Abstract
Purpose
Iridocorneal endothelial (ICE) syndrome-related glaucoma is often refractory to medical treatment, and traditional surgical treatment has lower success rates than typical for other types of glaucoma. We present a series of patients who were treated with XEN gel stent (Allergan plc, Dublin, Ireland) implantation.
Patients and Methods
Retrospective case series of 4 patients with ICE syndrome who underwent XEN with subconjunctival mitomycin C injection.
Results
Average preoperative IOP was 28.5 mmHg on 3.8 glaucoma medications, and average postoperative IOP was 10.5 mmHg on 1.0 medication. No patients required return to the operating room for additional procedures over an average of 6.9 months of follow-up. One patient had shallow anterior chamber that resolved with conservative management. Another had shallow anterior chamber that resolved with anterior chamber reformation with viscoelastic and developed non-appositional choroidal effusions that had resolved at most recent follow-up 7 months after surgery. No XEN implants have been occluded by membrane formation or peripheral anterior synechiae.
Conclusions
XEN is a safe and effective option for surgical management of ICE syndrome-related glaucoma. Further follow-up surveillance is necessary.
Keywords: XEN gel stent, ICE syndrome, minimally invasive glaucoma surgery
Précis
This case series reports safe, effective implantation of XEN gel stents to treat ICE syndrome. The stents continue to function well and have not been occluded by membranes or PAS, but continued follow-up is necessary.
Introduction
Iridocorneal endothelial (ICE) syndrome is subdivided into Chandler syndrome, essential/progressive iris atrophy, and Cogan-Reese/iris nevus syndrome subtypes. It has a 46–82% association with glaucoma.1 Elevated intraocular pressure (IOP) in ICE syndrome is thought to be caused by abnormal corneal endothelial cells migrating over the trabecular meshwork and causing peripheral anterior synechiae (PAS). ICE syndrome-related glaucoma is typically refractory to medical therapy and laser trabeculoplasty, and surgical outcomes are often worse than those for more common types of glaucoma. Historically, the most frequent surgeries for ICE syndrome-related glaucoma have been trabeculectomy and tube shunt implantation, and multiple surgeries are frequently necessary.2–9
Insertion of a XEN gel stent (Allergan plc, Dublin, Ireland) is a minimally invasive glaucoma surgery that involves placement of a 6mm tube of porcine collagen-derived gelatin cross-linked with glutaraldehyde. The internal lumen has a diameter of 45 μm and creates an alternative drainage pathway for aqueous from the anterior chamber to the subconjunctival space. A cross-linked gelatin microfistula with an internal lumen diameter of 140 μm, which was the XEN’s predecessor, showed safe and effective intraocular pressure control through up to 6 years of follow-up.10 This prototype gel stent was implanted without antimetabolites, but the current prevailing XEN technique is to use subconjunctival mitomycin C (MMC) injection. XEN is typically placed via an ab interno approach through a clear corneal incision, though ab externo approaches have been gaining popularity.11 It is most well studied in primary open-angle and pseudoexfoliative glaucoma.12–18 However, XEN has been reported to provide successful IOP control in a variety of other conditions, including corticosteroid-response glaucoma from intravitreal dexamethasone implants, previous failed trabeculectomy and tube shunts, uveitic glaucoma, and neovascular glaucoma.19–23 To date, there has been one case report of XEN used to treat glaucoma in ICE syndrome.24 That patient had undergone previous Descemet membrane endothelial keratoplasty (DMEK) and 3 cyclophotocoagulation procedures. Adjuvant 5-fluorouracil (5-FU) and anti-vascular endothelial growth factor (anti-VEGF) were used intraoperatively and at postoperative office visits. We present a series of 4 eyes with ICE syndrome-related glaucoma that were treated with XEN and highlight potential pitfalls and pearls for successful management.
Patients and Methods
We present 4 cases of eyes with ICE syndrome that were treated with ab interno XEN implantation with subconjunctival MMC injection. In each case, careful gonioscopy in the office and/or intraoperatively helped guide XEN implantation in a clock hour of the eye that was free of PAS. The XEN injector was aimed in standard fashion slightly anterior to the trabecular meshwork in order to avoid causing excessive bleeding. In all 4 cases, no eye had severe enough PAS to preclude XEN implantation in a PAS-free zone in the superior or superonasal subconjunctival space as is standard for traditional ab interno implantation technique. Unlike the previously reported case of ICE syndrome treated with XEN, no adjuvant 5-FU or anti-VEGF were used in our patients.
Results
Table S1 summarizes the results of the 4 cases. Average preoperative IOP was 28.5 mmHg on 3.8 glaucoma medications, and average postoperative IOP was 10.5 mmHg on 1.0 medication. Mean follow-up after XEN implantation was 6.9 months.
Case 1
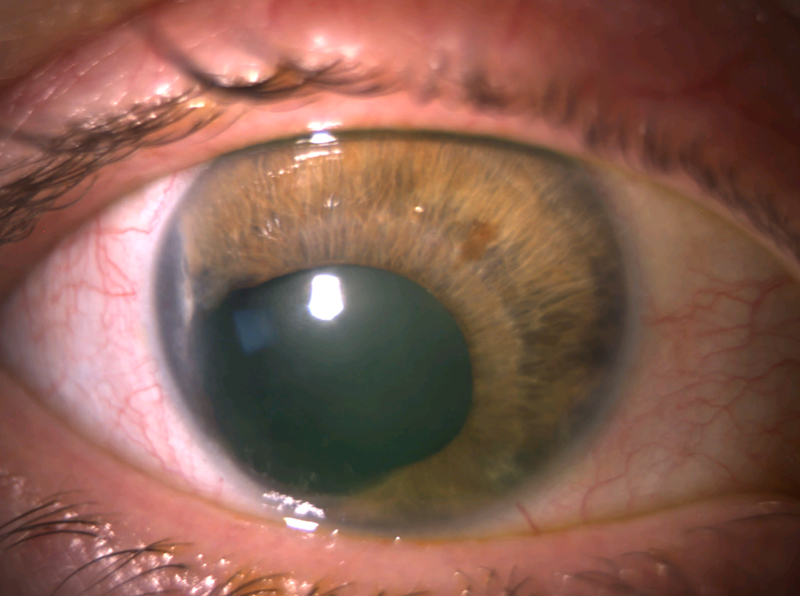
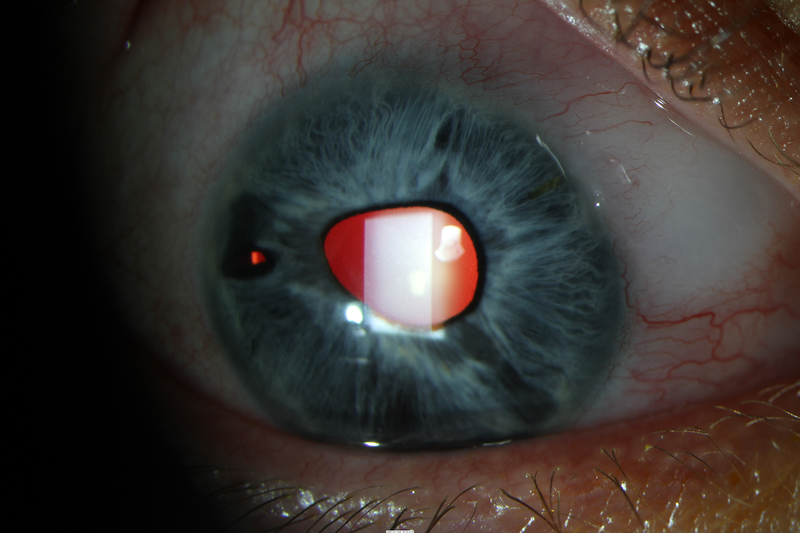
A 52-year-old phakic female with ICE syndrome of the right eye (OD) and a normal left eye (OS) was referred for uncontrolled IOP OD despite taking fixed combination dorzolamide/timolol twice daily, latanoprost nightly, and acetazolamide 250mg twice daily. She had a history of being unable to tolerate brimonidine due to excessive fatigue and sleepiness. Uncorrected visual acuity (VA) OD was 20/20, and IOP OD was 33 mmHg. Examination OD was notable for inferotemporal corectopia and iris-cornea touch temporally and inferonasally (Figure 1A). Gonioscopy revealed broad PAS throughout the angle, with 2 clock hours open to ciliary body superonasally. There was 1+ nuclear sclerosis. Cup-to-disc ratio (C/D) was 0.35 in both eyes, with an inferior notch in the optic nerve OD that corresponded to optical coherence tomography (OCT) of the retinal nerve fiber layer showing inferior thinning OD and visual field testing demonstrating an early superior arcuate visual field defect OD. The patient consented to XEN with subconjunctival MMC injection OD.
Figure 1.
Anterior segment photos of case 1. A) Preoperative photo showing corectopia extending inferotemporally. B) Postoperative month 4 photo showing superonasal XEN.
On postoperative day 1, VA was 20/20, and IOP was 3 mmHg. Examination revealed an elevated bleb that involved 360 degrees of conjunctiva, a well-positioned XEN, clear cornea, deep anterior chamber with 1+ cell and 1 mm hyphema, and no choroidal detachment. The patient was started on difluprednate and ofloxacin 4 times daily. On postoperative day 6, IOP remained 3 mmHg, and the bleb was now confined to the superonasal quadrant. There was only trace hyphema remaining, and ofloxacin was discontinued. At postoperative day 12, IOP increased to 8 mmHg, aqueous suppression with fixed combination dorzolamide/timolol once daily was started, and a difluprednate taper decreasing 1 drop every 2 weeks was initiated. The patient was seen locally in the interim, and the dorzolamide/timolol was increased to twice daily due to IOP of 18 mmHg. Figure 1B shows the eye at postoperative month 4. At most recent follow-up 10 months after surgery, uncorrected VA was 20/20, and IOP was 10 mmHg on fixed combination dorzolamide/timolol.
Case 2
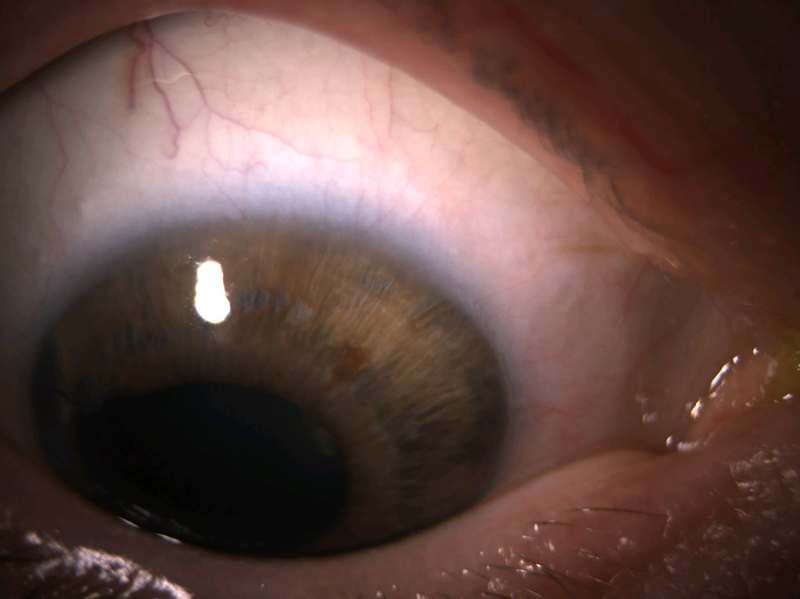
A 34-year-old phakic female with Chandler syndrome OD was referred for uncontrolled IOP. She had been diagnosed 7 years prior to presentation, had poor adherence to suggested follow-up visits scheduled with her local ophthalmologist, and reported a maximum recorded IOP of 66 mmHg. She was taking brinzolamide once daily, fixed combination brimonidine/timolol twice daily, and latanoprost nightly. Initial examination showed corrected VA of 20/50 with pinhole improvement to 20/20, IOP 19 mmHg, 2+ relative afferent pupillary defect OD, significant guttae, thinning of a blue iris, clear lens, and open-angle on gonioscopy but with 3 discrete isolated high PAS temporally and at 5, 6, and 11 o’clock. C/D was 0.65 with a sharp notch inferiorly OD, 0.1 OS. OCT showed diffuse thinning OD, and visual field testing showed a dense superior nasal step that was developing into a superior arcuate defect. Gonioscopy-assisted transluminal trabeculotomy (GATT) was recommended, with the thought that the mild PAS would not interfere, and the procedure would clear any membrane that had formed over the trabecular meshwork. However, intraoperatively, GATT was difficult to complete despite mild PAS, and IOP remained uncontrolled at 25 mmHg despite addition of fixed combination brimonidine/timolol and latanoprost. The patient underwent XEN with subconjunctival MMC injection 6 months after GATT.
On postoperative day 1, VA was 20/80, with pinhole improvement to 20/40. IOP was 3 mmHg. Examination showed a 360-degree bleb, grade 1 anterior chamber shallowing, and no choroidal detachment. The XEN was resting against the iris but was not occluded. One drop of cyclopentolate was administered in the clinic, and the patient was instructed to use difluprednate and ofloxacin 4 times daily. On postoperative day 4, IOP remained 3 mmHg, but the diffuse bleb had become more focal superonasally, and the anterior chamber was now only grade 0 shallow. By postoperative week 3, IOP had risen to 14 mmHg, the anterior chamber was deep, and difluprednate was tapered to twice daily for 1 week and once daily for 1 week before stopping. Figure 2 shows the eye at postoperative month 2. At most recent follow-up 5 months after XEN, corrected VA was 20/25, IOP was 13 mmHg, and there was a bleb with favorable morphology graded as H3E3V1S0 on the Indiana Bleb Appearance Grading Scale.25 The patient continues on fixed combination dorzolamide/timolol aqueous suppression once daily. The XEN remained close to the iris but was not occluded, and repeat gonioscopy revealed significant PAS superotemporally, inferotemporally, and superiorly near the XEN, while the nasal angle remained open with D40f Spaeth configuration.
Figure 2.
Anterior segment photo of case 2 at postoperative month 2. Arrow points to XEN and its bleb.
Case 3
An 80-year-old pseudophakic female with history of DMEK for ICE syndrome OD and bilateral pseudoexfoliation glaucoma was referred for decompensating DMEK and uncontrolled IOP of 26 mmHg despite using fixed combination latanoprost/timolol and brimonidine/brinzolamide. VA was 20/125. Extensive patchy PAS was noted on gonioscopic examination. C/D was 0.3 OD, 0.2 OS. The patient underwent XEN surgery without gonioscopic guidance, as the cornea was too hazy for an adequate view. Two passes of the XEN injector needle through the sclera were created because the first insertion exited too anteriorly. A small amount of Healon GV (Johnson & Johnson, Santa Ana, CA) was left in the anterior chamber at the conclusion of the surgery to prevent hypotony.
On postoperative day 1, IOP was 6 mmHg. On postoperative day 5, IOP decreased to 1 mmHg, and there was a shallow anterior chamber accompanied by choroidal effusion. Healon was used to reform the anterior chamber. The patient improved with tapering use of fixed combination prednisolone/phenylephrine and atropine, though non-appositional choroidal effusions persisted for over 6 months. At most recent follow-up 7 months after surgery, VA was 20/160, IOP was 7 mmHg on atropine 1% daily and prednisolone acetate 1% daily, the anterior chamber was deep, the choroidal effusions had resolved, and there was no hypotony maculopathy (Figure 3).
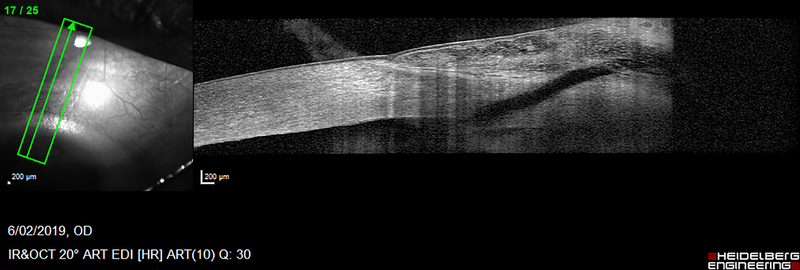
Figure 3.
Images of case 3 at postoperative month 7. A) Anterior segment photo showing diffuse bleb. B) Anterior segment optical coherence tomography image, with hyporeflective XEN stent draining to bleb.
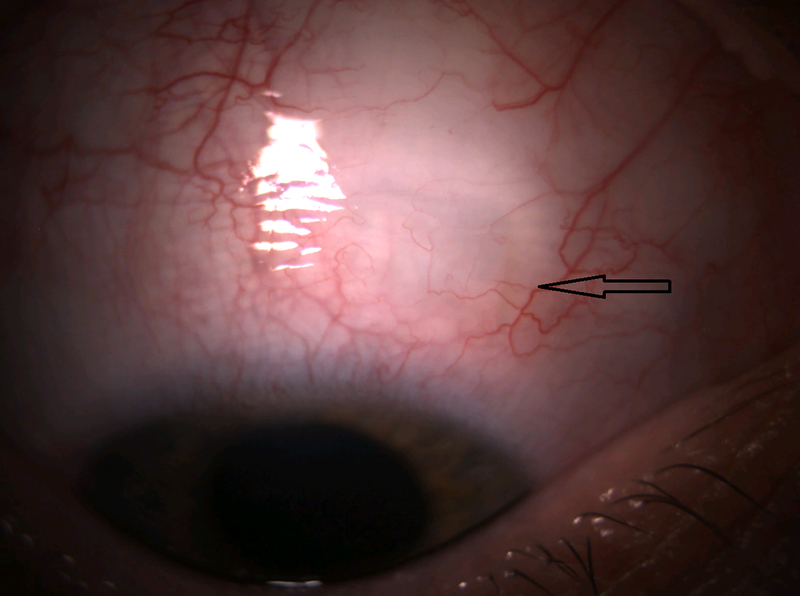
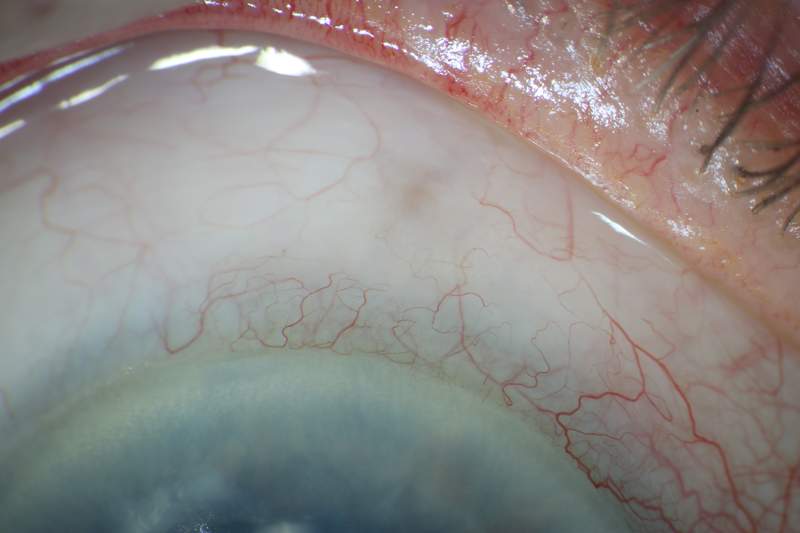
Case 4
A 24-year-old phakic male with Prader-Willi syndrome and ICE syndrome OD presented with initial IOP 62 mmHg OD. VA was 20/30. Examination showed sectoral PAS nasally and temporally, sectoral iris atrophy, and C/D 0.6 OD and 0.1 OS. He was successfully treated with timolol, brimonidine, and latanoprost. At subsequent visits, IOP fluctuated from 13 to 23 mmHg on an increasing medication regimen of timolol, brimonidine, brinzolamide, and bimatoprost. At 3 years after initial presentation, IOP increased to 30 mmHg, and examination showed increasing PAS, progressive corectopia, and increasing optic nerve rim loss inferiorly and superiorly that was confirmed by Heidelberg Retinal Tomography (Heidelberg Engineering, Heidelberg, Germany). The patient underwent XEN implantation with 11 μg MMC, with the XEN passing through Schwalbe’s line superonasally under gonioscopic control avoiding PAS.
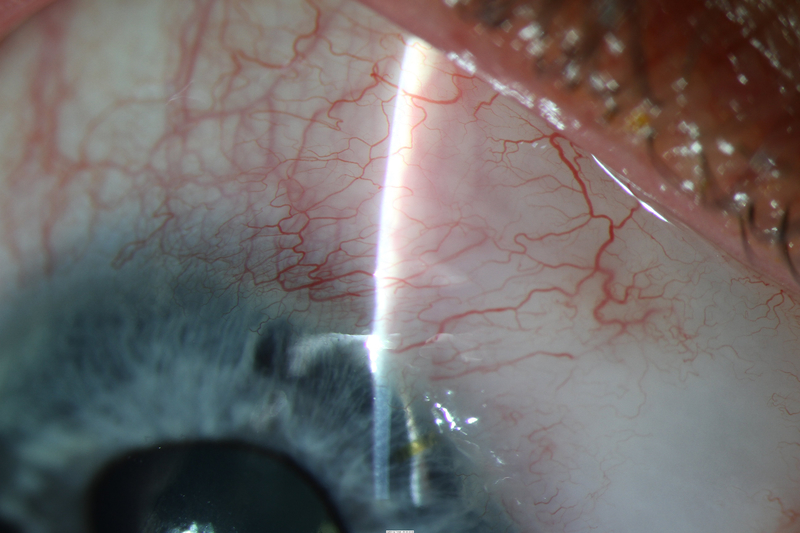
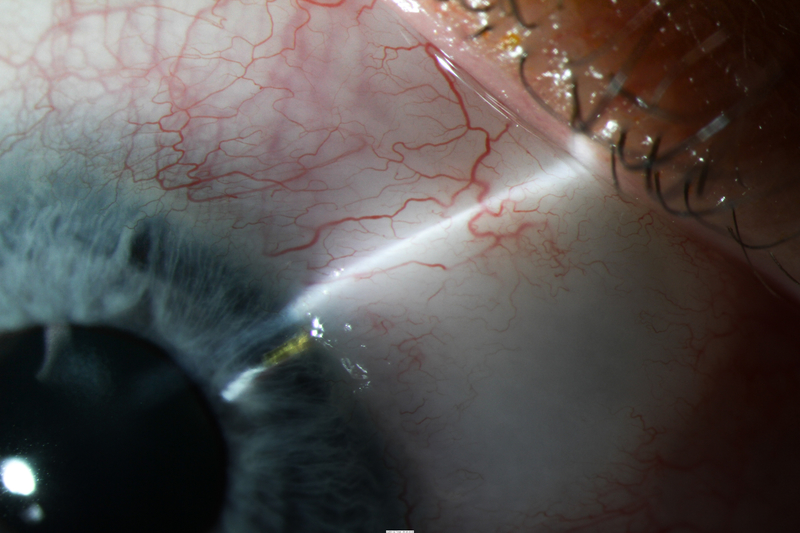
After surgery, IOP ranged from 11 to 16 mmHg while the patient was completing a routine postoperative taper of fixed combination prednisolone/phenylephrine, ketorolac, and chloramphenicol. IOP was 12 mmHg on no glaucoma medications at most recent follow-up 5.5 months postoperatively, with favorable bleb morphology (Figure 4).
Figure 4.
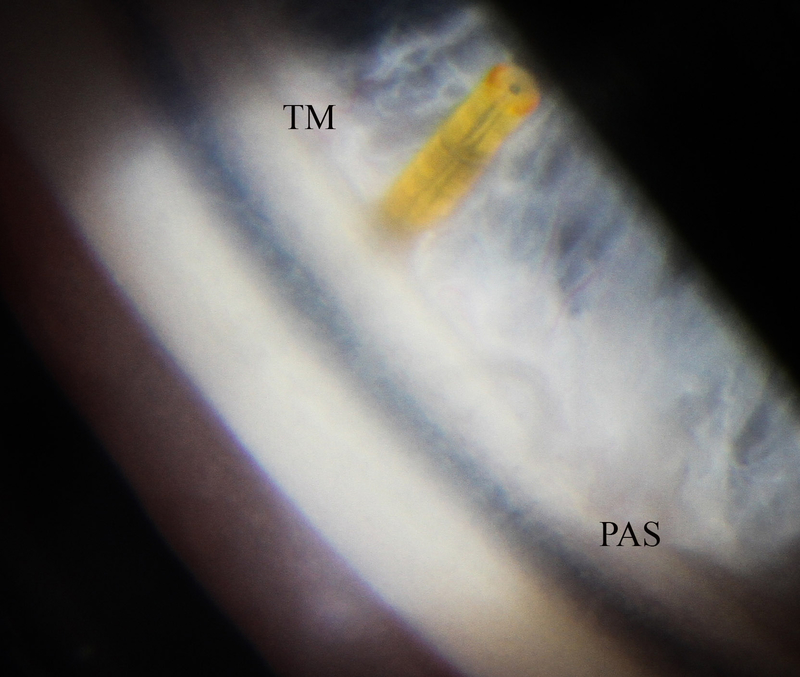
Photos of case 4 at postoperative month 5.5. A) Temporal iris atrophy and superonasal XEN bleb. B) and C) Slit lamp beams through XEN and bleb. D) Gonioscopic photograph of XEN placed such that it avoids nearby peripheral anterior synechiae. TM = trabecular meshwork. PAS = peripheral anterior synechiae.
Discussion
These 4 cases illustrate that XEN with MMC can be used successfully to treat uncontrolled IOP in ICE syndrome-related glaucoma. Unlike the one case that has previously been reported, our cases did not use adjuvant 5-FU or anti-VEGF. Failure of trabeculectomy in ICE syndrome has been thought to be due to occlusion of the ostium by a membrane or PAS, but as of latest follow-up in this series, no membrane has grown over the XEN lumens, and PAS has not worsened at the implant site or caused any XEN migration. Additionally, there has been no bleb scarring that has required surgical revision, but average follow-up for this series has been 6.9 months. In case 1, with the IOP rising slightly since surgery, the patient is being monitored closely, and the plan is to avoid manipulation of the eye if possible because procedures such as needling may cause inflammation and increased chance of membrane development covering the XEN. One surgeon (M.R.M.) prefers to keep patients on chronic aqueous suppression once IOP has reached 8 mmHg; the goal of aqueous suppressants is to decrease the volume of aqueous and number of inflammatory cells that the bleb is exposed to, which may enhance the survival of the bleb.
Cases 1 and 2 illustrate that early postoperative numerical hypotony of 3 mmHg can occur after XEN and may be well tolerated and managed conservatively. Low IOP in the immediate postoperative period may be due to peritubular flow or decreased aqueous production. Case 3 demonstrates that while XEN is thought to be a safer alternative to trabeculectomy, shallow anterior chamber and choroidal effusion can result. In this patient’s case, there were two full-thickness passes through the sclera with the XEN injector because the first pass was too anterior in the setting of poor visualization due to a failing DMEK graft. While the XEN limited flow in the second needle tract, the first needle tract may have allowed excessive unguarded aqueous flow in the early postoperative period before scarring ensued, though it would be expected that this extra tract would seal quickly, even in an eye that has been treated with MMC. However, chronic topical steroid due to history of DMEK may also have contributed to slower wound healing. It is atypical that the choroidal effusion in case 3 persisted for over 6 months, but other than hypotony and increased age of 80 years, there were no other specific risk factors; the patient did not have a history of nanophthalmos, uveitis, trauma, neoplasm, venous congestion, or predisposing systemic medication use.
Our series provides evidence to suggest that XEN may be used in more circumstances than those specifically outlined in the directions for use, which recommend the device for primary open-angle, pseudoexfoliative, and pigmentary glaucoma, but not angle-closure glaucoma where the angle has not been surgically opened.26 Despite PAS in our ICE syndrome cases, there were uninvolved areas where the XEN could be placed safely in the superonasal quadrant, and the implants remained well positioned in the postoperative period. XEN has also been reported to be successful in a case of neovascular glaucoma, and the authors were careful to avoid areas of PAS to prevent bleeding.22 Perhaps with use of viscoelastic and mechanical stretching to perform visco gonio synechiolysis in eyes with chronic angle-closure, XEN can also find judicious use in certain cases of angle-closure glaucoma.
Of note, some surgeons have adopted an ab externo approach to XEN implantation. However, in cases of ICE syndrome, surgical success may depend on careful placement of the XEN to avoid PAS, and this may be better achieved with a traditional ab interno approach combined with intraoperative gonioscopy, rather than an ab externo approach without the benefit of such visualization. While the simplicity and ease of the ab externo approach is appealing, the major drawback in cases of ICE is that the XEN injector may enter the anterior chamber through an area of PAS. This would require repositioning the injector in order to deploy the XEN in an area free of PAS, and the first scleral pass would pose a risk for complications of hypotony, as demonstrated by case 3 in our series.
The cases in this series will require continued close surveillance to monitor for development of XEN occlusion or bleb scarring, but XEN has performed well in ICE syndrome to date.
Supplementary Material
Table S1. (Supplemental) Summary of results for four cases of XEN gel stent implantation for iridocorneal endothelial syndrome-related glaucoma. IOP = intraocular pressure.
References
- 1.Silva L, Najafi A, Suwan Y, et al. The iridocorneal endothelial syndrome. Surv Ophthalmol. 2018;63(5):665–676. [DOI] [PubMed] [Google Scholar]
- 2.Shields MB, Campbell DG, Simmons RJ. The essential iris atrophies. Am J Ophthalmol. 1978;85(6):749–59. [DOI] [PubMed] [Google Scholar]
- 3.Kidd M, Hetherington J, Magee S. Surgical results in iridocorneal endothelial syndrome. Arch Ophthalmol. 1988;106(2):199–201. [DOI] [PubMed] [Google Scholar]
- 4.Wright MM, Grajewski AL, Cristol SM, et al. 5-Fluorouracil after trabeculectomy and the iridocorneal endothelial syndrome. Ophthalmology. 1991;98(3):314–6. [DOI] [PubMed] [Google Scholar]
- 5.Laganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Arch Ophthalmol. 1992;110(3):346–50. [DOI] [PubMed] [Google Scholar]
- 6.Kim DK, Aslanides IM, Schmidt CM Jr, et al. Long-term outcome of aqueous shunt surgery in ten patients with iridocorneal endothelial syndrome. Ophthalmology. 1999;106(5):1030–4. [DOI] [PubMed] [Google Scholar]
- 7.Doe EA, Budenz DL, Gedde SJ, et al. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology. 2001;108(10):1789–95. [DOI] [PubMed] [Google Scholar]
- 8.Chandran P, Rao HL, Mandal AK, et al. Outcomes of Primary Trabeculectomy With Mitomycin-C in Glaucoma Secondary to Iridocorneal Endothelial Syndrome. J Glaucoma. 2016;25(7):e652–6. [DOI] [PubMed] [Google Scholar]
- 9.Chandran P, Rao HL, Mandal AK, et al. Glaucoma associated with iridocorneal endothelial syndrome in 203 Indian subjects. PLoS One. 2017;12(3):e0171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan WH, Quill B, Cringle SJ, et al. Long-Term Results Using Gelatin Microfistulae Implantation without Antimetabolite. Ophthalmology. 2018;125(11):1828–1829. [DOI] [PubMed] [Google Scholar]
- 11.Myers JS, Zheng CX, Moster SJ, Lin MM. Short term outcomes of transconjunctival ab externo XEN 45 Gel Stent implantation. Association for Research in Vision and Ophthalmology Annual Meeting 2019 April 30, 2019 (Poster) [Google Scholar]
- 12.Grover DS, Flynn WJ, Bashford KP, et al. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am J Ophthalmol. 2017;183:25–36. [DOI] [PubMed] [Google Scholar]
- 13.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, Safety, and Risk Factors for Failure of Standalone Ab Interno Gelatin Microstent Implantation versus Standalone Trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. [DOI] [PubMed] [Google Scholar]
- 14.De Gregorio A, Pedrotti E, Russo L, et al. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38(3):1129–1134. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri K, Gillmann K, Rao HL, et al. Prospective Evaluation of XEN Gel Implant in Eyes With Pseudoexfoliative Glaucoma. J Glaucoma. 2018;27(10):869–873. [DOI] [PubMed] [Google Scholar]
- 16.Widder RA, Dietlein TS, Dinslage S, et al. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):765–771. [DOI] [PubMed] [Google Scholar]
- 17.Ibáñez-Muñoz A, Soto-Biforcos VS, Chacón-González M, et al. One-year follow-up of the XEN® implant with mitomycin-C in pseudoexfoliative glaucoma patients. Eur J Ophthalmol. 2018:1120672118795063 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Heidinger A, Schwab C, Lindner E, et al. A Retrospective Study of 199 Xen45 Stent Implantations From 2014 to 2016. J Glaucoma. 2019;28(1):75–79. [DOI] [PubMed] [Google Scholar]
- 19.Rezkallah A, Mathis T, Denis P, et al. XEN Gel Stent to Treat Intraocular Hypertension After Dexamethasone-Implant Intravitreal Injections: 5 Cases. J Glaucoma. 2019;28(1):e5–e9. [DOI] [PubMed] [Google Scholar]
- 20.Karimi A, Hopes M, Martin KR, et al. Efficacy and Safety of the Ab-interno Xen Gel Stent After Failed Trabeculectomy. J Glaucoma. 2018;27(10):864–868. [DOI] [PubMed] [Google Scholar]
- 21.Sandhu S, Dorey MW. Case Report: Xen Ab Interno Gel Stent Use in a Refractory Glaucoma Patient With Previous Filtration Surgeries. J Glaucoma. 2018;27(3):e59–e60. [DOI] [PubMed] [Google Scholar]
- 22.Sng CC, Wang J, Hau S, et al. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2018;46(4):339–345. [DOI] [PubMed] [Google Scholar]
- 23.Tailor R, Lalias T. A Case of Refractory Neovascular Glaucoma Treated With a XEN 45 Implant. J Glaucoma. 2018;27(10):929–930. [DOI] [PubMed] [Google Scholar]
- 24.Hohberger B, Welge-Lüen UC, Lämmer R. ICE-Syndrome: A Case Report of Implantation of a Microbypass Xen Gel Stent After DMEK Transplantation. J Glaucoma. 2017;26(2):e103–e104. [DOI] [PubMed] [Google Scholar]
- 25.Cantor LB, Mantravadi A, WuDunn D, et al. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12(3):266–71. [DOI] [PubMed] [Google Scholar]
- 26.Directions for the use for the XEN glaucoma treatment system. Available at: https://allergan-web-cdn-prod.azureedge.net/actavis/actavis/media/allergan-pdf-documents/labeling/xen/dfu_xen_glaucoma_treatment_system_us_feb2017.pdf. Accessed March 3, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. (Supplemental) Summary of results for four cases of XEN gel stent implantation for iridocorneal endothelial syndrome-related glaucoma. IOP = intraocular pressure.