Abstract
Purpose
To examine associations between near vision impairment (NVI) and frailty
Design
Cross-sectional study
Methods
Setting
Nationally representative sample of non-institutionalized United States civilians
Study Population
2,705 older adults ≥60 years from National Health and Nutrition Examination Survey (1999–2002)
Observation
Presenting NVI (PNVI)-near acuity worse than 20/40. Self-reported NVI (SNVI)-self-reported difficulty with near vision tasks
Main Outcome Measure(s)
5-item physical frailty; participants classified as frail (≥3 criteria) and pre-frail (1 or 2 criteria). Propensity score adjusted, and probability-weighted multinomial multivariable logistic regression was used to examine associations of PNVI and SNVI with frailty.
Results
Of 2,705 participants, 10%, 5%, and 3% had PNVI only, SNVI only, and PNVI+SNVI, respectively. In fully adjusted models, as compared to those without PNVI, participants with PNVI were more likely to be prefrail (OR=1.6; 95% CI=1.1,2.3) and frail (OR=2.5; 95% CI=1.4,4.3). As compared to those without SNVI, participants with SNVI were more likely to be prefrail (OR=2.9; 95% CI=1.8,4.7) and frail (OR=4.3; 95% CI=2.2,8.3). As compared to those without PNVI or SNVI, participants with PNVI+SNVI were more likely to be prefrail and frail (prefrail: OR=4.0; 95% CI=2.2,7.2 and frail: OR=4.5; 95% CI=1.7,12.7).
Conclusions
Older adults with PNVI and SNVI were more likely to be pre-frail and frail than those without respective NVI, suggesting that NVI is associated with frailty.
INTRODUCTION
Near vision impairment (NVI), broadly considered as difficulty with near vision tasks at a reading distance, and commonly defined as near vision of 20/40 or worse,1,2 increases with age.3 NVI is most commonly caused by presbyopia,4 a universal age-related diminution in elasticity and accommodative ability of the lens.5 Despite being easily correctable with reading glasses, NVI from presbyopia was estimated to affect 1.1 billion people globally in 2015, of whom 667 million (about 60%) were aged 50 years and older.6 While much less common, ocular diseases that impair distance vision, like cataract and age-related macular degeneration, among others, can also reduce near vision. Irrespective of whether NVI is related to presbyopia or other causes, it appears to play an important role on quality of life.7–10
Several studies have shown that NVI is associated with poorer vision and health-related quality of life.7,8,11 Furthermore, NVI is associated with decreased function across multiple functioning domains, including physical, cognitive, and psychosocial.12–14 Keller et al, reported that participants with NVI showed diminished functional status on Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) performance.12 NVI is also associated with greater cognitive decline,13 and worsening NVI is associated with greater incidence of nursing home residence and having 2 or more falls14. While these data collectively indicate that NVI may be reflecting several physiologic systems beyond visual functioning, research examining the impact of NVI on aging outcomes is limited.
Frailty is a geriatric syndrome characterized by multisystem dysfunction, decreased reserve and resistance to stressors, conferring a higher vulnerability to adverse health outcomes, including morbidity and mortality. Studies examining the relationship between vision impairment and frailty have, to date, largely focused on distance vision.16,17 There is a paucity of literature evaluating the relationship of NVI with frailty, despite evidence suggesting that NVI has many of the deleterious effects of distance vision impairment on quality of life.10 The only prior study to have examined the relationship between near vision and frailty found no association.18 However, it was conducted in a geriatric frailty clinic where most participants were pre-frail/frail, and therefore, may not have had a sufficient number of non-frail (i.e. robust) subjects to adequately examine the effect of vision on frailty status.
We sought to build on limited existing literature by using a large, nationally representative sample from the National Health and Nutrition Examination Survey (NHANES) to study the association between NVI, both presenting NVI and self-reported NVI, and frailty. We hypothesized that older adults with NVI are more likely to be frail than similarly-aged peers without NVI.
METHODS
Study Population
The NHANES utilizes cross-sectional probability samples of the United States (US) civilian non-institutionalized population, conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS), and comprises an interview and physical examination.19 The NCHS Institutional Review Board (IRB) prospectively approved all NHANES protocols, obtained written informed consent from all participants, and data were de-identified and publicly available. We limited our study population to older adults ≥60 years from NHANES cycles 1999–2002 with near vision and frailty data. The study sample included older adults eligible to have walking speed collected (one of the frailty criteria) (n=2,781 ), of whom 2.7% (n=76) were missing frailty or vision data, providing an analytic sample of 2,705.
Outcome Measure
The 5-item frailty phenotype, created by Fried et al comprises the following criteria.15 Individuals were considered pre-frail if they met 1 or 2 items, and frail if they met ≥3 items.
Poor endurance and energy, defined by the response “some difficulty” or “much difficulty” when participants were asked how much difficulty they have walking from one room to another on the same level by themselves without using special equipment.
Weakness, defined by the response “some difficulty”, “much difficulty”, or “unable to do” when asked how much difficulty they have lifting or carrying something as heavy as 10 pounds like a sack of potatoes or rice.
Low physical activity defined by the response “less active” when asked how active they were as compared to most men/women their age.
Shrinking, defined as ≥10 lb or ≥5% unintentional weight loss over 1 year based on self-report or body mass index (BMI) <18.5 kg/m2.
Slowness, defined as the slowest 20% of time completing a 20 ft walk, adjusted for sex and standing height.
Vision Measures
Presenting near vision impairment (PNVI)
Participants underwent presenting near vision testing using a near card with the following 5 lines: 20/400, 20/200, 20/63, 20/40, and 20/25. While wearing their usual near correction (if available) and keeping both eyes open, participants held the near chart at a comfortable reading distance of their choosing (measured from the center of the card to the examinee’s brow using a measuring tape; median: 16 inches, IQR: 14−18, range: 9−28 inches), and began reading at the 20/400 line. Participants continued to the next line if they correctly identified at least 4 out of 5 items on a line and presenting near vision acuity was recorded as the smallest line on which 4 of 5 items were correctly read. For analysis, PNVI was defined as presenting near vision worse than 20/40.
Self-reported near vision impairment (SNVI)
Participants answered two questions pertaining to difficulty in performing near vision tasks, based on the National Eye Institute (NEI) Visual Functioning Questionnaire (VFQ- 25).20 They were queried on how much difficulty they had 1) reading ordinary newsprint, and 2) doing work or hobbies requiring them to see well up close such as cooking, sewing, fixing things around the house, or using hand tools, while wearing routine refractive correction, if any. Response options included “no”, “a little”, “moderate”, or “extreme” difficulty, or “unable to do because of eyesight”. For analysis, SNVI was defined as a response of “moderate”, or “extreme” difficulty, or “unable to do because of eyesight” for either question.
Near vision impairment (NVI)
A third NVI variable combining PNVI and SNVI was coded as follows- 1) no PNVI or SNVI, 2) PNVI only, 3) SNVI only, and 4) both PNVI and SNVI present, in order to examine the association with isolated PNVI and SNVI, and co-existing presenting and self-reported vision impairments.
Other Measures
Other covariates collected included age, sex, race (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other Hispanic/other race), education (<high school, high school/equivalent, >high school), smoking, self-reported diabetes, and total number of self-reported comorbidities (0, 1–2, >2), which included angina, arthritis, cancer, congestive heart failure, coronary heart disease, high blood pressure, liver disease, myocardial infarct, and stroke.
Statistical Analysis
Socio-demographic and health characteristics were summarized across groups with and without PNVI and SNVI using Chi-squared and t-tests, and across the 4 NVI groups using Chi-squared tests and ANOVA (unweighted frequencies and weighted percentages reported). Propensity scores were used to ensure balancing of potential confounders, age in particular, across the groups with and without PNVI, SNVI, and NVI.21 Logistic regression models including variables hypothesized to be associated with frailty (age [cubic spine], sex, age*sex interaction term, race, BMI, education, smoking, diabetes, marital status, and each comorbid condition) were used to estimate the propensity scores as the predicted probability of a participant having PNVI, SNVI, and NVI. Based on data visualization (histograms and stem-and-leaf plots of the distribution of predicted probabilities) 18 participants were excluded from the PNVI model, 25 from the SNVI model, and 95 from the NVI model due to non-overlapping areas of predicted probability scores. In order to further restrict the analyses to participants with overlapping propensity score ranges across groups and to temper the impact of outliers, we implemented a 1% winsorizing procedure to reassign individuals above the 99.5th percentile and below the 0.5th percentile to the 99.5th and 0.5th percentiles, respectively.22 These truncated weights were used to fit the regression models.
Inverse probability weighted (survey weights*inverse propensity scores), multinomial, survey logistic regression models were used to examine associations of PNVI, SNVI, and NVI with frailty, adjusting for age (cubic spline), sex, race, education, smoking, diabetes, and total number of comorbidities. Additionally, a fourth model was used to evaluate the linear association of near visual acuity, as assessed as a numeric score, with log odds of frailty. Covariates were included based on clinical relevance and/or previous demonstration of impact on vision impairment and frailty. This method was used to simultaneously add independent variables to the regression models.
In sensitivity analyses, model results were similar with the inclusion of education, income or income and education. For parsimony, education was included in final models. All analyses accounted for the NHANES’ complex survey design, including stratification, oversampling, and non-response, and appropriate study-specific survey weights were applied. Taylor series linearization method was used for variance estimation. All analyses were conducted in STATA 14 (StataCorp, College Station, TX) and SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Population Characteristics
In this study population of 2,705 older adults, 82% had no PNVI or SNVI (n=2058), 10% had PNVI only (n=381), 5% had SNVI only (n=160), and 3% had both PNVI and SNVI (n=106). As compared to those without PNVI, participants with PNVI were older (71 ± 8 vs. 73 ± 8), less likely to be Non-Hispanic White (85% vs. 66%), more likely to have less than a high school education (26% vs. 57%), self-report diabetes (14% vs. 20%), and have a higher number of comorbid conditions (20% vs. 22% with >2 comorbidities) (p<0.01 for all), Table 1a. Similarly, when compared to those without SNVI, participants with SNVI were older (71 ± 8 vs. 72 ± 8), less likely to be Non-Hispanic White (84% vs. 72%), more likely to have less than a high school education (28% vs. 49%), and more likely to self-report diabetes (14% vs. 25%), and have a higher number of comorbid conditions (18% vs. 33% with >2 comorbidities) (p<0.01 for all), Table 1b. Supplemental Table 1c (Supplemental Material available at AJO.com) shows population demographics by the four NVI groups.
Table 1a:
Participant demographics by presenting near vision impairment status
| Total N= 2658 | No PNVIa n= 2171 (87%) | PNVIb n= 487 (13%) | P value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 71 (8) | 71 (8) | 73 (8) | <0.001 |
| Age in years, n (%) | ||||
| ≥60 to <65 | 695 (28) | 603 (29) | 92 (19) | |
| ≥65 to <70 | 555 (24) | 473 (25) | 82 (18) | |
| ≥70 to <75 | 521 (19) | 417 (18) | 104 (21) | <0.001 |
| ≥75 to <80 | 361 (15) | 287 (14) | 74 (17) | |
| ≥80 | 526 (14) | 391 (13) | 135 (25) | |
| Female, n (%) | 1328 (56) | 1108 (56) | 220 (52) | 0.23 |
| Race, n (%) | ||||
| Non-Hispanic White | 1542 (83) | 1340 (85) | 202 (66) | |
| Non-Hispanic Black | 441 (7) | 328 (7) | 113 (15) | <0.001 |
| Mexican American | 521 (3) | 389 (2) | 132 (5) | |
| Other Hispanics/other race | 154 (7) | 114 (6) | 40 (14) | |
| Education, n (%) | ||||
| < High school | 1101 (30) | 787 (26) | 314 (57) | |
| High school or equivalent | 631 (30) | 549 (31) | 82 (20) | <0.001 |
| > High school | 921 (40) | 831 (43) | 90 (23) | |
| Smoking, n(%) | ||||
| Never | 1256 (62) | 1000 (62) | 256 (65) | |
| Former | 549 (28) | 458 (29) | 91 (26) | 0.77 |
| Current | 170 (9) | 138 (9) | 32 (9) | |
| Diabetes, n (%) | 455 (15) | 338 (14) | 117 (20) | 0.005 |
| Total comorbid conditionsc, n (%) | ||||
| 0 comorbidities | 542 (20) | 444 (20) | 98 (17) | |
| 1–2 comorbidities | 1611 (60) | 1322 (60) | 289 (61) | 0.45 |
| >2 comorbidities | 505 (20) | 405 (20) | 100 (22) |
Note. Unweighted n (weighted %), Bold font: p<0.05
Presenting near visual acuity ≤20/40
Presenting near visual acuity >20/40
Total comorbid conditions include: angina, arthritis, cancer, congestive heart failure, coronary heart disease, high blood pressure, liver disease, myocardial infarct, and stroke.
Abbreviations: PNVI= presenting near visual impairment
Table 1b:
Participant demographics by self-reported near vision impairment statusa
| Total N= 2663 | No SNVI n= 2397 (92%) | SNVI n= 266 (8%) | P value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 71 (8) | 71 (8) | 72 (8) | 0.001 |
| Age in years, n (%) | ||||
| ≥60 to <65 | 700 (28) | 643 (29) | 57 (19) | |
| ≥65 to <70 | 555 (24) | 501 (24) | 54 (24) | |
| ≥70 to <75 | 521 (19) | 473 (19) | 48 (18) | 0.008 |
| ≥75 to <80 | 360 (15) | 325 (14) | 35 (15) | |
| ≥80 | 527 (14) | 455 (13) | 72 (25) | |
| Female, n (%) | 1330 (55) | 1201 (55) | 129 (58) | 0.48 |
| Race, n (%) | ||||
| Non-Hispanic White | 1548 (83) | 1425 (84) | 123 (72) | |
| Non-Hispanic Black | 440 (7) | 383 (7) | 57 (12) | 0.002 |
| Mexican American | 521 (3) | 454 (3) | 67 (4) | |
| Other Hispanics/other race | 154 (7) | 135 (6) | 19 (12) | |
| Education, n (%) | ||||
| < High school | 1092 (30) | 933 (28) | 159 (49) | |
| High school or equivalent | 638 (30) | 587 (30) | 51 (26) | <0.001 |
| > High school | 928 (40) | 872 (42) | 56 (25) | |
| Smoking, n (%) | ||||
| Never | 1257 (62) | 1143 (63) | 114 (58) | |
| Former | 555 (29) | 489 (28) | 66 (32) | 0.45 |
| Current | 168 (9) | 148 (9) | 20 (10) | |
| Diabetes, n (%) | 453 (15) | 384 (14) | 69 (25) | <0.001 |
| Total comorbid conditionsb, n (%) | ||||
| 0 comorbidities | 548 (20) | 514 (21) | 34 (8) | |
| 1–2 comorbidities | 1619 (60) | 1456 (61) | 163 (59) | <0.001 |
| >2 comorbidities | 496 (20) | 427 (18) | 69 (33) |
Note. Unweighted n (weighted %), Bold font: p<0.05
Based on self-reported difficulty reading newsprint and/or doing work or hobbies requiring them to see well up close
Comorbid conditions include: angina, arthritis, cancer, congestive heart failure, coronary heart disease, high blood pressure, liver disease, myocardial infarct, and stroke.
Abbreviations: SNVI= self-reported near visual impairment
Distribution of Frailty by Vision Impairment Status
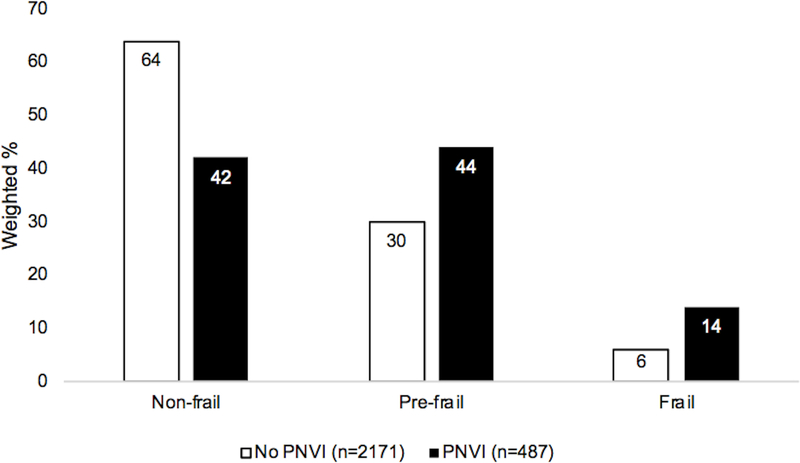
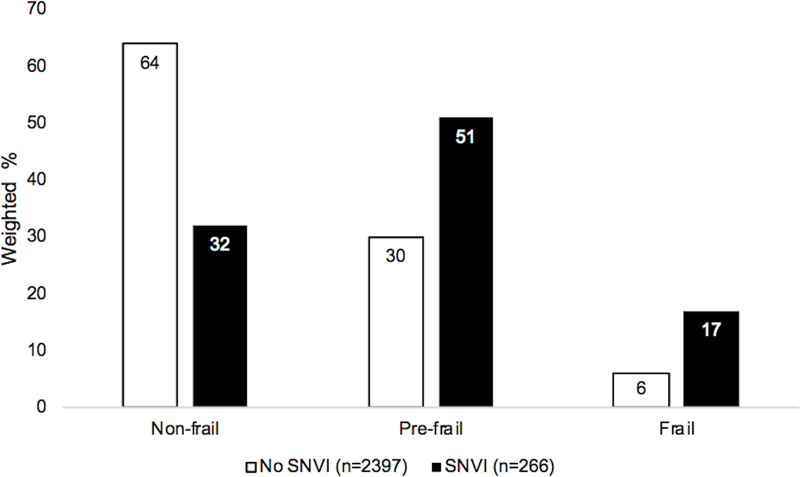
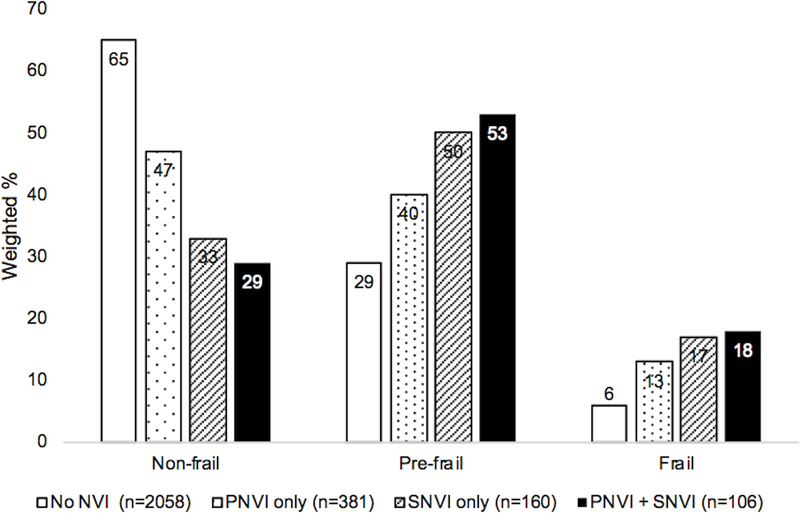
In the PNVI group, as compared to the group without PNVI, a lower proportion of individuals were non-frail (42% vs. 64%), and a higher proportion were prefrail and frail (44% vs. 30%, 14% vs. 6%, respectively, chi-squared p<0.001), Figure 1. Similarly, in the SNVI group, as compared to the group without SNVI, a lower proportion of individuals were non-frail (32% vs. 64%) and a higher proportion were prefrail and frail (51% vs. 30%, 17% vs. 6%, respectively, p<0.001), Figure 2. Those with PNVI only, SNVI only, and combined PNVI and SNVI, were less likely to be non-frail and more likely to be prefrail and frail, as compared to the group without PNVI and SNVI, Figure 3.
Figure 1. Frailty status by presenting near vision impairment.
Groups: No PNVI= near visual acuity 20/40 or better, PNVI= near visual acuity worse than 20/40
* chi-squared p<0.001
Abbreviations: PNVI= presenting near vision impairment
Figure 2. Frailty status by self-reported near vision impairment.
Groups: SNVI= self-reported difficulty with near vision tasks;
* chi-squared p<0.001
Abbreviations: SNVI= self-reported near vision impairment
Figure 3. Frailty status by near vision impairment status.
Groups: No NVI= near visual acuity 20/40 or better, and no self-reported difficulty with near vision tasks, PNVI only= near visual acuity worse than 20/40, SNVI only= selfreported difficulty with near vision tasks, PNVI+SNVI= near visual acuity worse than 20/40, and self-reported difficulty with near vision tasks
* chi-squared p<0.001
Abbreviations: PNVI= presenting near visual impairment, SNVI= self-reported near visual impairment, NVI= near visual impairment
Regression Analyses
In multivariable regression analysis, as compared to those without PNVI, participants with PNVI were more likely to be prefrail (OR=1.6; 95% CI=1.1,2.3; p=0.02) and frail (OR=2.5; 95% CI=1.4,4.3; p=0.001),Table 2. Similarly, as compared to those without SNVI, participants with SNVI were also more likely to be prefrail (OR=2.9; 95% CI=1.8,4.7; p=0.009) and frail (OR=4.3; 95% CI=2.2,8.3; p<0.001). In a third model with combined PNVI and SNVI categories, as compared to those without PNVI and SNVI, participants with PNVI only were not significantly more likely to be prefrail (OR=1.2; 95% CI=0.8,1.9; p=0.34) but were more likely to be frail (OR=2.4; 95% CI=1.2,4.9; p=0.02), while participants with SNVI only (prefrail: OR=2.3; 95% CI=1.2,4.5; p=0.02, and frail: OR=4.1; 95% CI=1.3,12.6; p=0.01), and PNVI+SNVI (prefrail: OR=4.0; 95% CI=2.2,7.2; p<0.001, and frail: OR=4.5; 95% CI=1.7,12.7; p=0.004) were more likely to be prefrail and frail. In sensitivity analysis, PNVI was reclassified after accounting for the testing distance of near vision card (PNVI * [test distance/16”]) and the results were similar.
Table 2.
Multinomial regression analysis: Frailty and near vision impairment
| Pre-fraila | Frailb | |||||||
|---|---|---|---|---|---|---|---|---|
| Interval | n | 95% CI | P value | OR | 95% CI | P value | ||
| Model 1. PNVI status | 2656 | OR | ||||||
| PNVI (>20/40) | No PNVI (≤20/40) | 1.6 | 1.1, 2.3 | 0.02 | 2.5 | 1.4, 4.3 | 0.001 | |
| Model 2. SNVI status | 2657 | OR | ||||||
| SNVI | No self-reported SNVI | 2.9 | 1.8, 4.7 | 0.009 | 4.3 | 2.2, 8.3 | 0.001 | |
| Model 3. NVI status | 2679c | OR | ||||||
| PNVI (>20/40) only | No PNVI or SNVI | 1.2 | 0.8, 1.9 | 0.34 | 2.4 | 1.2, 4.9 | 0.02 | |
| SNVI only | No PNVI or SNVI | 2.3 | 1.2, 4.5 | 0.02 | 4.1 | 1.3, 12.6 | 0.01 | |
| PNVI + SNVI | No PNVI or SNVI | 4.0 | 2.2, 7.2 | <0.001 | 4.5 | 1.7, 12.7 | 0.004 | |
| Model 4. Near Visual Acuity | 2656 | β | ||||||
| Near visual acuity | One line worse | 1.3 | 1.1, 1.4 | 0.001 | 1.4 | 1.1, 1.8 | 0.004 | |
Note: All models adjusted for age, sex, race, education, smoking status, diabetic status, and number of comorbidities.
Bold font: p<0.05
Pre-frail= ≤2 criteria
Frail= ≥3 criteria
Model 3 includes 10 individuals that had PNVI but were missing data on self-reported vision, and another 12 individuals that had SNVI but were missing data on presenting vison
Abbreviations: OR= odds ratio, CI= confidence interval, PNVI= presenting near visual impairment, SNVI= self-reported near visual impairment, NVI= near visual impairment
When presenting near visual acuity was assessed as a score having linear association with log odds of frailty, each one-line decrement in near acuity was associated with greater odds of prefrailty (OR=1.3 per 0.1 logMAR decrement; 95% CI: 1.1,1.4; p=0.001), and frailty (OR=1.4 per 0.1 logMAR decrement; 95% CI: 1.1,1.8; p=0.004).
DISCUSSION
In this sample of older adults, participants with presenting and self-reported NVI were more likely to be pre-frail and frail than those without these impairments. Further, older adults with both presenting and self-reported impairments had the greatest odds of frailty, suggesting that objective and subjective measures of vision may be needed to fully assess the vision-frailty relationship.
The strengths of our study include the use of a nationally representative sample, inclusion of objective and subjective measures of NVI in association with frailty, and the use of the frailty phenotype, a previously validated frailty measure shown to be linked to adverse health outcomes including disability and mortality.15 Although these survey data are not current, we don’t expect the cross-sectional relationship between NVI and frailty to have changed over time. We are unaware of data supporting temporal shifts in prevalence rates of NVI or frailty since 1999–2002, and therefore, surmise that this data is likely applicable to today’s population. The associations noted here underscore the need for longitudinal data examining the impact of uncorrected near vision on frailty to understand if near vision impairment is a risk factor that exacerbates frailty.
We noted substantial differences in race (85% vs 66% white, respectively) and education (57% vs 26% with less than high school education, respectively) between groups presenting with and without near vision impairment. The propensity score adjustment that we used in our analyses was meant to account for this imbalance between groups and minimize confounding by these variables. Similar group differences were noted in individuals with and without self-reported near vision impairment. These discrepancies are likely related to differences in health care access and health-seeking behavior across racial groups and levels of education.23–25
We found that older adults who reported difficulty with near vision tasks had greater odds of being prefrail and frail than individuals that presented with objectively measured impaired near vision (worse than 20/40). This difference in effect size between PNVI and SNVI may be due to perceived difficulty with vision having greater implications on function and frailty than objectively measured visual acuity. It is also important to note the possibility of same-source bias when interpreting the SNVI results since self-reported data were used for both the exposure (vision impairment) and outcome (frailty). For example, individuals reporting low physical activity or poor energy may be more likely to report having difficulty with near vision tasks, as responses may be influenced by factors such as overall perception of health.
Our study does not examine the mechanism of decreased near vision and it is therefore unclear what proportion of the NVI is due to presbyopia treatable with over the counter reading glasses, or prescription reading glasses, and what proportion is attributable to eye disease that cannot be treated with spectacle correction. However, almost all participants (99.5%) were wearing near correction for the presenting near acuity test. While this number should be considered with caution since there was substantial (20%) data missing for this variable, it regardless shows that most people are already using spectacle correction for reading/near work. Spectacles, especially reading eyeglasses, are inexpensive and widely available for easy purchase, for example in department stores in the US. Therefore, the NVI is possibly due to sub-optimal correction or eye disease, and older adults may potentially benefit from prescription glasses, and/or treatment of any co-existing eye conditions such as cataract.
These results are largely in agreement with the limited prior work exploring the relationship between visual impairment and frailty. Swenor et al. examined the association between distance vision impairment and frailty, also using NHANES data, and found that participants with visual impairment had 3.2 greater odds of prefrailty and 3.7 greater odds of frailty, than those without visual impairment.26 Complimentary longitudinal analyses using the Women’s Health and Aging Studies showed that older adults with visual impairment are also more likely to progress toward frailty (3.5 greater odds of incident frailty over 3 years) than those unimpaired, establishing temporality of this relationship.26 Klein et al. using data from the Beaver Dam Study reported that distance visual acuity and contrast sensitivity were significantly correlated with frailty as measured by their own 4-item frailty index including gait time, peak expiratory flow rate, handgrip strength, and chair stand.16 Ng et al. while developing a frailty risk index using data from the Singapore Longitudinal Ageing Studies (SLAS), identified distance visual impairment as one of 13 salient risk factors of frailty.27 Liljas et al. using data from the English Longitudinal Study of Ageing and the Fried phenotype reported that community-dwellers aged ≥60 years in England with poor self-reported vision had 2.5 greater odds of frailty.17 Additionally, non-frail participants with vision impairment had 2.1 greater odds of becoming frail over a 4-year follow-up period as compared to non-frail peers with no vision impairment.17 However, in this study, participants did not specify if they had difficulty with near or distance vision and likely included individuals with a combination of vision impairments. Of note, Soler et al., found that neither distance nor near vision impairment was associated with Fried’s frailty phenotype in a geriatric frailty clinic setting in France. However, as acknowledged by the authors, the lack of an association may be because they were not adequately powered to detect differences due to an imbalance in the groups. Given the study setting, they had relatively few robust patients with the majority (92.3%) being pre-frail or frail.18 Frailty studies limited to specific age-related eye diseases have reported associations between cataract28 and age-related macular degeneration,29 and some measures of frailty.
This study has some limitations. First, our estimates of most components of the frailty phenotype were based on self-reported data subject to recall bias. Our subjective definitions of poor endurance, weakness, and low physical activity differ from their definitions as originally intended, and the proxy for poor endurance is not a direct analog for the intended content. However, NHANES data has been previously used to define the frailty phenotype as we did.30 Second, while prior research has indicated that cognition and depression have an impact on frailty,15 we were unable to adjust for these factors since the data were not available in the NHANES subset used for these analyses. Third, the NHANES study population does not include residents in long-term care facilities, the incarcerated, and persons on active duty with the Armed Forces, and therefore is not generalizable to those specific populations. Fourth, the reading distance for the near acuity test was not standardized. It was designed to capture functional ability, and thus allowed testing at the participant’s preferred reading distance. However, we conducted sensitivity analysis reclassifying PNVI after accounting for test distance and the results were similar.
Finally, it is possible that NVI is simply a surrogate for health-related behaviors that may lead to frailty. For example, it may be that individuals with poorer healthcare access and health-seeking behavior are less likely to use reading glasses, as well as less likely to seek care for conditions contributing to frailty. Also, perhaps more frail individuals would be less likely to carry extra items such as reading glasses and may be less likely to have (or afford regularly replacing) progressive lenses where near acuity correction would be included. However, it may alternatively be possible that uncorrected near vision is a stressor that triggers or exacerbates the multisystem dysregulation that characterizes frailty. Given the magnitude of the associations observed in these analyses, further work is needed to evaluate if NVI is a risk factor for frailty, including longitudinal studies. Regardless of the mechanism, our results add to prior work indicating that uncorrected presbyopia is more than a trivial problem of late life, but rather may impact overall health and quality-of-life.7,8,11–14
In conclusion, we examined the association of NVI with frailty and found that older Americans with PNVI and SNVI were more likely to be frail than those without respective NVIs, and that individuals with co-existing PNVI and SNVI were most likely to be frail. Further studies are warranted to examine causal relationships using longitudinal data and explore the mechanism(s) underlying the relationship between NVI and frailty. Multi-pronged frailty interventions such as enhancing nutrition and physical activity have been proposed for older adults.31 Future investigations are needed to determine if interventions to optimize near vision in older adults may have the potential to deter the dysregulation process central to frailty and confer improved ability to deal with stressors leading to frailty.
Supplementary Material
ACKNOWLEDGMENTS
a. Funding/Support:
BKS supported by grants from the National Institute on Aging- NIA P30AG021334 & K01AG052640. The funding organization had no role in the design or conduct of this research.
Footnotes
b. Financial Disclosures:
Varshini Varadaraj- No financial disclosures Moon Jeong Lee- No financial disclosures Jing Tian- No financial disclosures Pradeep Y. Ramulu- No financial disclosures Karen Bandeen-Roche- No financial disclosuress Bonnielin K. Swenor- No financial disclosures
c. Other Acknowledgments: No other disclosures.
Supplemental Material available at AJO.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zebardast N, Friedman DS, Vitale S. The Prevalence and Demographic Associations of Presenting Near-Vision Impairment Among Adults Living in the United States. Am J Ophthalmol. 2017;174:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varma R, Ying-Lai M, Klein R, Azen SP, Los Angeles Latino Eye Study G. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004; 111 (6):1132–1140. [DOI] [PubMed] [Google Scholar]
- 3.He M, Abdou A, Naidoo KS, et al. Prevalence and correction of near vision impairment at seven sites in China, India, Nepal, Niger, South Africa, and the United States. Am J Ophthalmol. 2012;154(1):107–116 e101. [DOI] [PubMed] [Google Scholar]
- 4.The International Agency for the Prevention of Blindness Website. http://atlas.iapb.org/vision-trends/near-vision-impairment-presbyopia-also-deserves-attention/. Accessed 24 August, 2018.
- 5.Beers AP, van der Heijde GL. Age-related changes in the accommodation mechanism. Optom Vis Sci. 1996;73(4):235–242. [DOI] [PubMed] [Google Scholar]
- 6.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell PJ, Lee P, Spritzer K, Lindblad AS, Hays RD. Associations of presbyopia with vision-targeted health-related quality of life. Arch Ophthalmol. 2003; 121(11):1577–1581. [DOI] [PubMed] [Google Scholar]
- 8.Lamoureux EL, Fenwick E, Moore K, Klaic M, Borschmann K, Hill K. Impact of the severity of distance and near-vision impairment on depression and vision-specific quality of life in older people living in residential care. Invest Ophthalmol Vis Sci. 2009;50(9):4103–4109. [DOI] [PubMed] [Google Scholar]
- 9.Patel I, Munoz B, Burke AG, et al. Impact of presbyopia on quality of life in a rural African setting. Ophthalmology. 2006;113(5):728–734. [DOI] [PubMed] [Google Scholar]
- 10.Tahhan N, Papas E, Fricke TR, Frick KD, Holden BA. Utility and uncorrected refractive error. Ophthalmology. 2013;120(9):1736–1744. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Congdon N, He X, Murthy GV, Yang A, He W. Quality of life and near vision impairment due to functional presbyopia among rural Chinese adults. Invest Ophthalmol Vis Sci. 2011;52(7):4118–4123. [DOI] [PubMed] [Google Scholar]
- 12.Keller BK, Morton JL, Thomas VS, Potter JF. The effect of visual and hearing impairments on functional status. J Am Geriatr Soc. 1999;47(11):1319–1325. [DOI] [PubMed] [Google Scholar]
- 13.Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53(4):681–686. [DOI] [PubMed] [Google Scholar]
- 14.Klein BE, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110(4):644–650. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 16.Klein BE, Klein R, Knudtson MD, Lee KE. Relationship of measures of frailty to visual function: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2003;101:191–196; discussion 196–199. [PMC free article] [PubMed] [Google Scholar]
- 17.Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71(11): 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soler V, Sourdet S, Balardy L, et al. Visual Impairment Screening at the Geriatric Frailty Clinic for Assessment of Frailty and Prevention of Disability at the Gerontopole. J Nutr Health Aging. 2016;20(8):870–877. [DOI] [PubMed] [Google Scholar]
- 19.The NHANES website. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 25 October, 2018.
- 20.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-list-item national eye institute visual function questionnaire. J Archives of ophthalmology. 2001;119(7):1050–1058. [DOI] [PubMed] [Google Scholar]
- 21.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong JS, Collopy F. Error Measures for Generalizing About Forecasting Methods - Empirical Comparisons. Int J Forecasting. 1992;8(1):69–80. [Google Scholar]
- 23.Varadaraj V, Frick KD, Saaddine JB, Friedman DS, Swenor BK. Trends in Eye Care Use and Eyeglasses Affordability: The US National Health Interview Survey, 2008–2016. JAMA Ophthalmol. 2019;137(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr P, Barron Y, Schein OD, Rubin GS, West SK. Eye care utilization by older Americans: the SEE Project. Salisbury Eye Evaluation. Ophthalmology. 1999; 106(5):904–909. [DOI] [PubMed] [Google Scholar]
- 25.Owsley C, McGwin G, Scilley K, Girkin CA, Phillips JM, Searcey K. Perceived barriers to care and attitudes about vision and eye care: focus groups with older African Americans and eye care providers. Invest Ophthalmol Vis Sci. 2006;47(7):2797–2802. [DOI] [PubMed] [Google Scholar]
- 26.Swenor BK, Lee MJ, Tian T, Varadaraj V, Bandeen-Roche K. Visual Impairment and Frailty: Examining an understudied relationship. J Gerontol A Biol Sci Med Sci. Accepted for publication July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng TP, Feng L, Nyunt MS, Larbi A, Yap KB. Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc. 2014;15(9):635–642. [DOI] [PubMed] [Google Scholar]
- 28.Klein BE, Klein R, Knudtson MD. Frailty and age-related cataract. Ophthalmology. 2006;113(12):2209–2212. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Knudtson MD. Frailty and age-related macular degeneration: the Beaver Dam Eye Study. Am J Ophthalmol. 2005;140(1):129–131. [DOI] [PubMed] [Google Scholar]
- 30.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–470. [DOI] [PubMed] [Google Scholar]
- 31.Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.