Abstract
There has been growing interest on the effect of sleep problems on psychotic and prodromal symptoms. The current study investigated cross-sectional relations between sleep problems and attenuated psychotic symptoms in a large sample of 740 youth at Clinical High Risk (CHR) for psychosis in an attempt to replicate previous findings and assess whether findings from general population samples and psychotic samples extend to this CHR sample. Sleep problems were found to be significantly positively associated with attenuated psychotic symptom severity. Sleep problems were also found to be more closely associated with certain specific prodromal symptoms (e.g., suspiciousness and perceptual abnormalities) than other attenuated psychotic symptoms. Further, we found that depression mediated the cross-sectional association between sleep problems and paranoid symptoms only. This adds to a growing body of evidence suggesting the mediation role of depression is more pronounced for paranoid-type psychotic symptoms as compared to other psychotic symptoms (e.g., hallucinations).
Keywords: CHR, schizophrenia-spectrum, prodrome, prodromal, insomnia
1. Introduction
There is a growing interest and recognition of the role sleep problems play in the pathophysiology of psychiatric illness, including psychosis. Sleep problems are common in psychotic disorders with between 30 to 80% of patients with schizophrenia reporting significant sleep disturbance (Cohrs, 2008). In patients with schizophrenia, sleep disruption has been found to precede relapse (Benson, 2008). Retrospective studies of those with a first episode of psychosis show that sleep disturbance was among the most common prodromal symptoms of the disorder (Yung & McGorry, 1996). Recent prospective studies also suggest that sleep disturbance is common among individuals at clinical-high risk (CHR) for psychosis and is associated with more severe attenuated psychotic symptoms (e.g., sub-clinical hallucinations, delusions, grandiosity) (Poe et al., 2017; Lunsford-Avery et al., 2017). Additionally, there is preliminary evidence that certain aspects of sleep disturbance (e.g., specific kinds of circadian rhythm disruptions measured through actigraphy, and reduced sleep duration) may be associated with worsening prodromal symptoms over time (Lunsford-Avery et al. 2017; 2015; Reeve et al., 2018). These findings point to a potential role of sleep dysfunction in the pathophysiology of psychosis-spectrum disorders and highlight the need to better understand this relationship in CHR samples.
There is also evidence that sleep problems may be associated with specific types of psychotic-like symptoms. For example, epidemiological surveys have found that a diagnosis of insomnia is associated with a five times greater risk of paranoid thoughts (e.g., “a group of people are plotting to harm me”), and a four to five times greater likelihood of transient hallucinatory experiences (e.g., “hearing name being called when no-one was around”) (Freeman et al., 2012). In addition, sleep deprivation studies utilizing experimental and observational designs have documented associations between acute sleep loss and psychotic experiences. For example, Hurdiel and colleagues (2014) observed ultra-marathon runners who maintained wakefulness for approximately 46 hours and found that 4 of the 17 (24%) participants experienced hallucinatory experiences during their sleep deprived state. Sleep deprivation has also been associated with increased paranoid thoughts and perceptual distortions in experimental studies with healthy adults (Reeve, Emsley, Sheaves & Freeman 2017; Petrovsky et al., 2014). Taken together, the evidence from general population studies suggests that sleep disturbance may be specifically associated with transient suspiciousness/paranoid-type thoughts and perceptual abnormalities/hallucinations in otherwise healthy individuals. Given the idea that psychotic symptoms may exist on a continuum (Van Os, Linscott, Myin-Germeys, Delespaul & Krabbendam, 2009), we might expect to see the same pattern of psychotic-like symptoms affected by sleep problems in clinical samples as well is seen in healthy samples. Indeed, one recent study of 160 individuals at high risk for psychosis revealed that shorter sleep duration was associated with more severe delusional/persecutory ideas and hallucinations (Reeve et al., 2018). Together, this suggests that the same specificity of sleep and psychotic-like symptoms exists that exists in healthy populations also extends to those with the more enduring attenuated psychotic symptoms observed in CHR and patient populations.
Several studies have investigated potential mediators of the relationship between sleep problems and psychotic symptoms. A systematic review linking sleep dysfunction and psychotic experiences found that depression was the most commonly tested mediator and that it partially mediated the relationship between sleep dysfunction and psychotic experiences in a number of studies (Reeve, Sheaves & Freeman, 2015). The role of depression in the relationship between sleep and psychotic symptoms is consistent with cognitive models of persecutory delusions (Freeman et al., 2002) and hallucinatory experiences (Waters et al., 2012), in which negative affect (encompassing symptoms of depression and anxiety) is posited to play an important role in symptom formation. These models posit that negative beliefs about the self and the world, as well as negative affective states, result in certain biases when selecting explanations for and making meaning from anomalous internal experiences (e.g., perceptual abnormalities) and recent external experiences (e.g., ambiguous interpersonal interactions). Thus, ambiguous interpersonal interactions may be interpreted as threatening, fueling paranoia, and perceptual abnormalities are interpreted as malevolent (e.g., hearing voices saying personally insulting things). The role of depression in these associations is also relevant given the high rates of co-morbid depression observed in psychosis-spectrum populations. Between 40–50% of CHR and schizophrenia patients also meet criteria for a depressive disorder (Buckley, Miller, Lehrer & Castle, 2008). Thus, clarifying whether depression mediates the relationship between sleep and psychotic symptoms will have important implications for treatment approaches and targets with CHR individuals.
The current study investigated the associations between sleep disturbance, attenuated psychotic symptoms, and clinical outcomes in a large sample of CHR individuals drawn from the North American Prodrome Longitudinal Study (NAPLS-II). The aims were to 1) determine whether CHR individuals report greater sleep disturbance relative to healthy controls, 2) examine whether sleep problems are associated with specific types of attenuated psychotic symptoms, 3) test whether depression mediates the relationship between sleep problem and attenuated psychotic symptoms, and 4) explore whether sleep disturbance predicts subsequent worsening of prodromal psychotic symptoms and/or conversion to a psychotic illness.
Based on previous research in CHR samples (i.e., Poe et al., 2017; Lunsford-Avery et al., 2017), we predicted that sleep disturbance would be more severe in the CHR group in comparison to the healthy control (HC) group. Within the CHR group, we hypothesized that sleep disturbance would be positively associated with the severity of attenuated psychotic symptoms at baseline, and that these associations would be strongest for paranoia/suspiciousness and perceptual abnormalities symptoms. We also predicted that sleep disturbance would have an indirect effect on total attenuated psychotic symptoms through depressive symptoms. Finally, based on previous findings that sleep disturbance predicts worsening of psychotic symptoms over time (Lunsford-Avery et al. 2017), we hypothesized that sleep disruption reported at baseline would predict progression of the psychosis risk syndrome and/or conversion to a psychotic disorder at subsequent time points.
2. Methods
2.1. Participants
See Table 2. for detailed demographic and sample characteristics. The total sample included 1020 individuals between 12–30 years of age. Of the 1020 participants 740 (72.5%) met clinical-high risk (CHR) criteria for psychosis; 280 (27.5%) were healthy controls. All participants were recruited as part of the North American Prodrome Longitudinal Study (NAPLS-2). Specific details about ascertainment, inclusion, and exclusion criteria have been described in detail elsewhere (Addington et al. 2012).
Table 2.
Sample Characteristics
| CHR | Control | |
|---|---|---|
| N | 740 | 280 |
| Age, years (mean ± SD)** | 18.5 (± 4.26) | 19.7 (± 4.67) |
| Sex, n (%)* | ||
| Males | 424 (57.3%) | 141 (50.4%) |
| Females | 316 (42.7%) | 139 (49.6%) |
| Race, n (%) | ||
| White | 426 (57.6%) | 152 (54.3%) |
| Black | 111 (15%) | 49 (17.5%) |
| Interracial | 94 (12.7%) | 29 (10.4%) |
| Central/South American | 32 (4.3%) | 13 (4.6%) |
| East Asian | 19 (2.6%) | 15 (5.4%) |
| South Asian | 20 (2.7%) | 8 (2.9%) |
| Southeast Asian | 15 (2.0%) | 7 (2.5%) |
| First Nations | 13 (1.8%) | 4 (1.4%) |
| West/Central Asia and Middle East | 6 (0.8%) | 2 (0.7%) |
| Native Hawaiian or Pacific Islander | 3 (0.4%) | 1 (0.4%) |
| Ethnicity (Hispanic/Lantinx), n (%) | 136 (18.4%) | 50 (17.9%) |
| Attenuated Psychotic Symptoms, mean (SD)** | 11.86 (3.832) | 1.07 (1.67) |
| Negative Symptoms, mean (SD) ** | 11.89 (6.067) | 1.46 (2.23) |
| Sleep Disturbance, mean (SD) ** | 2.31 (1.568) | 0.48 (.904) |
Percentages add up to > 100% because one participant can score multiple items.
p <.05
p<.01
2.2. Assessments
2.2.1. CHR status
Clinical-high risk (CHR) designation was based on the Structured Interview for Psychosis-Risk Syndromes (SIPS) (McGlashan, Walsh & Woods, 2010). This semi-structured interview assesses prodromal symptoms across 4 symptom domains: 1.) Positive (e.g., unusual thought content/ideas, suspiciousness/persecutory ideas, grandiosity, perceptual abnormalities, and disorganized communication); 2.) Negative (e.g., social isolation, avolition, decreased expression of emotion, decreased experience of emotions and self, decreased ideational richness, and deterioration of role functioning), 3.) Disorganized (e.g., odd behavior of appearance, bizarre thinking, trouble with focus and attention, and impairment in personal hygiene or social attentiveness), and 4.) General (e.g., difficulty related to sleep disturbance, dysphoric mood, motor disturbances, and impaired tolerance to normal stress). After administration of the SIPS, each participant’s their attenuated psychotic symptoms are then rated on a 0–6 scale on the Scale of Prodromal Symptoms (SOPS). CHR status is based on the severity of the positive attenuated psychotic symptoms (see Addington et al., 2012 for details). The SIPS was administered at each study time point (i.e., 6m, 12m, 18m, 24m) to assess severity of prodromal symptoms and current clinic state.
2.2.2. Current clinical state
Current Clinical State was determined at each subsequent visit (6 months, 12 months, 18 months, 24 months) using symptom ratings from the SOPS. CHR participants are classified either as ‘symptomatic’ (i.e., scores between 3 and 5 on the SOPS positive symptoms with no recent changes in severity), ‘prodromal progression’ (i.e., scores between 3 and 5 on the SOPS positive symptoms with a recent increase in severity) ‘converted’ (i.e., currently meeting criteria for a psychotic disorder), or ‘in remission’ (i.e., scores of 2 or less on each of the SOPS positive symptoms). The last available clinical state classification was used as the outcome measure.
2.2.3. Sleep disturbance
Measures of sleep disturbance were obtained the general symptoms section of the SOPS, which includes an item assessing sleep patterns and concerns (G1). The Sleep Disturbance Item is rated from 0–6 based on the severity of the disturbance. The ratings are as follows: 0 – Absent/No sleep disturbance, 1 – Questionably Present (e.g., restless sleep), 2 – Mild (e.g., some difficulty falling asleep), 3 – Moderate (e.g., daytime fatigue due to difficulty sleeping), 4 – Moderately Severe (e.g., sleep pattern interfering with other aspects of functioning), 5 – Severe (e.g., day/night reversal and missing activities due to sleep problems), 6 – Extreme (e.g., unable to sleep for over 48 hours). Unlike the positive attenuated psychotic symptoms, there were no restrictions on the sleep item in terms of qualifying as CHR. Thus, scores on the sleep variable were allowed to vary across the full range (from 0–6) for all participants across the entire sample.
2.2.4. Depression symptoms
The Calgary Depression Scale for Schizophrenia
The CDSS (Addington, Addington & MatickaTyndale, 1993) was designed to measure depressive symptoms in patients with schizophrenia and shows good reliability and validity for measuring depressive symptoms in those at CHR of psychosis (Addington, Shah, Liu & Addington, 2014). It is administered by an experienced rater in the context of a clinical interview and assesses nine symptoms of depression that are summed to create a total score indicating severity of depressive symptoms.
2.3. Statistical analyses
All analyses were performed using SPSS version 25. Analysis of covariance (ANCOVA) was conducted to compare severity of sleep disturbance between groups (CHR versus HC). All subsequent analyses were performed only in the CHR group. Linear regression was used to examine the associations between baseline sleep disturbance and total positive symptom severity. A series of MANCOVA and ANCOVAs were used to examine the associations between sleep disturbance and specific attenuated psychotic symptoms (i.e., delusions, suspiciousness/paranoia, grandiosity, perceptual abnormalities, disorganized communication). To test for mediation, both direct and indirect effects were examined using a bootstrapping approach with the PROCESS macro for SPSS (Hayes, 2013). Confidence intervals that do not include zero indicate a significant effect. Finally, an ANCOVA was conducted to test whether the 4 clinical state outcome groups (remission, symptomatic, prodromal progression, and converted) differed on baseline sleep disturbance. All participants were coded as belonging to one of the 4 outcome groups using the last observation carried forward (LOCF) method.
Prior to analyses, we tested whether baseline medication status (psychotropic and sleep medications), cannabis use, and alcohol use were related to the sleep variable. We found no significant associations with any medication class or with alcohol use (see Table 3) and medication or alcohol use status was therefore not included as a covariate in the analyses. The lack of association between sleep disturbance and use of sleep medication likely indicates that the use of sleep medication is effective at reducing sleep disturbance. Lifetime cannabis use was significantly associated with the sleep variable [t(722)=−2.386, p=0.017] such that those who reported any cannabis use had higher mean scores on the sleep variable, indicating poorer sleep (n=399, M=2.43) and those who denied ever using cannabis had lower mean scores on the sleep variable, indicating better sleep (n=325, M=2.15). Thus, cannabis use was included as a covariate in all analyses.
Table 3.
Results of Correlations and T-tests comparing sleep disturbance rating in CHR participants with and without medication or alcohol use reported at baseline
| Type of Medication | Mean Sleep Disturbance Rating |
Correlation (rpb) | t-value | df | p-value | |
|---|---|---|---|---|---|---|
| Substance Use (n) | No substance use (n) | |||||
| Sleep Medication | 2.63 (42) | 2.29 (722) | .050 | −1.359 | 738 | .174 |
| Antipsychotic | 2.20 (213) | 2.36 (551) | −.046 | 1.239 | 738 | .216 |
| Antidepressant | 2.39 (324) | 2.25 (440) | .044 | −1.192 | 738 | .234 |
| Mood Stabilizer | 2.26 (77) | 2.32 (687) | −.011 | .302 | 738 | .763 |
| Stimulant | 2.20 (166) | 2.34 (598) | −.037 | 1.015 | 738 | .311 |
| Benzodiazepine | 2.58 (93) | 2.27 (671) | .065 | −1.778 | 738 | .076 |
| Any Psychotropic | 2.32 (464) | 2.29 (300) | .011 | −.287 | 738 | .774 |
| Alcohol | 2.33 (292) | 2.30 (444) | .009 | −.235 | 721 | .815 |
3. Results
Demographic and symptom characteristics of the sample are shown in in Table 2. The CHR group was younger (t=3.759, p<0.001) and had a larger proportion of males compared to the HC group (chi=3.960, p=0.047). There were no group differences in race or ethnicity. Age, sex, and cannabis use were included as covariates in analyses comparing CHR and HC differences. However, neither age nor sex were significantly associated with severity of sleep problems within the CHR group. CHR individuals reported higher levels of sleep disturbance [F(1,995)=306.975, p<.001] compared to controls.
3.1. Sleep disturbance and attenuated psychotic symptoms
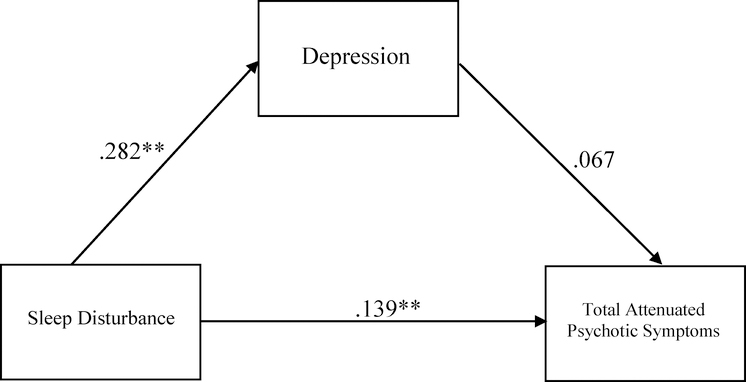
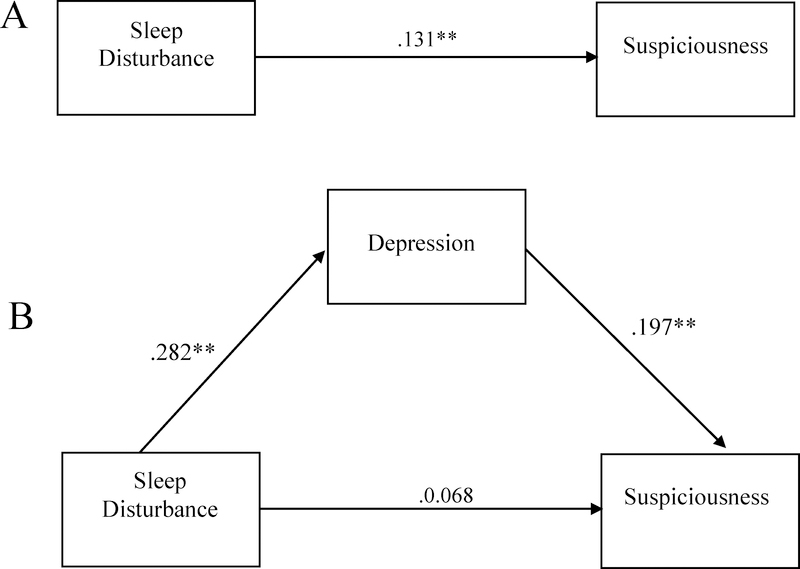
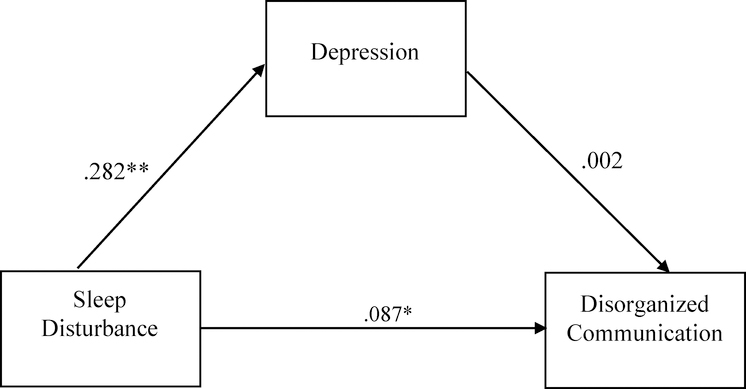
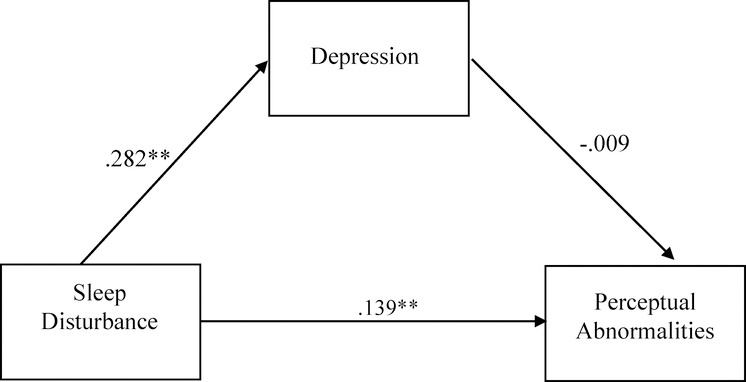
In the CHR group, sleep disturbance was positively associated with total positive symptom severity (R2=.032 F(2,720)=11.714, p<.01, sleep disturbance β=.160). Post-hoc analyses were conducted to determine whether this association differed between CHR individuals with a clinically significant sleep disturbance (i.e., rated as a 3 or above on the SOPS) from those without (i.e., rated as 0–2 on the SOPS). There was a group difference (clinical vs. nonclinical sleep disturbance) in the severity of total attenuated psychotic symptoms [F(1, 737)=12.365, p<.001], such that the clinical sleep disturbance group had higher levels of total attenuated psychotic symptoms. Follow-up analyses of specific attenuated psychotic symptoms showed significant group differences in severity of suspiciousness/paranoia [F(1,720)=7.54, p=.006), perceptual abnormalities/hallucinations [F(1,720)=10.719, p=0.001], and disorganized communication [F(1,720)=4.696, p=0.031]. No significant group differences were observed in the severity of unusual thought content/delusions or grandiosity symptoms. After applying the Bonferroni method to correct for multiple comparisons, only the association between sleep problems and suspiciousness/paranoia and the association between sleep problems and perceptual abnormalities/hallucinations remained significant. We then tested for an indirect effect of depression in the association between sleep disturbance and total attenuated psychotic symptoms, and we found no significant indirect effect (Figure 3). This was repeated for each specific attenuated psychotic symptom associated with sleep disturbance (i.e., suspiciousness, perceptual abnormalities, disorganized communication) (Figures 4–6). There was an indirect effect of sleep disturbance only on suspiciousness/persecutory symptoms through depression (b=.0537, CI (95%)= .0319 – .0787), but no significant indirect effect was found between sleep disturbance and perceptual abnormalities or disorganized communication.
Figure 3.
Standardized regression coefficients for the relationship between sleep disturbance and total attenuated psychotic symptoms (controlling for cannabis use). The indirect effect through depression was not significant (Unstandardized indirect effect: b = .0467, 95% CI [−.0064, .1028]). *p< .05, **p<.01
Figure 4.
Standardized regression coefficients for the direct effect between sleep disturbance and suspiciousness without depression (controlling for cannabis use) (A) and indirect effect between sleep disturbance and suspiciousness through depression (controlling for cannabis use) (B). The indirect effect was statistically significant (Unstandardized indirect effect: b = .0537, 95% CI [.0319, .0787]). *p<.05, **p<.01
Figure 6.
Standardized regression coefficients for the relationship between sleep disturbance and disorganized communication (controlling for cannabis use). The indirect effect through depression was not significant (Unstandardized indirect effect: b = .0005, 95% CI [−.0198, .0215]). *p< .05, **p<.01
3.2. Sleep disturbance and clinical outcome
There were no significant differences in baseline sleep disturbance among the four clinical outcome groups (remission, symptomatic, prodromal progression, conversion). This result remained both when controlling for cannabis use and without controlling for cannabis use.
4. Discussion
The current study examined the associations between sleep disturbance, attenuated psychotic symptoms and clinical outcomes in a large sample of CHR individuals. Consistent with our hypotheses, we found that CHR individuals reported greater sleep disturbance than healthy controls and that the severity of sleep disturbance was positively associated with concurrent severity of attenuated psychotic symptoms. We advance the current literature by demonstrating that these sleep disturbance and positive symptom associations are specific to suspiciousness (precursor of paranoia), perceptual abnormalities (precursor of hallucinations), and disorganized speech (indicator/precursor of thought disorder). Contrary to our hypothesis, depression did not mediate the association between sleep disturbance and total attenuated psychotic symptoms; however, it did mediate the association with suspiciousness. Additionally, baseline sleep disturbances did not differ among clinical outcomes groups.
Our finding that greater sleep disturbance is associated with more severe attenuated psychotic symptoms in CHR individuals is consistent with studies in the general population (Freeman et al., 2012) as well as those in at risk samples (Poe et al., 2017; Lunsford-Avery et al., 2017; Reeve et al., 2018). Specific associations between sleep disturbance and suspiciousness and between sleep disturbance and perceptual abnormalities have also been reported in previous literature and were replicated in the current study (Freeman et al., 2012; Reeve et al., 2018). We did not originally hypothesize that disorganized speech would be specifically associated with sleep disturbance, as this was not a consistent finding in the previous literature. However, two experimental studies separately found cognitive disorganization was one of the psychotic-like symptoms elicited by sleep deprivation in healthy subjects (Petrovsky et al., 2014; Reeve, Emsley, Sheaves & Freeman, 2017). In the current study, the association between sleep disturbance and disorganized speech did not remain significant after correcting for multiple comparisons. This fact combined with the generally sparse previous evidence of association suggests that if sleep problems and cognitive disorganization are indeed associated in healthy samples and/or CHR samples, the relationship is relatively weaker than the relationship between sleep disturbance and suspiciousness or perceptual abnormalities. Overall, when comparing the current findings with previous literature on healthy samples, it appears that the same pattern of association between sleep disturbance and psychotic-like symptoms is observed in both CHR individuals and healthy participants under conditions of sleep deprivation.
Interestingly, in our analysis of depression as a mediator between sleep disturbance and psychotic symptoms, we found no significant indirect effects on total positive symptom severity. This finding appears at odds with conclusions from a systematic review of the literature (Reeve et al., 2015) which reported that depression is consistently found to be a partial mediator in the relationship between sleep dysfunction and psychotic experiences. However, studies included in this review varied in their definition of „psychotic symptoms‟, and those that found a mediating effect of depression tended to focus on paranoid/suspicious dimension of psychotic symptoms (e.g., Freeman, Pugh, Vorontsova & Southgate, 2009; Freeman et al., 2012; Myers, Startup & Freeman, 2011). In addition, two recent investigations have found that depression/negative affect is a more significant mediator of the relation of sleep with paranoia, than with other psychotic symptoms (e.g., hallucinations) (Reeve, Emsley, Sheaves & Freeman, 2018; Reeve, Nickless, Sheaves & Freeman, 2018). Thus, our current findings are consistent with growing evidence that the mediation role of depression is more pronounced for paranoid-type psychotic symptoms. Further, our findings also lend support to the idea that sleep problems may be associated with different prodromal symptoms through different mechanisms (Reeve, Nickless, Sheaves & Freeman, 2018).
As previously mentioned, the cognitive model of persecutory delusions provides a framework for understanding how depression or negative affect may act as a mediator between sleep problems and paranoid thinking. This model posits that certain cognitive biases, often present in those with depression and anxiety, may predispose someone to select a threatening/suspicious explanation for neutral experiences (Freeman et al., 2002). Thus, if sleep disturbance increases depressive/anxious cognitive biases (as suggested in various studies including Gobin, Banks, Fins & Tartar, 2015 and Alfano, Zakem, Costa, Taylor & Weems, 2009), then suspiciousness/paranoid symptoms will also increase in severity. An increase in suspiciousness would, in turn, be expected to result in a state of hypervigilance which would be expected to further interfere with sleep. Preliminary support for the bidirectional association between sleep problems and paranoia was provided by a recent longitudinal investigation in 29 patients with psychosis (Reeve, Nickless, Sheaves & Freeman, 2018). The current study did not assess directionality of associations and focused only on the potential mediational role of depression in the relationship between sleep problems and psychotic symptoms. Future investigations testing different mediators, such as anxiety, and using longitudinal data are needed to better understand the specifics of the association of different psychotic symptoms and sleep problems over time. In addition, models and theories that could explain how sleep, depression and psychotic symptoms may be associated from a biological perspective may also help to clarify and guide future research directions in this area.
Finally, we did not find that baseline sleep disturbance predicted subsequent worsening of the prodromal syndrome and/or conversion to psychotic disorder. In fact, none of the outcome groups (conversion, prodromal progression, symptomatic, remission) differed in severity of baseline sleep disturbance. This null finding should be interpreted with caution given that this was not a longitudinal study. One explanation is that that these findings are due to the temporal lag between the baseline assessment of sleep and the subsequent designation of clinical outcome, which could have a duration of up to two years. The adverse effects of sleep disturbance on clinical status may be more proximal. Thus, it may be that sleep problems are related to psychotic symptom severity only contemporaneously, but are not predictive of continued worsening of symptoms over time. This is consistent with findings in the experimental sleep literature, which have found that acute sleep deprivation is associated with transient psychotic symptoms in a state-dependent way, such that psychotic-like symptoms are only present during the night of deprived sleep and dissipate during the day, or when recovery sleep is allowed (van Heugten-van der Kloet, Giesbrecht, & Merckelbach, 2015). However, this brings into question the nature of the association between sleep problems and psychotic symptoms over time and indicates the need for more detailed longitudinal investigations in a clinical sample. This is especially relevant given the recent interest in sleep problems as a potential target for preventative interventions for psychosis (e.g., Bradley et al., 2018; Waite, Bradley, Chadwick, Reeve, Bird & Freeman., 2018).
There are several important limitations in the current study that should be noted. First, sleep disturbance was assessed with a single item based largely on self-report, which has several inherent limitations. The sleep disturbance item was also assessed from the same scale used to measure attenuated psychotic symptoms. Future studies would benefit from using more independent and objective measures of sleep (e.g., actigraphy, sleep monitoring devices) that can also assess multiple aspects of sleep. Chronicity of sleep problems was also not assessed in this study, and it may be an important factor to consider when investigating the potential role of sleep problems in psychotic symptom exacerbation over time. For example, the neurological impact of a few bouts of insomnia over a lifetime may be negligible in comparison to long-term chronic insomnia. Finally, our analyses were cross-sectional in nature, and thus limit our ability to assess directionality of effects as well as our ability to assess true mediational effects over time. Additional longitudinal analyses of sleep and psychotic symptoms will be important to more fully understand the role of sleep problems in the development of psychotic disorders.
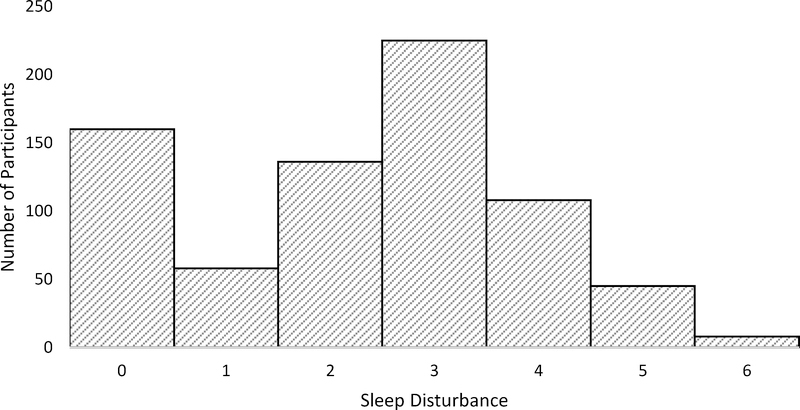
Figure 1.
Count data showing the distribution of healthy control participants across each level of sleep disturbance (0 = no sleep disturbance, 6 = extreme sleep disturbance)
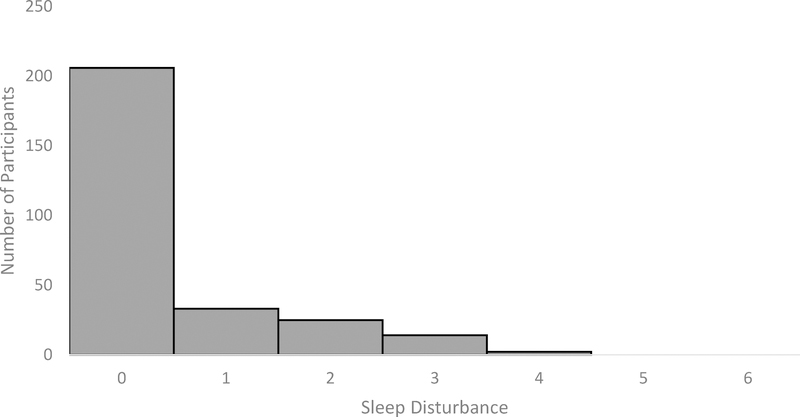
Figure 2.
Count data showing the distribution of clinical high risk participants across each level of sleep disturbance (0 = no sleep disturbance, 6 = extreme sleep disturbance)
Figure 5.
Standardized regression coefficients for the relationship between sleep disturbance and perceptual abnormalities (controlling for cannabis use). The indirect effect through depression was not significant (Unstandardized indirect effect: b = −.0026, 95% CI [−.0256, .0196]). *p< .05, **p<.01
Table 1.
G1 SIPS Sleep Disturbance Symptom, based on the SIPS (Miller et al., 1999)
| Symptom Scale | Descriptive Anchors* |
|---|---|
| 0 (Absent) | |
| 1 (Questionably Present) | Restless sleep. |
| 2 (Mild) | Some mild difficulty falling asleep or getting back to sleep. |
| 3 (Moderate) | Daytime fatigue resulting from difficulty falling asleep at night or early awakening. Sleeping more than considered average. |
| 4 (Moderately Severe) | Sleep pattern significantly disrupted and has intruded on other aspects of functioning (e.g. trouble getting up for school or work). Difficult to awaken for appointments. Spending a large part of the day asleep. |
| 5 (Severe) | Significant difficult falling asleep or awakening early on most nights. May have day/night reversal. Usually not getting to scheduled activities at all. |
| 6 (Extreme) | Unable to sleep at all for over 48 hours. |
Anchors in each scale are intended to provide guidelines and examples of signs for every symptom observed. It is not necessary to meet every criterion in any one anchor to assign a particular rating. Basis for ratings includes both interviewer observations and patient reports.
Table 4.
Summary of Univariate Analyses for Types of Attenuated Psychotic Symptoms
| Symptom | df | Mean Square | F | p-value | Group Mean (Sleep Disturbance) | Group Mean (No Sleep Disturbance) |
|---|---|---|---|---|---|---|
| Unusual Thought Content/Delusional Ideas | 1,720 | 2.682 | 1.529 | .217 | 3.40 | 3.26 |
| Suspiciousness/Persecutory Ideas | 1,720 | 16.941 | 7.540 | .006 | 2.89 | 2.57 |
| Grandiose Ideas | 1,720 | 1.649 | .970 | .325 | .94 | 1.03 |
| Perceptual Abnormalities/Hallucinations | 1,720 | 24.472 | 10.719 | .001 | 3.25 | 2.88 |
| Disorganized Communication | 1,720 | 10.043 | 4.696 | .031 | 1.82 | 1.58 |
Highlights.
Those at risk for psychosis have more sleep problems than controls
More severe sleep problems were associated with more severe psychotic symptoms
Sleep problems were specifically associated with paranoia and hallucinations
Depression mediated the relation between sleep problems and paranoia
Acknowledgements
This work was supported by a collaborative U01 award from the National Institute of Mental Health at the National Institutes of Health (MH081902 to TDC and CB; MH081857 to BAC; MH081988 to EW; MH081928 to LS; MH082004 to DP; MH081944 to KC; MH081984 to JA; MH082022 to SWW; and MH076989)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Addington D, Addington J, & Maticka-Tyndale E (1993). Assessing depression in schizophrenia: the Calgary Depression Scale. The British journal of psychiatry. [PubMed] [Google Scholar]
- Addington D, Addington J, & Schissel B (1990). A depression rating scale for schizophrenics. Schizophrenia research, 3(4), 247–251. [DOI] [PubMed] [Google Scholar]
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, … & Addington JA (2012). North American prodrome longitudinal study (NAPLS 2): overview and recruitment. Schizophrenia research, 142(1–3), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Epstein I, Liu L, French P, Boydell KM, & Zipursky RB (2011). A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia research, 125(1), 54–61. [DOI] [PubMed] [Google Scholar]
- Addington J, Shah H, Liu L, & Addington D (2014). Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophrenia research, 153(1–3), 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Zakem AH, Costa NM, Taylor LK, & Weems CF (2009). Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depression and anxiety, 26(6), 503–512. [DOI] [PubMed] [Google Scholar]
- Benson KL (2008). Sleep in schizophrenia. Sleep Medicine Clinics, 3(2), 251–260. [DOI] [PubMed] [Google Scholar]
- Bradley J, Freeman D, Chadwick E, Harvey AG, Mullins B, Johns L, … & Waite F (2018). Treating sleep problems in young people at ultra-high risk of psychosis: a feasibility case series. Behavioural and cognitive psychotherapy, 46(3), 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, & Castle DJ (2008). Psychiatric comorbidities and schizophrenia. Schizophrenia bulletin, 35(2), 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs S (2008). Sleep disturbances in patients with schizophrenia. CNS drugs, 22(11), 939–962. [DOI] [PubMed] [Google Scholar]
- Freeman D, Garety PA, Kuipers E, Fowler D, & Bebbington PE (2002). A cognitive model of persecutory delusions. British Journal of Clinical Psychology, 41(4), 331–347. [DOI] [PubMed] [Google Scholar]
- Freeman D, Pugh K, Vorontsova N, & Southgate L (2009). Insomnia and paranoia. Schizophrenia research, 108(1–3), 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Stahl D, McManus S, Meltzer H, Brugha T, Wiles N, & Bebbington P (2012). Insomnia, worry, anxiety and depression as predictors of the occurrence and persistence of paranoid thinking. Social psychiatry and psychiatric epidemiology, 47(8), 1195–1203. [DOI] [PubMed] [Google Scholar]
- Gobin CM, Banks JB, Fins AI, & Tartar JL (2015). Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. Journal of sleep research, 24(5), 535–542. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). The PROCESS macro for SPSS and SAS Software].
- Hurdiel R, Van Dongen HP, Aron C, McCauley P, Jacolot L, & Theunynck D (2014). Sleep restriction and degraded reaction-time performance in Figaro solo sailing races. Journal of sports sciences, 32(2), 172–174. [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Gonçalves BDSB, Brietzke E, Bressan RA, Gadelha A, Auerbach RP, & Mittal VA (2017). Adolescents at clinical-high risk for psychosis: circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophrenia research, 189, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, LeBourgeois MK, Gupta T, & Mittal VA (2015). Actigraphic-measured sleep disturbance predicts increased positive symptoms in adolescents at ultra high-risk for psychosis: a longitudinal study. Schizophrenia research, 164(1–3), 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, & A Mittal V (2013). Sleep dysfunction prior to the onset of schizophrenia: a review and neurodevelopmental diathesis–stress conceptualization. Clinical Psychology: Science and Practice, 20(3), 291–320. [Google Scholar]
- McGlashan T, Walsh B, & Woods S (2010). The psychosis-risk syndrome: handbook for diagnosis and followup. Oxford University Press. [Google Scholar]
- Myers E, Startup H, & Freeman D (2011). Cognitive behavioural treatment of insomnia in individuals with persistent persecutory delusions: a pilot trial. Journal of behavior therapy and experimental psychiatry, 42(3), 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Ettinger U, Hill A, Frenzel L, Meyhöfer I, Wagner M, … & Kumari V (2014). Sleep deprivation disrupts prepulse inhibition and induces psychosis-like symptoms in healthy humans. Journal of Neuroscience, 34(27), 9134–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe SL, Brucato G, Bruno N, Arndt LY, Ben-David S, Gill KE, … & Girgis RR (2017). Sleep disturbances in individuals at clinical high risk for psychosis. Psychiatry research, 249, 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve S, Emsley R, Sheaves B, & Freeman D (2017). Disrupting sleep: the effects of sleep loss on psychotic experiences tested in an experimental study with mediation analysis. Schizophrenia bulletin, 44(3), 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve S, Nickless A, Sheaves B, & Freeman D (2018). Insomnia, negative affect, and psychotic experiences: Modelling pathways over time in a clinical observational study. Psychiatry research, 269, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve S, Nickless A, Sheaves B, Hodgekins J, Stewart SLK, Gumley A, … & Freeman D (2019). Sleep duration and psychotic experiences in patients at risk of psychosis: A secondary analysis of the EDIE-2 trial. Schizophrenia research, 204, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve S, Sheaves B, & Freeman D (2015). The role of sleep dysfunction in the occurrence of delusions and hallucinations: a systematic review. Clinical psychology review, 42, 96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heugten–van der Kloet D, Giesbrecht T, & Merckelbach H (2015). Sleep loss increases dissociation and affects memory for emotional stimuli. Journal of behavior therapy and experimental psychiatry, 47, 9–17. [DOI] [PubMed] [Google Scholar]
- Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, & Krabbendam L (2009). A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychological medicine, 39(2), 179–195. [DOI] [PubMed] [Google Scholar]
- Waite F, Bradley J, Chadwick E, Reeve S, Bird JC, & Freeman D (2018). The Experience of Sleep Problems and Their Treatment in Young People at Ultra-High Risk of Psychosis: A Thematic Analysis. Frontiers in psychiatry, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, … & Vercammen A (2012). Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophrenia bulletin, 38(4), 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, & McGorry PD (1996). The initial prodrome in psychosis: descriptive and qualitative aspects. Australian and New Zealand Journal of Psychiatry, 30(5), 587–599. [DOI] [PubMed] [Google Scholar]