Abstract
Study
Carcinoma vulva is a rare cancer of the female genital tract. It mostly presents in postmenopausal women. The treatment of vulvar cancer is surgery, chemoradiation, radiotherapy or a combination of all modalities. Here, we present a study of 33 cases of carcinoma vulva over a period of 2 years at a Northeast India regional cancer institute describing its demographic features and treatment outcomes.
Methodology
A retrospective cohort study of vulvar cancer diagnosed at Northeast India regional cancer institute from January 2017 to December 2018.
Results
A total of 33 cases of biopsy proven carcinoma (Ca) vulva were studied. Maximum number of cases belonged to the age group: 60–69 years (39.4%). 66.67% cases had palpable inguinal lymph nodes at presentation, and 100% had squamous cell carcinoma on histopathology. Maximum number of cases belonged to stage III (44.8%), and least number of cases belonged to stage IV (10.3%) of FIGO 2009 staging of Ca vulva. 87.9% cases underwent treatment, and 12.1% were lost to follow-up. Out of the cases who underwent treatment, 55.2% cases were taken up for primary surgery and 44.8% cases for primary radiotherapy. 75% cases who underwent surgery received adjuvant radiotherapy. No complication was seen in patients post-radiation. But, 6.25% patients post-surgery developed lymphocyst and 18.75% patients developed wound necrosis (p > 0.05).
Conclusion
Vulvar cancer is not a common malignancy of the female genital tract that presents in sixth and seventh decades of life and often with palpable inguinal lymph nodes. Though early stages of Ca vulva are treated by surgery, the incidence of immediate postoperative complications in our study was more as compared to post-radiotherapy. Also, maximum patients in the present study post-surgery received adjuvant radiotherapy. Thus, radiotherapy can be considered as the primary treatment modality for patients with early as well as advanced vulvar carcinoma.
Keywords: Carcinoma vulva, Squamous cell carcinoma, Lymphocyst, Chemoradiation
Introduction
According to the latest GLOBACON data of 2018, the total number of cases of carcinoma vulva in the world is 44,235 and the age-standardized rate of the same is 0.9 [1]. Among all gynecologic cancers, Ca vulva ranks as the fourth commonest cancer that amounts to 5% of all female genital tract malignancies [2]. The most common histopathology seen in carcinoma vulva is squamous cell carcinoma [3]. Other histopathology includes basal cell carcinoma, melanoma, sarcoma, paget’s disease of the vulva, bartholin gland carcinoma and verrucous carcinoma. Prognosis of vulvar cancer depends upon the stage and type of histopathology report. For patients with grossly positive pelvic lymph nodes, Thaker et al. [4] reported a 5-year survival rate of 43% and disease-specific survival rates of 48%. Recurrence of the disease is commonly seen at the groin region which increases the mortality of patients to as high as nine times as compared to patients without a recurrence at the groin [5].
Aims and Objectives
To study the clinical profile and treatment outcomes of patients with vulvar cancer.
Methodology
A retrospective cohort study of cases diagnosed with vulvar cancer at Dr. B Borooah Cancer Institute, Guwahati, Assam, from January 2017 to December 2018. Thirty-three patients with histologically proven diagnosis of carcinoma vulva were studied. Patient profile, stage of disease, treatment modalities used and disease outcomes were analyzed and compared with the published literature.
Results
Age Distribution of 33 Cases (Table 1)
Table 1.
Age distribution of 33 cases
| Age group (years) | No. of patients | Percentage |
|---|---|---|
| 40–49 | 5 | 15.1 |
| 50–59 | 7 | 21.2 |
| 60–69 | 13 | 39.4 |
| 70–79 | 6 | 18.2 |
| 80 and above | 2 | 06.1 |
| Total | 33 | 100 |
It was seen that maximum number of our patients belonged to the age group of 60–69 years, that is, 39.4% followed by 50–59 years, that is, 21.2%. There were only 6.1% patients above 80 years and none below 40 years of age.
Performance Status of 33 Cases (Table 2)
Table 2.
Performance status of 33 cases
| Performance status | No. of patients | Percentage |
|---|---|---|
| ECOG 0 | 16 | 48.5 |
| ECOG 1 | 14 | 42.4 |
| ECOG 2 | 3 | 9.1 |
| Total | 33 | 100 |
Maximum number of our cases presented with ECOG 0 performance status, that is, 48.5% followed by ECOG 1, that is, 42.4% patients.
Presentation: All 33 cases presented with the chief complaint of swelling or mass over the vulvar region. Out of these 33 cases, 14 cases, that is, 42.4% presented with associated pruritus. 15.1% cases (five cases) had bleeding and discharge from the lesion, and 9.1% cases (three cases) had burning sensation at the tumor site.
Clinical Examination: Twenty-two cases, that is, 66.67% had palpable inguinal lymph nodes, and 11 cases, that is, 33.33% did not have any palpable lymph nodes. Among the 22 cases with palpable inguinal lymph nodes, only one case (3%) had enlarged pelvic lymph nodes on imaging.
Histopathology of 33 Cases: All cases had squamous cell carcinoma of vulva. 75.6% (25 cases) patients had well-differentiated squamous cell carcinoma, 15.1% had moderately differentiated SCC and 9% cases had poorly differentiated SCC.
Staging and Treatment Modality
Out of the 33 cases, 29 (87.9%) cases received treatment and four (12.1%) cases were lost to follow-up.
Staging of 29 Cases (Table 3)
Table 3.
Staging of 29 cases
| Stage | No. of patients | Percentage |
|---|---|---|
| I | 5 | 17.2 |
| IA | 1 | 3.4 |
| IB | 4 | 13.8 |
| II | 8 | 27.6 |
| III | 13 | 44.8 |
| III A | 3 | 10.3 |
| III B | 6 | 20.7 |
| III C | 4 | 13.8 |
| IV | 3 | 10.3 |
| IV A | 2 | 6.9 |
| IV B | 1 | 3.4 |
| Total | 29 | 100 |
Patients were staged according to FIGO staging system of 2009. It was seen that 17.2% cases belonged to stage I Ca vulva, 27.6% belonged to stage II, 44.8% belonged to stage III and 10.3% belonged to stage IV (Fig. 1).
Fig. 1.
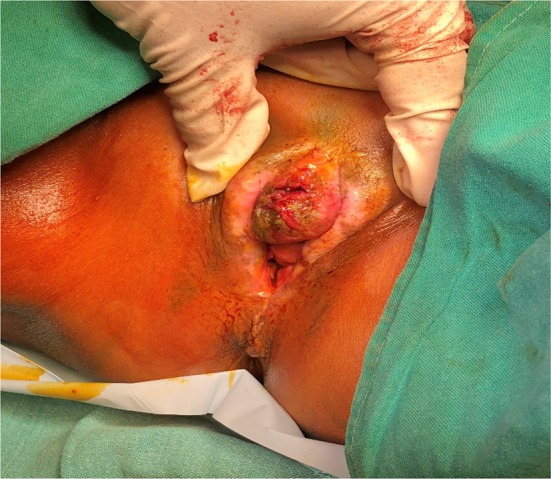
A case of Ca Vulva with involvement of clitoris
Treatment Received (Table 4)
Table 4.
Treatment received by 29 cases
| Treatment modality | No. of patients | Percentage |
|---|---|---|
| Primary surgery | 16 | 55.2 |
| Primary radiotherapy | 13 | 44.8 |
| Total | 29 | 100 |
In our study, patients with early stage disease were considered for surgery. The patients who presented with gross disease with distant metastasis were taken up for chemoradiation as the primary treatment modality.
55.2% patients were taken up for primary surgery, whereas 44.8% patients underwent primary radiotherapy.
Type of Surgery
Out of the 16 cases who underwent upfront surgery, one case (6.25%) underwent simple partial vulvectomy, five cases (31.25%) underwent wide local excision with bilateral superficial inguinal lymph node dissection (Fig. 2), and nine cases (56.25%) underwent modified radical vulvectomy with bilateral superficial inguinal lymph node dissection. Only one case (6.25%) underwent modified radical vulvectomy with bilateral inguinal lymph node dissection with bilateral pelvic lymph node dissection (Fig. 3).
Fig. 2.
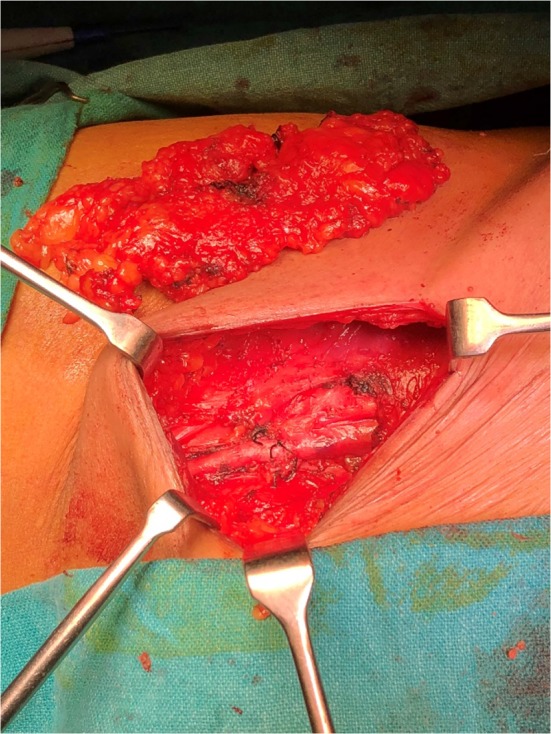
Inguinal lymph node dissection in a patient
Fig. 3.
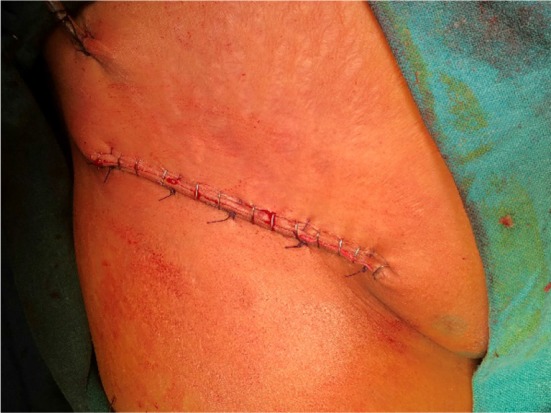
Post-inguinal lymph node dissection
Type of RT Received
Out of the 13 cases, ten cases (77%) received 50-Gy radiation in 25 fractions with concurrent chemotherapy and two cases (15.4%) received palliative radiation of 30-Gy dose in ten fractions. One case (7.6%) received two fractions of 8-Gy surface radiation.
Adjuvant RT Post-surgery
Adjuvant radiotherapy was given to 12 cases (75%) out of 16 who underwent primary surgery in view of increased depth of invasion by the tumor, and four cases (25%) were kept in observation.
Complications Post-treatment
Post-surgery: one case (6.25%) developed lymphocyst and three cases (18.75%) developed wound necrosis (p > 0.05) and later underwent secondary suturing.
Post-radiotherapy: no immediate or early complications were seen in patients who received radiotherapy.
The rate of complications was more post-surgery as compared to post-radiotherapy. This can be attributed to the fact that when patients went home following vulvar surgery, they were not able to maintain proper vulvar hygiene and apply regular compression bandaging, hence leading to wound necrosis and lymphocyst formation (Fig. 4), respectively.
Fig. 4.
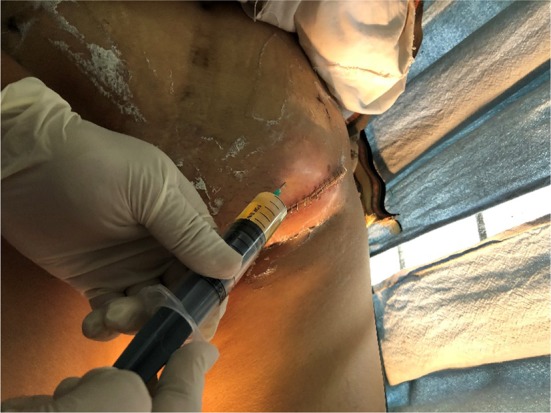
Aspiration of lymphocyst being done; appeared post-surgery on postoperative day 14
Discussion
In our hospital, the cases of vulvar cancer are increasing day by day. In the last two years (2017–2018), total number of cases diagnosed with carcinoma vulva were 33 as compared to 18 cases in the years 2006–2009 [6]. Ca vulva is commonly seen in postmenopausal women. The sixth and seventh decades of life show the highest incidence of the same [7]. In our study also, maximum number of cases, that is, 39.4% belonged to the age group of 60–69 years followed by 21.2% cases that belonged to the age group of 50–59 years. But, Okolo et al. showed a mean age of 49.7 years in their study of vulvar and vaginal cancers from Nigeria [8]. When compared to our study, the mean age was calculated to be as 65.2 years.
The most common histopathology seen in vulvar cancer patients is squamous cell carcinoma that is seen in 90% of the cases [9]. In our study, 100% cases presented with the same histopathology report. Deka et al. [6] also reported 100% cases of squamous cell carcinoma in their study with maximum number of cases (77.8%) belonging to well-differentiated squamous cell carcinoma group.
In advanced cases of Ca vulva, radiotherapy forms the basis of treatment. For locally advanced lesions, the choice between radiotherapy and surgery depends mainly on the ability of the surgeon to resect the tumor completely while preserving bowel and bladder symptoms [10]. In the present study, 55.2% cases were taken up for primary surgery and 44.8% cases were taken up for primary radiation therapy. Out of the 16 cases who underwent primary surgery, 75% cases were given adjuvant radiotherapy. 40–60% cure rates have been achieved for stages III and IV of Ca vulva with chemoradiation therapy alone [11].
Lymphoedema is a common complication seen in cases of vulvar cancer post-treatment. It is seen both after surgery as well as radiotherapy [10]. In our study, only one case developed lymphocyst post-surgery that was treated by aspiration and compression. To prevent the complication of lymphoedema and lymphocyst formation; Morotti et al. [11] used microsurgical lymphatic venous anastomosis and showed promising results.
Following radical surgery, significant morbidity is seen in patients that has led to the modification of various surgical techniques for both early and advanced cases of the disease. Pelvic lymphadenectomy is now being replaced by adjuvant radiotherapy for patients whose tumor has spread to more than one inguinal lymph node. Also, primary chemoradiation is now used to shrink large tumors following which surgical resection becomes possible [12].
All the treated cases of vulvar cancer in our hospital are in regular follow-up. Out of the 29 treated cases, one patient, that is, 3.4% has developed recurrence and is on palliative chemotherapy.
Timely diagnosis of Ca vulva is important for improving survival. Education and awareness of women on the same play a pivotal role. Self-examination and early evaluation for patients with chronic pruritus and vulvar ulcers should be encouraged and skin biopsy taken [13]. HPV vaccination can also be used for prevention of vulvar cancer. A study in Norway was conducted recently showing efficacy of HPV vaccination in reduction in vulvar cancer in the coming years among HPV-vaccinated communities [14].
Vulvar intraepithelial neoplasia or VIN is a known risk factor for invasive vulvar cancer. Early diagnosis and treatment of the same can help reduce the incidence of Ca vulva. Surgical excision with a margin of 1 cm is considered appropriate management option and helps to rule out invasive cancer [15].
Conclusion
Carcinoma vulva is not a common cancer of the female genital tract. The presentation of the same is mostly with a mass or swelling that is accompanied with itching and occasionally burning sensation over the lesion. Though early stages of Ca vulva are treated by surgery, the incidence of immediate postoperative complications in the present study was more as compared to post-radiotherapy. Also, maximum patients post-surgery received adjuvant chemoradiation. Thus, radiotherapy can be considered as the primary treatment modality for patients with early as well as advanced vulvar carcinoma. Since our sample size was less, our results were not significant. Further studies are needed over a longer follow-up period in order to recommend management protocols for carcinoma vulva.
Acknowledgements
I would like to thank my professors and seniors for helping me in publishing this manuscript and my parents for their continuous encouragement and support.
Dr. Megha Nandwani
is presently working as a fellow in the Department of Gynaecologic Oncology at Dr. B Borooah Cancer Institute, Guwahati, Assam. She is a research enthusiast and is an active participant at national as well as international conferences. She is also the winner of the Indumati Jhaveri Award for the best paper presentation in AICOG 2017.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Ethical Standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Footnotes
Dr. Megha Nandwani is a fellow in the Department of Gynaecologic Oncology at Dr. B Borooah Cancer Institute, Guwahati, Assam. D. Barmon is a HOD and Professor at Department of Gynaecologic Oncology. Dimpy Begum is a Senior Resident at Department of Gynaecologic Oncology. Haelom Liegise is a Fellowship trainee at Department of Gynaecology Oncology. A. C. Kataki is a Director.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Megha Nandwani, Email: megha.nandwani@gmail.com.
D. Barmon, Email: drdbarmon@gmail.com
Dimpy Begum, Email: drdimpyb@gmail.com.
Haelom Liegise, Email: haelomz@gmail.com.
A. C. Kataki, Email: dramalchkataki@yahoo.com
References
- 1.http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Gunther V, Alkatout I, Lez C, et al. Malignant melanoma of the urethra: a rare histologic subdivision of vulvar cancer with a poor prognosis. Case Rep Obstet Gynecol. 2012;2012:385175. doi: 10.1155/2012/385175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thaker NG, Klopp AH, Jhingran A, et al. Survival outcomes for patients with stage IVB vulvar cancer with grossly positive pelvic lymph nodes: time to reconsider the FIGO staging system? Gynecol Oncol. 2015;136(2):269–273. doi: 10.1016/j.ygyno.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nooij LS, Ongkiehong PJ, van Zwet EW, et al. Groin surgery and risk of recurrence in lymph node positive patients with vulvar squamous cell carcinoma. Gynecol Oncol. 2015;139(3):458–464. doi: 10.1016/j.ygyno.2015.09.081. [DOI] [PubMed] [Google Scholar]
- 6.Deka P, Barmon D, Shribastava S, et al. Prognosis of vulval cancer with lymph node status and size of primary lesion: a survival study. Journal of mid-life health. 2014;5:10–13. doi: 10.4103/0976-7800.127784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan C, Kirschner K, Truong M, et al. Multimodality imaging of vulvar cancer: staging, therapeutic response, and complications. AJR Am J Roentgenol. 2013;200(6):1387–1400. doi: 10.2214/AJR.12.9714. [DOI] [PubMed] [Google Scholar]
- 8.Okolo CA, Odubanjo MO, Awolude OA, et al. A review of vulvar and vaginal cancer in Ibadan, Nigeria. N Am J Med Sci. 2013;6(2):76–81. [Google Scholar]
- 9.Russell Anthony Henryk, Horowitz Neil S. Clinical Radiation Oncology. 2016. Cancers of the Vulva and Vagina; p. 1230-1263.e6. [Google Scholar]
- 10.Zweizig S, Korets S, Cain JM. Key concepts in management of vulvar cancer. Best Pract Res Clin Obstet Gynaecol. 2017;28(7):959–966. doi: 10.1016/j.bpobgyn.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Morotti M, Menada MV, Boccardo F, et al. Lymphedema microsurgical preventive healing approach for primary prevention of lower limb lymphedema after inguinofemoral lymphadenectomy for vulvar cancer. Int J Gynecol Cancer. 2013;23:769–774. doi: 10.1097/IGC.0b013e318287a8e8. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger TH, Hakim AA, Lee SJ, et al. Surgical management of vulvar cancer. J Natl Compr Canc Netw. 2017;15:121–128. doi: 10.6004/jnccn.2017.0009. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo AR, Fasolino C, Santoro G, et al. Evaluation of symptoms and prevention of cancer in menopause: the value of vulvar exam. Transl Med UniSa. 2016;15:74–79. [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen BT, Campbell S, Nygard M. Long-term incidence trends of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8:e019005. doi: 10.1136/bmjopen-2017-019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allbritton JI. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339–352. doi: 10.1016/j.ogc.2017.04.002. [DOI] [PubMed] [Google Scholar]


