Abstract
In this study, 34 Traditional Chinese Medicine (TCM) compounds were screened for potential anabolic and anti-inflammatory properties on human osteoarthritic (OA) chondrocytes. The anabolic effects were assessed by measuring the glycosaminoglycan (GAG) relative to the DNA content using a 3D pellet culture model. The most chondrogenic compounds were tested in an inflammatory model consisting of 3 days of treatment with cytokines (IL-1β/TNF-α) with or without supplementation of TCM compounds. The anti-inflammatory effects were assessed transcriptionally, biochemically and histologically. From the 34 compounds, Vanilic acid (VA), Epimedin A (Epi A) and C (Epi C), 2′′-O-rhamnosylicariside II (2-O-rhs II), Icariin, Psoralidin (PS), Protocatechuicaldehyde (PCA), 4-Hydroxybenzoic acid (4-HBA) and 5-Hydroxymethylfurfural (5-HMF) showed the most profound anabolic effects. After induction of inflammation, pro-inflammatory and catabolic genes were upregulated, and GAG/DNA was decreased. VA, Epi C, PS, PCA, 4-HBA and 5-HMF exhibited anti-catabolic and anti-inflammatory effects and prevented the up-regulation of pro-inflammatory markers including metalloproteinases and cyclooxygenase 2. After two weeks of treatment with TCM compounds, the GAG/DNA ratio was restored compared with the negative control group. Immunohistochemistry and Safranin-O staining confirmed superior amounts of cartilaginous matrix in treated pellets. In conclusion, VA, Epi C, PS, PCA, 4-HBA and 5-HMF showed promising anabolic and anti-inflammatory effects.
Keywords: osteoarthritis, traditional Chinese medicine compounds, osteoarthritic chondrocytes, anti-inflammatory effects, anabolic, catabolic
1. Introduction
Occurrence of age-related musculoskeletal disorders is increasing, with osteoarthritis (OA) being the most prevalent joint disorder. Approximately 20% of the adult population in Europe and the US is expected to be afflicted with OA by 2030 [1,2,3], resulting in excessive economic burdens on healthcare systems.
Development of OA results from a combination of genetic and environmental factors including obesity, ageing, acute joint trauma and inflammation, which cause an imbalance between chondrocyte anabolism and catabolism and primarily affect articular hyaline cartilage in load bearing joints [4,5,6,7,8]. Furthermore, OA can increase the risk of onset of other chronic diseases, making prevention and early effective treatment of OA a relevant research focus [9]. The avascular nature of cartilage, combined with low cell density and slow matrix turnover rate, results in a lack of effective methods to prevent or treat OA [7]. If conventional treatment such as physical therapy or weight reduction fails to alleviate symptoms, simple analgesics are initially recommended (e.g., acetaminophen). Additionally, non-steroidal anti-inflammatory drugs (NSAIDs), including specific or non-specific cyclooxygenase 2 (COX-2) inhibitors, are used frequently [10,11,12]. However, these systemic treatments are often insufficient and bear the risks of gastrointestinal, renal or cardiovascular side effects [13,14,15]. Alternative anti-inflammatory treatments include the inhibition of inducible nitric oxide synthase (iNOS) or nuclear factor kappa B (NFκB) signaling [16]. Furthermore, anti-cytokine therapy, such as anti-tumor necrosis factor (TNF)-α antibodies, can potentially suppress inflammation and prevent cartilage degradation in OA patients [17]. However, in addition to TNF-α, other pro-inflammatory cytokines such as interleukin (IL)-1, are also involved in the degenerative process [18]. Anti-matrix metalloproteinase (MMP) therapy faces a similar concern, as multiple enzymes contribute to the complex breakdown mechanism of the extracellular matrix. When the disease is progressing (Stage 3), intra-articular injection of hyaluronic acid, glucosamine or corticosteroids can be considered [19]. Though effects are often initially satisfactory, these injections only achieve short-term pain relief, and eventually surgical joint replacement is one of the last options remaining in the Stage 4 of OA [20]. Joint replacement is a highly invasive procedure with an extended rehabilitation period and limited options for revision when necessary [21]. Alternatively, arthroscopic lavage and debridement have been applied in OA patients with variable outcomes [22]. Since conventional treatment approaches aim at symptoms relief and do not address the catabolic component of the destructive OA disease [23], the development of innovative, disease modifying and low risk treatments for OA is an unmet clinical need.
Several plant-derived compounds have been identified that have the potential to inhibit NFκB signaling and inflammation [24,25,26,27,28]. Well known plant-derived compounds used for these purposes are curcumin and resveratrol [29]. In addition, extracts of Traditional Chinese Medicine (TCM) herbs have been investigated for their anti-inflammatory effects. For example, Caesalpinia sappan derived extracts were shown to suppress nitric oxide synthesis in osteoarthritic chondrocytes by down-regulating the iNOS mRNA expression. It was concluded that blockage of IL-1 induced NFκB signaling and its down-stream pro-inflammatory targets by Caesalpinia sappan extracts may counteract cartilage breakdown in OA [18]. Similarly, Honokiol, a low molecular weight natural product isolated from Magnolia officinalis, was able to prevent IL-1 induced inflammatory reactions and cartilage matrix breakdown in human OA chondrocytes [30].
Therapy with herbal Fufang is popular in TCM for prevention and treatment of osteoporosis and related bone diseases. Xianlinggubao formula (XLGB) was formed based on modification of the empirical “Miao minority” medicine, which was commonly used to tone the “kidney system” and nourish bones [31]. XLGB capsule was officially approved by the Chinese State Food and Drug Administration (cFDA) as the over-the-counter drug for treatment of osteoporosis [32], aseptic osteonecrosis [33], osteoarthritis [34] and bone fractures [32]. XLGB is composed of six kinds of herbs containing various compounds [32,33]. A total of 118 compounds were identified from XLGB extract [35]. Some of them, e.g., Icariin, had previously demonstrated extensive bioactivity and anti-inflammatory activity and were used as bioactive factors in tissue engineering for cartilage defect repair [36,37].
We hypothesized that, among the various compounds identified in TCM herbal extracts, there are distinct molecules with potent chondrogenic and anti-inflammatory effects. However, no comprehensive and systematic direct comparison of diverse TCM molecules in terms of combined chondrogenic and anti-inflammatory properties has been performed. The current study assessed the anabolic and anti-inflammatory effects of 34 relevant TCM compounds from XLGB on human osteoarthritic chondrocytes. The aim was to identify the most potent compounds in terms of cartilage matrix synthesis and counteraction of inflammatory responses.
2. Results
2.1. Toxicity Assay for the Compounds on Human Osteoarthritic Chondrocytes
Cytotoxicity assay of monolayer cultures showed that after 48 h of treatment with TCM compounds (1 µM, 10 µM, 25 µM, 50 µM), more than 75% of the cells were viable in all treatment groups. Furthermore, in different concentrations of dimethyl sulfoxide (DMSO) (0.01%, 0.1%, 0.25%, 0.5% v/v) as control vehicle group the numbers of viable cells were consistent. So, DMSO did not show any toxic effect on the cells in these concentrations. Furthermore, the viability of the cells in the un-treated control group was not different from the control vehicle group. All data are normalized to the control vehicle group (Table 1).
Table 1.
Relative viable cell count after treatment with the 34 compounds measured by WST-1 assay.
| Compound | % Cell Numbers (Normalized to Control Vehicle) | ||||
|---|---|---|---|---|---|
| Conc.[µM] | 1 | 10 | 25 | 50 | |
| 5-Hydroxymethylfurfural | 111.4 ± 6.6 | 107.0 ± 11.2 | 110.2 ± 9.4 | 107.9 ± 3.3 | |
| Protocatechuicaldehyde | 100.1 ± 4.2 | 96.0 ± 9.3 | 94.8 ± 6.4 | 81.2 ± 5.4 | |
| Vanilic acid | 102.1 ± 2.4 | 96.8 ± 3.7 | 115.7 ± 3.2 | 112.6 ± 2.1 | |
| 4-Hydroxybenzoic acid | 103.4 ± 2.5 | 102 ± 9.6 | 105 ± 3.4 | 78.6 ± 6.9 | |
| Chlorogenic acid | 77.8 ± 3.2 | 78.6 ± 4.8 | 77.8 ± 5.2 | 79.8 ± 5.6 | |
| Cryptochlorogenic acid | 86.2 ± 8.5 | 86 ± 7.9 | 93.8 ± 5.2 | 78.3 ± 8.4 | |
| Loganic acid | 85.6 ± 3.6 | 94.7 ± 3 | 91.5 ± 1.7 | 93.6 ± 4.9 | |
| Loganin | 107 ± 5.6 | 109.2 ± 5.1 | 110.6 ± 9.8 | 102.8 ± 3.6 | |
| Isobavachalcone | 102.1 ± 6.6 | 108.7 ± 5.7 | 105.7 ± 0.6 | 88.4 ± 8.8 | |
| Sweroside | 110.2 ± 2.3 | 106.7 ± 4.1 | 112.5 ± 1.8 | 107.7 ± 9.8 | |
| (+)-Cycloolivil | 101.5 ± 1.5 | 98. ± 2.1 | 105.6 ± 4.9 | 103.2 ± 8.2 | |
| Baohuoside I | 82.8 ± 6.3 | 76.3 ± 1.8 | 77.5 ± 0.4 | 79.8 ± 5.8 | |
| 2’-O-rhamnosylicariside II | 102.1 ± 6.6 | 105.5 ± 4.7 | 91.8 ± 3.2 | 102.9 ± 2.1 | |
| Epimedin A | 83.9 ± 5.6 | 84.8 ± 8.1 | 97.1 ± 6.9 | 111.3 ± 5.1 | |
| Epimedin B | 82.7 ± 9.2 | 88 ± 9.9 | 87.8 ± 9.1 | 94. ± 7.9 | |
| Epimedin C | 87.5 ± 0.6 | 89.5 ± 6.5 | 88.1 ± 3.7 | 89.8 ± 6.4 | |
| Isobavachin | 87.3 ± 1.3 | 91.5 ± 8.2 | 89.2 ± 0.8 | 85.7 ± 0.1 | |
| Bavachin | 109.1 ± 2.1 | 102 ± 8.4 | 89.2 ± 9.5 | 91.7 ± 2.1 | |
| Bavachinin | 96.4 ± 1.1 | 78.1 ± 6.6 | 79.9 ± 5.1 | 79.7 ± 3 | |
| Neobavaisoflavone | 84.4 ± 8.6 | 89.6 ± 1.9 | 99 ± 2.5 | 76.1 ± 2.2 | |
| Corylin | 89.6 ± 2.4 | 94 ± 3.7 | 87.8 ± 1.6 | 79.3 ± 3.1 | |
| Epimedin A1 | 95.48 ± 4.3 | 85.49 ± 0.5 | 88.21 ± 3.1 | 95.46 ± 2.4 | |
| Psoralen | 83.6 ± 5.1 | 80.3 ± 5.3 | 85.1 ± 6.4 | 84.8 ± 8.3 | |
| Isopsoralen | 86.2 ± 8.6 | 80.2 ± 1.9 | 83 ± 2.7 | 85.4 ± 3.2 | |
| (S)-Bukuchiol | 89.1 ± 4.4 | 94.4 ± 3.5 | 104.7 ± 4.2 | 88.4 ± 4.2 | |
| Psoralidin | 106.5 ± 6.3 | 84.7 ± 1.4 | 86.4 ± 5.9 | 85.1 ± 4.8 | |
| Asperosaponin VI | 93.9 ± 2.8 | 99 ± 9.2 | 103.8 ± 4.5 | 93.9 ± 4.8 | |
| Baohuoside II | 103.6 ± 5.9 | 96.4 ± 7.6 | 95 ± 5.2 | 79.7 ± 2.9 | |
| Epimedoside A | 89 ± 4.7 | 87.9 ± 3.1 | 90.6 ± 4.4 | 85.6 ± 3.7 | |
| Baohuoside V | 98.6 ± 8.7 | 100 ± 6.5 | 100.9 ± 7.8 | 78.5 ± 3.8 | |
| Corylifol A | 86.1 ± 3.3 | 85.3 ± 1.8 | 94.6 ± 0.9 | 103.8 ± 2.6 | |
| 4’-O-Methyl-broussochalcone | 81.4 ± 2.9 | 85.4 ± 2.4 | 95.4 ± 3.6 | 97.5 ± 2.3 | |
| Anhydroicaritin | 85.2 ± 2.2 | 83.4 ± 6.6 | 86.2 ± 1.5 | 81.8 ± 2.4 | |
| Icariin | 95.7 ± 3.6 | 101.4 ± 3.7 | 95.5 ± 3.2 | 101.5 ± 5.7 | |
The compounds were tested in different concentrations (1 µM, 10 µM, 25 µM, 50 µM) for 48 h. Data is normalized to the control vehicle group (cells with medium and DMSO).
2.2. Anabolic Effects of Traditional Chinese Medicine (TCM) Compounds on Glycosaminoglycan Production
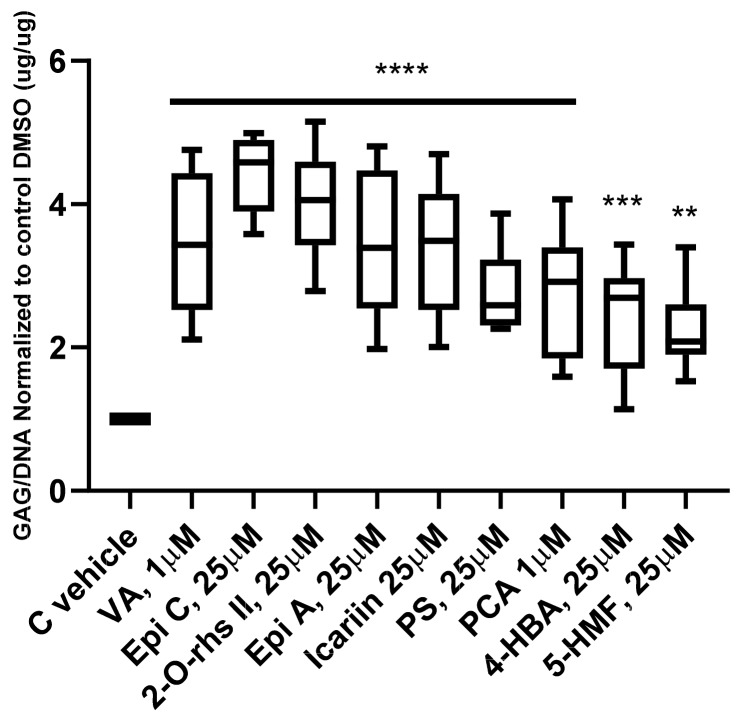
To determine the anabolic effect of the TCM compounds, all 34 compounds were tested in 3D pellet culture in four different concentrations (1 µM, 10 µM, 25 µM, 50 µM). Human OA chondrocytes from three donors were cultured in chondro-permissive medium with supplementation of compounds or with DMSO (0.01%, 0.1%, 0.25%, 0.5% v/v) (control vehicle group) for two weeks. Nine compounds, namely Vanilic acid (VA), Epimedin A (Epi A) and C (Epi C), 2′′-O-rhamnosylicariside II (2-O-rhs II), Psoralidin (PS), Icariin, Protocatechuicaldehyde (PCA), 4-Hydroxybenzoic acid (4-HBA) and 5-Hydroxymethylfurfural (5-HMF) significantly increased the GAG/DNA ratio compared to the control vehicle group in all three donors. All data are normalized to their respective control vehicle groups, and the compounds which were effective in all the different concentrations are shown in Figure 1 in their most effective dose. The complete data set from all compounds tested in four concentrations is shown in Supplementary Table S1. Epi A, Epi C, 2-O-rhs II and Icariin are all extracts of Epimedium medicinal herb with similar chemical structures and positive anabolic effects. Since Epi C showed the highest amount of matrix production among these four compounds, it was chosen as a representative of Epimedium herb extract for use in further experiments. Furthermore, VA, PS, PCA, 4-HBA and 5-HMF from the first screening in their most effective dose were selected for further studies in the inflammatory models.
Figure 1.
Glycosaminoglycan (GAG) production of 3D human osteoarthritic chondrocyte pellet cultures after two weeks in chondro-permissive medium supplemented with Traditional Chinese Medicine (TCM) compounds. Glycosaminoglycan content was normalized to the amount of DNA. The most effective doses of compounds which could promote GAG production versus control vehicle (C vehicle) group in 3/3 donors are shown; for each donor three experimental replicates were analyzed. For statistical analysis using Graphpad Prism, one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (multiple comparison) was applied. ** p < 0.001, *** p < 0.0005, **** p < 0.0001 versus control vehicle. Vanilic acid (VA), Epimedin A (Epi A) and C (Epi C), 2′′-O-rhamnosylicariside II (2-O-rhs II), Icariin, Psoralidin (PS), Protocatechuicaldehyde (PCA), 4-Hydroxybenzoic acid (4-HBA), 5-Hydroxymethylfurfural (5-HMF).
2.3. Effects of TCM Compounds on Pro-Inflammatory and Pro-Catabolic Gene Expression under Inflammatory Conditions
After one week of cartilage generation (short-term) in phase I, the pro-catabolic and pro-inflammatory genes in the pellets treated with IL-1β/TNF-α in phase II were significantly increased. To investigate the inhibitory effects of the selected compounds in phase II, compounds in their most effective dose were simultaneously added with inflammatory cytokines to the chondro-permissive culture media. We observed that after treatment with VA, Epi C, PS, PCA, 4-HBA and 5-HMF, the catabolic marker gene matrix metalloproteinase 13 (MMP13) was significantly downregulated in all treatment groups. In the groups treated with VA, Epi C, PCA, 4-HBA and 5-HMF, other catabolic marker genes matrix metalloproteinase 1, 3 (MMP1, MMP3) were also significantly inhibited compared with the control DMSO group. Furthermore, COX-2 was significantly downregulated in all the treatment groups except for PS and 4-HBA. In pellets treated with Epi C, in addition to downregulation of catabolic marker genes, the anabolic marker genes collagen type II (COL-II) and aggrecan (ACAN) were significantly upregulated (Figure 2a–f).
Figure 2.
Transcriptional level of catabolic genes matrix metalloproteinase (MMP) a) MMP1 b) MMP3 c) MMP13, pro-inflammatory gene d) cyclooxygenase 2 (COX-2) and anabolic marker genes e) collagen type II (COL-II) and f) aggrecan (ACAN) in Phase II (short term pellets) simultaneously treated with TCM compounds and IL-1β/TNF-α versus control vehicle (C vehicle) group in human OA chondrocytes. n = 3; n indicates the number of human OA donors; for each donor three experimental replicates were analyzed. Data are normalized to the levels of control groups. For statistical analysis using Graphpad Prism, one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (multiple comparisons) was applied. * p < 0.01, ** p < 0.001, *** p < 0.0005, **** p < 0.0001, ns (non-significant).
2.4. Effect of TCM Compounds on Matrix Production under Inflammatory Conditions
After two weeks of cartilage generation with chondrogenic medium in phase I (long term) the cells produced significant amounts of matrix as assessed by dimethylmethylene blue (DMMB) assay and histology. After induction of inflammation with inflammatory cytokines in phase II (IL-1β/TNF-α), biochemical analysis showed that the GAG/DNA ratio was decreased compared with phase I (Figure 3a). In phase III, after two weeks of treatment (long term), the GAG/DNA ratio did not recover in the control vehicle group in which the cells were treated with chondro-permissive medium supplemented with 0.01%, 0.1%, 0.25% v/v DMSO. In the control positive group (chondrogenic medium) the GAG/DNA ratio reached a similar level as before induction of inflammation (Figure 3a). In the groups treated with VA, Epi C, PS, PCA, 4-HBA and 5-HMF in phase III, the amount of GAG production per DNA increased as compared to the control vehicle group (Figure 3b).
Figure 3.
Biochemical analysis of the osteoarthritic chondrocyte pellets (long term) in the inflammatory model. a) GAG production per DNA in three different phases of the inflammatory model. b) The effect of TCM compounds on GAG/DNA ratio in human osteoarthritic chondrocytes in phase III after induction of inflammation normalized to control vehicle group (C vehicle). n = 3; n indicates the number of human OA chondrocytes donors; for each donor three experimental replicates were analyzed. For statistical analysis using Graphpad Prism, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test (multiple comparisons) for Figure 3a and Dunnett’s post hoc test (multiple comparisons) for Figure 3b were applied. * p < 0.01, ** p < 0.001, *** p < 0.0005, **** p < 0.0001.
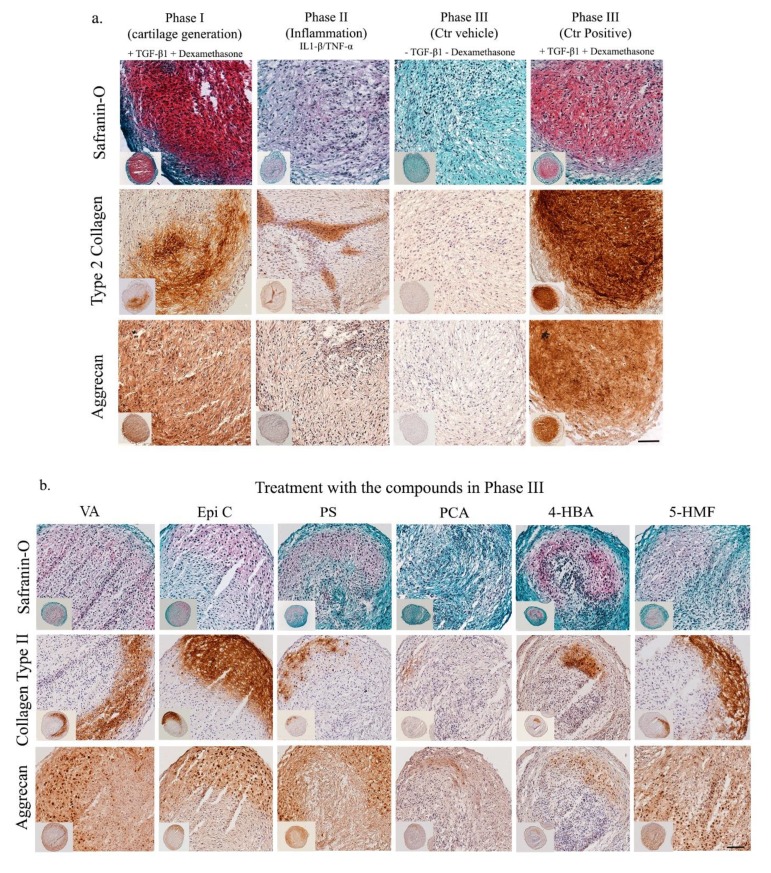
Histological analysis confirmed the observed biochemical results. In phase I, after two weeks of pellet culture in chondrogenic medium (long term), cartilage matrix was produced by osteoarthritic chondrocytes (Figure 4a). However, after induction of inflammation (phase II), the intensity of Safranin-O (Saf-O), COL-II and ACAN immunostaining significantly decreased. After two weeks of treatment (long term) in phase III, in the control positive group (chondrogenic medium), high intensity of Saf-O staining and immunostaining for COL-II and ACAN was observed, while staining for extracellular matrix (ECM) proteins in the control DMSO group was not noticeable (Figure 4a). After treatment with the compounds in the recovery phase III (long term), staining intensity for Saf-O, COL-II and ACAN in the groups treated with VA, Epi C, PS, PCA, 4-HBA and 5-HMF was preserved compared to phase II and stronger compared to the control vehicle group; though, the intensity of Saf-O staining and immunostaining for ECM proteins was still lower compared to phase I, and the compounds did not restore ECM to the same level as before induction of inflammation (Figure 4b).
Figure 4.
Histological and immunohistochemical characterization of pellets from OA chondrocytes in the inflammatory model (long term). a) Saf-O staining, COL-II and ACAN immunostaining of pellets in three different phases as the control groups (Phase I, Phase II, Phase III). b) and after 14 days of treatment with the TCM compounds in phase III (VA, Epi C, PS, PCA, 4-HBA, 5-HMF). Scale bars = 100 μm.
3. Discussion
For an effective biological therapy against OA, it is essential to counteract elevated catabolism while increasing anabolism. Furthermore, it is necessary to inhibit pro-inflammatory cytokines that are excessively abundant in osteoarthritic joints [18]. In the current study, for the first time, the anabolic and anti-inflammatory effects of 34 bioactive compounds existing in the over-the-counter XLGB formula for the treatment of osteoporosis [32] and osteoarthritis [34] were assessed in 3D pellet culture using human OA chondrocytes. Osteoporosis is recognized as one of the most common bone loss conditions in which the elevated amount of pro-inflammatory cytokines can cause a systemic inflammatory condition [38,39,40,41]. Since OA is considered an inflammatory disease, we hypothesized that the compounds which could decrease bone resorption and stimulate new bone formation in postmenopausal osteoporosis, could also have the potential for treatment of OA. In fact, a recent clinical study showed both efficacy and safety of XLGB herbal formula for treatment of OA [34].
In our study, the cytotoxicity of the TCM compounds in monolayer culture was tested at four different concentrations (1 µM, 10 µM, 25 µM, 50 µM). Cell viability was more than 75% for all the compounds and even at the highest concentration no cytotoxicity was seen. In previous in vitro studies, compounds with positive chondrogenic and anti-inflammatory effects with an optimal dose between 1 µM and 50 µM have been reported [42,43,44,45]. In an in vivo study in adult rabbits, Icariin which was added to the cell-hydrogel constructs at a final concentration of 10 µM showed potential effect in cartilage tissue regeneration [36]. Furthermore, in a rat model of experimental OA, injection of 0.3 mL of 20 µM Icariin reduced the number of cartilage lesions and the expression of MMP-13 [46]. Since most of the tested compounds in our study are novel in the orthopedic field, the reported concentrations of representative compounds from each herbal extract (Herba Epimedi, Radix ET Rhizoma Salviae, Fructus Psoraleae) were considered [47,48,49,50,51]. Therefore, the anabolic effects of all the compounds in 3D pellet culture in chondropermissive medium (deprived of TGF-β and dexamethasone) and supplemented with compounds in four concentrations (1 µM, 10 µM, 25 µM, 50 µM) were tested. Nevertheless, we cannot exclude that lower or higher concentrations of certain compounds may have shown more pronounced effects. Additional studies will be required to identify the optimal dose for each single drug.
Since TGF-β and dexamethasone could mask the anabolic and anti-inflammatory effect of the compounds, these two essential chondrogenic components were removed from our culture media and replaced with TCM compounds. Among them, we reported bioactivity for Epi A, Epi C, 2-O-rhsII, Icariin, VA, PS, PCA, 4-HBA and 5-HMF, which could increase matrix production in human OA chondrocytes at different doses. Furthermore, at the transcriptional level in the inflammatory model, in pellets treated with Epi C, the expression of anabolic marker genes including COL-II and ACAN was significantly increased compared to the vehicle group. There is limited literature available on the anabolic effect of these compounds on articular chondrocytes. Icariin, as a widely-studied TCM compound with similar chemical structure as Epi C, has been proposed as a potential promoting compound for cartilage repair and can be used as a substitute for growth factors [36].
In contrast, the anti-inflammatory effects of certain TCM compounds are better characterized in the literature. Since IL-1β and TNF-α are key regulators of inflammatory pathways and induce the synthesis of MMPs, prostaglandins, aggrecanases [52] and other pro-inflammatory cytokines, these two cytokines were used to induce inflammation in our in vitro inflammatory model. Biochemical characterization of pellets (long term) demonstrated that adding 1 ng/mL of IL-1β and TNF-α significantly decreased extracellular matrix production of the samples. Although a certain donor variation was noticed, the same trend in three independent donors in response to inflammatory cytokines and after treatment with the compounds was observed. We showed that pellets (short term) treated with VA, Epi C, PS, PCA, 4-HBA and 5-HMF could reduce the inflammatory response, and expression of MMP13, the critical target gene in OA progression, was significantly inhibited [53,54]. In the groups treated with VA, Epi C, PCA, 4-HBA and 5-HMF, other catabolic marker genes including MMP1 and MMP3 were downregulated. Additionally, COX-2 was inhibited in all the treatment groups except 4-HBA and PS. COX-2 is recognized as an inflammatory enzyme which can promote other inflammatory mediators including prostaglandins. Inhibition of COX-2 can provide relief from the symptoms of inflammation and pain [55]. The mechanism of action of acetylsalicylic acid (Aspirin®), the first NSAID, involves the inhibition of COX-2 and NFκB. Aspirin® is developed from salicylates as the active components of natural Willow bark [56,57]. Interestingly, the chemical structures of VA, PCA and 4-HBA which are the extracts of Radix ET Rhizoma Salviae, is very similar to salicylates. Hence, these currently investigated small molecules with anti-inflammatory effects are likely to be active through hindering the NFκB signaling pathway. An in vivo study using a murine model of inflammatory pain showed that VA could inhibit pro-inflammatory cytokine production by suppressing NFκB activity [49], which supports our hypothesis on the mechanism of action of VA, PCA and 4-HBA. O-Vanilic acid, a derivative of VA, reduced pro-inflammatory cytokine levels by inhibiting LPS-induced degradation of IκBα and nuclear translocation of NFκB in LPS-stimulated macrophages [58]. It has been shown that low daily oral doses of 3- or 4-HBA in mice were acting like Aspirin® and salicylic acids in terms of anti-inflammatory and analgesic effects [59]. PCA demonstrated promising anti-inflammatory and analgesic activity in rat models of both acute and chronic inflammation which was comparable with standard anti-inflammatory therapy [60]. Besides these compounds, we also reported Epi C and 5-HMF extracted from Herba Epimedi, as potential candidates with anti-inflammatory effects. It was demonstrated 5-HMF could suppress VCAM-1 expression in TNF-α stimulated HUVECs through inhibition of NFκB signaling pathway [45]. The anti-inflammatory effects of Epimedium plant extracts, such as Icariin, that act by inhibiting TNF-α and pro-inflammatory cytokines has been previously studied [61,62,63], and Epi C as a novel extract of this plant showed anti-inflammatory effects in our inflammatory model. Further study will be required to elucidate the exact signaling mechanisms of the compounds in human OA chondrocytes.
Histological and immunohistochemical staining showed that, after induction of inflammation by IL-1β/TNF-α, Saf-O staining and the staining intensity of ECM proteins, such as COL-II and ACAN, were significantly reduced. After treating pellets (long term) with VA, Epi C, PS, PCA, 4-HBA and 5-HMF, an increase in intensity for Saf-O, COL-II and ACAN in comparison with the control vehicle group was observed. These observations confirmed our inflammatory model, as well as previous results in which these compounds could preserve chondrogenic phenotype after induction of inflammation. After two weeks of treatment in phase III (recovery phase), the histological data of the control positive group treated with dexamethasone and TGF-β, showed that ECM matrix proteins including COL-II, ACAN and proteoglycans were significantly induced. However, it has been proved that in the presence of dexamethasone and TGF-β, the cells progress towards the hypertrophic phenotype [64]. Furthermore, an in vivo study in an osteoarthritic mouse model showed that the high concentration of active TGF-β1 in subchondral bone could initiate pathological changes leading to progression of OA, while inhibition of TGF-β1 activity in subchondral bone reduced the degradation of articular cartilage [65]. Since our compounds are less potent than TGF-β1, the parts of the pellets which were not exposed to the compound supplemented medium (due to culture in non-adherent v-bottom 96-well plates) demonstrated limited matrix production. To have an equal transport of drugs throughout the pellet, a trans-well system allowing all parts of the pellet to be simultaneously exposed to the compounds may be utilized. Additionally, in future studies, we will encapsulate the selected compound(s) in a hydrogel towards development of a local intra-articular delivery system.
In the current study, human OA chondrocytes were used, as they are the most clinically relevant source of cells for screening of potential OA treatments. However, due to use of human cells isolated from macroscopically evident OA regions of the joint, limited numbers of primary cells were obtained; therefore, passaged chondrocytes were used. Although, to minimize the cell de-differentiation an established expansion medium was used that promotes cell proliferation and maintains cell differentiation ability [66]. In further pre-clinical studies, the efficacy of the compounds will be validated in an osteochondral explant culture model using a cartilage bioreactor under mechanical stimulation [67] and finally tested in an osteoarthritic animal model.
In summary, after screening of anabolic and anti-inflammatory effects of 34 bioactive compounds in different in vitro models of human OA chondrocytes, VA, Epi C, PS, PCA, 4-HBA and 5-HMF showed anti-inflammatory and anti-catabolic effects and could inhibit matrix degradation after induction of inflammation. Additionally, Epi C also induced an anabolic effect. These bioactive compounds, and combinations thereof, can be considered as anti-inflammatory and anabolic drugs for treatment of early OA. A local drug delivery system will be envisioned, as the permeability and bioavailability of the drugs in the joint are limited.
4. Materials and Methods
4.1. Drugs Screening on Human Osteoarthritic Chondrocytes
Thirty-four compounds (listed in Table 2) were investigated for their anabolic and/or anti-inflammatory effect (Chengdu Herbpurify Co.LTD and Chengdu Push Bio-Technology Co.LTD, Chengdu, China). The extraction method is shown in Supplementary Figure S1 [68]. All compounds were dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 10 mg/mL and stored at 4 °C. Chondrocytes were treated with different concentrations of compounds (1 µM, 10 µM, 25 µM, 50 µM), as indicated in the specific experiments. These concentrations were selected from previous in vitro studies, where positive effects with doses between 1 µM and 50 µM had been reported [36,42].
Table 2.
List of 34 tested Traditional Chinese Medicine (TCM) compounds extracted from XLGB with their chemical formula, molecular weight and component herb.
| No | Name of the Compound | Molecular Formula | Molecular Weight | Component Herb |
|---|---|---|---|---|
| 1 | 5-Hydroxymethylfurfural | C6H6O3 | 126 | H.E |
| 2 | Protocatechuicaldehyde | C7H6O3 | 138 | R.S |
| 3 | Vanilic acid | C8H8O4 | 168 | R.S |
| 4 | 4-Hydroxybenzoic acid | C7H6O3 | 138 | R.S |
| 5 | Chlorogenic acid | C16H18O9 | 354 | H.E |
| 6 | Cryptochlorogenic acid | C16H18O9 | 354 | H.E |
| 7 | Loganic acid | C16H24O10 | 376 | R.D |
| 8 | Loganin | C17H26O10 | 390 | R.D |
| 9 | Isobavachalcone | C20H20O4 | 324 | F.P |
| 10 | Sweroside | C16H22O9 | 358 | R.D |
| 11 | (+)-Cycloolivil | C20H24O7 | 376 | H.E |
| 12 | Baohuside I | C27H30O10 | 514 | H.E |
| 13 | 2′′-O-rhamnosylicariside II | C33H40O14 | 660 | H.E |
| 14 | Epimedin A | C39H50O2 | 838 | H.E |
| 15 | Epimedin B | C38H48O19 | 808 | H.E |
| 16 | Epimedin C | C39H50O19 | 822 | H.E |
| 17 | Isobavachin | C20H20O4 | 324 | F.P |
| 18 | Bavachin | C20H20O4 | 324 | F.P |
| 19 | Bavachinin | C21H22O4 | 338 | F.P |
| 20 | Neobavaisoflavone | C20H18O4 | 322 | F.P |
| 21 | Corylin | C20H16O4 | 320 | F.P |
| 22 | Epimedin A1 | C39H50O20 | 838 | H.E |
| 23 | Psoralen | C11H6O3 | 186 | F.P |
| 24 | Isopsoralen | C11H6O3 | 186 | F.P |
| 25 | (S)-Bukuchiol | C18H24O | 256 | F.P |
| 26 | Psoralidin | C20H16O5 | 336 | F.P |
| 27 | Asperosaponin VI | C47H76O18 | 390 | R.D |
| 28 | Baohuside II | C26H28O10 | 500 | H.E |
| 29 | Epimedoside A | C32H38O15 | 662 | H.E |
| 30 | Baohuside V | C39H50O19 | 822 | H.E |
| 31 | Corylifol A | C25H26O4 | 390 | F.P |
| 32 | 4′-Methylbavachalcone | C21H22O4 | 338 | F.P |
| 33 | Icaitin | C21H20O6 | 368 | H.E |
| 34 | Icariin | C33H40O15 | 676 | H.E |
H.E.: Herba Epimedi; R.S.: Radix ET Rhizoma Salviae; R.D.: Radix Dipsaci; F.P.: Fructus Psoraleae.
4.2. Isolation of Human Osteoarthritic Chondrocytes and Cell Expansion
Cartilage tissue was obtained from three patients suffering from end stage OA and undergoing total knee arthroplasty (ages 47, 60 and 82 years; all female) at the university hospital of Basel under ethical agreement (Ethikkommission beider Basel, Ref.Nr. EK: 78/07, 20. March 2007). The cells were isolated as described elsewhere [69]. Briefly, tissue samples were minced with a scalpel into small pieces and were digested overnight in 0.2% collagenase II (300 U/mg, Worthington Biochemical Corp, Lakewood, NJ, USA) on an orbital shaker at 37 °C. Then, the isolated chondrocytes were expanded for three passages to 80% confluency in basal medium (BM, Dulbecco’s modified Eagle medium, high glucose (DMEM)), 10 mM HEPES, 1 mM sodium pyruvate, 1% penicillin/streptomycin (P/S), 100 μg/mL streptomycin, and 0.29 mg/mL glutamate (all from Gibco, Paisley, UK), supplemented with 10% fetal bovine serum (FBS), 5 ng/mL fibroblast growth factor-2 (FGF-2) and 1 ng/mL transforming growth factor (TGF)ß1 (both from Fitzgerald, Acton, MO, USA) in a humidified incubator (37 °C, 5% CO2).
4.3. Cell Toxicity Assay
Cytotoxicity of the compounds was assessed using the WST-1 reagent (Roche Applied Science, Mannheim, Germany) [70]. Human osteoarthritic chondrocytes were seeded in 96-well plates at a density of 2000 cells per well in 100 µL of DMEM containing 4.5 g/L glucose and supplemented with 5% FBS and 1% penicillin–streptomycin (P/S) (all products from Gibco). Cells were cultured at 37 °C, 5% CO2, 19% oxygen and 90% humidity. After 24 h of incubation, the cells were treated with TCM compounds in four different concentrations (1 µM, 10 µM, 25 µM, 50µM) or DMSO alone at concentrations of 0.01%, 0.1%, 0.25%, 0.5%, as control vehicle groups. After 48 h of treatment, medium was removed and 100 µL of a 10% v/v solution of WST-1 reagent in DMEM supplemented with 2.5% FBS was added to each well. After 4 h of incubation at 37 °C, absorbance of the samples was measured against a background control with a microplate reader (Victor 3, PerkinElmer, Waltham, MA, US) at 490 nm, with reference wavelength of 600 nm [71]. For this assay and further analyses, all treatment groups in different concentrations were normalized to the respective control vehicle group. Since DMSO in these concentrations (lower than 1%) did not show any significant effect on cells metabolism, normalization with the highest concentration of DMSO resulted in similar outputs [72].
4.4. Anabolic Effects of the TCM Compounds on Osteoarthritic Chondrocytes Pellet Cultures
Chondrogenic effect of TCM compounds was assessed by culturing the expanded chondrocytes at passage 3 in pellets. Standard BM (high glucose DMEM) was supplemented with 1.25 mg/mL human serum albumin (Gibco, Life Technologies Limited, Paisley, UK), ITS- Premix (Corning, Bedford, MA, USA), 0.1 mM ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), and 1% P/S. Since the standard chondrogenic supplements TGF-β and dexamethasone could mask the anabolic effect of the compounds, these two essential components for chondrogenic differentiation were omitted from our culture medium (referred to as chondropermissive medium) and were replaced with the TCM compounds. Briefly, cells were re-suspended in chondropermissive medium as described before [73]. Aliquots of 1.5 × 105 cells/150 μL were centrifuged at 400 g for 5 min in v-bottom non-adherent 96-well plates (Thermo Fisher scientific, Waltham, MA, US). Pellets were cultured for 2 weeks in a humidified incubator (37 °C, 5% CO2) with a change of medium twice a week. Medium was supplemented with TCM compounds in four different final concentrations (1 μM, 10 μM, 25 μM, 50 μM) or control vehicle group containing 0.01%, 0.1%, 0.25%, 0.5% v/v DMSO.
4.5. Anti-Catabolic Effects of the TCM Compounds on Osteoarthritic Chondrocytes Pellet Cultures
Passage 3 chondrocytes were centrifuged at 400 g for 5 min (2.5 × 105 cells per pellet in 250 μL medium) in v-bottom, non-adherent 96-well plates and cultured in pellets using standard chondrogenic medium (chondropermissive medium containing 10 ng/mL TGFβ and 10-7 M dexamethasone (Sigma Aldrich)). After one week of culture in chondrogenic medium (short term), pellets were harvested for transcriptional analysis, and after two weeks of culture in chondrogenic medium (long term), pellets were harvested for biochemical and histochemical analysis (phase I). The remaining pellets were exposed to IL-1β and TNF-α (both from Peprotech, London, UK), each at 1 ng/mL, for 72 h (phase II), using an established inflammatory pellet culture model [74]. During this inflammatory phase, pellets were cultured in chondropermissive medium (chondrogenic medium deprived of TGF-β1 and dexamethasone) with or without TCM compound. Pellets were then harvested for analysis (phase II) or cultured two more weeks (phase III) in chondropermissive medium supplemented with TCM compounds or DMSO as control vehicle group. The most potent compounds in terms of their anabolic effects were selected for testing in this inflammatory model, in 3 different concentrations (1 μM, 10 μM, 25 μM). The positive control (ctr +) group was supplemented with 10 ng/mL TGF-β1 and 10-7 M dexamethasone (phase III). Cell pellets were maintained at 37 °C, 5% CO2, with medium changes twice per week. The different experimental groups are summarized in Table 3.
Table 3.
Description of experimental groups.
| Pellet Culture | Group | Culture Phase I (Cartilage Generation) |
Culture Phase II (IL-1β/TNFα Exposure; Inflammatory phase) |
Culture Phase III (Treatment) |
||
|---|---|---|---|---|---|---|
| Time | Time | Compound | Time | Compound | ||
| Control (IL-1β/TNFα) | 1 week | 3 days | -- | n/a | ||
| Short term | Treatment group (IL-1β/TNFα + compound) | 1 week | 3 days | + | n/a | |
| Control (IL-1β/TNFα) | 2 weeks | 3 days | -- | 2 weeks | -- | |
| Long term | Treatment group (IL-1β/TNFα - compound) | 2 weeks | 3 days | + | 2 weeks | + |
4.6. Biochemical Analysis
Pellets (n = 3) from three donors were digested with 0.5 mL of 1 mg/mL proteinase K at 56 °C for 16 h (Roche, Mannheim, Germany). Using 1,9 dimethylmethylene blue (DMMB) assay, the amount of sulfated glycosaminoglycan (GAG) within the pellets was measured spectrophotometrically with chondroitin sulphate as a standard (Sigma-Aldrich) [75]. The DNA content was measured spectrofluorometrically using PicoGreen (Invitrogene, Eugene, OR, USA) dye with calf thymus DNA (LuBio Science, Zurich, Switzerland) as a standard and according to the manufacturer’s instruction. The GAG content was normalized to the DNA content in all samples.
4.7. Gene Expression Analysis
For total RNA extraction, 1 mL of TRI reagent (Molecular Research Center) was added to the pellet. After homogenization by a Tissue Lyser for 3 min and 5 Hz (Qiagen, Hilden, Germany), the RNA was extracted using phase separation by 1-Bromo-3-chloropropane (Sigma) in a volume ratio of 1:10 with the TRI reagent. Reverse transcription was performed with SuperScript VILO cDNA Synthesis Kit (Life Technologies, Carlsbad, CA). Quantitative real-time PCR (qPCR) was accomplished using TaqMan Universal Master Mix (Applied biosystems, Foster City, CA, USA) and the Quant Studio 6 Flex Instrument and software (Applied biosystems) were used for detection. The gene expression assays and sequences of human primers and TaqMan probes for human 18S rRNA, MMP1, MMP3, MMP13, COX-2, COL-II and ACAN are shown in Table 4 (a, b). The relative gene expression was calculated using the 2-ΔΔCT quantification method [76], with 18S ribosomal RNA as endogenous control.
Table 4.
Oligonucleotide primers and probes (human) used for Real-Time PCR.
a. Primers and probes (Applied Biosystems).
| Gene | Probe Type | Assay ID |
|---|---|---|
| MMP-1 | 5′ FAM-3′ NFQ | Hs00899658_m1 |
| MMP-3 | 5′ FAM-3′ NFQ | Hs00968305_m1 |
| 18s fast | 5′ FAM-3′ NFQ | Hs99999901_s1 |
MMP1: matrix metalloproteinase 1; MMP3: matrix metalloproteinase 3; FAM: Carboxyfluorescein; NFQ: nonfluorescent quencher a. Human Gene Expression Assays (ThemoFisher Scientific). b.
b. Custom Designed Primer/Probe (Microsynth, Balgach, Switzerland).
| Gene | Primer/Probe Type | Sequence |
|---|---|---|
| MMP-13 | Primer forward (5′-3′) Primer reverse (5′-3′) Probe (5′ FAM/3′ TAMRA) |
CGGCCACTCCTTAGGTCTTG TTTTGCCGGTGTAGGTGTAGATAG CTCCAAGGACCCTGGAGCACTCATGT |
| COX-2 | Primer forward (5′-3′) Primer reverse (5′-3′) Probe (5′ FAM/3′ TAMRA) |
TTGTACCCGGACAGGATTCTATG TGTTTGGAGTGGGTTTCAGAAATA GAAAACTGCTCAACACCGGAATTTTTGACAA |
| Col2a1 | Primer forward (5′-3′) Primer reverse (5′-3′) Probe (5′ FAM/3′ TAMRA) |
GGCAATAGCAGGTTCACGTACA GATAACAGTCTTGCCCCACTTACC CCTGAAGGATGGCTGCACGAAACATAC |
| ACAN | Primer forward (5′-3′) Primer reverse (5′-3′) Probe (5′ FAM/3′ TAMRA) |
AGTCCTCAAGCCTCCTGTACTCA CGGGAAGTGGCGGTAACA CCGGAATGGAAACGTGAATCAGAATCAACT |
COL2A1: collagen type II; MMP13: matrix metalloproteinase 13; COX-2: cyclooxygenase-2; ACAN: aggrecan; FAM: Carboxyfluorescein; TAMRA: Tetramethylrhodamine.
4.8. Histological and Immunohistochemical Analysis
Pellets were fixed in 70% methanol and incubated overnight in 5% sucrose solution at 4 °C. Then, the samples were embedded in cryo-compound and were sectioned with cryostat at 8 µm thickness. To visualize cells, proteoglycan and the collagen deposition, slides were first stained with Weigert’s Haematoxylin for 10 min. Sections were stained with 0.02% Fast green in ultrapure (ddH2O) water for 5 min to reveal collagen deposition, then stained with 0.1% Saf-O for 12 min to show proteoglycan deposition, and finally rinsed in dH2O and sequentially differentiated in 70%, 96% and 100% ethanol. The presence of ACAN and COL-II was identified immunohistochemically using antibodies against ACAN (1-C-6) and COL-II (CIIC1), both from Developmental Studies Hybridoma Bank, University of Iowa). Briefly, after enzyme treatment with hyaluronidase (Sigma-Aldrich, St. Louse, MO, USA) for COL-II and chondroitinase (Sigma-Aldrich) for 1C6, non-specific binding sites were blocked with horse serum (Vector Laboratories, Burlingame, CA, USA) for 1h at RT. The primary antibody (5 µg/mL) was incubated for 30 min and detected using secondary biotinylated anti-mouse antibody (Vector Laboratories) followed by incubation with avidin-biotin-peroxidase complex (Vectastain ABC Kit, Mouse IgG, Vector Laboratories). Peroxidase activity was visualized using diaminobenzidine (DAB) as a substrate (ImmPACT DAB, Substrate for Peroxidase, Vector Laboratories) [77].
4.9. Statistical Analysis
Statistical comparisons were performed using Graphpad Prism 7. One-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (multiple comparison) or Tukey’s post hoc test (multiple comparison) was applied as non-parametric test of three independent experiments with different human osteoarthritic chondrocytes. To assess statistical significance among the groups, differences were considered statistically significant at p < 0.05. All Graphs are displayed as box plots.
Acknowledgments
The CIICI and aggrecan antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA, USA.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/22/5745/s1.
Ethic Approval and Consent to Participate
Cartilage tissue was obtained from three patients suffering from end stage OA and undergoing total knee arthroplasty (ages 47, 60 and 82 years; all female) at the university hospital of Basel under ethical agreement (Ethikkommission beider Basel, Ref.Nr. EK: 78/07, 20. March 2007).
Author Contributions
R.Z., S.G. and M.A. had full access to all the data presented in this study and take responsibility for the integrity and accuracy of the data. Conception and design: R.Z., A.B., M.J.S., Z.L., I.M., X.-l.W., L.Q., M.A. and S.G. Collection and assembly of the data: R.Z., M.W. Analysis and interpretation of the data: R.Z., A.B., M.J.S., I.M., X.-l.W., L.Q., M.A. and S.G. Drafting of the article: R.Z. and S.G. Critical revision of the article for important intellectual content and final approval of the article: R.Z., A.B., M.J.S., Z.L., I.M., X.-l.W., L.Q., M.A. and S.G. Provision of study materials: R.Z., A.B., X.-l.W., L.Q., I.M.
Funding
The study was funded by the AO Foundation and the Swiss National Science Foundation (SNF) under the SSSTC program with grant number 156362.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A., Gabriel S., Hirsch R., Hochberg M.C., Hunder G.G., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace I.J., Worthington S., Felson D.T., Jurmain R.D., Wren K.T., Maijanen H., Woods R.J., Lieberman D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA. 2017;114:9332. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobasheri A. The future of osteoarthritis therapeutics: Emerging biological therapy. Curr. Rheumatol. Rep. 2013;15:385. doi: 10.1007/s11926-013-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shane Anderson A., Loeser R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010;24:15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carames B., Taniguchi N., Otsuki S., Blanco F.J., Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeser R.F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriatr. Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan Y., Grodzinsky A.J. Cartilage diseases. Matrix Biol. J. Int. Soc. Matrix Biol. 2018:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clockaerts S., Bastiaansen-Jenniskens Y.M., Runhaar J., Van Osch G.J., Van Offel J.F., Verhaar J.A., De Clerck L.S., Somville J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. Osteoarthr. Res. Soc. 2010;18:876–882. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Williams A., Kamper S.J., Wiggers J.H., O’Brien K.M., Lee H., Wolfenden L., Yoong S.L., Robson E., McAuley J.H., Hartvigsen J., et al. Musculoskeletal conditions may increase the risk of chronic disease: A systematic review and meta-analysis of cohort studies. BMC Med. 2018;16:167. doi: 10.1186/s12916-018-1151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tellegen A.R., Rudnik-Jansen I., Pouran B., de Visser H.M., Weinans H.H., Thomas R.E., Kik M.J.L., Grinwis G.C.M., Thies J.C., Woike N., et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018;25:1438–1447. doi: 10.1080/10717544.2018.1482971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cokelaere S.M., Plomp S.G.M., de Boef E., de Leeuw M., Bool S., van de Lest C.H.A., van Weeren P.R., Korthagen N.M. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur. J. Pharm. Biopharm. 2018;128:327–336. doi: 10.1016/j.ejpb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 12.van den Driest J.J., Pijnenburg P., Bindels P.J.E., Bierma-Zeinstra S.M.A., Schiphof D. Analgesic Use in Dutch Patients with Osteoarthritis: Frequent but Low Doses. J. Clin. Rheumatol. 2018 doi: 10.1097/RHU.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 13.Solomon D.H., Husni M.E., Libby P.A., Yeomans N.D., Lincoff A.M., Lupsilonscher T.F., Menon V., Brennan D.M., Wisniewski L.M., Nissen S.E., et al. The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial. Am. J. Med. 2017;130:1415–1422.e1414. doi: 10.1016/j.amjmed.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T., Koch G., Lei H., Mangal B., Sokka T., Moskowitz R., Wolfe F., Gibofsky A., Simon L., Zlotnick S., et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): Two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann. Rheum. Dis. 2004;63:931–939. doi: 10.1136/ard.2003.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz R.W., Abramson S.B., Berenbaum F., Simon L.S., Hochberg M. Coxibs and NSAIDs--is the air any clearer? Perspectives from the OARSI/International COX-2 Study Group Workshop 2007. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2007;15:849–856. doi: 10.1016/j.joca.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Marcu K.B., Otero M., Olivotto E., Borzi R.M., Goldring M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urech D.M., Feige U., Ewert S., Schlosser V., Ottiger M., Polzer K., Schett G., Lichtlen P. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNFa single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann. Rheum. Dis. 2010;69:443–449. doi: 10.1136/ard.2008.105775. [DOI] [PubMed] [Google Scholar]
- 18.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rovati L.C., Girolami F., Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: Efficacy, safety, and pharmacokinetic properties. Ther. Adv. Musculoskelet. Dis. 2012;4:167–180. doi: 10.1177/1759720X12437753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jevsevar D., Donnelly P., Brown G.A., Cummins D.S. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J. Bone Jt. Surg. Am. Vol. 2015;97:2047–2060. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 21.Clough T.M., Alvi F., Majeed H. Total ankle arthroplasty: What are the risks? Bone Jt. J. 2018:1352–1358. doi: 10.1302/0301-620X.100B10.BJJ-2018-0180.R1. [DOI] [PubMed] [Google Scholar]
- 22.Ronn K., Reischl N., Gautier E., Jacobi M. Current surgical treatment of knee osteoarthritis. Arthritis. 2011;2011:454873. doi: 10.1155/2011/454873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W., Moskowitz R.W., Nuki G., Abramson S., Altman R.D., Arden N., Bierma-Zeinstra S., Brandt K.D., Croft P., Doherty M., et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Zhu F., Ma X.H., Qin C., Tao L., Liu X., Shi Z., Zhang C.L., Tan C.Y., Chen Y.Z., Jiang Y.Y. Drug discovery prospect from untapped species: Indications from approved natural product drugs. PLoS ONE. 2012;7:e39782. doi: 10.1371/journal.pone.0039782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 26.Toegel S., Wu S.Q., Otero M., Goldring M.B., Leelapornpisid P., Chiari C., Kolb A., Unger F.M., Windhager R., Viernstein H. Caesalpinia sappan extract inhibits IL1beta-mediated overexpression of matrix metalloproteinases in human chondrocytes. Genes Nutr. 2012;7:307–318. doi: 10.1007/s12263-011-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussaieff A., Shohami E., Kashman Y., Fride E., Schmitz M.L., Renner F., Fiebich B.L., Munoz E., Ben-Neriah Y., Mechoulam R. Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-kappa B activation. Mol. Pharmacol. 2007;72:1657–1664. doi: 10.1124/mol.107.038810. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.H., Kismali G., Gupta S.C. Natural products for the prevention and treatment of chronic inflammatory diseases: Integrating traditional medicine into modern chronic diseases care. Evid. Based Complementary Altern. Med. eCAM. 2018;2018:9837863. doi: 10.1155/2018/9837863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobasheri A., Henrotin Y., Biesalski H.K., Shakibaei M. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int. J. Mol. Sci. 2012;13:4202–4232. doi: 10.3390/ijms13044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.J., Tsai K.S., Chan D.C., Lan K.C., Chen C.F., Yang R.S., Liu S.H. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 2014;32:573–580. doi: 10.1002/jor.22577. [DOI] [PubMed] [Google Scholar]
- 31.Gui L., Shen H. Application of Xianlinggubao in bone and arthrosis disease. Chin. J. New Drugs Clin. Rem. 2007;26:619–622. [Google Scholar]
- 32.Zhu H.M., Qin L., Garnero P., Genant H.K., Zhang G., Dai K., Yao X., Gu G., Hao Y., Li Z., et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. National Osteoporos. Found. USA. 2012;23:1317–1327. doi: 10.1007/s00198-011-1577-2. [DOI] [PubMed] [Google Scholar]
- 33.Li Z.R., Cheng L.M., Wang K.Z., Yang N.P., Yang S.H., He W., Wang Y.S., Wang Z.M., Yang P., Liu X.Z., et al. Herbal Fufang Xian Ling Gu Bao prevents corticosteroid-induced osteonecrosis of the femoral head-A first multicentre, randomised, double-blind, placebo-controlled clinical trial. J. Orthop. Transl. 2018;12:36–44. doi: 10.1016/j.jot.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F., Shi L., Zhang Y., Wang K., Pei F., Zhu H., Shi Z., Tao T., Li Z., Zeng P., et al. A traditional herbal formula xianlinggubao for pain control and function improvement in patients with knee and hand oSteoarthritis: A multicenter, randomized, open-label, controlled trial. Evid. Based Complementary Altern. Med. eCAM. 2018;2018:1827528. doi: 10.1155/2018/1827528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y., Tu F.J., Yao Z.H., Ding B., Xu W., Qiu X.H., Yao X.S. Rapid identification of chemical constituents in traditional Chinese medicine fufang preparation xianling gubao capsule by LC-linear ion trap/Orbitrap mass spectrometry. Am. J. Chin. Med. 2013;41:1181–1198. doi: 10.1142/S0192415X13500808. [DOI] [PubMed] [Google Scholar]
- 36.Li D., Yuan T., Zhang X., Xiao Y., Wang R., Fan Y., Zhang X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2012;20:1647–1656. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Li C., Li Q., Mei Q., Lu T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015;126:57–68. doi: 10.1016/j.lfs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Pietschmann P., Mechtcheriakova D., Meshcheryakova A., Foger-Samwald U., Ellinger I. Immunology of Osteoporosis: A Mini-Review. Gerontology. 2016;62:128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginaldi L., Di Benedetto M.C., De Martinis M. Osteoporosis, inflammation and ageing. Immun. Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faienza M.F., Ventura A., Marzano F., Cavallo L. Postmenopausal osteoporosis: The role of immune system cells. Clin. Dev. Immunol. 2013;2013:575936. doi: 10.1155/2013/575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapir-Koren R., Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone. 2017;103:102–115. doi: 10.1016/j.bone.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Mathy-Hartert M., Jacquemond-Collet I., Priem F., Sanchez C., Lambert C., Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009;58:899–908. doi: 10.1007/s00011-009-0063-1. [DOI] [PubMed] [Google Scholar]
- 43.Csaki C., Mobasheri A., Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X., Shi D., Shen Y., Xu Z., Dai J., Chen D., Teng H., Jiang Q. Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res. Ther. 2015;17:20. doi: 10.1186/s13075-015-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H.K., Choi Y.W., Lee E.N., Park J.K., Kim S.G., Park D.J., Kim B.S., Lim Y.T., Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFalpha-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-kappaB activation. Phytother. Res. PTR. 2011;25:965–974. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- 46.Zeng L., Wang W., Rong X.F., Zhong Y., Jia P., Zhou G.Q., Li R.H. Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model. Int. Immunopharmacol. 2014;18:175–181. doi: 10.1016/j.intimp.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Wang P., Zhang F., He Q., Wang J., Shiu H.T., Shu Y., Tsang W.P., Liang S., Zhao K., Wan C. Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1alpha in Chondrocytes and Promotes Articular Cartilage Repair. PLoS ONE. 2016;11:e0148372. doi: 10.1371/journal.pone.0148372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kankala R.K., Lu F.J., Liu C.G., Zhang S.S., Chen A.Z., Wang S.B. Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials. 2018;11 doi: 10.3390/ma11081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calixto-Campos C., Carvalho T.T., Hohmann M.S., Pinho-Ribeiro F.A., Fattori V., Manchope M.F., Zarpelon A.C., Baracat M.M., Georgetti S.R., Casagrande R., et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFkappaB Activation in Mice. J. Nat. Prod. 2015;78:1799–1808. doi: 10.1021/acs.jnatprod.5b00246. [DOI] [PubMed] [Google Scholar]
- 50.Rao Z., Wang S., Wang J. Protective effects of psoralidin on IL1betainduced chondrocyte apoptosis. Mol. Med. Rep. 2018;17:3418–3424. doi: 10.3892/mmr.2017.8248. [DOI] [PubMed] [Google Scholar]
- 51.Cao H.J., Li C.R., Wang L.Y., Ziadlou R., Grad S., Zhang Y., Cheng Y., Lai Y.X., Yao X.S., Alini M., et al. Effect and mechanism of psoralidin on promoting osteogenesis and inhibiting adipogenesis. Phytomedicine. 2019;61:152860. doi: 10.1016/j.phymed.2019.152860. [DOI] [PubMed] [Google Scholar]
- 52.Echtermeyer F., Bertrand J., Dreier R., Meinecke I., Neugebauer K., Fuerst M., Lee Y.J., Song Y.W., Herzog C., Theilmeier G., et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nature Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 53.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.J., Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H., Wang D., Yuan Y., Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017;19:248. doi: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laine L., White W.B., Rostom A., Hochberg M. COX-2 selective inhibitors in the treatment of osteoarthritis. Semin. Arthritis Rheum. 2008;38:165–187. doi: 10.1016/j.semarthrit.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Rainsford K.D. Anti-inflammatory drugs in the 21st century. Sub-Cell. Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 57.Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 58.Lee J.-K., Lee S.L., Shin T.-Y.S., Khang D.K., Kim S.-H.K. Anti-inflammatory effect of o-vanillic acid on lipopolysaccharide-stimulated macrophages and inflammation models. J. Food Nutr. Res. 2018;6:227–233. doi: 10.12691/jfnr-6-4-4. [DOI] [Google Scholar]
- 59.Khan S.A., Chatterjee S.S., Kumar V. Low dose aspirin like analgesic and anti-inflammatory activities of mono-hydroxybenzoic acids in stressed rodents. Life Sci. 2016;148:53–62. doi: 10.1016/j.lfs.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 60.Lende A.B., Kshirsagar A.D., Deshpande A.D., Muley M.M., Patil R.R., Bafna P.A., Naik S.R. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology. 2011;19:255–263. doi: 10.1007/s10787-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 61.Ti H., Wu P., Xu L., Wei X. A novel icariin type flavonoid from Epimedium pseudowushanense. Nat. Prod. Res. 2018:1–8. doi: 10.1080/14786419.2018.1481840. [DOI] [PubMed] [Google Scholar]
- 62.Oh Y.C., Jeong Y.H., Cho W.K., Ha J.H., Lee S.J., Ma J.Y. Inhibitory Effects of Epimedium Herb on the Inflammatory Response In Vitro and In Vivo. Am. J. Chin. Med. 2015;43:953–968. doi: 10.1142/S0192415X1550055X. [DOI] [PubMed] [Google Scholar]
- 63.Liu M.H., Sun J.S., Tsai S.W., Sheu S.Y., Chen M.H. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr. Res. 2010;30:57–65. doi: 10.1016/j.nutres.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 64.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 65.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., Askin F.B., Frassica F.J., Chang W., Yao J., et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakob M., Demarteau O., Schafer D., Hintermann B., Dick W., Heberer M., Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::AID-JCB1051>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 67.Vainieri M.L., Wahl D., Alini M., van Osch G., Grad S. Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies. Acta Biomater. 2018;81:256–266. doi: 10.1016/j.actbio.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 68.Tu F., Dai Y., Yao Z., Wang X., Yao X., Qin L. Flavonol glycosides from Epimedium pubescens. Chem. Pharm. Bull. 2011;59:1317–1321. doi: 10.1248/cpb.59.1317. [DOI] [PubMed] [Google Scholar]
- 69.Müller S., Acevedo L., Wang X., Karim M.Z., Matta A., Mehrkens A., Schaeren S., Feliciano S., Jakob M., Martin I., et al. Notochordal cell conditioned medium (NCCM) regenerates end-stage human osteoarthritic articular chondrocytes and promotes a healthy phenotype. Arthritis Res. Ther. 2016;18:125. doi: 10.1186/s13075-016-1026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S.Q., Saijo K., Todoroki T., Ohno T. Induction of human autologous cytotoxic T lymphocytes on formalin-fixed and paraffin-embedded tumour sections. Nat. Med. 1995;1:267–271. doi: 10.1038/nm0395-267. [DOI] [PubMed] [Google Scholar]
- 71.Li Z., Lang G., Chen X., Sacks H., Mantzur C., Tropp U., Mader K.T., Smallwood T.C., Sammon C., Richards R.G., et al. Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials. 2016;84:196–209. doi: 10.1016/j.biomaterials.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 72.de Abreu Costa L., Henrique Fernandes Ottoni M., Dos Santos M.G., Meireles A.B., Gomes de Almeida V., de Fatima Pereira W., Alves de Avelar-Freitas B., Eustaquio Alvim Brito-Melo G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-alpha, IFN-gamma, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules. 2017;22 doi: 10.3390/molecules22111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neumann A.J., Gardner O.F., Williams R., Alini M., Archer C.W., Stoddart M.J. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS ONE. 2015;10:e0136229. doi: 10.1371/journal.pone.0136229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scotti C., Osmokrovic A., Wolf F., Miot S., Peretti G.M., Barbero A., Martin I. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1beta and low oxygen. Tissue Eng. Part A. 2012;18:362–372. doi: 10.1089/ten.tea.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitley C.B., Ridnour M.D., Draper K.A., Dutton C.M., Neglia J.P. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin. Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 76.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 77.Milz S., Aktas T., Putz R., Benjamin M. Expression of extracellular matrix molecules typical of articular cartilage in the human scapholunate interosseous ligament. J. Anat. 2006;208:671–679. doi: 10.1111/j.1469-7580.2006.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.