Abstract
Context
Enteral iron supplementation in preterm infants is recommended to supply sufficient iron for growth and development without increasing the risk of iron overload. However, the current recommendations date from 2010 and are based on limited evidence.
Objective
This systematic review aimed to investigate the effects of enteral iron supplementation on iron status, growth, neurological development, and adverse clinical outcomes in preterm (<37 weeks’ gestation) and low-birth-weight (LBW, <2500 g) infants.
Data sources
The PubMed/Medline and Cochrane Library databases were searched to 31 October 2018.
Data extraction
Of the 684 records identified, 27 articles, describing 18 randomized controlled trials (RCTs) plus 4 nonrandomized interventions, were included. Using the Cochrane Collaboration’s criteria, study quality was found to be poor to fair overall.
Results
Most articles (23/27) reported iron status indices; supplementation for ≥8 weeks resulted in increased hemoglobin and ferritin concentrations and a reduction in iron deficiency and anemia. No article reported on iron overload. Growth-related parameters reported in 12 articles were not affected by supplementation. Among the 7 articles on neurological development, a positive effect on behavior at 3.5 and 7 years was observed in one Swedish RCT. No association was found between supplementation and adverse clinical outcomes in the 9 articles reporting on studies in which such data was collected.
Conclusions
Long-term iron supplementation appears to result in improved iron status and a reduction in iron deficiency and anemia in preterm and LBW infants. However, high-quality evidence regarding the long-term effects of supplementation on functional health outcomes is lacking. Iron overload has largely been ignored. Well-designed, long-term, dose-response RCTs are required to ascertain the optimal dose and delivery method for the provision of dietary iron in preterm infants, with consideration of short- and long-term health effects.
Systematic Review Registration
PROSPERO registration no. CRD42018085214.
Keywords: growth, iron supplementation, low birth weight infants, neurological development, preterm infants
INTRODUCTION
Iron deficiency in early life adversely affects the growth and functioning of multiple organ systems.1 The effects of iron deficiency on the developing brain are permanent and life-altering.2,3 Preterm infants are deprived of the significant iron accretion that occurs in the third trimester of pregnancy and have reduced iron stores at birth compared with term infants.4,5 Pregnancy complications, including gestational diabetes mellitus and fetal growth restriction, as well as maternal lifestyle factors such as smoking and obesity, also compromise infant iron stores.6,7 Apart from the considerable impact of preterm birth itself, infants delivered preterm are at high risk of iron deficiency owing to the increased postnatal iron required to facilitate rapid growth and an earlier onset of erythropoiesis, which occurs 1–3 months earlier than in term infants.8,9
Iron supplementation is recommended for preterm infants, although extreme caution is warranted, as the potential risk of iron overload is high. High iron doses from supplementation or blood transfusions can result in excess iron, leading to free circulating Fe2+ ions, which contribute to the production of reactive oxygen species that can affect sensitive organ systems, such as the heart, liver, pancreas, and developing brain.10 Therefore, a balance in the provision of adequate iron is required to ensure optimal growth and development without contributing to iron overload.
To achieve this balance, international intake recommendations for enteral iron in preterm infants are 2–3 mg/kg/d from the age of 2–6 weeks until at least 6–12 months.11,12 Higher iron doses (>5 mg/kg/d) are only recommended for infants who are receiving erythropoietin treatment or for those that have experienced significant, uncompensated blood losses.11 These recommendations, made in 2010, were based on a limited evidence basis, with significant heterogeneity in trial design and iron treatment doses among studies that primarily focused on the effects of supplementation in relation to hematological parameters. Systematic reviews in 2012 highlighted the lack of evidence regarding the effect of iron supplementation on functional health outcomes, particularly long-term growth and neurological development.13,14
Following the 2010 recommendations and 2012 reviews, the availability of relevant intervention studies has increased. Therefore, the primary objective of the present work was to perform an updated systematic review to examine the effect of enteral iron supplementation on health outcomes in preterm and low-birth-weight (LBW, <2500 g) infants. The main health outcomes of interest were iron status, growth, and neurological development. A secondary objective was to investigate associations between supplementation and adverse clinical outcomes.
METHODS
The protocol for this systematic review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered prospectively with PROSPERO (CRD42018085214).
Search strategy
The PubMed/Medline and Cochrane Library databases were searched up to October 31, 2018. A structured search strategy was devised, incorporating the Medical Subject Headings (MeSH) search terms “Infant, Low Birth Weight,” “Infant, Extremely Low Birth Weight,” “Infant, Very Low Birth Weight,” “Infant, Premature,” “Infant, Extremely Premature,” “Premature Birth” or text words “preterm,” “premature” AND MeSH terms “Iron,” “Iron, Dietary,” “ferrous” or text words “iron intake,” “iron supplementation,” “iron supplements,” “ferrous sulphate/fumarate/sulfate,” “dietary iron intake.” Eligible studies were limited to English language articles only. The bibliographies of identified studies and key review (narrative and systematic/meta-analyses) articles were searched manually and incorporated into the current review in accordance with the eligibility criteria. Clinical trial registries were also searched for ongoing or recently completed trials.
Eligibility criteria
Studies in infants born premature (<37 wk’ gestation) or with a birth weight <2500 g investigating the effect of enteral iron supplementation on health outcomes, including iron status, growth, neurological development, and adverse clinical outcomes, were eligible for inclusion (Table 1). Studies in which enteral iron supplementation was provided with concurrent erythropoietin treatment were not eligible. Narrative reviews, case reports, comments, and editorials were also excluded.
Table 1.
PICOS criteria for inclusion of studies
| Criterion | Description |
|---|---|
| Participants | Infants born premature (<37 wk’ gestation) or with a birth weight <2500 g |
| Intervention | Iron supplementation through the enteral route, ie, medicinal iron, infant formula, human milk fortifier |
| Comparison | Different regimens of enteral iron supplementation, with respect to dose, duration, and timing of initiation of supplementation |
| Outcomes | 1. Iron status during and/or post the intervention period: |
| a) Indices reflecting storage iron (ferritin), transport iron (iron, transferrin, TIBC, transferrin saturation, soluble transferrin receptors), and functional iron (MCV, hematocrit, hemoglobin) | |
| b) Prevalence of iron deficiency, iron deficiency anemia, and iron overload (as defined by study investigators) | |
| 2. Growth-related parameters during and/or post the intervention period, including weight, length, and head circumference | |
| 3. Measures of neurological development during and/or post the intervention period, assessed through standardized neurodevelopmental assessments performed at predefined time points | |
| 4. Incidence of adverse short-term clinical outcomes (including NEC, ROP, CLD, PVL, oxidative stress, and sepsis) as defined by study investigators during and/or post the intervention period | |
| Study design | Any intervention study, including RCTs and nonrandomized trials |
Abbreviations: CLD, chronic lung disease; MCV, mean corpuscular volume; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia; RCT, randomized controlled trial; ROP, retinopathy of prematurity; TIBC, total iron-binding capacity.
Data collection
Following removal of duplicates, the titles and abstracts of all records retrieved from the search were screened independently by two researchers (E.K.M. and M.E.K.) to assess compliance with the eligibility criteria. Following exclusion of unsuitable records, the full texts of the remaining records were evaluated by all authors and the final list of included papers was agreed upon following discussion.
Information on study design/methodology, participant characteristics (eg, gestational age [GA], birth weight [BW], feeding method), interventions (dose, duration, timing initiation, comparators), outcome assessments (type, timing), and main findings were extracted from each study using an internally piloted, standardized data collection form. The duration of supplementation was stratified into short-term (<8 wk) and long-term (≥8 wk). To ensure uniformity, the iron supplementation dose was standardized to mg/kg/d, using the published mean birth weight as the body weight for this calculation. For trials of infant formula, an estimate of the daily intake of formula of 160 mL/kg/d was applied.13
The risk of bias in study design was assessed independently by the reviewers using the criteria set out by the Cochrane Handbook for Systematic Reviews of Interventions.15 These criteria were used to assess both randomized controlled trials (RCTs) and nonrandomized trials, and further assessment of the risk of selection bias was performed for the nonrandomized trials.
RESULTS
Study selection
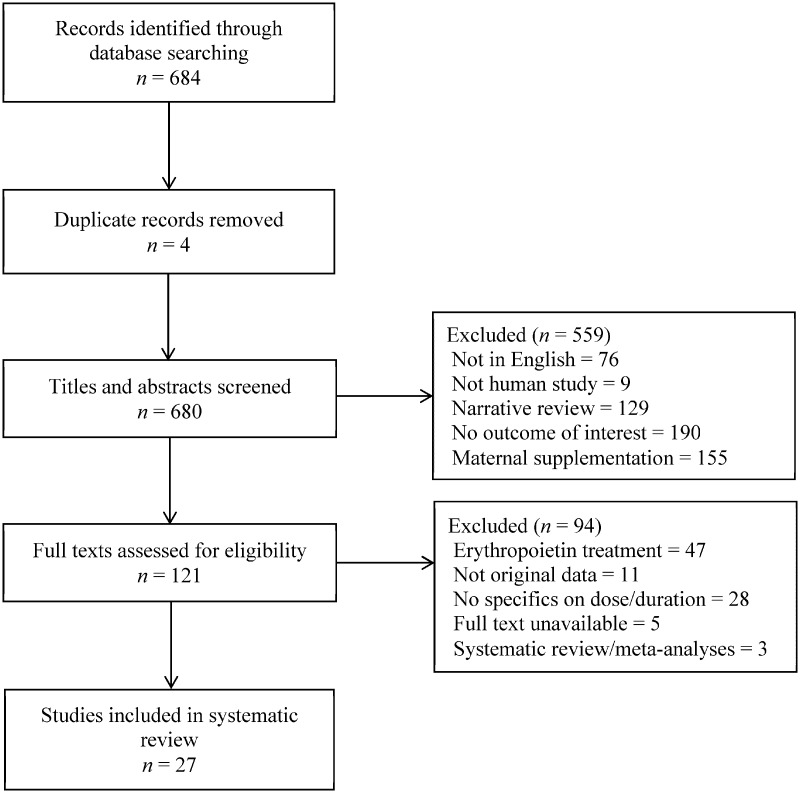
Following the database searches, 684 records were identified and 4 duplicates were removed (Figure 1). Of the 680 records screened, 559 were excluded, mainly owing to no reported outcome of interest or a focus on maternal supplementation. Following full-text review of the remaining records, 27 papers were eligible for inclusion in the systematic review.16–42
Figure 1.
Flow diagram of the literature selection process.
Study characteristics
The 27 included articles, characterized in Table 2,16–42 were published between 1974 and 2018 (including 7 since 2012), and most studies were conducted in Europe and North America. In total, there were 18 RCTs, as 5 publications16,17,22–24 were derived from a single trial conducted in Sweden.24 There were 4 nonrandomized interventions.27,29,37,42 Ten RCTs were placebo-controlled trials involving iron supplementation vs no supplementation/human milk as placebo19,21,24,25,30,31,35,36,38,41; 4 trials compared 2 or more iron doses20,32,34,39; and 4 compared early (age ≤2 wk) to late (4–8 wk) initiation of supplementation.18,28,33,40 The study by Steinmacher et al26 is a follow-on to the trial described by Franz et al.33 The intervention dose provided in most studies was 2–4 mg/kg/d, although doses of 6–24.2 mg/kg/d were also reported20,27,29,36,38,42 and the duration of supplementation ranged from 1 week to 1 year.
Table 2.
Characteristics of the included studies examining the effects of enteral iron supplementation on health outcomes in preterm and low-birth-weight infants
| Reference (country) | Eligibility | Sample sizea | Enteral iron dose | Age when iron supplementation was initiatedb | Duration of intervention | Follow-up ages and outcomesb |
|---|---|---|---|---|---|---|
| Berglund et al (2018)16 (Sweden) | BW: 2000–2500 g |
|
|
6 wk | 4.5 mo |
|
| Berglund et al (2015)17 (Sweden) | BW: 2000–2500 g |
|
|
6 wk | 4.5 mo |
|
| Joy et al (2014)18 (India) |
|
|
Both groups 2 mg/kg/d |
|
|
|
| van de Lagemaat et al (2014)19 (Netherlands) |
|
|
|
Term age | 6 mo |
|
| Miller et al (2013)20 (USA) | GA: 27–30 wk |
|
|
>1 wk (average age ∼ 3 wk) | 3 wk |
|
| Taylor and Kennedy (2013)21 (USA) | BW: <1500 g |
|
|
Range: 6–49 d (<32 wk PMA) | 4–5 wk |
|
| Berglund et al (2013)22 (Sweden) | BW: 2000–2500 g |
|
|
6 wk | 4.5 mo |
|
| Berglund et al (2011)23 (Sweden) | BW: 2000–2500 g |
|
|
6 wk | 4.5 mo |
|
| Berglund et al (2010)24 (Sweden) | BW: 2000–2500 g |
|
|
6 wk | 4.5 mo |
|
| Sankar et al (2009)25 (India) | BW: <1500 g |
|
|
14 d | 6–7 wk |
|
| Steinmacher et al (2007)26 (Germany) | BW: <1301 g |
|
|
|
NR |
|
| Braekke et al (2007)27 (Norway) |
|
21 | Median dose: 9.4 mg/kg/d | 6 wk | 1 wk |
|
| Arnon et al (2007)28 (Israel) | GA: ≤32 wk |
|
Both groups 5 mg/kg/d |
|
4–6 wk (to 8 wk of age) |
|
| Miller et al (2006)29 (USA) | GA: 24–32 wk |
|
|
Range: 7–60 d | 2–3 wk |
|
| Aggarwal et al (2005)30 (India) |
|
|
|
Range: 50–80 d | 8 wk |
|
| Berseth et al (2004)31 (USA) |
|
|
|
Range: 13–16 d | 28 d |
|
| Friel et al (2001)32 (Canada) | BW: <2500 g |
|
|
mean: 32-wk GA (receiving full enteral feeds) | 1 year |
|
| Franz et al (2000)33 (Germany) | BW: <1301 g |
|
|
|
NR |
|
| Griffin et al (1999)34 (UK) |
|
|
|
35–37 d | 6 mo |
|
| Hall et al (1993)35 (USA) |
|
|
|
8–10 d | 34–44 d (to hospital discharge) |
|
| Barclay et al (1991)36 (UK) | BW: <2500 g | 55 |
|
28 d | 16 wk |
|
| Iwai et al (1986)37 (Japan) | BW: 1000–2499 g |
|
|
3 mo | IFF for 3 mo |
|
| Halliday et al (1983)38 (UK) | GA: 28–36 wk |
|
|
7 d | 1 y |
|
| Rudolph et al (1981)39 (USA) |
|
|
|
5–6 d | 5 wk |
|
| Jansson et al (1979)40 (Sweden) |
|
|
|
|
4–5 mo (to 6 mo of age) |
|
| Lundstrom et al (1977)41 (Finland) | BW: 1050–2000 g |
|
|
2 wk | 5.5 mo |
|
| Brozovic et al (1974)42 (UK) |
|
47 | 24.2 mg/kg/d | 5 wk | 8 mo |
|
Sample size included in data analysis.
Chronological age, unless otherwise stated.
Abbreviations: BW, birth weight; C, control group; CA, corrected age; CRP, C-reactive protein; EI, early intervention; GA, gestational age; HM, human milk; IFF, iron-fortified formula; LI, late intervention; MCV, mean corpuscular volume; NR, not reported; PD, post discharge; PMA, postmenstrual age; RBC, red blood cells; RDW, red blood cell distribution width; T, treatment group; ZnPP/H, zinc protoporphyrin to heme ratio.
Outcomes from the 27 articles analyzed were described individually because, although there were 22 individual trials, each paper assessed health outcomes independently. This is a challenging and vulnerable population to study. Although the overall study quality was poor to fair, some of the recent studies were of high quality (Table 316–42). The main source of bias in the RCTs was a lack of allocation concealment during the intervention (12/18 RCTs), although most did employ an appropriate method of randomization (16/18). Incomplete outcome data was another limitation of many of the papers, with significant, but understandable, attrition of participants in studies involving long-term follow-up. Given the large degree of heterogeneity in study design, a quantitative comparison or meta-analysis was inappropriate and not feasible. Particular limitations that precluded such an analysis were the variability in participant characteristics with respect to GA and BW; differences in intervention doses, timing, and duration of supplementation; and variability in both outcomes and timing and methods of outcome assessment.
Table 3.
Assessment of risk of bias in the studies reported on in the 27 articles included in the present review
| Reference | Random sequence generation | Allocation concealment | Blinding of participants/ personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Berglund et al (2018)16 | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Berglund et al (2015)17 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Joy et al (2014)18 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| van de Lagemaat et al (2014)19 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Miller (2013)20 | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Taylor and Kennedy (2013)21 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Berglund et al (2013)22 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Berglund et al (2011)23 | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Berglund et al (2010)24 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sankar et al. (2009)25 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Steinmacher et al (2007)26 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Braekke et al (2007)27a | High risk | High risk | High risk | High risk | Low risk | Low risk |
| Arnon et al (2007)28 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Miller et al (2006)29a | High risk | High risk | High risk | High risk | Low risk | Low risk |
| Aggarwal et al (2005)30 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Berseth et al (2004)31 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Friel et al (2001)32 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Franz et al (2000)33 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Griffin et al (1999)34 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Hall et al (1993)35 | Low risk | Unclear | Low risk | Low risk | High risk | Low risk |
| Barclay et al (1991)36 | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk |
| Iwai et al (1986)37a | High risk | High risk | High risk | High risk | Unclear | Low risk |
| Halliday et al (1983)38 | Low risk | Unclear | Low risk | Unclear | Unclear | Low risk |
| Rudolph et al (1981)39 | Low risk | Unclear | High risk | High risk | High risk | Low risk |
| Jansson et al (1979)40 | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Lundstrom et al (1977)41 | High risk | High risk | Unclear | Unclear | Low risk | Low risk |
| Brozovic et al (1974)42a | High risk | High risk | High risk | High risk | Low risk | Low risk |
Nonrandomized trial – risk of selection bias also assessed in these studies.
Effects of enteral iron supplementation
Iron status indices
The effect of supplementation on iron status indices was assessed in 23 papers.17–21,24,25,27–42 In 8 trials, duration of supplementation was <8 weeks, and in 11 trials duration of supplementation was ≥8 weeks, while another 4 trials investigated the timing of supplementation initiation. While 2 studies,27,35 including a small nonrandomized trial of high-dose iron (9.4 mg/kg/d) in very-low-birth-weight (VLBW, <1500 g) infants,27 observed a small effect of short-term supplementation on iron indices, the other studies on short-term supplementation reported no effect.20,21,25,29,31,39 Treatment with medicinal iron, formula, or human milk fortifier at 2–4 mg/kg/d was found to have no effect on ferritin, hematocrit, or hemoglobin concentrations in VLBW infants.21,25,31,39 Additionally, high-dose (average 7–10 mg/kg/d) supplementation in infants born at less than 32 weeks’ gestation had no effect on ferritin, iron, transferrin saturation, transferrin receptors, or total iron-binding capacity (TIBC).20,29
Of the 11 studies in which duration of supplementation was ≥8 weeks, 8 reported an effect on iron status.17,19,24,30,36,37,41,42 In marginally LBW infants, a dose of 2 mg/kg/d increased circulating iron, ferritin, transferrin saturation, transferrin receptors, mean corpuscular volume (MCV), and hemoglobin at 6 months compared to the placebo group.24 Ferritin concentrations remained significantly higher at 12 months, but not at 3.5 years, in the marginally LBW infants compared with the placebo group.17 Supplementation with high-dose (∼7.1 and 24.2 mg/kg/d) medicinal iron resulted in higher serum ferritin and iron concentrations.36,42 Long-term formula interventions also reported beneficial effects on ferritin, MCV, and hemoglobin concentrations.19,37 Three studies observed no effect with long-term supplementation,32,34,38 including 2 dose-comparison formula interventions, where consumption of a higher-iron formula had no effect on ferritin, transferrin, hematocrit, or hemoglobin concentrations compared with a lower-iron formula.32,34
Four studies examined the effect of early vs late initiation of supplementation on iron status.18,28,33,40 Short-term interventions by Joy et al18 and Arnon et al28 reported higher ferritin, iron, transferrin receptors, and hemoglobin in infants initiated at 2 weeks vs 4–6 weeks of age. In the only long-term trial, no differences in ferritin or hemoglobin were observed between infants that received 2–3 mg/kg/d from the age of 3 weeks and those that received the same dose from 8 weeks.40
Prevalence of iron deficiency, iron deficiency anemia, and iron overload
Nine articles reported the effect of iron supplementation on the prevalence of iron deficiency or iron deficiency anemia.17,19,24,32–35,37,41 It is worth noting that no article reported on iron overload. In the only short-term intervention, treatment with a high-iron formula (∼2.4 mg/kg/d, delivering 15 mg/L) resulted in a lower prevalence of low serum ferritin (<19 ng/mL) (32% in the high-iron vs 79% in the low-iron group) and anemia (hemoglobin <9 g/dL) (26% vs 70%) than treatment with a low-iron formula (∼0.5 mg/kg/d, delivering 3 mg/L).35 The prevalence of low ferritin among infants receiving fortified human milk (delivering 1.7 mg/L) was 23%, and 77% were anemic.
In 2 long-term RCTs looking at formula milk, a reduced incidence of iron deficiency was observed in the formula-fed groups compared to the human milk–fed groups.19,37 Long-term medicinal iron supplementation was also associated with a reduced prevalence of iron deficiency and/or iron deficiency anemia.17,24,33,41 Marginally LBW infants supplemented with 2 mg/kg/d had a lower prevalence of iron depletion, iron deficiency, and anemia at both 6 and 12 months than those in the placebo group.17,24 Comparable findings were reported in an initiation timing trial; 15% of infants supplemented at ∼1 week of age had iron deficiency compared to 40% of those initiated at ∼8.5 weeks.33
Growth
Twelve papers investigated the effects of iron supplementation on growth-related parameters – namely, weight, length, and head circumference.17–21,24,25,30–32,35,36 In 6 short-term interventions, no effect of medicinal iron, formula, or fortifier on any growth-related parameters was observed.18,20,21,25,31,35 In the only short-term intervention with long-term follow-up, high-dose (average 10.4 mg/kg/d) iron had no effect on weight, length, or head circumference up to 24 months corrected age (CA).20 Six articles reported that long-term supplementation had no effect on growth,17,19,24,30,32,36 including a high-dose (∼7.1 mg/kg/d) medicinal iron RCT in LBW infants.36 Long-term supplementation of 1–2 mg/kg/d had no effect on long-term growth to 3.5 years of age in marginally LBW infants.17,24
Neurological development
Seven articles reported on studies that explored the effect of iron supplementation on neurological development, as summarized in Table 4.16,18,20,22,23,26,32 Short-term supplementation had no effect on neurological development in 2 RCTs,18,20 including a high-dose (average 10.4 mg/kg/d) trial with outcomes assessed to 2 years of age.20 In an initiation timing trial of 2–4 mg/kg/d, while no effect on cognition, motor function, visual impairment, or behavior was observed at 5 years CA, there was a trend towards benefit of early initiation; 35% of the late initiation group had an abnormal neurological examination result compared to 19% of the early initiation group.26 While no effect of long-term supplementation on neurophysiological functioning – assessed using auditory brainstem responses – was observed at 6 months in a Swedish RCT in marginally LBW infants,23 supplemented children had a significantly lower prevalence of behavioral problems than those in the placebo group (3% vs 13%, respectively) at 3.5 years22 and had significantly lower scores in externalizing-type behaviors (aggression/attention seeking) at 7 years.16
Table 4.
Effect of enteral iron supplementation on neurological development in preterm and low-birth-weight infants
| Reference | Duration of intervention | Age at follow-upa | Outcome assessment | Main findings |
|---|---|---|---|---|
| Berglund et al (2018)16 | 4.5 mo | 7 y | Wechsler Scale of Intelligence | No significant differences between treatment groups in Wechsler Scale or Five-to-Fifteen scores. |
| Child Behavior Checklist | ||||
| Five-to-Fifteen questionnaire | Children in placebo group had significantly higher Child Behavior Checklist scores for externalizing problems than did supplemented groups. | |||
| Joy et al (2014)18 | 6–10 wk | 12 wk | Hammer-Smith neurological examination | No significant difference between treatment groups in prevalence of neurological deficits. |
| Miller et al (2013)20 | 3 wk | 6 and 24 mo CA | Bayley Scales of Infant Development (3rd Edition) | No significant difference between treatment groups in scores at 6 or 24 mo CA. |
| Berglund et al (2013)22 | 4.5 mo | 3.5 y | Wechsler Scale of Intelligence | No significant difference between treatment groups on Wechsler Scale. |
| Child Behavior Checklist | Significantly higher prevalence of Child Behavior Checklist total problem scores > US subclinical cutoff in placebo group compared to supplemented groups. | |||
| Berglund et al (2011)23 | 4.5 mo | 6 mo | Auditory brainstem responses | No significant difference between treatment groups in auditory brainstem response wave V latencies. |
| Central conduction time significantly higher in treatment group but not correlated to iron intake. | ||||
| Steinmacher et al (2007)26 | NR | 5 y CA | Gross Motor Functioning Classification Scale | Significantly higher prevalence of abnormal results on standard neurological examination in late group compared to early group. |
| Lincoln-Oseretsky Scale | No significant differences between groups in any other developmental measures. | |||
| Kaufman Assessment Battery | ||||
| Visual impairment | ||||
| Child Behavior Checklist | ||||
| Friel et al (2001)32 | 1 y | 3, 6, 9, 12 mo CA | Griffiths Mental Development Assessment | No significant difference between treatment groups in assessment scores at any time point. |
Chronological age, unless otherwise stated.
Abbreviation: CA, corrected age; NR, not reported.
Adverse clinical outcomes
Nine articles reported the effect of supplementation on the incidence of short-term adverse clinical outcomes, including necrotizing enterocolitis, retinopathy of prematurity, chronic lung disease, periventricular leukomalacia, oxidative stress, and sepsis (Table 518,21,25,27–29,31,32,36). Short-term supplementation with medicinal iron or human milk fortifier at 2–4 mg/kg/d had no effect in 4 RCTs.18,21,25,31 High-dose (≥5 mg/kg/d) iron for <8 weeks also had no effect on adverse outcomes, including measures of oxidative stress, in 1 timing RCT and 2 nonrandomized trials.27–29
Table 5.
Effect of enteral iron supplementation on incidence of adverse short-term clinical outcomes in preterm and low-birth-weight infants
| Reference | Necrotizing enterocolitis | Retinopathy of prematurity | Chronic lung disease | Periventricular leukomalacia | Oxidative stress | Sepsis |
|---|---|---|---|---|---|---|
| Joy et al (2014)18 | No effect | No effect | Not reported | No effect | Not reported | Not reported |
| Taylor and Kennedy (2013)21 | No effect | Not reported | No effect | Not reported | Not reported | No effect |
| Sankar et al (2009)25 | No effect | No effect | No effect | No effect | Not reported | No effect |
| Braekke et al (2007)27 | Not reported | Not reported | Not reported | Not reported | No effect | Not reported |
| Arnon et al (2007)28 | No effect | No effect | No effect | No effect | Not reported | No effect |
| Miller et al (2006)29 | Not reported | Not reported | Not reported | Not reported | No effect | Not reported |
| Berseth et al (2004)31 | No effect | Not reported | Not reported | Not reported | Not reported | No effect |
| Friel et al (2001)32 | Not reported | Not reported | Not reported | Not reported | Glutathione peroxidase higher in high iron group | Not reported |
| Barclay et al (1991)36 | Not reported | Not reported | Not reported | Not reported | Superoxide dismutase lower in high iron group | Not reported |
Measures of antioxidant activity/oxidative stress were reported in 2 long-term RCTs.32,36 Erythrocyte superoxide dismutase activity decreased in the high-iron (∼7.1 mg/kg/d) group during a 16-week RCT, with no changes observed in the low-iron (∼3.6 mg/kg/d) or no-iron groups, although no effect was seen with other markers of copper metabolism or plasma zinc.36 In 1 year-long RCT, glutathione peroxidase activity was slightly higher in the high-iron formula (∼3.4 mg/kg/d) group than in the normal (∼2.1 mg/kg/d) formula group at study-end, but no differences in superoxide dismutase activity, malondialdehyde, or catalases were observed.32
DISCUSSION
This systematic review provides an important update on the evidence regarding the effect of enteral iron supplementation on health outcomes in preterm and LBW infants. Since the 2010 recommendations for preterm infants, an additional 8 papers have been published, based on 5 trials. Despite this new evidence, questions about the long-term health benefits or risks of enteral iron supplementation in preterm and LBW infants, over and above improved iron status, still remain.
Consistent with the evidence in term-born infants and children,43,44 iron supplementation for ≥8 weeks appears to result in improved iron status in preterm and LBW infants, with ferritin and hemoglobin the most frequently reported indices. There is little to no benefit of short-term supplementation (<8 wk) in this population. Worryingly, the long-term effect of supplementation remains unclear; only 1 trial, published in 2015, measured iron indices beyond 1 year of age.17 Long-term supplementation was associated with a decreased prevalence of iron deficiency and anemia in preterm and LBW infants, although, only 9 of the articles in this review reported data on the prevalence of iron deficiency. The reporting of prevalence data enables meaningful comparisons across studies; investigating a supplementation effect on individual indices in isolation is of limited benefit in terms of understanding the clinical condition itself. However, to achieve this, the current uncertainty regarding the appropriate diagnostic criteria for iron deficiency in this population should be addressed as a matter of urgency.
A U-shaped curve of risk is associated with iron, as both deficiency and overload can lead to impaired health outcomes.43,45 Excess iron has been associated with decreased growth, impaired cognitive development, and an increased risk of infection, with evidence also emerging of altered gut microbiota in infants and young children.45,46 Preterm infants are particularly vulnerable to iron overload; however, the issue of overload was largely not addressed by the articles in this review. Further consideration of overload and its potential consequences in this vulnerable population is warranted, especially in very preterm infants who often receive multiple blood transfusions during their hospital stay.47 As hemoglobin concentrations alone do not provide enough insight into the development of iron overload, clinical management should also include the routine measurement of ferritin concentrations, particularly during the inpatient period. This would facilitate the establishment of more personalized and tailored supplementation regimens; for example, in infants with elevated ferritin concentrations (>300µg/L), supplementation could be delayed or ceased temporarily until their ferritin concentrations return to normal.9,48 Further investigation into the suitability of other iron indices, such as transferrin receptors, in this population is also necessary.
While excess free iron has been postulated to play a role in the etiology of some neonatal diseases,49–51 this review found no reported evidence of an increased risk of necrotizing enterocolitis, retinopathy of prematurity, chronic lung disease, periventricular leukomalacia, or sepsis with supplementation. Two RCTs observed slight changes in oxidative stress markers following long-term supplementation, but the clinical significance of these changes are unclear as long-term follow-up was not implemented.32,36 No other studies of high-dose supplementation reported adverse effects,27–29 although it is important to note that the evidence to date is inadequate, particularly given the wide variability in the monitoring of adverse outcomes in trials, in both the frequency and type of clinical data collected. Trials need to include the systematic collection of adverse outcome data, thereby enabling an accurate assessment of the potential risks of supplementation, which is yet to be achieved.
Adverse effects of iron supplementation for growth in iron-replete infants and young children have been reported.45 In this review, there was no evidence that short- or long-term iron supplementation had any adverse or beneficial effects on growth in preterm and LBW infants. There is still insufficient evidence regarding the effect on long-term growth: only 2 trials assessing growth-related parameters beyond the first year of life were published in the intervening years since the recommendations.17,20 Most studies included measurements of weight, length, and head growth only, with actual or z-score changes in growth parameters reported by relatively few of the included articles. Moreover, the potential effect of iron supplementation on body composition in this population is yet to be investigated.
The evidence regarding the effect of iron supplementation on neurological development in preterm and LBW infants is limited. Only 7 articles reported on studies that evaluated neurological development; while 5 of these articles were published since the 2010 recommendations, 3 were based on the same trial in marginally LBW infants. There was no evidence of an effect of supplementation on cognition in preterm and LBW infants; however, long-term supplementation was suggested to have a positive effect on behavior, albeit only in that trial of marginally LBW infants.16,22 The effect of supplementation in the most vulnerable infants, particularly those born very preterm or with a birth weight <1500 g, is yet to be elucidated. This paucity of data, combined with the significant methodological differences in assessments, have made it difficult to draw meaningful conclusions in this review. Further, the difficulty in determining the effect of iron supplementation, above and beyond the potential effect of other comorbidities, is a significant challenge in this vulnerable population.
The extensive search strategy and prospective protocol registration are strengths of this review, although the search was limited to English language articles only. The generalizability of this review’s findings is limited to high-resource settings, where the studies were predominately conducted.
While limited evidence was available on which to base the 2010 recommendations, this review has not identified sufficient quantities of appropriate, consistent evidence published since then to formulate revised recommendations from the perspectives of dosing or duration of supplementation. The substantial heterogeneity in trial design with respect to participant characteristics, intervention regimens, outcome assessments, and follow-up has limited study comparisons. In particular, the iron dose administered in trials conducted both pre and post the 2010 recommendations has been variable, making it difficult to truly assess the benefits or risks of higher doses. The most effective delivery method for iron in this population remains unclear, especially given the lack of consideration given by many investigators to the variability in iron concentration and bioavailability of different feeding methods. The significant difference in participants’ underlying medical care is another challenge that is often unaccounted for, particularly with respect to placental transfusion methods, use of blood transfusions, nutritional management, or other nutrient supplementation.
CONCLUSION
Long-term iron supplementation appears to result in improved iron status, coupled with a decrease in iron deficiency and anemia in preterm and LBW infants. No beneficial or adverse effects of supplementation on short-term growth outcomes have been reported. However, there remains a paucity of high-quality evidence on the effect of iron supplementation on other health outcomes, in particular with respect to long-term growth and neurological development. The potential adverse effects of supplementation, particularly the risk of iron overload, require further consideration.
The 2010 recommendations for enteral iron supplementation in preterm infants should continue to be implemented. However, adjustments in clinical practice should be carefully considered. Tailored supplementation regimens for individual patients, based on routine monitoring of iron status indices, particularly hemoglobin and ferritin, should be formulated for all hospitalized preterm and LBW infants, particularly those in receipt of multiple blood transfusions.
Advancement of this research field is necessary before the current recommendations can be updated. However, the ethical constraints of conducting a placebo-controlled trial in this vulnerable population group present a significant challenge. Therefore, well-designed, long-term, dose-response RCTs, comparing 2 or more iron doses and including a fortified human milk control group, are required to ascertain the optimal dose and delivery method for the provision of iron in preterm infants. Follow-up of short-, medium-, and long-term health outcomes is essential, while further consideration of the most vulnerable infants, particularly those born very preterm, is also needed.
Acknowledgments
Author contributions. The authors’ responsibilities were as follows: E.K.M. and M.E.K. designed the research; E.K.M. conducted the literature search, analyzed the data, and drafted, reviewed, and revised the manuscript; M.E.K. analyzed the data and critically reviewed and revised the manuscript; E.M.D. provided a critical review of the manuscript; and all authors read and approved the final manuscript.
Funding. E.K.M. is supported by the Science Foundation Ireland–funded PiNPoINT (Personalised Nutrition for the Preterm Infant) project (13/SP INFANT/B2888). This work was carried out at the Irish Centre for Fetal and Neonatal Translational Research (INFANT) and was funded in part by Science Foundation Ireland (12/RC/2272). No funding agencies had any role in the design, analysis, or writing of this article.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Domellof M, Braegger C, Campoy C, et al. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr. 2014;58:119–129. [DOI] [PubMed] [Google Scholar]
- 2. Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozoff B, Smith JB, Kaciroti N, et al. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr. 2013;163:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singla PN, Gupta VK, Agarwal KN.. Storage iron in human foetal organs. Acta Paediatr Scand. 1985;74:701–706. [DOI] [PubMed] [Google Scholar]
- 5. Lackmann GM, Schnieder C, Bohner J.. Gestational age-dependent reference values for iron and selected proteins of iron metabolism in serum of premature human neonates. Biol Neonate. 1998;74:208–213. [DOI] [PubMed] [Google Scholar]
- 6. Rao R, Georgieff MK.. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarthy EK, Kenny LC, Hourihane JO, et al. Impact of maternal, antenatal and birth-associated factors on iron stores at birth: data from a prospective maternal-infant birth cohort. Eur J Clin Nutr. 2017;71:782–787. [DOI] [PubMed] [Google Scholar]
- 8. Rao R, Georgieff MK.. Neonatal iron nutrition. Semin Neonatol. 2001;6:425–435. [DOI] [PubMed] [Google Scholar]
- 9. Domellof M, Georgieff MK.. Postdischarge iron requirements of the preterm infant. J Pediatr. 2015;167(suppl 4):S31–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wessling-Resnick M. Excess iron: considerations related to development and early growth. Am J Clin Nutr. 2017;106(suppl 6):1600s–1605s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. [DOI] [PubMed] [Google Scholar]
- 12. Baker RD, Greer FR.. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040–1050. [DOI] [PubMed] [Google Scholar]
- 13. Mills RJ, Davies MW.. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst Rev. 2012;3:CD005095.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long H, Yi JM, Hu PL, et al. Benefits of iron supplementation for low birth weight infants: a systematic review. BMC Pediatr. 2012;12:99.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S.. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester, England: Wiley; 2011. Available at: www.handbook.cochrane.org. Accessed September 24, 2018. [Google Scholar]
- 16. Berglund SK, Chmielewska A, Starnberg J, et al. Effects of iron supplementation of low birth weight infants on cognition and behavior at 7 years – a randomized controlled trial. Pediatr Res. 2018;83:111–118. [DOI] [PubMed] [Google Scholar]
- 17. Berglund SK, Westrup B, Domellof M.. Iron supplementation until 6 months protects marginally low-birth-weight infants from iron deficiency during their first year of life. J Pediatr Gastroenterol Nutr. 2015;60:390–395. [DOI] [PubMed] [Google Scholar]
- 18. Joy R, Krishnamurthy S, Bethou A, et al. Early versus late enteral prophylactic iron supplementation in preterm very low birth weight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2014;99:F105–F109. [DOI] [PubMed] [Google Scholar]
- 19. van de Lagemaat M, Amesz EM, Schaafsma A, et al. Iron deficiency and anemia in iron-fortified formula and human milk-fed preterm infants until 6 months post-term. Eur J Nutr. 2014;53:1263–1271. [DOI] [PubMed] [Google Scholar]
- 20. Miller SM. Iron supplementation in premature infants using the zinc protoporphyrin to heme ratio: short- and long-term outcomes. J Perinatol. 2013;33:712–716. [DOI] [PubMed] [Google Scholar]
- 21. Taylor TA, Kennedy KA.. Randomized trial of iron supplementation versus routine iron intake in VLBW infants. Pediatrics. 2013;131:e433–e438. [DOI] [PubMed] [Google Scholar]
- 22. Berglund SK, Westrup B, Hagglof B, et al. Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics. 2013;131:47–55. [DOI] [PubMed] [Google Scholar]
- 23. Berglund SK, Westrup B, Haraldsson E, et al. Effects of iron supplementation on auditory brainstem response in marginally LBW infants. Pediatr Res. 2011;70:601–606. [DOI] [PubMed] [Google Scholar]
- 24. Berglund S, Westrup B, Domellof M.. Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics. 2010;126:e874–e883. [DOI] [PubMed] [Google Scholar]
- 25. Sankar MJ, Saxena R, Mani K, et al. Early iron supplementation in very low birth weight infants – a randomized controlled trial. Acta Paediatr. 2009;98:953–958. [DOI] [PubMed] [Google Scholar]
- 26. Steinmacher J, Pohlandt F, Bode H, et al. Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: neurocognitive development at 5.3 years’ corrected age. Pediatrics. 2007;120:538–546. [DOI] [PubMed] [Google Scholar]
- 27. Braekke K, Bechensteen AG, Halvorsen BL, et al. Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. J Pediatr. 2007;151:23–28. [DOI] [PubMed] [Google Scholar]
- 28. Arnon S, Shiff Y, Litmanovitz I, et al. The efficacy and safety of early supplementation of iron polymaltose complex in preterm infants. Am J Perinatol. 2007;24:95–100. [DOI] [PubMed] [Google Scholar]
- 29. Miller SM, McPherson RJ, Juul SE.. Iron sulfate supplementation decreases zinc protoporphyrin to heme ratio in premature infants. J Pediatr. 2006;148:44–48. [DOI] [PubMed] [Google Scholar]
- 30. Aggarwal D, Sachdev HP, Nagpal J, et al. Haematological effect of iron supplementation in breast fed term low birth weight infants. Arch Dis Child. 2005;90:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berseth CL, Van Aerde JE, Gross S, et al. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics. 2004;114:e699–e706. [DOI] [PubMed] [Google Scholar]
- 32. Friel JK, Andrews WL, Aziz K, et al. A randomized trial of two levels of iron supplementation and developmental outcome in low birth weight infants. J Pediatr. 2001;139:254–260. [DOI] [PubMed] [Google Scholar]
- 33. Franz AR, Mihatsch WA, Sander S, et al. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics. 2000;106:700–706. [DOI] [PubMed] [Google Scholar]
- 34. Griffin IJ, Cooke RJ, Reid MM, et al. Iron nutritional status in preterm infants fed formulas fortified with iron. Arch Dis Child Fetal Neonatal Ed. 1999;81:F45–F49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall RT, Wheeler RE, Benson J, et al. Feeding iron-fortified premature formula during initial hospitalization to infants less than 1800 grams birth weight. Pediatrics. 1993;92:409–414. [PubMed] [Google Scholar]
- 36. Barclay SM, Aggett PJ, Lloyd DJ, et al. Reduced erythrocyte superoxide dismutase activity in low birth weight infants given iron supplements. Pediatr Res. 1991;29:297–301. [DOI] [PubMed] [Google Scholar]
- 37. Iwai Y, Takanashi T, Nakao Y, et al. Iron status in low birth weight infants on breast and formula feeding. Eur J Pediatr. 1986;145:63–65. [DOI] [PubMed] [Google Scholar]
- 38. Halliday HL, Lappin TR, McClure BG.. Do all pre-term infants need iron supplements? Ir Med J. 1983;76:430–432. [PubMed] [Google Scholar]
- 39. Rudolph N, Preis O, Bitzos EI, et al. Hematologic and selenium status of low-birth-weight infants fed formulas with and without iron. J Pediatr. 1981;99:57–62. [DOI] [PubMed] [Google Scholar]
- 40. Jansson L, Holmberg L, Ekman R.. Medicinal iron to low birth weight infants. Acta Paediatr Scand. 1979;68:705–708. [DOI] [PubMed] [Google Scholar]
- 41. Lundstrom U, Siimes MA, Dallman PR.. At what age does iron supplementation become necessary in low-birth-weight infants? J Pediatr. 1977;91:878–883. [DOI] [PubMed] [Google Scholar]
- 42. Brozovic B, Burland WL, Simpson K, et al. Iron status of preterm low birthweight infants and their response to oral iron. Arch Dis Child. 1974;49:386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iannotti LL, Tielsch JM, Black MM, et al. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pasricha SR, Hayes E, Kalumba K, et al. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health. 2013;1:e77–e86. [DOI] [PubMed] [Google Scholar]
- 45. Lonnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr. 2017;106(suppl 6): 1681s–1687s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paganini D, Zimmermann MB.. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr. 2017;106(suppl 6): 1688s–1693s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (pint) study: a randomized, controlled trial of a restrictive (LOW) versus liberal (HIGH) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149: 301–307. [DOI] [PubMed] [Google Scholar]
- 48. Uauy R, Koletzko B.. Defining the nutritional needs of preterm infants. World Rev Nutr Diet. 2014;110:4–10. [DOI] [PubMed] [Google Scholar]
- 49. Inder TE, Clemett RS, Austin NC, et al. High iron status in very low birth weight infants is associated with an increased risk of retinopathy of prematurity. J Pediatr. 1997;131:541–544. [DOI] [PubMed] [Google Scholar]
- 50. Hirano K, Morinobu T, Kim H, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F188–F193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lackmann GM, Hesse L, Tollner U.. Reduced iron-associated antioxidants in premature newborns suffering intracerebral hemorrhage. Free Radic Biol Med. 1996;20:407–409. [DOI] [PubMed] [Google Scholar]