Abstract
Volunteer-led surveys are increasingly used to collect ecological information and may represent a means for obtaining the tree measurement datasets necessary to calculate carbon stocks in tropical forests in order to justify funding like REDD+. However, the accuracy of tree measurements collected by volunteers remains unassessed. Here, we examine how tree measurements collected by student volunteers vary compared to measurements collected by trained ecologists using identical methods. Measurements by both teams were collected at 11 habitat plots on Buton Island, Indonesia. Both teams counted similar numbers of trees per plot and obtained positively correlated circumference-at-breast-height measurement values at plot and individual tree scales of aggregation. Volunteer and ecologist-generated median carbon stock estimates differed by just 1.1%. We therefore suggest that with sufficient training and supervision volunteers can be used to obtain accurate tree measurement data for carbon stock calculations.
Keywords: Citizen science, Habitat, Indonesia, Rainforest, REDD+
Introduction
Tropical forests continue to experience the highest rates of destruction and degradation of all terrestrial ecosystems globally (Hansen et al. 2013), with numerous ecological and socio-economic consequences (Fearnside 2005; Bradshaw et al. 2009; Van der Werf et al. 2009). In response, ambitious attempts to mitigate these impacts have been developed, such as the United Nations REDD+ programme, which is facilitating provision of billions of dollars to arrest trends in tropical deforestation (Agrawal et al. 2011; Goetz et al. 2015). However, while major funds for these projects have already been pledged (McNeill 2015), effectively allocating them can be inhibited by the data ‘vacuum’ inherent in most tropical forest ecosystems (Gardner et al. 2007). Funding applications to systems such as REDD+ invariably require large ecological datasets to provide evidence of the proposed project area’s value in terms of biodiversity and carbon stocks (Verified Carbon Standard 2012; Narasimhan et al. 2014). Assembling such datasets is, however, time-consuming and expensive, a major limitation considering the paucity of financial resources available in the tropics (James et al. 1999).
One potential means of addressing this issue is the mobilisation of volunteer ‘citizen scientists’ within tropical forest ecosystems. Many examples of ecological projects utilising large volunteer teams to collect diverse forms of biological data have been documented in recent decades (e.g. Prater 1981; Brown et al. 2001; Niinioja, et al. 2004; Dickinson et al. 2010). Various advantages and disadvantages of these volunteer-led surveys have been described. Common limitations include an inability to effectively identify taxa to a species level (Brandon et al. 2003; Kremen et al. 2011), as well as difficulties measuring complex variables effectively (Galloway et al. 2006). Conversely, numerous studies have also demonstrated that data from volunteer-led surveys can be highly accurate, consistent, and of comparable quality to that produced by professionals, provided that the goals of these surveys are sufficiently simple (e.g. Darwall and Dulvy 1996; Newman et al. 2003; Boudreau and Yan 2004; Delaney et al. 2008; Roman et al. 2017). While challenges in their use remain (Dickinson et al. 2010), the scale of volunteer-generated datasets is swiftly growing (Silvertown 2009), due to greater appreciation of volunteer-led data collection models within the scientific community and an increased desire by funding bodies for scientists to incorporate volunteers into their research (Dickinson et al. 2012).
The growth of volunteer-led surveys has seen a corresponding increase in citizen scientist projects focussing on habitat structure measurements, as well as numerous evaluations of the effectiveness of these projects (e.g. Brandon et al. 2003; Galloway et al. 2006; Butt et al. 2013). However, very few of these studies have focussed specifically on tropical forest ecosystems, and those that have (e.g. Holck 2008) did not specifically investigate the measurement of habitat structure variables used in ground-based methods for collecting the carbon stock data required for applications to large-scale funding systems such as REDD+. This is an important research gap, as while allometric carbon calculations themselves can be complex, most tend to use a single simple measurement derived from tree circumference-at-breast-height (CBH) as a starting point to determine above-ground biomass, and hence ultimately carbon stocks (e.g. Brown 1997; Chave et al. 2005; Verified Carbon Standard 2012; Narasimhan et al. 2014). Demonstrating that volunteers can accurately record tree measurements may therefore have implications for how baseline data for programmes like REDD+ are collected. Here, we investigate this issue by comparing volunteer-led tree measurement data from a tropical forest with data generated by a team of trained ecologists working at the same survey sites using identical methodologies. We analyse the differences between these two datasets to determine how accurate volunteer-led surveys can be in providing the baseline data necessary for generating carbon stock measurements, with an additional outcome of testing whether increased time collecting project data makes volunteer surveyors more reliable.
Materials and methods
Study area
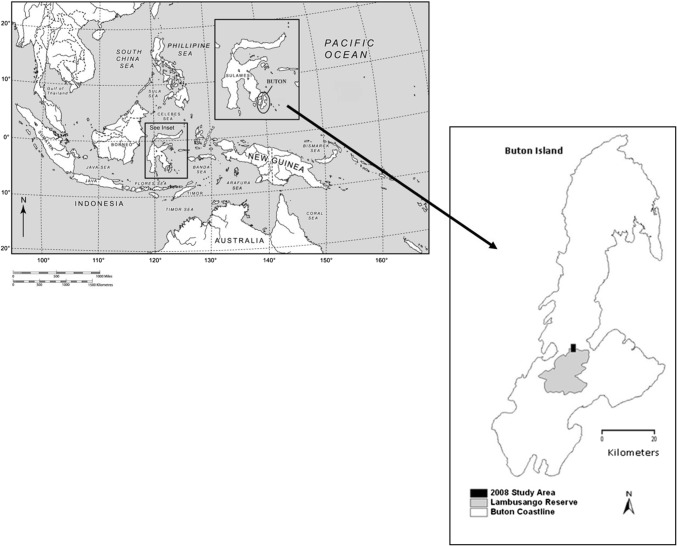
Fieldwork was conducted over a 6-week period between June and July 2008 on Buton Island, Southeast Sulawesi, in the Indonesian archipelago (between 123°12′–122°33′E and 5°44′–4°21′S) (Fig. 1). Buton experiences a tropical monsoon climate with a dry season from August to October and a wet season from November to April. The mean annual rainfall of 2012 mm peaks between April and June, and mean annual temperatures range from 25 to 27 °C (Whitten et al. 2002). Altitude varies from 0 to 200 m in coastal areas to between 400 and 800 m along the island’s central spine, with isolated peaks reaching up to 1000 m (O’Donovan 2001). Most of the island’s low-lying areas are underlain by uplifted karstic limestone, while the mountainous interior is a complex mix of sandstone, chert and ultramafic soils overlying ophiolitic rock (Milsom et al. 1999).
Fig. 1.
Map showing the location of Buton within the Indonesian archipelago, and the location of our study area on the northern boundary of Lambusango Forest Reserve
The climax vegetation cover for most of the island is seasonal lowland tropical forest, with mangroves occurring in coastal areas (Martin et al. 2012). These forests hold large carbon stocks and support a high biodiversity, including many globally endemic and endangered species (Martin et al. 2015) although due to inadequate protection, they continue to be deforested at a rate of 0.54% per annum (Martin et al. 2015).
Habitat surveys
We established 11 randomly placed 50 m × 50 m survey plots within a 2 km × 2.8 km area of lowland (220–280 m asl) tropical forest straddling the northern boundary of the Lambusango Forest Reserve in central Buton (Fig. 1). Each randomly generated plot co-ordinate corresponded to the south-west corner of that plot. We reached this location using a GPS (Garmin GPSMAP 60), and then set out the north–south orientated perimeter of the plot using a compass and four 50-m tape measures. To allow relocation of the plot perimeter, the trees closest to the plot corners were marked with brightly coloured spray paint. Each of these plots was then subdivided, using eight 50-m ropes laid out at 10-m intervals along both the north–south and east–west axes of the plot, into 25 10 m x 10 m subplots. Plots were either laid out by trained ecologists alone or volunteers under the supervision of the ecologists, depending on the survey treatment of the plot (see below). Our methodological protocols required that two variables be recorded (using a pencil and paper) in each of these subplots: a count of ‘large’ trees (as opposed to saplings), defined as any tree species with a height of > 1.3 m and a CBH of ≥ 30 cm, and CBH measurements of all these ‘large’ trees. Following recommendations provided by Phillips et al. (2008), all CBH measurements were taken at 1.3 m above the ground; where buttresses were present, measurements were taken immediately above the buttress, at a height slightly greater than 1.3 m (if possible) or just below 1.3 m (if not possible). Measurements were also taken immediately above abnormal growths or branchings if these were present at 1.3 m. Where trees were leaning or growing on a steep slope, measurements were taken 1.3 m up the trunk in the direction of the lean or in the direction of the slope, respectively. If the tree split below 1.3 m, then CBH was measured below the split. If multiple stems occurred from the ground up, the CBH of all stems were measured. Once a tree was measured, it was marked with a Latchsbacher tree tag to allow it to be reliably relocated during both sets of measurements. We did not attempt to identify the species of the trees in our dataset as this does not improve the accuracy of carbon stock assessment in our study area. Although species-specific allometric equations exist for many tree species (e.g. Cole and Ewel 2006; Xiang et al. 2016), these are largely lacking in the poorly studied Wallacea region. This is quantitatively demonstrated by the fact that, of the 222 tree species known to occur on Buton (Martin et al. 2015), only 1.7% possess a species-specific equation on the authoritative Globallometree database (Globallometree 2017).
Our overall sample size equated to 275 subplots for tree count data and 250 subplots for CBH measurements for both volunteers and ecologists (which together comprised 1044 individual tree CBH measurements). The sample size analysed for CBH measurements was lower than that for tree counts because the measurements from one 50 m × 50 m plot (equalling 25 subplots) represented an extreme outlier in the dataset. The average difference in CBH values between volunteers and ecologists in this plot (10.2 cm) was over four times larger than the average difference in the other ten plots (2.5 cm). We believe this to be due to a data entry error, rather than an issue inherent to unusually shaped trees or other environmental factors (given that this anomaly was exclusive to this one plot, while trees with unusually large buttresses, multiple stems, and other complex shapes are found throughout the study area) and so excluded these data points from further analysis.
The variables within each plot were sampled twice during the study period, once by a team of two student volunteers (aged between 16 and 18) operating under the close supervision of a team of trained ecologists (two British scientists) who observed and directed but did not measure any variables, and once by the ecologists working alone. The two student volunteers assigned to tree measurements were part of a larger team which included six more students tasked with collecting other habitat structure and physical geography variables unrelated to carbon stock assessments. The data from these other teams were not used in this study, but we highlight that the supervisory attention of the two ecologists was split between eight students spread out across the 50 m × 50 m plot. These two ecologists constituted a team leader, who possessed 6 months’ experience of conducting habitat surveys in tropical forests using the described methodologies, and an assistant with 8 weeks’ experience completing habitat surveys in temperate woodland, although no prior tropical experience. Before volunteers completed any survey work, each team was given 6 hours training by the ecologists, following minimum requirements recommended, e.g. by Holck (2008), in which a ‘practice plot’ was completed. Once training was completed, each student team completed two plots before being replaced by a new volunteer team.
Data analysis
Data analysis was focussed towards comparing data quality between volunteers and ecologists, with an assumption that data collected by ecologists are ‘correct’ and that the closer volunteer-derived data correlate with ecologist-derived data, the more accurate it can be considered to be. While this assumption provides a practically useful framework for statistically analysing the performance of volunteers, it should be acknowledged that variability is also often present within ‘expert-derived’ data (Bloniarz and Ryan 1996; Lewandowski and Specht 2015) and this should be taken into consideration with regard to interpreting our results.
Once fieldwork was completed, we first compared congruence between tree counts and median tree CBH measurements (both aggregated by subplot) collected by volunteers and ecologists using Pearson correlations (Zar 2010). For tree CBH measurements, we also completed a finer-scale Pearson correlation analysis comparing congruence between volunteer and ecologist measurements for all individual trees in our dataset. We then tested if any significant differences occurred between the values of tree count and CBH measurements collected by volunteers and ecologists (also aggregated by subplot, with an additional individual tree-scale analysis for CBH values) using Wilcoxon signed-rank tests (Zar 2010). We also examined whether volunteers’ measurements for both variables matched ecologists’ measurements more closely with increased time spent recording data for the project, using Kendall rank correlations (Zar 2010) to assess changes in similarity of volunteer and ecologist data between each volunteer group’s first and second survey plot. This analysis was aggregated on a plot (rather than subplot) scale, to provide a view of overall convergence of volunteer vs. ecologist surveys that would be less affected by fine-grained subplot-level values (and as such was not completed at all for the CBH comparisons of individual trees).
Finally, we used the CBH measurements generated by volunteers and ecologists to calculate per plot carbon stock estimates for both groups. This scale of analysis was used as plot-scale aggregations of carbon data are the base unit of measurement recommended for calculating a project area’s carbon stocks in guidelines used for REDD+ projects (i.e. Verified Carbon Standard 2012). Given the lack of species-specific allometric equations for most Wallacean tree species (see above), we chose the general allometric equation for calculating above-ground biomass (AGB) provided by Brown (1997), which was empirically designed for use in moist tropical forest and has been authoritatively applied in several other locations in the tropics, including within South-East Asia (Foody et al. 2001; Widayati and Carlisle 2012), and its use has been recommended by organisations such as the United Nations Development Programme (Watson 2009) and the Center for International Forestry Research (Kurnianto and Murdiyarso 2010). This equation is expressed as AGB = exp{− 2.134 + 2.530*ln(D)} where exp is the exponent and ln is the natural logarithm. AGB is expressed in Kg, and D is diameter at breast height (DBH) expressed in cm. DBH was calculated by dividing all tree CBH measurements by π. Once AGB was calculated, a root-to-shoot ration of 0.26 was applied to determine an estimate of below-ground biomass (BGB) (Cairns et al. 1997; Ravindranath and Ostwald 2008; Watson 2009). These AGB and BGB values were then added to calculate a total biomass estimate for each tree, expressed in Kg. The biomass values were then converted into carbon estimates using the standard conversion rate of multiplying biomass scores by 0.5 (IPCC 2000). While proportions of carbon-as-biomass vary between different species of tropical trees (Martin and Thomas 2011), using the standard assumption of 50% carbon-as-biomass ratio was deemed a pragmatic approach given the poorly studied nature of Wallacean forests.
Results
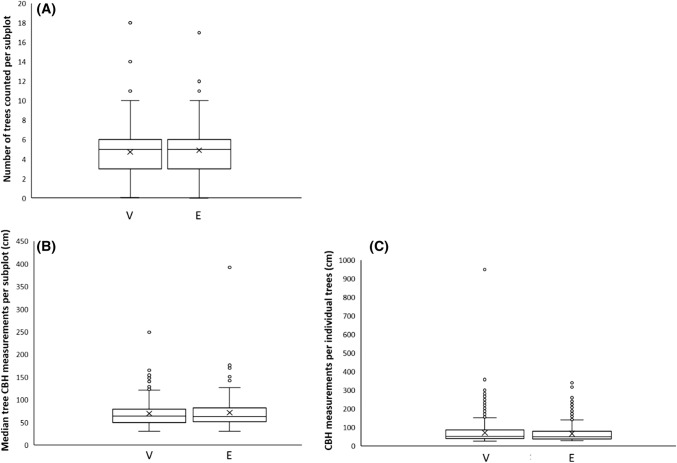
Our results indicate that counts of large trees per subplot by volunteers and ecologists were significantly correlated (R = 0.618, P ≤ 0.001), as were CBH measurements aggregated by subplot (R = 0.473, P ≤ 0.001) and CBH measurements for individual trees (R = 0.790, P ≤ 0.001) (Table 1). We also found no significant difference between volunteer and ecologist measurements of both median tree count values (V = 9454, P = 0.189) and median CBH values aggregated on a subplot scale (V = 13 675, P = 0.856). However, we found differences in CBH values of individual trees to be strongly significant (V = 150 067, P ≤ 0.001) (Table 1). The median number of trees counted per subplot was identical for volunteers and ecologists (five trees each), while the average difference in median CBH measurements for volunteers compared to the ecologist measurements was just 1 cm when aggregated on the subplot level, and 3 cm when aggregated on the level of individual trees (Table 1). Figure 2 provides graphical representations of these results. Carbon stock estimates were also similar between volunteers (45 426 kg per plot) and ecologists (44 930 kg per plot)—an average difference of 1.1%. However, results also indicate little change in congruence between student and ecologist data with increasing time students spent collecting data, both for tree counts (Kendall’s Tau = 0.087 P = 0.734) and for CBH measurements (Kendall’s Tau = 0.001 P = 0.098) (Table 1).
Table 1.
Summary data for comparisons of habitat structure data generated by volunteers and ecologists within 11 50 m × 50 m survey plots on Buton Island, Indonesia. Median numbers of trees and circumference-at-breast-height (CBH) values per subplot have values of 1 standard deviation provided in parenthesis. The Wilcoxon Signed-rank column displays Wilcoxon Signed-rank test values for comparisons of median tree count and CBH values generated by volunteers and ecologists. The Pearson’s R and Kendall’s Tau columns show correlation co-efficient values for comparisons between volunteer and ecologist-derived datasets. Test statistics denoted * are significant (P < 0.05). For Wilcoxon Signed-rank tests and Pearson’s correlations, data were aggregated by 10 m × 10 m subplots (N = 275 for tree count analysis and N = 250 for CBH measurement analysis). The same tests were applied to all individual trees in our dataset (N = 1044). As these test statistics are based on the whole tree dataset, and not median values, these are reported in a separate row. For Kendall’s Tau correlations, data were aggregated on a plot scale (N = 11) for tree count analysis and N = 10 for CBH measurement analysis)
| Volunteers | Ecologists | Wilcoxon signed-rank V | Pearson’s R | Kendall’s Tau | |
|---|---|---|---|---|---|
| Median number of trees counted per subplot | 5 (± 2.69) | 5 (± 2.75) | 9454 | 0.618* | 0.087 |
| Median value of CBH measurements generated (cm) per subplot | 64 (± 28.22) | 63 (± 32.45) | 13 675 | 0.473* | 0.001 |
| Median value of CBH measurements generated (cm) per tree | 52 (± 54.14) | 49 (± 46.21) | N/A | N/A | N/A |
| Test statistics for comparisons of individual tree CBH values (cm) | N/A | N/A | 150 067* | 0.791* | N/A |
Fig. 2.
Box and whisker diagrams showing distribution of values generated by volunteers and ecologists on Buton Island, Indonesia for a number of trees counted per subplot, b median circumference-at-breast-height (CBH) values aggregated by subplot, c circumference-at-breast-height (CBH) values for individual trees. Box plots underscored by a ‘V’ indicate values generated by volunteers, while box plots underscored by an ‘E’ indicate values generated by ecologists
Discussion
Our results indicate student volunteers, when provided with basic training, can produce tree counts and CBH measurements statistically similar to those produced by trained ecologists, at least when aggregated on the spatial scales generally used in the analysis of carbon stocks. However, finer-scale analysis examining CBH measurements of individual trees shows that volunteer and ecologist measurements, while positively correlated, still possess significant discrepancies in values. This may be a result of particular plots or unusually shaped trees being difficult to survey, individual survey teams being less motivated than others, or potentially shorter volunteers instinctively taking measurements lower than the 1.3 m to which they were trained. The differences observed in this fine-scale analysis indicate a source of error in volunteer-derived datasets that requires further research to clarify and develop appropriate mitigation strategies against. However, at a broader scale of analysis that is more pertinent to how carbon stock data are generally calculated for the purposes of REDD+ and similar projects, these fine-scale differences seem to be less pronounced, and our overall results are similar to those reported from studies completed in temperate forest ecosystems. For example, the 3-cm average difference between volunteer and ecologist measurements we report from our individual tree scale analysis of median CBH is comparable to the 2.54-cm difference reported in Roman et al. (2017), while the 1.1% deviation of carbon stock estimates between our two treatments is similar to the 1.7% difference in biomass estimates between teams of volunteers and experts reported in Butt et al. (2013). Our finding that volunteers can, at least on broad ecological scales, source forest structure data of similar quality to ecologists also matches results reported from temperate forest ecosystems more broadly (e.g. Galloway et al. 2006) and corroborates previous evaluations that volunteers can generate valuable data when set sufficiently simple tasks (Boudreau and Yan 2004; Delaney et al. 2008). Our results may suggest that volunteer surveyors could represent a means of collating ground-based tree measurement data for producing robust carbon stock estimates for prospective REDD+ and other similar funding applications. However, some care should be taken with this assumption given that seemingly low-cost citizen scientist projects often require extensive ‘hidden costs’ in order to operate effectively (Wiggins 2013). Cost assessments of large-scale volunteer-driven carbon survey projects in tropical forests therefore represent an important avenue for future research. It should also be noted that the applications of volunteer-led carbon stock surveys in tropical forests could change in the future, as the growth of species-specific allometric equation libraries (e.g. Globallometree 2017) may see standard requirements for carbon calculations changing to incorporate species-level identifications as well as tree volume measurements. This would in likelihood be beyond the abilities of most volunteer surveyors to provide, as has been demonstrated to be the case even in less speciose temperate ecosystems (e.g. Brandon et al. 2003; Roman et al. 2017; Bancks et al. 2018). However, given the current shortfall of these species-specific equations for locations in many tropical regions, simple volume-based equations should remain standard for most carbon stock calculations, for the near future at least.
While all REDD+ projects are by necessity long-term endeavours requiring regular monitoring of carbon stocks to provide evidence that emissions reductions targets are being met (Narasimhan et al. 2014), volunteer-led surveys are perhaps best positioned to make contributions to the initial application process, where ground-based methods to estimate existing carbon stocks within prospective project areas are needed to prepare applications. However, if volunteer surveyors were to be used for regular monitoring throughout a project’s lifespan, our finding that volunteers are as capable of completing similar tree counts within established plots as ecologists is encouraging, given that monitoring programmes require the same trees to be measured over time to determine growth rates and associated carbon stock changes. This would, however, assume that volunteer researchers are capable of relocating specific plot and tree locations multiple times, often with many months between each sampling period—a potentially difficult issue that was not tested in this short-term study, and might represent another useful area for future research.
While our results suggest that, generally, volunteers are capable of producing tree measurement data sufficiently accurate to calculate carbon stocks in tropical forests, the significant difference in CBH values at the scale of individual trees highlights that volunteers are capable of making substantial errors, at least on a local scale. Volunteer and ecologist datasets are also not extremely strongly correlated (particularly CBH measurements aggregated by subplot, which possessed weaker r values than tree counts and CBH measurements aggregated by individual tree). Additionally, in contrast to patterns often seen in citizen scientist surveys (Kosmala et al. 2016), these discrepancies also did not improve with increased time volunteers spent collecting data, albeit in the limited context of a single repetition per volunteer team. It should also be noted, though, that increased time collecting data for a project may not always correspond to better quality data, and that other factors, such as fatigue from working in particularly difficult plots, may have a confounding effect on data quality from more experienced fieldworkers—see (Burg et al. 2015). These findings suggest that some errors can always be expected to be made by volunteer surveyors, even when measuring simple variables, and that careful training and supervision will always be necessary to ensure highly accurate results. A particularly common volunteer error observed, which was also documented in Roman et al. (2017), involved the incorrect following of methodologies for dealing with atypical trees (with large buttresses, leaning trunks, etc.) and we highlight the importance of specific training for dealing with these non-standard measurements, and careful observation of volunteers measuring these trees. One means of improving volunteer training with regard to dealing with specific issues such as this, as well as for following survey methodologies correctly in general, could be the development of smartphone-based instructional videos, a technique that has proved effective in other citizen scientist projects (Starr et al. 2014).
Conclusion
This study demonstrates that, at broad scales of data aggregation, volunteer surveyors are capable of generating basic tree measurements required for ground-based carbon stock calculations that are of similar quality to those generated by trained ecologists, and that mobilising large teams of volunteers may represent a means of generating the extensive datasets required for the submission of large conservation funding applications to systems such as REDD+. That is not to say that all data necessary for these applications can be collected by volunteers; detailed biodiversity and social science datasets are also typically required (Narasimhan et al. 2014) which rely on specialist expertise during collection. However, carbon stock calculations are arguably the single most important consideration for emissions-orientated funding bodies and ground-based approaches are often labour-intensive. The use of properly trained volunteer surveyors may thus represent a useful means of increasing the number of prospective funding applications for tropical forests, which in turn could help yield major conservation outcomes in these high-priority ecosystems. However, we highlight that careful training and close supervision of such volunteer teams will in likelihood always be necessary to obtain good quality data, and that further research into the influence of physical factors (such as the architecture of individual trees) and intrinsic factors (such as volunteer motivation and fatigue) on data quality may identify ways in how to further improve the effectiveness of citizen science projects in tropical forest ecosystems.
Acknowledgements
The authors thank Mick McCarthy and Bruce Carlisle for guidance, and Plume School, Sedburgh School, Coulsden College, Harrogate Ladies College and Newcastle College for their surveying efforts. We also thank RISTEK Indonesia for the necessary permits to complete this research, and two anonymous reviewers for their constructive and helpful comments.
Biographies
Barnabas Harrison
is a sustainability consultant at Accenture Strategy. His research interests include habitat structure of tropical forests, and the application of citizen science projects to monitor change in habitat structure.
Tom Martin
is a conservation biologist at Operation Wallacea. His research interests include tropical ornithology and the biogeography of tropical islands.
Abdul Haris Mustari
is a biology lecturer at Institut Pertanian Bogor. His research interests include the ecology and conservation of Indonesian mammal species.
Contributor Information
Barnabas Harrison, barnabas.harrison@googlemail.com.
Thomas Edward Martin, Phone: +44 (0)1790 763194, Email: tom_martin_2010@yahoo.co.uk.
Abdul Haris Mustari, Email: haris.anoa@yahoo.com.
References
- Agrawal A, Nepstad B, Chhatre A. Reducing emissions from deforestation and forest degradation. Annual Review of Environment and Resources. 2011;36:373–396. doi: 10.1146/annurev-environ-042009-094508. [DOI] [Google Scholar]
- Bancks N, North EA, Johnson GR. An analysis of agreement between volunteer- and researcher-collected urban tree inventory data. Arboriculture & Urban Forestry. 2018;44:73–86. [Google Scholar]
- Bloniarz DV, Ryan HDP., III The use of volunteer initiatives in conducting urban forest resource inventories. Journal of Aboriculture. 1996;22:75–82. [Google Scholar]
- Boudreau SA, Yan ND. Auditing the accuracy of a volunteer-based surveillance program for an aquatic invader bythotrephes. Environmental Monitoring and Assessment. 2004;91:17–26. doi: 10.1023/B:EMAS.0000009228.09204.b7. [DOI] [PubMed] [Google Scholar]
- Bradshaw CJA, Sodhi NS, Brook BW. Tropical turmoil: A biodiversity tragedy in progress. Frontiers in Ecology and the Environment. 2009;7:79–87. doi: 10.1890/070193. [DOI] [Google Scholar]
- Brandon A, Spyreas G, Molano-Flores B, Carroll C, Ellis J. Can volunteers provide reliable data for forest vegetation surveys? Natural Areas Journal. 2003;23:254–262. [Google Scholar]
- Brown S. Estimating biomass and biomass change of tropical forests: A primer. Rome: Food and Agriculture Organization; 1997. [Google Scholar]
- Brown WT, Krasny ME, Schoch N. Volunteer monitoring of non-indigenous invasive plant species in the Adirondack Park, New York, USA. Natural Areas Journal. 2001;21:189–196. [Google Scholar]
- Burg S, Rixen C, Stöckli V, Wipf S. Observation bias and its causes in botanical surveys on high-alpine summits. Journal of Vegetation Science. 2015;26:191–200. doi: 10.1111/jvs.12211. [DOI] [Google Scholar]
- Butt N, Slade E, Thompson J, Malhi Y, Riutta T. Quantifying the sampling error in tree census measurements by volunteers and its effect on carbon stock estimates. Ecological Applications. 2013;23:936–943. doi: 10.1890/11-2059.1. [DOI] [PubMed] [Google Scholar]
- Cairns MA, Brown S, Helmer EH, Baumgardner GA. Root biomass allocation in the world’s upland forests. Oecologia. 1997;111:1–11. doi: 10.1007/s004420050201. [DOI] [PubMed] [Google Scholar]
- Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus QD, Fölster H, Fromard F, et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia. 2005;145:87–99. doi: 10.1007/s00442-005-0100-x. [DOI] [PubMed] [Google Scholar]
- Cole TG, Ewel JJ. Allometric equations for four valuable tropical tree species. Forest Ecology and Management. 2006;229:351–360. doi: 10.1016/j.foreco.2006.04.017. [DOI] [Google Scholar]
- Darwall WRT, Dulvy NK. An evaluation of the suitability of non-specialist volunteer researchers for coral reef fish surveys. Mafia Island, Tanzania—A case study. Biological Conservation. 1996;78:223–231. doi: 10.1016/0006-3207(95)00147-6. [DOI] [Google Scholar]
- Delaney DG, Sperling CD, Adams CS, Leung B. Marine invasive species: Validation of citizen science and implications for national monitoring networks. Biological Invasions. 2008;10:117–128. doi: 10.1007/s10530-007-9114-0. [DOI] [Google Scholar]
- Dickinson JL, Shirk J, Bonter D, Bonney R, Crain RL, Martin J, Phillips T, Purcell K. The current state of citizen science as a tool for ecological research and public engagement. Frontiers in Ecology and the Environment. 2012;10:291–297. doi: 10.1890/110236. [DOI] [Google Scholar]
- Dickinson JL, Zuckerberg B, Bonter DN. Citizen science as an ecological research tool: Challenges and benefits. Annual Review of Ecology Evolution and Systematics. 2010;41:149–172. doi: 10.1146/annurev-ecolsys-102209-144636. [DOI] [Google Scholar]
- Fearnside PM. Deforestation in Brazilian Amazonia: History, rates, and consequences. Conservation Biology. 2005;19:680–688. doi: 10.1111/j.1523-1739.2005.00697.x. [DOI] [Google Scholar]
- Foody GM, Cutler ME, McMorrow J, Pelz D, Tangki H, Boyd DS, Douglas I. Mapping the biomass of Bornean tropical rain forest from remotely sensed data. Global Ecology and Biogeography. 2001;10:379–387. doi: 10.1046/j.1466-822X.2001.00248.x. [DOI] [Google Scholar]
- Galloway AWE, Tudor MT, Haegen WMV. The reliability of citizen science: A case study of Oregon White Oak stand surveys. Wildlife Society Bulletin. 2006;34:1425–1429. doi: 10.2193/0091-7648(2006)34[1425:TROCSA]2.0.CO;2. [DOI] [Google Scholar]
- Gardner TA, Barlow J, Parry LW, Peres CA. Predicting the uncertain future of tropical forest species in a data vacuum. Biotropica. 2007;39:25–30. doi: 10.1111/j.1744-7429.2006.00228.x. [DOI] [Google Scholar]
- Globallometree. 2017. Globallometree: Assessing volume, biomass and carbon stocks of trees and forests. http://www.globallometree.org/. Accessed 23 Aug 2017
- Goetz SJ, Hansen M, Houghton RA, Walker W, Laporte N, Busch J. Measurement and monitoring needs, capabilities and potential for addressing reduced emissions from deforestation and forest degradation under REDD+ Environmental Research Letters. 2015;10:123001. doi: 10.1088/1748-9326/10/12/123001. [DOI] [Google Scholar]
- Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- Holck M. Participatory forest monitoring: An assessment of the accuracy of simple cost–effective methods. Biodiversity and Conservation. 2008;17:2023–2036. doi: 10.1007/s10531-007-9273-4. [DOI] [Google Scholar]
- Intergovernmental Panel on Climate Change . Land use, land-use change, and forestry. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- James AN, Gaston KJ, Balmford A. Balancing the Earths accounts. Nature. 1999;401:323–324. doi: 10.1038/43774. [DOI] [PubMed] [Google Scholar]
- Kosmala M, Wiggins A, Swanson A, Simmons B. Assessing data quality in citizen science. Frontiers in Ecology and the Environment. 2016;14:551–560. doi: 10.1002/fee.1436. [DOI] [Google Scholar]
- Kremen C, Ullmann KS, Thorp RW. Evaluating the quality of citizen-scientist data on pollinator communities. Conservation Biology. 2011;25:607–617. doi: 10.1111/j.1523-1739.2011.01657.x. [DOI] [PubMed] [Google Scholar]
- Kurnianto S, Murdiyarso D. Forest carbon database: A web-based carbon stock data repository and exchange system. CIFOR: Bogor, Indonesia; 2010. [Google Scholar]
- Lewandowski E, Specht H. Influence of volunteer and project characteristics. on data quality of biological surveys. Conservation Biology. 2015;29:713–723. doi: 10.1111/cobi.12481. [DOI] [PubMed] [Google Scholar]
- Martin TE, Harrison B, Wheeler PM. The case for REDD+ funding for the forests of Buton Island, SE Sulawesi, Indonesia—A summary. Old Bolingbroke, UK: Operation Wallacea; 2015. [Google Scholar]
- Martin TE, Kelly DJ, Keogh NT, Heriyadi D, Singer H, Blackburn GA. The avifauna of the Lambusango Forest Reserve, Buton Island, South-East Sulawesi (with additional sightings from Southern Buton) Forktail. 2012;28:107–112. [Google Scholar]
- Martin AR, Thomas SC. A reassessment of carbon content in tropical trees. PLoS One. 2011 doi: 10.1371/journal.pone.0023533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill D. Norway and REDD+ in Indonesia: The art of not governing? Forum for Development Studies. 2015;42:113–132. doi: 10.1080/08039410.2014.997791. [DOI] [Google Scholar]
- Milsom J, Ali J, Sudarwono. Structure and collision history of the Buton continental fragment, Eastern Indonesia. AAPG Bulletin. 1999;83:1666–1689. [Google Scholar]
- Narasimhan P, Starr I, Hayward J, Noponen M, Durbin J. Guidance for the use of the CCB standards. Arlington, Virginia: Climate, Community and Biodiversity Alliance and the Rainforest Alliance; 2014. [Google Scholar]
- Newman C, Buesching CD, Macdonald DW. Validating mammal monitoring methods and assessing the performance of volunteers in wildlife conservation - “Sed quis custodiet ipsos custodies?”. Biological Conservation. 2003;113:189–197. doi: 10.1016/S0006-3207(02)00374-9. [DOI] [Google Scholar]
- Niinioja R, Holopainen AL, Lepistö L, Rämö A, Turkka J. Public participation in monitoring programmes as a tool for lakeshore monitoring: the example of Lake Pyhäjärvi, Karelia, Eastern Finland. Limnologica - Ecology and Management of Inland Waters. 2004;34:154–159. doi: 10.1016/S0075-9511(04)80035-5. [DOI] [Google Scholar]
- O’Donovan G. Report on the botanical and ecological status of the Kakenauwe and Lambusango Nature Reserves on Buton Island, Sulawesi. Old Bolingbroke, UK: Operation Wallacea; 2001. [Google Scholar]
- Phillips, O., T. Baker, T. Feldpausch, and R. Brienen. 2008. RAINFOR field manual for plot establishment and remeasurement. http://www.geog.leeds.ac.uk/projects/rainfor/manuals/RAINFOR%20field%20manual%20version%202008%20ENG.pdf. Accessed 14 June.
- Prater AJ. Estuary Birds of Britain and Ireland. Calton, UK: T & AD Poyser; 1981. [Google Scholar]
- Ravindranath NH, Ostwald M. Carbon inventory methods: Handbook for greenhouse gas inventory, carbon mitigation and roundwood production projects. Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- Roman LA, Scharenbroch BC, Östberg JPA, Mueller LS, Henning JG, Koeser AK, Sanders JR, Betza DR, et al. Data quality in citizen science urban tree inventories. Urban Forestry and Urban Greening. 2017;22:124–135. doi: 10.1016/j.ufug.2017.02.001. [DOI] [Google Scholar]
- Silvertown J. A new dawn for citizen science. Trends in Ecology & Evolution. 2009;24:467–471. doi: 10.1016/j.tree.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Starr J, Schweik CM, Bush N, Fletcher L, Finn J, Fish J, Bargeron CT. Lights, camera…citizen science: assessing the effectiveness of smartphone-based video training in invasive plant identification. PLoS One. 2014 doi: 10.1371/journal.pone.0111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf GR, Morton DC, DeFries RS, Olivier JGJ, Kasibhatla PS, Jackson RB, Collatz GJ, Randerson JT. CO2 emissions from forest loss. Nature Geoscience. 2009;2:737–738. doi: 10.1038/ngeo671. [DOI] [Google Scholar]
- Verified Carbon Standard. 2012. VCS Standard. Version 3. Washington DC: VCS.
- Watson C. Forest carbon accounting: overview and principals. London: United Nations Development Programme; 2009. [Google Scholar]
- Whitten T, Mustafa M, Henderson GS. The ecology of Sulawesi. 2. Yogyakarta: Gadjah Mada University Press; 2002. [Google Scholar]
- Widayati A, Carlisle B. Impacts of rattan cane harvesting on vegetation structure and tree diversity of Conservation Forest in Buton, Indonesia. Forest Ecology and Management. 2012;266:206–215. doi: 10.1016/j.foreco.2011.11.018. [DOI] [Google Scholar]
- Wiggins, A. 2013. Free as in puppies: Compensating for ICT constraints in citizen science. In Proceedings of the ACM International conference on computer-supported cooperative work and social computing. San Antonio, USA.
- Xiang W, Zhou J, Ouyang S, Zhang S, Lei P, Li J, Deng X, Fang X, et al. Species-specific and general allometric equations for estimating tree biomass components of subtropical forests in southern China. European Journal of Forest Research. 2016;135:963–979. doi: 10.1007/s10342-016-0987-2. [DOI] [Google Scholar]
- Zar JH. Biostatistical analysis. 5. Englewood Cliffs, NJ: Prentice-Hall; 2010. [Google Scholar]