Introduction
Pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome is a rare autosomal dominant disorder with variable expressivity [1] and results from mutations in the PSTPIP1 gene. The PSTPIP1 protein is a cytoskeletal protein within hematopoietic cells that serves as a scaffold for the binding of other cellular proteins, such as pyrin, protein tyrosine phosphatases, c-Abl, CD2, and WASP. Through these interactions, PSTPIP1 regulates several cellular functions including IL-lβ release, cytoskeleton organization, cell migration, and T-cell activation [2, 3]. Depending on the mutation location within the PSTPIP1 gene and consequent alterations in protein-protein interactions, a spectrum of autoinflammatory disorders may result and are collectively referred to as PSTPIP1-associated inflammatory diseases (PAID). While the underlying pathogenesis is not completely understood, the PAID spectrum is characterized by dysregulated IL-lβ release and neutrophil responses [4] and clinically manifests as recurrent bouts of untriggered inflammation involving various organ systems [5]. PAPA syndrome is only one entity within the PAID spectrum and classically presents with sterile pyogenic arthritis, pyoderma gangrenosum (PG), and cystic acne. Due to heterogeneous presentations, delays in diagnosis and misdiagnoses as autoimmune or immunodeficiency conditions are common.
Given the rarity of PAPA syndrome and other disorders in the PAID spectrum, no guideline-based treatment approaches exist and previous reports highlight the significant variability in responses to monotherapy with two of the most common long-term therapies utilized: IL-1β antagonists and TNF-α inhibitors [2, 6]. Other anti-inflammatory agents are reserved for treatment failures, and dual therapy, except in combination with steroids, is rarely reported for treatment of PAPA syndrome [3, 6]. Early diagnosis and initiation of effective treatment to induce remission, however, are vital to decreasing the morbidity and mortality associated with these autoinflammatory disorders. We present here a case of an adolescent female referred to our immunology clinic for suspected immunodeficiency, but who was subsequently diagnosed with and treated for PAPA syndrome with combination adalimumab and tacrolimus therapy. To our knowledge, this is the first report of the effective and safe use of tacrolimus in combination with adalimumab for PAPA syndrome.
Case Presentation
A 12-year-old female was referred to our immunology clinic for evaluation of a possible immunodeficiency given her history of recurrent infections since 1 year of age. Her pertinent clinical history is summarized in Figure 1a. She initially developed recurrent purulent otitis media (OM), requiring multiple sets of tympanostomy tubes. At 5-years-old, she developed erythema, bruising, swelling, and pain of her left ankle that was initially attributed to trauma. Over several months, her symptoms extended to involve her bilateral ankles, knees, wrists, and hands. Following a rheumatology evaluation, she was diagnosed with seronegative polyarticular juvenile idiopathic arthritis (JIA). After failing therapy with naproxen, methotrexate was attempted but discontinued after four months due to an increase in OM. Her arthritis interestingly resolved spontaneously over the next several months without further treatment.
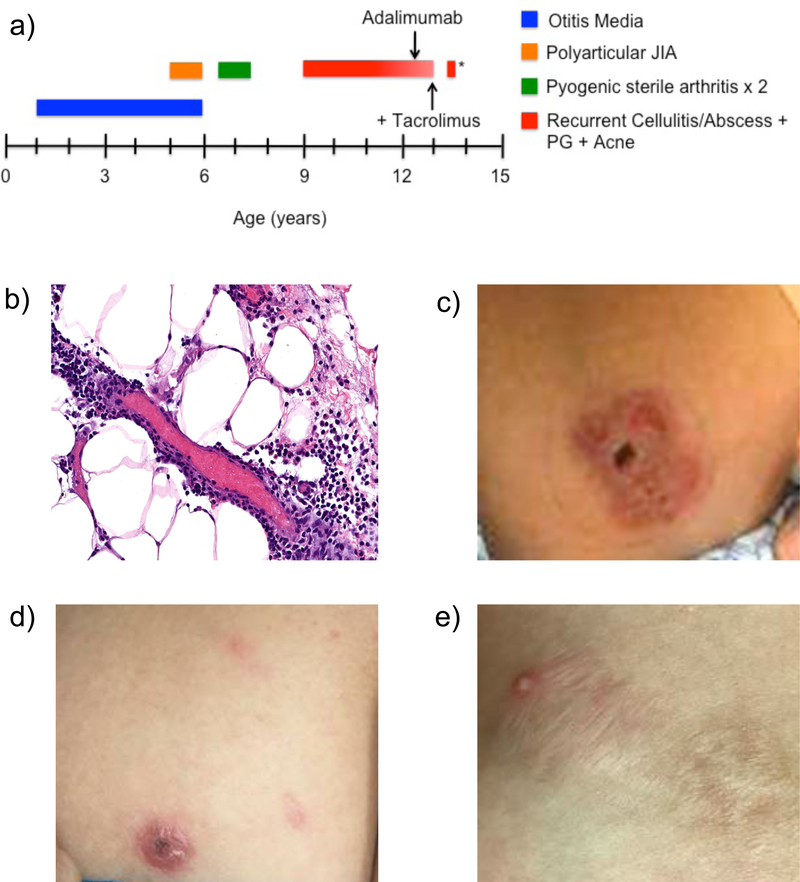
Figure 1:
a) Our patient’s clinical history was significant for recurrent episodes of purulent otitis media, polyarticular juvenile idiopathic arthritis (JIA), two episodes of pyogenic sterile arthritis and osteomyelitis of the right elbow, and various cutaneous manifestations including recurrent episodes of cellulitis and abscesses, pyoderma gangrenosum (PG), and nodulocystic acne. Arrows indicate initiation of adalimumab and addition of tacrolimus. * represents PG flare during lapse of adalimumab therapy for 6–8 weeks. b) Dermatopathology from biopsy of left breast lesion revealed a mixed lobular panniculitis with interstitial neutrophils and lymphocytes and features consistent with a neutrophilic dermatosis. c) Erythematous, violaceous ulcerated plaque that represents PG lesion prior to treatment. d) PG lesion following treatment with adalimumab with reduction in erythema, ulceration, and size. e) PG lesion following addition of tacrolimus to adalimumab has healed into a hypertrophic scar.
Between 6–7 years of age, she developed two episodes of presumed right elbow septic arthritis and osteomyelitis. With each episode, joint fluid studies revealed 100,000–200,000 white blood cells (WBCs) with >95% neutrophils but negative gram stain and culture. Additional evaluation during each episode was notable for normal peripheral WBCs and differential count and often minimal to no elevation in acute phase reactants. Synovial tissue biopsies revealed marked acute and chronic synovitis. Poor wound healing followed each incision and drainage (I&D) procedure, and she showed, at best, only slow clinical improvement with antibiotics. Also noteworthy is a lack of true fevers throughout this clinical course.
An immune work-up performed during her initial episode of right elbow septic arthritis was reassuring and revealed polyclonal hypergammaglobulinemia; protective antibody titers to diphtheria, tetanus, and 6/12 testable pneumococcal serotypes in Prevnar 13; positive isohemagglutinins with an O+ blood type; normal CH50; and normal oxidative burst.
Finally, our patient also had a history of recurrent episodes of presumed cellulitis and cutaneous abscesses beginning at a young age but increasing in frequency with age. Despite multiple I&Ds, no microbes were isolated from these abscesses, and clinically, no fever was noted. At 9-years-old, she developed a left preseptal cellulitis and abscess and again had a normal peripheral WBC count with differential and normal inflammatory markers. Symptoms resolved slowly with minimal response to antibiotic treatment. In the 6 months prior to presentation to our clinic, she developed recurrent presumed abscesses over her breast that initially began as small pustules but rapidly progressed to deeply erythematous, violaceous papules and ulcerated plaques. Dermatopathology from punch biopsies revealed lobular panniculitis with interstitial neutrophils and lymphocytes and features consistent with a neutrophilic dermatosis (Figure 1b). Cultures from the biopsy were negative, so a short course of systemic steroids was initiated for a diagnosis of PG (Figure 1c) with rapid improvement. Additional clinical history was pertinent for plantar warts and nodulocystic acne.
Case Discussion and Treatment
Several salient features of this case pointed toward an autoinflammatory disorder rather than an immunodeficiency. Most notably, despite an impressive history of presumed infections, no organisms were ever isolated. She also often lacked fever, leukocytosis, or left shift in differential cell count, all features typically seen in acute infectious processes. Finally, she had minimal to no response to appropriate antibiotics. Instead, this history of pyogenic, sterile joint inflammation and polyarticular JIA that resolved with minimal therapy prior to adolescence coupled with cutaneous manifestations of PG and nodulocystic acne increasing in frequency after puberty was highly suggestive of PAPA syndrome. Our patient underwent PSTPIP1 gene sequencing, which revealed heterozygosity for a known pathogenic mutation (c.688G>A, p.A230T) and confirmed the diagnosis.
Treatment with TNF-α inhibitor adalimumab 40 mg every two weeks was initiated and favored over IL-1β antagonist anakinra due to its advantage of biweekly administration. This resulted in partial clinical improvement of PG lesions (Figure 1d) and acne. After the interval development of new PG lesions, decision was made to pursue combination therapy. Given our group’s experience in treating recalcitrant PG with oral tacrolimus [7] and variable response of cutaneous manifestations with anakinra [6], slow titration of oral tacrolimus up to 3 mg twice daily was initiated. In combination with adalimumab, tacrolimus resulted in greater improvement of PG lesions (Figure 1e). She has now been on adalimumab and tacrolimus for 18 months with excellent disease control and absolutely no adverse events. Rare onset of new PG lesions are controlled with topical dapsone and/or topical steroids. While monotherapy with tacrolimus was considered, a lapse in treatment with adalimumab for 6–8 weeks resulted in a significant flare of PG, suggesting the ongoing need for dual therapy.
This case emphasizes the need for a high index of suspicion for an autoinflammatory disorder in the setting of sterile sites of recurrent inflammation. Early-onset pyogenic, sterile, destructive arthritis and later-onset cutaneous manifestations of acne and PG as seen in our patient are classic manifestations for PAPA syndrome. While dysregulated IL-1β release is a known factor in disease pathogenesis, Holzinger and colleagues recently highlighted a myriad of cytokine and inflammatory protein alterations in patients with PAPA syndrome and other diseases among the PAID spectrum [8]. This underscores the pathogenic complexity of these disorders and adds to the difficulty in developing well-defined treatment approaches.
Our case further highlights the potential need for dual therapy when monotherapy with either TNF-α inhibitors and/or IL-1β antagonists are ineffective. To our knowledge, ours is the first report on the effective and safe use of adalimumab in combination with tacrolimus for treatment of PAPA syndrome. A similar regimen consisting of infliximab and cyclosporine was successfully used in treatment of PG, acne, suppurativa hidradenitis (PASH), another disorder among the PAID spectrum [9]. This suggests that the combined use of TNF-α inhibitors and calcineurin inhibitors may adequately suppress the overactive inflammatory pathways resulting from PSTPIP1 mutations and warrants further study in PAID.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clinic proceedings. 1997;72(7):611–5. [DOI] [PubMed] [Google Scholar]
- 2.Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH Syndromes: Pathophysiology, Presentation and Treatment. American journal of clinical dermatology. 2017;18(4):555–62. [DOI] [PubMed] [Google Scholar]
- 3.Holzinger D, Roth J. Alarming consequences - autoinflammatory disease spectrum due to mutations in proline-serine-threonine phosphatase-interacting protein 1. Current opinion in rheumatology. 2016;28(5):550–9. [DOI] [PubMed] [Google Scholar]
- 4.Mistry P, Carmona-Rivera C, Ombrello AK, Hoffmann P, Seto NL, Jones A, et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Annals of the rheumatic diseases. 2018;77(12):1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzano AV, Damiani G, Ceccherini I, Berti E, Gattorno M, Cugno M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). The British journal of dermatology. 2017;176(6):1588–98. [DOI] [PubMed] [Google Scholar]
- 6.Demidowich AP, Freeman AF, Kuhns DB, Aksentijevich I, Gallin JI, Turner ML, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis and rheumatism. 2012;64(6):2022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crouse L, McShane D, Morrell DS, Wu EY. Pyoderma gangrenosum in an infant: A case report and review of the literature. Pediatric dermatology. 2018;35(5):e257–e61. [DOI] [PubMed] [Google Scholar]
- 8.Holzinger D, Fassl SK, de Jager W, Lohse P, Rohrig UF, Gattorno M, et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. The Journal of allergy and clinical immunology. 2015;136(5):1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staub J, Pfannschmidt N, Strohal R, Braun-Falco M, Lohse P, Goerdt S, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015;29(11):2243–7. [DOI] [PubMed] [Google Scholar]