Abstract
Perks and Pierce consider a new study in worms showing that touch intensity is encoded by the quantal activity of mechanoreceptors.
Researchers have already determined the molecular identity of many mechanoreceptive ion channels. The next major challenge is to determine how these channels work together in a sensory neuron to transform mechanical stimuli into depolarizing currents in a way that can encode different stimuli. For instance, how do these channels operate to distinguish shallow and deep forms of touch? Increasing levels of pressure may be conveyed with a continuous code whereby the same set of channels increase their activity in proportion to the stimulus. Alternatively, increasing pressure may be conveyed with a quantal code whereby additional channels activate across a widening area of epithelial strain. In a recent JGP paper, Katta et al. combine in vivo patch-clamp electrophysiology, high-speed mechanical stimulation, and mathematical modeling to address this question. They discover that touch intensity in Caenorhabditis elegans is conveyed with a quantal code in which more mechanoreceptive ion channels are activated as the skin is depressed further.
We can get by without many of our senses, including vision, hearing, taste, smell, and even pain. However, sensing mechanical pressure is essential to both human viability and reproduction. Our unconscious response to pressure changes within internal tissues maintains our cycle of breathing. Unconscious modulation of smooth muscle tone within vessels regulates our blood pressure. The act of copulation also necessitates sensing of pressure changes. Our ability to consciously sense touch with our largest sensor organ, skin, is a nonessential process, but it is critical nonetheless to daily life.
Most of the excitement in the study of this essential sensory modality has surrounded the molecular identity of mechanosensors, especially the mechanoreceptive ion channels (Ranade et al., 2015). Over the past three decades, numerous mechanoreceptive ion channels have been revealed. This includes Piezo channels, identified by a reverse in vitro genetic screen, and certain TRP channels, identified by candidate genetic screens in C. elegans and Drosophila (Li et al., 2006; Coste et al., 2010; Kim et al., 2012; Yan et al., 2013). However, the pioneering screen for mechanoreceptive ion channels was performed in C. elegans. Chalfie and Sulston performed a saturating genetic screen to reveal a set of 13 genes required for the worm to flinch when tickled with an eyelash (Chalfie and Sulston, 1981). Mutants that continued to move forward, unperturbed after a gentle prodding of the head by the experimenter’s eyelash, signified the existence of corresponding genes that are required for the development or function of the six so-called “touch neurons” (Chalfie and Thomson, 1979). One of these genes, mec-4, was represented by distinct loss-of-function and gain-of-function alleles. Loss-of-function mutants had normal looking touch neurons, while gain-of-function mutants had touch neurons that degenerated due to excess ion flux (Chalfie and Sulston, 1981; Driscoll and Chalfie, 1991). These results suggested that mec-4 encodes an ion channel critical for transducing mechanical pressure in touch neurons. Later, in vivo patch-clamp recordings of mechanoreceptive currents in worm touch neurons revealed conclusive evidence that MEC-4 constitutes an essential portion of the mechanosensitive ion channel (O’Hagan et al., 2005). Because missense mutations in MEC-4 altered ion selectivity of touch-induced currents, the MEC-4 protein must comprise the pore-forming portion of the mechanoreceptive channel in worm touch neurons (O’Hagan et al., 2005). The MEC-4 channel has paralogs in worm as well as orthologues in other animals, including ENaC, DEG, and ASIC channels, many of which are mechanoreceptive (Eastwood and Goodman, 2012; Omerbašić et al., 2015). Like other members of this ion channel family, the MEC-4 protein is thought to trimerize by itself or with a similar MEC-10 protein (Carnally et al., 2008; Jasti et al., 2007).
Touch neurons in C. elegans have a simple shape. They each have a cell body from which a long dendrite extends to sit beneath the basement membrane under the skin (cuticle). The rod-shaped dendrite contains an array of microtubules (Chalfie and Thomson, 1979) and labeled MEC-4 ion channel complexes appear in puncta ∼2 µm apart along its length (Fig. 1; Chelur et al., 2002; Cueva et al., 2007; Emtage et al., 2004). This spatial arrangement of channels is practical as it permits the worm to sense touch at any location along the dendritic sensory field in order to avoid predators or collisions. In this study, Katta et al. (2019) reinvestigated the detailed distribution of MEC-4 using the bright mNeoGreen tag. They determined that channel density is highest near the cell body (every 0.8–5 µm) and decreases with distance along the dendrite (every 1.2–8 µm). Future studies may be able test if this nonuniform spatial gradient of MEC-4 distribution allows higher spatial resolution and fidelity for the detection and encoding of mechanical stimuli applied close to the cell body.
Figure 1.
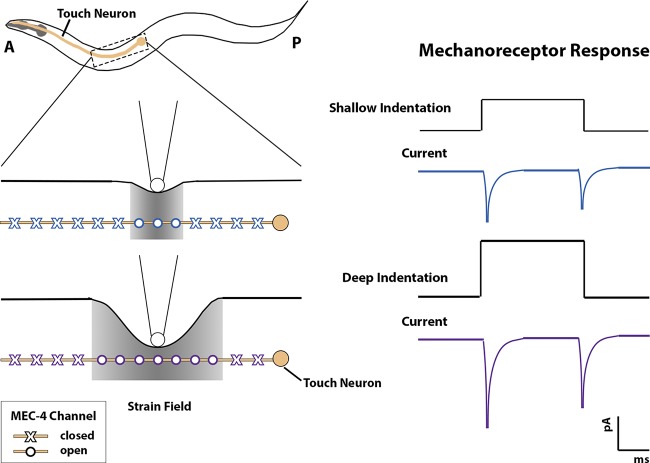
The quantal code of touch. Touch receptor neurons encode indentation depth via the discrete number of activated MEC-4 channels that contribute to the peak mechanoreceptive current. MEC-4 channels are distributed ∼2 µm apart along the length of the touch neuron’s dendrite (shown in beige). Shallow indentation (in blue) produces a limited strain field and opens a small number of channels within this field, resulting in a small phasic current. In comparison, deep indentation (in purple) widens the strain field and activates more channels, resulting in a larger current.
The physiology of touch neurons and MEC-4 channels are also well described. At the whole-cell level, patch-clamp recordings of mechanotransduction currents show that increasing pressure and indentation of the skin results in a transient depolarizing current upon depression, and again on release, of a prodding stimulus (Fig. 1; O’Hagan et al., 2005). The magnitude of the whole-cell current relates directly to the amount of applied pressure (O’Hagan et al., 2005). Tiny indentations cause tiny currents (∼5 pA) whereas deep depressions cause large currents (∼100 pA). At the single-channel level, in vitro recordings show that heterologously expressed MEC-4 channels pass a maximal single channel current of −1.6 pA, matching estimates from in vivo recordings (O’Hagan et al., 2005; Brown et al., 2007).
With these detailed snapshots of touch neuron shape and physiology in hand, Katta et al. (2019) aimed to compose a more comprehensive picture of how C. elegans sense mechanical stimuli at the level of individual MEC-4 channels, while taking the force transduced through the worm’s skin into account. The Goodman laboratory had previously modeled how a single MEC-4 channel might respond to touch as a viscoelastic mechanical filter (Eastwood et al., 2015). Their new study, however, offers the first realistic model to consider how multiple MEC-4 channels respond after incorporating sampled measures for touch neuron geometry, MEC-4 channel spatial distribution, single channel properties, and mechanical strain through the epithelial tissue.
Katta et al. (2019) initially asked if, given these empirically measured properties, the peak whole-cell mechanoreceptive current could be explained simply by the number of single MEC-4 channels activated by strain through the skin? To explore this, they recorded touch neurons while transiently prodding the skin with a 22-µm diameter probe and confirmed that peak current increased with indentation depth. They also found that experimental and simulated peak whole-cell mechanoreceptive current and total charge matched extremely well for different touch stimuli.
The model also afforded the opportunity to ask how individual MEC-4 channels respond to these different stimuli. As expected, simulated MEC-4 channels closest to the stimulus were the most likely to activate. Likewise, deeper indentations produced wider strain through the epithelia, which activated a larger number of channels. Because individual MEC-4 channels quickly saturate in activity, however, a combination of the two phenomena produced a less intuitive result, namely that the size of the indentation and peak current corresponded directly with the number of active MEC-4 channels. Peak currents increased nonlinearly across a range of indentations relevant to the worm’s experience. The model showed that shallow indentations (0.5 µm) activated only a few MEC-4 channels, while deeper indentations (12 µm or ∼30% of the worm’s width) activated ∼100 MEC-4 channels. The model robustly maintained an identical indentation current relation even after decreasing the density of simulated MEC-4 channels (every 1.4 to 4 µm). For indentation stimuli greater than 2 µm, MEC-4 channels were predicted to stay open rather than dwelling in subconductance states. This saturated response means that the size of the peak mechanoreceptive current simply reflects the number of MEC-4 channels activated. Interestingly, even with the largest indentation, whole-cell mechanoreceptive current and the number of MEC-4 channels predicted to contribute to net current did not saturate. Thus, touch intensity is encoded by the number of MEC-4 channels activated: a quantal code.
Katta et al. (2019) next asked what happens when the worm is touched behind the touch neuron, 50 µm away from the anatomical field of the dendrite. They found that the threshold indentation to cause a macroscopic current was less than a micron when pressed directly above the dendrite, but 4 µm at this distal location. The model accurately reproduced displacement-current relations for simulated indentations close to and far from the dendrite. Overall, the model suggests that larger indentations produce larger field strains that can reach more channels than smaller indentations. Thus, touch intensity outside the anatomical field of the touch neuron similarly follows a quantal code.
How does the speed of mechanical deformation affect how these channels activate with touch? Katta et al. (2019) found that indenting the skin the same depth but faster caused larger macroscopic mechanoreceptive currents. Both transient onset and offset currents grew larger. The initial transient current also showed a shorter latency to activate and a faster rise to peak current. The faster onset could be accounted for by an increase in the synchrony of MEC-4 channel activation. These differences in macroscopic current suggest a plausible code for stimulus speed.
With a new understanding of how simple prodding is encoded, they next asked how more complicated vibratory stimuli are encoded. Unlike other mechanoreceptive ion channels such as Piezo and TRPs, the MEC-4-related family of channels respond to a single prod with two current transients. This dual response may be ideal for responding to vibratory stimuli (Eastwood and Goodman, 2012). Katta et al. (2019) found that the touch neurons’ macroscopic currents increase with increasing frequency of vibratory stimuli. In contrast to prodding stimuli that elicit phasic transient mechanoreceptive currents, they found that vibratory “buzz” stimuli produced strong steady currents that did not adapt. Steady-state responses began to reach a maximum at 100–200 Hz. This roughly matches the optimal 90–150 Hz detection range for ENaC-expressing mechanoreceptor organs in human fingers, lips, and genitals. Katta et al. (2019) were able to fit the current-frequency curve of the real response with two hypothetical values for an elastic filament in their model. These results suggest a general principle of how distinct mechanical stimuli are encoded: by simply recruiting additional MEC-4 channels.
The Goodman and Vergassola laboratories have recently published a companion study (Sanzeni et al., 2019) that further addresses important details regarding mechanosensory coding in worm touch neurons. As in the Katta et al. study, Sanzeni et al. used a model that integrates body mechanics and the spatial recruitment of various channels. They found that this model could account for macroscopic currents generated in response to nontrivial spatial patterns, such as extended or multiple contacts that could not be addressed otherwise. Together, the two studies argue that neuroscientists should not focus solely on the neuron, but also consider the critical contribution of the skin to complex mechanosensory responses. Future studies in C. elegans may empirically test how changing the properties of skin through mutation and transgenic expression of epithelial components affects the skin-touch neuron transfer function.
The combined approach of physiology and modeling employed by Katta et al. (2019) and Sanzeni et al. (2019) may prove to be an effective way to discern how more complex mechanosensory neurons operate. In C. elegans, the touch neurons are the simplest mechanosensory neuron with their ball and stick shape. Other mechanosensory neurons in the worm are more complex. The PVD neuron features ornate primary, secondary, tertiary, and quaternary processes that branch off in a tiled pattern, resembling a series of candelabra used to sense painful mechanical stimuli (Inberg et al., 2019). The FLP neuron extends dendrites in a web pattern beneath the skin of the worm’s head, possibly to sense the mechanical stretch that accompanies changes in humidity (Russell et al., 2014). The IL mechanosensory neuron sprouts a simple cilium under the tip of the worm’s nose during most life stages, but unexpectedly elaborates a complex dendritic tip during the alternate dauer stage (Schroeder et al., 2013). Many of these neurons house putative or characterized ENaC/MEC or TRP mechanosensory channels that could be analyzed in the same way that the Goodman and Vergassola laboratories have elucidated the worm touch neurons. Future studies may also adapt their approach to examine how force is transduced through mammalian skin, and across the onion-like layers of epithelia in Pacinian corpuscles, to activate channels in the embedded mechanosensory neuron. Interestingly, these mammalian mechanosensory neurons express orthologues of MEC-4 and display rapidly adapting, phasic responses similar to worm touch neurons (Geffeney and Goodman, 2012).
Acknowledgments
Richard W. Aldrich served as editor.
Funds were provided by a CurePSP Fellowship, as well as National Institutes of Health grants R01-AA020992 and RF1-AG057355.
The authors declare no competing financial interests.
References
- Brown A.L., Fernandez-Illescas S.M., Liao Z., and Goodman M.B.. 2007. Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. J. Gen. Physiol. 129:161–173. 10.1085/jgp.200609672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnally S.M., Dev H.S., Stewart A.P., Barrera N.P., Van Bemmelen M.X., Schild L., Henderson R.M., and Edwardson J.M.. 2008. Direct visualization of the trimeric structure of the ASIC1a channel, using AFM imaging. Biochem. Biophys. Res. Commun. 372:752–755. 10.1016/j.bbrc.2008.05.100 [DOI] [PubMed] [Google Scholar]
- Chalfie M., and Sulston J.. 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans Dev. Biol. 82:358–370. 10.1016/0012-1606(81)90459-0 [DOI] [PubMed] [Google Scholar]
- Chalfie M., and Thomson J.N.. 1979. Organization of neuronal microtubules in the nematode Caenorhabditis elegans J. Cell Biol. 82:278–289. 10.1083/jcb.82.1.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur D.S., Ernstrom G.G., Goodman M.B., Yao C.A., Chen L., O’ Hagan R., and Chalfie M.. 2002. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 420:669–673. 10.1038/nature01205 [DOI] [PubMed] [Google Scholar]
- Coste B., Mathur J., Schmidt M., Earley T.J., Ranade S., Petrus M.J., Dubin A.E., and Patapoutian A.. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 330:55–60. 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva J.G., Mulholland A., and Goodman M.B.. 2007. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 27:14089–14098. 10.1523/JNEUROSCI.4179-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., and Chalfie M.. 1991. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 349:588–593. 10.1038/349588a0 [DOI] [PubMed] [Google Scholar]
- Eastwood A.L., and Goodman M.B.. 2012. Insight into DEG/ENaC channel gating from genetics and structure. Physiology (Bethesda). 27:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A.L., Sanzeni A., Petzold B.C., Park S.J., Vergassola M., Pruitt B.L., and Goodman M.B.. 2015. Tissue mechanics govern the rapidly adapting and symmetrical response to touch. Proc. Natl. Acad. Sci. USA. 112:E6955–E6963. 10.1073/pnas.1514138112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage L., Gu G., Hartwieg E., and Chalfie M.. 2004. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron. 44:795–807. 10.1016/j.neuron.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Geffeney S.L., and Goodman M.B.. 2012. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron. 74:609–619. 10.1016/j.neuron.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inberg S., Meledin A., Kravtsov V., Iosilevskii Y., Oren-Suissa M., and Podbilewicz B.. 2019. Lessons from Worm Dendritic Patterning. Annu. Rev. Neurosci. 42:365–383. 10.1146/annurev-neuro-072116-031437 [DOI] [PubMed] [Google Scholar]
- Jasti J., Furukawa H., Gonzales E.B., and Gouaux E.. 2007. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 449:316–323. 10.1038/nature06163 [DOI] [PubMed] [Google Scholar]
- Katta S., Sanzeni A., Das A., Vergassola M., and Goodman M.B.. 2019. Progressive recruitment of distal MEC-4 channels determines touch response strength in C. elegans J. Gen. Physiol. 151:1213–1230. 10.1085/jgp.201912374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Coste B., Chadha A., Cook B., and Patapoutian A.. 2012. The role of Drosophila Piezo in mechanical nociception. Nature. 483:209–212. 10.1038/nature10801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Feng Z., Sternberg P.W., and Xu X.Z.. 2006. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 440:684–687. 10.1038/nature04538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R., Chalfie M., and Goodman M.B.. 2005. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8:43–50. 10.1038/nn1362 [DOI] [PubMed] [Google Scholar]
- Omerbašić D., Schuhmacher L.N., Bernal Sierra Y.A., Smith E.S., and Lewin G.R.. 2015. ASICs and mammalian mechanoreceptor function. Neuropharmacology. 94:80–86. 10.1016/j.neuropharm.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Ranade S.S., Syeda R., and Patapoutian A.. 2015. Mechanically Activated Ion Channels. Neuron. 87:1162–1179. 10.1016/j.neuron.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Vidal-Gadea A.G., Makay A., Lanam C., and Pierce-Shimomura J.T.. 2014. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans Proc. Natl. Acad. Sci. USA. 111:8269–8274. 10.1073/pnas.1322512111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzeni A., Katta S., Petzold B., Pruitt B.L., Goodman M.B., and Vergassola M.. 2019. Somatosensory neurons integrate the geometry of skin deformation and mechanotransduction channels to shape touch sensing. eLife. 8:e43226 10.7554/eLife.43226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder N.E., Androwski R.J., Rashid A., Lee H., Lee J., and Barr M.M.. 2013. Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr. Biol. 23:1527–1535. 10.1016/j.cub.2013.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhang W., He Y., Gorczyca D., Xiang Y., Cheng L.E., Meltzer S., Jan L.Y., and Jan Y.N.. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 493:221–225. 10.1038/nature11685 [DOI] [PMC free article] [PubMed] [Google Scholar]