Abstract
Background:
Fetal growth patterns in pregnancy-associated hypertensive disorders is poorly understood since prospective longitudinal data are lacking.
Objective:
To compare longitudinal fetal growth trajectories between normotensive women and those with pregnancy-associated hypertensive disorders.
Study Design:
This is a study based on data from a prospective longitudinal cohort study of fetal growth performed at 12 U.S. sites (2009-2013). Project gestational age was confirmed by ultrasound between 8w0d and 13w6d and up to six ultrasounds were performed across gestation. Hypertensive disorders were diagnosed based on 2002 American College of Obstetricians and Gynecologists guidelines and grouped hierarchically as severe preeclampsia (including eclampsia or HELLP syndrome), mild preeclampsia, severe gestational hypertension, mild gestational hypertension or unspecified hypertension. Women without any hypertensive disorder constituted the normotensive group. Growth curves for estimated fetal weight and individual biometric parameters including biparietal diameter, head circumference, abdominal circumference, femur and humerus length were calculated for each group using linear mixed models with cubic splines. Global and weekly pairwise comparisons were performed between women with a hypertensive disorder compared with normotensive women to analyze differences while adjusting for confounding variables. Delivery gestational age and birthweights were compared among groups.
Results:
Of 2,462 women analyzed, 2296 (93.3%) were normotensive, 63 (2.6%) had mild gestational hypertension, 54 (2.2%) mild preeclampsia, 32 (1.3%) severe preeclampsia, and 17 (0.7%) unspecified hypertension. Compared with normotensive women, those with severe preeclampsia had estimated fetal weights that were reduced between 22 and 38 weeks (all weekly pair-wise P values <.008). Women with severe preeclampsia compared to those without hypertension also had significantly smaller fetal abdominal circumference between 23 to 31 and 33 to 37 weeks’ gestation (weekly pair-wise P values <.04). Scattered weekly growth differences were noted on other biometric parameters between these two groups. The consistent differences in estimated fetal weight and abdominal circumference were not observed between women with other hypertensive disorders and those who were normotensive. Women with severe preeclampsia delivered significantly earlier (mean gestational age 35.9 ± 3.2 weeks) than the other groups (global P<.0001). Birthweights in the severe preeclampsia group were also significantly lower (mean −949.5 g (95% confidence interval (95% CI) −1117.7, −781.2 g); P <.0001) than in the normotensive group.
Conclusion:
Among women with pregnancy-associated hypertensive disorders, only those destined to develop severe preeclampsia demonstrated a significant and consistent difference in fetal growth (i.e., smaller estimated fetal weight and abdominal circumference) when compared to normotensive women.
Keywords: Pregnancy-associated hypertensive disorders, Fetal growth trajectory, Preeclampsia, Gestational hypertension, Biometric parameters
Introduction
Pregnancy-associated hypertensive disorders including preeclampsia and gestational hypertension are major contributors to severe maternal and perinatal morbidity and mortality.1 Preeclampsia occurs in 4% to 8% of pregnant women while gestational hypertension, often viewed as a transitory condition, progresses to preeclampsia in almost half of affected pregnancies.2–4 Failure of deep placentation is a central pathophysiologic feature of new-onset hypertension in pregnancy.5–9 Impaired physiological transformation of spiral arteries seen in deep placentation disorders is characterized by poor trophoblastic invasion, persistent endothelial cells, arterial endothelial activation, and frequently acute atherosis5,6 leading to utero-placental hypoperfusion, oxidative stress, intravascular inflammation, and angiogenic imbalance.5–9
The association of fetal growth restriction (FGR) and pregnancy-associated hypertensive disorders is complex, but has mainly been attributed to placental vascular dysfunction, a common pathologic feature shared by both disorders.5,6,10,11 Preeclampsia is associated with FGR; however, fetal growth remains normal in most pregnancies complicated by this disorder.10,11,13–17 Severe placental abnormalities including excessive villous regression and extensive infarction secondary to atherosis are predominant in pregnancies complicated by FGR.11 On the other hand, placental involvement is minimal and fetal growth has been found to be unaffected in pregnancies with milder manifestations of the disease.11 Tay et al demonstrated recently that compared with normotensive pregnancies, maternal cardiac output is high and peripheral vascular resistance (PVR) is low in women with preeclampsia without FGR, but CO is low and PVR is elevated when fetal growth is restricted by preeclampsia.13 Others have postulated that women with preeclampsia have a greater degree of vascular inflammation, endothelial dysfunction, and metabolic abnormalities than those with FGR only.10
Patterns of fetal growth in pregnancy-associated hypertensive disorders are poorly understood given the lack of prospective studies that compare longitudinal fetal growth between women with and without hypertension. Current evidence is conflicting and limited by several factors including retrospective design,15–17 use of birthweight as a proxy for fetal growth,13, 15–18 use of small for gestational age (SGA) birthweight as a proxy for FGR,13,15–17 and several studies were conducted in populations different from the current U.S. pregnant population.15–17 Srinivas et al found that women diagnosed with preeclampsia were at increased risk of having a fetus with SGA, defined as birthweight <10th percentile (adjusted odds ratio (AOR) 2.7; 95% confidence interval (CI) 1.94, 3.86) or birthweight <5th percentile (AOR 4.3; 95% CI 2.58, 7.17).13 A retrospective European population-based study found a 12% mean birthweight reduction, defined as the ratio between the observed and the expected birthweight, in severe preeclampsia and 23% in early-onset severe preeclampsia (< 32 weeks’ gestation).15 Another large European study found a significant association of asymmetric SGA, defined as ponderal index (100 × g/cm3) <10th or <2.5th percentile, with early-onset preeclampsia (< 37 weeks’ gestation), whereas both small and large birthweights were seen in late-onset preeclampsia.16 Also, a large retrospective Chinese cohort study found associations of both small and large birthweights in women with gestational hypertension and preeclampsia, compared with normotensive women.17 A study conducted in 15 U.S. centers found that birthweight at term was significantly lower by 60.7 g in women who developed preeclampsia compared with the customized standard group (P <.01).18 It is important to note that birthweight reported in these studies is an index of size, not necessarily growth.
The NICHD Fetal Growth Study was a contemporaneous prospective multisite observational study that established standards of normal fetal growth for singleton gestations in a racially/ethnically diverse pregnant population.19 Importantly, that study excluded women with factors, such as medical co-morbidities before entering pregnancy, adverse environmental exposures, and previous abnormal pregnancy outcomes, which potentially were associated with abnormal intrauterine growth. Our objectives were to establish fetal growth trajectories for estimated fetal weight (EFW) and individual fetal anthropometric parameters from the NICHD Fetal Growth Studies cohort in pregnancies complicated by hypertensive disorders of pregnancy compared with normotensive pregnancies, and to determine the gestational timing of any potential differences. This research work was presented at the 37th Annual Meeting Society for Maternal Fetal Medicine, Las Vegas, NV, 2017. Poster #210.20
Materials and Methods
Study Protocol
The study was based on data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies – Singletons, a prospective study conducted in 12 U.S. health care centers between July 2009 and January 2013.19,21 Non-obese (body mass index (BMI) 19.0 to <30.0 kg/m2) women with spontaneous conceptions without medical co-morbidities including chronic hypertension, adverse environmental exposures (smoking, alcohol, and illicit drugs) or recognized obstetrical risk factors for abnormal fetal growth were eligible for enrollment. To improve generalizability, women with a BMI 30.0 - 45.0 kg/m2 were also recruited, although the inclusion and exclusion criteria were not as strict given that obesity is associated with many co-morbidities as well as alterations in fetal growth.22 Recalled pre-gravid weight and self-reported height were used for the calculation of BMI at enrollment. Human subjects’ approval was obtained from all participating sites, the NICHD, and the data coordinating center. All women provided informed consent prior to any data collection. Of 2,802 women enrolled, we excluded 168 who deactivated from the study (33 were lost to follow-up, 93 refused to continue, 29 moved, 9 had voluntary termination of the pregnancy, and 4 for other reasons), 17 ineligible after enrollment, 86 who had structural fetal abnormalities, 5 who had fetal chromosomal abnormalities, 11 with pregnancy loss <20 weeks’ gestation, 19 with fetal demise, and 29 with missing data, leaving 2,467 who are included in the descriptive analysis (Table 1). For the analyses, we further excluded 5 women with severe gestational hypertension, given that their small number precluded meaningful analysis, leaving a total of 2,462 pregnancies.
Table 1.
Maternal characteristics among normotensive and pregnancy-associated hypertensive disorders. P-values reported are for global tests.
| Severe Preeclampsia | Mild Preeclampsia | Severe Gestational Hypertensiona | Mild Gestational Hypertension | Unspecified Hypertension | Normotensive | All | P-value | |
|---|---|---|---|---|---|---|---|---|
| n = 32 | n = 54 | n = 5 | n = 63 | n = 17 | n = 2296 | N = 2467 | ||
| Maternal age, years - mean, ± SD | 26.6 (6.0) | 26.2 (5.8) | 29.0 (6.8) | 28.0 (5.5) | 25.6 (5.4) | 28.3 (5.4) | 28.2 (5.5) | 0.0081d |
| Race/ethnicity, n (%) | <.0001d | |||||||
| White/non-Hispanic | 9 (28.1%) | 14 (25.9%) | 1 (20.0%) | 22 (34.9%) | 4 (23.5%) | 634 (27.6%) | 684 (27.7%) | |
| Black/non-Hispanic | 14 (43.8%) | 22 (40.7%) | 1 (20.0%) | 25 (39.7%) | 10 (58.8%) | 602 (26.2%) | 674 (27.3%) | |
| Hispanic | 6 (18.8%) | 18 (33.3%) | 1 (20.0%) | 14 (22.2%) | 3 (17.6%) | 667 (29.1%) | 709 (28.7%) | |
| Asian | 3 (9.4%) | 2 (40.0%) | 2 (3.2%) | 393 (17.1%) | 400 (16.2%) | |||
| Gravidity, n (%) | 0.1360 | |||||||
| 1 | 15 (46.9%) | 24 (44.4%) | 3 (60.0%) | 28 (44.4%) | 5 (29.4%) | 729 (31.7%) | 804 (32.6%) | |
| 2 | 8 (25.0%) | 16 (29.6%) | 18 (28.6%) | 5 (29.4%) | 713 (31.0%) | 760 (30.8%) | ||
| ≥3 | 9 (28.1%) | 14 (25.9%) | 2 (40.0%) | 17 (27.0%) | 7 (41.2%) | 854 (37.2%) | 903 (36.6%) | |
| Parity, n (%) | <.0001f | |||||||
| 0 | 24 (75.0%) | 39 (72.2%) | 4 (80.0%) | 40 (63.5%) | 10 (58.8%) | 1040 (45.3%) | 1157 (46.9%) | |
| 1 | 4 (12.5%) | 10 (18.5%) | 15 (23.8%) | 4 (23.5%) | 807 (35.1%) | 840 (34.0%) | ||
| ≥2 | 4 (12.5%) | 5 (9.3%) | 1 (20.0%) | 8 (12.7%) | 3 (17.6%) | 449 (19.6%) | 470 (19.1%) | |
| Prepregnancy BMIb (kg/m2), n (%) | <.0001g | |||||||
| < 25.0 | 10 (31.3%) | 15 (28.3%) | 2 (40.0%) | 20 (31.7%) | 5 (31.3%) | 1327 (58.2%) | 1379 (56.3%) | |
| 25.0 - 30.0 | 13 (40.6%) | 20 (37.7%) | 2 (40.0%) | 19 (30.2%) | 4 (25.0%) | 591 (25.9%) | 649 (26.5%) | |
| ≥30.0 | 9 (28.1%) | 18 (34.0%) | 1 (20.0%) | 24 (38.1%) | 7 (43.8%) | 361 (15.8%) | 420 (17.2%) | |
| Marital statusc, n (%) | 0.0011h | |||||||
| Not married | 13 (40.6%) | 22 (40.7%) | 1 (20.0%) | 21 (33.3%) | 8 (47.1%) | 559 (24.4%) | 624 (25.3%) | |
| Married or living with partner | 19 (59.4%) | 32 (59.3%) | 4 (80.0%) | 42 (66.7%) | 9 (52.9%) | 1735 (75.6%) | 1841 (74.7%) | |
| Education, n (%) | 0.2239 | |||||||
| < High school | 4 (12.5%) | 8 (14.8%) | 8 (12.7%) | 1 (5.9%) | 262 (11.4%) | 283 (11.5%) | ||
| High school/equivalent | 4 (12.5%) | 14 (25.9%) | 11 (17.5%) | 6 (35.3%) | 415 (18.1%) | 450 (18.2%) | ||
| Some college/associate | 11 (34.4%) | 20 (37.0%) | 3 (60.0%) | 17 (27.0%) | 6 (35.3%) | 680 (29.6%) | 737 (29.9%) | |
| Bachelors degree | 11 (34.4%) | 7 (13.0%) | 1 (20.0%) | 19 (30.2%) | 1 (5.9%) | 548 (23.9%) | 587 (23.8%) | |
| Postgraduate degree | 2 (6.3%) | 5 (9.3%) | 1 (20.0%) | 8 (12.7%) | 3 (17.6%) | 391 (17.0%) | 410 (16.6%) | |
| Family incomec, n(%) | 0.0899 | |||||||
| <$30,000 | 6 (21.4%) | 19 (39.6%) | 17 (32.1%) | 7 (41.2%) | 566 (28.3%) | 615 (28.6%) | ||
| $30,000-$74,999 | 15 (53.6%) | 16 (33.3%) | 3 (60.0%) | 14 (26.4%) | 6 (35.3%) | 617 (30.9%) | 671 (31.2%) | |
| $75,000-$99,999 | 6 (12.5%) | 5 (9.4%) | 270 (13.5%) | 281 (13.1%) | ||||
| $100,000 or more | 7 (25.0%) | 7 (14.6%) | 2 (40.0%) | 17 (32.1%) | 4 (23.5%) | 544 (27.2%) | 581 (27.0%) | |
| Insurance, n (%) | 0.2847 | |||||||
| Other | 11 (34.4%) | 27 (50.0%) | 22 (34.9%) | 8 (47.1%) | 840 (36.6%) | 908 (36.8%) | ||
| Private or managed care | 21 (65.6%) | 27 (50.0%) | 5 (100.0%) | 41 (65.1%) | 9 (52.9%) | 1456 (63.4%) | 1559 (63.2%) | |
| Full-time employment/student statusc, n (%) | 0.0689 | |||||||
| No | 5 (15.6%) | 17 (31.5%) | 1 (20.0%) | 12 (19.0%) | 2 (11.8%) | 677 (29.5%) | 714 (29.0%) | |
| Yes | 27 (84.4%) | 37 (68.5%) | 4 (80.0%) | 51 (81.0%) | 15 (88.2%) | 1618 (70.5%) | 1752 (71.0%) | |
| Infant sexc, n (%) | 0.9368 | |||||||
| Male | 15 (46.9%) | 26 (48.1%) | 1 (20.0%) | 30 (47.6%) | 9 (52.9%) | 1174 (51.4%) | 1255 (51.1%) | |
| Female | 17 (53.1%) | 28 (51.9%) | 4 (80.0%) | 33 (52.4%) | 8 (47.1%) | 1111 (48.6%) | 1201 (48.9%) | |
| Gestational age at deliveryc weeks - mean, ± SD | 35.9 (3.2) | 38.8 (1.6) | 37.9 (2.4) | 39.2 (1.4) | 39.5 (1.1) | 39.3 (1.7) | 39.2 (1.7) | <.0001i |
Due to its small n, Severe Gestational Hypertension was not included in statistical comparisons among hypertension groups.
BMI = Body Mass Index
Not included in the totals are missing data: BMI (n=19), Martial status (n=2), Income (n=319, 299 from Normotensive, 10 from Mild Gestational Hypertension, 6 from Mild Preeclampsia, 4 from Severe Preeclampsia groups), Full-time employment/student status (n=1), Infant sex (n=11), Gestational age at delivery (n=12).
Although the overall ANOVA was significant no pairwise comparisons indicated significant differences in age between groups at the 0.05 level.
The distribution of racial/ethnic groups was significantly different in the Normotensive group compared to all other groups except Severe Preeclampsia
The distribution of parity categories was significantly different in the Normotensive group compared to all other groups except Unspecified Hypertension.
The distribution of BMI categories was significantly different in the Normotensive group than all other groups.
The proportion of those married or living with a partner was significantly different in the Normotensive group than for all other groups except Mild Gestational Hypertension.
Gestational age at delivery was significantly smaller in the Severe Preeclampsia group than that for all other hypertensive groups.
Sonographic Population
Ultrasound screening between 8w0d and 13w6d was performed to ensure dating consistency with the patient reported first day of the last menstrual period. Following a standardized ultrasound between 10w0d and 13w6d, women were randomized to one of four sonogram schedules with 5 additional planned study visits (16-22, 24-29, 30-33, 34-37, and 38-41 gestational weeks). By design, this mixed longitudinal randomization scheme captured weekly fetal growth data without exposing women to weekly ultrasound examinations. Study sonographers underwent training and credentialing prior to enrollment. At each ultrasound, biometric measurements for biparietal diameter (BPD; outer to inner), humerus length (HL), and femur length (FL) using the linear function and for head circumference (HC) and abdominal circumference (AC) using the ellipse function were performed using standard protocols and identical equipment. Study sonographers were blinded to both the gestational age at the measurements and the patient’s clinical condition. EFW was computed from HC, AC and FL using a Hadlock formula23. A detailed study protocol has been published elsewhere.19,21,24
Hypertensive Groups
Prospectively, the study protocol guidebooks instructed the research nurse abstractors to document the presence and severity of pregnancy-associated hypertensive disorders as recorded in the medical record. Diagnosis of pregnancy-associated hypertensive disorders was made by clinicians at participating centers following the 2002 ACOG criteria.14 Gestational hypertension was defined as elevated blood pressure (systolic blood pressure (SBP) ≥ 140 mm Hg or a diastolic blood pressure (DBP) ≥ 90 mm Hg) after 20 weeks of gestation without proteinuria in a women with previously normal blood pressure. Severe gestational hypertension was defined as gestational SBP ≥ 160 mm Hg or DBP ≥ 110 mm Hg without proteinuria. Preeclampsia was defined as new-onset hypertension after 20 weeks with proteinuria (≥ 0.3 grams of protein in a 24-hour urine specimen). The criteria for severe preeclampsia included SBP ≥ 160 mm Hg or DBP ≥ 110 mm Hg, proteinuria ≥ 5 grams or > 3+ on two random urine samples, oliguria < 400 mL in 24 hours, cerebral or visual disturbances, pulmonary edema or cyanosis, epigastric or right upper-quadrant pain, impaired liver function, thrombocytopenia, and fetal growth restriction.14 the postpartum discharge diagnoses were as listed in the medical record and abstracted by research nurses: mild gestational hypertension, severe gestational hypertension, mild preeclampsia, severe preeclampsia (including eclampsia or HELLP syndrome) and unspecified hypertension (all were recorded as mild but lacked sufficient modifiers to differentiate between mild gestational hypertension and mild preeclampsia). For the present analysis, we grouped the hypertensive disorders in a hierarchical manner as severe preeclampsia, mild preeclampsia, severe gestational hypertension, mild gestational hypertension, and unspecified hypertension. Women without any hypertensive disorder recorded as a discharge diagnosis were categorized as normotensive.
Statistical Analysis
Baseline and clinical data were compared for participants by type of hypertensive disorder. Overall differences (P <.05) were determined using Chi-square or ANOVA for categorical and continuous data, respectively. Where overall tests indicated significant differences across the no-hypertension and hypertension groups, pairwise Chi-square or Tukey’s Studentized Range (HSD) Test were used to determine significant differences between these groups.
Fetal growth curves for the ultrasound biometric measures BPD, HC, AC, FL, and HL, and calculated EFW were generated for each hypertensive group (severe preeclampsia, mild preeclampsia, mild gestational hypertension, unspecified hypertension, and normotensive).
The ultrasound biometric measures and EFW were log-transformed to stabilize variance across gestational ages and to optimize approximations for the error structures.24 Linear mixed models with cubic splines for the fixed effects were used for the primary analysis to estimate specific fetal growth curves.25 Three-knot points (25th, 50th, 75th percentiles) were selected at gestational ages that evenly split the distributions. Percentiles (5th, 50th, and 95th) were estimated for EFW and each biometric parameter in the studied groups from the 11th to 40th week of gestation based on the assumed normal distribution of the random effects on error structure.
The overall differences in the curves of EFW and each biometric measure for each group were calculated using a likelihood-ratio test. When the global test was significant (P <.05) for differences in the overall curves, week-specific differences were tested using the Wald test at each gestational week. Where the weekly global test was significant (P <.05) for differences among hypertensive groups, pairwise tests between hypertensive groups were performed. These tests were conducted on the estimated curves with and without adjustment for maternal characteristics: age, self-reported race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and Asian or Pacific Islander), self-reported pre-gravid weight and height, parity (0, 1 and ≥ 2) , full-time employment/student status (yes/no), marital status (married/living as married vs not), insurance (private/managed vs Medicaid/other), education, and infant sex. Education was analyzed categorically: <high school, high school or equivalent, some college or associate degree, bachelor’s degree, and postgraduate degree. ANCOVA was used to analyze birthweight data adjusting for the same variables described above for the fetal growth trajectory models.
All analyses were performed using SAS (version 9.4; SAS Institute Inc, Cary, NC) or R (version 3.1.2; http://www.R-project.org).
Results
Patient Demographics
Of the 2,462 women eligible for analysis, 2,296 (93.3%) were normotensive, 63 (2.6%) had mild gestational hypertension, 54 (2.2%) mild preeclampsia, 32 (1.3%) severe preeclampsia, and 17 (0.7%) unspecified hypertension. Maternal age varied across hypertension groups (global analysis P =.008), but no pair-wise comparison was statistically significant (Table 1). The distribution of racial/ethnic groups was significantly different between the normotensive group and all other groups, except severe preeclampsia (P <.02 for pair-wise comparisons). Parity was significantly different between the normotensive group and all other groups, except unspecified hypertension (P <.02 for pair-wise comparisons). Other maternal characteristics results are presented in Table 1.
Compared with the other groups, normotensive women had the lowest obesity rate (15.8%). Women with severe preeclampsia delivered significantly earlier than women with other hypertensive conditions and those without hypertension (Table 1; global P<.0001). Birthweights of infants born to women without hypertension (mean = 3352.9 g, standard deviation (SD) = 494.4 g) were significantly higher than the infant birthweights of women with severe preeclampsia (mean = 2297.2 g, SD = 741.0 g). Birthweight mean difference between these groups was −949.5 g (95% confidence interval (95% CI) −1117.8, −781.2 g); P <.0001). In contrast, neonates born to women with mild hypertensive disorders had similar birthweights to those born to women with no hypertension (P values >.05).
Fetal Growth trajectories for EFW
Fetal growth trajectories for EFW including the 5th, 50th, and 95th percentiles for the normotensive and hypertension groups were statistically different (global P <.0001) and remained significant after adjustment for confounders (Figure 1). Compared with fetuses of normotensive women, fetuses of women with severe preeclampsia had a median EFW significantly higher at 11 weeks’ gestation and became progressively smaller from 22 to 38 weeks’ gestation (Table 2). The median EFW difference for gestational age was 31 g at 22 weeks, 74 g at 28 weeks, 136 g at 32 weeks, and 474 g at 38 weeks (P <.008 for all weekly pairwise comparisons). Furthermore, the median percent EFW difference for gestational age between these two groups was 6.5% at 22 weeks, 6.4% at 28 weeks, 7.2% at 32 weeks, and 15.0% at 38 weeks. By the third trimester, EFW curves in women with severe preeclampsia were characterized by flattening at the 5th and 50th percentiles and progressively separating from the fetal growth curves of women with no hypertension, mild gestational hypertension, and mild preeclampsia.
Figure 1. Distribution of estimated fetal weight by hypertensive condition and gestation, NICHD Fetal Growth Studies - singletons.
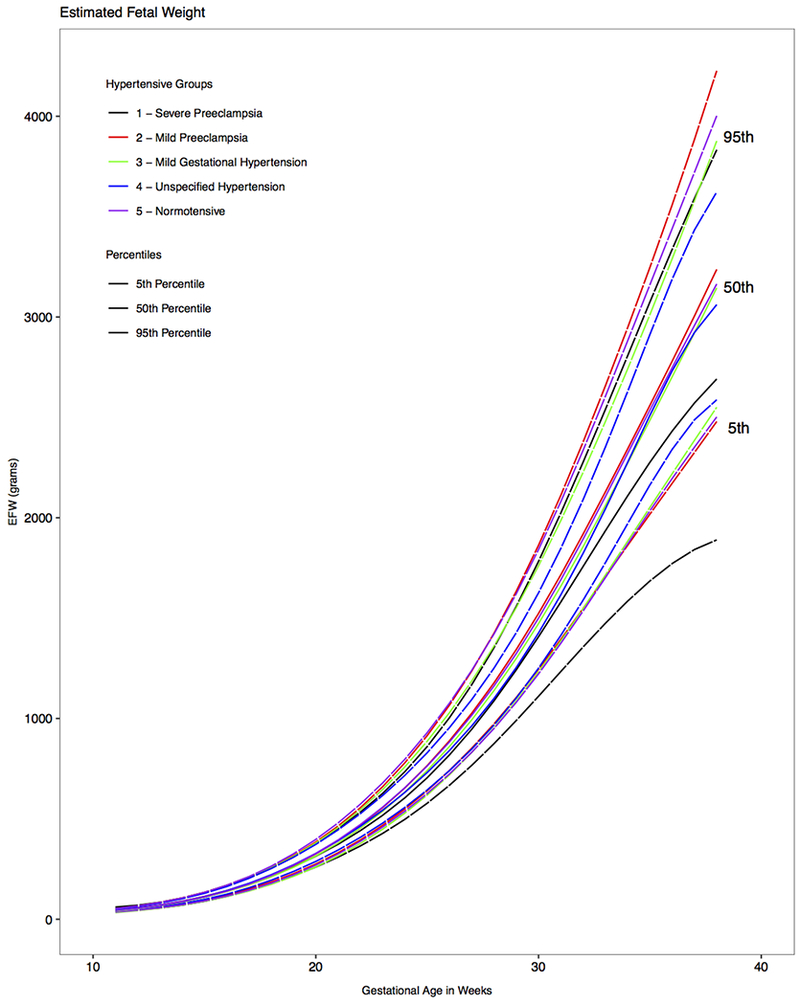
Estimated 5th, 50th and 95th percentiles for fetal weight by hypertensive condition, as estimated from linear mixed models with log-transformed outcomes and cubic splines.
EFW, estimated fetal weight; GA, gestational age
Table 2.
Estimated fetal weight* by hypertension group, n = 2462.
| Median Estimated Fetal Weight, grams | |||||
|---|---|---|---|---|---|
| GA, weeks | Normotensive (n = 2296) | Severe Preeclampsia (n = 32) | Mild Preeclampsia (n = 54) | Mild Gestational Hypertension (n = 63) | Unspecified Hypertension (n = 17) |
| 11 | 44 | 48† | 43 | 41† | 44 |
| 12 | 55 | 56 | 55 | 53 | 55 |
| 13 | 69 | 69 | 71 | 68 | 70 |
| 14 | 88 | 86 | 90 | 86 | 89 |
| 15 | 111 | 109 | 113 | 109 | 113 |
| 16 | 140 | 139 | 142 | 137 | 143 |
| 17 | 175 | 174 | 177 | 171 | 179 |
| 18 | 218 | 215 | 219 | 211 | 222 |
| 19 | 269 | 261 | 268 | 259 | 271 |
| 20 | 328 | 314 | 326 | 315 | 328 |
| 21 | 395 | 374 | 392 | 379 | 392 |
| 22 | 472 | 441† | 469 | 454† | 463 |
| 23 | 558 | 518† | 556 | 538† | 543 |
| 24 | 655 | 605† | 654 | 634† | 632† |
| 25 | 763 | 705† | 765 | 741† | 731† |
| 26 | 883 | 818† | 889 | 860 | 841† |
| 27 | 1016 | 946† | 1026 | 993 | 963† |
| 28 | 1162 | 1088† | 1177 | 1139 | 1100† |
| 29 | 1323 | 1242† | 1343 | 1299 | 1254† |
| 30 | 1500 | 1407† | 1523 | 1473 | 1426† |
| 31 | 1691 | 1580† | 1716 | 1659 | 1616† |
| 32 | 1893 | 1757† | 1918 | 1856 | 1822† |
| 33 | 2105 | 1935† | 2128 | 2061 | 2044 |
| 34 | 2321 | 2109† | 2343 | 2272 | 2276 |
| 35 | 2536 | 2276† | 2560 | 2486 | 2510 |
| 36 | 2748 | 2432† | 2780 | 2702 | 2732 |
| 37 | 2958 | 2571† | 3005 | 2921 | 2923 |
| 38 | 3165 | 2691† | 3237 | 3145 | 3062 |
Computed from head circumference, abdominal circumference and femur length
Value differed significantly from the no hypertension group (global and weekly pairwise p<0.05, obtained by the Wald test with adjustment for maternal age, self-reported height and prepregnancy weight, parity, racial ethnic group, full-time job or student status, marital status, insurance, education, and infant sex)
Median EFW growth trajectories did not differ significantly between the fetuses of normotensive women and those whose mother had mild preeclampsia (Table 2, P >.05 for all weekly pair-wise comparisons). Compared with the normotensive group, fetuses of mothers with mild gestational hypertension exhibited a significantly lower median EFW at 11 weeks and from 22 to 25 weeks’ gestation (P <.04 for all weekly pair-wise comparisons), but the difference was not statistically significant at later gestational ages.
Fetal Growth Trajectories for AC
Growth curves for AC (Figure 2) were statistically significantly different between the fetuses of normotensive mothers and those of mothers with severe preeclampsia at 11 weeks, from 23 to 31 weeks, and from 33 to 37 weeks’ gestation (P <.04 for all weekly pair-wise comparisons). Compared with fetuses of normotensive mothers, the median AC of fetuses whose mothers had severe preeclampsia was 4.9 mm, 6.3 mm, 11.9 mm, and 17.6 mm smaller at 22, 28, 34, and 37 weeks’ gestation, respectively (Table 3). The median percent AC difference for gestational age was 2.8% at 22 weeks, 2.6% at 28 weeks, 3.9% at 34 weeks and 5.3% at 37 weeks.
Figure 2. Distribution of fetal abdominal circumference by hypertensive condition and gestation, NICHD Fetal Growth Studies - singletons.
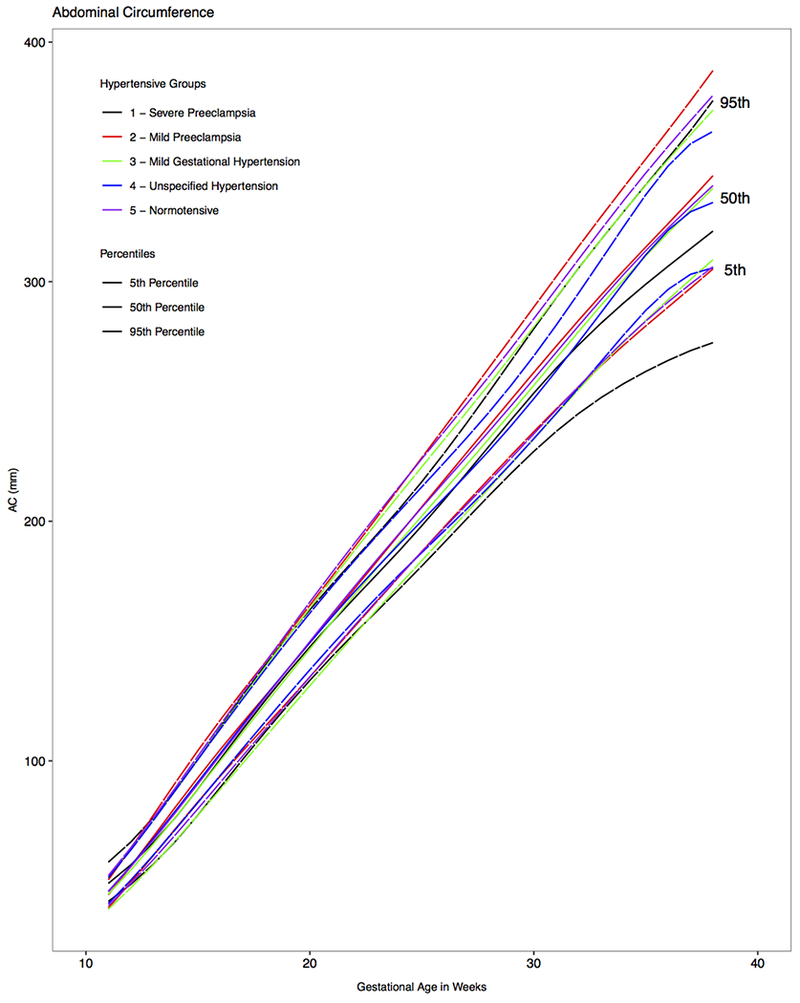
Estimated 5th, 50th and 95th percentiles for fetal abdominal circumference by hypertensive condition, as estimated from linear mixed models with log-transformed outcomes and cubic splines.
GA, gestational age
Table 3.
Median abdominal circumference by hypertension group, n = 2462.
| Median Abdominal Circumference, mm | |||||
|---|---|---|---|---|---|
| GA, weeks | Normotensive (n = 2296) | Severe Preeclampsia (n = 32) | Mild Preeclampsia (n = 54) | Mild Gestational Hypertension (n = 63) | Unspecified Hypertension (n = 17) |
| 11 | 45.4 | 48.8† | 44.1 | 44.4 | 45.5 |
| 12 | 55.7 | 56.6 | 55.9 | 54.4 | 56.1 |
| 13 | 66.8 | 65.8 | 68.3† | 65.2† | 67.4 |
| 14 | 78.4 | 76.4 | 80.8† | 76.5† | 79.2 |
| 15 | 90.3 | 88.2 | 93.0† | 88.2† | 91.2 |
| 16 | 102.3 | 100.7 | 104.7† | 100.0† | 103.2 |
| 17 | 114.3 | 113.1 | 116.0 | 111.9 | 115.0 |
| 18 | 126.2 | 125.2 | 127.0 | 123.6 | 126.7 |
| 19 | 138.1 | 136.7 | 138.0 | 135.2 | 138.2 |
| 20 | 149.9 | 147.6 | 149.3 | 146.7 | 149.4 |
| 21 | 161.5 | 158.0 | 160.6 | 158.1 | 160.2 |
| 22 | 172.9 | 168.0 | 171.9 | 169.4 | 170.7 |
| 23 | 184.1 | 177.9† | 183.3 | 180.5† | 180.8 |
| 24 | 195.0 | 187.8† | 194.7 | 191.4† | 190.6† |
| 25 | 205.8 | 198.2† | 206.1 | 202.2† | 200.2† |
| 26 | 216.4 | 209.0† | 217.3 | 212.9† | 209.7† |
| 27 | 226.9 | 220.1† | 228.6 | 223.6† | 219.4† |
| 28 | 237.6 | 231.3† | 239.8 | 234.5 | 229.4† |
| 29 | 248.4 | 242.5† | 251.0 | 245.5 | 240.0† |
| 30 | 259.4 | 253.5† | 262.1 | 256.7 | 251.2† |
| 31 | 270.5 | 263.9† | 273.1 | 268.0 | 262.8† |
| 32 | 281.6 | 273.6 | 283.9 | 279.2 | 274.8 |
| 33 | 292.4 | 282.7† | 294.4 | 290.1 | 287.0 |
| 34 | 303.0 | 291.1† | 304.5 | 300.7 | 299.4 |
| 35 | 312.9 | 298.9† | 314.5 | 310.8 | 311.2 |
| 36 | 322.4 | 306.5† | 324.2 | 320.4 | 321.5 |
| 37 | 331.4 | 313.8† | 334.1 | 329.8 | 329.2 |
| 38 | 340.1 | 321.1 | 344.3 | 339.0 | 333.1 |
Value differed significantly from the no hypertension gioup (gloual and weekly panwise p<0.05, obtained by tbe Wald test with adjustment for maternal age, self-reported height and prepregnancy weight, parity, racial ethnic group, full-time job or student status, marital status, insurance, education, and infant sex)
AC growth in fetuses of mothers with mild preeclampsia differed only from 13 to 16 weeks’ gestation compared with normotensive mothers (Figure 2; P <.05 for all weekly pair-wise comparisons), but the difference became not significant at later gestational ages (P >.05 for all weekly pair-wise comparisons). As observed with EFW growth trajectory, AC was significantly smaller in the fetuses of mothers with mild gestational hypertension group compared with those of normotensive mothers from 13 to 16 weeks and from 23 to 27 weeks’ gestation (P <.04 for all weekly pair-wise comparisons).
Fetal Growth Trajectories for Other Individual Biometric Parameters
Growth trajectories of all other biometric measurements, BPD, HC, FL, and HL (Figure 3 a–d), had scattered weekly growth differences in the first and second trimesters between the normotensive, severe preeclampsia, and the other hypertensive disorder groups.
Figure 3. Distribution of fetal biparietal diameter, head circumference, femur length, and humerus length by hypertensive condition and gestation, NICHD Fetal Growth Studies - singletons.
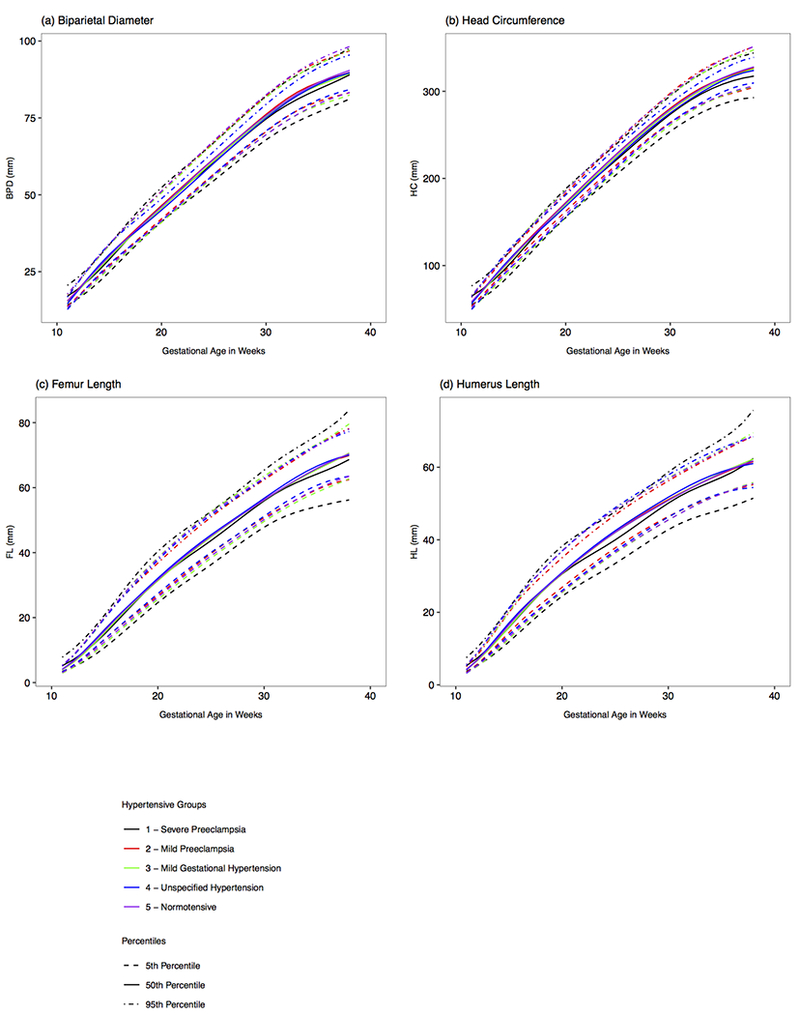
Estimated 5th, 50th, and 95th percentiles for fetal biparietal diameter, head circumference, femur length, and humerus length by hypertensive condition (a-d), as estimated from linear mixed models with log-transformed outcomes and cubic splines.
GA, gestational age
Comment
Principal Findings
Our analysis of the NICHD Fetal Growth Study-Singletons found significant and consistent reductions in fetal growth during the second and third trimesters among women who developed severe preeclampsia compared with normotensive pregnancies. With severe preeclampsia, EFW growth differences occurred consistently beginning 22 weeks’ gestation and continued through 38 weeks, with significantly smaller AC measurements observed between 23 to 31 weeks and from 33 to 37 weeks’ gestation. The EFW differences were corroborated by birthweight, which was lower for the newborns of women who developed severe preeclampsia compared to women without hypertension. Our study demonstrated normal fetal growth patterns, except for temporal differences on AC growth from 13 to 16 weeks’ gestation, in women who developed mild preeclampsia similar to those women without hypertension suggesting that utero-placental function is preserved to a greater extent in this milder form of preeclampsia. Women who developed mild gestational hypertension pregnancies had a transitory deceleration of EFW and AC growth in the second trimester, but subsequently normalized fetal growth at later gestational ages.
Results in Context of Other Studies
Our results demonstrate different fetal growth patterns across the hypertensive groups. Although fetal growth patterns in pregnancy-associated hypertensive disorders have not been previously evaluated in a longitudinal fashion, abnormalities of neonatal size associated with pregnancy-associated hypertensive disorders have been previously reported with conflicting results. For example, Srinivas et al showed a higher risk for SGA (birthweight <10th percentile for gestational age) among women with severe preeclampsia compared to normotensive women (AOR 1.87; 95% CI 1.11, 2.97).13. A European study demonstrated that birth size was lower with increasing preeclampsia severity.15 Others have reported an association between small as well as large birthweights with preeclampsia and gestational hypertension.16,17 Our study showed an abnormal asymmetric fetal growth pattern in women with severe preeclampsia. In this respect, Rasmussen et al reported at increased risk for asymmetric SGA with early-onset preeclampsia (< 37 weeks).16
Biological Mechanisms
Fetal growth depends on the efficient transport of nutrients in the uteroplacental circulation. Reduced uteroplacental blood flow is a result of placental vascular pathologic changes highly associated with FGR and preeclampsia.5–11 Impaired spiral arterial remodeling and deficient extravillous trophoblast invasion are highly characteristic abnormalities of these disorders.5–11 Deficient remodeling might be precipitated by inadequate histotrophic nutrition in the first trimester,11 excessive apoptosis in the placental bed26 reducing the number of extravillous trophoblast cells,5,6,9,11 or failure of interstitial trophoblasts to penetrate the arterial wall.9 A greater gradient of aberrant remodeling at the junctional zone and myometrial segment are highly associated with preeclampsia with FGR.11 Atherotic changes with accumulation of foam cells and arterial narrowing distal to the junctional zone significantly restricts blood flow to the placenta leading to oxidative stress, endoplasmic reticulum (ER) stress, proinflammatory cytokines production, and apoptosis.5,6,9,11
Reduced volume and surface area of the placenta are characteristic features of pregnancies with FGR.11 Downregulated function of the protein kinase B/ mechanistic target of rapamycin (AKt/ mtOR) signaling pathway reduces placental growth and reduces activity of placental transporters in FGR cases.27,28 Unfolded protein response (UPR) pathways are activated in response to hypoxic environment.28,29 A greater degree of activation of UPR stimulates the release of proinflammatory cytokines and apoptosis implicated in endothelial cell activation, a pathognomonic feature of preeclampsia with FGR, but not of FGR alone.28,29
Emerging findings from genetic studies further elucidated pathogenic mechanisms on preeclampsia and FGR.30–41 For instance, Pleckstrin homology like domain family A member 2 (PHDA2) that inhibits growth is upregulated whereas mesoderm specific transcript (MESt) that promotes growth is downregulated in FGR placentas.33,34 A recent metanalysis identified 20 miRs associated with cell death/apoptosis and cell movement in placentas with preeclampsia.35 Other studies have identified miR-20b, miR-151, miR-524-3p, and miR-34c-5p associated with angiogenic pathways in preeclampsia.36 Four genes associated with growth and metabolism (IGFBP-1, PRL, LEP, and CHR) have been found in FGR placentas by MRNA transcriptome analysis.37,38 Microarray studies in preeclampsia have identified gene expression of LEP, HTRA1, INHA, INHBA, PAPP2, and FSTL3 involved in cell signaling, lipid response, apoptosis, hypoxia, immune, inflammation and oxidative stress pathways.30,39–41 The expression of a group of 10 genes: LEP, FTRA4, FSTL3, LHD, TREM1, ENG, PAPP2, FLT-1, IHBA, and INHBA has been found to be higher in early-onset preeclampsia (<34 weeks) than later-onset preeclampsia and also higher in late-onset preeclampsia with SGA than without it.32
Clinical Implications
Investigators debate whether preeclampsia with and without FGR are distinct entities.10,11,12,13,15–17 Our study provides new insights about the relationship between fetal growth and pregnancy-associated hypertensive disorders. We highlight the following findings of clinical relevance: 1) abnormal fetal growth is identifiable early in gestation in severe preeclampsia and likely precedes its clinical manifestations; 2) a pattern of asymmetric fetal growth, primarily driven by diminished AC growth, is characteristic of severe preeclampsia. Delayed AC growth begins sooner than the physiological acceleration of AC growth (27-31 weeks) described in normal pregnancies42 preventing the fetus from achieving normal growth and development. The observed differences of AC growth between normotensive pregnancies and those with severe preeclampsia are corroborated with neonatal size differences noted among these groups ; and 3) fetal growth is predominantly normal in mild hypertensive disorders suggesting preserved placental function in these pregnancies.
The presence or absence of fetal growth abnormality distinguished in the studied pregnancy-associated hypertensive disorders might depend on a variety of maternal and placental vascular abnormalities reported since early gestation, from severe deficiency of spiral artery remodeling with excessively impaired extravillous trophoblastic migration and formation of atherotic lesions leading to placental infarcts (preeclampsia with FGR) to minimal or inexistent placental involvement (preeclampsia without FGR).11 Recently described distinctive maternal cardiovascular profiles12 between pregnancies complicated by preeclampsia only (high CO and low PVR), FGR only (unaltered CO, high PVR) and preeclampsia with FGR (low CO and high PVR) might have repercussions on uteroplacental blood flow. Compared with normal pregnancies, placental perfusion calculated by MRI has been found to be significantly lower in women with early-onset preeclampsia (<34 weeks), but significantly higher in women with late-onset preeclampsia.43 The reduction of placental perfusion was more drastic with the presence of FGR.
A recent large European study found that the risk of SGA (birthweight <2.5%tile of birthweight z-score stratified by infant sex) was almost 3 times higher in women with preeclampsia with small placenta (lowest 10% of placenta weight) compared to normotensive women with small placenta weight.44 Conversely, the association of the highest 10% of placenta weight and LGA (≥ 97.5th percentile) was stronger in the preeclampsia group than in the controls. In a recent prospective case –control study, women with preeclampsia and FGR, as compared to women with preeclampsia without FGR, had more severe clinical manifestations, more placental morphologic abnormalities, and lower placental weight and thickness.45 Conversely, decidual vasculopathy was similar in preeclampsia with and without FGR. This suggests that changes in the vascular bed are characteristic of preeclampsia whereas placental villus abnormalities are more strongly correlated with FGR, but both are highly prevalent in severe preeclampsia. Altogether, preeclampsia is manifested in two-well defined clinical phenotypes marked be the presence or absence of FGR, which is ultimately determined by the degree of maternal/ placental hemodynamic abnormalities as well as placental functional and structural changes. Our study support the association of poor fetal growth with severe preeclampsia and the lack of fetal growth abnormality in mild preeclampsia.
Research Implications
This study is unique because the analyzed cohort is composed of a low risk population highly representative of the U.S. contemporaneous pregnant population. With this prospective observational design, we were able to identify the timing in gestation when changes of fetal growth occurred, the progression of these changes throughout pregnancy, and the temporary association of fetal growth with hypertensive disorders.
The reduction of fetal growth identified in women with severe preeclampsia is in contrast with the similarity of fetal growth patterns observed between women with mild hypertensive disorders and normotensive women. Further investigation is necessary to elucidate the mechanisms that elicit development of preeclampsia with and without FGR. Our findings prompt the investigation to determine whether the elaborated fetal growth charts for pregnancies later complicated by severe preeclampsia, perhaps in association with biomarkers and other sonographic parameters, may be used effectively to predict the later onset of clinically evident disease. We also highlight the need to determine fetal growth velocities in normotensive versus pregnancy-associated hypertensive disorders and their association with neonatal size and pregnancy outcomes.
Strengths and Limitations
Our study is strengthened by the prospective design describing longitudinal patterns of fetal growth as well as individual anthropometric parameters in pregnancies complicated by gestational hypertensive disorders in a demographically and racially/ethnically diverse U.S. obstetrical population. Our data collection used a standardized protocol across the twelve centers. The sonographic examinations were performed by experienced and certified sonographers and the methodology used to analyze the data has been previously validated.24 Although, we included a sub-group of obese women (BMI: 30.0-45.0 kg m2) other underlying chronic medical conditions such as chronic hypertension and maternal/fetal risks factors were excluded. In addition, our findings remain significant after controlling for major confounders.
We also acknowledge several limitations. Information on the characteristics used to define the hypertensive categories was not collected. However, we feel that our categories are clinically relevant since these were the diagnoses that were used in clinical practice. This study pre-dated the widespread implementation of the new 2013 ACOG criteria for the diagnosis and classification of preeclampsia.46 It is believed unlikely that use of the newer classification system would significantly alter our findings. In 2016, Kallela et al47 re-analyzed the diagnosis of preeclampsia from a nationwide Finnish database using both the ACOG 2002 and 2013 criteria. The number of women diagnosed with preeclampsia only increased 0.8% (1457 versus 1447) when the new 2013 criteria was applied.
Possible biases can exist in the diagnosis assignment. Classification of our patients was based on the discharge diagnosis summaries from each participating center and lacked standardization across all centers. Any potential misclassification is likely to be non-differential, resulting in a bias towards the null. The low incidence rates of hypertensive disorders in pregnancy in our cohort likely reflects a healthy pregnant population; however, the small sample size in these groups could still potentially result in type-II errors. In addition, we did not observe individual changes in growth at every gestational week, so that differences in measurements per week are extrapolated. The linear mixed models with cubic splines for the fixed effects are flexible enough to allow for a robust calculation of growth at any point in gestation.
Conclusions
This is the first prospective longitudinal study of fetal growth trajectories in pregnancy-associated hypertensive disorders compared with normotensive pregnancies in a low risk, demographically diverse U.S. pregnant population. Compared with normotensive pregnancies, diminished EFW and AC growth was identifiable as early as 22 and 23 weeks of gestation respectively in women with severe preeclampsia. These mid-second trimester fetal growth abnormalities likely anticipate clinical manifestations of severe preeclampsia. We also highlight the similar fetal growth curves in mild pregnancy-associated hypertensive disorders compared to normotensive women.
Supplementary Material
Condensation:
Fetal growth in pregnancy-associated hypertensive disorders is abnormal only in severe preeclampsia and is characterized by asymmetric growth reduction identifiable as early as mid-gestation.
AJOG at a Glance:
A. Why was this study conducted?
To establish fetal growth trajectories in pregnancy-associated hypertensive disorders.
To determine gestational timing for any differences of fetal growth in women with pregnancy-associated hypertensive disorders compared with normotensive women.
B. What are the key findings?
Women with severe preeclampsia demonstrated reduced estimated fetal weights and abdominal circumferences from as early as 22-23 weeks’ gestation compared to women without hypertension.
Fetal growth was similar between mild preeclampsia and normotensive groups.
Fetuses of women with mild gestational hypertension had transitory fetal growth delays that normalized by the third trimester.
C. What does this study add to what is already known?
Women destined to develop severe preeclampsia experienced persistent reduction in fetal growth with significant divergence from normotensive pregnancies as early as 22 weeks’ gestation.
Our study confirms reductions in fetal growth are related to disease severity.
Acknowledgement/Funding:
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health and included ARRA funding (Contract Numbers: HHSN275200800013C; HHSN275200800002I; HHSN27500006; HHSN275200800003IC; HHSN275200800014C; HHSN275200800012C; HHSN275200800028C; HHSN275201000009C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov Identifier:
Disclosure: Deborah A. Wing has been a consultant for Parsagen, for which she received no compensation. The other authors did not report any potential conflicts of interest.
Publisher's Disclaimer: Disclaimer: K.L. Grantz, J. Grewal, P.S. Albert, S. Kim, C. Zhang, and G.M. Buck Louis are U.S. federal government investigators; please see accompanying cover sheet.
This study was presented the 37th Annual Meeting Society for Maternal Fetal Medicine, Las Vegas, NV, 2017. Poster #210.
References
- 1.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019. January;133(1):e1–e25. [DOI] [PubMed] [Google Scholar]
- 2.Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95(1):24–28. [DOI] [PubMed] [Google Scholar]
- 3.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013. September;170(1):1–7. [DOI] [PubMed] [Google Scholar]
- 4.Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105(11):1177–1184. [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Brosens JJ, Muter J, Puttermans P, Benagliano G. Preeclampsia: the role of persistent endothelial cells in uteroplacental arteries. Am J Obstet Gynecol. 2019. February 6 [DOI] [PubMed] [Google Scholar]
- 6.Labarrere CA, DiCarlo HL, Bammerlin E, et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol. 2017. March;216(3):287.e1–287.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R Pre-eclampsia. Lancet. 2010;376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 8.Moll W, Nienartowicz A, Hees H, Wrobel KH, Lenz A. Blood flow regulation in the uteroplacental arteries. Trophoblast Res.1988;3:83–96. [Google Scholar]
- 9.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblastic invasion in preeclampsia and fetal growth restriction. Hypertension. 2013;62:1046–1054. [DOI] [PubMed] [Google Scholar]
- 10.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006. July;195(1):40–9. [DOI] [PubMed] [Google Scholar]
- 11.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018. February;218(2S):S745–S761. [DOI] [PubMed] [Google Scholar]
- 12.Tay J, Foo L, Masini G, Bennett PR, McEniery CM, Wilkinson IB, Lees CC. Early and late preeclampsia are characterized by high cardiac output, but in the presence of fetal growth restriction, cardiac output is low: insights from a prospective study. Am J Obstet Gynecol. 2018. May;218(5):517.e1–517.e12. [DOI] [PubMed] [Google Scholar]
- 13.Srinivas SK, Edlow AG, Neff PM, Sammel CM, Elovitz MA. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol. 2009. October;29(10):680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002. January;99(1):159–67. [DOI] [PubMed] [Google Scholar]
- 15.Ødegărd RA, Vatten LJ, Nilsen ST, Salvessen KA, Austgulen R Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–955. [PubMed] [Google Scholar]
- 16.Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003;101:575–83. [DOI] [PubMed] [Google Scholar]
- 17.Xiong X, Mayes D, Demianczuk N, et al. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999;180:207–213. [DOI] [PubMed] [Google Scholar]
- 18.Gardosi J, Francis A. A customized standard to assess fetal growth in a US population. Am J Obstet Gynecol. 2009. July;201(1):25.e1–7. [DOI] [PubMed] [Google Scholar]
- 19.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449.e1–449.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateus J, Newman R, Kim S, et al. Fetal growth Patterns in hypertensive disorders in pregnancy: the NICHD fetal growth studies. Am J Obstet Gynecol. 2017;216 (1): S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grewal J, Grantz KL, Zhang C, et al. Cohort Profile: NICHD Fetal Growth Studies – Singletons and Twins. International Journal of Epidemiology. 2018; 47(1):25–25l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Hediger ML, Albert PS, et al. Association of maternal obesity with longitudinal ultrasonographic measures of fetal growth: Findings from the NICHD Fetal Growth Studies. JAMA Pediatrics 2018; 172(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight gain with the use of head, body, and femur measurements: a prospective study. Am J Obstet Gynecol 1985;151:333–337. [DOI] [PubMed] [Google Scholar]
- 24.Hediger ML, Fuchs KM, Grantz KL, et al. Ultrasound quality assurance for singletons in the National Institute of Child Health and Human Development Fetal Growth Studies. J Ultrasound Med. 2016;35(8):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pincheiro JC, Bates DM. Mixed-effects models in S and S-plus. New York: Springer Science-Business Media, New York, 2000. [Google Scholar]
- 26.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–8. [DOI] [PubMed] [Google Scholar]
- 27.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol. 2007;582:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yung HW, Calabrese S, Hynx D, et al. Evidence of placental translation inhibition of endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173:451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung HW, Cox M, Tissot Van Patot M, Burton GJ. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J 2012;26:1970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizawa H, Ota S, Suzuki M et al. Comparative gene expression profiling of placentas from patients with severe pre-eclampsia and unexplained fetal growth restriction. Reprod Biol Endocrinol. 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead CL, Walker SP, Ye L, et al. Placental specific mRNA in the maternal circulation are globally dysregulated in pregnancies complicated by fetal growth restriction. J Clin Endocrinol Metab. 2013;98E429–36. [DOI] [PubMed] [Google Scholar]
- 32.Cox B, Leavey K, Nosi U, Wong F, Kingddom. Placental trancriptome in development and pathology: expression, function, and methods of analysis. Am J Obstet Gynecol. 2015. October; 231 (4 suppl): S138–51. [DOI] [PubMed] [Google Scholar]
- 33.McMinn J, Wei M, Schupf N, et al. , Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27:540–9. [DOI] [PubMed] [Google Scholar]
- 34.Diplas AI, Lambertini L, Lee MJ et al. Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics 2009;4:235–40. [DOI] [PubMed] [Google Scholar]
- 35.Betoni JS, Derr K, Pahl MC et al. MicroRNA analysis in placentas from patients with pre-eclampsia: comparison of new and published results. Hypertens Pregnancy. 2013;32:321–39. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Feng L, Zhang H, et al. Preeclampsia up-regulates angiogenesis-associated microRNA (ie,miR-17, -20a, and -20b) that target ephrin B2 and EPHB4 in human placenta. J Clin Endocrinol Metab. 2012;97:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Struwe E, Berzi G, Schild R, et al. Microarray analysis of placental tissue in intrauterine growth restriction. Clin Endocrinol (Oxf). 2010;72:241–7. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy C, Cotter FE, McElwaine S, et al. Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. Am J Obstet Gynecol. 2007;196:1–6. [DOI] [PubMed] [Google Scholar]
- 39.Tsai S, Hardison NE, James A, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals dysregulation of sialic acid acetylesterase and immune signaling pathways. Placenta. 2011;32:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng T, Chen H, Sun M, Wang H, Zhao G, Wang X Identification of differential gene expression profiles in placentas form preeclamptic pregnancies versus normal pregnancies by DNA microarrays, OMICS. 2012;16:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishizawa H, Pryor-Koishi K, Kato T, Kowa H, Kurashashi H, Udagawa Y Microarray analysis of differential expressed fetal genes in placental tissue derived from early and late onset severe pre-eclampsia. Placenta. 2007:28:487–97. [DOI] [PubMed] [Google Scholar]
- 42.Grantz KL, Kim S, Grobman W, et al. Fetal Growth Velocity: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2018;219(3):285.e1–285.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohberg S, Mulic-Lutvica A, Lindgren P, Ortiz-Nieto F, Wikström AK, Wikström J. Placental perfusion in normal pregnancy and early and late preeclampsia: a magnetic resonance imaging study. Placenta. 2014;35:(3) 202–206. [DOI] [PubMed] [Google Scholar]
- 44.Eskild A, Romundstad PR, Vatten L. Placental weight and birthweight: does the association differ between pregnancies with and without preeclampsia? Am J Obstet Gynecol. 2009;201:595.e1–5. [DOI] [PubMed] [Google Scholar]
- 45.Milosevic-Stevanovic J, Krstic M, Radovic-Janosevic D, Stefanovic M, Antic V, Djordjevic I. Preeclampsia with and without intrauterine growth restriction-Two pathogenetically different entities? Hypertension in Pregnancy 2016;35(4):573–582. [DOI] [PubMed] [Google Scholar]
- 46.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologist’s Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;1122–1131. [DOI] [PubMed] [Google Scholar]
- 47.Kallela J, Jääskeläinen T Kortelainen E, et al. The diagnosis of pre-eclampsia using the revised classifications in the Finnish Pre-eclampsia Consortium (FINNPEC) cohort. BMC Pregnancy Childbirth. 2016;16:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


