Abstract
Nitric oxide is a signalling molecule with an extensive range of functions in both health and disease. Discovered in the 1980s through work that earned the Nobel prize, nitric oxide is an essential factor in regulating cardiovascular, immune, neurological and haematological function in normal homeostasis and in response to infection. Early work implicated exaggerated nitric oxide synthesis as a potentially important driver of septic shock; however, attempts to modulate production through global inhibition of nitric oxide synthase were associated with increased mortality. Subsequent work has shown that regulation of nitric oxide production is determined by numerous factors including substrate and co-factor availability and expression of endogenous regulators. In sepsis, nitric oxide synthesis is dysregulated with exaggerated production leading to cardiovascular dysfunction, bioenergetic failure and cellular toxicity whilst at the same time impaired microvascular function may be driven in part by reduced nitric oxide synthesis by the endothelium. This bench to bedside review summarises our current understanding of the ways in which nitric oxide production is regulated on a tissue and cellular level before discussing progress in translating these observations into novel therapeutic strategies for patients with sepsis.
Keywords: Nitric oxide, Sepsis, Septic Shock, Arginine, Asymmetric dimethylarginine, Citrulline, Tetrahydrobiopterin
Background
The discovery of nitric oxide
Nitric oxide (NO) was the first endogenous gaseous signalling molecule to be discovered and was the product of work undertaken over an extended period by many scientists tackling questions about vascular and immune cell function. The culmination of this work included the demonstration by Robert Furchgott that rapid reductions in smooth muscle tone were driven by production of an endothelial cell dependent mediator. Described initially as endothelial derived relaxing factor (EDRF) [1], Salvador Moncada went on to demonstrate that EDRF was in fact identical to NO [2]. In parallel, the discovery by Ferid Murad that nitric oxide (NO) was a potent activator of soluble guanylate cyclase [3] ultimately led to the confirmation by Louis Ignarro that NO was the second messenger molecule responsible for reducing smooth muscle tone through this mechanism. This work secured Furchgott, Ignarro and Murad the Nobel prize for physiology or medicine in 1998 [4], an award that was built on a body of work going back many years across many disciplines.
NO synthesis and actions
NO is synthesised by nitric oxide synthase (NOS) from the guanidino group of arginine through oxygen and NADPH dependent oxidation producing NO, with citrulline as a by-product. Three isoforms of nitric oxide synthase regulate the production and vary in their tissue distributions. Neuronal NOS (nNOS) is predominantly found in the nervous and enteric systems but also in vascular smooth muscle [5] and cardiac myocytes [6]. Endothelial NOS (eNOS) is found predominantly in the endothelium and cardiac mycoytes [7]. The inducible isoform (iNOS) is widely expressed in response to inflammatory stress although is found constitutively at low levels in some tissues.
The actions of NO can be broadly divided into direct and indirect. Classically, NO acts as a second messenger through direct activation of soluble guanylate cyclase (sGC) resulting in increased synthesis of cyclic guanosine monophosphate (cGMP) and reduced vascular tone. In addition to the direct action on sGC, NO directly binds to heme moieties on a range of proteins and leads to a number of processes including modulation of the mitochondrial electron transport chain at the level of complex IV and inhibition of cytochrome P450–mediated metabolism.
Indirectly, NO mediates its effects through its role as a free radical. NO rapidly interacts with other free radicals such as superoxide to form secondary metabolites such as peroxynitrite (ONOO−). Through similar reactions it can intercept lipid oxidation products (peroxy radicals) and act as chain-breaking antioxidant [8, 9].
The impact of the NO production in sepsis is extensive; however, the literature and in some respects its interpretation are conflicting. A detailed review of the function of NO is beyond the scope of this review; however, NO is essential to the maintenance of normal cardiovascular and immune responses to infection. It has diverse effects including maintenance of microvascular function, regulation of platelet aggregation and leukocyte activity, adhesion and transport [10]. Similarly, NO is directly toxic to most bacteria and has free radical scavenging action which reduces tissue injury and bioenergetic dysfunction. However, in sepsis and septic shock, these processes become dysregulated to variable degrees. Exaggerated NO production has been implicated in the development of macrovascular compromise [11], myocardial dysfunction [12], reduced responsiveness to adrenergic stimuli [13, 14], direct cellular toxicity and bioenergetic failure [15, 16]. Conversely, impaired local NO production in the endothelium has been cited as a cause of the microvascular dysfunction and impaired regional perfusion seen in septic shock.
Regulation of NO synthesis
The balance of harmful and beneficial effects is determined by a series of regulatory processes that determine the site and degree of NO synthesis that arises in response to an inflammatory stimulus.
The active isoforms of NOS are homodimers and depend upon a range of co-factors for their activity. Dimerisation is essential for activation and leads to the sequestration of iron and creation of binding sites for arginine and the essential cofactor tetrahydrobiopterin (BH4). In addition, dimerisation facilitates electron transfer between two key domains linked by a calmodulin binding motif. In eNOS and nNOS, the binding of calmodulin to the NOS homodimer is calcium dependent. Conversely, calmodulin is tightly bound to iNOS and therefore displays calcium independent activation. Enzymatic activity also depends upon, haem, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) availability as well as oxygen and NADPH as co-substrates (Fig. 1).
Fig. 1.
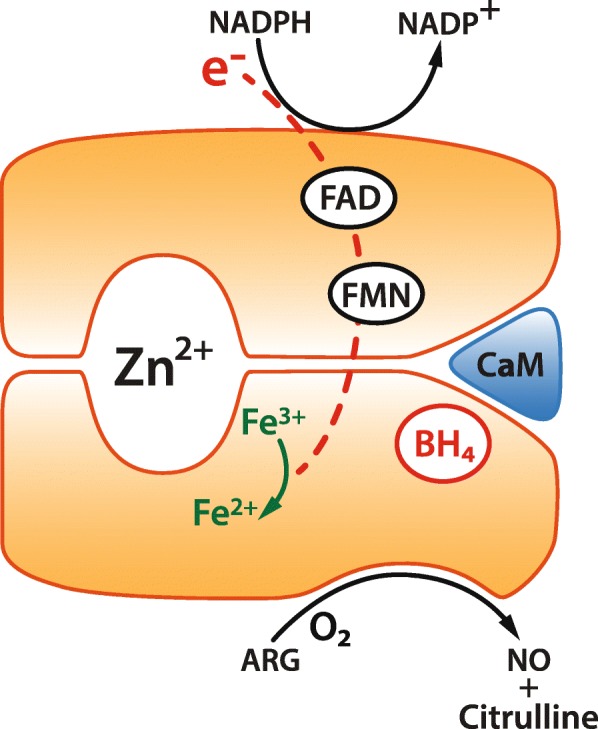
Nitric oxide synthase (NOS) cofactors and activity. NOS enzymes function as homodimers within subunits structurally supported by a Zinc ion (Zn2+). Following sequestration of iron, the binding site for arginine is expressed. Electron transfer also depends on flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) availability and the binding of calmodulin (CaM) which is calcium dependent for eNOS and nNOS and independent for iNOS. NADPH, oxygen and tetrahydrobiopterin (BH4) are essential cofactors for the synthesis of nitric oxide
Differential expression of the NOS isoforms within the cell or in tissues also influences NO synthesis. eNOS is commonly found bound to caveolin, a specialised anchoring protein within the caveolae of the endothelial cell membrane [17] which may itself regulate NO production [18]. This conformation has been implicated in the development of reduced NO synthesis in the septic microvasculature. In myocytes, the three NOS isoforms are differentially distributed with eNOS predominantly found in endothelium, nNOS in the presynaptic nerves and both found in different portions of the sarcoplasmic reticulum. iNOS by contrast, when induced by pro-inflammatory stress is most commonly found in the cytoplasm where it generates a two or three log fold higher increases in NO synthesis compared to the constitutive isoforms.
Therapeutic modulation of NO in sepsis
In the years following its discovery came a burgeoning recognition that NO played an important role in the development of the vasoplegia and hypotension that is the hallmark of septic shock. This led first to small scale studies of inhibition of sGC activity by methylene blue [19, 20], and subsequently, clinical trials of the non-specific small molecule inhibitor of all three NOS isoforms, L-NMMA. Phase II results [21] showed promise as L-NMMA demonstrated improved systemic vascular resistance and blood pressure in patients with septic shock. However, a phase III trial was terminated early due to excess mortality in the treatment group [22]. Questions regarding dose selection, blood pressure management and treatment strategy meant that interest in the area was not extinguished, and subsequent work has recognised the plethora of roles that NO has in regulating organ function in both health and disease.
In recent years, potential approaches to modulating NO production in sepsis have been developed targeting pathways beyond direct NOS inhibition. The remainder of this review will focus on a selection of these strategies which include modulating endogenous regulators of NOS activity, NO scavenging or donation and optimising substrate availability.
Nitric oxide inhibition
Endogenous regulators of NOS activity
Tetrahydrobiopterin (BH4) is an essential cofactor for NOS-mediated NO synthesis through facilitation of electron transfer to L-arginine. Reduced BH4 bioavailability results in the diversion of electron flow to molecular oxygen and production of free radical superoxide rather than N O[23], a process also known as NOS uncoupling. Reduced BH4 bioavailability has been implicated in the development of chronic cardiovascular diseases through this mechanism.
Whilst in sepsis, pro-inflammatory stress has been shown to increase production of BH4 [24], coexistent oxidative stress and reactive oxygen species cause conversion of BH4 to BH2. In addition to depleting BH4 scores, BH2 binds to NOS with equal affinity but promotes the uncoupling process [25] thereby reducing NO production. In malaria, increasing severity of disease is associated with reduced BH4 levels and BH4:BH2 ratio [26] and has been proposed as a driver of microcirculatory dysfunction in this population.
Supplementation of a BH4 analogue appears to reduce haemodynamic impairment, organ dysfunction and improve survival in rat models of acute inflammation and sepsis [27–29]. In an ovine model of septic shock secondary to faecal peritonitis, supplemental tetrahydrobiopterin therapy was associated with preservation of the sublingual microcirculation, reduced cardiovascular compromise and severity of lung and renal injury [30]. Furthermore, In animal models, ascorbate supplementation has been shown to restore capillary blood flow and eNOS activity in a BH4 dependent manner [31]. This has been postulated as one of the mechanisms underlying the potential benefits of Vitamin C supplementation in sepsis.
A further example of potential therapeutic approaches includes increasing the expression of endogenous inhibitors of NO synthesis. Derived from the methylation of arginine residues by the Protein Arginine Methyl Transferases (PRMTs), the methylarginines have an unusual physiological role in regulating the synthesis of NO. The three methylarginines are asymmetric dimethylarginine (ADMA), symmetric dimethyl arginine (SDMA) and, occurring at approximately a tenth of the concentration, monomethylarginine (L-NMMA). The asymmetric pair, ADMA and L-NMMA, acts as equipotent competitive inhibitors of arginine binding to the all three NOS isoforms. By contrast, SDMA has no direct action on NOS activity. Elevated levels of both ADMA and SDMA are associated with poor outcome in patients with sepsis; however, an increase in the ratio of ADMA to SDMA is associated with reduced mortality in patients with septic shock [32], suggesting there may be a disconnection between the association of elevated ADMA levels and the mechanisms responsible for dysregulated NO production.
The dominant method of methylarginine handling is enzymatic and driven by one of the two dimethylarginine dimethylaminohydroxylases (DDAH1 and DDAH2). Approximately 80% of ADMA and L-NMMA are metabolised to dimethylarginine (DMA) and L-citrulline by the DDAH isoforms, with the remaining 20% excreted by the kidney [33] (Fig. 2).
Fig. 2.
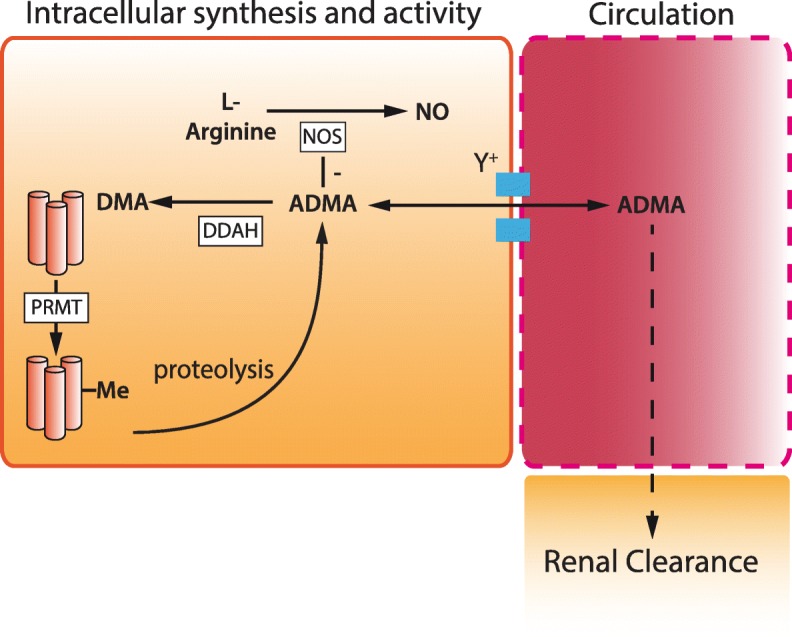
Methylarginine-mediated nitric oxide synthase inhibition. Representative image of the synthesis and regulation of ADMA. Protein Arginine Methyl Transferases (PRMT) catalyse the methylation of arginine containing protein residues to ADMA and SDMA which are released upon proteolysis. ADMA and SDMA are transported via the y+ cationic amino acid transporter into and out of the circulation. ADMA is metabolised by the two isoforms of dimethylarginine dimethylaminohydrolase (DDAH) in a wide range of tissues. ADMA acts intracellularly to inhibit nitric oxide synthase (NOS). ADMA is largely metabolised by DDAH to dimethylamine (DMA), a small amount is cleared unchanged through the kidney. nb. L-NMMA (monomethylarginine) is considered to have the same synthetic pathway, activity, metabolism and clearance as ADMA but is present in only 10% of the concentration
In addition to expression in the vasculature [34], DDAH1 is readily identified in the liver, kidney, brain, skeletal muscle and pancreas [35]. Small amounts of DDAH1 have also been found in the pulmonary vasculature [36] and in placental [37] tissues. The tissue distribution of DDAH2 differs markedly from that of DDAH1. Whilst both isoforms are found in blood vessels, liver and kidney, DDAH2 predominates in the placenta and is not found in the central nervous system. It is also the only isoform found in cardiomyocytes and in immune tissues [34, 35, 38]. This has led to the suggestion it may play an important role in the response to inflammatory or immune mediated conditions.
In rodent lipopolysaccharide (LPS) and polymicrobial models, both genetic and therapeutic inhibition of DDAH1, has been shown to offer improved survival [39]. In addition, DDAH1 inhibitor therapy was found to reduce the severity of hypotension and requirement for noradrenaline to maintain normal blood pressure. These effects still occurred when treatment was delayed until after the onset of shock symptoms. Furthermore, DDAH1 inhibition was shown to improve indices of renal and hepatic function, preserve microvascular flow and improve survival without any effect on immune cell function [39]. This favourable balance of improved vascular function without the deleterious impacts on the cardiac or immune system has led to DDAH1 inhibition being postulated as a potential therapeutic approach in septic shock.
Nitric oxide scavenging
The recognition that NO has essential functions in sepsis and that global inhibition of NO synthesis could have harmful effects, led to the hypothesis that scavenging excess NO might prove an effective therapeutic strategy [40]. One of these approaches was the development of pyridoxylated haemoglobin polyoxyethylene (PHP), which was originally developed as a candidate oxygen delivery substitute. PHP is pyridoxylated to remove its oxygen carrying capacity and then combined with polyoxyethylene to increase the apparent molecular weight of the compound. The resulting product has a high affinity for NO which it rapidly oxidises to the more stable nitrate as well as reducing peroxynitrite concentrations and regulating the redox state of the cell [41].
Preclinical ovine models of sepsis demonstrated that PHP improved systemic vascular resistance [42] and that treatment was not associated with impaired tissue perfusion. This led to a phase II study in of 62 patients with septic shock [43]. In this study, those treated with PHP displayed trends towards reduced requirements for vasopressors and ventilatory support. This was followed by the phase III PHOENIX study [44], which recruited 377 patients. The study showed no survival benefit following PHP therapy at 28 days but evidence of increased mortality in patients with a SOFA score of > 13 on inclusion. Despite this, the previously observed improvements in systemic haemodynamics persisted, with survivors displaying reduced duration of vasopressor therapy. The authors concluded that regional variation in NO synthesis in the microcirculation could have been exacerbated in the PHP treated patients thereby worsening organ perfusion and accounting for the worse outcomes observed. This coupled with the inability to identify which patients had more substantially elevated systemic NO levels based on noradrenaline requirement meant that selecting patients that might benefit from this approach based on clinical indices was not possible.
Increasing nitric oxide bioavailability
Substrate bioavailability
Arginine is the substrate for NOS enzymes and its availability is a dominant factor in the regulation of NO production. Whilst most bioavailable arginine is derived from the diet, the intracellular concentrations are governed by a range of processes including transmembrane transport, enzymatic synthesis and metabolism.
In sepsis, arginine becomes a conditionally essential amino acid with reduced bioavailability resulting from a variety of factors. Exaggerated protein turnover leads to an increase in stored arginine release however in patients with sepsis, reduced dietary intake of arginine and citrulline [45, 46] is coupled with increased turnover by NOS and arginase enzymes [47]. This coupled with reduced de novo synthesis at the tissue level in organ failure [48] means that circulating arginine concentrations are substantially reduced regardless of the infectious cause [49–51] with direct consequences for the production of NO and the microcirculation [52]
Extensive investigation has been undertaken exploring the potential role of exogenous arginine supplementation in sepsis. A number of animal models have produced promising results with a murine [52] and pretreatment porcine inflammatory model [53] showing improved microvascular function and organ perfusion mediated by increased NO production. In healthy volunteers, arginine supplementation is well tolerated at doses less than 8g/day; however, supplementation at doses above this was associated with gastrointestinal side effects [54]. In patients with critical illness, arginine supplementation has not consistently demonstrated clinically relevant improvements in outcome. There is some trial evidence suggesting supplementing arginine in addition to other nutrients may result in a reduced hospital length of stay [55] or improved organ function [56]. Preiser et al. [57] successfully increased plasma arginine concentrations via enteral supplementation in a small randomised controlled trial of a mixed ICU population. Whilst this was associated with some signal of increased arginase activity, no increase in plasma markers of NO production was detected. In a subsequent randomised trial of 597 critically ill patients, an immunonutrition approach including supplementation of arginine as well as glutamine and omega-3 was not associated with improved clinical outcomes [58]. A further study of arginine containing enteral immunonutrition versus parenteral therapy was stopped after an interim analysis suggested an excess mortality in patients receiving enteral supplementation [59]. This has led to the recommendation in the surviving sepsis guidelines that arginine supplementation is not used to treat patients with sepsis [60].
In addition to dietary uptake, arginine can be synthesised through the conversion of citrulline by a pair of enzymes that are found in the cytosol, argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) [61] (Fig. 3). The majority of bioavailable citrulline is synthesised by enterocytes with some derived from the diet [62] and a small proportion synthesised in the kidney, liver and enterocytes from ornithine by ornithine transcarbamoylase [63]. Citrulline bioavailability is also reduced in sepsis. Observed in patient populations to be associated with increased mortality [64], a low citrulline concentration is likely multifactorial. In addition to reduced dietary intake, reduced uptake of the citrulline precursor glutamine from the gut may play a role [65]. Compensatory increases in the expression of ornithine transcarbamoylase which synthesises citrulline from ornithine in the kidney may mitigate this effect to some degree [66].
Fig. 3.
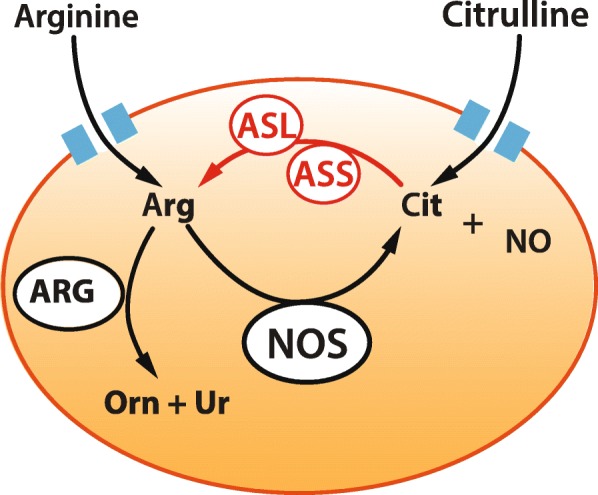
Arginine bioavailability. Arginine (ARG) is transported into the cell via cationic amino acid transporters and is converted to citrulline (Cit) and NO. Citrulline derived from a number of sources including diet can be converted to arginine by argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) increasing the available intracellular arginine. Arginase (ARG) metabolises arginine to ornithine (Orn) and urea (Ur)
A number of cell types use citrulline conversion by ASS and ASL to generate endogenous arginine. These include vascular smooth muscle [67], macrophages [68], neural cells [69] and endothelial cells [70, 71]. Importantly, in endothelial cells, ASS and ASL co-locate with eNOS and play an important role in regulating endogenous NO synthesis [72].
In sepsis, ASS expression is elevated in both the vasculature and immune cell populations, possibly in order to facilitate local NO production by iNOS [73, 74]. In animal models, heterozygote and tissue specific knockout of ASS are associated with worse outcomes due to reduced immune cell NO synthesis [75]. Supplementation with recombinant ASS mitigates the lethal effects of LPS in animal models although the mechanism of efficacy may also be related to direct binding to Gram negative bacteria reducing organism pathogenicity rather than the production of increased arginine [74].
With the similar aim of augmenting arginine and subsequent NO bioavailability and with some evidence from animal models [52], citrulline supplementation has also been proposed as a possible treatment strategy in sepsis. Whilst there is some evidence of association between low citrulline concentrations and the development of ARDS [46] and gut mucosal integrity in patients with sepsis [76], there is no prospective evidence to recommend citrulline supplementation at this stage.
The isoforms of the arginase enzymes degrade arginine to ornithine and urea rendering it an important regulator substrate availability and therefore of NO synthesis in the cell (Fig. 3). The arginase isoforms have differing tissue distributions, with arginase 1 expressed in endothelial cells, immune cells and the liver. Arginase 2 is also found in the endothelium [77] and immune cells [78] but is also detected in the kidney, brain and small intestine.
The expression of arginase isoforms during sepsis is a dynamic process that points to a role in regulating the initiation and resolution of inflammation [79–81] by limiting arginine availability. After an acute inflammatory stress, arginase induction typically arises some hours after peak iNOS induction [45]. This is responsible in part for the downregulation of NO synthesis and is best characterised in immune cells where it defines the transition of the macrophage from the pro-inflammatory M1 to the restorative M2 phenotype where arginase mediates the synthesis of proline and polyamines crucial to the process of wound healing [82].
Arginase 1 knockout immune cells and mice deficient in arginase 1 in endothelial and haematopoietic cells are prone to exaggerated NO synthesis in LPS models of inflammation with an associated microvascular dysfunction seen in murine models [83]. In patients, limited data suggests that levels of the enzyme in the circulation are elevated in sepsis but is not directly related to clinical indices of illness severity [84]. Although no therapeutic evaluation of arginase modulation is underway, the role of the enzymes in regulating systemic and local arginine concentrations is undoubtedly important and further understanding of the balance between NOS and arginase expression may yield novel therapeutic approaches.
Targeting microcirculatory dysfunction
The wide recognition of the importance of NO in maintaining the microcirculation, combined with the negative outcomes of trials of NOS inhibition has also led to suggestions that rather than reducing synthesis of NO, attention should focus on the use of NO donors to improve microvascular function, reduce shunting and optimise tissue perfusion in septic shock. Two distinct approaches have been proposed. The first involves optimising eNOS activity within the vascular endothelium which, as described above, is known to be impaired in sepsis due to the uncoupling of NO production due in part to reduced BH4 availability, lack of availability of substrate and elevated oxidative stress.
The alternative therapeutic strategy is to deliver exogenous compounds which can release NO at the microcirculatory level in order to optimise small vessel function. There are a number of proposed functions of the microcirculation that could be improved through NO modulation which include smooth muscle relaxation, reduced platelet aggregation and microthrombus formation and reduced endothelial leak and interstitial oedema. In addition, there is evidence that inorganic nitrites may offer some protection from ischaemia reperfusion injury and have a cardioprotective effect through a range of actions on mitochondrial and cellular function [85, 86].
This approach was tested in a small study of 8 patients as early as 2002 with nitroglycerin infusion which led to reported improvements in the sublingual microcirculation [87] although a subsequent randomised controlled trial published in 2010 randomised 70 patients to receive nitroglycerin infusion or placebo and found that this was not associated with improved microvascular flow in any category of vessel or interestingly on systemic haemodynamics, suggesting that it may not have been having the desired pharmacodynamic effect [88]. One potential reason for this could be the failure to effectively convert nitroglycerin to NO in vivo due to dysfunction of aldehyde dehydrogenase type 2 in capacitance vessels, which may not function effectively in patients with sepsis and leads to reduced nitroglycerin bioactivation [89]. This however remains only one endogenous route of activation [90] and therefore may not entirely explain this apparent result.
Based on a similar methodological approach, the direct administration of NO via an inhaled route has recently been trialled in patients with sepsis [91]. In a randomised trial of 50 patients, direct NO administration was able to increase circulating nitrite levels consistent with the hypothesis that pulmonary delivery of NO could lead to increased systemic availability; however, no impact on sublingual microvascular or organ function was detected.
Conclusions
Extensive research has led to the recognition that NO synthesis is regulated by a range of mechanisms which determine the production and actions of NO. Substrate and co-factor availability all contribute to NOS activity, which also determined on a tissue level by protein expression, cellular localisation and the presence of endogenous regulators.
In the last decade therapeutic research into the modulation of NO in critical illness has taken on a Janusian quality as it explores the competing challenges of overproduction of NO in some systems and impaired synthesis in others. Exaggerated NO production is responsible for cardiac, macrovascular and cellular dysfunction; however, microvascular function due to reduced eNOS activity and NO synthesis also appears to play an important role in outcome. The development of novel treatments in this area must mitigate the pathological consequences of at least one of these without exacerbating the other or impairing the essential physiological NO synthesis that is core to the response to infection. This challenge must be addressed if an improved understanding of the mechanistic role of NO in sepsis can be translated into therapies that improve outcomes for patients.
Acknowledgements
None
Abbreviations
- ADMA
Asymmetric dimethylarginine
- ASL
Arginosuccinate lyase
- ASS
Arginosuccinate synthetase
- BH4
Tetrahydrobiopterin
- cGMP
Cyclic guanosine monophosphate
- DDAH
Dimethylaminohydroxylases
- DMA
Dimethylarginine
- EDRF
Endothelial derived relaxing factor
- FAD
Flavin adenine dinucleotide
- FMN
Flavin mononucleotide
- L-NMMA
Monomethylarginine
- LPS
Lipopolysaccharide
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PHP
Pyridoxylated haemoglobin polyoxyethylene
- PRMTs
Protein Arginine Methyl Transferases
- RNS
Reactive nitrogen species
- SDMA
Symmetric dimethyl arginine
- sGC
Soluble guanylate cyclase
- SOFA
Sequential Organ Failure Assessment
Authors’ contributions
SL was the sole contributor to this work. The author read and approved the final manuscript.
Authors’ information
SL is a clinician specialising in anaesthesia and intensive care medicine. His research is focussed on translational studies exploring the mechanisms of vascular dysfunction in sepsis.
Funding
SL is funded by a NIHR Clinical Lecturer post at Cambridge University
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
SL is a founding director of Critical Pressure Ltd, a biotechnology company developing small molecule modulators of vascular nitric oxide synthesis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986;78:1–5. doi: 10.1172/jci112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith O. Nobel Prize for NO research. Nat Med. 1998;4:1215. doi: 10.1038/3182. [DOI] [PubMed] [Google Scholar]
- 5.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.res.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loughran, P.A., Z. Lei, L. Xu, M. Deng, and T.R. Billiar, Chapter 22 - Nitric oxide in sepsis and hemorrhagic shock: beneficial or detrimental?, in Nitric oxide (Third Edition), L.J. Ignarro and B.A. Freeman, Editors. 2017, Academic Press. p. 289-300.
- 11.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–565. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 12.Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29:500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Tsuneyoshi I, Kanmura Y, Yoshimura N. Nitric oxide as a mediator of reduced arterial responsiveness in septic patients. Crit Care Med. 1996;24:1083–1086. doi: 10.1097/00003246-199606000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36:2019–2029. doi: 10.1007/s00134-010-2045-8. [DOI] [PubMed] [Google Scholar]
- 15.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies N, Cooper C, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 16.Dyson A, Bryan NS, Fernandez BO, Garcia-Saura MF, Saijo F, Mongardon N, Rodriguez J, Singer M, Feelisch M. An integrated approach to assessing nitroso-redox balance in systemic inflammation. Free Radic Biol Med. 2011;51:1137–1145. doi: 10.1016/j.freeradbiomed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial Nitric Oxide Synthase Targeting to Caveolae: SPECIFIC INTERACTIONS WITH CAVEOLIN ISOFORMS IN CARDIAC MYOCYTES AND ENDOTHELIAL CELLS. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 18.Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Ren Physiol. 2003;285:F178–F190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- 19.Kirov MY, Evgenov OV, Evgenov NV, Egorina EM, Sovershaev MA, Sveinbjornsson B, Nedashkovsky EV, Bjertnaes LJ. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. 2001;29:1860–1867. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Andresen Max, Dougnac Alberto, Diaz Orlando, Hernandez Glen, Castillo Luis, Bugedo Guillermo, Alvarez Manuel, Dagnino Jorge. Use of methylene blue in patients with refractory septic shock: Impact on hemodynamics and gas exchange. Journal of Critical Care. 1998;13(4):164–168. doi: 10.1016/S0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 21.Bakker Jan, Grover Robert, McLuckie Angela, Holzapfel Laurent, Andersson Jan, Lodato Robert, Watson David, Grossman Steven, Donaldson Jill, Takala Jukka. Administration of the nitric oxide synthase inhibitor NG-methyl-l-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: Results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002)*. Critical Care Medicine. 2004;32(1):1–12. doi: 10.1097/01.CCM.0000105118.66983.19. [DOI] [PubMed] [Google Scholar]
- 22.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 23.Yuyun MF, Ng LL, Ng GA. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res. 2018;119:7–12. doi: 10.1016/j.mvr.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Werner ER, Werner-Felmayer G, Mayer B. Tetrahydrobiopterin, cytokines, and nitric oxide synthesis. Proc Soc Exp Biol Med. 1998;219:171–182. doi: 10.3181/00379727-219-44331. [DOI] [PubMed] [Google Scholar]
- 25.Hye-Lim, K., amp, amp, and P. Young Shik (2010) Maintenance of cellular tetrahydrobiopterin homeostasis. BMB Rep 43:584-592 [DOI] [PubMed]
- 26.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Weinberg JB, Hyland K, Granger DL, Anstey NM. Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog. 2015;11:e1004667. doi: 10.1371/journal.ppat.1004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzal F, Redl H, Strohmaier W, Werner ER, Bahrami S. A 4-amino analogue of tetrahydrobiopterin attenuates endotoxin-induced hemodynamic alterations and organ injury in rats. Shock. 2002;18:158–162. doi: 10.1097/00024382-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Bahrami S, Fitzal F, Peichl G, Gasser H, Fuerst W, Banerjee A, Strohmaier W, Redl H, Werner-Felmayer G, Werner ER. Protection against endotoxemia in rats by a novel tetrahydrobiopterin analogue. Shock. 2000;13:386–391. doi: 10.1097/00024382-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Dumbarton TC, Maxan A, Farah N, Sharawy N, Zhou J, Nantais J, Lehmann C. Tetrahydrobiopterin improves microcirculation in experimental sepsis. Clin Hemorheol Microcirc. 2017;67:15–24. doi: 10.3233/ch-160207. [DOI] [PubMed] [Google Scholar]
- 30.He X, Su F, Velissaris D, Salgado DR, de Souza Barros D, Lorent S, Taccone FS, Vincent JL, De Backer D. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med. 2012;40:2833–2840. doi: 10.1097/CCM.0b013e31825b88ba. [DOI] [PubMed] [Google Scholar]
- 31.Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med. 2008;36:2355–2362. doi: 10.1097/CCM.0b013e31818024f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambden S, Tomlinson J, Piper S, Gordon AC, Leiper J. Evidence for a protective role for the rs805305 single nucleotide polymorphism of dimethylarginine dimethylaminohydrolase 2 (DDAH2) in septic shock through the regulation of DDAH activity. Crit Care. 2018;22:336. doi: 10.1186/s13054-018-2277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran Cam T.L, Leiper James M, Vallance Patrick. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003;4(4):33–40. doi: 10.1016/S1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 34.Tran CT, Fox MF, Vallance P, Leiper JM. Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics. 2000;68:101–105. doi: 10.1006/geno.2000.6262. [DOI] [PubMed] [Google Scholar]
- 35.Leiper JM. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem.J. 1999;343:209–214. doi: 10.1042/bj3430209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucio Iannone LZ, Dubois O, Duluc L, Rhodes CJ, Wharton J, Wilkins MR, Leiper J, Wojciak-Stothard B. miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J. 2014;462:103–112. doi: 10.1042/BJ20140486. [DOI] [PubMed] [Google Scholar]
- 37.Anderssohn M, Maass LM, Diemert A, Luneburg N, Atzler D, Hecher K, Boger RH. Severely decreased activity of placental dimethylarginine dimethylaminohydrolase in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2012;161:152–156. doi: 10.1016/j.ejogrb.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Lambden S, Kelly P, Ahmetaj-Shala B, Wang Z, Lee B, Nandi M, Torondel B, Delahaye M, Dowsett L, Piper S, Tomlinson J, Caplin B, Colman L, Boruc O, Slaviero A, Zhao L, Oliver E, Khadayate S, Singer M, Arrigoni F, Leiper J. Dimethylarginine dimethylaminohydrolase 2 regulates nitric oxide synthesis and hemodynamics and determines outcome in polymicrobial sepsis. Arterioscler Thromb Vasc Biol. 2015;35:1382–1392. doi: 10.1161/atvbaha.115.305278. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Lambden S, Taylor V, Sujkovic E, Nandi M, Tomlinson J, Dyson A, McDonald N, Caddick S, Singer M, Leiper J. Pharmacological inhibition of DDAH1 improves survival, hemodynamics and organ function in experimental septic shock. Biochem J. 2014;460:309–316. doi: 10.1042/BJ20131666. [DOI] [PubMed] [Google Scholar]
- 40.Harbrecht BG. Therapeutic use of nitric oxide scavengers in shock and sepsis. Curr Pharm Des. 2006;12:3543–3549. doi: 10.2174/138161206778343000. [DOI] [PubMed] [Google Scholar]
- 41.Privalle Christopher, Talarico Todd, Keng Teresa, DeAngelo Joseph. Pyridoxalated hemoglobin polyoxyethylene: a nitric oxide scavenger with antioxidant activity for the treatment of nitric oxide-induced shock. Free Radical Biology and Medicine. 2000;28(10):1507–1517. doi: 10.1016/S0891-5849(00)00260-4. [DOI] [PubMed] [Google Scholar]
- 42.Bone HG, Schenarts PJ, Fischer SR, McGuire R, Traber LD, Traber DL. Pyridoxalated hemoglobin polyoxyethylene conjugate reverses hyperdynamic circulation in septic sheep. J Appl Physiol. 1998;84:1991–1999. doi: 10.1152/jappl.1998.84.6.1991. [DOI] [PubMed] [Google Scholar]
- 43.Kinasewitz GT, Privalle CT, Imm A, Steingrub JS, Malcynski JT, Balk RA, DeAngelo J. Multicenter, randomized, placebo-controlled study of the nitric oxide scavenger pyridoxalated hemoglobin polyoxyethylene in distributive shock*. Crit Care Med. 2008;36:1999–2007. doi: 10.1097/CCM.0b013e31817bfe84. [DOI] [PubMed] [Google Scholar]
- 44.Vincent J-L, Privalle CT, Singer M, Lorente JA, Boehm E, Meier-Hellmann A, Darius H, Ferrer R, Sirvent J-M, Marx G. Multicenter, randomized, placebo-controlled phase III study of pyridoxalated hemoglobin polyoxyethylene in distributive shock (PHOENIX) Crit Care Med. 2015;43:57–64. doi: 10.1097/CCM.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 45.Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care. 2003;6:223–228. doi: 10.1097/01.mco.0000058594.27240.12. [DOI] [PubMed] [Google Scholar]
- 46.Ware LB, Magarik JA, Wickersham N, Cunningham G, Rice TW, Christman BW, Wheeler AP, Bernard GR, Summar ML. Low plasma citrulline levels are associated with acute respiratory distress syndrome in patients with severe sepsis. Crit Care. 2013;17:R10. doi: 10.1186/cc11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau T, Owen W, Yu YM, Noviski N, Lyons J, Zurakowski D, Tsay R, Ajami A, Young VR, Castillo L. Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest. 2000;105:1217–1225. doi: 10.1172/jci7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis JS, Anstey NM. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Crit Care Med. 2011;39:380–385. doi: 10.1097/CCM.0b013e3181ffd9f7. [DOI] [PubMed] [Google Scholar]
- 50.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–152. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 51.Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond) 2009;117:23–30. doi: 10.1042/cs20080444. [DOI] [PubMed] [Google Scholar]
- 52.Wijnands KA, Vink H, Briede JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS One. 2012;7:e37439. doi: 10.1371/journal.pone.0037439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poeze M, Bruins MJ, Kessels F, Luiking YC, Lamers WH, Deutz NE. Effects of L-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am J Clin Nutr. 2011;93:1237–1247. doi: 10.3945/ajcn.110.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boger RH. The pharmacodynamics of L-arginine. Altern Ther Health Med. 2014;20:48–54. [PubMed] [Google Scholar]
- 55.Bower RH, Cerra FB, Bershadsky B, Licari JJ, Hoyt DB, Jensen GL, Van Buren CT, Rothkopf MM, Daly JM, Adelsberg BR. Early enteral administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med. 1995;23:436–449. doi: 10.1097/00003246-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Beale RJ, Sherry T, Lei K, Campbell-Stephen L, McCook J, Smith J, Venetz W, Alteheld B, Stehle P, Schneider H. Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med. 2008;36:131–144. doi: 10.1097/01.CCM.0000297954.45251.A9. [DOI] [PubMed] [Google Scholar]
- 57.Preiser JC, Berre PJ, Van Gossum A, Cynober L, Vray B, Carpentier Y, Vincent JL. Metabolic effects of arginine addition to the enteral feeding of critically ill patients. JPEN J Parenter Enteral Nutr. 2001;25:182–187. doi: 10.1177/0148607101025004182. [DOI] [PubMed] [Google Scholar]
- 58.Kieft H, Roos AN, van Drunen JD, Bindels AJ, Bindels JG, Hofman Z. Clinical outcome of immunonutrition in a heterogeneous intensive care population. Intensive Care Med. 2005;31:524–532. doi: 10.1007/s00134-005-2564-x. [DOI] [PubMed] [Google Scholar]
- 59.Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, Zanforlin G, Tognoni G. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29:834–840. doi: 10.1007/s00134-003-1711-5. [DOI] [PubMed] [Google Scholar]
- 60.Rhodes Andrew, Evans Laura E., Alhazzani Waleed, Levy Mitchell M., Antonelli Massimo, Ferrer Ricard, Kumar Anand, Sevransky Jonathan E., Sprung Charles L., Nunnally Mark E., Rochwerg Bram, Rubenfeld Gordon D., Angus Derek C., Annane Djillali, Beale Richard J., Bellinghan Geoffrey J., Bernard Gordon R., Chiche Jean-Daniel, Coopersmith Craig, De Backer Daniel P., French Craig J., Fujishima Seitaro, Gerlach Herwig, Hidalgo Jorge Luis, Hollenberg Steven M., Jones Alan E., Karnad Dilip R., Kleinpell Ruth M., Koh Younsuk, Lisboa Thiago Costa, Machado Flavia R., Marini John J., Marshall John C., Mazuski John E., McIntyre Lauralyn A., McLean Anthony S., Mehta Sangeeta, Moreno Rui P., Myburgh John, Navalesi Paolo, Nishida Osamu, Osborn Tiffany M., Perner Anders, Plunkett Colleen M., Ranieri Marco, Schorr Christa A., Seckel Maureen A., Seymour Christopher W., Shieh Lisa, Shukri Khalid A., Simpson Steven Q., Singer Mervyn, Thompson B. Taylor, Townsend Sean R., Van der Poll Thomas, Vincent Jean-Louis, Wiersinga W. Joost, Zimmerman Janice L., Dellinger R. Phillip. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Medicine. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 61.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Phys. 1990;259:E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437. [DOI] [PubMed] [Google Scholar]
- 62.van de Poll MC, Ligthart-Melis GC, Boelens PG, Deutz NE, van Leeuwen PA, Dejong CH. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol. 2007;581:819–827. doi: 10.1113/jphysiol.2006.126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr. 2012;142:572–580. doi: 10.3945/jn.111.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galban C, Montejo JC, Mesejo A, Marco P, Celaya S, Sanchez-Segura JM, Farre M, Bryg DJ. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med. 2000;28:643–648. doi: 10.1097/00003246-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol Endocrinol Metab. 2013;304:E1359–E1364. doi: 10.1152/ajpendo.00628.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr. 2010;29:545–551. doi: 10.1016/j.clnu.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Wileman SM, Mann GE, Pearson JD, Baydoun AR. Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS and interferon-gamma. Br J Pharmacol. 2003;140:179–185. doi: 10.1038/sj.bjp.0705407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nussler AK, Billiar TR, Liu ZZ, Morris SM., Jr Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. Implications for regulation of nitric oxide production. J Biol Chem. 1994;269:1257–1261. [PubMed] [Google Scholar]
- 69.Schmidlin A, Fischer S, Wiesinger H. Transport of L-citrulline in neural cell cultures. Dev Neurosci. 2000;22:393–398. doi: 10.1159/000017468. [DOI] [PubMed] [Google Scholar]
- 70.Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 71.Hilderman RH, Casey TE, Pojoga LH. P(1),P(4)-Diadenosine 5’-tetraphosphate modulates l-arginine and l-citrulline uptake by bovine aortic endothelial cells. Arch Biochem Biophys. 2000;375:124–130. doi: 10.1006/abbi.1999.1643. [DOI] [PubMed] [Google Scholar]
- 72.Flam BR, Eichler DC, Solomonson LP. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide. 2007;17:115–121. doi: 10.1016/j.niox.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Kawahara K, Gotoh T, Oyadomari S, Kajizono M, Kuniyasu A, Ohsawa K, Imai Y, Kohsaka S, Nakayama H, Mori M. Co-induction of argininosuccinate synthetase, cationic amino acid transporter-2, and nitric oxide synthase in activated murine microglial cells. Brain Res Mol Brain Res. 2001;90:165–173. doi: 10.1016/S0169-328X(01)00100-0. [DOI] [PubMed] [Google Scholar]
- 74.Prima V, Wang A, Molina G, Wang KK, Svetlov SI. Inhibition of LPS toxicity by hepatic argininosuccinate synthase (ASS): novel roles for ASS in innate immune responses to bacterial infection. Int Immunopharmacol. 2011;11:1180–1188. doi: 10.1016/j.intimp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Wijnands KA, Castermans TM, Hommen MP, Meesters DM, Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7:1426–1463. doi: 10.3390/nu7031426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crenn Pascal, Neveux Nathalie, Chevret Sylvie, Jaffray Patrick, Cynober Luc, Melchior Jean-Claude, Annane Djillali. Plasma l-citrulline concentrations and its relationship with inflammation at the onset of septic shock: A pilot study. Journal of Critical Care. 2014;29(2):315.e1-315.e6. doi: 10.1016/j.jcrc.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Buga GM, Singh R, Pervin S, Rogers NE, Schmitz DA, Jenkinson CP, Cederbaum SD, Ignarro LJ. Arginase activity in endothelial cells: inhibition by NG-hydroxy-L-arginine during high-output NO production. Am J Phys Heart Circ Phys. 1996;271:H1988–H1998. doi: 10.1152/ajpheart.1996.271.5.H1988. [DOI] [PubMed] [Google Scholar]
- 78.Morris SM, Jr, Kepka-Lenhart D, Chen L-C. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. American Journal of Physiology-Endocrinology and Metabolism. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z, Ming X-F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014;5:533. doi: 10.3389/fimmu.2014.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cowburn AS, Crosby A, Macias D, Branco C, Colaço RDDR, Southwood M, Toshner M, Crotty Alexander LE, Morrell NW, Chilvers ER, Johnson RS. HIF2α–arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci. 2016;113:8801–8806. doi: 10.1073/pnas.1602978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Phys. 1998;274:R775–R782. doi: 10.1152/ajpregu.1998.274.3.R775. [DOI] [PubMed] [Google Scholar]
- 82.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532–532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wijnands KAP, Hoeksema MA, Meesters DM, van den Akker NMS, Molin DGM, Briedé JJ, Ghosh M, Köhler SE, van Zandvoort MAMJ, de Winther MPJ, Buurman WA, Lamers WH, Poeze M. Arginase-1 deficiency regulates arginine concentrations and NOS2-mediated NO production during endotoxemia. PLoS One. 2014;9:e86135–e86135. doi: 10.1371/journal.pone.0086135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darcy CJ, Woodberry T, Davis JS, Piera KA, McNeil YR, Chen Y, Yeo TW, Weinberg JB, Anstey NM. Increased plasma arginase activity in human sepsis: association with increased circulating neutrophils. Clin Chem Lab Med. 2014;52:573–581. doi: 10.1515/cclm-2013-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu L-Y, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 88.Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, Buter H, Bruins N, Egbers PH, Gerritsen RT, Koetsier PM, Kingma WP, Kuiper MA, Ince C. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38:93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- 89.Huellner MW, Schrepfer S, Weyand M, Weiner H, Wimplinger I, Eschenhagen T, Rau T. Inhibition of aldehyde dehydrogenase type 2 attenuates vasodilatory action of nitroglycerin in human veins. FASEB J. 2008;22:2561–2568. doi: 10.1096/fj.07-098830. [DOI] [PubMed] [Google Scholar]
- 90.Janero DR, Bryan NS, Saijo F, Dhawan V, Schwalb DJ, Warren MC, Feelisch M. Differential nitros(yl)ation of blood and tissue constituents during glyceryl trinitrate biotransformation in vivo. Proc Natl Acad Sci U S A. 2004;101:16958–16963. doi: 10.1073/pnas.0406075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, Roberts BW, Chansky ME, Parrillo JE, Hollenberg SM. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis*. Crit Care Med. 2014;42:2482–2492. doi: 10.1097/ccm.0000000000000549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


