Abstract
Background:
Epidemiologic studies have reported associations between prenatal and early postnatal air pollution exposure and autism spectrum disorder (ASD); however, findings differ by pollutant and developmental window.
Objectives:
We examined associations between early life exposure to PM2.5 and ozone in association with ASD across multiple US regions.
Methods:
Our study participants included 674 children with confirmed ASD and 855 population controls from the Study to Explore Early Development, a multi-site case–control study of children born from 2003 to 2006 in the United States. We used a satellite-based model to assign air pollutant exposure averages during several critical periods of neurodevelopment: 3 months before pregnancy; each trimester of pregnancy; the entire pregnancy; and the first year of life. Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CIs), adjusting for study site, maternal age, maternal education, maternal race/ethnicity, maternal smoking, and month and year of birth.
Results:
The air pollution–ASD associations appeared to vary by exposure time period. Ozone exposure during the third trimester was associated with ASD, with an OR of 1.2 (95% CI: 1.1, 1.4) per 6.6 ppb increase in ozone. We additionally observed a positive association with PM2.5 exposure during the first year of life [OR = 1.3 (95% CI: 1.0, 1.6) per 1.6 μg/m3 increase in PM2.5].
Conclusions:
Our study corroborates previous findings of a positive association between early life air pollution exposure and ASD, and identifies a potential critical window of exposure during the late prenatal and early postnatal periods.
Keywords: Air pollution, autism spectrum disorder, particulate matter, ozone
Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders marked by impairments in social interaction and communication, and repetitive behaviors. The Centers for Disease Control and Prevention (CDC) estimates that 1 in 59 children has been identified with ASD.1 The etiology of ASD is poorly understood; however, studies suggest contributions from both genetic and environmental factors.2-4 Studies have suggested the prenatal and early postnatal periods to be critical windows of susceptibility for environmental toxicant exposure in relation to ASD.5,6
Particulate matter and ozone are among the most ubiquitous criteria air pollutants and have been shown to induce inflammation and oxidative stress,7-9 both of which have been implicated in the development of ASD.10,11 Several epidemiologic studies have reported associations between prenatal and early postnatal air pollution exposure and ASD; however, findings have differed by pollutant and developmental window.12 Particulate matter ≤2.5 and ≤10 μm in diameter (PM2.5 and PM10) exposure during the prenatal13-16 and postnatal15,17 developmental periods has been associated with ASD in studies in the US, with two studies suggesting the third trimester may be a potential critical window of susceptibility.14,16 In addition, one previous study found a positive association between prenatal ozone exposure and ASD.13 Further, there is geographic variation in the composition of air pollution and may be geographic variation in associations between air pollution and ASD, however few studies have had the capacity to explore this potential heterogeneity. Additionally, few studies to date have assessed associations between air pollution and ASD across different geographic locations using uniform exposure and outcome assessment methods.
To address these limitations, we investigated the association between early life exposure to PM2.5 and ozone in association with ASD using data from the Study to Explore Early Development (SEED). We assessed associations with exposures during critical periods of neurodevelopment, including the period three months before pregnancy, the entire pregnancy period, by trimester, and during the first year of life. We additionally investigated whether the association with PM2.5 and ozone differed for ASD with or without a co-occurring intellectual disability, by ASD severity, and by state of birth (SEED study site).
Methods
Study Population
SEED is a multi-site case–control study with study sites for the first phase located within California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania.18 Both cases and controls were eligible to participate in SEED if they were born in a study catchment area between September 2003 and August 2006 and resided there at 30-68 months of age. Children with possible ASD were ascertained through multiple sources serving or evaluating children with developmental problems, including: early intervention programs, special education programs, clinics, and individual providers. Population controls were identified by randomly sampling state birth records of children born in the specified date range to mothers that resided in the study catchment area at the time of delivery. Eligibility was based on residence, birthdate, and availability of a knowledgeable caregiver to participate in English or Spanish (California and Colorado only). An introductory letter was first sent to potential participants and those reached were then screened for eligibility through a follow-up recruitment call. For most, we were unable to determine the validity of their address, thus whether the target sample received mailed recruitment materials and were eligible, therefore our ability to determine response rates was limited.
Institutional review boards at each study site and at CDC approved SEED. Informed consent was obtained from all enrolled participants.
Outcome Ascertainment
Upon telephone enrollment, caregivers of all children were administered the Social Communication Questionnaire (SCQ).19 Any children who screened positive (SCQ score ≥11) or reported a previous ASD diagnosis received a comprehensive developmental assessment to determine final ASD classification. This assessment included two gold standard instruments, 1) the Autism Diagnostic Observation Schedule (ADOS),20 2) the Autism Diagnostic Interview-Revised (ADI-R).21,22 Final ASD case classification was based on the results from the ADOS and ADI-R. ASD severity was additionally assessed using the ADOS calibrated severity score, which was calculated using the ADOS total score, ADOS language level, and chronological age at evaluation.23 The ten-point scale was dichotomized into low/moderate (scores 4-7) and more severe (8-10).
All children participated in a general developmental assessment that included Mullen Scales of Early Development. Children who did not have an indication of possible ASD (negative SCQ screen, no previous ASD diagnosis and no ASD-specific service classification) received only this general developmental assessment. Children with a Mullen Early Learning Composite Score of <70 were classified as having an intellectual disability.24
Exposure Assessment
Exposure to air pollution was determined using data from satellite-based models linked to address at birth. Study participants’ dates of birth and addresses at birth were identified using birth certificates. To ensure participant’s privacy, all dates, including dates of birth, were randomly shifted by 0-14 days in either direction. For a given participant, the shift was the same for all dates in order to maintain the relation among dates. We calculated start date of pregnancy by subtracting the child’s gestational age from their date of birth, using calculated LMP as a proxy for time of conception. Analyses used the clinical estimate of gestational age reported on birth certificates. Addresses at birth were geocoded in ArcGIS using the ESRI Maps street database. Geocoding match rates ranged from 95%-100% across study sites.
Average PM2.5 and ozone concentration estimates were derived at a daily temporal resolution and a 1×1 km spatial resolution using an exposure prediction model for the study exposure period years (2002-2007). This prediction model has been thoroughly described elsewhere25,26 and has previously been used in a study of air pollution and mortality in the US.27 Briefly, the prediction model incorporated data from a chemical transport model that simulates atmospheric chemistry, and is driven by meteorologic input from the Goddard Earth Observing System of the NASA Global Modeling and Assimilation Office (GEOS-Chem).28 GEOS-Chem predictions were calibrated using monitored data. Land use terms (percentage of urban areas, population density, road density, and elevation), meteorologic variables (such as air temperature, precipitation, and wind speed), and satellite data were used to help calibrate GEOS-Chem outputs and to aid in downscaling to a finer spatial resolution. This previously developed model used a neural network to calibrate all the predictors to monitored PM2.5 and ozone and was trained and validated with ten-fold cross-validation.
Participants were matched to the centroid of the nearest grid cell based on their residence at birth. Daily PM2.5 and ozone concentrations were averaged for several periods, including the 1st (weeks 1-13 of pregnancy), 2nd (weeks 14-26), and 3rd trimesters (weeks 27 to birth), preconception (3 months prior to conception), entire pregnancy, and year post birth.
Covariates
We obtained information to assess potential confounders from the caregiver interview and birth certificates. We used a directed acyclic graph to identify the covariate adjustment set to be included in the model that would result in the least biased estimate (see eFigure 1).29 The final adjustment set consisted of the following variables: maternal age (continuous), maternal race/ethnicity (non-Hispanic white, other race/ethnicity), maternal education (< bachelor’s degree, ≥ bachelor’s degree), maternal smoking (any smoking three months before conception or during pregnancy), and year of birth (2003-2004, 2005-2006). Season of birth is related to the levels of air pollution and has been linked with ASD in some previous studies. Therefore, analyses additionally included an indicator variable for month of birth to control for seasonal trend. All models included an indicator for study site (California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania). We excluded participants from the current analyses if they did not have a geocoded address (n=44) or were missing data on key covariates (n=32). In total, 16 cases and 16 controls were missing covariate data on maternal tobacco use and race/ethnicity.
Statistical Analyses
We used logistic regression to estimate adjusted odds ratios (OR) and corresponding 95% confidence intervals (CI) for the associations between PM2.5 and, separately, ozone with ASD, with the population group serving as the control group for all analyses. We first modeled the exposure–response function between air pollution and ASD graphically using natural cubic spline models (eFigure 2). We analyzed PM2.5 and ozone exposures as continuous measures because a continuous term fit better than categorical coding (quartiles), per the lower Akaike Information Criterion value and because continuous coding allows comparability with the results from previous studies. Effect estimates are scaled to the interquartile range (IQR) value for the entire pregnancy period, averaged across study sites. This approach allows the comparison of effect estimates across pollutants, study sites, and developmental windows. We report results for each developmental window modeled separately and also mutually adjusted for the other windows, in order to isolate potentially critical windows of susceptibility. Specifically, results are presented for each trimester mutually adjusted for the other trimesters and, for the preconception, pregnancy, and postnatal windows, mutually adjusted for the other developmental windows.
We assessed effect measure modification by study site for each of the developmental windows in order to assess possible heterogeneity in the PM2.5 and ozone and ASD associations by geographic location. We conducted likelihood ratio tests by comparing models with and without interaction terms for study site and used a p<0.10 as a threshold for presence of modification on the multiplicative scale.
We additionally investigated if PM2.5 and ozone and ASD associations differed for children with ASD depending on the presence of a co-occurring intellectual disability. Further, we compared associations by ASD severity using a dichotomized ASD severity score.23 We compared the two severity subgroups to the population controls using multinomial logistic regression.
We conducted several sensitivity analyses. Ozone may confound relationships between PM2.5 and ASD.30 Therefore, we report results from two-pollutant models for each developmental window by controlling for the other pollutant in each exposure model. We additionally report results adjusted for monitored levels of nitrogen dioxide (NO2), as a marker of traffic-related air pollution. Air pollution exposure has been associated with preterm birth31 and preterm birth is a risk factor for ASD.32 Therefore, we did not control for preterm birth was due to its potential mediating role.33 Instead, we assessed the potential mediating role of preterm birth by comparing air pollution and ASD models without an indicator for preterm birth status to those adjusted for preterm birth. Finally, we used ordinal logistic regression to assess associations between air pollution exposure and ASD severity using the ordinal measure of the severity score. Since controls were not given a severity score, these analyses were among ASD cases only.
Results
The final sample consisted of 1,529 mother–child pairs with complete outcome classification information based on a completed developmental assessment and geocoded address data available. This included 674 cases of children with ASD and 855 population controls. Compared to the population controls, children with ASD were more likely to be boys, born preterm, and born to non-white mothers and mothers with lower level of education (Table 1). These differences were consistent across SEED study sites (eTable 1).
Table 1.
Characteristics of ASD Cases and Population Controls.
| Characteristic | ASD Cases (n=674) |
Controls (n=855) |
|---|---|---|
| Child sex, N (%) | ||
| Male | 551 (82) | 453 (53) |
| Female | 123 (18) | 402 (47) |
| Birth year, N (%) | ||
| 2003-2004 | 273 (41) | 393 (46) |
| 2005-2006 | 401 (59) | 462 (54) |
| Maternal race/ethnicity, N (%) | ||
| Non-Hispanic white | 378 (56) | 611 (71) |
| Othera | 296 (44) | 244 (29) |
| Maternal education, N (%) | ||
| <Bachelor’s | 331 (49) | 289 (34) |
| ≥Bachelor’s | 343 (51) | 566 (66) |
| Maternal age at birth (years), N (%) | ||
| <35 | 486 (72) | 589 (69) |
| ≥35 | 188 (28) | 266 (31) |
| Maternal smoking, N (%) | ||
| Yes | 112 (17) | 79 (9) |
| No | 562 (83) | 776 (91) |
| Preterm, N (%) | ||
| Yes | 111 (16) | 82 (10) |
| No | 563 (84) | 773 (90) |
| SEED study site, N (%) | ||
| California | 96 (14) | 134 (16) |
| Colorado | 139 (21) | 185 (22) |
| Georgia | 130 (19) | 160 (19) |
| Maryland | 107 (16) | 126 (15) |
| North Carolina | 100 (15) | 146 (17) |
| Pennsylvania | 102 (15) | 104 (12) |
| PM2.5 (μg/m3), mean ± SD | ||
| Pregnancy | 12.8 ± 2.7 | 12.7 (2.6) |
| First year of life | 12.7 ± 2.5 | 12.5 (2.5) |
| Ozone (ppb), mean ± SD | ||
| Pregnancy | 36.8 ± 5.7 | 37.0 ± 5.7 |
| First year of life | 37.8 ± 4.4 | 38.1 ± 4.3 |
ASD indicates Autism Spectrum Disorder; PM2.5, particulate matter <2.5 μm; SEED, Study to Explore Early Development; SD, standard deviation.
Includes African American, Asian, Hispanic, multiracial, and all others.
PM2.5 and ozone levels varied both spatially and temporally within and across the SEED study areas, which were generally centered around large urban areas (Figure 1; also see eFigure 3). During the study years, PM2.5 peaked during the summer months for the Eastern study areas and during the winter months for the California study site. There was less temporal variation in PM2.5 in the Colorado study area. In contrast, ozone concentrations peaked in the summer months for all of the study areas.
Figure 1.
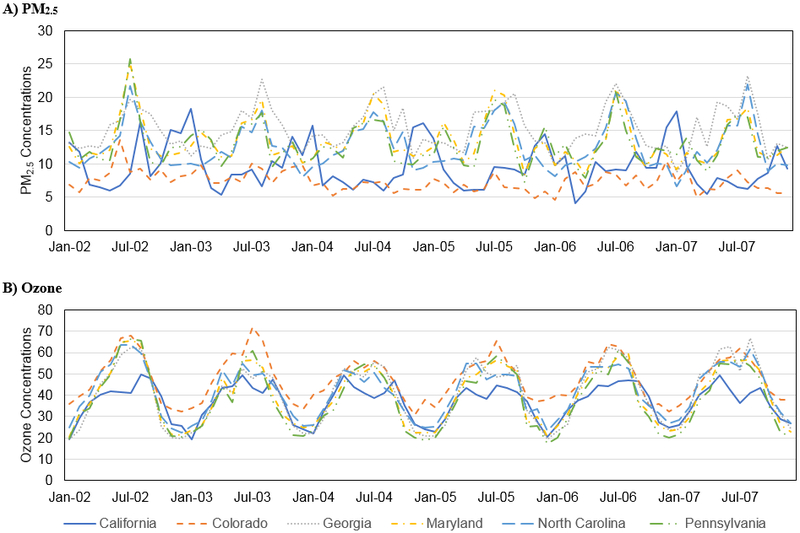
Monthly (A) PM2.5 (μg/m3) and (B) ozone (ppb) concentrations from 2002-2007, by SEED study site.
The overall PM2.5 average for the pregnancy period was 12.7 μg/m3 (range: 4.9 – 18.6 μg/m3) among all controls (Table 2), and varied considerably across study sites, ranging from 9.0 μg/m3 in CO to 15.5 μg/m3 in GA. The overall ozone average for the pregnancy period was 37.1 ppb, and was lowest in California (29.5 ppb) and highest in Colorado (39.8 ppb). Mean PM2.5 levels were strongly correlated across several of the developmental periods (eTable 2), particularly for pregnancy and first year of life (correlation of 0.92). Pollutant levels were often inversely correlated between the first and third trimester; e.g. for PM2.5 in California (−0.64), Georgia (−0.65), and North Carolina (−0.71), and for ozone in Colorado (−0.78), Georgia (−0.86), Maryland (−0.85), North Carolina (−0.89), and Pennsylvania (−0.86).
Table 2.
PM2.5 (μg/m3) and Ozone (ppb) Exposure Distribution [mean (interquartile range width, IQRw)] by Study to Explore Early Development Study Site and Developmental Windows, among Controls Only.
| All sites Mean (IQRW) |
California Mean (IQRW) |
Colorado Mean (IQRW) |
Georgia Mean (IQRW) |
Maryland Mean (IQRW) |
North Carolina Mean (IQRW) |
Pennsylvania Mean (IQRW) |
|
|---|---|---|---|---|---|---|---|
| Ozone (ppb) | |||||||
| Preconception | 37.7 (18.8) | 31.6 (9.4) | 39.8 (20.8) | 37.5 (21.5) | 37.5 (21.3) | 40.3 (13.5) | 39.1 (17.9) |
| Entire pregnancy | 37.1 (8.5) | 29.5 (4.5) | 39.8 (7.9) | 39.3 (7.5) | 36.8 (7.8) | 39.7 (4.4) | 35.3 (7.5) |
| First trimester | 36.1 (21.2) | 28.8 (13.1) | 39.8 (21.3) | 36.9 (25.1) | 34.2 (16.6) | 40.2 (21.7) | 34.7 (25.4) |
| Second trimester | 37.2 (20.0) | 29.1 (13.9) | 40.6 (18.6) | 40.5 (18.8) | 36.2 (21.9) | 40.0 (14.3) | 33.7 (17.2) |
| Third trimester | 38.2 (20.8) | 30.7 (11.7) | 39.2 (21.3) | 41.1 (21.9) | 40.3 (14.1) | 38.6 (21.6) | 38.0 (24.1) |
| First year of life | 38.2 (5.0) | 31.1 (3.2) | 40.0 (5.3) | 40.0 (3.4) | 38.2 (3.5) | 40.5 (2.6) | 38.3 (3.3) |
| PM2.5 (μg/m3) | |||||||
| Preconception | 12.7 (5.0) | 10.7 (5.2) | 9.1 (2.2) | 15.8 (4.8) | 15.3 (3.9) | 12.9 (3.3) | 13.3 (3.2) |
| Entire pregnancy | 12.7 (3.9) | 11.6 (1.8) | 9.0 (1.6) | 15.5 (1.7) | 14.8 (1.8) | 13.0 (1.5) | 12.9 (1.1) |
| First trimester | 12.6 (5.2) | 11.9 (7.7) | 9.0 (2.3) | 15.0 (3.0) | 14.3 (2.5) | 13.3 (4.8) | 13.0 (3.7) |
| Second trimester | 12.7 (4.7) | 12.1 (7.9) | 8.9 (2.3) | 15.6 (4.8) | 14.7 (2.4) | 13.0 (3.9) | 12.6 (2.1) |
| Third trimester | 12.7 (5.6) | 10.8 (5.8) | 9.1 (2.3) | 16.1 (4.8) | 15.5 (5.0) | 12.9 (4.8) | 13.1 (2.7) |
| First year of life | 12.5 (3.9) | 11.3 (1.1) | 8.7 (1.5) | 15.5 (0.8) | 14.5 (1.6) | 13.1 (0.9) | 13.0 (0.8) |
Adjusted associations between air pollutants and ASD differed across developmental periods. For both ozone and PM2.5, we observed null associations with ASD for exposure during preconception, the pregnancy average, and the first and second pregnancy trimesters (Table 3). We observed elevated associations with ASD for ozone exposure during the third trimester: OR=1.2 per the interquartile range width (IQRW) of 6.6 ppb (95% CI: 1.1, 1.4), and for PM2.5 exposure during the first year of life: OR=1.3 (95% CI: 1.0, 1.6) per 1.6 μg/m3 increase in PM2.5 (Table 3; also see eTable 3 for unadjusted results). Results were similar, but with wider CIs, comparing two-pollutant to single pollutant models (eTable 4). Results were additionally similar in models adjusted for preterm birth (eTable 3) and monitored levels of NO2 (eTable 5). Finally, results were generally similar across the developmental windows and pollutants when adjusting for sex of the child (eTable 3).
Table 3.
Adjusteda Associations between PM2.5 and Ozone and ASD, for Single and Mutual Window Adjustment Models, among Study to Explore Early Development Participants.
| Ozone OR (95%CI) |
PM2.5 OR (95%CI) |
|
|---|---|---|
| First Trimester | ||
| Modeled separately | 0.94 (0.83, 1.1) | 0.98 (0.91, 1.1) |
| Mutual adjustment for exposures during trimesters 2 and 3 | 0.98 (0.85, 1.1) | 1.0 (0.93, 1.1) |
| Second Trimester | ||
| Modeled separately | 0.96 (0.85, 1.1) | 0.97 (0.91, 1.0) |
| Mutual adjustment for exposures during trimesters 1 and 3 | 0.89 (0.77, 1.0) | 0.96 (0.90, 1.0) |
| Third Trimester | ||
| Modeled separately | 1.2 (1.1, 1.4) | 1.1 (0.98, 1.1) |
| Mutual adjustment for exposures during trimesters 2 and 3 | 1.3 (1.1, 1.5) | 1.1 (0.98, 1.2) |
| Preconception | ||
| Modeled separately | 0.92 (0.82, 1.1) | 1.0 (0.97, 1.1) |
| Mutual adjustment for exposures during pregnancy and the year post birth | 1.0 (0.87, 1.2) | 1.0 (0.92, 1.1) |
| Entire pregnancy | ||
| Modeled separately | 1.1 (0.85, 1.3) | 1.0 (0.88, 1.2) |
| Mutual adjustment for exposures during preconception and the year post birth | 1.4 (1.0, 1.8) | 0.95 (0.77, 1.2) |
| First year of life | ||
| Modeled separately | 0.79 (0.60, 1.0) | 1.3 (1.0, 1.6) |
| Mutual adjustment for exposures during preconception and pregnancy | 0.62 (0.41, 0.94) | 1.3 (0.98, 1.7) |
Effect estimates are scaled to the interquartile range width value for the entire pregnancy period, averaged across study sites (1.6-μg/m3 for PM2.5 and 6.6-ppb for ozone).
ASD indicates autism spectrum disorder; CI, confidence interval; OR, odds ratio; PM2.5, particulate matter <2.5 μm.
All models are adjusted for maternal age, maternal education, maternal race/ethnicity, maternal smoking, study site, month of birth, and year of birth.
Table 3 also includes results from models for trimester-specific associations mutually adjusted for the other trimesters, as well as from models mutually adjusted for the preconception, entire pregnancy, and first year of life exposure periods. The elevated OR for third trimester ozone exposure increased in models mutually adjusted for exposure during trimesters 2 and 3 [OR=1.3 (95% CI: 1.1, 1.5)] (Table 3). In models mutually adjusted for the preconception period and the first year of life, an association between ozone exposure during pregnancy and ASD was observed [OR=1.4 (95% CI: 1.0, 1.8). Adjusting for exposures during preconception and pregnancy, the OR for PM2.5 during the first year of life remained similar [OR: 1.3 (95% CI: 0.98, 1.7)].
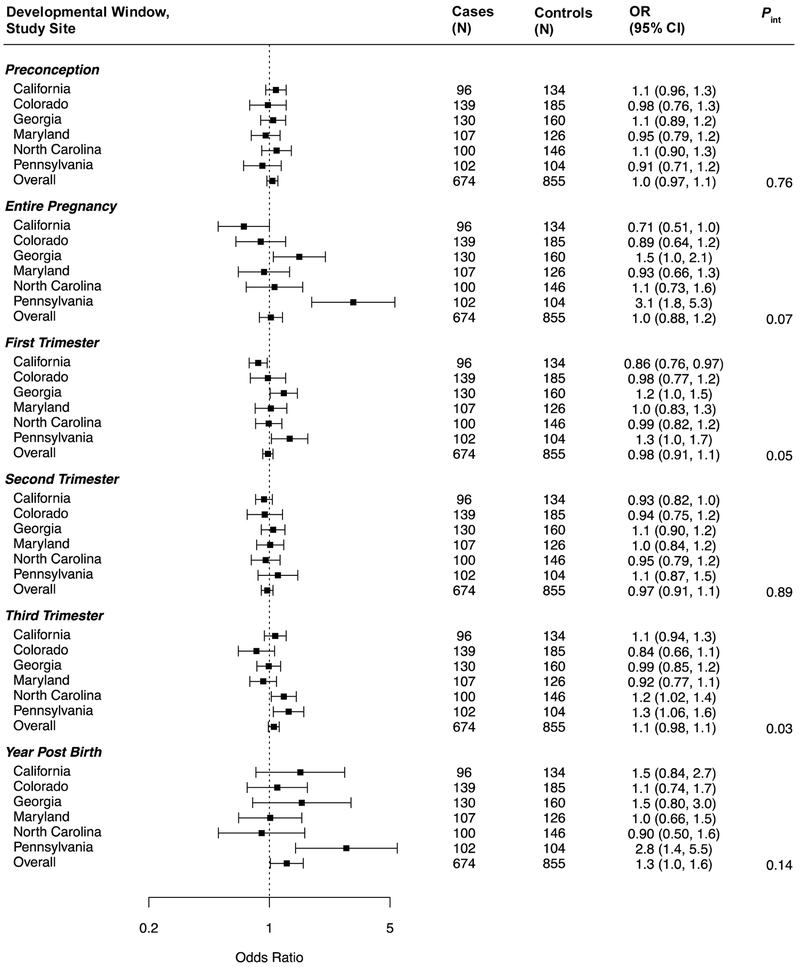
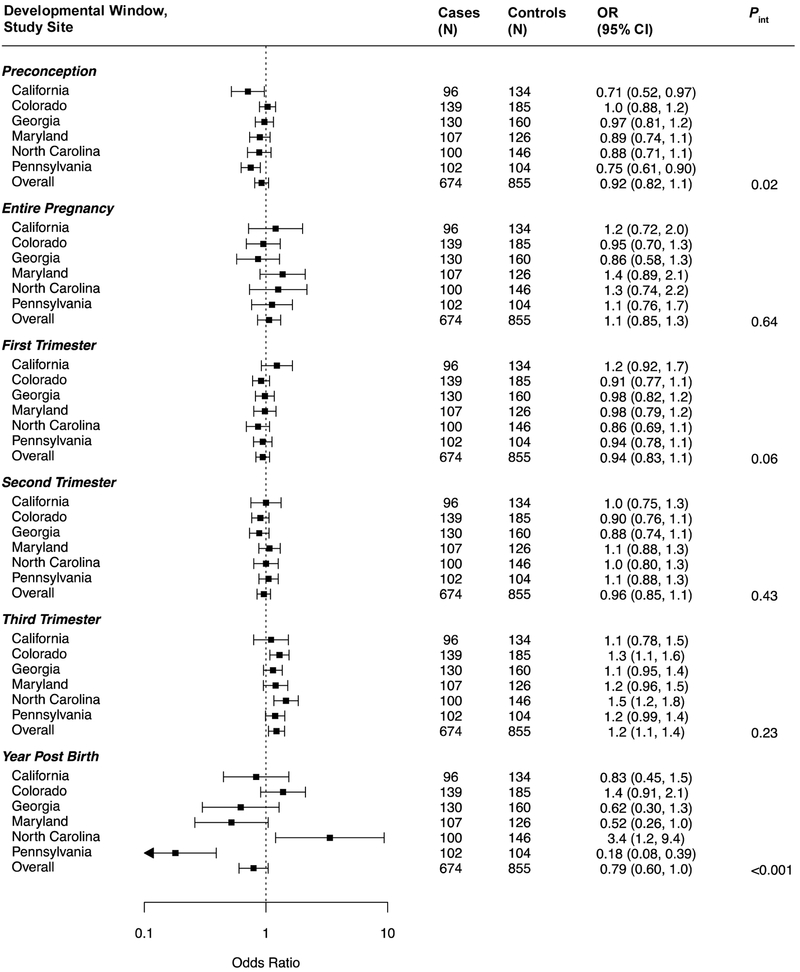
The association between ASD and PM2.5 exposure during the first year of life did not vary across study sites (P = 0.14) (Figure 2; also see eTable 6 for numeric data). However, there did appear to be effect modification by site for the associations with PM2.5 during the first (P = 0.05) and third (P = 0.03) trimesters, as well entire pregnancy exposures (P = 0.07). As results for the Pennsylvania site appeared to be outliers, we re-ran models excluding them (eTable 7); the overall association of first year of life PM2.5 exposure and ASD was somewhat driven by this site, but less so for the model that also adjusted for the preconception and pregnancy exposure periods. The association observed between third trimester ozone exposure and ASD did not vary across sites (Figure 3) and was slightly strengthened by excluding PA data. Modification by study site was suggested for associations with ozone exposure during the preconception period (P = 0.02), first trimester (P = 0.06), and first year of life (P <0.001). The inverse associations of ozone exposure observed during the first year of life (overall or adjusted for preconception and pregnancy exposures) appeared to be largely driven by the Pennsylvania study site (eTable 7).
Figure 2.
Site-specific and overall adjusted odds ratios (OR) and 95% confidence intervals (CI) for the associations between PM2.5 and ASD, by developmental window. Results are reported per 1.6-μg/m3 increase in PM2.5. All models are adjusted for maternal age, maternal education, maternal race/ethnicity, maternal smoking, month of birth, and year of birth. Interaction p-values for each developmental window are additionally included.
Figure 3.
Site-specific and overall adjusted odds ratios (OR) and 95% confidence intervals (CI) for the associations between ozone and ASD, by developmental window. Results are reported per 6.6-ppb increase in ozone. All models are adjusted for maternal age, maternal education, maternal race/ethnicity, maternal smoking, month of birth, and year of birth. Interaction p-values for each developmental window are additionally included.
When exploring whether associations differed for phenotypic case groups: severe versus mild ASD symptoms, and, separately, the co-occurrence of an intellectual disability, result patterns were generally similar across the groups (Table 4 and eTable 8). Additionally, there were no consistent associations between air pollution and ASD severity among ASD cases (eTable 9).
Table 4.
Adjusteda Odds Ratios and 95% Confidence Intervals for the Associations between PM2.5 and Ozone Exposure and ASD Severity.
| Low/moderate Severity OR (95%CI)b (n=401) |
High Severity OR (95%CI)c (n=272) |
|
|---|---|---|
| Ozone | ||
| Preconception | 0.93 (0.81, 1.1) | 0.91 (0.77, 1.1) |
| Entire pregnancy | 0.99 (0.77, 1.3) | 1.2 (0.88, 1.6) |
| First Trimester | 0.91 (0.78, 1.1) | 0.98 (0.83, 1.2) |
| Second Trimester | 0.95 (0.82, 1.1) | 0.98 (0.83, 1.2) |
| Third Trimester | 1.2 (0.99, 1.4) | 1.3 (1.1, 1.6) |
| First year of life | 0.77 (0.55, 1.1) | 0.82 (0.57, 1.2) |
| PM2.5 | ||
| Preconception | 1.0 (0.94, 1.1) | 1.1 (0.98, 1.2) |
| Entire pregnancy | 1.1 (0.92, 1.3) | 0.92 (0.76, 1.1) |
| First Trimester | 1.0 (0.96, 1.1) | 0.90 (0.82, 1.0) |
| Second Trimester | 0.97 (0.90, 1.1) | 0.97 (0.88, 1.1) |
| Third Trimester | 1.0 (0.95, 1.1) | 1.1 (0.98, 1.2) |
| First year of life | 1.4 (1.1, 1.8) | 1.1 (0.82, 1.5) |
Effect estimates are scaled to the IQRW value for the entire pregnancy period, averaged across study sites (1.6-μg/m3 for PM2.5 and 6.6-ppb for ozone).
ASD indicates autism spectrum disorder; CI, confidence interval; OR, odds ratio; PM2.5, particulate matter <2.5 μm.
Models are adjusted for study site, maternal age, maternal education, maternal race/ethnicity, maternal smoking, month of birth, and year of birth.
Low/moderate severity includes ASD cases with an ADOS Calibrated Severity Score of 4-7.
High severity includes ASD cases with an ADOS Calibrated Severity Score of 8-10.
Discussion
In this US-based multisite case–control study, PM2.5 exposure during the first year of life was associated with increased odds of autism spectrum disorder. Our trimester-specific analyses also revealed the third trimester as a potentially critical window of susceptibility for ozone. These findings held in models mutually adjusted for the other developmental windows, as well as two-pollutant models. Associations for third trimester ozone and first year of life PM2.5 exposure were fairly consistent across study sites. A growing number of studies have noted associations with air pollution exposure during the third trimester or early postnatal period.16,34 This reflects a period of development where the brain structures are formed, but neuronal maturity (myelination and synaptic density) is rapidly increasing.35
There are several plausible mechanistic pathways for late prenatal and early postnatal air pollution to contribute to the development of ASD. Synapse formation and neurotransmitter receptor formation both occur during the third trimester of development.35 Additionally, particulate matter and ozone are established immune toxicants.36,37 Inflammatory cytokines, produced from the mother in response to both ozone and PM2.5 exposure, can cross the placenta, enter fetal circulation, and induce systemic inflammation and oxidative stress.38 Pollutants directly inhaled by the infant postnatally can additionally induce a systemic inflammatory response and perturb normal development of the immune system. These pro-inflammatory cytokines may reach the developing brain, cross the blood brain barrier, resulting in neuroinflammation, neuron damage/loss, microglia activation, and DNA damage.9 Of emerging interest is the mediating role of microglia activation in the association between early life toxicant exposure and ASD.39 Alterations in microglial development by late prenatal and early postnatal inflammation may alter synaptic pruning,40,41 resulting in altered neuronal connectivity, and disruption of normal brain development.
The finding that PM2.5 exposure specifically during the first year of life is associated with increased odds of ASD is consistent with recent studies in California,15 Pennsylvania,17 Ohio,42 and Denmark43 despite some differences in exposure levels (see eFigures 4 and 5 for comparison of studies). The observation that the first year of life may be a specifically susceptible window for exposure during development is consistent with a recent study based in Israel that found associations with postnatal NO2 exposure, specifically when also adjusting for pregnancy averages.34 There were additionally slight increases in odds of ASD for PM2.5 exposure during the third trimester, though results were imprecise; this finding is consistent with two recent US-based studies of PM2.514 and PM1016 exposure in relation to ASD. The association with ozone exposure was higher when exposure occurred in the third trimester. In a previous California-based study, Becerra et al.13 found associations between ozone exposure during the entire pregnancy and ASD in single and multi-pollutant models additionally adjusted for PM2.5.
We assessed the association between PM2.5 and ozone and ASD using similar exposure assessment and outcome ascertainment methods across study sites from different geographic regions in the US. Although associations for third trimester ozone and first year of life PM2.5 exposure did not vary across different geographic locations in our study, there was heterogeneity in site-specific findings for several of the other windows. For instance, for entire pregnancy exposure averages, a few of the study sites had inverse associations, while we observed elevated odds ratios for sites such as Georgia and Pennsylvania. Exposure levels varied between sites, with the highest PM2.5 levels in Georgia, and the lowest in Colorado and California. In addition, on the east coast PM2.5 is dominated by sulfates from power plants and other regional sources; in contrast, nitrates make up the largest composition of PM2.5 on the west coast.44 Thus, the regional differences in particulate matter composition and exposure levels could contribute to differences in the estimated associations between air pollution and ASD from different geographic regions described in the literature, and across sites in our analysis.
In order to correct for the inherent correlation of exposure among developmental windows,45 we present results mutually adjusted for the other developmental windows of interest. Our finding of a direct association for ozone exposure during pregnancy and an inverse association during the first year of life, particularly in mutually adjusted models, may be partially explained by the complex correlation structure between different developmental windows of both the same and different pollutants, i.e. the negative correlation between ozone and PM2.5 and markers of traffic-related air pollution. This was particularly true for the Pennsylvania study site for the year post birth (correlation of −0.54 between ozone and PM2.5 and −0.69 between ozone and monitored NO2). These findings additionally highlight the complexity of identifying critical windows of susceptibility across pollutants with differing spatial and temporal variability.
The stronger signal of association during the late prenatal and early postnatal developmental windows could also reflect more accurate exposure measures during these windows. We assigned exposure from just before pregnancy to one year after birth based on only the residential address at birth, which assumes limited mobility during pregnancy and the first year of life. Further, if women in this study spent more time away from home during the earlier pregnancy periods,46 then the exposure estimates may not have been an accurate proxy for their actual exposure during these periods.47 Therefore, it is possible that exposure misclassification for earlier pregnancy periods may have attenuated effect estimates for these windows. Previous studies have assessed the extent of air pollution exposure misclassification when using complete residential history versus only the address on the birth certificate. Findings from these studies showed little change in exposure assignment48 and risk effect estimation49 when the full address history was incorporated. In addition, Warren et al.50 assessed the impact of incomplete residential history on identification of critical windows of susceptibility for air pollution exposure and found minimal exposure misclassification and impact on the identification of critical windows.
We were unable to explore shorter windows of exposure because of procedures introduced to ensure privacy of participants. The dates of birth of SEED participants were shifted by up to two weeks in either direction; this shift in date of birth did not impact longer exposure average periods (trimester, year post birth, etc.), based on the sensitivity analyses in the one site for which both actual and shifted data were available, but prevented us from confidently studying shorter exposure periods (such as weeks).
ASD represents a heterogeneous group of neurodevelopmental disorders. Because of this heterogeneity, we attempted to refine the case definition of ASD by conducting analyses with individuals grouped by their severity score level and also by presence of a co-occurring intellectual disability. Our goal in these analyses was to compare the refined case definitions of ASD (moderate/ high severity and co-occurring ID) to the population control group, similar to our approach when using the standard dichotomized ASD measure. These analyses did not reveal marked differences by level of severity or presence of a co-occurring intellectual disability. We additionally assessed associations between PM2.5 and ozone and ASD severity among ASD cases only. These analyses revealed no consistent associations between air pollution and the continuous calibrated severity score. This finding is consistent with a recent study that found associations between early life air pollution exposure and deficits in cognitive and adaptive function among children with ASD, but not ASD severity.51
Although we adjusted for various markers of socioeconomic status in our analyses, including maternal age and education, there is still the potential for residual confounding by socioeconomic status or place of residence. Our adjustment for study site produced estimates from models that accounted for the multi-site study design, which also further adjusts for any residual confounding due to between-site variability in air pollution exposure.
SEED aimed to include mother–child pairs from diverse population subgroups by recruiting children with disabilities who were recruited from multiple clinical and education sources and sampling population-based controls from birth certificates. A limitation of this approach is that many targeted families could not be located, potentially because they had moved, possibly outside of the study area, and information about these families was limited. We recognize the importance of considering the influence of selection bias when interpreting results. While participants in all groups were recruited from defined geographic catchment areas, population controls were, in general, more educated and affluent than ASD participants.52 Maternal age and education were associated with participation in the Georgia site; the only site with the ability to fully assess predictors of non-response.53 Consequently, we adjusted for maternal age and education in our analyses; still some residual influence of sample selectivity is possible and the direction of such bias is indeterminable.
Our approach involved numerous statistical models, across developmental windows, pollutants, study sites, etc. Some of the conclusions, for example, whether associations with air pollutants differed by autism severity, were limited by a modest sample size in subgroups. Furthermore, the large number of statistical comparisons increases the overall chance of false-positive findings, and for this reason we emphasize results that were robust across several modeling approaches rather than relying solely on statistical significance testing.
Finally, we assessed associations with satellite-based modeled estimates of PM2.5 and ozone in relation to ASD. Given that previous studies have found associations between NO2 and ASD15,34, it would have been of interest to incorporate modeled NO2 estimates in the current study. Unfortunately, that data was not available at the time of our analyses.
This study has several strengths. First, it is the first to assess associations between PM2.5 and ozone and ASD using an air pollution model that incorporates satellite-based data at a fine spatial resolution, as well as land-use terms, meteorologic variables, and data from a chemical transport model. Land-use regression models have adequate spatial resolution, but often have poor temporal resolution since the land-use terms are usually time-invariant.25 The use of satellite data helps to improve temporal resolution in the resulting exposure predictions, and allows the inclusion of rural locations further away from monitors.25
SEED study participants were drawn from a variety of settings serving children with disabilities across six different study sites located in the western, central, and eastern US. This ensures greater representation of ASD than previous clinically oriented studies. The geographic variability also provides the necessary exposure variability to assess health-related associations from exposure to PM2.5 and ozone. SEED additionally provided rich covariate data, allowing thorough adjustment for potential confounding. In addition to standard confounders, we additionally adjusted for year and month of birth in all analyses to account for seasonal trends related to both air pollution exposure and ASD.54,55 Finally, SEED used gold standard outcome assessment tools, including ADOS and ADI-R, therefore reducing any potential outcome misclassification. This allowed us to investigate the heterogeneity of ASD and to distinguish associations by core ASD symptom severity.
Conclusions
In summary, our study corroborates previous findings of a positive association between early life exposure to PM2.5 and ozone and ASD, and also confirms prior findings of potential late prenatal and early postnatal windows of susceptibility. Given some heterogeneity in results across our study sites representing multiple regions of the United States, future research to explore the influence of variable PM composition by geographic region is warranted.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the families and children who participated in SEED. This work was supported by the Centers for Disease Control and Prevention (cooperative agreements U10DD000180, U10DD000181, U10DD000182, U10DD000183, U10DD000184, and U10DD000498). Additional support was provided by the National Institutes of Health (T32ES007018 and T32HD049311).
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the U.S. Environmental Protection Agency (EPA). The EPA does not endorse any commercial products, services, or enterprises.
Footnotes
Conflicts of interest: The authors declare they have no actual or potential competing financial interests.
Data and Code availability: All code used for the analyses described in this paper can be available upon request. The outcome data are not available for replication due to the sensitive nature of the outcome. Air pollution exposure data are available upon request.
References
- 1.CDC. Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2014. https://www.cdc.gov/mmwr/volumes/67/ss/ss6706a1.htm Accessed 5 May 2018. [DOI] [PMC free article] [PubMed]
- 2.Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care 2014;44(10):277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health 2007;28:235–58. [DOI] [PubMed] [Google Scholar]
- 4.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68(11):1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodier PM. The early origins of autism. Sci Am 2000;282(2):56–63. [DOI] [PubMed] [Google Scholar]
- 6.Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med 2014;370(13):1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 2003;60(8):612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller P, Danielsen PH, Karottki DG, et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res Rev Mutat Res 2014;762:133–66. [DOI] [PubMed] [Google Scholar]
- 9.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 2009;32(9):506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol 2006;80(1):1–15. [DOI] [PubMed] [Google Scholar]
- 11.Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunol Lett 2015;163(1):49–55. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ Res 2016;151:763–776. [DOI] [PubMed] [Google Scholar]
- 13.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 2013;121(3):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz R, Roberts AL, Lyall K, et al. Autism Spectrum Disorder and Particulate Matter Air Pollution before, during, and after Pregnancy: A Nested Case–control Analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect 2015;123(3):264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013;70(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalkbrenner AE, Windham GC, Serre ML, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 2015;26(1):30–42. [DOI] [PubMed] [Google Scholar]
- 17.Talbott EO, Arena VC, Rager JR, et al. Fine particulate matter and the risk of autism spectrum disorder. Environ Res 2015;140:414–20. [DOI] [PubMed] [Google Scholar]
- 18.Schendel DE, Diguiseppi C, Croen LA, et al. The Study to Explore Early Development (SEED): a multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. J Autism Dev Disord 2012;42(10):2121–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 20.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007;37(4):613–27. [DOI] [PubMed] [Google Scholar]
- 21.Rutter M, A. LC, Lord C. ADI-R: The Autism Diagnostic Interview-Revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 22.Wiggins LD, Reynolds A, Rice CE, et al. Using standardized diagnostic instruments to classify children with autism in the study to explore early development. J Autism Dev Disord 2015;45(5):1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiggins LD, Barger B, Moody E, Soke G, Pandey J, Levy S. Brief Report: The ADOS Calibrated Severity Score Best Measures Autism Diagnostic Symptom Severity in Pre-School Children. J Autism Dev Disord 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullen EM. Mullen scales of early learning Circle Pines, MN: American Guidance Service Inc., 1995. [Google Scholar]
- 25.Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing PM2.5 Exposures with High Spatiotemporal Resolution across the Continental United States. Environ Sci Technol 2016;50(9):4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc 2017;67(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Q, Wang Y, Zanobetti A, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med 2017;376(26):2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bey I, Jacob D, Yantosca R, et al. Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation. Journal of Geophysical Research 2001;106(D19):23073–23095. [Google Scholar]
- 29.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10(1):37–48. [PubMed] [Google Scholar]
- 30.Luben TJ, Buckley BJ, Patel MM, et al. A cross-disciplinary evaluation of evidence for multipollutant effects on cardiovascular disease. Environ Res 2018;161:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–11. [DOI] [PubMed] [Google Scholar]
- 32.Schieve LA, Clayton HB, Durkin MS, Wingate MS, Drews-Botsch C. Comparison of Perinatal Risk Factors Associated with Autism Spectrum Disorder (ASD), Intellectual Disability (ID), and Co-occurring ASD and ID. J Autism Dev Disord 2015;45(8):2361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ. On the relative nature of overadjustment and unnecessary adjustment. Epidemiology 2009;20(4):496–9. [DOI] [PubMed] [Google Scholar]
- 34.Raz R, Levine H, Pinto O, Broday DM, Yuval, Weisskopf MG. Traffic-Related Air Pollution and Autism Spectrum Disorder: A Population-Based Nested Case–control Study in Israel. Am J Epidemiol 2018;187(4):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108 Suppl 3:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin RB, McDonnell WF, Mann R, et al. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol 1991;4(1):72–81. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 2003;111(4):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slama R, Darrow L, Parker J, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect 2008;116(6):791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanamsagar R, Bilbo SD. Environment matters: microglia function and dysfunction in a changing world. Curr Opin Neurobiol 2017;47:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paolicelli RC, Ferretti MT. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front Synaptic Neurosci 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333(6048):1456–8. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman JA, Wright JM, Rice G, Connolly N, Bowers K, Anixt J. Ambient ozone and fine particulate matter exposures and autism spectrum disorder in metropolitan Cincinnati, Ohio. Environ Res 2019;171:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritz B, Liew Z, Qi Y, et al. Air pollution and autism in Denmark. Environ Epidemiol 2018;2(4):e028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect 2007;115(7):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am J Epidemiol 2017;186(11):1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nethery E, Brauer M, Janssen P. Time-activity patterns of pregnant women and changes during the course of pregnancy. J Expo Sci Environ Epidemiol 2009;19(3):317–24. [DOI] [PubMed] [Google Scholar]
- 47.Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr Environ Health Rep 2015;2(4):430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res 2010;110(2):162–8. [DOI] [PubMed] [Google Scholar]
- 49.Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: The influence of residential mobility in pregnancy. Environ Res 2016;147:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren JL, Son JY, Pereira G, Leaderer BP, Bell ML. Investigating the Impact of Maternal Residential Mobility on Identifying Critical Windows of Susceptibility to Ambient Air Pollution During Pregnancy. Am J Epidemiol 2018;187(5):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerin T, Volk H, Li W, et al. Association Between Air Pollution Exposure, Cognitive and Adaptive Function, and ASD Severity Among Children with Autism Spectrum Disorder. J Autism Dev Disord 2018;48(1):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiGuiseppi CG, Daniels JL, Fallin DM, et al. Demographic profile of families and children in the Study to Explore Early Development (SEED): Case–control study of autism spectrum disorder. Disabil Health J 2016;9(3):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schieve LA, Harris S, Maenner MJ, Alexander A, Dowling NF. Assessment of demographic and perinatal predictors of non-response and impact of non-response on measures of association in a population-based case control study: findings from the Georgia Study to Explore Early Development. Emerg Themes Epidemiol 2018;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res 2010;3(4):185–90. [DOI] [PubMed] [Google Scholar]
- 55.Lee LC, Newschaffer CJ, Lessler JT, Lee BK, Shah R, Zimmerman AW. Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatr Perinat Epidemiol 2008;22(2):172–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.