Abstract
Tetraspanin CD151 is increasingly implicated as a multifaceted mediator of cancer development and progression. Here we investigated the role of CD151 in breast cancer in the context of the Wnt oncogenic activation. Our data showed that removal of one or both of CD151 alleles in the MMTV-Wnt1 model significantly decreased the tumor-free survival of mice from 34 weeks on average to 22 weeks and 18 weeks, respectively. This effect coincided with an accelerated tumor growth and an increased number of Ki-67+ proliferative cells. Mechanistically, the CD151-deficient tumors were largely ER+, and exhibited hyperactivation of the Wnt pathway as reflected by a marked upregulation in β-catenin and Cyclin D1, and their target genes. In addition, E-cadherin displayed a cytosolic distribution and transcription factor Snail was markedly upregulated. Collectively, this data implies that CD151 suppresses the Wnt1-driven tumorigenesis, at least in part, via counteracting the epithelial-mesenchymal transition (EMT)-like program in luminal epithelial cells. Meanwhile, the proportion of tumor cells expressing CK5 or p63, the biomarkers of myoepithelial/basal cells, markedly decreased in the absence of CD151. This change was accompanied by a decreased invasiveness of tumors and their incompetence to form a long-term cell culture. Consistent with this basal cell-linked role, the CD151 downregulation impairs mammosphere formation in MCF-10A cells and the defect was rescued by re-expression of intact CD151 ORF, but not its integrin binding-defective mutant. Overall, our study suggests that CD151 is a key player in the Wnt oncogene-driven tumorigenesis and impacts breast cancer malignancy in a cell type-dependent manner.
Introduction
It is increasingly evident that the Wnt pathway plays a key role in breast tumorigenesis and cancer progression [1], [2]. Extensive clinical studies have shown that a number of Wnt ligands, along with key components of their receptor complexes, including a number of receptors (e.g., Frizzles) and co-receptor (e.g., LRP5/6), and effectors or target genes, are frequently overexpressed in breast tumors [1], [2], [3]. These deregulations are associated with poor clinical response to the current therapies and early recurrence of the disease [2]. In addition, there are mutations or deletions of E-cadherin and APC, the prominent repressors of the canonical Wnt pathway, in breast primary tumors [4]. Mechanistically, activation of the canonical Wnt pathway appears to promote expression of target genes involved in cell proliferation, survival and metabolism [5]. It is also implicated in regulating the capability of breast tumor-initiating cells or cancer stem cells (CSCs) [6], [7]. More recently, high susceptibility of differentiated and non-differentiated epithelial cells to the oncogenic targeting of the Wnt pathway has also been implicated in intrinsic intratumoral heterogeneity of breast cancer, particularly the triple-negative subtype (TNBC) [8]. Hence, understanding how the Wnt pathway is regulated during tumorigenesis may provide a new biological basis for therapeutic targeting of breast cancer malignancy.
Integrins, a class of extracellular matrix-binding adhesion receptors, are strongly implicated in regulation of the Wnt pathway in breast cancer [9]. To date, most of the integrins appear to promote Wnt signaling [10], [11]. Notably, members of the RGD based integrin subfamily, such as α 2 β 1 and α 5 β 1 integrins, along with their signaling effectors (e.g., ILK), appear to act cooperatively with the Wnt pathway to affect tumorigenic or metastatic processes [12]. Meanwhile, we and others have recently found that α 3 β 1, a laminin-binding (LB) integrin, appears to act as a suppressor of the Wnt pathway via stabilizing the E-cadherin-mediated cell to cell contact during oncogenic transformation [13], [14], [15], [16]. To a certain extent, this functional link is reminiscent of the genetic or epithelia-mesenchymal transition (EMT)-associated change, making β -catenin available to sustain the Wnt signaling [17], [18], [19]. Hence, integrins may regulate the Wnt oncogenic pathway-initiated tumorigenic process via diverse molecular mechanisms.
The close functional link between integrins and the Wnt signaling in breast cancer is also highlighted by recent studies of tetraspanins, a group of integrin-associated cell surface molecules [20]. Multiple members of the tetraspanin family, including CD151, CD9, CD82 and TSPAN12, are implicated in regulating tumorigenesis in the context of the Wnt signaling [21], [22], [23], [24]. Interestingly, some of these molecules, like the α 3 β 1 integrin, appears to antagonize the tumorigenic activity of the Wnt pathway via regulating the E-cadherin-dependent cell to cell adhesion [15], [25]. In contrast, others, such as TSPAN12, appear directly to participate in the activation of the Wnt1 receptor complexes and subsequent action of β –catenin [26], [27]. A similar scenario appears to occur in CD151, a strong regulator of LB integrin function and signaling [28], [29], [30]. Notably, CD151 promotes tumor growth in some breast cancer subtypes [31], [32]. It also suppresses tumor growth by mediating the stability of the E-cadherin/ β -catenin complexes [15], [25]. Conceivably, tetraspanin CD151, along with its integrin partners, may represent a unique class of regulators for cellular functions and signal transduction of the Wnt pathway in human cancer.
In the current study, we investigated functional and signaling roles of the integrin-associated CD151 in the de novo mammary tumorigenesis driven by the Wnt1 oncogene. The effect of CD151 deletion on tumor formation and growth was analyzed with the MMTV-Wnt1 transgenic model. The underlying mechanisms were dissected by analyzing the effect of CD151 ablation/knockdown or mutants on cellular activities and signaling in breast cancer cells. Our data shows that removal of CD151 gene leads to hyperactivation of the canonical Wnt/β-catenin signaling pathway, thereby accelerating growth of the luminal epithelial cell-derived mammary tumors. The CD151 deletion also appears to concomitantly affect development of myoepithelial/basal cell-derived mammary tumors in vivo. This effect coincides with altered cell survival, the tumor-initiating capability and activation of the Ras/MAPK pathway. Overall, our study demonstrates a strong epithelial cell type-dependent role of CD151 in the Wnt oncogene-driven tumorigenesis, and provides a new basis for targeting the tetraspanin-integrin complexes for breast cancer treatment.
Materials and methods
Mice and analyses of de novo tumor formation
The MMTV-Wnt1 (Mouse mammary tumor virus -Wnt1) transgenic mouse tumor model was obtained from The Jackson Laboratory (Bar Harbor, ME). The generation CD151-targeted mouse was described in a prior study [33]. Mouse littermates used for whole-mount experiments included the offspring of the intercrossing of CD151+/- FVB/Sv129 mice which were then backcrossed onto FVB background for two to three consecutive generations as described in our prior study [31]. Genotyping was done at the beginning and end of in vivo experiments for CD151 and Wnt1 genes. At the end of in vivo tumor growth assessment, all experimental animals were humanely sacrificed by CO2 inhalation in accordance to the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky.
Antibodies, reagents and cell culture
The primary antibodies used for the studies were described in Table S1. Antibodies for Cytokeratin (CK) 5/6 and CK-14 were purchased from Covance (Princeton, NJ) and CK-18 from Abcam (Cambridge, MA). The antibodies against smooth muscle actin (SMA) and Ki67 were obtained from Sigma-Aldrich (St. Louis, MO) and Thermo-Fisher Scientific (Waltham, MA), respectively. Antibodies to Snail, Slug, Axin1, cleaved Caspase 3 and β -catenin, as well as to total and/or phosphorylated forms of Erk1/2 and p38MAPK, were obtained from Cell Signaling Technology (Danvers, MA). Antibodies to human integrins and tetraspanins were raised in house [34]. Rabbit polyclonal antibody against human α3 cytoplasmic tails was raised in house [35].
The immortalized MCF-10A cells were maintained in a F12/DMEM medium supplemented 5% horse serum, 20 ng/ml EGF, 0.25 µg/ml Hydrocortisone, 100 ng/ml Cholera toxin and 10 µg/ml Insulin. Breast cancer cell lines (HCC1937 and MDA-MB-231) were purchased from ATCC (Manassas, VA) and cultured in defined medium [34] or RPMI-1640 medium (Life Technologies, Carlsbad, CA) supplemented with 10% FBS and 10 mM HEPES. All cell cultures were performed at 37 °C and 5% CO2. Hydrocortisone and insulin were obtained from Sigma-Aldrich; bFGF and EGF were from R&D Systems (Minneapolis, MN).
Analysis of cell survival, tumorsphere formation and generation of mouse tumor cell lines
Cell viability, cell cycle, apoptosis and signaling
To evaluate apoptotic cell death, tumor cells were stained with a combination of propidium iodide and APC-conjugated Annexin V (10 μg/ml, BioLegend), and analyzed by flow cytometry at the core facility. For the tumorsphere assay, MCF-10A cells were allowed to grow in suspension (24–well ultra-low adhesion plate) in a stem cell culture medium, which consisted of mammary epithelial basal medium (MEBM) (Lonza, Basel, Switzerland) supplemented with B27 mixture (Life Technologies, Carlsbad, CA), 20 ng/ml bFGF, 20 ng/ml EGF and 4 µg/ml Heparin, over a span of 5–7 days, and followed by imagining microscopically. The size, number and morphologies of mammosphere or colonies were quantified by analyzing the photographed representative fields using Nikon NIS-Elements Advanced Analysis Software [31].
To generate stable cell lines from primary tumors in the MMTV-Wnt1 mice, fresh tumor tissues were digested in collagenase overnight. After filtering with 40 µm strainers, cell mixtures were cultured for 3–5 generations in a F12/DMEM medium supplemented with 5% fetal bovine serum, 10 ng/ml EGF, 0.25 µg/ml hydrocortisone, 100 ng/ml Cholera toxin and 10 µg/ml insulin.
Transfection, cell sorting and immunofluorescence
Stable knockdown of > 90% of CD151 proteins in human immortalized or tumorigenic cell line (MCF-10A, MDA-MB-231 and HCC1937) were carried out using lentiviral infection, and confirmed by FACS analysis or immunoblotting with CD151 antibodies [34]. For immunofluorescence (IF) analyses, cells were stained alive on ice with antibodies and then fixed in 2% paraformaldehyde, according to protocols previously described [34]. The stained cells were imaged under confocal microscope.
Signaling and immunoblot analyses
The protein samples were prepared by lysing cultured cells or mammary tumor tissues in RIPA buffer supplemented with 1 mM NaV3O4, PMSF and protease inhibitor cocktail (Roche, Basel, Switzerland) [34]. Protein concentrations were determinate using the DC Protein Assay Kit (Bio-Rad, Hercules, CA). Western blotting was conducted by separating proteins on SDS-PAGE, transferring onto nitrocellulose membrane prior and detecting with the indicated antibodies and Amersham ECL Detection kit (GE Healthcare Life Sciences, Piscataway, NJ).
Gene expression profiling analysis
The mammary tumor tissues from CD151 wildtype and null mice (n = 3–5) were snap-frozen and extracted for total RNA by using TRIZOL method (Invitrogen). After quality control analysis, RNA samples were analyzed with Affymetrix 430_0.2 mouse DNA array chips. The difference between control and null groups were assessed by the paired t-test.
Whole-mount and IHC analyses of mammary glands and tumor tissues
The fourth and/or ninth inguinal mammary glands from 6 week old female virgin mice were subjected to the whole-mount analysis. In brief, freshly isolated mammary glands were mounted onto glass slides and then fixed in Carnoy’s solution overnight, followed by washing, de-fatting and imaging. For IHC analysis, tumor tissues or murine internal organs were fixed in 10% neutral formalin (Sigma-Aldrich, St. Louis, MO). Fixed kidney tissues were then rinsed and stored in 70% ethanol for hematoxylin and eosin (H&E) or IHC analyses to verify the CD151 phenotype. During the IHC analysis, the antigen retrieval was conducted using 0.1% EDTA. Tissue slices were stained with primary antibodies and biotin-conjugated secondary antibodies, followed by incubation with Avidin and detection (BD Biosciences, San Jose, CA). All IHC images were taken with a Nikon Automated Eclipse Ti-E inverted microscope.
Statistical analyses
The log-rank test was applied for analyses of differences in tumor-free survival between mice with varying CD151 genotype, and the correlation/association between CD151 and related pathways. The differences in cell proliferation index were assed with the paired t-test.
Results
Effect of CD151 ablation on latency and growth of mammary tumors in the MMTV-Wnt1 model
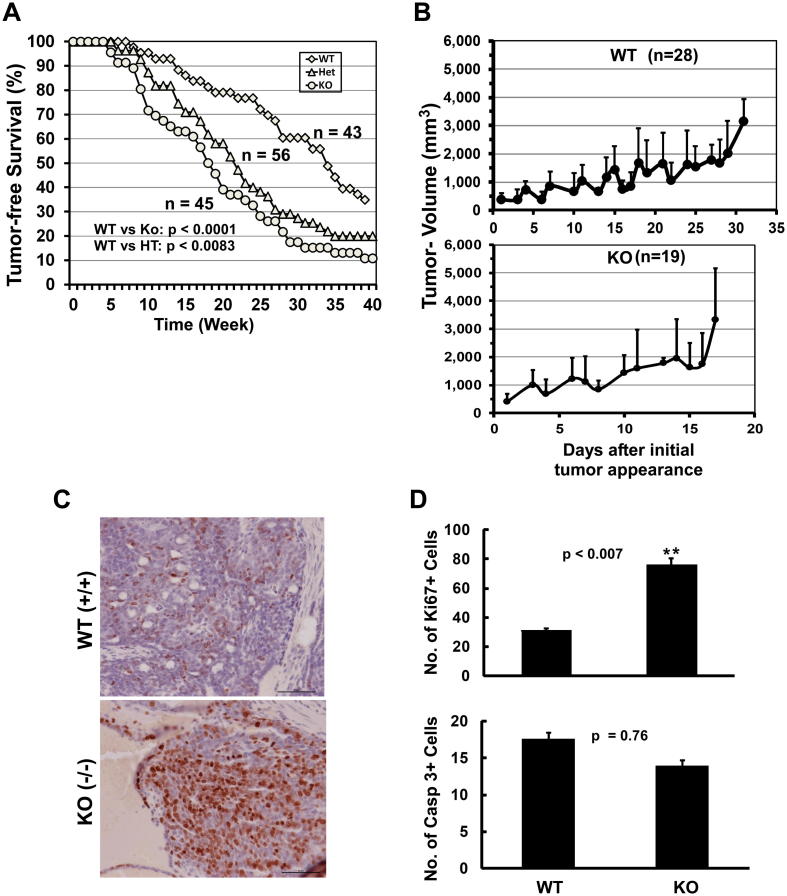
To evaluate the link between tetraspanin, CD151, and the Wnt signaling pathway during breast cancer development, we examined the effects of CD151 deletion on mammary tumor development in the MMTV-Wnt1 transgenic model. Compared to the control group, removal of one or both Cd151 alleles decreased tumor latency from 34 weeks to 17 weeks (Fig. 1A). Also, on average, the mammary tumors from the CD151-null mouse cohort grew to 3,300 mm3 within 17 days, while the control group took 32 days to grow the same volume (Fig. 1B). In line with this growth-repressive effect of CD151, the number of Ki-67-positive cells in the CD151-deficient mammary tumors was significantly higher than the control group (Fig. 1C, D). By comparison, there was a minimal difference in the number of cleaved Caspase 3-positive tumor cells (Fig. 1D). Collectively, this data illustrates a strong suppressive role of CD151 in the Wnt1 oncogene-driven mammary tumor formation/growth, and the effect is primarily associated with a deregulation of tumor cell proliferation.
Fig. 1.
Effect of CD151 deletion on de novo formation and growth of mammary tumors in the Wnt1-driven tumor model. A The Kaplan-Meier’s plot of CD151 removal and the tumor-free survival. The 3 week-old MMTV-Wnt1-expressing mice varying in CD151 genotypes, that is, wild-type (WT), heterozygous (Het or HT), knockout (KO) were subjected to the weekly examination for palpable mammary tumors. The survival time of tumor-bearing mice was calculated as the duration from the birth date to the time needed for tumors reaching 2 cm in length. Differences in survival of mice from three groups were statistically analyzed using the log-rank test. The numbers of mice being analyzed (n) and p values were indicated. B Plot of tumor size (mm3) versus time (days). The palpable tumors in individually tagged mice were measured since their initial appearance. Tumor volumes (Mean ± SEM, mm3): calculated using length-width-height × 0.52 for CD151 wild-type (WT) and knockout (KO) mice, respectively. C and D: IHC analysis of Ki-67 and caspase 3–positive cells (Mean ± SEM). Numbers of Ki67- positive cells per field were calculated for 5 mice for each CD151 genotype (C,; D, top panel)). Cleaved caspase 3–positive cells represent 1.5% and 7.5% of total cells in areas counted for WT and KO mice, respectively (D). p values were indicated. Scale bar: 50 μm.
It is worth noting that even prior to tumor development, number of β -catenin-positive mammary epithelial cells in the Wnt1-expressing mice markedly increased in the absence of CD151 (Fig. S1), consistent with its role in mammary gland development [36]. In addition, CD151 removal had a minimum impact on the kidney pathology of mice throughout our in vivo study, consistent with its sex-linked and strain-dependent roles in normal physiology or kidney function, as described in prior studies [31], [37]. There was also a lack of difference in the multiplicity of tumors between wild-type and the CD151-null groups.
The signaling basis of CD151 function in the Wnt1-driven tumorigenesis
Next, we investigated the molecular mechanism underlying the tumor-suppressive role of CD151 in the MMTV-Wnt1 model. Upon the CD151 removal, the protein levels of β -catenin and cyclin D1 in tumor tissues, the key signaling intermediates of the canonical Wnt pathway, exhibited a > 20 fold increase (Fig. 2A). These changes were corroborated by a marked increase in the nuclear staining of β -catenin and cyclin D1 in CD151-deficient tumor tissues, compared to their counterparts (Fig. 2B). By comparison, there was only about a 1.5-fold increase in the Axin-1. A similar change in the phosphorylated form of LRP6, a key co-receptor for the Wnt pathway, was detected (data not shown).
Fig. 2.
The signaling effect of CD151 deletion in the Wnt1-driven mammary tumors. A Analysis of key signaling intermediates of the Wnt pathway. Four individual tumors from CD151 wildtype (+/+) and null (−/−) mice were lyzed in RIPA buffer and immunoblotted for indicated proteins. B Representative images of the IHC staining of β -catenin and cyclin D1 in mammary tumors (n = 5). CD151 wildtype: a,c; null group: b,d. Scale bar; 50 μm.
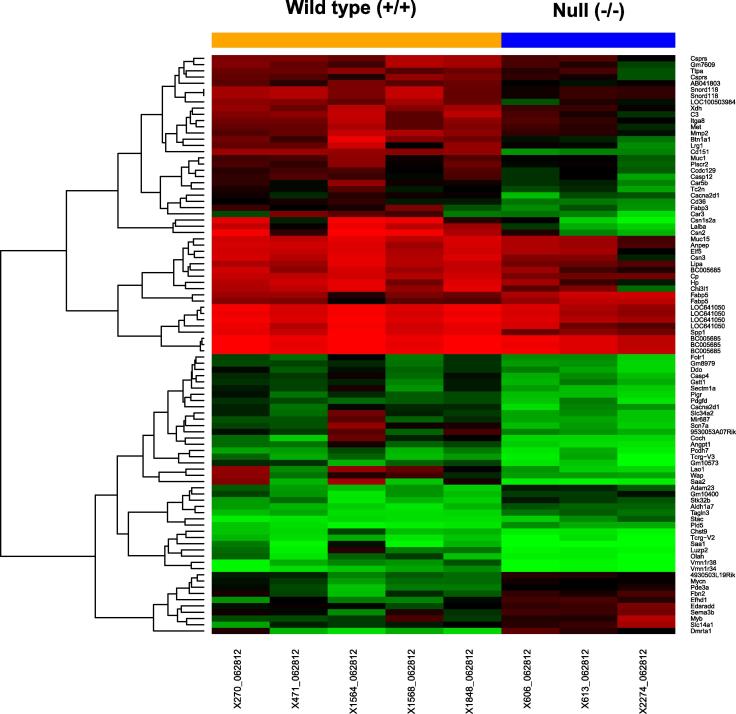
The link between CD151 and the Wnt pathway was also assessed by comparing gene expression profile in mammary tumor tissues. A number of well-recognized Wnt target genes, including N-Myc, Myb and Fibrinogen 2, exhibited 1.5–3-fold increase at the mRNA level upon CD151 deletion (Fig. 3), consistent with the signaling effect described above. However, there was a lack of marked change in the mRNA expression of β -catenin or cyclin D1. Interestingly, there was a marked difference in the pathways being associated with candidate stromal cells, including macrophages, fibroblasts and endothelial cells (Fig. 3 and Table S1). Combined, this data demonstrates that the tumor–suppressive role of CD151 in the MMTV-Wnt1 model is linked to the repression of the canonical Wnt/ β -catenin signaling.
Fig. 3.
Comparison of gene expression profile of mammary tumors between CD151 wildtype and null mouse groups. The heat map: differential expression of main genes between groups; Red: high expression; green: low expression. The mRNA samples were prepared from murine mammary tumors from control (n = 5) and null-mice (n = 3). FDR < 0.05.
A cell to cell contact-dependent role of CD151 in luminal epithelial cell-derived tumors
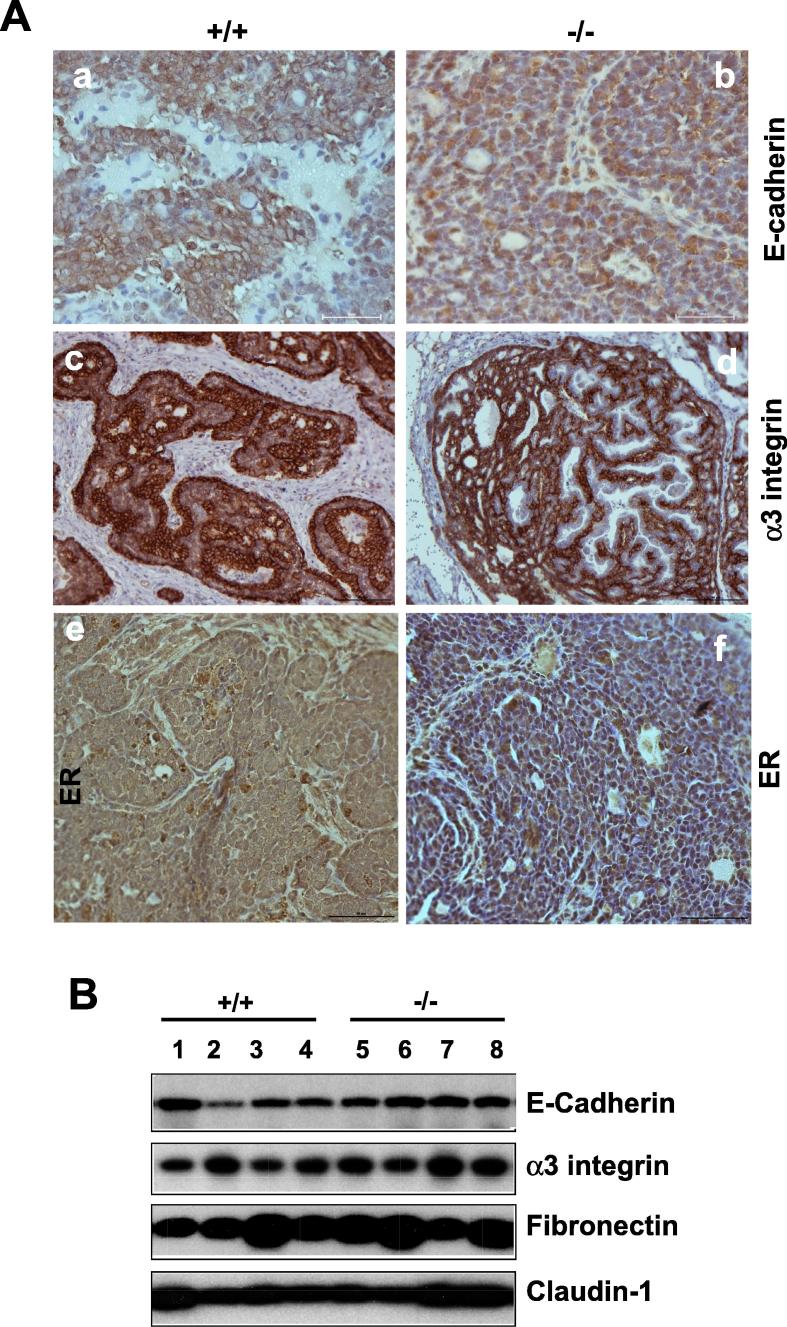
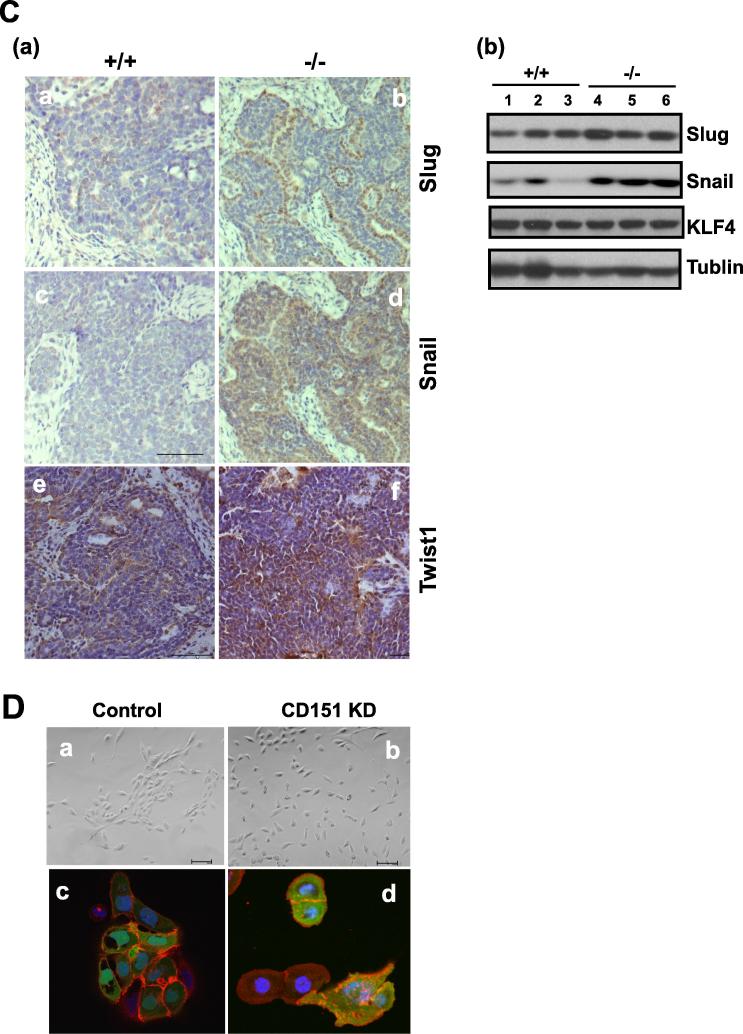
Next, we investigated the cellular basis for the tumor-suppressive role in the MMTV-Wnt1 model. The data from the combination of signaling analysis and gene expression suggest that the effect of CD151 deletion on mammary tumorigenesis occurs at the post-transcriptional level. This notion is also supported by our observation that CD151 removal had a minimal impact on the activation of EGFR or FAK, two key integrin-related signaling molecules (Fig. S2). Based on these observations, we speculated that the anti-tumor effect of CD151 in the MMTV-Wnt1 model may be associated with aberrant regulation of the cell to cell adhesion in mammary tumors. Indeed, in contrast to the membrane localization in the control group, E-cadherin was largely cytosolic in CD151-null mammary tumors, reminiscent of the epithelial-mesenchymal (EMT)-linked alteration (Fig. 4A). The total level of E-cadherin protein in tumor tissues, however, remained unchanged, according to the immunoblotting analysis (Fig. 4B). In line with this observation, Snail, a dominant EMT-inducing transcription factor, was also markedly upregulated at protein level in CD151-deficient tumors, based on immunoblotting and IHC analyses (Fig. 4C).
Fig. 4.
The fictional link of CD151 to the phenotype of the luminal epithelial cell-derived mammary tumors. A Expression and distribution of E-cadherin, α 3 integrin and ER proteins in mammary tumors. B Analysis of expression of CD151-related adhesion molecules by immunoblotting. C: Recapturing of CD151-mediated cell interaction in breast cancer by the impact of its knockdown on the morphology of cultured MCF-10A cells in 2D (B) or 3D (C). C (a). Immunoblotting of Snail, Slug, Twist1 and KLF4 in mammary tumors. (b) IHC staining. D Image of human TNBC cells (HCC1937) with or without stable CD151 knockdown. Top panel, The-EMT-like change; bottom panel, confocal images of β -catenin staining in tumor cells. The specificity of CD151 shRNA was validated in a prior study [43]. Scale bar: 50 μm.
In addition, the large portions of tumor cells in CD151-deficient tumor tissues appeared estrogen receptor (ER)-positive, while there was a lack of apparent difference in CD151-associated α 3 integrin between two groups (Fig. 4A). This suppressive role of CD151 in the luminal cell-associated tumorigenesis is in line with our recent observation on the effect of the RNAi-mediated CD151 downregulation in HCC1937, an E-cadherin-positive breast cancer cell line [36]. Intriguingly, even though CD151 downregulation coincided with an EMT-like change, we failed to detect a marked increase in the nuclear distribution of β -catenin in cultured TNBC cells (Fig. 4D). Collectively, these data illustrate the tumor-suppressive role of CD151 in the MMTV-Wnt1 model, and it is partially linked to regulation of the Wnt1 oncogene-targeting pool of the ER+ luminal epithelial cells in the mammary gland.
Impact of CD151 deletion on development of basal/stem cell lineage-associated tumors
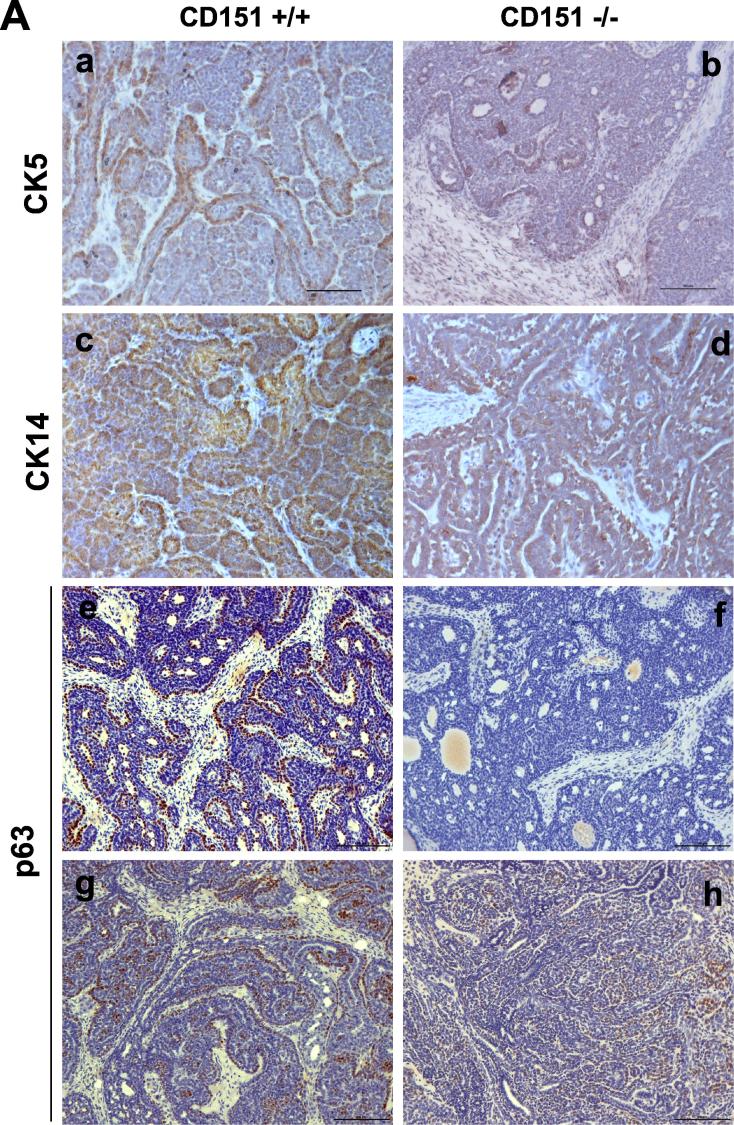
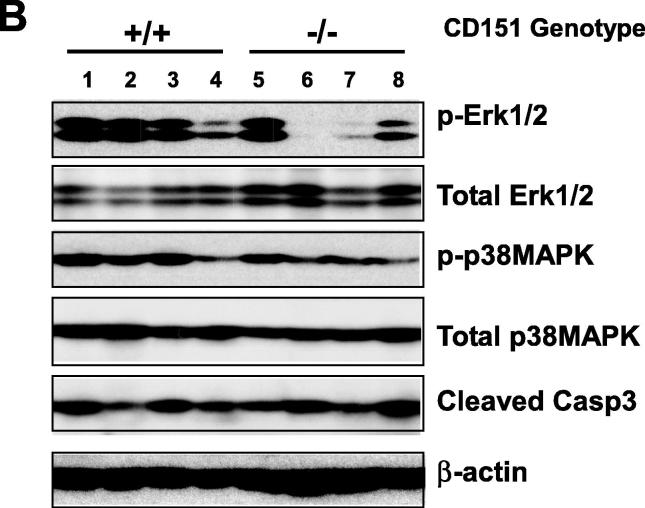
Mammary tumors in the MMTV-Wnt1 model are known to arise from the oncogenic targeting of both basal/stem and luminal epithelial cell populations [8]. In addition, such effect is strongly linked to the activation of Oncogenic H-Ras-mediated Erk1/2 pathway [38]. Indeed, this feature was recaptured by the development of mammary tumors from the CD151 wild type/control mice, as indicated by expression of CK5 and CK14, typical basal cell or stem markers, according to IHC analysis (Fig. 5A). In addition, there was a differential staining of p63, a bonnie fide marker for mammary basal/stem epithelial cells. It was also indirectly supported by a concomitant increase in the expression of ER, a typical luminal cell marker (Fig. 4A). Furthermore, the portion of individual tumors exhibiting the activated RAS/MAPK pathway, as indicated by the robust phosphorylation of Erk1/2, markedly decreased upon CD151 deletion (Fig. 5B). Such effect of CD151 downregulation also occurred in p38, a kinase being implicated in its signaling in breast cancer [39]. Combined, these results demonstrate that the CD151 removal skews or diminishes the representation of the basal/stem cell population-derived mammary tumors in the MMTV-Wnt1 model.
Fig. 5.
A role of CD151 in the formation of basal/stem cell-derived mammary tumors in the MMTV-Wnt1 mosaic model. A Images of IHC staining of basal/stem cell-oriented molecular markers in mammary tumors, CK5, CK14 and p63 gene. Staining tumors from the Cd151-wildtype: a, c, e .g and i.; CD151-null tumors; b, d, f, h and j. B Effect of Cd151 deletion on the RAS/MAPK pathway. Levels of total, phosphorylated Erk1/3 adnp38 from individual tumors (n = 4) were analyzed by immunoblotting. Scale bar: 50 μm.
A link to the tumor-initiating capability and survival of mammary tumor cells
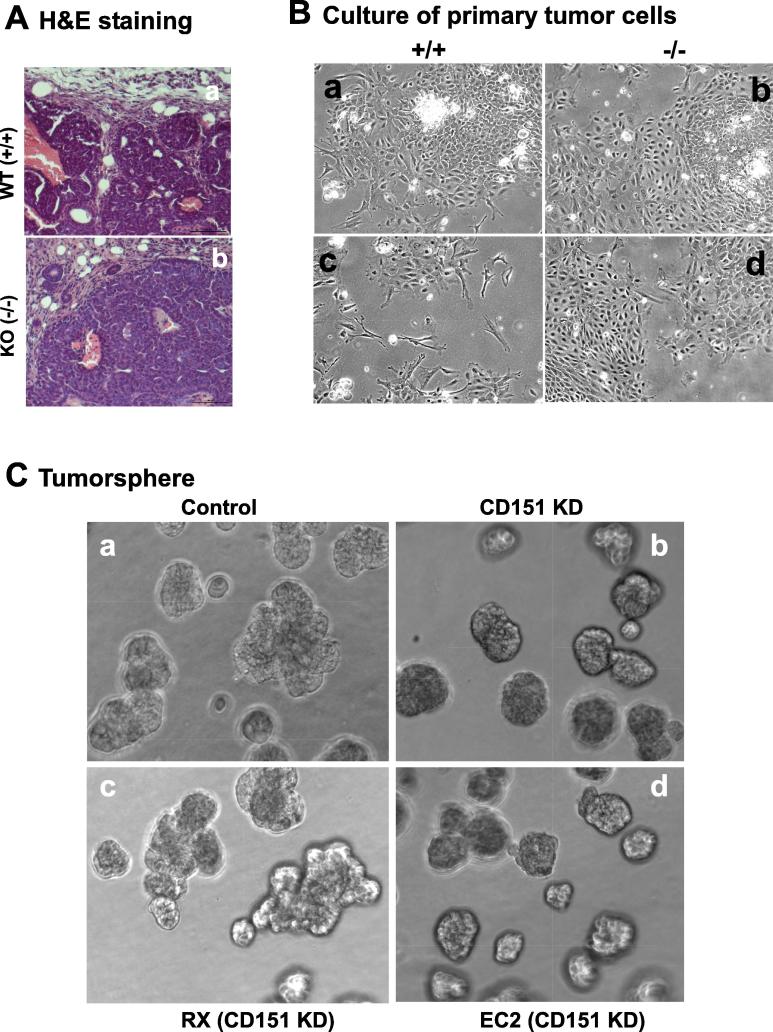
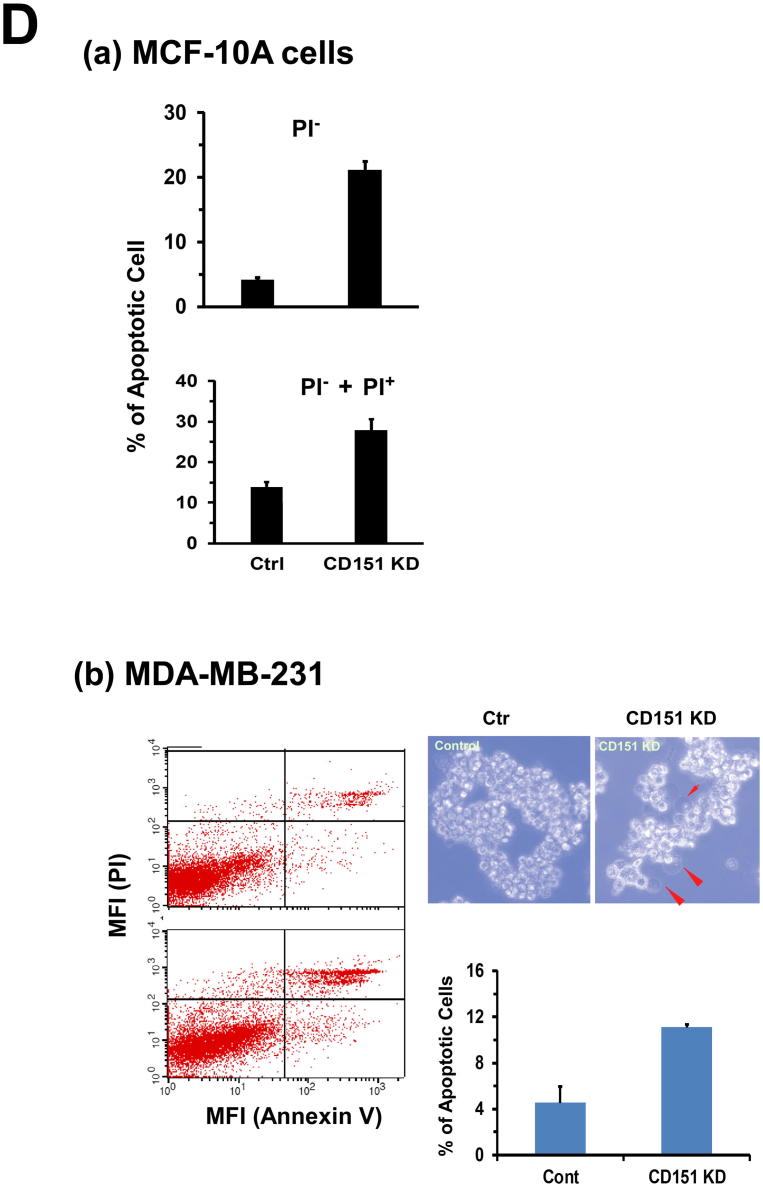
We subsequently investigated how the representation of the basal/stem cell lineage-derived mammary tumors in the MMTV-Wnt1 model was compromised in the absence of CD151 gene. Our H&E staining showed that the protrusive leading edge of mammary tumors was markedly impaired in the absence of CD151 (Fig. 6A). A similar phenomenon was recaptured by our in vitro culture of primary tumor cells, where the protrusive or spike-like structures were present in the tumor cells from the control group only (Fig. 6B). Furthermore, we failed to establish any long-term cell culture or stable cell lines from the CD151-deficient primary tumors compared to the control group (0/10 versus 5/5). Additionally, the RNAi-mediated CD151 downregulation decreased the capability of MCF-10A cells to form tumorspheres under ultra-low adhesion conditions (Fig. 6C, a-b). This effect was rescued by re-expressing functionally-intact CD151 gene, but not its mutant being defective in the integrin association (Fig. 6C, c-d). Moreover, the CD151 downregulation led to a marked increase in apoptotic cell death in multiple basal or TNBC cell lines, including MCF-10A and MDA-MB-231 (Fig. 6D). Considered together, our data indicates that CD151 supports the development of basal cell-derived mammary tumors in the MMTV-Wnt1 model, underscoring its role in the maintenance of the TNBC malignancy-linked tumor-initiating cells.
Fig. 6.
A link between CD151 and the tumor-initiating capability of breast cancer cells. A H&E staining of mammary tumor tissues. Scale bar, 100 μm. B Images of primary cell culture of mammary tumors from the MMTV-Wnt1 mice. The fresh tumor tissues were surgically removed, digested overnight in collagenase, filtered with 40 µm strainer, followed by additional 3 days of culture prior to the imaging. C Images of tumorspheres formed by MCF-10A cells. MCF-10A cells were infected with control (a) and CD151-specific shRNA-expressing (b) lentiviral vector to express CD15 shRNA (KD). The Cd151 KD cells were further infected with retroviral-based CD151 wildtype (RX) and an integrin binding-defective mutant (EC2). Scale bar: 50 μm. D Effect of CD151 knockdown (KD) on cell survival. Cells with or without CD151knckdown were analyzed for difference in apoptotic cell death by flow cytometry-based analysis of cell staining of APC-conjugated Annexin V and PI. on: (a) MCF-10 cells were cultured for 24 hrs prior to the analysis. (b) MDA-MB-231 cells were kept under ultra-low adhesion for 24hrs, and imaged or analyzed on flow cytometry for apoptosis. Images of typical apoptotic cells, arrow-pointed. Values: mean ± SEM (n = 3). *: p < 0.05.
Discussion
With gene-targeted mice and the MMTV-Wnt1 transgenic model, we have investigated functional and signaling links between tetraspanin CD151 and the Wnt1 pathway in breast cancer. Our data demonstrate that CD151 represses growth of the luminal epithelial cell-origin mammary tumors largely through affecting the stability of the E-cadherin/ β -catenin complexes on the cell surface. Meanwhile, this tetraspanin also promotes the development of basal cell-derived mammary tumors by affecting cell survival and ability to invade neighboring stroma. These unexpected observations, together with the data from recent studies with the MMTV-ErbB2 model and in vitro cell culture systems [25], [32], [36], [31], [40], pinpoint a cell lineage and oncogenic context-dependent role of CD151 in breast cancer development and progression.
The luminal epithelial cell-dependent role of CD151 in the Wnt1-driven mammary tumorigenesis
Our current study has revealed a strong anti-tumorigenic role of CD151 in the context of the luminal epithelial cell lineage, bolstering its importance in multiple breast cancer subtypes [31], [41]. Our data indicate that this functional role is associated with the maintenance of cell to cell contacts or counteracting of the EMT-like process (Fig. 3, Fig. 4, Fig. 5, Fig. 6). This finding is also consistent with our recent analyses of CD151 in mammary gland development or other cancer type [42], [43], [44], [45], and a recent study of CD151 and α 3 integrin in human lobular-type breast cancer [15]. Accordingly, there is a strong likelihood that part of increased growth of CD151-deficient mammary tumors stems from expansion of a larger pool of the Wnt 1oncogene-susceptible luminal progenitor cells in mammary glands (Fig. S1).
Our data also suggest that the anti-proliferative role of CD151 results from the repression of the canonical Wnt β -catenin pathway (Fig. 2, Fig. 3). Besides perturbing the E-cadherin-mediated cell to cell adhesion, CD151 deletion may impact the transcription of a set of target genes of the canonical Wnt/ β -catenin pathway (i.e., N-Myc and Myb), and the Snail-associated network (Fig. 3, Fig. 4, Fig. 5). Thus, the hyperactivation of β -catenin and cyclin D1 in CD151-deficient tumors may reflect a simultaneous deregulation of multiple molecular networks in mammary luminal epithelial or progenitor cells at both transcriptional and post-transcriptional levels.
The pro-malignant role of CD151 in stem/basal cell-associated breast cancer
One of the hidden findings in our current study is the evidence on the critical role of CD151 in the maintenance of basal cell-derived mammary tumors (Fig. 1, Fig. 2, Fig. 3, Fig. 4). Based on our analyses, this finding is strongly associated with the pro-survival role of CD151 in the tumor cells with the basal/stem epithelial cell lineage, consistent with prior studies of this gene and associated integrins in human TNBC cell lines [32]. Also, this functional property is highly reminiscent of the tumor-initiating capability of cancer stem cells or its role in recurrence and metastatic progression of breast cancer [46], [47].
More observations, however, are still needed in order to define a direct role of CD151 in such subset of malignant tumor cells and associated clinical malignancy in the TNBC subtype.
Additionally, our study reveals a strong pro-invasive role of CD151 in the Wnt oncogene-driven mammary tumors (Fig. 6C). This observation is consistent with in vitro studies of CD151 function in metastatic breast cancer cells [20], [32], [48], and analysis with the MMTV-ErbB2 model [31]. In addition, this effect may be associated with tumor stroma or microenvironments, according to our gene expression profiling analysis. Notably, expression of several myeloid cell-associated genes, including CD36 and MMP2, is markedly downregulated in CD151-deficient mammary tumors (Fig. 3). Consistent with this observation, activation of the Wnt pathway has been linked to recruitment of macrophages during mammary tumorigenesis [47]. Thus, it will be of interest to determine if CD151 deletion alters the infiltration of macrophages or other immune cells in the future study, thereby uncovering a novel link of CD151 to mammary tumor progression.
It is worth noting that our gene expression data support the link between CD151 and endothelial cells during mammary tumor development (Table S1). This observation is also consistent with prior study of this molecule in cultured endothelial cells or related pathological processes [49], [50], [51].
A potential link to cellular metabolism
Our gene expression profiling data implicates a link between CD151 and cellular metabolism (Fig. 3 and Table S1). Notably, expression of Cel, a gene involved in the lipid esterification, is affected by CD151 (Fig. 3). There is also a potential link between CD151 and the Twist1-mediated lipid metabolism signature, as well as transcription factors, including LXR/RXR and FXR/RXR and others (DMRTA1 and Myb) (Table S1). This observation is in line with the link to stromal myeloid cells, such as macrophages and granulocytes, which are known contributors of lipid metabolism. Collectively, this set of data implicates that CD151 may regulate breast cancer development via orchestrating tumor cell metabolism.
CD151 as a candidate therapeutic target
To date, a number of tetraspanins are directly implicated in affecting the receptor complexes and activation of the Wnt pathways, notably CD9, CD82 and Tspan12 [20], [27]. This molecular link appears consistent with the prominent role of CD151 in the survival of the basal/stem cell lineage -related tumor cells. If this is proven in the future study, it should serve as an important basis for targeting CD151 to mitigate the malignancy of TNBC subtype.
Overall, our study unveils a differential role of CD151 in the Wnt1 oncogene-driven mammary tumors arising from basal and luminal epithelial cells. CD151 acts cooperatively with the Wnt pathway to promote tumorigenesis and cell survival in the context of oncogenic targeting of mammary basal/stem cells. It plays a suppressive role in the luminal cell-originated mammary tumors by sequestering the canonical Wnt/ β -catenin signaling. These findings highlight the potential of targeting CD151 to mitigate the intra-tumoral heterogeneity and disease recurrence of breast cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was supported in part by a pilot project grant from American Cancer Society #IRG 85-001-25 and a pilot project from NIH COBRE grant (5P20GM121327-03) to XH Yang, The Chinese National Natural Science Foundation grant (81572877) to Song Chen, and The Medical Science and Technology Development Foundation, Department of Health, Nanjing/Jiangsu & The Chinese National Natural Science Foundation grant (81502623) to Junfong Shi. The Biospecimen Core was supported by the National Cancer Institute (P30CA177558). The plasmids containing ORF (RX) and integrin binding-defective mutant (EC2) of CD151 were kindly provided by Dr. Christopher Stipp.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2019.08.005.
Contributor Information
Song Chen, Email: schen@zzu.edu.cn.
Hai Qian, Email: qianhai24@cpu.edu.cn.
Binhua P. Zhou, Email: peter.zhou@uky.edu.
Pao Xu, Email: xup@ffrc.cn.
Xiuwei H. Yang, Email: xiuwei-yang@uky.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhang J., Li Y., Liu Q., Lu W., Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2010;29:539–549. doi: 10.1038/onc.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prosperi J.R., Goss K.H. A Wnt-ow of opportunity: targeting the Wnt/beta-catenin pathway in breast cancer. Curr Drug Targets. 2010;11:1074–1088. doi: 10.2174/138945010792006780. [DOI] [PubMed] [Google Scholar]
- 3.Khramtsov A.I. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens P.J. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anastas J.N., Moon R.T. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 6.Schuijers J., Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenz H.J., Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105:1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary A.S., Leonard T.L., Gestl S.A., Gunther E.J. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508:113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidi H., Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oloumi A. Cooperative signaling between Wnt1 and integrin-linked kinase induces accelerated breast tumor development. Breast Cancer Res: BCR. 2010;12:R38. doi: 10.1186/bcr2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. dev.027805 [pii] 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudjadi S., Beaulieu J.F. MYC and integrins interplay in colorectal cancer. Oncoscience. 2016;3:50–51. doi: 10.18632/oncoscience.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell K. Suppression of integrin alpha 3 beta 1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and cross-talk to endothelial cells. Cancer Res. 2010;70:6359–6367. doi: 10.1158/0008-5472.CAN-09-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zevian S.C. CD151 promotes alpha3beta1 integrin-dependent organization of carcinoma cell junctions and restrains collective cell invasion. Cancer Biol Ther. 2015;16:1626–1640. doi: 10.1080/15384047.2015.1095396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanska H.M. Lack of CD151/integrin alpha3beta1 complex is predictive of poor outcome in node-negative lobular breast carcinoma: opposing roles of CD151 in invasive lobular and ductal breast cancers. Br J Cancer. 2015;113:1350–1357. doi: 10.1038/bjc.2015.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roela RA, Brentani MM, Katayama ML, Reis M, Federico MH. Simultaneous changes in the function and expression of beta 1 integrins during the growth arrest of poorly differentiated colorectal cells (LISP-1). Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 36, 1091–1099 (2003). [DOI] [PubMed]
- 17.Micalizzi D.S., Farabaugh S.M., Ford H.L. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi A., Kishida S., Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. 200602281 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Cheriyamundath S, Ben-Ze'ev A. Cell-cell adhesion: linking Wnt/beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 7, doi:10.12688/f1000research.15782.1 (2018). [DOI] [PMC free article] [PubMed]
- 20.Hemler M.E. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14:49–60. doi: 10.1038/nrc3640. [DOI] [PubMed] [Google Scholar]
- 21.Bocchinfuso W.P., Hively W.P., Couse J.F., Varmus H.E., Korach K.S. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- 22.Li Y., Hively W.P., Varmus H.E. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 23.Teissedre B, et al. MMTV-Wnt1 and -Delta N89 beta-Catenin Induce Canonical Signaling in Distinct Progenitors and Differentially Activate Hedgehog Signaling within Mammary Tumors. PloS one 4, -, doi:Artn E4537Doi 10.1371/Journal.Pone.0004537 (2009). [DOI] [PMC free article] [PubMed]
- 24.Jue S.F., Bradley R.S., Rudnicki J.A., Varmus H.E., Brown A.M. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol. 1992;12:321–328. doi: 10.1128/mcb.12.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson J.L., Winterwood N., DeMali K.A., Stipp C.S. Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J Cell Sci. 2009;122:2263–2273. doi: 10.1242/jcs.045997. jcs.045997 [pii]10.1242/jcs.045997. [DOI] [PubMed] [Google Scholar]
- 26.Knoblich K. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits beta-catenin degradation. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junge H.J. TSPAN12 regulates retinal vascular development by promoting norrin- but not wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X.A. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stipp C.S., Hemler M.E. Transmembrane-4-superfamily proteins CD151 and CD81 associate with alpha 3 beta 1 integrin, and selectively contribute to alpha 3 beta 1-dependent neurite outgrowth. J Cell Sci. 2000;113(Pt 11):1871–1882. doi: 10.1242/jcs.113.11.1871. [DOI] [PubMed] [Google Scholar]
- 30.Yauch R.L., Kazarov A.R., Desai B., Lee R.T., Hemler M.E. Direct extracellular contact between integrin alpha(3)beta(1) and TM4SF protein CD151. J Biol Chem. 2000;275:9230–9238. doi: 10.1074/jbc.275.13.9230. [DOI] [PubMed] [Google Scholar]
- 31.Deng X. Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset and metastasis. Neoplasia. 2012;14:678–689. doi: 10.1593/neo.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roselli S. Deletion of Cd151 reduces mammary tumorigenesis in the MMTV/PyMT mouse model. BMC Cancer. 2014;14:509. doi: 10.1186/1471-2407-14-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda Y. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–1532. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X.H. CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization. Cancer Res. 2008;68:3204–3213. doi: 10.1158/0008-5472.CAN-07-2949. 68/9/3204 [pii] 10.1158/0008-5472.CAN-07-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X.H. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 2010;70:2256–2263. doi: 10.1158/0008-5472.CAN-09-4032. 0008-5472.CAN-09-4032 [pii] 10.1158/0008-5472.CAN-09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin Y. CD151 represses mammary gland development by maintaining the niches of progenitor cells. Cell Cycle. 2014;13:2707–2722. doi: 10.4161/15384101.2015.945823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q. Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene. 2012 doi: 10.1038/onc.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podsypanina K., Li Y., Varmus H.E. Evolution of somatic mutations in mammary tumors in transgenic mice is influenced by the inherited genotype. BMC Med. 2004;2:24. doi: 10.1186/1741-7015-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadej R. Tetraspanin CD151 regulates transforming growth factor beta signaling: implication in tumor metastasis. Cancer Res. 2010;70:6059–6070. doi: 10.1158/0008-5472.CAN-09-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldwin L.A. CD151-alpha3beta1 integrin complexes suppress ovarian tumor growth by repressing slug-mediated EMT and canonical Wnt signaling. Oncotarget. 2014 doi: 10.18632/oncotarget.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roselli S. Deletion of Cd151 reduces mammary tumorigenesis in the MMTV/PyMT mouse model. BMC Cancer. 2014;14:509. doi: 10.1186/1471-2407-14-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazarov A.R., Yang X., Stipp C.S., Sehgal B., Hemler M.E. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–1309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winterwood N.E., Varzavand A., Meland M.N., Ashman L.K., Stipp C.S. A critical role for tetraspanin CD151 in alpha3beta1 and alpha6beta4 integrin-dependent tumor cell functions on laminin-5. Mol Biol Cell. 2006;17:2707–2721. doi: 10.1091/mbc.E05-11-1042. E05-11-1042 [pii]10.1091/mbc.E05-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterk L.M. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- 45.Yang X. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J Cell Biol. 2004;167:1231–1240. doi: 10.1083/jcb.200404100. jcb.200404100 [pii]10.1083/jcb.200404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polyak K., Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harbor Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo E.J. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74:2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stipp C.S. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12 doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F. Tetraspanin CD151 maintains vascular stability by balancing the forces of cell adhesion and cytoskeletal tension. Blood. 2011;118:4274–4284. doi: 10.1182/blood-2011-03-339531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanez-Mo M. MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood. 2008;112:3217–3226. doi: 10.1182/blood-2008-02-139394. [DOI] [PubMed] [Google Scholar]
- 51.Takeda Y. Diminished metastasis in tetraspanin CD151-knockout mice. Blood. 2011;118:464–472. doi: 10.1182/blood-2010-08-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.