Abstract
Background:
Racial disparities in U.S. adult pneumococcal vaccination rates persist despite reduced barriers to access. Consequently, racial and ethnic minorities experience pneumococcal disease at higher rates than whites. This study examined prevalence of high risk conditions and pneumococcal hospitalizations among U.S. black and non-black populations aged ≥50 years.
Methods:
National Health Interview Survey, National Center for Health Statistics and National Inpatient Sample data were used to create black and non-black population cohorts, determine risk factors for pneumococcal disease (pneumococcal vaccine indications) and assess the impact of pneumococcal hospitalization. Each racial cohort was segmented into groups based on the presence of immunocompromising or other pneumococcal high-risk conditions. Persons without those conditions were separated into smokers (also a pneumococcal vaccine indication) and nonsmokers. Mortality was estimated from NCHS life table data. NIS data provided length of stay and costs (calculated from cost to charge ratios) for admissions related to pneumococcal disease including bacteremia, meningitis and nonbacteremic pneumonia.
Results:
There were similar proportions of immunocompromised (<5%) and smokers (14%) in both racial cohorts. Likelihood of non-immunocompromising pneumococcal high-risk conditions was higher for blacks than non-blacks at age 65, but higher for non-blacks than blacks at age 80 years (P<0.001). Age-specific relative likelihood of mortality was 1.1%−12% higher in blacks than non-blacks (P<0.001). Length of stay was significantly longer for blacks than non-blacks in all age and discharge status groups for non-bacteremic pneumonia and for blacks discharged alive with invasive pneumococcal disease. Costs were higher for blacks 65 years or older with invasive pneumococcal disease.
Conclusion:
Marked differences exist between U.S. black and non-black populations in likelihood of conditions conferring a high-risk of pneumococcal disease, and for length of stay and costs of pneumococcal disease hospitalizations. Further research is recommended to identify cost-effective policies or interventions to increase vaccine uptake in higher risk populations.
Introduction
Current adult pneumococcal vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC) are determined by age and health status. Adults aged ≥65 years are recommended to receive both pneumococcal vaccines: the 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 Adults aged <65 years with immunocompromising conditions are also recommended to receive both vaccines, while those younger than 65 years with certain high-risk conditions, such as chronic heart or lung disease and those who smoke cigarettes, should receive PPSV23.1 Despite increased pneumococcal disease risk stemming in part from chronic illness comorbidities in minority populations, vaccination rates among black adults are lower than in other US adult populations.2 Furthermore, black adults are at increased risk of pneumococcal disease.2,3
This study compared the likelihoods of chronic health conditions, specifically those that confer an increased risk of pneumococcal disease, of U.S. black and non-black populations aged ≥50 years. Lengths of stay and costs of hospitalization related to pneumococcal disease admissions were also compared across these racial groups.
Methods
Data were analyzed in 2017. IRB approval was not needed because all data were available in public data sets. Population health status data from the 2014 National Health Interview Survey (NHIS) and 2012 U.S. Life Tables from the National Center for Health Statistics (NCHS)4 were divided into black and non-black general population cohorts and segmented into 5-year age groups, beginning at ages 50–54 years and ending with ages 80+ years. CDC definitions for conditions conferring high risk of pneumococcal disease were used to categorize the population subgroups into those with immunocompromising conditions (HIV infection, chronic renal failure, nephrotic syndrome, leukemia, lymphoma, Hodgkin disease, generalized malignancy, iatrogenic immunosuppression, solid organ transplant, or multiple myeloma); other high-risk comorbid conditions (chronic heart, lung, or liver disease, diabetes mellitus, alcoholism, asthma, cirrhosis); current cigarette smokers, and those with none of these conditions.5
Within the 2014 NHIS dataset, high-risk individuals with immunocompromising were combined with individuals with other high-risk conditions. Therefore, to define the proportion with immunocompromising conditions in 5-year age increments, we used linear regression applied to the prevalence of immunocompromised individuals in 10-year age increments from a CDC cross-sectional analysis of noninstitutionalized civilian adults in the United States.6 These computed values were then subtracted from the likelihood of being within the inclusive chronic disease category for the respective age group, to calculate the likelihood of high-risk conditions in each age group. This procedure places individuals in the immunocompromised health state without regard to the presence or number of other high-risk conditions.
To determine age-specific mortality risks, we multiplied the relative likelihood of being in each age group’s health states (immunocompromised, other high-risk condition, smoker, healthy) by the proportion alive in each age group, using NCHS life tables for black and non-black U.S. populations. These proportions formed the basis for age-and race-specific cohort size and health state likelihood in our analysis.4 The proportion of each population subgroup in each health state were plotted and compared at age 65 and 80 years using Chi-squared tests.
Age group-specific pneumococcal vaccination rates by race for high-risk individuals were determined from 2014 NHIS data. For individuals who were 50–64 years, high-risk was defined as having one or more of the following: alcoholism, chronic heart disease, chronic lung disease, chronic liver disease, diabetes, immunocompromising condition, cancer, cigarette smoking. All individuals age 65 years and over were considered high-risk.
National Inpatient Sample (NIS) data (2014) were used to determine length of stay and costs for pneumococcal disease hospitalizations. NIS data include a 20% stratified sample of all discharges from U.S. community hospitals for a given year. The sample of all adults ≥50 years was divided into black and non-black populations for analysis. Patients were divided into three age groups – 50–64 years, 65–79 years, and 80 years and older. Patients were selected who had a discharge diagnosis of bacteremia (ICD-9 codes = 041.2, 038.2, 790.7) and meningitis (ICD-9 codes = 036.0, 036.1, 053.0, 320), which were combined into invasive pneumococcal disease, and nonbacteremic pneumonia (ICD-9 codes = 480–486, 487.0). Inpatient charges were adjusted by cost-to-charge ratios to yield cost of hospitalizations. Outcome measures were discharge status (alive vs. died in hospital), length of stay measured in days and total costs (USD), calculated by dividing total charges by cost-to-charge ratios. Data were analyzed using SAS 9.3 (SAS Institute, Cary NC) and SAS callable SUDAAN 11.0 (Research Triangle Institute, Research Triangle Park, NC) software. Statistical significance was set at alpha ≤0.05.
Results
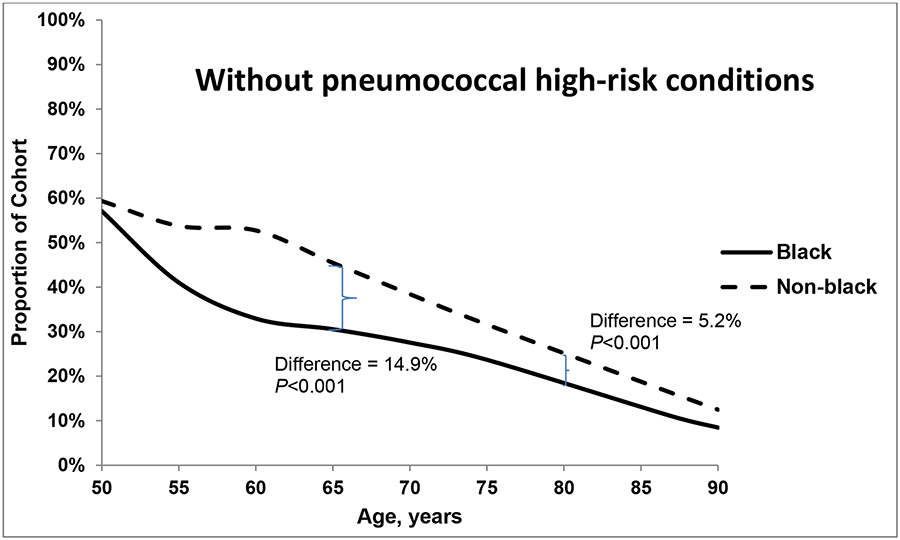
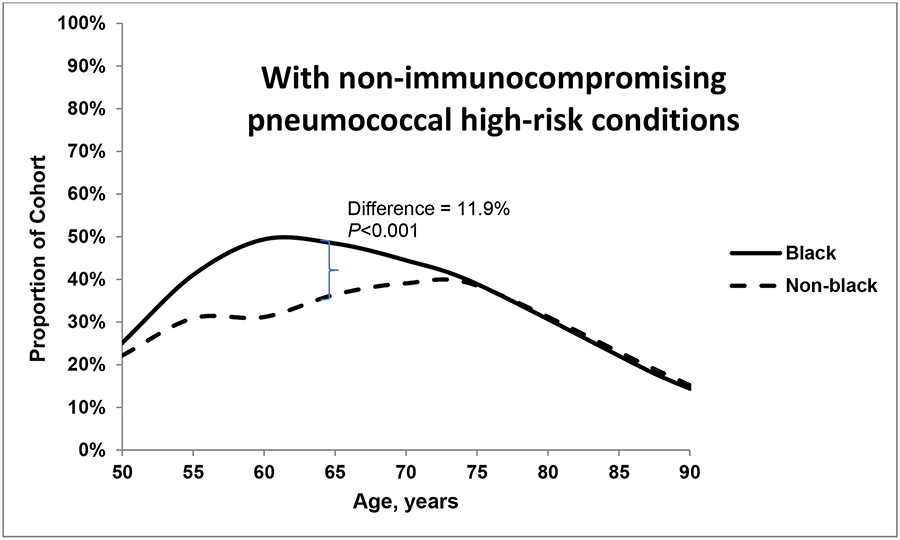
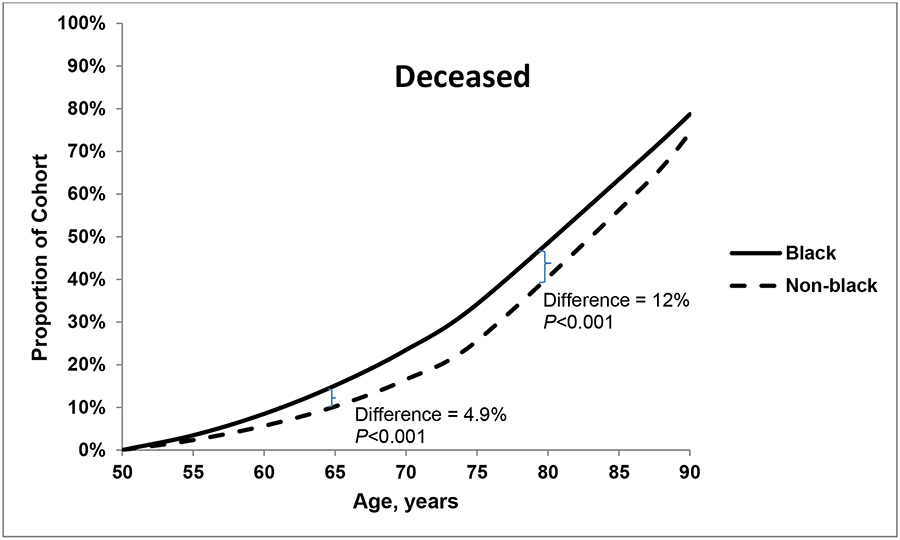
The proportions of individuals who were immunocompromised (<5%) and smokers with no high-risk conditions (14%) were similar for both groups at age 50 years and throughout the lifespan (data not shown). Figures 1a–c plot the proportion of individuals in each racial group with each of three health states – without pneumococcal risk conditions (a); with non-immunocompromising pneumococcal high-risk conditions (b); and deceased (c). In both black and non-black populations at age 50 years, approximately 60% of the population had no high-risk indications for pneumococcal vaccination (Figure 1a) and 23%−28% had one or more non-immunocompromising high-risk indication (Figure 1b). The likelihood of non-immunocompromising pneumococcal high-risk conditions increased with age, which, notably, was 12% higher among blacks than non-blacks at age 65 years. At age 80 years, the prevalence is higher among non-blacks (19.4%) than blacks (18.7%; P<0.001). The proportion who were deceased was larger among blacks than among non-blacks at age 65 years (15.1% vs. 10.2%, respectively; P<0.001) and at age 80 years (48.4% vs. 36.4%, respectively; P<0.001). Over all age groups, the age-specific relative likelihood of mortality was 1.1%−8.6% higher in the black population than in the non-black population.
Figures 1.
a, b and c. Proportion of black and non-black populations who had (a) no pneumococcal high-risk conditions, (b) non-immunocompromising high-risk conditions; and (c) died, by age.
Pneumococcal vaccination rates (data not shown) among all high-risk blacks were 29.8% for 50–64-year-olds, 44.4% for 65–79-year-olds and 55.2% for those 80 years and older. Among non-blacks, rates were 28.2% for 50–64-year-olds, 57.7% for 65–79-year-olds and 66.6% for those 80 years and older. Differences between racial groups for each age grouping were significant at P<0.001.
The demographic characteristics of the population ≥50 years old who were hospitalized with pneumococcal disease are shown in Table 1. In general, black hospitalized pneumococcal disease patients were significantly more likely to be younger, female, to have longer lengths of stay and higher costs associated with their hospitalizations.
Table 1.
Demographic characteristics of patients hospitalized with pneumococcal disease – NIS 2014*
| Characteristic | Total (N=2,193,296) | Black (N=236,620) | Non-Black (N=1,956,676) | P Value |
|---|---|---|---|---|
| 80+ | 32.4% | 19.1% | 34.0% | |
| Male | 49.3% | 47.1% | 49.6% | |
| Public/self-pay | 86.0% | 85.8% | 86.0% | |
| Dead | 8.0% | 8.1% | 8.0% | |
| Length of stay (days), mean (SE) | 7.7 (0.05) | 9.1 (0.10) | 7.5(0.05) | <0.001 |
| Cost, $US, mean (SE) | 18,154 (203) | 20,733 (306) | 17,844 (203) | <0.001 |
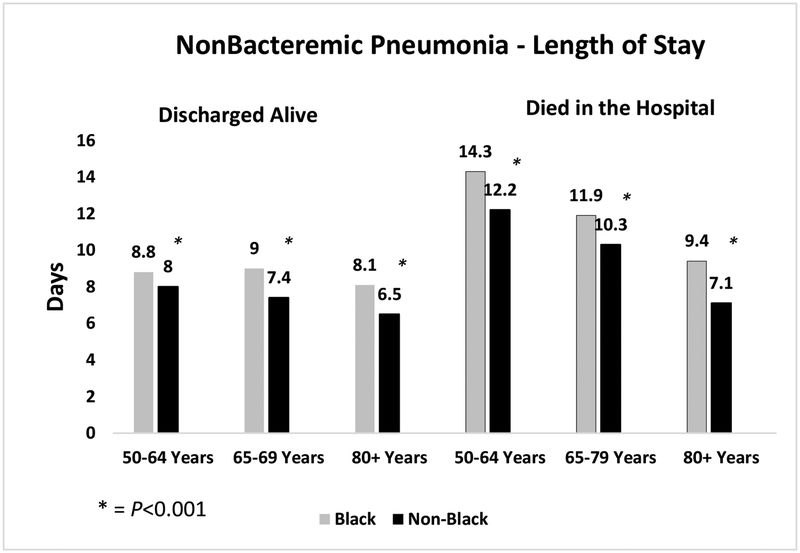
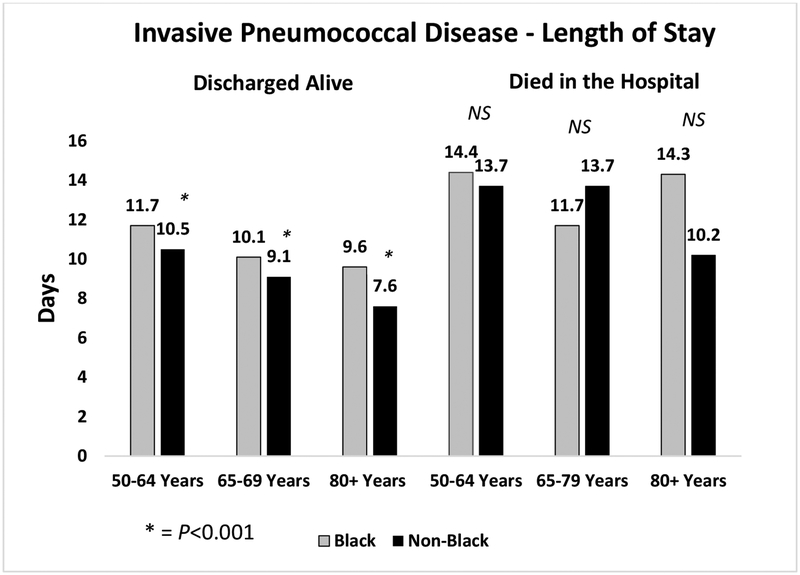
While overall lengths of stay and costs were higher for all pneumococcal hospitalizations for blacks than non-blacks, Figures 2a and b show differences in length of stay by age group, diagnosis and discharge status (alive or not). Figure 2a shows significantly longer lengths of stay for black versus non-black patients in all age groups for those discharged alive and those who died in the hospital for those with non-bacteremic pneumonia. This pattern was also observed for those discharged alive with invasive pneumococcal disease. However, there were no significant differences in length of stay between blacks and non-blacks for those who died in the hospital with invasive pneumococcal disease.
Figures 2.
a and b depict the differences in length of stay for black and non-black populations of patients hospitalized with (a) non-bacteremic pneumonia; and (b) invasive pneumococcal disease, by age group.
The costs associated with these hospitalizations (Table 2) were generally not different for invasive pneumococcal disease, with the exception of the oldest blacks (80+ years) who incurred higher costs than their non-black counterparts (P=0.01). Whereas, black patients ≥65 years with non-bacteremic pneumonia incurred significantly higher costs than non-black patients (P<0.05); there were no differences in costs among younger adults 50–64 years.
Table 2.
Cost ($US) of Pneumococcal Hospitalization
| Invasive Pneumococcal Disease | |||
|---|---|---|---|
| Age, years | Black | Non-black | P-value |
| Discharged alive | |||
| 50–64 | $26,135 | $25,961 | 0.868 |
| 65–79 | $21,562 | $20,576 | 0.195 |
| 80+ | $17,567 | $15,265 | 0.010 |
| Died in the hospital | |||
| 50–64 | $49,066 | $49,066 | 1.000 |
| 65–79 | $38,396 | $42,665 | 0.459 |
| 80+ | $32,487 | $28,937 | 0.746 |
| Nonbacteremic Pneumonia | |||
| Age, years | Black | Non-black | P-value |
| Discharged alive | |||
| 50–64 | $19,780 | $19,648 | 0.689 |
| 65–79 | $19,460 | $17,087 | <0.001 |
| 80+ | $15,427 | $12,843 | <0.001 |
| Died in the hospital | |||
| 50–64 | $46,012 | $46,388 | 0.841 |
| 65–79 | $37,171 | $34,275 | 0.041 |
| 80+ | $23,681 | $19,440 | <0.001 |
Discussion
This analysis found striking differences between U.S. black and non-black populations in age of onset and proportion with high-risk conditions that are pneumococcal vaccine indications. These differences extend to length of stay and costs associated with pneumococcal disease hospitalizations. A similar analysis, examining proportions of the U.S. population at higher risk of influenza,7 noted significantly greater likelihood of high-risk conditions in black adults ≥50 years compared to white adults and significant increases in health burden in low income adults aged ≥50 years.
The longer length of stay for blacks with invasive pneumococcal disease who were discharged alive and those with non-bacteremic pneumonia, regardless of discharge status, compared with non-blacks may be a reflection of earlier onset of pneumococcal high-risk conditions with their attendant side effects. More frequent or more severe complications may affect both the length of stay and the cost of the hospitalization.
Overall pneumococcal vaccination rates among high-risk adults continue to be suboptimal (24% for adults 19–64 years and 67% for adults ≥65 years) and racial disparities are evident.8 Addressing racial disparities in immunization rates was a priority in Healthy People 2010 as was increasing immunization pneumococcal polysaccharide vaccine uptake. According to Healthy People 2010, 15% of the targeted change for high risk adults 18–64 years of age was achieved.9 While progress is gratifying, much needs to be done because rates did not achieve the 2010 target of 60%. The racial differences in pneumococcal vaccine uptake and the morbidity and mortality burdens among blacks that occur 15 years earlier than in non-blacks suggest a need for new strategies to address race-based health disparities. Changes to pneumococcal vaccination recommendations that decrease race- or income-based barriers to vaccine uptake, or implementation of programs shown to improve vaccine uptake in at-risk populations, might reduce risk and racial disparities in pneumococcal morbidity and mortality. While pneumococcal vaccine uptake was slightly, but significantly higher among blacks than non-blacks at ages 50–64 years, by age 65 years, the difference in uptake was significantly larger and in favor of non-whites. A better understanding of why the differences in pneumococcal vaccine uptake begin to appear after age 50 may help to focus these efforts. These race-based data may provide valuable input for computational modeling studies of the cost-effectiveness of race-based vaccination recommendations.
Strengths and Limitations
While this analysis examines racial differences in the likelihood and age of onset of pneumococcal vaccine indications, it does not address underlying factors and their potential causative effects on the increased and prolonged burdens experienced by the black population. Furthermore, this analysis did not attempt to determine the potential additive effects of smoking combined with high-risk conditions, but was limited to discrete categorization of risk groups. Because the proportion of smokers with no other high-risk conditions was similar for both racial groups, the effect was likely similar for both groups and would not change the differences observed between them with respect to high-risk medical conditions. Other limitations are that data were derived from three data sets, did not reflect the same individuals and could not be cross-validated. However, these data came from large, national samples with appropriate weighting applied, thus are considered to be representative of the population as a whole. The proportion of immunocompromised by race in our study was obtained from a CDC cross-sectional analysis where a sample of individuals self-reported their immunocompromised status.6 Those authors indicated that self-reports of immunosuppression may be prone to misclassification. Moreover, the study did not assess individual conditions such as sickle cell disease and HIV, which the CDC has determined predispose to pneumococcal infection.10 The NHIS data are self-reported and are not verified by medical record review, but are widely reported and used for secondary analyses. Finally, this analysis did not examine risk in other racial and ethnic groups due to small sample sizes.
Conclusions
The marked differences between U.S. black and non-black populations in age of onset and proportion with high-risk conditions suggest that changes in pneumococcal vaccination policy or implementation of current policies may be required to address differential pneumococcal risk based on differences in health status. The fact that pneumococcal vaccination rates are similar at age 50, but begin to diverge thereafter, this age group (50–64 years) may be an ideal focus of intervention programs. Further research is needed to identify cost-effective policies and/or intervention programs aimed at increasing vaccine uptake in populations at greater risk of disease.
Contributor Information
Mary Patricia Nowalk, University of Pittsburgh School of Medicine, Pittsburgh PA, USA;
Angela R. Wateska, University of Pittsburgh School of Medicine, Pittsburgh PA, USA;
Chyongchiou Jeng Lin, University of Pittsburgh School of Medicine, Pittsburgh PA, USA;
William Schaffner, Vanderbilt University School of Medicine, Nashville TN, USA;.
Lee H. Harrison, University of Pittsburgh School of Medicine, Pittsburgh PA, USA;
Richard K. Zimmerman, University of Pittsburgh School of Medicine, Pittsburgh PA, USA;
Kenneth J. Smith, University of Pittsburgh School of Medicine, Pittsburgh PA, USA
References
- 1.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged > 65 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2014;63(37):822–825. [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DC, Flannery B, Bennett NM, et al. Socioeconomic and Racial/Ethnic Disparities in the Incidence of Bacteremic Pneumonia Among US Adults. Am. J. Public Health 2010;100(10):1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wortham JM, Zell ER, Pondo T, et al. Racial Disparities in Invasive Streptococcus pneumoniae Infections, 1998–2009. Clin. Infect. Dis 2014;58(9):1250–1257. [DOI] [PubMed] [Google Scholar]
- 4.Arias E, Heron M, Xu J. United States Life Tables, 2012. Natl. Vital Stat. Rep 2016;65(8). [PubMed] [Google Scholar]
- 5.Centers for Disease Contol and Prevention (CDC). Adult Conditions Immunization Schedule. National Center for Immunization and Respiratory Diseases. 2016; http://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. Accessed 6/15/2016.
- 6.Harpaz R, Dahl RM, Dooling KL. Prevalence of Immunosuppression Among US Adults, 2013. JAMA. 2016;316(23):2547–2548. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman RK, Lauderdale DS, Tan SM, Wagener DK. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 2010;28(39):6470–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung M-C, Williams WW, Lu P-J, et al. Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2016. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html#pneumo.
- 9.National Center for Health Statistics. Healthy People 2010 Final Review. Hyattsville, MD: 2012. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review.pdf. [Google Scholar]
- 10.Centers for Disease Contol and Prevention (CDC). (2017). Pneumococcal Vaccination: Summary of Who and when to Vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html.