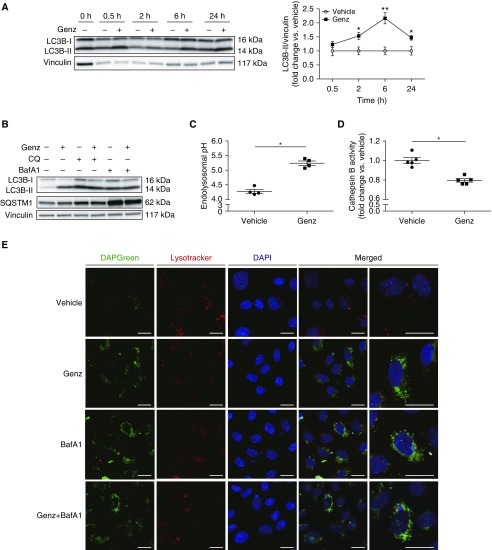
Because GCS inhibition markedly repressed mTOR, which is integral to the lysosomal nutrient-sensing complex that provides key input into autophagy, we examined the effect of GCS inhibition on autophagy. After Genz or vehicle treatment for up to 24 hours, we assessed LC3B-II protein, a marker for autophagosome formation (31). Genz significantly increased LC3B-II levels, with a peak at 6 hours (Figure 4A). Elevations of LC3B-II may also reflect decreased lysosomal fusion and degradation of autophagosomes (known as flux) (31). We therefore assessed the autophagic flux by cotreating HLMVECs with Genz (2 μM, 6 h) and lysosomal inhibitors, CQ (chloroquine; 20 μM, 6 h) or BafA1 (bafilomycin A1; 5 nM, 6 h), followed by Western blotting for p62-SQSTM1 (sequestosome-1), an autophagosome cargo protein involved in selective autophagy (31), as well as LC3B-II. Treatment with Genz alone increased both LC3B-II and SQSTM1 (Figure 4B and Figures E4A and E4B), suggesting decreased lysosomal degradation. Cotreatment with either CQ or BafA1 significantly increased SQSTM1 and LC3B-II above Genz treatment alone, suggesting that the inhibition of autophagic flux by Genz was submaximal (Figure 4B and Figures E4A and E4B). siRNA-mediated GCS knockdown had similar effects as Genz on autophagic flux in HLMVECs (Figure E4C). To explore potential mechanisms that link GCS inhibition to autophagic flux impairment, we focused our studies on lysosomal function. GCS inhibition with Genz significantly increased endolysosomal pH (Figure 4C) and decreased cathepsin B activity in HLMVECs (Figure 4D), suggesting that GCS is necessary for lysosomal homeostasis. As a complementary study of autophagy flux, we used immunofluorescence with DAPGreen and LysoTracker deep red to visualize autophagosomes and lysosomes, respectively. HLMVECs treated with Genz for 6 hours exhibited increased abundance of green punctate vesicles (autophagosomes) and reduced number of red punctate vesicles (lysosomes), with sparse colocalization of autophagosomes with lysosomes (Figure 4E). A similar pattern was observed after either BafA1 or combined Genz and BafA1 treatment. Because LysoTracker deep red accumulates in highly acidic organelles (32), and cathepsin B requires an acidic pH for proteinase activity, our results suggest that Genz-induced lysosomal dysfunction is associated with impaired lysosomal acidification.
Figure 4.
Effect of GCS inhibition on lysosomal function and autophagic flux in human lung microvascular endothelial cells (HLMVECs). (A and B) Representative Western blots and densitometry of (A) LC3B measured in HLMVECs treated with vehicle (DMSO) or Genz (2 μM) for up to 24 hours (n = 3–5) and (B) SQSTM1 in HLMVECs treated with vehicle (DMSO) or Genz (2 μM; 6 h) while exposed to vehicle (DMSO), CQ (chloroquine; 20 μM), or BafA1 (bafilomycin A1; 5 nM) for 6 hours (n = 3). Vinculin was used as loading control. (C) Endolysosomal pH measured in HLMVECs treated with vehicle or Genz (2 μM; 6 h) using Lysosensor yellow/blue (5 μM; 5 min; 37°C) (n = 4). (D) Cathepsin B activity measured in HLMVECs treated with vehicle or Genz (2 μM; 6 h) using Magic Red Cathepsin B detection kit (n = 5). (E) Representative immunofluorescence images of HLMVECs treated with vehicle, Genz (2 μM), BafA1 (5 nM), or Genz (2 μM) plus BafA1 (5 nM) for 6 hours. After treatments, cells were incubated with DAPGreen (50 nM) and LysoTracker deep red (25 nM) for 30 minutes. Scale bars, 20 μm. Western blots and immunofluorescence images are representative of at least three independent experiments. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by Mann-Whitney U test. GCS = glucosylceramide synthase; Genz = Genz-123346.
CS Inhibits GCS Activity in HLMVECs
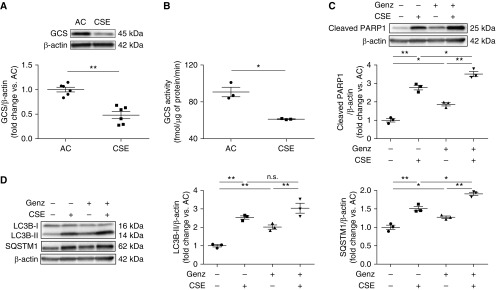
Having shown that GCS is required for HLMVECs homeostasis, we assessed whether CS exposure directly inhibits GCS in HLMVECs. Compared with extract from AC, exposure to aqueous CSE (3%) significantly decreased both GCS protein (Figure 5A) and GCS activity (Figure 5B). We then determined if CS exposure recapitulates the effects on GCS inhibition in HLMVECs. Similar to Genz, 3% CSE decreased cell viability and increased LDH release, Bax/Bcl-2 ratio, cleaved caspase-3 (Figures E5A and E5D), and cleaved PARP1; addition of Genz to CSE further increased PARP1 cleavage (Figure 5C). Furthermore, 3% CSE markedly decreased S6 phosphorylation (Figure E5E) and increased LC3B-II and SQSTM1 (Figure 5D), suggesting impairment of autophagic flux, which was confirmed using BafA1 treatment and fluorescence microscopy (Figures E5F and E5G). Addition of Genz to CSE further augmented SQSTM1 (Figure 5D), suggesting increased inhibition of lysosomal degradation in CS-exposed HLMVECs. These results indicate that inhibition of GCS activity in HLMVECs may be a mechanism by which CSE perturbs mTOR signaling, triggers autophagy with impaired autophagic flux, and increases apoptosis.
Figure 5.
Effect of cigarette smoke extract (CSE) on GCS levels, apoptosis, and autophagy in human lung microvascular endothelial cells (HLMVECs). (A) Representative Western blots and densitometry analysis of GCS in HLMVECs exposed to ambient air control (AC) or CSE (3%) for 6 hours (n = 6). (B) GCS activity measured by liquid chromatography with tandem mass spectrometry in HLMVECs exposed to AC or CSE (3%, 18 h) (n = 3). (C and D) Representative Western blots and densitometry of cleaved PARP1 (C) and LC3B and SQSTM1 (D) in HLMVECs exposed to AC, 3% CSE, Genz (2 μM), or 3% CSE plus Genz (2 μM) for 6 hours (n = 3). β-Actin was used as loading control. Western blots are representative of at least three independent experiments. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by one-way ANOVA with Tukey’s multiple comparisons test or Mann-Whitney U test. GCS = glucosylceramide synthase; Genz = Genz-123346; n.s. = not significant.
GlcCer Treatment Attenuates Apoptosis in GCS-inhibited or CS-exposed HLMVECs
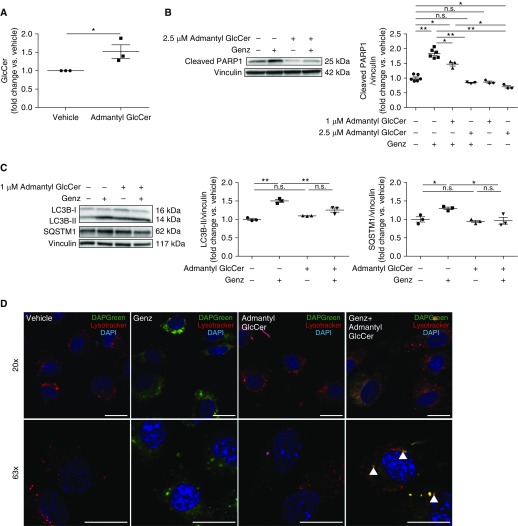
GCS inhibition can both increase ceramide and decrease GlcCer. Whereas ceramide accumulation has been known to cause lung endothelial cell death (4), the effect of the net loss of GlcCer on lung endothelial cell fate is unknown. To increase intracellular GlcCer (33), HLMVECs were treated with N-(1-adamantaneacetyl)-GlcCer (admantyl GlcCer) (2.5 μM, 1 h or 1.0 μM, 24 h) or vehicle (ethanol), and then exposed to Genz (2 μM) or CSE (3%) for 6 hours. Using LC-MS/MS, we first confirmed that admantyl GlcCer treatment increased intracellular GlcCer levels (Figure 6A and Figure E6A). Admantyl GlcCer treatment decreased cleaved PARP1 levels in both GCS-inhibited (Figure 6B and Figure E6B) and CS-exposed HLMVECs (Figure E6C). Whereas GlcCer treatment preserved the increase of LC3B-II in response to CS exposure (Figure E6D), it mitigated the increase of SQSTM1 in both GCS-inhibited (Figure 6C) and CS-exposed (Figure E6D) HLMVECs. Fluorescence microscopy studies of autophagic flux showed that GlcCer treatment increased the colocalization of autophagosomes with lysosomes in both GCS-inhibited (Figure 6D) and CS-exposed cells (Figure E6E). These results indicate that GlcCer is necessary for proper lysosomal function, and that supplementation with GlcCer is sufficient to attenuate apoptosis in both GCS-inhibited and CS-exposed HLMVECs.
Figure 6.
Effect of glucosylceramide (GlcCer) treatment on apoptosis and impaired autophagic flux in human lung microvascular endothelial cells (HLMVECs) exposed to GCS inhibitor. (A) Endogenous GlcCer levels measured by liquid chromatography with tandem mass spectrometry in HLMVECs treated with vehicle (ethanol) or admantyl GlcCer (1 μM; 30 h) (n = 3). (B) Representative Western blots and densitometric analysis of cleaved PARP1 in HLMVECs incubated with either vehicle or admantyl GlcCer (2.5 μM for 1 h or 1 μM for 24 h) and exposed to vehicle or Genz (2 μM; 6 h) (n = 3–6). (C) Representative Western blots and densitometric analysis of LC3B and SQSTM1 in HLMVECs treated with vehicle or admantyl GlcCer (1 μM; 24 h) and exposed to vehicle or Genz (2 μM; 6 h) (n = 3). (D) Representative immunofluorescence images of HLMVECs incubated with vehicle or admantyl GlcCer (1 μM; 24 h) and exposed to vehicle or Genz (2 μM; 6 h). Cells were then incubated with DAPGreen (50 nM) and LysoTracker deep red (25 nM) for 30 minutes. Arrowheads indicate colocalization of autophagosome and lysosome. Scale bars, 20 μm. Western blots and immunofluorescence images are representative of three independent experiments. Vinculin was used as loading control. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by one-way ANOVA with Tukey’s multiple comparisons test or Mann-Whitney U test. GCS = glucosylceramide synthase; Genz = Genz-123346; n.s. = not significant.
GCS Overexpression Mitigates Apoptosis and Impaired Autophagic Flux in CS-exposed HLMVECs
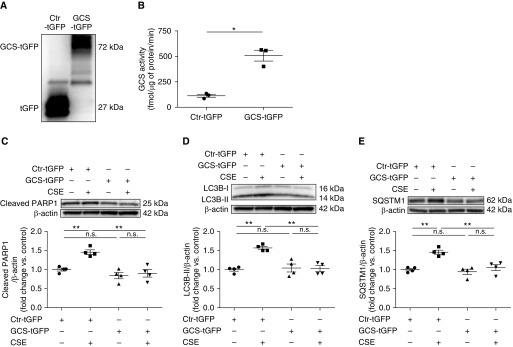
Because exogenous supplementation with GlcCer may have different effects than those obtained by increasing endogenous GlcCer, we assessed the effect of GCS overexpression on CS-exposed HLMVECs. Cells were transfected with GCS overexpression vector with 1 μg of turbo GFP (green fluorescent protein) tag (GCS-tGFP) or an empty control vector with tGFP tag (Ctr-tGFP) for 24 hours and exposed to AC or 3% CSE for 6 hours. GCS overexpression was verified by Western blot detection of GCS-tGFP fusion protein in transfected cells (Figure 7A). Using LC-MS/MS, we determined that GCS-tGFP transfection markedly increased GCS activity and GlcCer compared with control (Figures 7B and E7A). Besides S1P, which was modestly elevated, ceramide, sphingomyelin, and sphingosine levels were unaffected by GCS overexpression (Figures E7B–E7E). Importantly, GCS overexpression significantly decreased cleaved PARP1, LC3B-II, and SQSTM1 in CS-exposed cells to levels similar to those in control cells (Figures 7C–7E). These results implicate GCS inhibition as a mechanism by which CS triggers autophagy-related apoptosis in lung endothelial cells.
Figure 7.
Effect of GCS overexpression on apoptosis and impaired autophagic flux in CSE-exposed human lung microvascular endothelial cells (HLMVECs). (A and B) Representative Western blots of GCS–turbo GFP fusion protein (A) and GCS activity (B) measured by liquid chromatography with tandem mass spectrometry in HLMVECs transfected with 1 μg of GCS overexpression vector with turbo GFP tag (GCS-tGFP) or 1 μg of empty control vector with turbo GFP tag (Ctr-tGFP) for 24 hours (n = 3–4). Whole-cell extract was probed with anti–turbo GFP antibody. (C–E) Representative Western blots and densitometric analysis of cleaved PARP1 (C), LC3B (D), and SQSTM1 (E) in HLMVECs transfected with 1 μg of GCS-tGFP or Ctr-tGFP for 24 hours and treated with AC or CSE (3%; 6 h; n = 4). Western blots are representative of at least three independent experiments. All graphs show mean ± SEM; *P < 0.05 and **P < 0.01 by one-way ANOVA with Tukey’s multiple comparisons test or Mann-Whitney U test. AC = air control; CSE = cigarette smoke extract; Ctr = control; GCS = glucosylceramide synthase; n.s. = not significant.
Impact of COPD on GCS Levels in Lungs
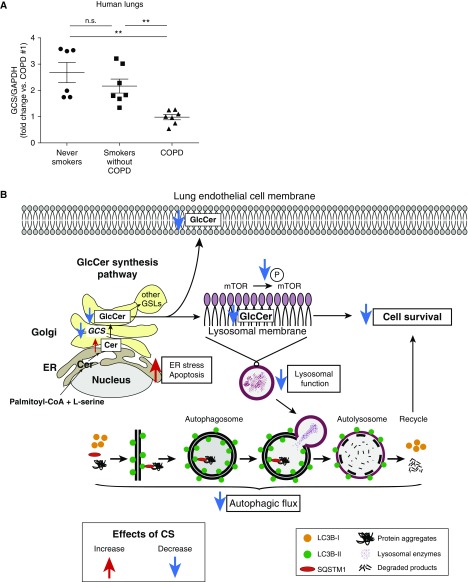
To determine the clinical relevance of our findings, we measured GCS in lung tissue from subjects with COPD compared with nondiseased smokers and nonsmokers. The subjects’ clinical characteristics are summarized in Table E1. Consistent with our results in mouse lungs and HLMVECs, GCS levels in COPD-diseased lungs were markedly reduced compared with those in lungs of nondiseased individuals (Figure 8A and Figures E8A and E8B).
Figure 8.
Decreased GCS levels in chronic obstructive pulmonary disease (COPD) lungs. (A) Densitometric analysis of GCS normalized to GAPDH as loading control in the lungs from healthy subjects (never-smokers) versus smokers without COPD versus smokers with COPD (mean ± SEM; **P < 0.01 one-way ANOVA with Tukey’s multiple comparisons test; n = 6–7). (B) Schematic of proposed mechanism by which GCS contributes to lung endothelial cell fate during homeostasis (black arrows) and during CS exposure (red or blue arrows). CS exposure inhibits GCS activity in human lung endothelial cells. Inhibition of GCS causes increased endoplasmic reticulum (ER) stress and decreased mTOR signaling, triggering autophagy with decreased autophagic flux associated with lysosomal dysfunction and apoptosis. CS = cigarette smoke; GCS = glucosylceramide synthase; GlcCer = glucosylceramide; mTOR = mammalian target of rapamycin; n.s. = not significant.
Discussion
These studies are the first to demonstrate that decreased GlcCer levels are associated with CS-induced emphysema, and that GCS inhibition is sufficient to cause ER stress as well as impairment of both mTOR signaling and autophagic flux, culminating in apoptosis in lung microvascular endothelial cells (Figure 8B). Our data indicate that approaches to increase GCS activity or its product, GlcCer, attenuate CS-induced HLMVEC death, whereas further inhibition of GCS augments the injurious effects of CS exposure in HLMVECs. Corroborating our previous report that plasma GSL levels detected by nontargeted metabolomics are negatively associated with emphysema (5), we showed, for the first time, a significant decrease in lung GCS in murine models of emphysema and in human COPD, and a negative correlation between airspace size and GlcCer levels in the BALF of mice.
GlcCer is a metabolite of ceramide, the hub of sphingolipid metabolism that has been implicated in COPD pathogenesis (1). In particular, increased ceramide levels, such as those induced by CS exposure or oxidative stress, cause pulmonary endothelial cell apoptosis (1, 29) and murine emphysema-like disease (1, 34). However, the mechanisms of ceramide accumulation that are associated with emphysema are incompletely understood, and they may involve insufficient metabolism of ceramide to other sphingolipids, such as GSLs. GCS catalyzes the first glycosylation step in the biosynthesis of GSLs from ceramide. Genetic deletion of GCS causes marked imbalance of ceramide/GSLs (35), and GCS knockout mice exhibit embryonic lethality due to massive apoptosis (9). In turn, GCS overexpression provides resistance against ceramide-induced cell death in human cancer cells (36–38). Our results demonstrate that GCS inhibition is sufficient to increase ER stress and interfere with lysosomal function, triggering autophagy with impaired flux that culminates with apoptosis in HLMVECs. These results suggest that, by controlling the ceramide/GlcCer balance, GCS plays a key role in lung endothelial cell fate. It is notable that, compared with the effects of GCS inhibition, CS more potently induced both autophagy and apoptosis. This may reflect more complex effects of CS beyond those directly linked to increased ceramide and decreased GlcCer. Nevertheless, GCS overexpression markedly improved autophagic flux during CS exposure and reduced CSE-induced apoptosis in HLMVECs. These results indicate that GCS inhibition is detrimental for lung endothelial cell function, and that GCS might be a critical regulator of cellular activities by controlling the balance of ceramide/GlcCer in HLMVECs.
Another novel aspect of our study is that we, for the first time, mechanistically investigated the role of the first step in GSL metabolism in lung cell fate, such as mTOR signaling and autophagy, ER stress, and apoptosis. Although mTOR signaling and autophagy are increasingly recognized as important in COPD pathogenesis (16, 39), the mechanisms by which CS regulates mTOR signaling and autophagy in lung cells remain incompletely elucidated. Our study suggests that GCS inhibition may be a mechanism by which CS triggers its effects on mTOR and autophagy, by involving lysosomal function. This notion is supported by the key role that the lysosome plays in mTOR signaling and autophagy (16, 40). Our findings indicate that GCS inhibition markedly altered lysosome function, decreasing lysosomal enzyme activity and increasing lysosomal pH. Our report is consistent with reports that GCS inhibition increased endolysosomal pH in mouse peritoneal macrophages (41) and increased lysosomal membrane permeability in neurons (42). The precise molecular mechanism by which GCS maintains lysosomal function is still unclear, but GSLs are essential components of the lysosomal membrane (43), which is also the site of mTOR docking and activation. It is therefore compelling to envision that changes in GCS activity may be rapidly sensed by signaling pathways that inform the function of this essential cellular organelle.
In this study, we found that GCS levels in lung lysates from subjects with COPD were significantly reduced compared with nondiseased smokers and nonsmokers. Because endothelial cells comprise approximately 30% of cells in the human lung parenchyma (44), it is possible that CS exposure decreases GCS levels in other lung cells, a question that could be addressed in future studies.
Because low levels of plasma GlcCer have been recently associated with a higher incidence of myocardial infarction (45), our findings in HLMVECs may inform the pathogenesis of other vascular pathologies associated with CS exposure. Our mechanistic studies in human lung cells suggest that GCS and GlcCer are important determinants of cell fate in the pathogenesis of CS-related endothelial injury, and that GSL metabolism might be a novel, therapeutically targetable pathway in emphysema.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Drs. Kelly S. Schweitzer, Susan M. Majka, Moumita Ghosh, and Katsuyuki Takeda for technical assistance and helpful discussions.
Footnotes
Supported by NIH contract grants R01HL077328 (I.P.), P20HL113445 (I.P.), and R03HL095440 (I.P. and E.V.B.), a Banyu Life Science Foundation International Fellowship (K.K.), and an American Lung Association Biomedical Research grant (K.K.).
Author Contributions: K.K., E.V.B., and I.P. conceived and designed experiments; K.K., E.V.B., A.M.M., and K.N. performed experiments; K.K., E.V.B., A.M.M., I.A.B., A.S.B., J.P.J., E.L.B., and I.P. analyzed data; K.K., A.M.M., E.L.B., D.C., A.K.S., K.A.S., and I.P. contributed reagents/materials/analysis tools; and K.K., E.V.B., and I.P. wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2311OC on July 2, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrache I, Berdyshev EV. Ceramide signaling and metabolism in pathophysiological states of the lung. Annu Rev Physiol. 2016;78:463–480. doi: 10.1146/annurev-physiol-021115-105221. [DOI] [PubMed] [Google Scholar]
- 3.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike K, Berdyshev EV, Bowler RP, Scruggs AK, Cao D, Schweitzer KS, et al. Bioactive sphingolipids in the pathogenesis of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15:S249–S252. doi: 10.1513/AnnalsATS.201809-592MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowler RP, Jacobson S, Cruickshank C, Hughes GJ, Siska C, Ory DS, et al. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med. 2015;191:275–284. doi: 10.1164/rccm.201410-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodas M, Min T, Vij N. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis. 2015;20:725–739. doi: 10.1007/s10495-015-1098-0. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki N, Toyoda M. Glycoconjugates and related molecules in human vascular endothelial cells. Int J Vasc Med. 2013;2013:963596. doi: 10.1155/2013/963596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, et al. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96:9142–9147. doi: 10.1073/pnas.96.16.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giussani P, Bassi R, Anelli V, Brioschi L, De Zen F, Riccitelli E, et al. Glucosylceramide synthase protects glioblastoma cells against autophagic and apoptotic death induced by temozolomide and Paclitaxel. Cancer Invest. 2012;30:27–37. doi: 10.3109/07357907.2011.629379. [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Henry AG, Paumier KL, Li L, Mou K, Dunlop J, et al. Inhibition of glucosylceramide synthase stimulates autophagy flux in neurons. J Neurochem. 2014;129:884–894. doi: 10.1111/jnc.12672. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, et al. Autophagy protein microtubule–associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke–induced emphysema. Proc Natl Acad Sci USA. 2010;107:18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, et al. Egr-1 regulates autophagy in cigarette smoke–induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, et al. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahira K, Pabon Porras MA, Choi AM. Autophagy in pulmonary diseases. Am J Respir Crit Care Med. 2016;194:1196–1207. doi: 10.1164/rccm.201512-2468SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike K, Berdyshev EV, Bronova IA, Mikosz AM, Beatman EL, Petrache I. Role of glucosylceramide synthase in cigarette smoke–induced lung endothelial injury [abstract] Am J Respir Crit Care Med. 2018;197:A1192. [Google Scholar]
- 18.Koike K, Schweitzer KS, Beatman EL, Mikosz AM, Justice MJ, Bronova IA, et al. Requirement of glucosylceramide synthase for human lung microvascular endothelial cell survival [abstract] Am J Respir Crit Care Med. 2017;195:A7041. [Google Scholar]
- 19.Clauss M, Voswinckel R, Rajashekhar G, Sigua NL, Fehrenbach H, Rush NI, et al. Lung endothelial monocyte–activating protein 2 is a mediator of cigarette smoke–induced emphysema in mice. J Clin Invest. 2011;121:2470–2479. doi: 10.1172/JCI43881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizumura K, Justice MJ, Schweitzer KS, Krishnan S, Bronova I, Berdyshev EV, et al. Sphingolipid regulation of lung epithelial cell mitophagy and necroptosis during cigarette smoke exposure. FASEB J. 2018;32:1880–1890. doi: 10.1096/fj.201700571R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks DL, Paul P, Kamisaka Y, Pagano RE. Methods for studying glucosylceramide synthase. Methods Enzymol. 2000;311:50–59. doi: 10.1016/s0076-6879(00)11066-3. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler S, Haberkant P, Bhardwaj M, Tongue P, Ferraz MJ, Halter D, et al. Cytosolic glucosylceramide regulates endolysosomal function in Niemann-Pick type C disease. Neurobiol Dis. 2019;127:242–252. doi: 10.1016/j.nbd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Koike K, Beatman EL, Schweitzer KS, Justice MJ, Mikosz AM, Ni K, et al. Subcutaneous administration of neutralizing antibodies to endothelial monocyte-activating protein II attenuates cigarette smoke–induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2019;316:L558–L566. doi: 10.1152/ajplung.00409.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwashita H, Sakurai HT, Nagahora N, Ishiyama M, Shioji K, Sasamoto K, et al. Small fluorescent molecules for monitoring autophagic flux. FEBS Lett. 2018;592:559–567. doi: 10.1002/1873-3468.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–792. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai L, McLaren RP, Byrne A, Chuang WL, Huang Y, Dufault MR, et al. The chemosensitizing activity of inhibitors of glucosylceramide synthase is mediated primarily through modulation of P-gp function. Int J Oncol. 2011;38:701–711. doi: 10.3892/ijo.2010.888. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, et al. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 28.Csordas A, Kreutmayer S, Ploner C, Braun PR, Karlas A, Backovic A, et al. Cigarette smoke extract induces prolonged endoplasmic reticulum stress and autophagic cell death in human umbilical vein endothelial cells. Cardiovasc Res. 2011;92:141–148. doi: 10.1093/cvr/cvr165. [DOI] [PubMed] [Google Scholar]
- 29.Petrusca DN, Van Demark M, Gu Y, Justice MJ, Rogozea A, Hubbard WC, et al. Smoking exposure induces human lung endothelial cell adaptation to apoptotic stress. Am J Respir Cell Mol Biol. 2014;50:513–525. doi: 10.1165/rcmb.2013-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 31.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamani M, Mylvaganam M, Tian R, Rigat B, Binnington B, Lingwood C. Adamantyl glycosphingolipids provide a new approach to the selective regulation of cellular glycosphingolipid metabolism. J Biol Chem. 2011;286:21413–21426. doi: 10.1074/jbc.M110.207670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane–cystic fibrosis transmembrane conductance regulator–dependent ceramide signaling in lung injury and emphysema. J Immunol. 2011;186:602–613. doi: 10.4049/jimmunol.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingwood CA. Glycosphingolipid functions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouazé V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, et al. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 37.Liu YY, Han TY, Yu JY, Bitterman A, Le A, Giuliano AE, et al. Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. J Lipid Res. 2004;45:933–940. doi: 10.1194/jlr.M300486-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Patwardhan GA, Zhang QJ, Yin D, Gupta V, Bao J, Senkal CE, et al. A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PLoS One. 2009;4:e6938. doi: 10.1371/journal.pone.0006938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuder RM. Bringing light to chronic obstructive pulmonary disease pathogenesis and resilience. Ann Am Thorac Soc. 2018;15:S227–S233. doi: 10.1513/AnnalsATS.201808-583MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sillence DJ. Glucosylceramide modulates endolysosomal pH in Gaucher disease. Mol Genet Metab. 2013;109:194–200. doi: 10.1016/j.ymgme.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Wei J, Fujita M, Nakai M, Waragai M, Sekigawa A, Sugama S, et al. Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies. Am J Pathol. 2009;174:1891–1909. doi: 10.2353/ajpath.2009.080680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze H, Kolter T, Sandhoff K. Principles of lysosomal membrane degradation: cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta. 2009;1793:674–683. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 45.Deguchi H, Navarro S, Payne AB, Elias DJ, Dowling NF, Austin HD, et al. Low level of the plasma sphingolipid, glucosylceramide, is associated with thrombotic diseases. Res Pract Thromb Haemost. 2017;1:33–40. doi: 10.1002/rth2.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.