Abstract
Motoneurons are known to be an essential component of central pattern generators in invertebrates, but it is only recently that they have been shown to play a similar role in vertebrate locomotor circuits. Here, we review early experiments implicating motoneurons in the genesis of spontaneous motor activity in development and more recent experiments identifying motoneurons as important regulators of locomotor activity in the adult zebrafish and in the neonatal mouse spinal cord. We discuss the mechanisms responsible for these actions, the experimental challenges in studying the role of motoneurons in the mammalian spinal cord and the functional significance of the excitatory influence of motoneuron activity on locomotor behavior.
Introduction
The vertebrate spinal cord contains the essential circuitry for generating locomotion. The core component of this circuitry—the central pattern generator (CPG)—can produce the essential features of locomotor behavior in the absence of descending or afferent inputs [1]. It has long been assumed that the CPG for locomotion is comprised exclusively of spinal interneurons and that motoneurons function primarily as the output of the spinal cord [2]. This view was supported by anatomical and physiological evidence in the lamprey and zebrafish, showing that axial motoneurons possess few or no axon collaterals [3,4]. Consistent with this anatomical evidence, experiments in the isolated lamprey spinal cord showed that antidromic stimulation of the ventral roots had no effect on fictive swimming induced by NMDA [5]. By contrast, in limbed vertebrates, motoneurons have recurrent collaterals that synapse onto other motoneurons [6] and onto inhibitory interneurons (called Renshaw cells in mammals, R-interneurons in the chick embryo) within the spinal cord [7–9]. Because the target interneurons are inhibitory, they were not thought to have a significant role in locomotor rhythmogenesis.
Motoneurons regulate rhythmic motor activity in the developing spinal cord
In the developing spinal cord, motoneurons have been shown to play an active role in regulating motor activity. In the isolated spinal cords of the chick [10] and mouse [11] embryos, stimulation of a ventral root can trigger an episode of rhythmic bursting. In the chick embryo, ventral root stimulation activated the spinal network through the synaptic connections of motoneurons with the avian homologue of the mammalian Renshaw cell [10]. In addition, at the onset of spontaneous episodes of rhythmic activity, calcium, and voltage sensitive dye imaging revealed that the earliest optical signals were detected in the ventrolateral part of the cord and spread throughout the cord from there [10,12,13]. The first indication that motoneurons might have access to the vertebrate locomotor CPG came from experiments on the tadpole showing that rhythmically active spinal interneurons, presumed to be part of the swimming CPG, received cholinergic synapses that were proposed at the time to originate from motoneurons [14]. However, the later demonstration that some spinal, glutamatergic interneurons also release acetylcholine rendered this assumption uncertain [15].
Motoneuron activity regulates locomotion and locomotor-like activity
The demonstration that motoneurons influence the mammalian locomotor CPG came from experiments in the neonatal mouse spinal cord, showing that stimulation of a ventral root or the sciatic nerve (sensory dorsal roots cut) could trigger an episode of locomotor-like activity [16] (Figure 1a). Surprisingly, the phenomenon was not blocked by cholinergic antagonists which could be partly explained by the finding that motoneurons release a second excitatory transmitter—that binds to glutamatergic receptors—in addition to acetylcholine at their central synapses with Renshaw cells [16,17]. Whether the transmitter is glutamate is not completely settled, because the expression of the known vesicular glutamate transporters at motoneuron terminals remains controversial [16–19] and aspartate is present at a higher level than glutamate [20]. Motoneurons also have excitatory connections with other motoneurons (at least in neonatal and juvenile mice) that have been reported to be mediated by both cholinergic and glutamatergic receptors [17] or exclusively by glutamate receptors [21•]. The difference in the findings may be due to the age of the animals which were older in the study finding exclusively glutamate-like transmission [21•]. Further evidence for an excitatory effect of motoneurons on locomotor networks came from studies in the neonatal rat [22] and mouse [23] showing that stimulation of a ventral root could accelerate locomotor-like activity induced by drugs, although in the rat this occurred rarely and only in the presence of noradrenaline. Machacek and Hochman [22] proposed that noradrenaline unmasked a connection between motoneurons and an unknown class of excitatory interneuron. They also showed that ventral root stimulation could sometimes entrain disinhibited bursting, another manifestation of the excitatory effects of ventral root stimulation on spinal networks. The ability of ventral root stimulation to entrain disinhibited bursting was also demonstrated in the neonatal mouse spinal cord by Bonnot et al. [24] who used calcium imaging to show that, following a ventral root stimulus, optical activity often began in the vicinity of the motor nuclei and propagated dorso-medially from there to encompass the whole cord.
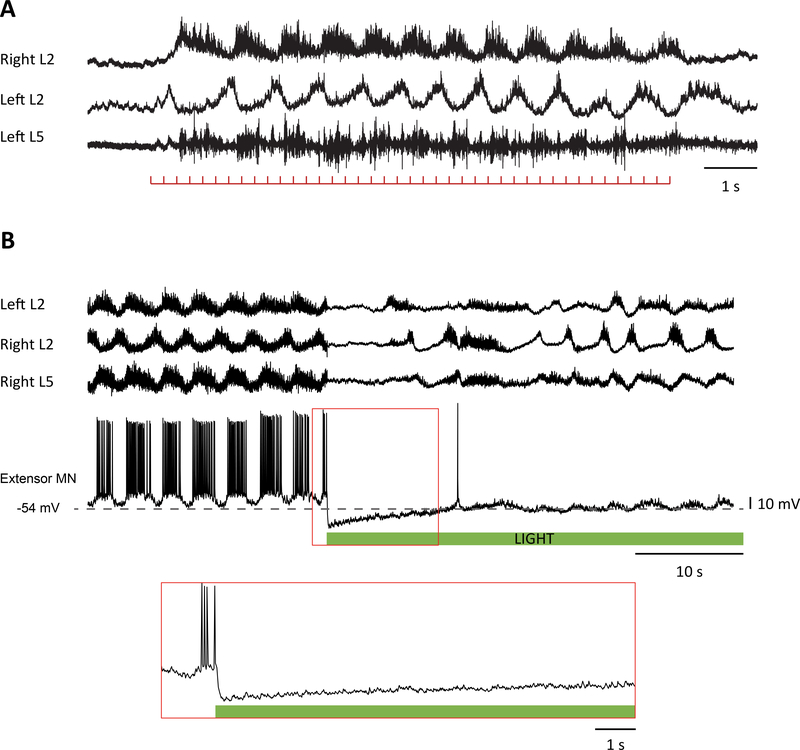
Figure 1.
(a) Motoneurons can trigger locomotor-like activity. Fictive locomotion evoked by ventral root stimulation (100 μA, 250 μs, 4 Hz) recorded from the right L2 and left L2 and L5 ventral roots in a P3 Wild type spinal cord. The signals were filtered to remove the stimuli artifacts and were high pass filtered at 0.1 Hz. The red trace below the recordings shows the train of stimuli. (b) Hyperpolarization of cholinergic neurons in a ChAT-Archaerhodopsin spinal cord transiently abolished the rhythmic synaptic drive to motoneurons and decreases the frequency of the rhythm. Locomotor-like activity evoked by 5 μM NMDA and 10 μM 5-HT recorded from the left L2 and right L2 and L5 ventral roots together with an extensor motoneuron in the right L5 segment. The green bar below the intracellular recording indicates the duration of the light. The part of the intracellular record delineated by the red rectangle has been expanded in the panel below to show the absence of rhythmic drive for the first 10–15 s after the light turns on. The data in (b) are adapted from Ref. [27••].
An important advance in understanding the role of motoneurons in modulating locomotor function came from experiments in the zebrafish showing that optogenetic hyperpolarization of motoneurons reduced the frequency and duration of swimming bouts in 3–4 week old zebrafish [25••]. In this animal, V2a interneurons project monosynaptically to motoneurons and are an important source of their locomotor synaptic drive [26]. A majority of the V2 neurons connect to motoneurons via bidirectional gap junctions at mixed-chemical electrical synapses. Through these gap junctions, motoneuron membrane potential can modulate transmitter release and the firing of the V2a interneurons. Accordingly, retrograde hyperpolarization of the V2a population by optogenetically hyperpolarizing motoneurons depresses V2a spiking activity and transmitter release thereby slowing and curtailing the swimming episode [25••]. For technical reasons, the demonstration that blockade of gap junctions eliminated the motoneuronal modulation of the swim bouts was not performed.
Using a similar optogenetic approach, it was shown that lumbar motoneurons can also regulate drug-induced locomotor-like activity in the neonatal mouse spinal cord. Falgairolle et al. [27••] expressed the inhibitory opsin archaerhodopsin into cholinergic neurons and in separate experiments into islet-1 expressing neurons. In both preparations, illumination of the lumbar spinal cord with green/yellow light slowed the locomotor rhythm (Figure 1b). Mixed chemical/electrical synapses are reported to be common in the adult mammalian spinal cord [28], so the question arises as to whether the regulation of locomotor-like activity in the neonatal mouse spinal cord also employs this mechanism of retrograde control. However, in contrast to the findings in the zebrafish, this effect did not appear to be mediated by gap junctions because it persisted in the presence of the gap junction blocker carbenoxolone, consistent with the earlier demonstration that carbenoxolone did not abrogate ventral root-evoked locomotor-like activity [16]. In addition, it has been shown that V2a neurons in the neonatal mouse spinal cord are not electrically coupled to motoneurons [21•] and that the modulatory effects of motoneuron activity on the locomotor-like rhythm appear to be mediated, in part, through a glutamatergic mechanism [27••].
Possible mechanisms for ventral root-evoked locomotor-like activity
The recent discovery that neonatal motoneurons have excitatory projections to spinal neurons other than Renshaw cells provides a potential mechanism for the excitatory effects of motoneurons on locomotor activity (Figure 2). Chopek et al. have shown that motoneurons have reciprocal, monosynaptic excitatory connections with V3 glutamatergic interneurons [29••]. The existence of these connections may explain the earlier observations of Ichinose and Miyata [30] and Schneider and Fyffe [31] who recorded ventral root-evoked EPSPs in motoneurons that had latencies similar to that of the recurrent IPSP, consistent with a disynaptic connection.
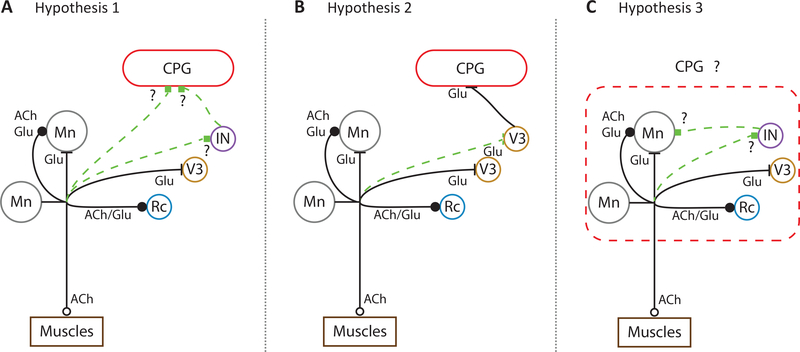
Figure 2.
Hypothesized connections of motoneurons to the CPG in neonatal mice. Circles represent neurons: motoneurons (Mn), Renshaw Cell (Rc), V3 interneurons (V3) and an unknown interneuronal population (IN). Each axon has been labeled with their known transmitter(s): acetylcholine (ACh) and glutamate (Glu). Motoneuronal to motoneuronal connections show 2 axon collaterals to encompass different results in the literature [17,21•]. (a) Hypothesis 1. Motoneurons connect to the CPG either directly or through a projection to an unidentified interneuron (IN). (b) Hypothesis 2. V3 interneurons have been shown to receive monosynaptic inputs from motoneurons [29••] and to modulate the CPG [32]. Here we hypothesized that the ability of motoneurons to trigger locomotor-like activity and target the CPG is mediated through this pathway. (c) Hypothesis 3. Motoneurons and the CPG are traditionally seen as separate modules. In this schematic, we hypothesize that motoneurons are part of the CPG and that they might be playing a crucial role in activating and generating locomotion.
Whether or not these novel excitatory connections are responsible for the excitatory effects of motoneuron activity on the CPG is unclear. When V3 neurons are acutely silenced using the allatostatin receptor system, the locomotor-like rhythm is degraded so that the duration of the left and right flexor bursts becomes more variable as does the cycle period [32]. Furthermore, it is not known if activation of this population can initiate locomotor-like activity or the extent to which they regulate the frequency of locomotor-like activity. It is important to emphasize even if a particular cell class targeted by motoneurons does not underlie the excitatory effects of motoneurons on locomotion, the novel connection is likely to be functionally significant.
The excitatory effects of motoneuron activity on the locomotor CPG represent a form of positive feedback because motoneuronal activity increases the frequency of locomotor-like activity [22,23,27••]. Such a system is potentially unstable and may be balanced by an inhibitory pathway. The well-known connection of motoneurons to inhibitory Renshaw cells may serve this function although there is only indirect evidence to support such a role. This comes from experiments in mice in which a vesicular transporter for glutamate (VGluT2) was knocked out [23]. This transporter underlies ~90% of excitatory glutamatergic transmission in the spinal cord, and despite its absence, the isolated cord can still generate locomotor-like activity in response to drugs [23]. In this preparation, stimulation of a ventral root can block drug-induced locomotor-like activity indicating the potential presence of a negative feedback loop that may counteract the excitatory effects of motoneuron activity on the CPG.
Challenges in studying the feedback effects of motoneuron activity on spinal networks
One of the difficulties in studying the effects of motoneuronal effects on locomotor networks is that a unique molecular marker for motoneurons does not exist, which precludes the selective expression of the opsins into motoneurons. Song et al. [25••] used a Gal4s1020t zebrafish line in which the Gal-4 driver of halorhodopsin was expressed in motoneurons and also in GABAergic Kolmer-Agduhr cells. Optogenetic activation of the Kolmer-Agduhr cells can trigger swimming bouts in the zebrafish embryo [33]. Their influence in the experiments of Song et al. was minimized by the presence of the GABAA antagonist gabazine in the bathing medium. In the ChAT-Archaerhodopsin mouse spinal cords used by Falgairolle et al. [27••], archaerhodopsin was expressed in autonomic preganglionic neurons and cholinergic interneurons in addition to motoneurons. To minimize the contribution of these non-motoneuronal cells to the light-induced effects on the locomotor rhythm, the experiments were repeated in the presence of nicotinic and muscarinic cholinergic antagonists. In these blockers, the inhibitory effects of light on the frequency of the locomotor-like rhythm were still present. Although this procedure would have also blocked cholinergic motoneuronal synapses, motoneurons also release an excitatory amino acid so that they could still exert an action on spinal networks. Of course, a similar argument applies to cholinergic interneurons that co-release acetylcholine and glutamate [15,19].
A second challenge in studying the excitatory actions of motoneurons on spinal and locomotor networks in the neonatal mouse cord is that the phenomenon is variable during the neonatal period and is not detectable in every experiment [22,24,34]. One possibility is that the effects may depend on the neuromodulatory state of the isolated cord. For example, Machacek and Hochman [22] showed that the excitatory effects of motoneuron stimulation were enhanced by noradrenaline and inhibited by serotonin. Humphreys and Whelan [34] showed that dopamine could block ventral root activation of the CPG and ventral root entrainment in the disinhibited cord. Why the neuromodulatory state of the cord should vary from one experiment to another is not clear, but it could depend on how the animals are raised and handled. Another contributing factor may be that the motoneuronal connections with the CPG are immature in the neonatal period. Unfortunately, it has been difficult to determine if the excitatory effects of motoneurons on locomotor activity are present in older cords because they lose viability when maintained in vitro. Nevertheless, it should be possible to establish if motoneuron projections to V3 interneurons are present in slice preparations of the adult cord.
Outstanding questions
There are many outstanding questions that remain to be addressed before we can achieve a complete understanding of how motoneurons interact with the mammalian locomotor CPG. For example, we do not know whether all classes of motoneuron are involved. Previous work reported that high stimulus intensities applied to the ventral roots were necessary to evoke locomotor-like activity, raising the possibility that the motoneurons responsible were of the slow type or were even gamma motoneurons [35•].
A second issue concerns how motoneuron stimulation triggers locomotor-like activity. V3 interneurons that are targeted by motoneurons have not been directly implicated in rhythmogenesis. Zhang et al. [32] reported that rhythmic motor activity persists when the population is chronically or acutely silenced. Moreover, intracellular recordings from V3 neurons do not reveal the presence of electrical properties consistent with cellular rhythmogenesis [32]. Of course, the V3 population is likely to be heterogeneous and perhaps the subpopulation targeted by motoneurons does exhibit these properties and is responsible for the ability of motoneurons to evoke fictive locomotion when stimulated (Figure 2b). Alternatively, another, as yet unknown, population of interneurons that is also targeted by motoneurons may be responsible (Figure 2a). Furthermore, unless this connection is not always present, it is difficult to account for the variability in evoking motor activity in either the normal or the disinhibited cord [22,24].
As discussed above, it is not known whether the excitatory effects of motoneuron activity on the CPG persist into adulthood. Early in the development of the tadpole and the zebrafish, motoneurons release glutamate at the neuromuscular junction [36,37], and this phenomenon is abolished or reduced later in development.
However, at motoneuron-Renshaw synapses in the adult rodent cord, glutamate or a glutamate-like neurotransmitter is co-released with acetylcholine, indicating that activation of glutamate receptors by motoneuron activity is conserved through development [38]. Determining the extent to which motoneurons regulate locomotion in adult animals, particularly in vivo, will be experimentally challenging. In the moving animal, any manipulations of motoneuronal activity will be complicated by the altered afferent feedback that accompanies changes in muscle activity.
An important and unresolved question is the extent to which motoneurons contribute to locomotor rhythmogenesis. Motoneurons are known to exhibit TTX-resistant, NMDA-induced oscillations of membrane potential [39], plateau potentials [40,41] and persistent inward and outward currents that can induce bursting [42]. Furthermore, none of the genetic disruptions and deletions of the canonical interneuronal precursors abolishes the locomotor rhythm. In some experiments, in which neonatal motoneurons were optogenetically hyperpolarized, rhythmic activity recorded from the ventral roots and intracellularly from individual motoneurons was abolished (Figure 1b), consistent with the idea that motoneurons might contribute to rhythmogenesis (Figure 2c). Resolution of this issue is a complicated, because it is difficult to distinguish between motoneuronal input as a requirement for CPG function compared to motoneurons as the origin of the rhythm. This difficultly applies not just to motoneurons but also to all classes of interneurons.
Like animals, human locomotion is thought to be generated by a spinal CPG, and it is likely the spinal circuitry is organized in a similar way [43]. If motoneurons do regulate motor function in humans, this will be of major significance for neurodegenerative motoneuron disorders and the recovery of locomotor function after spinal injury.
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the NINDS, NIH.
Footnotes
Conflict of interest statement
Nothing declared.
Contributor Information
Melanie Falgairolle, Section on Developmental Neurobiology, NINDS, NIH, Bethesda MD 20892, USA.
Michael J. O’Donovan, Section on Developmental Neurobiology, NINDS, NIH, Bethesda MD 20892, USA
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Brown TG: The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B 1911, 84:308–319. [Google Scholar]
- 2.Kiehn O: Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 2016, 17:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ampatzis K, Song J, Ausborn J, El Manira A: Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish. J Neurosci 2013, 33:10875–10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmykhova NM, Adanina VO, Karamian OA, Kozhanov VM, Vesselkin NP, Clamann HP: Comparative study of spinal motoneuron axon collaterals. Brain Res Bull 2005, 66:381–386. [DOI] [PubMed] [Google Scholar]
- 5.Wallen P, Lansner A: Do the motoneurones constitute a part of the spinal network generating the swimming rhythm in the lamprey. J Exp Biol 1984, 113:493–497. [Google Scholar]
- 6.Cullheim S, Kellerth JO: A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol 1978, 281:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eccles JC, Fatt P, Koketsu K: Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol 1954, 126:524–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renshaw B: Influence of discharge of motoneurons upon excitation of neighboring motoneurons. J Neurophysiol 1941, 4:167–183. [Google Scholar]
- 9.Wenner P, O’Donovan MJ: Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. J Neurosci 1999, 19:7557–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenner P, O’Donovan MJ: Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol 2001, 86:1481–1498. [DOI] [PubMed] [Google Scholar]
- 11.Hanson MG, Landmesser LT: Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci 2003, 23:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai Y, Mentis GZ, Wu JY, O’Donovan MJ: Ventrolateral origin of each cycle of rhythmic activity generated by the spinal cord of the chick embryo. PLoS One 2007, 2:e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donovan M, Ho S, Yee W: Calcium imaging of rhythmic network activity in the developing spinal cord of the chick embryo. J Neurosci 1994, 14:6354–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrins R, Roberts A: Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus embryos. J Neurophysiol 1995, 73:1013–1019. [DOI] [PubMed] [Google Scholar]
- 15.Li WC, Soffe SR, Roberts A: Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci U S A 2004, 101:15488–15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ: Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A 2005, 102:7344–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O: Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A 2005, 102:5245–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog E, Landry M, Buhler E, Bouali-Benazzouz R, Legay C, Henderson CE, Nagy F, Dreyfus P, Giros B, El Mestikawy S: Expression of vesicular glutamate transporters, VGLUT1 and VGLUT2, in cholinergic spinal motoneurons. Eur J Neurosci 2004, 20:1752–1760. [DOI] [PubMed] [Google Scholar]
- 19.Liu TT, Bannatyne BA, Jankowska E, Maxwell DJ: Cholinergic terminals in the ventral horn of adult rat and cat: evidence that glutamate is a cotransmitter at putative interneuron synapses but not at central synapses of motoneurons. Neuroscience 2009, 161:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards DS, Griffith RW, Romer SH, Alvarez FJ: Motor axon synapses on Renshaw cells contain higher levels of aspartate than glutamate. PLoS One 2014, 9:e97240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhumbra GS, Beato M: Recurrent excitation between motoneurones propagates across segments and is purely glutamatergic. PLoS Biol 2018, 16:e2003586.•Previous work in the adult cat revealed that motoneurons have axon collaterals projecting to other motoneurons, but that these appeared to be non-functional. This study shows that in young mice, these synapses are present and active, and that this recurrent excitation is mediated exclusively by an excitatory amino acid.
- 22.Machacek DW, Hochman S: Noradrenaline unmasks novel self-reinforcing motor circuits within the mammalian spinal cord. J Neurosci 2006, 26:5920–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talpalar AE, Endo T, Low P, Borgius L, Hagglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O: Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 2011, 71:1071–1084. [DOI] [PubMed] [Google Scholar]
- 24.Bonnot A, Chub N, Pujala A, O’Donovan MJ: Excitatory actions of ventral root stimulation during network activity generated by the disinhibited neonatal mouse spinal cord. J Neurophysiol 2009, 101:2995–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Ampatzis K, Bjornfors ER, El Manira A: Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 2016, 529:399–402.•• In this study, the authors show by using dual patch clamp recordings that a large fraction of V2a interneurons have a hybrid chemical/electrical synapse with motoneurons in the zebrafish spinal cord. Hyperpolarizing motoneurons leads to a decrease in the frequency of fictive swimming that was mediated through the retrograde voltage changes in the V2a population.
- 26.Eklof-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlen P, Higashijima S, El Manira A: Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci U S A 2012, 109:5511–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falgairolle M, Puhl JG, Pujala A, Liu W, O’Donovan MJ: Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse. eLife 2017, 6.••In the neonatal mouse spinal cord, decreasing or increasing the firing of motoneurons resulted in a decrease or an increase in frequency of drug-evoked locomotor-like activity. The authors went on to show that the effects were not abolished when blocking either cholinergic synapses or gap junctions, but only when blocking AMPA receptors.
- 28.Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T: Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci U S A 1996, 93:4235–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chopek JW, Nascimento F, Beato M, Brownstone RM, Zhang Y: Sub-populations of spinal V3 interneurons form focal modules of layered pre-motor microcircuits. Cell Rep 2018, 25:146–156 e143.••Motoneurons were known to connect synaptically to only one class of interneurons (the Renshaw cells) in the mammalian spinal cord. In this paper, the authors show that motoneurons synapse with a class of glutamatergic commissural interneurons (a subset of V3 interneurons) located in the ventral part of the spinal cord that have been shown to be involved in stabilizing fictive locomotion.
- 30.Ichinose T, Miyata Y: Recurrent excitation of motoneurons in the isolated spinal cord of newborn rats detected by whole-cell recording. Neurosci Res 1998, 31:179–187. [DOI] [PubMed] [Google Scholar]
- 31.Schneider SP, Fyffe RE: Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol 1992, 68:397–406. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S et al. : V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 2008, 60:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY: Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 2009, 461:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphreys JM, Whelan PJ: Dopamine exerts activation-dependent modulation of spinal locomotor circuits in the neonatal mouse. J Neurophysiol 2012, 108:3370–3381. [DOI] [PubMed] [Google Scholar]
- 35.Pujala A, Blivis D, O’Donovan MJ: Interactions between dorsal and ventral root stimulation on the generation of locomotor-like activity in the neonatal mouse spinal cord. eNeuro 2016, 3.•In this study, the authors show, by using stimulus summation, that motoneurons elicit fictive locomotion through a different pathway than that engaged by sensory afferents, although both inputs ultimately converge onto the same CPG.
- 36.Bertuzzi M, Chang W, Ampatzis K: Adult spinal motoneurons change their neurotransmitter phenotype to control locomotion. Proc Natl Acad Sci U S A 2018, 115:E9926–E9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert FM, Cardoit L, Courty E, Bougerol M, Thoby-Brisson M, Simmers J, Tostivint H, Le Ray D: Functional limb muscle innervation prior to cholinergic transmitter specification during early metamorphosis in Xenopus. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamotte d’Incamps B, Bhumbra GS, Foster JD, Beato M, Ascher P: Segregation of glutamatergic and cholinergic transmission at the mixed motoneuron Renshaw cell synapse. Sci Rep 2017, 7:4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochman S, Jordan LM, Schmidt BJ: TTX-resistant NMDA receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J Neurophysiol 1994, 72:2559–2562. [DOI] [PubMed] [Google Scholar]
- 40.MacLean JN, Schmidt BJ, Hochman S: NMDA receptor activation triggers voltage oscillations, plateau potentials and bursting in neonatal rat lumbar motoneurons in vitro. Eur J Neurosci 1997, 9:2702–2711. [DOI] [PubMed] [Google Scholar]
- 41.Bouhadfane M, Tazerart S, Moqrich A, Vinay L, Brocard F: Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats. J Neurosci 2013, 33:15626–15641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manuel M, Li Y, Elbasiouny SM, Murray K, Griener A, Heckman CJ, Bennett DJ: NMDA induces persistent inward and outward currents that cause rhythmic bursting in adult rodent motoneurons. J Neurophysiol 2012, 108:2991–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grillner S: Human locomotor circuits conform. Science 2011, 334:912–913. [DOI] [PubMed] [Google Scholar]