Abstract
Background
Tumor size has historically been used to stage breast cancer and guide treatment recommendations. The importance of tumor biology in long-term outcomes is increasingly being acknowledged. No large studies have examined the relative roles of tumor size and receptor status on response to neoadjuvant chemotherapy (NAC) in breast cancer.
Methods
The National Cancer Database was queried for women who underwent NAC and surgery for unilateral clinical stage 1–3 (cT1–3) invasive breast cancer from 2010–2013. Multivariable logistic regression models assessed the relation between receptor status, tumor size, and pathologic complete response (pCR) while controlling for other biologic, sociodemographic, diagnosis, and treatment factors.
Results
38,864 women were included in this study, the majority presenting with cT2 disease (55%). Patients predominantly had ER/PR+Her2- (45%) or ER-PR-Her2- (28%) disease. Nineteen percent had a pCR. cT3 (OR 0.64 CI 0.59–0.70) but not cT2 cancers (OR 0.95 CI 0.89–1.02) were associated with lower pCR rates in comparison to cT1 disease. Her2+ (ER/PR+Her2+: OR 2.94 CI 2.72–3.18; ER−PR−Her2+: OR 6.45 CI 5.92–7.02) and ER−PR−Her2− cancers (OR 3.94 CI 3.68–4.22) were more likely to experience pCR than ER/PR+Her2− cancers. Receptor status was more strongly associated with pCR than tumor size.
Conclusions
Tumor size is independently associated with pCR following NAC after controlling for receptor status, although the effect of receptor status is stronger. These data reinforce the importance of receptor status as well as tumor size, each of which may be acting as surrogates for tumor biology, in setting expectations for outcomes in patients undergoing NAC.
Microabstract
The impact of tumor size on response to neoadjuvant chemotherapy in patients with stage I-III breast cancer remains an open question. In this national study, tumor size is found to be an independent predictor of complete pathologic response in patients with invasive breast cancer. However, cancer biology has a greater impact than tumor size.
Introduction
Neoadjuvant chemotherapy (NAC) has increasingly become a first-line treatment choice for some patients with node positive breast cancer as well as those individuals with Her-2/neu (Her2) positive and estrogen and progesterone receptor (ER and PR) negative malignancies.1 The benefits of NAC to the patient include acting as an in vivo test of the tumor’s susceptibility to systemic therapies, which is highly prognostic and strongly associated with survival.2,3 Additionally, NAC may increase the ability to perform breast conservation and less extensive axillary surgery.4 Finally, patients with residual disease after NAC may have the opportunity to either enroll in clinical trials evaluating novel adjuvant therapies or receive evidence based additional adjuvant therapies.5,6
The most recent consensus guidelines on NAC state that age, tumor size, nodal status, grade, proliferation, ER, PR, and Her2 status all influence the likelihood of a complete pathologic response (pCR) following NAC.1 Tumor receptors (ER, PR, and Her2) are currently the best indicators of the biologic function of invasive breast cancer. When NAC is administered, less than 20% of ER+ or PR+ (ER/PR+) Her2− cancers experience a pCR, in contrast to upwards of 30–40% of patients with triple negative cancers and 20–50% of Her2+ cancers regardless of ER or PR status.3,7,8
Although tumor size is often considered when selecting patients for NAC, it is less clear how tumor size impacts the likelihood of pCR after tumor receptors are considered. Recent studies have provided conflicting results. Baron et al9 found no contribution of tumor size when tumor biology was controlled for in a subset of the Neoadjuvant Breast Symphony Trial, while Goorts et al10 did see a difference between these two tumor characteristics in their multivariable analysis of data from the Netherlands cancer registry. When clinicians discuss the potential for a pathologic complete response with their patients they often refer more to the biologic or receptor subtype rather than size11, yet there is no data directly comparing the relative contributions of tumor size and receptor status to pCR. We sought to evaluate whether tumor size was independently associated with pCR post NAC after controlling for receptor status, utilizing the National Cancer Database (NCDB).12 Based on the importance of breast cancer biology and the associated response to chemotherapeutic agents we hypothesized that the underlying tumor biology (ER, PR, Her2 status) would have the strongest association with pCR in patients undergoing NAC, but that tumor size would still be an independent predictor.
Methods
The NCDB is a hospital-based cancer registry that is estimated to capture approximately 70% of all malignancies diagnosed in the United States.12,13 All patient information in the participant user file is de-identified and was exempt from institutional review board review.
Study population
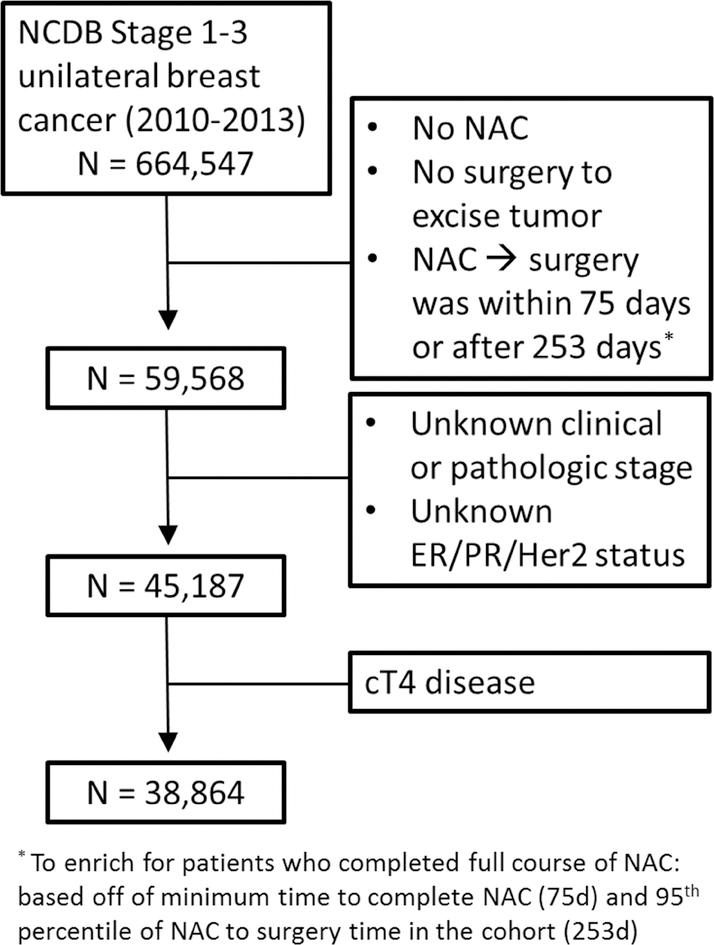
The patient population included women ≥ 18 years with clinical stage 1–3 unilateral breast cancer who were diagnosed in 2010–2013 and underwent neoadjuvant chemotherapy (NAC) with subsequent surgical excision of the breast excluding excisional breast biopsy (N=64,252). This timeframe was selected to ensure consistent reporting of Her2 status and represents a time period when patients were most likely to have received guideline-recommended Her2 targeted therapy. The NCDB therapy timing variable that includes NAC treatment relies on the planned treatment and not what the patient actually received. To ensure women in this study received chemotherapy prior to surgery we defined NAC as patients who were coded as receiving chemotherapy prior to undergoing surgical excision. We further restricted the study cohort to the subgroup of women who were most likely to have completed their NAC regimen, by only including women with 75–252 days between starting NAC and undergoing surgical excision (N=59,568). This lower limit was selected based on the minimum amount of time to complete NAC (Cyclophosphamide every 14 days for four cycles14) and schedule surgery (14 days). The upper limit was determined by the 95th percentile of NAC timing for women in the cohort. We also excluded women missing clinical T and N stage, pathologic T stage, and ER, PR and Her2neu status as well as women with cT4 disease as the T4 designation does not include any indication as to tumor size. The final sample size was 38,864.
The primary outcome variable was complete pathologic response (pCR) following NAC. Patients with a pCR were defined as no identifiable tumor in the breast (ypT0). Patients with residual in situ disease were classified as not experiencing a pCR. Nodal response was not included in the definition of pCR used in this study. The primary predictor variables were receptor status and tumor size. ER/PR and Her2 receptor statuses were combined into one categorical variable with 4 groups: ER/PR+Her2−, ER/PR+Her2+, ER−PR−Her2− (triple negative), and ER−PR−Her2+.15 Tumor size was approximated using clinical T stage. Control variables associated with tumor size, receptor status, or pCR included age, race (white, nonwhite and unknown), ethnicity (Hispanic and non-Hispanic), insurance, facility type for reporting (community, academic, cancer center, integrated center, or unknown), Charlson (comorbidity) index (categorized as 0, 1, ≥2), income, education, and clinical nodal stage as a dichotomous variable (positive/negative).
Analysis
Descriptive statistics assessed sociodemographic, diagnosis, and treatment characteristics overall. Multivariable logistic regression was used to assess the relation between clinical tumor stage and pCR following NAC controlling for receptor status. Additional models that stratified patients by receptor status were also constructed. Control variables in the model included age, race, ethnicity, insurance, facility type, Charlson (comorbidity) index, income, education, and clinical nodal stage. Odds ratios, p values, 95% confidence intervals, and pseudo-R2 values were calculated for each model. In addition, a model was estimated that included the interaction between tumor size and receptor status. In order to determine the comparative effect of tumor size and receptor status the percent change in logistic regression pseudo-R2 (% ΔR2) was calculated. This was performed by calculating the pseudo-R2 for the model including all covariates (R2tot), and then repeating the pseudo-R2 calculation for each of three different models that excluded either cT stage or receptor status individually or the two variables in combination. The % ΔR2 was then calculated using the formula: (R2tot-R2x)/R2tot where R2x is the pseudo-R2 value for the model excluding the variable of interest.16 Relative contributions of tumor size and receptor status were evaluated by comparing the calculated % ΔR2 for the model with both variables excluded and models lacking the variables individually. All statistics were performed using STATA 14.0 (StataCorp LP, College Station, TX).
Results
The CONSORT diagram for included patients who received NAC for stage 1–3 invasive breast cancer prior to definitive surgery is presented in Figure 1. We identified 38,864 individuals within the NCDB who fit our inclusion criteria (Table 1). The average age of these women was 52 years old and the majority were non-Hispanic and white with private insurance. Over half of these women had cT2 tumors (2–5cm) and a slight majority had nodal disease prior to NAC. Approximately 63% of breast cancers in this cohort were ER/PR+ and 28% were triple negative. Within the total cohort the pCR rate was 19% following NAC. The pCR rate for women with cT1 and cT2 disease was 20% and 21% respectively while it was 15% for tumors >5cm. Nine percent of women with ER/PR+Her2− cancers experienced pCR compared with 22% for ER/PR+Her2+, 27% for triple negative and 38% for ER−PR−Her2+. Surgery type was stratified by cT stage and is presented in Table 2. Women with cT3 tumors underwent the lowest percentage of breast conserving surgery (BCS; 22% vs 38% for cT1 and 44% for cT2).
Figure 1:
CONSORT diagram of study criteria
Table 1:
Patient demographics
| All, # (%) N= 38,864 | pCR, # (% of All) N=7,432 | ||
|---|---|---|---|
| cT Stage | 1 | 7,958 (21) | 1,631 (20) |
| 2 | 21,406 (55) | 4,393 (21) | |
| 3 | 9,500 (24) | 1,408 (15) | |
| Receptor status | ER/PR+Her2− | 17,424 (45) | 1,519 (9) |
| ER/PR+Her2+ | 6,871 (18) | 1,537 (22) | |
| ER−PR−Her2− | 10,756 (28) | 2,934 (27) | |
| ER−PR−Her2+ | 3,813 (10) | 1,442 (38) | |
| Clinical Nodal disease | Absent | 16,783 (43) | 3,086 (18) |
| Present | 22,081 (57) | 4,346 (20) | |
| Age | <45 | 10,819 (28) | 2,277 (21) |
| 45–55 | 13,783 (36) | 2,610 (19) | |
| >55 | 14,262 (37) | 2,545 (18) | |
| Race | White | 29,731 (77) | 5,637 (19) |
| Non-White | 8,805 (23) | 1,734 (20) | |
| Unknown | 325 (1) | 61 (19) | |
| Ethnicity | Non-Hispanic | 36,035 (93) | 6,885 (19) |
| Hispanic | 2,829 (7) | 547 (19) | |
| Insurance | Private | 25,889 (67) | 5,147 (20) |
| Uninsured | 1,612 (4) | 323 (20) | |
| Medicaid | 4,649 (12) | 820 (18) | |
| Gov’t/Medicare | 6,257 (16) | 1,058 (17) | |
| Unknown | 457 (1) | 84 (18) |
Table 2:
Definitive surgery type and clinical tumor stage, number (percentage)
| cT1 | cT2 | cT3 | |
|---|---|---|---|
| BCS* | 3,000 (38) | 9,353 (44) | 2,041 (21) |
| Mastectomy | 4,958 (62) | 12,051 (56) | 7,459 (79) |
| Unknown | 0 (0) | 2 (0) | 0 (0) |
BCS: Breast conserving surgery
Tumors > 5cm were independently associated with a decrease in likelihood of pCR following NAC (OR 0.64 CI 0.59, 0.70) in our multivariable analysis (Table 3). Receptor status was also strongly associated with likelihood of pCR with OR of 2.94 (CI 2.72, 3.18), 3.94 (CI 3.68, 4.22), and 6.45 (CI 5.92, 7.02) for ER/PR+Her2+, triple negative, and ER/PR−Her2+ cancers respectively. Clinical stage three tumors, >5cm, were associated with decreased likelihood of pCR with ORs of 0.52 (CI 0.44, 0.62) for ER/PR+Her2−, 0.68 (CI 0.57, 0.82) for ER/PR+Her2+, 0.67 (CI 0.58, 0.77) for triple negative and 0.75 (CI 0.61, 0.92) for ER/PR−Her2+ disease.
Table 3:
Multivariable logistic regression models evaluating clinical tumor stage and pCRa
| All patients, n=38,732 | ER/PR+Her2−, n=17,424 | ER/PR+Her2+, n=6,871 | ER/PR−Her2−, n=10,756 | ER/PR−Her2+, n=3,813 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | |
| cT Stage | ||||||||||
| 1 | REF | ** | REF | ** | REF | ** | REF | ** | REF | ** |
| 2 | 0.95 (0.89, 1.02) | 0.89 (0.78, 1.01) | 0.96 (0.84, 1.11) | 0.96 (0.86, 1.08) | 1.02 (0.85, 1.21) | |||||
| 3 | 0.64 (0.59, 0.70) | 0.52 (0.44, 0.62) | 0.68 (0.57, 0.82) | 0.67 (0.58, 0.77) | 0.75 (0.61, 0.92) | |||||
| Receptors | ||||||||||
| ER/PR+Her2− | REF | ** | ||||||||
| ER/PR+Her2+ | 2.94 (2.72, 3.18) | |||||||||
| ER−PR−Her2− | 3.94 (3.68, 4.22) | |||||||||
| ER−PR−Her2+ | 6.45 (5.92, 7.02) | |||||||||
| Age | ||||||||||
| <45 | REF | ** | REF | ** | REF | ns | REF | ** | REF | * |
| 45–55 | 0.89 (0.82, 0.97) | 0.80 (0.68, 0.95) | 0.93 (0.78, 1.12) | 0.91 (0.80, 1.05) | 0.93 (0.74, 1.17) | |||||
| >55 | 0.85 (0.78, 0.93) | 0.70 (0.58, 0.83) | 1.05 (0.87, 1.28) | 0.74 (0.64, 0.86) | 1.17 (0.93, 1.49) | |||||
| Clinical Nodal Disease | ||||||||||
| No | REF | ** | REF | ** | REF | ** | REF | ** | REF | * |
| Yes | 1.23 (1.16, 1.30) | 1.20 (1.07, 1.34) | 1.33 (1.18, 1.50) | 1.21 (1.10, 1.32) | 1.19 (1.03, 1.37) | |||||
Ethnicity, race, insurance, facility type, education, income, Charlson index, and geographic location were also included in these models.
p-values: ns not significant
p<0.05
p<0.005
The relative contributions of cT stage and receptor status to pCR are shown in Table 4. Receptor status had the largest impact on pCR as evidenced by a % ΔR2 of 88.7% compared to cT stage (% ΔR2=5.1%). There was no statistically significant interaction between tumor size and receptor status (X2=8.91, p=0.18).
Table 4:
Relative contributions of staging variables to pCR following NAC
| Baseline Model R2 = 0.0780 | |||
|---|---|---|---|
| Variable(s) Removed | R2 | ΔR2 | % ΔR2 |
| cT stage | 0.0740 | 0.0040 | 5.1% |
| Receptor status | 0.0088 | 0.0692 | 88.7% |
| cT and Receptor status | 0.0041 | 0.7390 | 94.7% |
Discussion
This study demonstrates that tumors >5cm are associated with a lower likelihood of having a pCR following NAC even when accounting for receptor status. Not surprisingly, receptor status has a larger impact on pCR than tumor size. This is the largest study specifically examining the role of tumor size in response to NAC performed in the modern era.
Two recently published studies examining this issue demonstrated conflicting results. When controlling for tumor biology (receptor status and tumor grade), Goorts et al10 identified cT3/cT4 disease as an independent predictor of decreased pCR likelihood whereas Baron et al9 found no association between tumor size and pCR in their multivariable analysis. Neither of these studies compared cT1 to cT2 disease and both utilized smaller homogenous populations. Goorts et al10 looked at data from a time period (2005–2008) where breast cancer treatments, specifically the adoption of Her2-targeted therapies, was inconsistent. This study from the Netherlands also examined ER, PR, and Her2 receptor status as three independent variables and reported an association between PR and Her2 status, but not ER status, with pCR; whereas our study combined receptor status into one variable and identified an interaction between the composite of ER, PR and Her2 with pCR. Through analysis of the NCDB we were able to independently compare cT1, cT2 and cT3 stages in a broad range of breast cancer patients from across the United States during the modern era of breast cancer treatment and highlighted that though tumor size has a smaller effect on pCR than receptor status, both were influential.
Recent studies developing predictive models for axillary pCR following NAC have also evaluated the impact of tumor size on pCR17–20. These studies differ from ours in several important features. Their outcome of interest, axillary pCR (ypN0) was different from ours (ypT0). Different definitions of pCR are associated with differences in patient outcomes21 and in-breast and nodal response rates to NAC differ likely reflecting distinct biological niches within each anatomic location as well as biological heterogeneity.22 Studies examining axillary response to NAC either only include node positive patients18–20 or analyze cN0 patients separately from patients with nodal disease17 while we included patients with and without nodal involvement in our models. Additionally, these studies were not designed to answer the question of whether tumor size impacts pCR, they were designed to develop tools to help clinicians predict which patients may not require axillary dissection and using results of individual covariates from these models can fall into the table two fallacy.23 The study presented here was specifically designed to examine the impact of tumor size on in breast pCR following NAC, a question of clinical importance that has not been fully addressed in the literature to date.
Though the intrinsic biology of a breast cancer is perhaps best understood through its receptor status, tumor size may also provide a different or additive window into a cancer’s internal biology. Breast cancer screening studies have highlighted the improved outcomes for patients whose cancers are identified at an earlier stage.24 While tumor size may simply be a function of a cancer that has been existent in the breast for a longer period of time and therefore less responsive to cytotoxic treatments, it may more importantly provide an understanding of biologic properties that are not reflected in the receptors. A meta-analysis of circulating tumor cells (CTCs) in early stage breast cancer patients undergoing NAC revealed that 25.2% of patients had CTCs prior to NAC and the presence of CTCs was associated with tumor size but not receptor status.25 Increasing numbers of CTCs resulted in a decrease in overall survival as well as locoregional relapse-free survival but was not associated with pCR.25 This highlights the fact that though receptor status is a key component of a cancer’s response to therapy it is not the only component that will influence treatment response. Further studies into how a cancer achieves a certain size in its breast microenvironment will assist in both the expectations for response to NAC as well as guide future changes in NAC treatment options.
This study has several limitations. NCDB collects data on whether patients receive chemotherapy, but does not record whether they completed their course or what agents were received. We attempted to enrich our study population with individuals who had completed NAC by ensuring that only patients with at least 75 days between starting NAC and undergoing surgery were included. We were unable to evaluate whether women with Her2+ cancers received trastuzumab as NCDB classified it with other chemotherapy and did not enter it as a separate category until 2013. We addressed this potential limitation by including patients diagnosed with breast cancer after 2010 when Her2 status was a required measure in the NCDB and Her2− directed therapies were widely administered. Other limitations to this study resulted from how variables were coded in NCDB. We were not able to include tumor grade in our analysis as that variable is most commonly taken from the surgical pathology and not the initial biopsy report. Lower tumor grade has been associated with a decrease in pCR following NAC9 and it is possible that the larger cancers in our study were disproportionately lower grade as well. We were unable to use the continuous variable tumor size and relied on the categorical cT stage for our variable of interest as tumor size in NCDB was sometimes populated from post-NAC pathologic evaluation and it is not possible to know when that occurred. We were still able to compare tumors <1cm to those 2–5cm and >5cm, a more clinically useful comparison than the 5cm cutoff previously published.9,10
Conclusion
This study demonstrates the importance of continuing to incorporate tumor size into the shared decision making discussion around expected outcomes for NAC in patients with nonmetastatic invasive breast cancer. For patients with smaller tumors that are more responsive to chemotherapy, such as those with triple negative and Her-2 positive cancers, a higher likelihood of pCR can be described during decision making discussions with the patient. Patients with large tumors however would benefit from counseling that despite the lower likelihood of a pCR with NAC, this treatment approach offers the opportunity to perform more minimally invasive surgery and the lack of a pCR may or may not reflect a worse long term outcome.
Clinical Practice Points
With the growing understanding of the important role tumor receptors and tumor biology play in breast cancer response rates to neoadjuvant chemotherapy (NAC), the contributions of tumor size to pathologic complete response (pCR) rates remains unknown. This large national study examines the influence of clinical tumor stage and receptor status on pCR rates and finds that patients with cT3, but not cT2, tumors are less likely to experience pCR while hormone and Her2 receptor status have a much larger impact on pCR. These data reinforce the importance of receptor status as well as tumor size, each of which may be acting as surrogates for tumor biology, in setting expectations for outcomes in patients undergoing NAC. This study demonstrates the continued importance of incorporating tumor size into the shared decision making discussion regarding NAC in patients with non-metastatic invasive breast cancer.
Acknowledgements
“The NCDB is a joint project of the American College of Surgeons Commission on Cancer and the American Cancer Society. The hospitals participating in the NCDB are the source of de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis of the conclusions derived by the authors.”
Portions of this study were presented at the 2018 American Society of Breast Surgeons conference in Orlando, Florida.
Funding: This work was supported by the National Institutes of Health (T32 CA090217 to DLR, TSD, and KV) and American College of Surgeons (Resident Research Scholarship to DLR).
Disclosures: LGW is a founder of Elucent Medical. CCG is a consultant for Johnson & Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 2.Penault-Llorca F, Abrial C, Raoelfils I, et al. Comparison of the prognostic significance of Chevallier and Sataloff’s pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum Pathol. 2008;39(8):1221–1228. doi: 10.1016/J.HUMPATH.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 4.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights Breast Cancer, Version 1.2016. J Natl Compr Canc Netw. 2015;13(12):1475–1485. doi: 10.6004/JNCCN.2015.0176. [DOI] [PubMed] [Google Scholar]
- 5.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2018. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 7.Cortazar P, Geyer CE. Pathological Complete Response in Neoadjuvant Treatment of Breast Cancer. Ann Surg Oncol. 2015;22(5):1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 8.Houssami N, MacAskill P, Von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Baron P, Beitsch P, Boselli D, et al. Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2015;(April 2015):1522–1529. doi: 10.1245/s10434-015-5030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goorts B, van Nijnatten TJA, de Munck L, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2017;163(1):83–91. doi: 10.1007/s10549-017-4155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss A, King TA, Hunt KK, Mittendorf EA. Incorporating Biologic Factors into the American Joint Committee on Cancer Breast Cancer Staging System: Review of the Supporting Evidence. Surg Clin North Am. 2018;98(4):687–702. doi: 10.1016/j.suc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons. National Cancer Database. https://www.facs.org/qualityprograms/cancer/ncdb.
- 13.Raval M V, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99(8):488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 14.Breast Cancer Clinical Guidelines. NCCN Clinical Practice Guidelines in Oncology. doi: 10.1136/bmj.290.6471.855-b. [DOI] [Google Scholar]
- 15.Cossetti RJD, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2014;33(1):65–73. doi: 10.1200/JCO.2014.57.2461. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg CC, Lipsitz SR, Hughes ME, et al. Institutional variation in the surgical treatment of breast cancer: a study of the NCCN. Ann Surg. 2011;254(2):339–345. doi: 10.1097/SLA.0b013e3182263bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy BL L. Hoskin T, Heins CDN, Habermann EB, Boughey JC. Preoperative Prediction of Node-Negative Disease After Neoadjuvant Chemotherapy in Patients Presenting with Node-Negative or Node-Positive Breast Cancer. Ann Surg Oncol. 2017;24(9):2518–2525. doi: 10.1245/s10434-017-5872-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim WH, Kim HJ, Park HY, et al. Axillary Pathologic Complete Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Breast Cancer Patients: A Predictive Model Integrating the Imaging Characteristics of Ultrasound Restaging with Known Clinicopathologic Characteristics. Ultrasound Med Biol. 2019;45(3):702–709. doi: 10.1016/j.ultrasmedbio.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Shin MS, Kim CJ, et al. Improved Model for Predicting Axillary Response to Neoadjuvant Chemotherapy in Patients with Clinically Node-Positive Breast Cancer. J Breast Cancer. 2017;20(4):378. doi: 10.4048/jbc.2017.20.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantor O, Sipsy LMN, Yao K, James TA. A Predictive Model for Axillary Node Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol. 2018;25(5):1304–1311. doi: 10.1245/s10434-018-6345-5. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, Untch M, Blohmer J-UU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 22.Fleming CA, McCarthy K, Ryan C, et al. Evaluation of Discordance in Primary Tumor and Lymph Node Response After Neoadjuvant Therapy in Breast Cancer. Clin Breast Cancer. 2018;18(2):e255–e261. doi: 10.1016/j.clbc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Westreich D, Greenland S. The Table 2 Fallacy: Presenting and Interpreting Confounder and Modifier Coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Autier P, Héry C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009;27(35):5919–5923. doi: 10.1200/JCO.2009.22.7041. [DOI] [PubMed] [Google Scholar]
- 25.Bidard FC, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: A Meta-analysis. J Natl Cancer Inst. 2018;110(6):560–567. doi: 10.1093/jnci/djy018. [DOI] [PubMed] [Google Scholar]