Abstract
Background/Objective
Evidence from non-human species indicate that hydration and arginine vasopressin (AVP) influence fuel selection, energy expenditure (EE), and food intake, but these relationships are unclear in humans. We sought to assess whether hydration biomarkers [24-h urine volume (UVol) and urine urea nitrogen concentration (UUN)] and copeptin (a surrogate for AVP) are associated with 24-h EE, respiratory quotient (RQ), and daily energy intake (DEI).
Subjects/Methods
In a secondary analysis of collected data, we selected healthy adults (Group 1, n = 177) who had 24-h whole-room indirect calorimetry measurements in energy balance with 24-h urine collection and fasting copeptin measurements (n=117), followed by 3 days ad libitum food intake. A separate group (Group 2, n=284) with hydration markers and calorimetry measurements was also studied. The main outcome measures were 24-h RQ, 24-h EE, DEI, substrate oxidation.
Results
In Group 1, lower 24-h UVol and higher 24-h UUN, indicating lower hydration, were correlated with lower 24-h RQ (r = 0.35, p <0.0001, and r = −0.29, p = 0.0001, respectively; results similar in Group 2) and predicted subsequent reduced DEI (r = 0.20, p = 0.01, and r = −0.27, p = 0.0003, respectively), adjusted for confounders. Copeptin was independently associated with 24-h lipid oxidation (r = −0.23, p = 0.01). In Group 2, lower hydration was associated with reduced 24-h EE (24-h UVol: r = 0.29, p <0.0001; 24-h UUN: r = −0.25, p <0.0001).
Conclusions
Hydration biomarkers were associated with metabolic differences characterized by altered food intake, fuel selection, and possibly EE. Independently, copeptin was associated with higher lipid oxidation.
Introduction
Both water and food are essential for human life. The physiological systems responsible for body water homeostasis and energy metabolism, rather than being independent of one another, are interconnected [1, 2]. Hydrational status influences various aspects of metabolism which are linked to weight gain [3–7]. In humans, higher respiratory quotient (RQ), which primarily indicates preference for carbohydrate over lipids as an oxidative fuel source, predicts overeating and weight gain [3–6]. Likewise, lower-than-expected 24-hour energy expenditure (24-h EE) and basal metabolic rate predict both weight gain [5, 7] and fat mass (FM) in Native Americans [5]. Though understanding hydrational factors which may influence metabolic fuel selection, energy expenditure (EE), and energy intake (EI), is important, it is unclear how hydration relates to these factors [8].
Non-human species in a water-conserving state demonstrate a shift towards greater fat oxidation [9, 10]. In humans, whether hydration influences fuel selection and EE is unclear. Water ingestion has variously been reported to increase, decrease, or have no effect on EE and RQ [11–15] However, these prior studies have involved small sample sizes, have been short-term (< 3 hours), and mostly relied upon a ventilated hood technique for EE measurements. Whether hydration influences fuel selection and EE over an entire day in a metabolic chamber during eucaloric feeding is unclear.
The impact of hydration on food intake has been studied in animal models. Multiple studies involving non-human species have shown diminished food intake in response to hypertonic saline [16, 17] and water restriction [18, 19]. In humans, the relationship between hydration and food intake is less clear. Studies have shown a temporal association between eating and drinking [20–22]. In a small study, water-restricted men reduced ad libitum food intake, unrelated to food palatability [20].
The internal cues linking hydrational status and EI or metabolic fuel selection are unclear. Arginine vasopressin (AVP), a crucial hormone regulating water balance [1], reduces food intake [23, 24] and influences lipid and glucose metabolism [25] in animal models. Copeptin, the C-terminal part of the AVP prohormone, shows good ex vivo stability in contrast to AVP, and is a surrogate for AVP [26]. Although both higher circulating copeptin [27] and higher RQ [28] each predict obesity, the relationship between copeptin and RQ is uncertain. In addition, whether copeptin concentration is associated with 24-h EE and food intake is also unclear.
Both 24-h urine volume (24-h UVol) and 24-h urine urea nitrogen concentration (24-h UUN) are well correlated with daily fluid intake volume [29–31]. In the present observational study, our first aim was to evaluate the relationship between these hydration biomarkers and both 24-hour EE and RQ measured in a metabolic chamber, during which diet is controlled but water intake is ad libitum. We studied 177 healthy adult participants identified a priori (Group 1) of varied racial/ethnic background (majority Native American) and found that lower 24-h UVol and higher 24-h UUN (both indicating a less hydrated state) were associated with reduced 24-h respiratory quotient (24-h RQ). Next, we confirmed this finding in a separate, larger group (n = 284, Group 2). Our second aim was to investigate (Group 1) whether these hydrational biomarkers, reflecting a behavioral tendency to drink, predict subsequent EI using a 3-day vending machine paradigm, a reproducible measure of ad libitum food intake [32], and found that both lower 24-h UVol and higher 24-h UUN were associated with reduced food intake. The third aim was to evaluate if copeptin is an internal hormonal signal contributing to the findings observed related to the first two aims. Accordingly, we then measured circulating copeptin in a subgroup of Group 1 (n = 117) and evaluated the relationship between copeptin and 24-h EE, 24-h RQ, and ad libitum EI.
Subjects and methods
This analysis included participants who had enrolled in two ongoing natural history studies of energy balance. This is a secondary analysis of this collected data. Volunteers were healthy adults recruited from the Phoenix Area, Arizona, USA, and admitted to the clinical research unit for participation in one of two observational studies investigating risk factors for obesity and diabetes as previously described [33, 34]. All volunteers had measurements of 24-h UVol and 24-h UUN, and 24-h EE by indirect calorimetry via the metabolic chamber. Volunteers in Group 1 also had measurement of ad libitum EI. During this inpatient study, participants were not permitted to drink alcohol, smoke, or take any medications, but were allowed ad libitum fluid intake. While on the research unit, physical activity was restricted to light activities (e.g. playing pool, television, arts, crafts) during the entire time course of the study. Written informed consent was obtained prior to participation. The studies were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
On admission to the clinical research unit, participants received, for at least three days, a standard diet to meet weight maintaining energy needs (WMEN; daily kcal: 20% protein, 30% fat and 50% carbohydrates) that was estimated based on body weight at admission and sex [35]. Subsequently, all volunteers underwent a 75-g oral glucose tolerance test after an overnight fast and were included if they were without diabetes, according to American Diabetes Association criteria [36].
Automated food-selection system
Ad libitum food intake was measured over 3 consecutive days using an automated vending machine paradigm as previously described [32, 37]. Briefly, food preferences were determined using a Food Preferences Questionnaire. Individuals rated each food by using a 9-point Likert scale with 1 = dislike extremely; 5 = neutral; 9 = like extremely; the possibility to rate pleasantness of food items as unknown was also included. During the 3 days of food intake assessment on the clinical research unit, participants self-selected all food items using a vending machine system. Each day vending machines were individually stocked with 40 food items and subjects had 23.5h ad-libitum access to the machines. Volunteers were asked to eat whenever and whatever they desired and to prepare and consume all foods only in a separate eating area. The weights of the consumed foods were used to calculate daily energy intake (DEI, kcal/d) and intake of individual macronutrients. This was done by using the CBORD Professional Diet Analyzer Program (CBORD, Inc., Ithaca, New York, USA) and the Food Processor database (ESHA version 10.0.0, ESHA Research, Salem, Oregon, USA) modified to reflect the nutrient content of specific food items as indicated by the manufacturer. Calories derived from each individual macronutrient intake were calculated as 4 kcal/gram for protein and carbohydrates and 9 kcal/gram for fat.
Body composition
After an overnight fast, body composition was assessed by underwater weighing with simultaneous determination of residual lung volume by helium dilution as previously described [38] or by total body dual energy x-ray absorptiometry (DPX-L; Lunar Radiation, Madison, Wisconsin, USA) as previously described [39]. Absorptiometry measures were converged to comparable underwater weighing values as previously described [39].
Measurements in the metabolic chamber
Participants completed 24-h EE and spontaneous physical activity (SPA) measurements in a whole-room indirect calorimeter as previously described [38, 40]. Prescribed EI in the metabolic chamber provided 4 balanced meals and was reduced approximately 80% to account for restricted physical activity inside the chamber [41]. The meal times and foods for the standardized diet within the chamber are shown in the supplementary table. Participants were directed not to exercise (e.g. push-ups) while in the metabolic chamber, but may otherwise move freely about the chamber. 24-h energy balance (24-h EB) during the stay in the metabolic chamber was calculated as 24-EI minus the 24-h EE. Participants, who were allowed ad libitum water intake, were instructed to void before chamber entry and exit. EE-related measures and urine volume, measured volumetrically, were collected for 23.5 hours and were extrapolated to 24 hours. Rates of 24-h carbohydrate oxidation (24-h carbox) and 24-h lipid oxidation (24-h lipox) were calculated from 24-h RQ, accounting for protein oxidation [42, 43].
Analytical procedures
Plasma glucose was measured by the glucose oxidase method (Beckman Instruments, Fullerton, California, USA). UUN was measured by the local hospital laboratory using the urease/glutamate dehydrogenase method. Fasting samples were obtained from blood collected in plasma heparin or serum EDTA tubes from volunteers immediately after exiting the chamber and stored at −70 °C. Copeptin was measured on stored samples using a commercially available ELISA kit (Phoenix Pharmaceuticals, Burlingame, California, USA); the intraassay and interassay coefficients of variation were 5.7% and 6.8% respectively
Statistical Analysis
SAS Software (SAS Institute Inc., version 9.4, Cary, NC, USA) was used for statistical analysis. A p-value <0.05 was considered statistically significant. Non-normally distributed data were log (natural) transformed. Differences in mean between groups (e.g. sex) were compared using the Student’s t-test. To test the main aims, Pearson’s correlation coefficient was used to quantify associations between biomarkers of interest [i.e. 24-h UVol, 24-h UUN (natural log), and copeptin] and residuals of outcome measures (i.e. 24-h RQ, 24-h EE, and DEI) adjusted for predictors (i.e. the difference between observed minus predicted outcome measure using a linear function of predictors). As in Group 1, similar statistical methods were applied to subjects in Group 2 which was used to confirm findings involving 24-h RQ and 24-h EE from Group 1. Residual 24-h RQ was adjusted for age, sex, percentage body fat (%fat), 24-h EB, and race (Group 1 only since Group 2 included only Native American volunteers). Residual 24-h EE was adjusted for age, sex, race (Group 1 only), FM, fat-free mass (FFM), and SPA. Residual DEI was adjusted for age, sex, race, FM, FFM, and residual 24-h EE, with and without 24-h RQ. Residual 24-h carbox, 24-h lipox, and 24-h protein oxidation were calculated adjusting for age, sex, race, 24-h EB, FM, FFM, and SPA. Means of 24-EE, DEI, carbox, lipox, and protein oxidation were added to their residuals for representation in the figures.
Results
The characteristics of the study groups are shown in Table 1. In the main study group (Group 1), the average 24-h UVol was 3.100 L/d ± 1.352 L/d. The median 24-h UUN concentration was 402 mg/dl (interquartile range, 322 mg/dl to 561 mg/dl). As anticipated, 24-h UVol and 24-h UUN (log) were inversely related (r = −0.63, p < 0.0001). Twenty-four-h UVol was greater in men (3.352 l/d ± 1.444 l/d) than women (2.677 l/d ± 1.062 l/d) (p = 0.0005). There were no racial differences in 24-h UVol and UUN. Twenty-four-h UVol was not associated with FM, FFM, and weight, each adjusted for sex. Both 24-h UVol and 24-h UUN were associated with %fat (r = −0.17, p = 0.03, and r = 0.19, p = 0.01) but not after adjusting for sex.
Table 1–
Characteristics of study participants
| Characteristic | Group 1: n=177 | Group 2: n=284 |
|---|---|---|
| Age (years), mean (SD) | 35.8 (10.1) | 30.0 (7.5) |
| Sex, n | 111 M, 66 F | 163 M, 122 F |
| Race/ethnicity, n | 109 NA, 36 W, 7 B, 25 O/H | 284 NA |
| Body weight (kg), mean (SD) | 90.3 (22.2) | 95.5 (24.3) |
| Body mass index (kg/m2), mean (SD) | 31.5 (7.5) | 34.2 (8.4) |
| Body fat (%), mean (SD) | 31.0 (8.7) | 33.2 (7.6) |
| Fat mass (kg), mean (SD) | 28.8 (12.6) | 32.3(13.8) |
| Fat-free mass (kg), mean (SD) | 61.5 (13.4) | 62.8 (13.3) |
| Glucose, fasting (mg/dl), mean (SD) | 92.5 (8.8) | 87.9 (8.9) |
| Glucose, 2-hour (mg/dl), mean (SD) | 131.4 (35.3) | 123.4 (32.8) |
| 24-h EE (kcal/d), mean (SD) | 2229.1 (417.4) | 2373.9 (402.6) |
| 24-h RQ, mean (SD) | 0.85 (0.03) | 0.85 (0.03) |
| 24-h Non-protein RQ, mean (SD) | 0.87 (0.05) | 0.86 (0.03) |
| 24-h Protein oxidation (kcal/d), mean (SD) | 344.1 (115.9) | 312.7 (92.6) |
| 24-h Carbox (kcal/d), mean (SD) | 1047.7 (298.3) | 1061.4 (253.2) |
| 24-h Lipox (kcal/d), mean (SD) | 875.2 (397.8) | 964.6 (322.3) |
| 24-h Energy balance (kcal/d), mean (SD) | −65.7 (325.6) | −122.0 (245.3) |
| 24-h Protein balance (kcal/d), mean (SD) | 101 (124) | 138 (94) |
| 24-h Carb balance (kcal/d), mean (SD) | 64 (260) | 59 (220) |
| 24-h Lipid balance (kcal/d), mean (SD) | −207 (385) | −286 (296) |
| SPA (%), median (IQR) | 7.5 (5.9, 9.2) | 7.1 (4.9, 7.8) |
| Ad libitum food intake (kcal/d), mean (SD) | 3966.6 (1266.6) | - |
| Soda intake (kcal/d), median (IQR) | 336 (144, 528) | - |
| 24-h urine volume (l), mean (SD) | 3.100 (1.352) | 2.627 (1.132) |
| 24-h UUN (mg/dl), median (IQR) | 402 (322, 561) | 493 (356, 589) |
| Copeptin (ng/ml), median (IQR) a | 0.64 (0.44, 0.86) | - |
| Copeptin storage (years), median (IQR)a | 6.4 (3.8, 14.2) | - |
SD, standard deviation; IQR, interquartile range; EE, energy expenditure; RQ, respiratory quotient; carbox, carbohydrate oxidation; lipox, lipid oxidation; SPA, spontaneous physical activity; UUN, urine urea nitrogen; NA, Native American; W, white; B, black; O/H, other/Hispanic;
n = 117
Relationship between hydration biomarkers and metabolic chamber measures
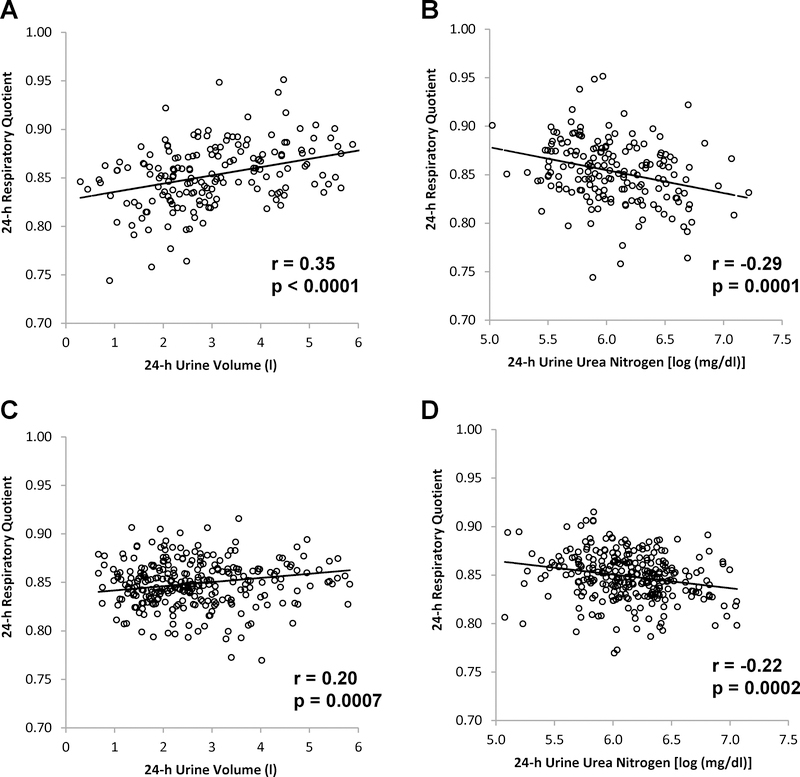
In Group 1, reduced 24-h UVol and higher 24-h UUN concentration were associated with reduced unadjusted (r = 0.36, p < 0.0001 and r = −0.25, p = 0.0006, respectively) and residual 24-h RQ (r = 0.35, p <0.0001, Figure 1A and r= −0.29, p = 0.0001, Figure 1B, respectively). Analysis of data restricted to chambers in which 24-h EE was within 20% or 10% of EI, the results were unchanged; 24-h UVol and UUN each remained associated with adjusted 24-h RQ (for within 20%: r = 0.42, p <0.0001, and r = −0.27, p = 0.0007, respectively; for within 10%: r = 0.45, p < 0.0001, and r = −0.26, p = 0.009). Similarly, when the analysis included only Native Americans, both 24-h UVol and UUN remained associated with adjusted 24-h RQ (r = 0.25, p = 0.008, and r = −0.27, p = 0.004, respectively). Both 24-h UVol and UUN were not associated with 24-h EE or SPA or average 24-h chamber temperature (median 24.2°C, interquartile range 23.3°C to 25.3°C).
Figure 1.
Relationships between 24-h respiratory quotient and 24-h urine volume (A, Group 1; C, Group 2) and 24-h urine urea nitrogen concentration (B, Group 1; D, Group 2). Pearson’s correlation coefficient (r) is reported along with its significance (p). 24-h respiratory quotient is adjusted for age, sex, race (Group 1), body fat percentage, and 24-h energy balance.
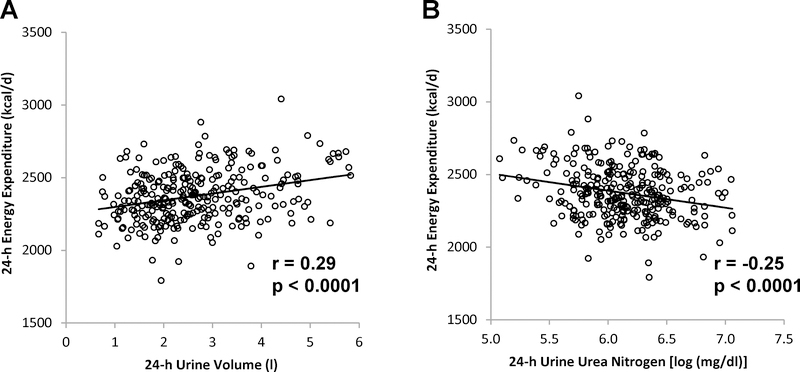
To verify the association between biomarkers of hydration with 24-h RQ, similar relationships were assessed in a larger, separate group of Native American participants (Group 2, Table 1). Lower 24-h UVol was again associated with lower unadjusted and adjusted 24-h RQ (r = 0.18, p = 0.003 and r = 0.20, p = 0.0007, Figure 1C). Likewise, higher 24-h UUN was associated with lower unadjusted and adjusted 24h RQ (r = −0.21, p = 0.0004 and r = −0.22, p =0.0002, Figure 1D). In Group 2, lower 24-h UVol and higher 24-h UUN each were also associated with lower residual 24-h EE adjusted for age, sex, FM, FFM, and SPA (Figure 2, A and B). There was no association with SPA.
Figure 2.
Relationships between 24-h urine volume and urea nitrogen concentration, and 24-h energy expenditure. 24-h energy expenditure is adjusted for age, sex, fat-mass, fat-free mass, and spontaneous physical activity. Pearson’s correlation coefficient (r) is reported along with its significance (p).
Relationship between copeptin and metabolic chamber measures
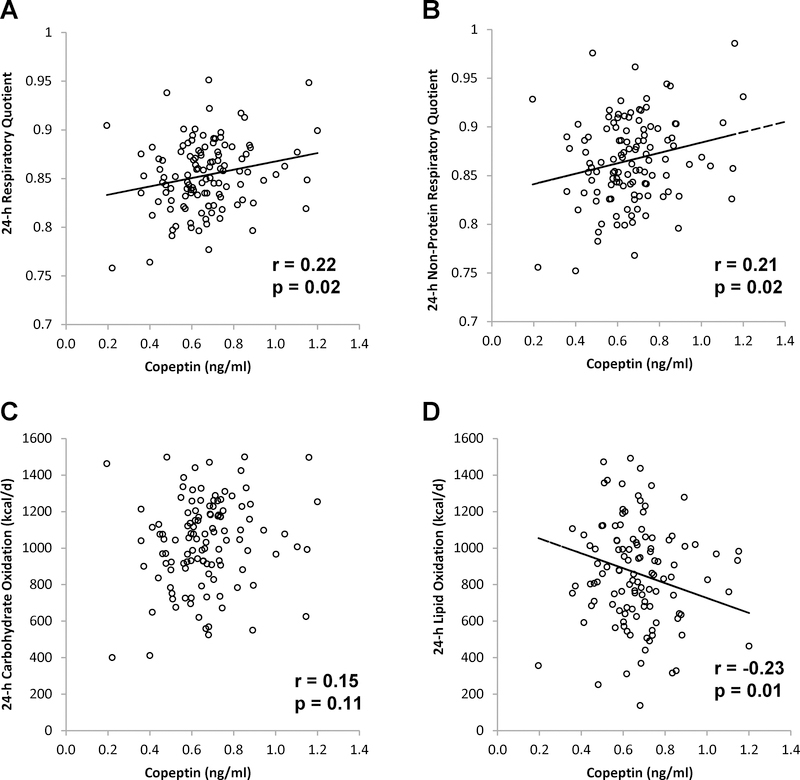
Since hydrational biomarkers were associated with 24-h RQ, we then measured copeptin in a subgroup of Group 1 (n=117) to assess the relationship between this surrogate for a key regulator (i.e. AVP) of water balance and 24-h RQ. Since mean copeptin concentration from serum samples (0.82 ng/ml ± 0.20 ng/ml) was greater (p < 0.0001) than mean copeptin concentration from plasma (0.43 ng/ml ± 0.14 ng/ml), copeptin concentration was adjusted for specimen type. There was no association between adjusted copeptin and storage time. Copeptin was not associated with age, body-mass index, FM, FFM, or %fat. Copeptin concentrations were not associated with 24-h UVol or 24-h UUN.
Copeptin concentration was associated with unadjusted and adjusted 24-h RQ (r = 0.20, p = 0.03, and r = 0.22, p = 0.02, Figure 3A, respectively) and 24-h non-protein RQ (r = 0.19, p = 0.04, and r = 0.21, p = 0.02, Figure 3B, respectively). Copeptin concentration was not associated with either 24-h protein oxidation or carbox (Figure 3C) but was associated with 24-h lipox such that higher copeptin was associated with lower unadjusted and adjusted 24-h lipox (r = −0.20, p = 0.03, and r = −0.23, p = 0.01, Figure 3D, respectively). Further adjustment for lipid intake did not change the association (r = −0.21, p = 0.02)
Figure 3.
Relationships between copeptin and 24-h respiratory quotient (A), non-protein respiratory quotient (B), carbohydrate oxidation (C), and lipid oxidation (D). 24-h respiratory quotient and non-protein respiratory quotient are adjusted for age, sex, race, body fat percentage, and 24-h energy balance. Pearson’s correlation coefficient (r) is reported along with its significance (p).
When 24-h UVol and copeptin was included in same linear model, both 24-h UVol (beta = 0.01 per liter, p < 0.0001) and copeptin (beta = 0.04 per ng/ml, p = 0.01) remained independently associated with residual 24-h RQ adjusted for age, sex, race, %fat, and 24-h EB (r2 = 0.20). Similarly, when 24-h UUN was included with copeptin in the same linear model, each remained associated with residual 24-h RQ (beta = −0.01 per 50% difference in 24-h UUN, p = 0.0004, and beta = 0.04 per ng/ml of copeptin, p = 0.01, respectively; r2 = 0.15).
Relationship between hydration and food intake
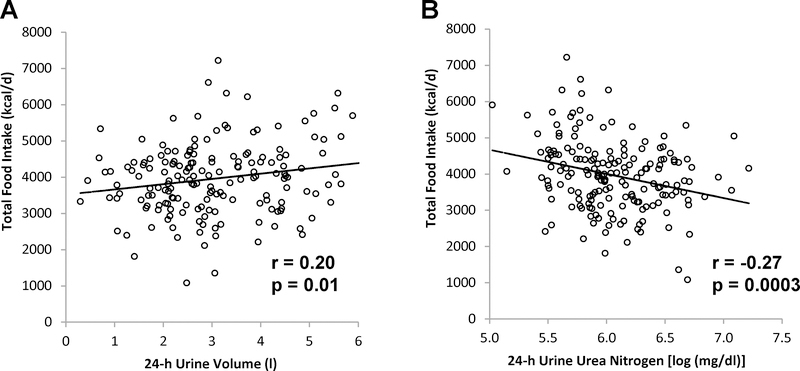
In bivariate analysis, both higher 24-h UVol and lower 24-h UUN predicted subsequent greater ad libitum DEI (r = 0.25, p = 0.0009, and r = −0.26, p =0.0005, respectively). When DEI was adjusted for age, sex, race, FM, FFM, and residual 24-h EE, 24-h UVol and 24-h UUN each predicted subsequent DEI (r = 0.20, p = 0.01, and r = −0.27, p = 0.0003, respectively; Figure 4, A and B). When 24-h RQ was further included in the model, the 24-h urine volume was no longer significantly associated with DEI. On the other hand, the association between 24-h UUN and DEI was attenuated but remained significant (r = −0.18, p =0.02) when DEI was further adjusted for 24-h RQ. 24-h UVol and UUN were associated with residual daily carbohydrate intake adjusted for age, sex, FM, FFM and residual 24-h EE (r = 0.22, p = 0.003 and r = −0.32, p < 0.0001, respectively), but not associated with EI from soda, fat, or protein. Copeptin concentration was also not associated with DEI.
Figure 4.
Relationships between 24-h urine volume and urea nitrogen concentration, and total ad libitum food intake. Energy intake is adjusted age, sex, race, fat mass, fat-free mass, and residual 24-h energy expenditure (A and B). Energy intake also adjusted for 24-h respiratory quotient (B). Pearson’s correlation coefficient (r) is reported along with its significance (p).
Discussion
This study demonstrated that hydration biomarkers which reflect the tendency to conserve water (i.e. lower 24-h UVol and higher 24-h UUN) were associated with lower RQ. This association was observed in a large group, confirmed in a second larger group, and found to be independent of copeptin levels. Moreover, copeptin, independent of those hydration biomarkers, was associated with higher 24-h RQ and lower 24-h lipox. To our knowledge, the relationship between these markers of water balance (i.e. copeptin, 24-h UVol, and 24-h UUN) and these measurements from the metabolic chamber over a 24-h period has not been reported in humans. Lastly, we showed that volunteers with biomarkers indicating lower fluid intake, also subsequently ate less using an objective measure of DEI, further highlighting the interdependency of food and fluid intake.
Fluid restriction reduces ad libitum EI without reducing food palatability indicating that there are shared mechanisms that regulate both food and water intake [20]. There may be an adaptive reason for a positive association between water and EI. A reduction in EI diminishes water movement into the gut for digestion and reduces intake of osmolytes, potentially worsening cellular dehydration and hyperosmolemia [17].
We observed a metabolic shift to lower RQ in association with body water conservation (i.e. higher 24-h UUN and lower 24-h UVol), indicating an increased reliance upon fat oxidation when volunteers are less hydrated. A similar reorganization in fuel selection is seen in animals under pressure to retain body water, usually in hot, arid environmental conditions [44]. Estivation is the term used to denote a variety of preservation strategies used to conserve water and forestall death. Animals display evidence of fuel switching during dehydration stress. The desert snail Otala lactea, estivating in the heat, inhibits lipogenesis and glycogen synthesis, resulting in a switch to fuel reserves for energy production [45]. Mice fed high-salt food and given salinized drinking water, exhibited an estivation-like phenotype and predominantly rely upon fat oxidation for energy production [10]. These mice also had increased protein catabolism and ureagenesis to supply urea to the renal medullary interstitium to support the kidney’s ability to concentrate urine. Since ureagenesis and gluconeogenesis are energy intensive and are in competition, the shift away from carbohydrate oxidation was interpreted as sparing hepatic energy for ureagenesis. In the current study, a shift towards lower RQ in our volunteers who were less hydrated may be evidence of an estivation-like phenotype. Moreover, the metabolic shift to lower RQ and using on-board fuel reserves may also be advantageous if food availability is low, as is typical during times of drought.
Estivation is also characterized by a state of light dormancy that is rapidly reversible (unlike hibernation) and associated with hypometabolism as a life-extending strategy [44]. A reduction in energy expenditure may benefit an organism in times of drought to reduce water loss from pulmonary respiration and reduce the need for food. We observed a lower 24-h energy expenditure in association with lower 24-h UVol and higher 24-h UUN only in Group 2 but not in Group 1. The discrepant finding may be due to differences between the groups. Group 2 was overall less hydrated than Group 1 and had a larger sample size.
Although copeptin as a marker of AVP production was a plausible mediator between hydration markers and substrate oxidation, we found that the associations between and 24-h RQ and hydration biomarkers were independent of copeptin. However, higher copeptin itself was associated with higher 24-h RQ and lower 24-h lipox, independent of 24-h urinary hydration markers. This result is consistent with known anti-lipolytic effects of AVP that may occur via alteration in blood flow to different fat depots which influences release of fatty acids into circulation [46]. Consistent with an effect in decreasing lipid oxidation, copeptin predicts incident abdominal obesity in humans [27]. Copeptin has a circadian rhythm in individuals [47] which was controlled for in our study as samples were collected fasting and at the same time in the morning after exiting the metabolic chamber. In our study, copeptin concentration was not correlated with 24-h UVol, consistent with what others have found in other populations [48]. On the other hand, a large study detected a relatively weak correlation (r ≈ −0.25) between copeptin and 24-h UVol [49]. The differences may be due to differences in sample size or copeptin assay used.
Though 24-h UVol varied widely among our participants, the average volume was greater than what is reported by others [50]. One possible explanation is the hot arid climate of Phoenix, Arizona. Alternatively, confinement in the metabolic chamber may have led to greater fluid intake, compared with free-living conditions. Nevertheless, we had adequate variability on our hydration markers to analyze associations with metabolic parameters.
A limitation of the current study is that actual water intake was not measured. Though 24-h UVol and UUN are well correlated with fluid intake [29, 30], they are nonetheless surrogate measures of water intake. It is unclear how other surrogate measures such as urine osmolality would compare with the urine biomarkers chosen in this study though we suspect that results would not differ since 24-h UVol, UUN, and urine osmolality are similarly well-correlated with fluid intake [29, 30]. In addition, hydration can be defined in terms of total body water or the balance between extracellular and intracellular water. It is unclear how hydration defined in this way would have performed in this study since they were not assessed. Moreover, correlations between hydration biomarkers and 24-h RQ, 24-h EE, and DEI ranged from weak to moderate. Thus, these results should be confirmed by other laboratories. We also acknowledge that the study population were primarily Native Americans living in the desert, possibly limiting the generalizability of our results.
In summary, hydration markers, indicating a tendency to drink less, were associated with reduced ad libitum EI, supporting the interdependency between food and water intake. Hydration markers indicating lower hydration status were also associated with a reduced RQ and possibly lower 24-h EE. Together, lower hydration status is associated with metabolic alterations that may be indicative of an adaptive survival strategy when faced with inadequate environmental water. Copeptin concentration did not account for this metabolic reorganization though was associated with a metabolic shift to higher lipid oxidation. The results of this study highlight the importance of the link between water balance and energy metabolism.
Supplementary Material
Acknowledgments
We thank the nursing, clinical, and dietary staffs and laboratory technicians of the clinical research center for their valuable assistance and care of the volunteers.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- 24-h EE
24-hour energy expenditure
- 24-h EB
24-hour energy balance
- 24-h NPRQ
24-hour non-protein respiratory quotient
- 24-h RQ
24-hour respiratory quotient
- 24-h UUN
24-hour urine urea nitrogen concentration
- 24-h UVol
24-hour urine volume
- 24-h carbox
24-hour carbohydrate oxidation
- 24-h lipox
24-hour lipid oxidation
- AVP
arginine vasopressin
- DEI
daily energy intake
- EE
energy expenditure
- EI
energy intake
- FFM
fat-free mass
- FM
fat mass
- RQ
respiratory quotient
- SPA
spontaneous physical activity
- %fat
percentage body fat
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Mavani GP, DeVita MV, Michelis MF. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front Med (Lausanne). 2015;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enhorning S, Melander O. The Vasopressin System in the Risk of Diabetes and Cardiorenal Disease, and Hydration as a Potential Lifestyle Intervention. Ann Nutr Metab. 2018;72 Suppl 2:21–7. [DOI] [PubMed] [Google Scholar]
- 3.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba B, Raz I, Saad M, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. American Journal of Physiology-Endocrinology And Metabolism. 1990;259(5):E650–E7. [DOI] [PubMed] [Google Scholar]
- 4.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. The Journal of Clinical Endocrinology & Metabolism. 2015;100(8):3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The Journal of Clinical Endocrinology & Metabolism. 2013;98(4):E703–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. The American journal of clinical nutrition. 2007;86(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. New England Journal of Medicine. 1988;318(8):467–72. [DOI] [PubMed] [Google Scholar]
- 8.Stookey JJ. Negative, Null and Beneficial Effects of Drinking Water on Energy Intake, Energy Expenditure, Fat Oxidation and Weight Change in Randomized Trials: A Qualitative Review. Nutrients. 2016;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohenegger M, Laminger U, Om P, Sadjak A, Gutmann K, Vermes M. Metabolic effects of water deprivation. J Clin Chem Clin Biochem. 1986;24(5):277–82. [DOI] [PubMed] [Google Scholar]
- 10.Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017;127(5):1944–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocelak P, Zak-Golab A, Rzemieniuk A, Smetek J, Sordyl R, Tyrka A, et al. The influence of oral water load on energy expenditure and sympatho-vagal balance in obese and normal weight women. Arch Med Sci. 2012;8(6):1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubnov-Raz G, Constantini NW, Yariv H, Nice S, Shapira N. Influence of water drinking on resting energy expenditure in overweight children. Int J Obes (Lond). 2011;35(10):1295–300. [DOI] [PubMed] [Google Scholar]
- 13.Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, Jordan J. Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab. 2007;92(8):3334–7. [DOI] [PubMed] [Google Scholar]
- 14.Boschmann M, Steiniger J, Hille U, Tank J, Adams F, Sharma AM, et al. Water-induced thermogenesis. J Clin Endocrinol Metab. 2003;88(12):6015–9. [DOI] [PubMed] [Google Scholar]
- 15.Sharief NN, Macdonald I. Differences in dietary-induced thermogenesis with various carbohydrates in normal and overweight men. Am J Clin Nutr. 1982;35(2):267–72. [DOI] [PubMed] [Google Scholar]
- 16.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19(14):6111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts AG, Boyle CN. The functional architecture of dehydration-anorexia. Physiol Behav. 2010;100(5):472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolles RC. The interaction of hunger and thirst in the rat. J Comp Physiol Psychol. 1961;54:580–4. [DOI] [PubMed] [Google Scholar]
- 19.Finger FW, Reid LS. The effect of water deprivation and subsequent satiation upon general activity in the rat. J Comp Physiol Psychol. 1952;45(4):368–72. [DOI] [PubMed] [Google Scholar]
- 20.Engell D Interdependency of food and water intake in humans. Appetite. 1988;10(2):133–41. [DOI] [PubMed] [Google Scholar]
- 21.Phillips PA, Rolls BJ, Ledingham JG, Morton JJ. Body fluid changes, thirst and drinking in man during free access to water. Physiol Behav. 1984;33(3):357–63. [DOI] [PubMed] [Google Scholar]
- 22.de Castro JM. A microregulatory analysis of spontaneous fluid intake by humans: evidence that the amount of liquid ingested and its timing is mainly governed by feeding. Physiol Behav. 1988;43(6):705–14. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura M, Nishimura K, Nishimura H, Sonoda S, Ueno H, Motojima Y, et al. Activation of endogenous arginine vasopressin neurons inhibit food intake: by using a novel transgenic rat line with DREADDs system. Sci Rep. 2017;7(1):15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer AH, Langhans W, Scharrer E. Vasopressin reduces food intake in goats. Q J Exp Physiol. 1989;74(4):465–73. [DOI] [PubMed] [Google Scholar]
- 25.Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92(4):1813–64. [DOI] [PubMed] [Google Scholar]
- 26.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9. [DOI] [PubMed] [Google Scholar]
- 27.Enhorning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, et al. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmo Diet and Cancer Study cardiovascular cohort. Int J Obes (Lond). 2013;37(4):598–603. [DOI] [PubMed] [Google Scholar]
- 28.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5 Pt 1):E650–7. [DOI] [PubMed] [Google Scholar]
- 29.Perrier E, Vergne S, Klein A, Poupin M, Rondeau P, Le Bellego L, et al. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr. 2013;109(9):1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier E, Rondeau P, Poupin M, Le Bellego L, Armstrong LE, Lang F, et al. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur J Clin Nutr. 2013;67(9):939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong LE, Johnson EC, Munoz CX, Swokla B, Le Bellego L, Jimenez L, et al. Hydration biomarkers and dietary fluid consumption of women. J Acad Nutr Diet. 2012;112(7):1056–61. [DOI] [PubMed] [Google Scholar]
- 32.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91(2):343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes (Lond). 2014;38(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329(27):1988–92. [DOI] [PubMed] [Google Scholar]
- 35.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53(6):1368–71. [DOI] [PubMed] [Google Scholar]
- 36.Genuth S, Alberti K, Bennett P, Buse J, DeFronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes care. 2003;26(11):3160–8. [DOI] [PubMed] [Google Scholar]
- 37.Votruba SB, Kirchner H, Tschop M, Salbe AD, Krakoff J. Morning ghrelin concentrations are not affected by short-term overfeeding and do not predict ad libitum food intake in humans. Am J Clin Nutr. 2009;89(3):801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78(6):1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. The American journal of clinical nutrition. 1995;62(4):730–4. [DOI] [PubMed] [Google Scholar]
- 40.Chang DC, Piaggi P, Krakoff J. A Novel Approach to Predict 24-Hour Energy Expenditure Based on Hematologic Volumes: Development and Validation of Models Comparable to Mifflin-St Jeor and Body Composition Models. J Acad Nutr Diet. 2017;117(8):1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott WG, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255(3 Pt 1):E332–7. [DOI] [PubMed] [Google Scholar]
- 42.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. [DOI] [PubMed] [Google Scholar]
- 44.Storey KB, Storey JM. Aestivation: signaling and hypometabolism. J Exp Biol. 2012;215(Pt 9):1425–33. [DOI] [PubMed] [Google Scholar]
- 45.Ramnanan CJ, McMullen DC, Groom AG, Storey KB. The regulation of AMPK signaling in a natural state of profound metabolic rate depression. Mol Cell Biochem. 2010;335(1–2):91–105. [DOI] [PubMed] [Google Scholar]
- 46.Rofe AM, Williamson DH. Mechanism for the ‘anti-lipolytic’ action of vasopressin in the starved rat. Biochem J. 1983;212(3):899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beglinger S, Drewe J, Christ-Crain M. The Circadian Rhythm of Copeptin, the C-Terminal Portion of Arginine Vasopressin. J Biomark. 2017;2017:4737082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77(1):29–36. [DOI] [PubMed] [Google Scholar]
- 50.Enhorning S, Tasevska I, Roussel R, Bouby N, Persson M, Burri P, et al. Effects of hydration on plasma copeptin, glycemia and gluco-regulatory hormones: a water intervention in humans. Eur J Nutr. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.