Abstract
An increasing number of patients are being diagnosed with neurological diseases, but are rarely cured because of the lack of curative therapeutic approaches. This situation creates an urgent clinical need to develop effective diagnosis and treatment strategies for repair and regeneration of injured or diseased neural tissues. In this regard, biodegradable functional biomaterials provide promising solutions to meet this demand owing to their unique responsiveness to external stimulation fields, which enable neuro-imaging, neuro-sensing, specific targeting, hyperthermia treatment, controlled drug delivery, and nerve regeneration. This review discusses recent progress in the research and development of biodegradable functional biomaterials including electroactive biomaterials, magnetic materials and photoactive biomaterials for the management of neurological disorders with emphasis on their applications in bioimaging (photoacoustic imaging, MRI and fluorescence imaging), biosensing (electrochemical sensing, magnetic sensing and opical sensing), and therapy strategies (drug delivery, hyperthermia treatment, and tissue engineering). It is expected that this review will provide an insightful discussion on the roles of biodegradable functional biomaterials in the diagnosis and treatment of neurological diseases, and lead to innovations for the design and development of the next generation biodegradable functional biomaterials.
Keywords: neurological disorders, functional biomaterials, degradation, bioimaging, biosensing
Graphical abstract
1. Introduction
Neurological disorders are diseases of brain, spine and nerves that connect them. There are more than 600 diseases of the nervous system, such as Alzheimer’s disease, Parkinson's disease, traumatic brain injuries, brain tumors, epilepsy, and stroke, as well as less familiar ones such as frontotemporal dementia [1, 2]. The number of diagnosed refractory neurological diseases has been growing every year. According to the report by U.S. Pharmacist (Jan 2018), 20 million Americans experience neuropathy, and 16% of U.S. households have an individual with brain impairment. Each year, of the 1.2 million mostly diagnosed adult-onset brain disorders, 51.3% and 21% are caused by stroke and Alzheimer’s disease, respectively. Annually, the total number of newly diagnosed episodes of Parkinson’s disease and traumatic brain injury equals the total number of epilepsy episodes (135 million). As stated in the Central Brain Tumor Registry of the United States (CBTRUS) statistical report (2018), the overall estimated number of new cases of brain and other central nervous system (CNS) tumors in the U.S. for 2018 and 2019 are 85,440 and 86,970, respectively [3]. Many of these neurological diseases lack curative therapeutic approaches, causing the suffering and economic burden of patients. The challenges associated with the management of neurological disorders are numerous, including the poor understanding of the mechanism underlying the diseases, lack of the specificity in the diagnosis and treatment, the limited regenerative capability of adult neuron cells, especially in central nervous system (CNS), and the difficulty in drug administration across the blood brain barrier (BBB) effectively into brain [4-10].
The clinical need necessitates the development of innovative and effective diagnosis and treatment strategies for repair and regeneration of injured or diseased neurological tissues and organs. Recent progresses made in the bioimaging have enabled the detection of anatomical, biochemical and physiological conditions in different nervous systems, allowing a better understanding and more precise diagnosis of neurological diseases [11-16]. The bioimaging strategy can also provide post-treatment monitoring of neurological disorders to investigate treatment effects. In addition, advanced biosensing techniques that allow quantitative detection of neurotransmitters and biomarkers appears to be desired to improve the diagnosis and treatment process of neurological disorders [17-19]. As many neurological diseases are associated with changes of certain neurotransmitters and biomarkers in biological fluid such as urine, plasma, serum and cerebrospinal fluids (CSF) [20-23]. Furthermore, the treatment for neurological disorders requires certain physical support to guide the growth and differentiation of nerve cells, as well as nanocarriers to facilitate the delivery of drugs, neuroprotective agents, and growth factors to across BBB and target to disease sites [8-10].
To fulfill the above mentioned demands for the diagnosis and treatment of neurological disorders, increasing efforts are being devoted to the development of advanced biomaterials [24]. Among all developed biomaterials, functional biomaterials including electroactive biomaterials, magnetic biomaterials and photoactive biomaterials are attracting more attentions. Nerve cells are intrinsically responsive to external stimulations such as electric field, magnetic field and light [25-28]. With the assistance of these functional biomaterials, external stimulations could be located to targeted nerve injuries. So the functional biomaterials can be popularly used as supporting scaffolds to guide nerve cell growth and tissue regeneration, especially under external stimulations [29]. In addition, with the latest evolution of nanobiotechnology, functional nanobiomaterials are developed and widely applied for neurological disease treatment. For instance, all the aforementioned three types of functional biomaterials can generate heat under external stimulation, so they are able to perform hyperthermia treatment and heat-triggered drug release [30, 31]. The electroactive biomaterials and magnetic biomaterials can also be precisely guided and collected in a specific location by an external electric field and magnetic field, respectively, enabling the potential to guide drugs to cross BBB and reach targeting disease sites [32]. Moreover, these functional biomaterials could work as imaging contrast agents, allowing photoacoustic imaging (electroactive biomaterials) [33], magnetic resonance imaging (MRI) (magnetic biomaterials) [34], and fluorescence imaging (photoactive biomaterials) [35] to facilitate precise diagnosis and efficient therapy for neurological disorders. Despite of all these advantages described above, most functional biomaterials are claimed to be biocompatible but usually are not biodegradable, which may cause chronic inflammation and need for surgical removal. Recently, an increasing number of studies have been conducted on the development of biodegradable functional biomaterials for the bioimaging [36-39], biosensing [40], and treatment of neurological disease [36, 41, 42]. In this manuscript, we focus on the roles of advanced biodegradable functional biomaterials including electroactive biomaterials, magnetic biomaterials and photoactive biomaterials in the understanding and treatment of neurological disorders. We particularly highlight their capabilities in bioimaging, biosensing and therapy strategies of neurological diseases.
2. Neurological Disorders
2.1. Nervous system and major disorders of the nervous system
The nervous system is the most complex and essential system in humans, and controls the sensory input, information integration, and motor output of the entire body. It is divided into two main parts: the central nervous system (CNS) consisting of the brain and spinal cord, which integrates and processes the information sent by nerves; and the peripheral nervous system (PNS) consisting mainly of nerves, which are enclosed bundles of long fibers, that carry sensory messages to the central nervous system (sensory nerves) and send commands from the CNS to every other part of the body (motor nerves). At the cellular level, the nervous system is composed of two main types of cells: neurons and their supporting cells known as glial cells. As the structural and functional building blocks of the nervous system, neurons are organized and grouped into nerve bundles surrounded by protective connective tissues called myelin sheath, to conduct electrochemical signals rapidly and precisely via nerve impulse, and to release neurotransmitters (e.g. acetylcholine, dopamine and glutamate) that regulate the activation of muscles and glands via synapse. In addition, various types of glial cells (e.g. oligodentrocyts, astrocytes, ependymal cells, and microglia in CNS; Schwann cells and satellite cells in PNS) are present in the nervous system to protect and nourish the neurons.
Neurological disorders refer to any disorder of the nervous system, that is, any structural, biochemical or electrochemical abnormalities in the brain, spinal cord or other peripheral nerves that result in a range of severe symptoms with high mortality, morbidity and disability, representing a major public health problem [2]. There is a long list of neurological disorders, which can be classified according to the primary location affected, such as brain disorder, spinal cord disorder and peripheral neuropathy, or according to the primary type of dysfunction, such as structural disorder (e.g. traumatic injuries and brain tumor), biochemical disorder (e.g. Parkinson's disease) and electrochemical disorder (e.g. epilepsy seizure). Among over 600 different kinds of neurological diseases, we focus on the most common and costly neurological diseases, including traumatic brain injuries [43-47], brain tumor [48-51], Alzheimer's disease [5, 52-55], Parkinson's disease [4, 52], epilepsy [56-57], and stroke [58-60], which represent significant societal burden with parallel broad unmet needs of management.
2.2. Challenges and opportunities in the management of neurological disorders
The challenges associated with the management of neurological disorders are numerous, including the poor understanding of the mechanism underlying the diseases, lack of the specificity in the diagnosis and treatment, the limited regenerative capability of adult neuron cells, especially in CNS, and the difficulty in drug administration across the BBB effectively into brain.
First, the most significant success of disease management is expected to be based on a detailed understanding of the mechanism and the pathological molecular details of neurological diseases [10]. Unfortunately, the fundamental mechanism for most of the neurological disorders, particularly for neurodegenerative diseases, remains as undetermined due to their intrinsic complexity and heterogeneity [4, 5]. As a result, early diagnosis of neurological diseases is still lack of high specificity, and treatments up to now are merely symptomatic, leading to the fact that most neurological diseases are still not curable [10]. Fortunately, advances in molecular biology, genomics and proteomics are increasing and refreshing our understanding of the diseases, providing opportunity to uncover the underlying pathology and to identify disease-specific biomarkers. At the same time, although the traumas to the CNS arise from different forms and even from unknown reasons, there is a growing agreement that the injury processes share numerous key molecular mechanisms [44], such as the release of excitatory transmitters glutamate, calcium influx into cells, mitochondrial dysfunction, free radical formation, cell apoptosis and the inflammatory reaction. Currently, there are well-defined molecular targets of neuroprotection, such as anti-inflammation therapy [9], anti-oxidant treatment, and anti-apoptotic agents [61]. However, to improve the accuracy of diagnosis and treatment effect of neurological diseases, more advanced bioimaging and biosensing technologies are desired to unfold the structure complexity and pathological molecular details of nervous system.
Additionally, the regeneration and reconstruction of nerve network remain as a challenge, especially for the CNS. The neurons in CNS have poorer self-healing capability compared with those in PNS, especially when additional structural (e.g. glial scars) and chemical obstructions (e.g. myelin-associated inhibitors [6]) in CNS have been found to further lower their intrinsic nerve regrowth potential [7]. Therefore, stem cell based therapy has received numerous attention to promote nerve regeneration, either by replacing the damaged or lost nerve cells, or by modulating the injury microenvironment through paracrine signaling to encourage endogenous repairing [62]. However, a few issues, such as tracking of stem cell migration and their survival, directional and controlled differentiation of the delivered stem cells, as well as physical support to guide transplanted stem cells and newly regenerated nerve cells, remain as unsolved to greatly slow down the translation of stem cell therapy.
Lastly, BBB acting as the barrier protecting the brain, not only blocks the bloodborne pathogens but also prevents most therapeutic drugs, neuroprotective agents and growth factors from entering the brain. Therefore, the design of powerful nanoparticles, which can deliver drugs, gene and small molecules (chemical stimulus or inhibitors) across BBB with minimal disruption to its structure and function, is a major objective for the treatments of a wide range of neurological disorders [8-10]. Despite the progresses, more advanced strategies and technologies are still required for the nanoparticle design to endow high targeting specificity, controlled releasing, and intracranial imaging-guided therapy.
To alleviate challenges mentioned above, a wide variety of biomaterials have offered great opportunities for diagnosis and treatment of neurological disorders, including nanoparticles for neuro-imaging and neuro-sensing, scaffolds for promoting nerve regeneration, systems for promoting stem-cell-mediated therapy, nanocarriers for enabling BBB crossing of drugs or gene. Among all applied biomaterials, biodegradable functional biomaterials including biodegradable electroactive biomaterials, biodegradable magnetic biomaterials and biodegradable photoactive biomaterials are attracting more attentions. The biodegradable functional biomaterials are superior to regular biomaterials because they are intrinsically responsive to external stimulation fields like electric field, magnetic field and light. This special property of biodegradable functional biomaterials provide them with improved interactions with nerve cells, higher targeting accuracy, and better imaging capability than regular biomaterials. More detailed and specific discussions will be introduced in the following section.
3. Biodegradable functional biomaterials
The functional biomaterials we discuss in this review paper are focusing on electroactive biomaterials, magnetic biomaterials and photoactive biomaterials. These functional biomaterials usually response to external stimulations from electric field, magnetic field and light, which in turn could adjust their interactions with nerve cells to modulate cellular activities. Functional biomaterials also have the ability to interact with neurotransmitters and biomarkers, enabling neuro-sensing that increases diagnosis accuracy. In addition, the functional biomaterials are able to generate heat under external stimulation fields, allowing heat-triggered controlled release and hyperthermia treatment. With the help of external fields, functional biomaterials can also be guided to a targeted location, enabling the delivery of functional agents to pass through BBB and reach disease sites. Furthermore, these functional biomaterials possess intrinsic imaging capability, including photoacoustic imaging (electroactive biomaterials), magnetic resonance imaging (MRI) (magnetic biomaterials), and fluorescence imaging (photoactive biomaterials), providing noninvasive neuro-imaging to facilitate diagnosis and treatment of neurological disorders. In addition to all these advantages of functional biomaterials for neurological disorders, biodegradable functional biomaterials possess extra benefits. The applied biodegradable functional biomaterials can be degraded in vivo, and then absorbed by or removed from human body after finishing their usage, which strongly eliminates the concern of chronic inflammation and the need for surgical removal of implants. The cytocompatibility and biocompatibility of biodegradable functional biomaterials have been widely proved. For example, Guo et al. reported that many biodegradable conductive polymers were verified with cytocompatibility in cell culturing studies with nerve cell lines such as PC12 cells and Schwann cells [63]. Studies also demonstrated magnetic poly(lactic acid) (PLA)-SPION nanocomposites with high levels of encapsulated magnetite achieve efficient labeling (≥90%) of primary neural stem cells without significant toxicity [64]. In addition, biodegradable photoactive biomaterials such as a group of citrate-based BPLPs have been confirmed with excellent biocompatibility through both in vitro cytocompatibility and in vivo foreign body response studies [65]. Therefore, the studies conducted on biodegradable functional biomaterials for the imaging, sensing, and treatment of neurological disease are increasing. In this section, we discuss the mechanisms, properties and functionalities of biodegradable functional biomaterials that utilized in the field of neurological disorders. Detailed applications in neuro-imaging, neuro-sensing and treatment of neurological diseases will be summarized in following sections.
3.1. Biodegradable electroactive biomaterials
Existing studies show that living organisms are complex electrochemical systems where each cell generates cell-type specific membrane potential producing endogenous electric fields throughout the biological system. The typical value of the potential is usually between −60mV and −100Mv [66]. Endogenous electric fields in the human body play an integral role in maintaining biological functions, such as muscle contraction, neural signaling, wound healing, embryogenesis, and tissue regeneration [67]. Endogenous electric fields (5–18 mV/mm) was observed to polarize the nerve system along the rostral–caudal axis and guide nerve cell growth [25]. In addition, major activities of nerve cells are usually accompanied by electrical changes. For instance, when the signaling process of the nerve system is active, the transmembrane potential changes from negative to positive [66]. Regarding the electrical properties of nerve cells, exogenous electrical stimulation strongly affects their activities. Initial studies investigating the effect of electrical stimulation on nerve cells were conducted on Xenopus neurons by exposing them to electric fields from 0.1 to 10 V/cm, proving that electric fields were able to influence the orientation and length of neurite growth [25]. Later, Wood et al. also demonstrated that the application of a 25 V/m DC electrical stimulation for 10 min enhanced overall neurite outgrowth over controls for up to 48 h [68]. Moreover, Yamada et al. reported that mild electrical stimulation could influences embryonic stem cells to differentiate into neuronal cells [69]. Several theories have been suggested to explain the mechanism of electrical stimulation on nerve cells. For instance, it was proposed that electrical stimulation alters protein synthesis in transected sciatic nerve segments and promotes neurite outgrowth. Kimura et al. suggested that electrical stimulation were able to electrically activate gene expression for nerve growth factor (NGF) for rat neuronal pheochromocytoma cells (PC12 cells) [66]. Recently, Chan et al. claimed that electrical stimulation boosts neuronal expression of neurotrophic factors and their receptors. The elevated neurotrophic factor levels cause an upregulation of cAMP level, enabling increased expression of nerve regeneration-associated genes such as tubulin, actin and GAP-43 [70].
Despite the advantages of direct exogenous electric fields for nerve stimulation, it’s hard to be applied to targeted nerve tissues. In order to provide more precise control over stimulation conditions, electroactive polymers have become very important tools. Conductive polymers were first reported in 1977 when researchers developed the conductive polyacetylene [29]. Conductive polymers are a new generation of organic materials that characteristically have a conjugated backbone structure with a high degree of π-orbital overlap. The π-conjugated polymers in general are not conductive, and only turn to a conductive state after a doping process. Doping is the process of oxidizing (p-doping) or reducing (n-doping) the neutral conjugated polymer and providing a counter anion or cation, respectively. In this process, charge carriers, in the form of charged polarons and bipolarons, are introduced into the polymer chain. Ordered movement of these charge carriers along the conjugated backbone generates electrical conductivity. Conductive polymers possess similar electrical and optical properties to those of metals and inorganic semiconductors, but have better processability, designability and biocompatibility. Based on their electroactivity and strong light absorption, conductive polymers have been greatly investigated for modulation of cellular activities, controllably release of drugs and biological molecules [71], detection of neurotransmitters and biochemical reactions [72], as well as photoacoustic imaging [73]. Various conductive polymers, such as polyaniline (PANI) [74], polypyrrole (PPy) [75], and poly(3,4-ethylene dioxythiophene) (PEDOT) [76], have been investigated for alleviating neurological disorders and nerve regeneration.
One of the greatest drawbacks of conductive polymers for in vivo applications is their inherent inability to degrade, which may cause chronic inflammation and need for surgical removal [63]. To address this limitation, attempts to develop biodegradable conductive polymers have been carried out. One applied strategy is making polymer blends or composites with conductive polymers and biodegradable polymers. For instance, nerve conduits were fabricated with the composite of PPy and poly (D,L-lactic acid) (PDLLA) to facilitate nerve defect regeneration. The obtained PPY/PDLLA composite nerve conduits were proved to support the differentiation of rat pheochromocytoma 12 (PC12) cells under electrical stimulation in vitro and promote nerve regeneration for a rat sciatic nerve defect [41]. Another widely studied method for preparing biodegradable conductive polymers is to develop copolymers with conductive oligomers and biodegradable polymers. Wu et al. developed a biodegradable conductive polyurethane by polycondensation of poly(glycerol sebacate) and aniline pentamer, which significantly enhanced Schwann cells' myelin gene expression and neurotrophin secretion for peripheral nerve tissue regeneration [77], Recently, biodegradable conductive polymers are greatly replacing traditional non-degradable conductive polymers in the field of neurological disorders, including neuro-imaging, neurosensing, and treatment.
3.2. Biodegradable magnetic biomaterials
Magnetism is an intrinsic property of every atom. Substantial evidences have shown that all living organisms contain magnetic particles and can act as magnetic receptors [26]. Magnetotactic bacteria, a phylogenetically diverse group of aquatic bacteria, are perhaps the first living organisms to orient themselves with the earth’s magnetic field [78]. These bacteria align along magnetic fields with the help of a chain of organelles containing magnetosomes, and swim along the field lines with the help of their flagella. The magnetosomes are magnetic nanoparticles such as magnetite (Fe3O4) and greigite (Fe3S4). The haemoglobin in our blood is an iron (Fe) containing protein. In the 1930s, haemoglobin was found with magnetic properties that differed depending on whether oxygen was carried. The haemoglobin carrying no oxygen was found to be more sensitive to magnetic field than oxygenated blood [79]. These results established that the magnetic field and magnetic materials have a significant role to play in healthcare and biological applications. In particular, much effort has been devoted to the design and synthesis of magnetic nanoparticles, due to their multifunctional properties including small size, high operational surface areas, and unusual superparamagnetic behavior. Magnetic nanoparticles are typically classified into pure metals (Fe, Co, Ti, Ni, etc.), metal oxides (Fe3O4, maghemite (γ-Fe2O3), etc.), ferrites (BaFe12O19, SrFe12O19, CuFe2O4, NiFe2O4, MnFe2O4, etc.), and metal alloys (CoPt, FePt, etc.) [30, 80]. Among all magnetic nanoparticles, iron oxide nanoparticles, typically Fe3O4 and γ-Fe2O3, have been most extensively studied and applied in biomedicine due to their low toxicity [81]. Magnetic iron oxide nanoparticles exhibit fast change of magnetic state under an external magnetic field (high magnetic susceptibility) and loss of magnetization after removal of the magnetic field (superparamagnetism) are usually desired for biomedical applications. In order to achieve superparamagnetism, magnetic iron oxide nanoparticles typically with sizes below 20 nm, and are named superparamagnetic iron oxide nanoparticles (SPIONs) [82, 83]. However, particle sizes should not be too small (< 10 nm). Otherwise, they would have rapid renal clearance and low values of saturation magnetization (SM). SM represents the maximum level of achievable magnetization of SPIONs under an external magnetic field, and it also determines their capability of producing heat [84].
The appropriate functionalization of the SPIONs for biomedical applications is ruled not only by their intrinsic magnetic properties but also by their biophysical properties. Modifications with biomolecules, targeting agents, and biocompatible materials are suggested to enable SPIONs with required biocompatibility, targeting specificity, pharmacokinetics, biodistribution, and cell interaction processes [85, 86]. For example, the surface modification of biocompatible polymers renders SPIONs with multiple advantages such as longer circulation time, better protection of drugs, and less undesired accumulation in important organs to improve drug delivery efficiency. It was reported that polyethylene glycol (PEG) functionalized SPIONs demonstrated double the amount of circulation time and reduced nanoparticle accumulation in untargeted organs [87]. In order to develop biodegradable magnetic materials, SPIONs have been incorporated with biodegradable polymers through various strategies including surface coating, encapsulation, and chemical conjugation.
With the latest evolution of nanobiotechnology and biomaterials, biodegradable magnetic materials are attracting increasing attention due to their potential to improve conventional diagnostic and therapeutic procedures. Due to their superparamagnetism, biodegradable magnetic nanoparticles can be precisely guided and collected in a desired location by an external magnetic field [32]. In order to realize specific and controlled drug delivery, the drug molecules can be connected to the nanoparticles through a cleavable linker or encapsulation. Once they reach the target site under the external magnetic field, drugs can be released locally either via enzymatic cleavage or changes in the physiological environment. For instance, pH triggered nanoparticle drug release system was developed by a biodegradable poly(ethylene glycol)-b-poly(2-[diisopropylamino]ethyl aspartate) block copolymer loaded with doxorubicin and SPIONs. Once the magnetic nanoparticles reached acidic microenvironment of tumors, the drug release rate was approximately ten-times higher than that in a neutral environment [88]. Moreover, SPIO nanoparticles are being developed for hyperthermia treatment and heat-triggered drug release as a result of their ability to convert magnetic energy into heat [30]. Kim et al. used a multifunctional thermo-responsive poly(N-isopropylacrylamide-co-acrylamide)-block-poly(ε-caprolactone) (P(NIPAAm-co-AAm)-b-PCL) copolymer to encapsulate doxorubicin and SPIONs, and fabricated a magnetic hyperthermia-mediated payload release micelle system. Taking advantage of the magnetic hyperthermia of SPIONs and thermal sensitivity of the polymer, the nanoparticles were able to release drugs under the regulation of external magnetic field [89]. Also, biodegradable magnetic materials exhibit synergic effect on cell adhesion, proliferation, and differentiation, so they have also been investigated for tissue engineering applications. Wei et al. developed biodegradable magnetic nanofibrous membranes by electrospinning of Fe3O4/chitosan (CS)/poly vinyl alcohol (PVA), which significantly promoted the adhesion and proliferation of MG63 cells [90]. It is well known that biodegradable magnetic materials can be used as magnetic resonance imaging (MRI) contrast agents for bioimaging purpose. The imaging performance is affected by many conditions including the strength of applied magnetic field, the acquisition time, the SM of the nanoparticles, and concentration of the nanoparticles. A water-dispersible and tumor-targeted MRI contrast agent was manufactured through encapsulation of nonclustered SPIONs with folate modified copolymers of poly(ethylene glycol) and poly(ε-caprolactone) (Fa-PEG-PCL). The resulting small-sized (35 nm) nanomicelles (Fa–PEG4.3k–PCL1k–SPION) displayed efficient MRI effect within the tumor section [91]. Regarding the multifunctionality of biodegradable magnetic materials, Ye et al. developed biodegradable poly(lactic-co-glycolic acid) (PLGA) vesicles containing magnetic nanoparticles, quantum dots (QDs) and anticancer drugs as a nanocarrier system for both bioimaging and anticancer drug delivery [92]. Therefore, biodegradable magnetic materials can serve as a multifunctional system to simultaneously provide specific targeting, drug administration, tissue regeneration, and in vivo real-time therapeutic response monitoring, enabling the potential for precise personalized medical care applications [93].
3.3. Biodegradable photoactive biomaterials
Light is readily modulated in space, time, and intensity, providing an inexpensive and specific tool for mediating biological behaviors of various cells such as nerve cells, muscle cells, stem cells, and macrophage. The infrared neural stimulation (INS) has been shown to induce both excitation and inhibition of neural activity depending on the stimulation condition [27, 28]. Effective INS was demonstrated on CNS [94] and PNS [95]. enabling more understanding and better theranostic strategies for neurological diseases. Wang et al. investigated light stimulation effects with different lights on human adipose derived stem cells (hASCs). One of their studies demonstrated that blue (415 nm) and green (540 nm) wavelengths were more effective in stimulating osteoblast differentiation of hASCs than red (660 nm) and (NIR (810 nm) lights, because blue/green lights produced a bigger increase in intracellular calcium and reactive oxygen species (ROS). In another study, they found that blue/green lights inhibit the proliferation of hASCs, while red/NIR lights stimulates the process. The reason is that blue/green wavelengths decrease cellular ATP, while red/NIR increase ATP in a biphasic manner [96]. Kang et al. also reported the ability of NIR light to regulate intracellular calcium in order to modulate macrophage polarization [97]. Among all the probable mechanisms of light stimulation effects, including electric field mediation, photo-mechanical transform and photo-thermal transform, the most likely appears to be photo-thermal transform [98, 99]. The absorption of light pulse by the tissue’s water causes a rapid local increase in temperature. This heating alters the electrical capacitance of plasma membrane, depolarizing the target cells. This mechanism is reversible and broadly applicable to most cell membranes [100]. In addition, optogenetics is a technique whereby light-responsive ion channels are introduced into target cells, enabling unprecedented precision and specificity for neuron modulation. This technology has even been involved in the treatment of diseases such as Parkinson’s disease, hereditary blindness, and epilepsy with chronic light stimulation [101-103].
Regarding the significant role of light regulation played in neurobiology, photoactive biomaterials are becoming promising tools due to their capability of photo-thermal conversion, fluorescence and light guidance. In the following sections, photoactive biomaterials and related devices, including photothermal biomaterials, fluorescent biomaterials and optical waveguides, will be outlined, discussing their material design, properties and possible applications.
Photothermal materials are a family of photoactive materials that release thermal energy after light radiation. The thermal energy can be used to change material properties to release chemicals and drugs, stimulate surrounding cells, and kill cancer cells. Conductive and semiconductive polymers are among the most popular photothermal biomaterials for biomedical applications. Li et al. demonstrated that PPY microgels could be used for light-controlled release of neurotransmitters when triggered by NIR irradiation [31]. Nanoparticles made by a NIR-excited semiconducting copolymer, poly(cyclopentadithiophene-alt-diketopyrrolopyrrole), were reported to be able to control thermosensitive ion channels in neurons, instigating neuron depolarization [104]. Another semiconductive polymer poly(3-hexylthiophene-2,5-diyl) (P3HT) also demonstrated great photothermal effect, which has been utilized to regulate membrane hyperpolarization of hippocampal neurons and human embryonic kidney (HEK-293) cells [28, 105]. In addition to conductive and semiconductive polymers, magnetic nanoparticles are another kind of widely used photothermal biomaterials. Chu et al. investigated the hyperthermia treatment effect for esophageal cancer by Fe3O4 nanoparticles coated with carboxyl-terminated poly (ethylene glycol)-phospholipid. It was found that the surface-functionalized Fe3O4 nanoparticles had no obvious toxicity to esophageal cancer cells without NIR irradiation, but presented effective suppression on in vitro cell viability and significant inhibition on in vivo tumor growth with NIR irradiation [106]. Chen et al. also demonstrated that the highly crystallized iron oxide nanoparticles (HCIONPs) coated with a polysiloxane-containing copolymer could be used as effective and biodegradable mediators for photothermal therapy of SUM-159 breast cancer [107].
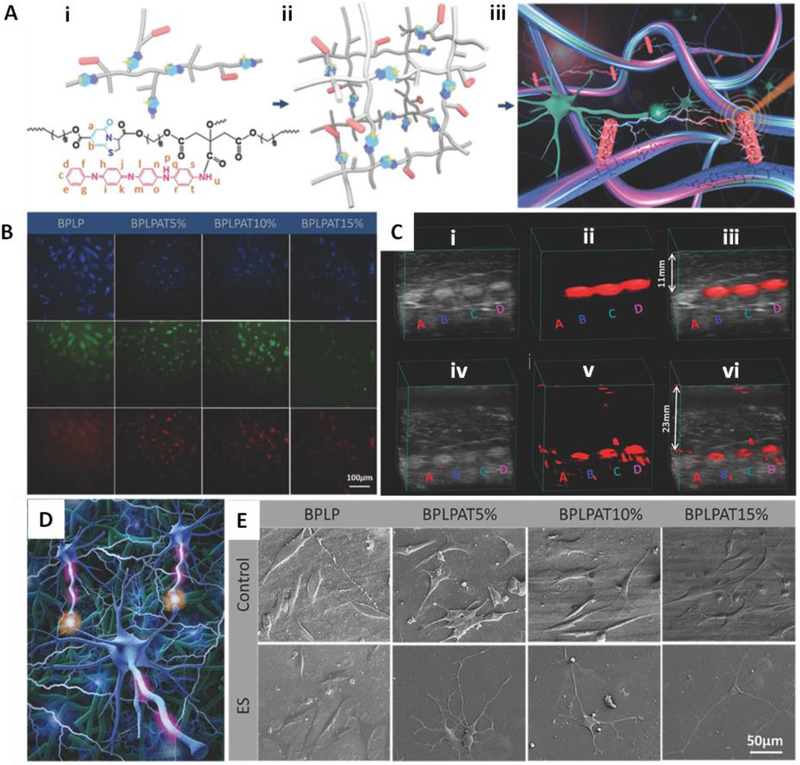
Fluorescent materials are a special group of photoactive materials. A large amount of biocompatible and biodegradable fluorescent materials have attracted significant attentions in biomedical fields of theranostic and regenerative medicine [108]. Most reported biodegradable fluorescent materials are made through the encapsulation or conjugation of fluorescent organic dyes [109] or inorganic QDs [110, 111] with biodegradable polymers. However, the photobleaching of organic dyes and toxicity of QDs have largely limited their in vivo applications and motivated the design of fully biodegradable and biocompatible fluorescent materials. Benefitting from the reactive nature of citric acid, Yang’s lab developed a series of citrate-based biodegradable polymers through a convenient and cost-effective thermal polycondensation reaction of citric acid and biocompatible diols [112-116], Among the citrate-based biomaterials, a group of biodegradable photoluminescent polymers (BPLPs) were created by introducing amino acids to the system [114, 117-121]. In the reaction, citric acid and amino acids contributed to both the formation of molecular fluorophores and development of the polymer backbones, while the diol is mainly responsible for the construction of polymer backbones. The distinctive fluorescence properties of BPLPs were tunable by changing amino acids, monomer feeding ratios and even excitation wavelengths [119]. Among all kinds of BPLPs, BPLP-Cys (using L-cysteine) displayed excitation-independent fluorescence with a strong emission (~ 430 nm) and h a high quantum yield of 62.3%. It also possessed a high photostability with photobleaching of about 2% after 3h of continuous UV excitation, while the traditional organic dye rhodamine-B had about 10% photobleaching under the same excitation condition. BPLP-Ser (using L-serine), which had a QY of 26.0%, was an exemplified BPLP that showed an excitation-dependent emission from blue to red and even NIR by increasing excitation from 310 to 573 nm. This unique fluorescence property not only allows BPLP-Ser to be a promising material for deep tissue imaging, but also gives it potential for multiplex optical imaging. In addition to their fluorescence properties, BPLPs demonstrated high designability due to their reactive side groups (carboxyl group, hydroxyl group, and amino group). These reactive groups have facilitated BPLPs to be modified with different chemical structures, physical properties, and biological functionalities, enabling a wide variety of applications including biological labeling and imaging [122, 123], biosensing [124], drug delivery [123, 125-127], and regenerative medicine [119, 122, 125]. Recently, by doping BPLP-Cys with conductive aniline tetramer, a citrate-based fluorescence/photoacoustic dual-imaging enabled biodegradable polymer (BPLPAT) has been developed. The BPLPAT nanoparticles were applied for label PC12 nerve cells and performing deep tissue detection [36].
Due to significant scattering and absorption loss, light cannot be efficaciously delivered to or collected from target regions within deep tissue, significantly hindering the execution of in vivo light stimulation. A traditional strategy is to apply long wavelength NIR or infrared radiation to provide a relatively deep penetration up to about a couple cm, which is still insufficient for full body applications [128]. Another effective method for alleviating this problem is implanting optical waveguide in tissues or organs for light delivery. To date, silica glass remains the mainstream optical material platform because of their capability to achieve efficient light delivery with low loss [129]. However, silica fibers often suffer from mechanical fragility and brittleness, limited biological functionalities, as well as nondegradability, hindering their application as implantable devices. Benefiting from the recent progress of polymeric biomaterials, many optical waveguides with high optical efficiency and flexible mechanics have been developed with advanced polymeric biomaterials, including bacteria cell-based biomaterials [130, 131, naturally derived biomaterials [132-134], and synthetic biomaterials [135, 136]. Among the optical waveguides, those made from synthetic biodegradable polymer are more attractive candidates due to their excellent biocompatibility, programmable degradation, adjustable mechanics, and flexible designability [137]. For instance, Shan et al. reported a biocompatible and biodegradable step-index optical fiber fabricated from citrate-based polymeric elastomers. The designability and processability of citrate-based biomaterials enable ultra-fine tuning of refractive index difference between the core and the cladding layers, while maintain homogenous biodegradation rate and identical mechanical properties to yield high device integrity. A 0.4 dB/cm loss allows the flexible optical fiber to perform efficient image transmission and in vivo deep tissue optical imaging [136]. Fu et al. also developed biodegradable PLLA fibers with high mechanical flexibility and optical transparency using a thermal drawing process. The fibers were implemented in vivo as optical neural interfaces for intracranial light delivery and detection, allowing deep brain fluorescence sensing and optogenetic interrogation [38].
4. Biodegradable functional biomaterials for neuro-imaging
Neurological disorders that combine cognitive, motor, and behavior abnormalities are often mistakenly diagnosed as diseases such as depression, motor impairments, and lethargy that follow debilitating immune suppression [138]. A better understanding of their symptom and pathology is desired to facilitate precise diagnosis of the diseases at early stages before overt symptoms. Recent progresses made in bioimaging have enabled the detection of anatomical, biochemical and physiological conditions in nervous systems. Moreover, the bioimaging strategy can also provide post-treatment monitoring of neurological disorders to investigate treatment effects [11]. Many of the well-established techniques such as photoacoustic imaging, MRI, optical imaging, positron emission tomography (PET), single photon emission tomography (SPECT), and computer tomography (CT) have gained considerable interest and applicability in neurological diagnostic studies. In order to increase the precision and practicality of neuro-imaging, various biocompatible and biodegradable materials have been used as contrast agents and carriers of contrast agents in the imaging process. In the following sections, imaging modalities that involve biodegradable functional biomaterials will be discussed.
4.1. Photoacoustic imaging for neurological disorders
Photoacoustic imaging is a hybrid imaging modality that combines optical image contrast and ultrasound detection principles [139, 140]. Illuminated by non-ionizing laser pulses, targeted subjects absorb the light and convert it into heat, leading to transient thermoelastic expansion and thus wideband ultrasonic acoustic signal. The generated ultrasonic waves are then detected by transducers to form images. Photoacoustic imaging can overcome this primary challenge of shallow penetration of optical techniques because of the lower ultrasonic scattering coefficients of absorbents compared with their optical equivalents, so the propagation of the photons in the diffuse regime enables photoacoustic imaging with a penetration depth up to ~50 mm with a resolution of <1 mm. Overall, photoacoustic imaging combines the good contrast and multiplexing capabilities of optical imaging and the deep penetration and 3D imaging capability of ultrasound imaging.
It has been widely used for neuro-imaging. The image contrast is primarily determined by the light absorption properties of the tissue, and the imaging frame rate is controlled by the sound speed in tissue (typically about 1.5 mm/μs) and the pulse repetition rate of laser (10 Hz to 5 kHz) [141]. There are some endogenous substances with special light absorption such as hemoglobin, melanin, and lipid, have been used as photoacoustic contrast agents [142]. In brain tissues, hemoglobin can be used as a photoacoustic contrast agent for label-free vascular imaging. As oxyhemoglobin and deoxyhemoglobin have different light absorption spectra, photoacoustic imaging is able to quantitatively measure the total hemoglobin concentration and oxygen saturation, which are difficult to be measured with many other neuroimaging modalities. In addition to endogenous contrast agents, exogenous contrast agents, such as nanoparticles [143], organic dyes [144], and fluorescence proteins [145], are used to further enhance the sensitivity, specificity and contrast of photoacoustic imaging, allowing for molecular neuro-imaging. For instance, conjugated polymer nanoparticles demonstrated highly efficient photoacoustic imaging of orthotopic brain tumors [12] and brain vascular imaging [146]. NIR dye (Prussian blue) labeled Mesenchymal Stem Cells also demonstrated successful photoacoustic imaging for the detection of brain injury and rehabilitation [13]. In order to relieve safety concerns for in vivo applications, biocompatible and/or biodegradable functional biomaterials have also been applied as exogenous photoacoustic contrast agents for neuro-imaging. Zha et al. developed a biocompatible conductive monodispersed PPy nanoparticles (~46 nm) with strong absorption in NIR range through a facile aqueous dispersion method. The PPy nanoparticles demonstrated great photoacoustic imaging ability with deep penetration (~4.3 cm under chicken breast muscle) and low background noise, allowing a better imaging efficiency than that of hemoglobin in blood. After intravenous administration, the polyvinyl alcohol (PVA)-stabilized PPy nanoparticles exhibited clear brain vascular imaging of mouse [33]. Recently, a biodegradable conductive aniline tetramer doped citrate-based polymer with strong photoacoustic effect after excitation with NIR light was synthesized. Combined with its significant promotion of the proliferation and differentiation of nerve cells, the newly developed biodegradable conductive materials are a promising candidate for use in neuro-imaging and nerve regeneration [36].
4.2. MRI for neurological disorders
MRI is based on the relaxation process of water protons generated by a contrast agent in the presence of external electric field. When a proper radio frequency is applied and absorbed by the latters, it leads to spin flipping. Once the external electric field is removed, the proton spins relax. During the relaxation process, a weak radio frequency is generated and detected by the receiver coils, enabling the image creation by the equipment software [147, 148]. Contrast agents play an important role by enhancing the contrast and improving the sensitivity of MRI [149]. The relaxation process occurs in a longitudinal (T1) and a transverse (T2) plane and therefore contrast agents for MRI can be classified into T1 and T2 agents. The former are positive contrast agents, typically contain paramagnetic gadolinium (Gd) derivatives, to reduce proton longitudinal relaxation time (T1), thereby enhancing the MRI signal. Another are negative contrast agents, which are usually composed of superparamagnetic nanoparticles, for reducing the transverse relaxation time (T2) of surrounding water protons, thus decreasing signal intensity [150]. MRI can penetrate deep into tissues, and conduct non-invasive entire body imaging with relatively high spatial resolution (~50 μm) and sequential repeatability [151]. Various superparamagnetic nanoparticles and Gd derivatives based nanoparticles have been developed for molecular, cellular and tissue imaging applications [152-155].
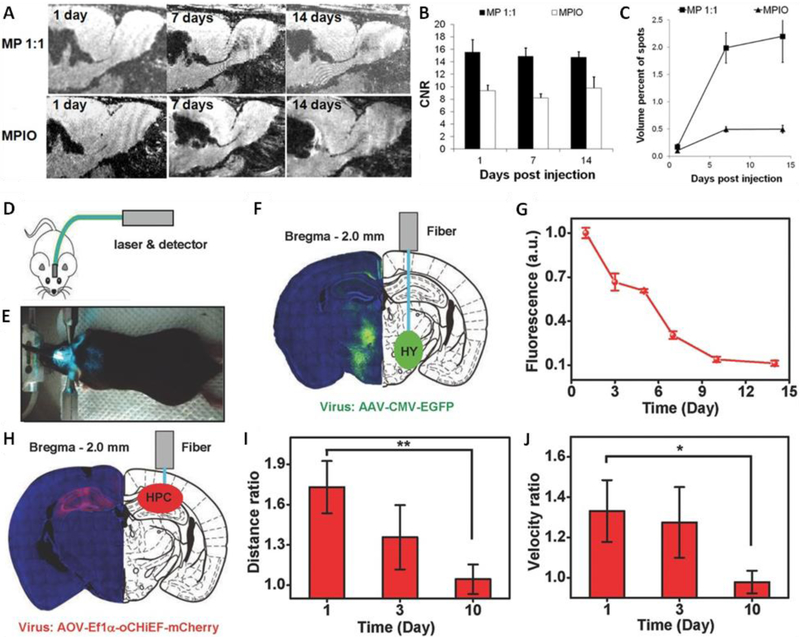
As one of the most powerful techniques in modern clinical diagnostics, MRI has widely been involved as a great imaging tool during the diagnosis, therapy and post-treatment monitoring processes of neurological disorders including Parkinson’s disease [156, 157], Alzheimer’s disease [14], Wilson’ s disease [158], stroke [159], brain tumors [160], and neuronal intranuclear inclusion disease [161]. For example, SPIONs have been utilized to detect amyloid plaques and Aβ plaques in Alzheimer’s disease transgenic mice with MRI [34]. Stroh et al. also demonstrated a long term tracking of SPIONs labeled stem cells with MRI in a Rat Model of Parkinson disease, providing evaluation of stem cell-based therapies for the disease [156]. To improve the in vivo circulating time and biocompatibility of MRI contrast agents, biodegradable polymer encapsulated magnetic nanoparticles have been fabricated. Norman et al. developed wheat germ agglutinin coated magnetite nanoparticles, and used them for neural stem cell labeling [162]. Furthermore, Granot et al. prepared biodegradable magnetic PLGA nanoparticles (~100 nm) and microparticles (1-2 μm) for MRI-based cell tracking. The magnetic microparticles enabled in vivo imaging of the migration of endogenous neural progenitor cells in rat brain over 2 weeks, providing higher contrast to noise ratio than inert micron-sized iron oxide particles (Fig. 1A-C) [37].
Fig. 1.
Biodegradable functional materials for neuro-imaging. (A-C) In vivo MRI of the migration of endogenous neural progenitor cells in rat brain. (A) MRI montage of same animal at level of SVZ –RMS –OB injected with microparticles (MP) or inert MPIOs. (B) CNR measurement of dark contrast within RMS. (C) Volume of dark contrast in the OB. (D-J) In vivo fluorescence photometry and optogenetic experiments with PLLA optical fibers. (D) Schematic cartoon of the experiment design and setup. E) Photographic image of the experiment setup. F) Left: confocal microscopic image of a coronal section containing EGFP. Right: schematic illustration of the fiber implanted into the HY. G) Fluorescence signals recorded via PLLA optical fibers (standard deviation, n = 6) normalized to those measured via silica fibers. H) Left: confocal microscopic image of a coronal section containing oCHiEF protein. Right: schematic illustration of the fiber implanted into the HPC. I) Ratio of travelling distance with the laser on and off. J) Ratio of travelling velocity with the laser on and off. Abbreviations: SVZ, subventricular zone; RMS, rostral migratory stream; OB, olfactory bulb; MP, microparticle; MPIO, micron sized iron oxide particle; CNR, contrast to noise ratio; EGFP, enhanced green fluorescence protein; HY, hypothalamus; HPC, hippocampus. Reproduced from Ref. [38] with permission of John Wiley and Sons.
4.3. Optical imaging for neurological disorders
Optical imaging is performed based on the interactions of lights (infrared (300 GHz-430 THz, 700 nm-1 mm), visible (430-790 THz, 380-700 nm), and ultraviolet light (790 THz-30 PHz, 10-300 nm)) with detecting objects [163]. Optical imaging techniques measure scattering, absorption, and luminescence of light that is either transmitted through or reflected out of objects. Light properties, both wavelength and intensity, determine the imaging penetration depth and size. Optical imaging provides high temporal resolution, but reduced spatial resolution with increased penetration depth. Also, it is often limited in penetration depths (< 1 cm), so traditional fluorescence imaging have been mostly confined to in vitro applications such as cell labeling, immunohistochemistry, and flow cytometry. However, recent development of fluorescent agents with longer excitation/emission wavelengths (e.g. near-infrared (NIR)) as well as advancement of biocompatible optical waveguides have allowed light to be delivered deeper into tissues, greatly improving the viability of optical imaging for in vivo applications [164-166].
Optical imaging has unique advantages associated with low cost, simplicity, and easy-to-operate equipment, offering a variety of interrogations from intrinsic functional information on blood oxygenation to molecular sensing [167]. As such, optical imaging is applied a useful tool for neuro-imaging. Neural stem cells-mediated therapy is emerging as a promising approach to treat a wide variety of neurological diseases [15, 16]. However, their clinical applications are severely constrained by challenges such as tracking the survival rate and migration of the stem cells, evaluating their “stemness” and directional differentiation, and monitoring their therapy effect [62, 168]. To address these challenges, Wang et al. developed an optical imaging system. In particular, they created biodegradable PLGA nanovehicles loaded with fluorescent 6-courmarin and retinoic acid (one of the most potent morphogens to induce stem cell differentiation into neuron subtypes) using a nanoprecipitation method, enabling real-time tracking of the differentiation dynamics of transplanted neural stem cells after traumatic brain injuries [35].
As an effective way to alleviate the light penetration problem of optical imaging, optical waveguides have also been utilized in the field of neuro-imaging to provide organ-scale light delivery and collection. For instance, Fu et al. developed implantable and biodegradable PLLA fibers that can be used for optical neural interfaces [38]. Specifically, the PLLA optical fiber was implanted into the hypothalamus, which had been stimulated with a high expression of enhanced green fluorescence protein (EGFP), of a freely moving mouse. Later, an excitation light (488 nm) from a laser source was delivered via the optical fiber into the hypothalamus. The green fluorescence from EGFP was able to be collected and guided by the same fiber, and then received by a standard detector. The detected fluorescence signals decrease with time, revealing the degradation of PLLA optical fibers in vivo. The same optical fiber was also used to demonstrate optogenetic interrogation in vivo (Fig. 1D-J).
4.4. Multimodality imaging for neurological disorders
The nervous system and the pathologies of neurological diseases are extremely complex. To allow better understanding of the system and more accurate diagnosis of neurological disorders, improved imaging strategies are desired to provide comprehensive and detailed information of both healthy and diseased nervous systems. As such, an ideal neuro-imaging technique is expected to possess high temporal and spatial resolution, ability for histological and functional analysis, availability for long-term tracking, and high specificity to the targeted areas. However, each imaging modality has its own merits and limits, and there is no single imaging technique that fulfills all of the above conditions. Thus, the current mainstream approach is to combine multiple imaging modalities to achieve optimal imaging strategies. For instance, MRI is excellent for spatial resolution and soft-tissue contrast, but is far less sensitive than optical imaging. The integration of these two complementary imaging modalities would improve neuro-imaging effect. By incorporating Fe3O4 nanoparticles and Cy5.5-labled lactoferrin into pH/temperature sensitive nanogels, Jiang et al. developed a nano-system with both dual modality MR/fluorescence imaging capability and brain tumor (Glioma) targeting specificity. The resulting multifunctional nanogels demonstrated efficient MR/fluorescence imaging with high sensitivity and specificity for in vivo studies on rats bearing in situ glioma [169]. Another multimodal imaging study was conducted by Qiao et al. where MRI and photoacoustic imaging were combined for brain tumor imaging. The researchers developed gold-coated SPIONs (SPIO@Au), and used them to label bone marrow-derived human mesenchymal stem cells. Then the MSCs loaded with SPIO@Au were injected in mice bearing orthotopic U87 brain tumors via the carotid artery. Both MRI and photoacoustic imaging were applied for tracking the migration and distribution of MSCs in vivo, showing the possibility for real-time monitoring during stem cell-mediated therapy for brain tumors [170]. Furthermore, there were a few studies were conducted on biodegradable functional biomaterials that were able to perform multimodality neuro-imaging. Koffie et al. prepared biodegradable nanocarrier systems made of poly(n-butyl cyanoacrylate) dextran polymers coated with polysorbate 80 (PBCA nanoparticles). The BBB-impermeable fluorophores and MRI contrast agents labeled PBCA nanoparticles were allowed to cross the BBB into the brain for targeted molecular neuroimaging. Using the advanced PBCA nanocarrier system realized brain cellular imaging, in vivo visualization of amyloid plaques of Alzheimer’s disease, as well as whole brain MRI [39]. Shan et al. also developed a citrate-based fluorescence/photoacoustic dual-imaging enabled biodegradable polymer (BPLPAT). The biodegradable dual-imaging BPLPAT nanoparticles were able to label PC12 nerve cells for fluorescence imaging and perform deep tissue detection with photoacoustic imaging. BPLPAT scaffolds demonstrated 3D high-spatial-resolution deep tissue photoacoustic imaging, as well as promoted the growth and differentiation of PC12 nerve cells. As such, the biodegradable dual-imaging-enabled electroactive BPLPAT will be a promising candidate for neuro-imaging and neurological disease therapy [36].
5. Biodegradable functional biomaterials for neuro-sensing
Neurological disorders generally accompany changes of certain neurotransmitters and biomarkers in the nerve system. Neurotransmitters are endogenous chemicals that are released from synaptic vesicles in synapses into the synaptic cleft to transmit signals from one neuron to another target neuron. Heretofore, more than 100 neurotransmitters have been identified, and they can be categorized according to their functions into excitatory and inhibitory neurotransmitters. The excitatory neurotransmitters (e.g., glutamate) stimulate a nerve cell to produce an action potential to transmit signals, while inhibitory neurotransmitters (e.g., γ -amino butyric acid (GABA)) try to prevent the signal-transmission process. However, some neurotransmitters possess both excitatory and inhibitory properties (e.g., dopamine). Neurotransmitters play significant role in many brain functions such as behavior and cognition. They adjust muscle tone and heart rate, as well as regulate sleeping, appetite, consciousness, mood and memory [20]. Changes in the concentration of neurotransmitters have been associated with many mental and physical disorders. Acetylcholine was the first neurotransmitter discovered in the CNS and PNS. It is determined as the transmitter at the neuromuscular junction that connects motor nerves to muscles. It activates skeletal muscles in the somatic nervous system, and may either excite or inhibit internal organs in the autonomic system. Acetylcholine is also vital to enhance learning and memory ability, improve alertness, and sustain attention [171 -163]. Damage to the cholinergic system, which produces acetylcholine, in the brain has been demonstrated to be associated with Alzheimer's disease related memory deficits [174, 175]. Glutamate is the most common excitatory neurotransmitter, which is excitatory at well over 90% of the synapses in the human brain [21]. Dynamics of glutamatergic excitation is vital in normal physiological processes including synaptic plasticity, long-term potentiation, differentiation and apoptosis. However, abnormalities in glutamate transport are associated with many illnesses. Excessive glutamate release can overstimulate the brain and cause excitotoxicity, triggering neuronal degeneration and cell death. The excitotoxicity has been implicated in many neurological diseases including Parkinson's disease, Alzheimer's disease, Huntington disease, seizures, stroke, epilepsy, and amyotrophic lateral sclerosis [176, 177]. Dopamine is another vital neurotransmitter that regulates the reward and pleasure centers of the brain, controls the release of various hormones, and affects motor behaviors. The concentration of endogenous dopamine has been determined in the range of 0.01–1 μM in the brain of bovine, rats, and humans [178]. Various neurological disorders are related with the dysfunction of the dopamine system. For instance, Parkinson's disease has been associated with low levels of dopamine, and schizophrenia has been linked to high levels of dopamine [22, 23]. In addition to neurotransmitters, certain biomarkers are also measurable indicators of neurological states, demonstrating normal or pathogenic biological processes. Most nerve cells of interest are often not directly observable in typical clinical settings, so validated biomarkers are critically essential to help the diagnosis of neurological disorders, and track the response to therapeutic intervention [179]. There are many validated biomarkers that have been determined to support the diagnosis and therapy for neurological disorders. For instance, the deposition of β -amyloid peptides (A β) in plaques in brain tissue has been proposed to cause the neurodegeneration in Alzheimer's disease. Among the various A β species, Aβ42 (a peptide with 42 amino acids) is the major constituent of the abnormal plaques in the brains of patients with Alzheimer's disease. It was found that most of the patients with Alzheimer's disease had decreased levels of A β 42 in their cerebrospinal fluids (CSF) [180, 181]. MHC class II, as another example, is a biomarker of microglial activation. Studies have shown that the expression of MHC class II to be higher in the putamen and substantia nigra, as well as in the hippocampus, temporal cortex, transentorhinal cortex, and cingulate cortex of the brains of patients with Parkinson's disease [179].
Based on the above discussions, quantitative detection of neurotransmitters and biomarkers in biological environments of urine, plasma, serum and CSF appears to be able to improve the diagnosis and treatment process of neurological disorders. Surveys of literatures showed that functional biomaterials including electroactive biomaterials, magnetic biomaterials and photoactive biomaterials have been widely studied in the application of biosensing for neurotransmitters and biomarkers. For instance, Qian et al. developed conjugated polymer-based fluorescent nanoparticles with phenylboronic acid (PBA) on the surface for optical sensing of dopamine. The PBA works as binding sites for dopamine targeting. The nanoparticles, referred to as PFPBA-NPs, were utilized for fluorescence detection of neurotransmitter dopamine in both nerve cells PC12 and brain of zebrafish larvae. A good linear relationship was established between the fluorescence intensity ratio (I0 - I)/I0 of PFPBA nanoparticles and logarithmic concentration of dopamine in the range of 0.025–10 μM (where I0 and I represent the maximum fluorescence intensity in the absence and presence of dopamine, respectively). The PFPBA-NPs also demonstrated a low detection limit of 38.8 nM, as well as a high specificity with minimum interference from other endogenous molecules such as glucose, ascorbic acid, tyramine, tyrosine, epinephrine, and norepinephrine. Therefore, the fabricated nanoparticles displayed high sensitivity and selectivity, allowing accurate detection of dopamine for diagnosis and therapy of dopamine related neurological diseases [182]. Kergoat et al. reported conducting poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate)/platinum nanoparticle composites based organic electrochemical transistors (PEDOT:PSS/Pt NPs OECTs) for the detection of glutamate and acetylcholine. For glutamate detection, the PEDOT:PSS/Pt NPs OECT sensors presented a sensitivity of 4.3 A mol−1 L1 cm−2 and a detection limit of 5 μM. For acetylcholine sensing, the OECT sensors demonstrated a sensitivity of 4.1 A mol−1 L1 cm−2 and a detection limit of 5 μM. The detection limit of the OECT sensors was sufficient for the detection of glutamate in the extracellular fluid (typically in the low μM range), while not low enough for the sensing of acetylcholine in the extracellular fluid (typically in the nM range). The authors’ ongoing work regarding the optimization of the nanoparticles dispersion in the PEDOT:PSS matrix is expected to improve the detection sensitivity and limit [183]. Li et al. applied magnetic nitrogen-doped graphene (MNG) to modify the Au electrode, and developed a reusable biosensor for the detection of amyloid-beta peptide 1–42 (Aβ42). To improve the detection specificity, antibodies of Aβ 1–28 (Aβab) that serve as the biorecognition element for Aβ42 were conjugated on the surface of MNG. The reusable biosensor showed presented a linear calibration curve within the range from 5 to 800 pg mL−1, covering the cut-off level of Aβ42. It also achieved a detection limit of 5 pg mL −1. The results demonstrated that fabricated biosensor possesses many advantages such as simplicity, high sensitivity and selectivity, quick response time, low cost, reproducibility, and stability, enabling a potential candidate for early diagnosis of Alzheimer’s disease [18].
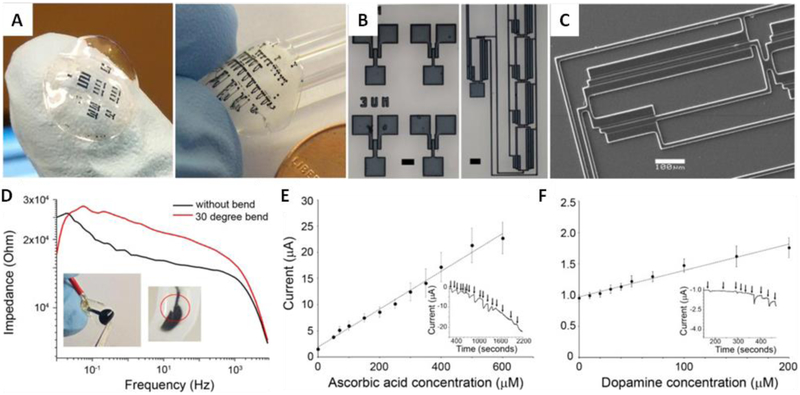
So far, very few biodegradable functional biomaterials have been involved in the application of biosensing of neurological disorders. Flexible and biodegradable electrochemical sensors reported by Pal et al. can be an example (Fig. 2) [40]. The sensors were fabricated using a benchtop photolithographic setup to format PEDOT:PSS micropattern on a flexible and fully biodegradable silk protein fibroin substrate. The resulting devices are mechanically flexible and optically transparent. Applying the conductive PEDOT:PSS micropatterns as working electrodes, the biosensors exhibited excellent electrochemical activity and stability over three days. The biosensors were applied for non-specific detection of dopamine and ascorbic acid. For dopamine detection, the sensors showed a lowest detectable concentration of 15.21 μM and a quantitation limit of 46.1 μM. For ascorbic acid sensing, they demonstrated a lowest detectable concentration of 15.47 μM and a quantitation limit of 46.87 μM. The amperometric response curve of the dopamine exhibited a linear range from 10 to 200 μM, with a sensitivity of 45.9 nA μM−1 cm−2. While the amperometric response curve of ascorbic acid had a linear range from 10 to 600 μM, with a sensitivity of 256.5 nA μM”1 cm−2. The response time (time taken to reach 95% of steady state current) for both dopamine and ascorbic acid was ~30 s. The linear and dynamic ranges for detection of dopamine and ascorbic acid of the fabricated sensors were comparable with other reported sensors. These sensors also presented high stability under mechanical stress. They were able to retain their physical integrity and electrochemical properties under repeated mechanical deformations of 150 bending cycles. Furthermore, the sensing devices were completely biodegradable under enzymatic action, and were able to be designed with programmable degradation rate through controlling the film crosslinking. Therefore, the reported technique is able to be used for the development of robust, biodegradable, sensitive, and inexpensive biosensors for dopamine and ascorbic acid detection. With further modification or incorporation with other electroactive species, biosensors with more specificity and sensitivity for the detection of neurotransmitters and biomarkers are expected to be developed to improve the diagnosis and treatment for neurological diseases.
Fig. 2.
Biodegradable functional materials for neuro-sensing. (A) Large area micropatterns of PEDOT:PSS formed on flexible and conformable silk fibroin sheets via photolithography. (B) Optical micrographs and (C) SEM images of PEDOT:PSS micropatterns on glass. (D) Mechanical bending and corresponding effect on impedance after 150 bending cycles. Non-specific sensing of (E) ascorbic acid and (F) dopamine biosensing, showing the linear ranges of both sensors (R2=0.989 and 0.979 respectively with n=3 sensors ). The insets show the chronoamperometric response with addition for one sensor as an example. Reproduced from Ref. [40] with permission of Elsevier.
6. Biodegradable functional biomaterial mediated therapy for neurological disorders
Functional biomaterials play essential roles in the treatment for neurological disorders by supporting and guiding the growth and differentiation of neuron cells in forms of scaffolds, by facilitating the delivery of drugs, neuroprotective agents, and growth factors across BBB in forms of nanocarriers [24, 184, 185]. Compared with traditional biomaterials, functional biomaterials based therapy could further provide physical stimuli using optical, electrical and magnetic methods to either regulate cell signaling directly or to control drug delivery with high precision and accuracy. Here, we focus on recent advances in the development of biodegradable functional biomaterials that may lead to strategies for nerve regeneration, neuroprotection, and brain tumor treatment. We highlight studies using conductive materials for electric stimulation of neuron activity and for electrochemical controlled drug delivery; magnetic materials for magnetic stimulation of neurons and hyperthernia treatment for brain tumor; photoactive materials for light stimulation of nerve activity and for optical fiber to deliver light; as well as examples of multifunctional biomaterials for theranostic applications. Lastly, the materials with intrinsic neuroprotective potential for neurological diseases will be discussed.
6.1. Biodegradable electroactive biomaterials for the treatment of neurological disorders
Electrically conductive materials have received marked attention in our effort towards promoting neuroregeneration, by means of either electric stimuli delivery or controlled release of therapeutic drugs, given the specific electric nature of neuron cells. In fact, electrical stimulation has long been applied in clinics with its therapeutic effect proven to improve a wide range of neurological disorders, including stroke, Parkinson's disease, Alzheimer's disease [186], seizure [187], as well as to facilitate the functional rehabilitation after traumatic injuries either in CNS or in PNS [188]. Established modalities of electric stimulation therapy, non-invasive [186] (transcranial electric stimulation for CNS disorders and transcutaneous stimulation for PNS disorders) or invasive [189] (deep brain stimulation for CNS disorders and direct low frequency electric stimulation for PNS disorders), in combination with commercialized devices are available to promote neuron axon growth, to influence neurotrophic factors production, and to modulate neuron activity [190].
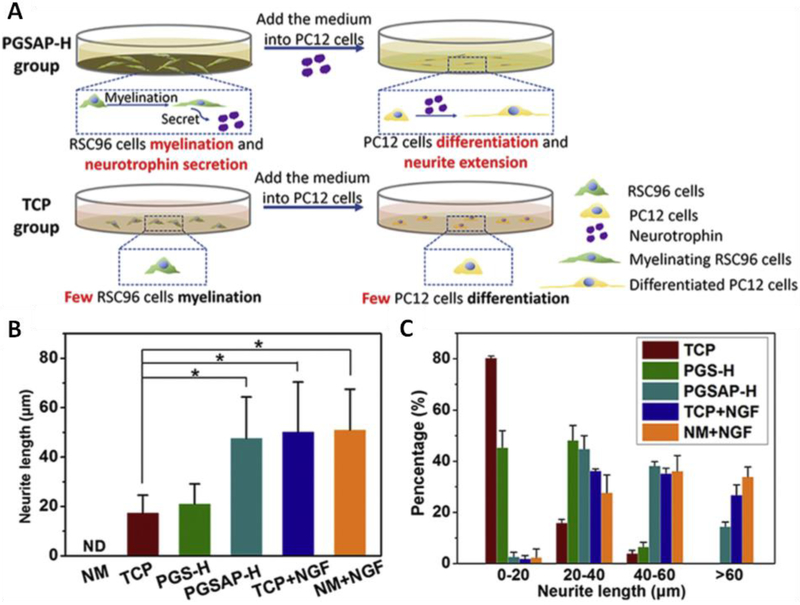
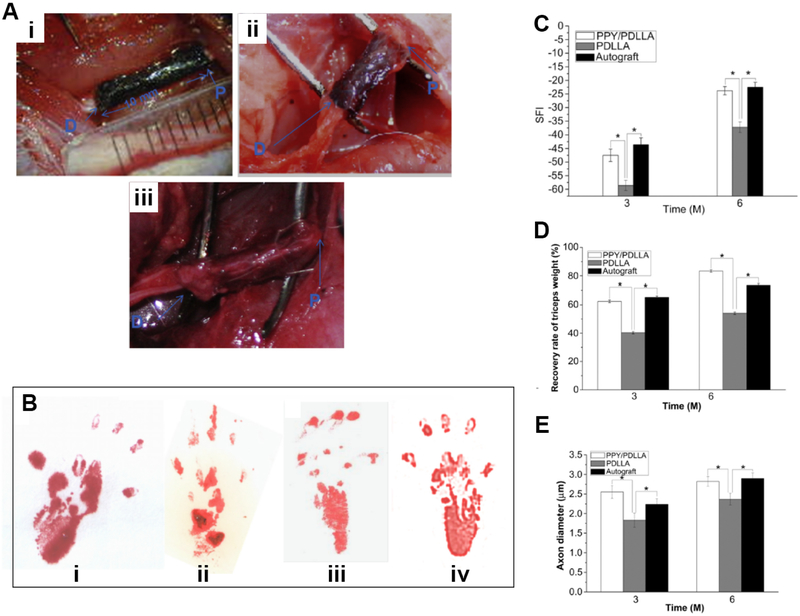
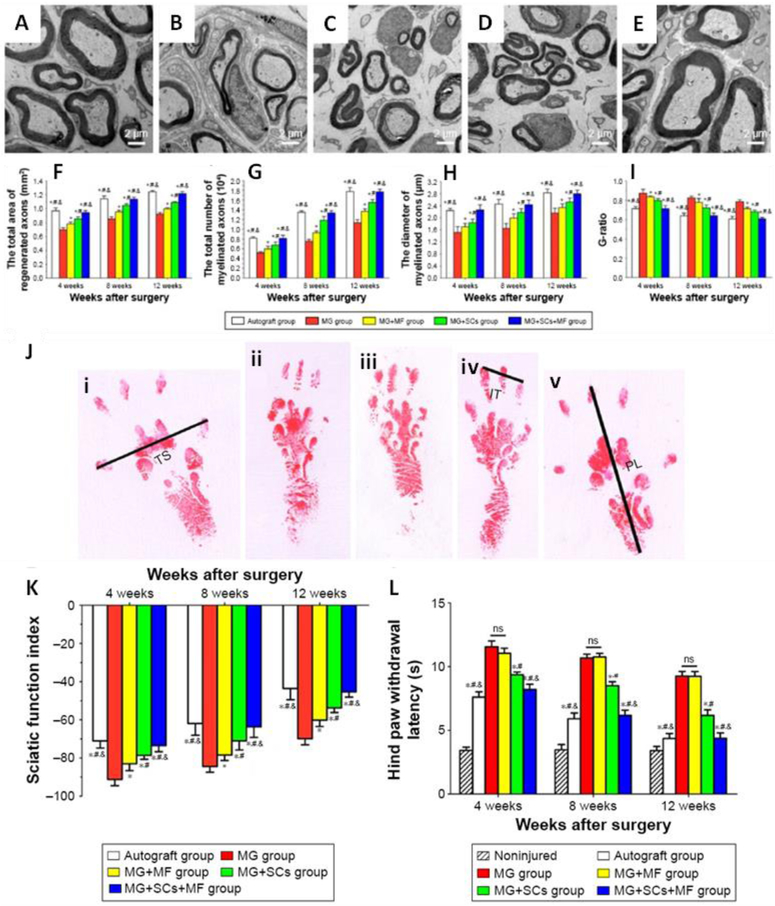
The noted beneficial effect of electrical stimulation makes the conductive materials an attractive candidate for functionalized scaffold, which allows the local delivery of electrical stimulus with temporal and spatial control, while supporting and guiding cell growth. Numerous evidence has shown that the locally delivered electrical stimulus is beneficial for nerve regeneration. For example, the direct current electric fields stimulation of 100 mV/cm delivered by the polypyrrole-block-polycaprolactone (PPy-PCL) biodegradable substrate for 2 h already increased the axon growth of the attached dorsal root ganglia by 21% (±3%) [191]. The axon outgrowth from neuron stem cell (NSCs) could also be guided in a controlled manner by combining a micropatterned PEG hydrogel with silver nanowire (AgNW), which led to a higher rate of axon directional outgrowth after electrical stimulation [182]. Moreover, rat PC12 cells cultured on both PPy doped poly (D,L-lactic acid) (PPy/PDLLA) [41] and aniline tetramer doped citrate based materials (BPLPAT) [36] have shown a marked increase in both the percentage of neurite-bearing cells and their median neurite length. Besides, Schwann cells that cultured on conductive poly (glycerol sebacate)-co-aniline pentamer (PGS-AP) exhibited enhanced myelin gene expression and neurotrophin secretion, which subsequently induced the neurite growth of PC12 cells (Fig. 3) [77]. The mechanism underlying the benefit of electrical stimulation through conductive materials is not fully understood. Different hypotheses have been proposed, and one compelling hypothesis is that electric stimulation might alter the protein adsorption behavior to conductive materials, thereby affecting cell-materials interaction [193]. Also, one recent study pointed out that conductive films affected the intracellular calcium level, which led to altered CaSR and PLCβ pathways for induced neurotrophin secretion [77]. Of note, up to now the application of those biodegradable conductive materials has mainly focused on the regeneration of peripheral nerves, and encouragingly, the conductive PPy/PDLLA guidance conduit has shown promising performance as evaluated by walking track, triceps weight, electrophysiological and histological assessment, similar to autologous graft in the functional repairing of rat sciatic nerve defects (Fig. 4) [41]. At the same time, conductive hydrogel-based materials are being explored as a coating for neural electrodes, which are used widely in the invasive electric stimulation therapy, to regulate the electrode modulus and to improve its biocompatibility. Although currently non-degradable hydrogels are focused for this coating purpose, efforts have been made towards the development of degradable coating hydrogels. For instance, a biodegradable conductive hydrogel composed of degradable agarose and aniline petramer (AP) [194] was developed. The resultant hydrogels presented enhanced ions and electrons mobility, as well as improved cytocompatiblilty, serving as a potential candidate for different applications in neuron tissue engineering.
Fig. 3.
Schwann cells-laden conductive PGSAP-H films inducing PC12 cells differentiation. (A) Schematic diagram depicting PC12 cells differentiation and neurite extension when culture in medium suspension from Schwann cells (RSC96) on conductive films. (B) Quantitative analysis of neurite length of PC12 cells supplemented with the normal medium (NM) group, TCP group, PGS-H group, PGSAP-H group, TCP + NGF group, and NM + NGF group. (d) Frequency of neurite length distribution of PC12 cells of each group. *P < 0.05. Reproduced from Ref. [77] with permission of Elsevier.
Fig. 4.
Conductive PPy/PLLA conduit for the regeneration of 10-mm sciatic nerve defects. (A) Intraoperative photographs of the PPY/PDLLA nerve conduit implantation. “P” indicates the proximal end and “D” indicates the distal end. i) Immediately after grafting. ii) 3 months postoperatively. iii) 6 months postoperatively. (B) Footprint stamps in walking track analysis after 6 months of implantation. Groups: i) PPY/PDLLA. ii) PDLLA. iii) Autograft. iv) Normal left leg. (C) Sciatic function index (SFI) as a function of implantation time. (D) Triceps weight (%) evaluation after 3 and 6 months post-operation (n= 6, * indicates p < 0.05). (E) Average axon diameter of regenerated myelinated nerve fibers. Reproduced from Ref. [41] with permission of Elsevier.
In addition to modulating cell activity, conductive materials have also shown their potential to electrochemically control the release of drugs based on the ability to electrically switch the oxidized and reduced state of the polymer, leading to uptake and expulsion of charged drugs from the bulk materials [195]. Many therapeutic and neuroprotective drugs, including neurotransmitter dopamine, antiinflammatory factor dexamethasone, as well as neuroregenerative neurotrophin-3 and brain-derived neurotrophic factor (BDNF), have already been bound and released from PPy-based conductive polymers [196]. There is also ongoing effort to develop biodegradable conductive materials that are capable of electrochemically controlled drug release. For example, biodegradable multiblock copolymer composed of oligoaniline and PEG or poly(ε-caprolactone) (PCL) has been shown to possess a drug loading as high as 31 wt%, and deliver dexamethasone phosphate triggered by potential cycling between 0.7 V and −0.5 V [196]. Also, the release profile can be tailored by simple modulation of the electrical input and the stimulation cycle numbers. Similarly, a voltage-dependent releasing of dexamethasone was achieved by using the conductive agarose-AP hydrogel [194]. Together with the proven cytocompatibility, this new series of conductive polyesters and hydrogels possess the potential to develop an advanced system for future management of neurological disorders by integrating electrochemically controlled drug delivery system with electrical stimulation enabled tissue scaffolds.
6.2. Biodegradable magnetic biomaterials for the treatment of neurological disorders
Direct techniques to alter neural activity also include magnetic stimulation by applying magnetic fields near the scalp to induce electric fields within the brain to modulate neuron activities [188], in addition to electrical stimulation. Disorders such as Parkinson's disease, epilepsy and stroke rehabilitation were treated with the magnetic stimulation and have shown therapeutic efficacy [186]. In fact, magnetic techniques offer distinct benefits for in vivo applications, given magnetic fields can penetrate deeply into biological tissue without significant attenuation [197]. Importantly, in combination with magnetic nanoparticles, magnetic field could also be transformed into heat [198, 199], as well as into mechanical force [200].
Increasing evidence has shown that magnetic iron oxide nanoparticles can also serve as potent photothermal agents to modulate neuron cell activity, evidenced by an exciting study using superparamagnetic ferrite nanoparticles to bind to specific cells expressing the temperature sensitive ion channel TRPV1 [198, 199]. After applying a radiofrequency magnetic field, the nanoparticles heating led to the opening of TRPV1 channels and calcium influx was observed. A similar strategy using polymer encapsulated magnetic manganese ferrite nanoparticles, when binding to the neurons in the motor cortex which were transfected to express TRVP1 channels [199], has confirmed the capability of opening TRVP1 channel via heating the nanoparticles, which further resulted in deep brain stimulation in the striatum caused rotation. This study provided proof of principle for the magnetic-based deep brain control of movement for the treatment of motor nerve derived neurological diseases, such as amyotrophic lateral sclerosis (ALS). Of interest, the electromagnetic fields (EMF) at specific range generated by electrically charged subject, can also influence cell function and even guide cell differentiation. Specifically, a specific frequency and intensity of EMF stimulation (2 ϗ 10−3 T/100 Hz) has been found to dramatically improve the efficacy of direct lineage reprogramming of somatic fibroblasts [201], in the presence of reprogramming transcription factor, into induced dopaminergic neurons both in vitro and in vivo, which represents a viable and promising therapeutic strategy to replace lost neurons in neurodegenerative diseases such as Parkinson's disease with high efficiency, low toxicity and high controllability. Moreover, the underlying mechanism for the EMF mediated cell reprogramming was due to the promoted chromatin remodeling which enabled the transcriptional changes necessary for the reprogramming process.
Magnetic field induced heat has also been applied in the hyperthermia therapy, named magnetic hyperthermia, for the treatment of glioblastoma, based on the fact that tumor cells are more sensitive to sudden increases in temperature than the normal surrounding cells, leading to selectively tumor cell death [200]. The magnetic hyperthermia offers advantages in generating a homogenous distribution of therapeutic temperature in deep and inaccessible tissues. Evidence has shown that SPIONs are appropriate intratumoral hyperthermia agents. Their design requirements, in vitro application, as well as preclinical and clinical application have been reviewed elsewhere [200]. In addition to the magnetothermal effect, magnetic field on nanoparticles can also be transformed into mechanical force. Together with the surface modification with targeted ligand, the nanoparticles could generate a force usually in the femtonewton range on the membrane receptors or could lead to receptor clustering, thereby influencing cell function. For example, by conjugating with monoclonal antibody for DR4 receptors, zinc-doped iron oxide magnetic nanoparticles selectively bound to the DR4 receptor on cancer cells, which induced clustering of the DR4s by using a magnetic field in a non-invasive manner [202], leading to the activation of apoptotic signaling pathways. Although still at the early stage of development, this new technology offers the potential of applying magnetic techniques in spatially and temporally activation of cell death or other signaling pathways at the target region for the treatment of neurological diseases, such as glioblastoma.
With all above emerging studies showing the controllability and feasibility for the therapy design and precise imaging of neurological disorder that is brought by magnetic materials, it would be promising to further functionalize the magnetic nanoparticles with other types of functional biomaterials, to not only improve cytocompatibility and introduce additional functionality, but also allow easy loading and controlled releasing of therapeutic drugs [203-205]. Coating of magnetic nanoparticles with temperature sensitive polymers [203], could serve as a good example, as the increased temperature generated through magnetic nanoparticles was found to increase the releasing of doxorubicin controllably in a temperature-dependent manner. A coating of polymers on magnetic nanoparticles could also provide an easier way for further surface modification to prepare "smart" nanoparticles. For example, after encapsulating magnetic nanoparticles using (dodecyl-grafted-poly-(isobutylene-alt-maleic-anhydride) (PMA) polymer [199], the polymer coating was further modified with NeutrAvidin, which mediated the binding of the resultant nanoparticles to biotinylated neuronal cell membrane. Dylight 550 fluorophores was also conjugated to the polymer as molecular thermometers, to monitor the cell surface temperature changes. Similarly, by coating magnetic nanoparticles with cationic polyamine dendrimer, it allowed better siRNA loading while aiding the escape of siRNA from endosomes [206]. More importantly, a marked increase in transfection efficiency to promote neural stem cell differentiation was achieved by applying external magnetic field, named magnetic facilitated drug delivery. Given the critical role of NSCs in nerve regeneration and treatment of neurological diseases, the above studies strongly suggest the promising application and the essential role of those composite magnetic materials, especially those in combination with biodegradable functional biomaterials, for the future treatment of neurological disorders. By composting magnetic nanoparticles with a biodegradable chitosan-glycerophosphate polymer, Liu et al. developed a new magnetic nanocomposite scaffold with tunable magnetization and degradation rate (Fig. 5). The resultant scaffold could be synergized with applied magnetic field, not only to enhance the viability of Schwann cells in vitro, but also to promote the nerve regeneration and functional recovery of a 15 mm sciatic nerve defect in rats [42], representing as a promising therapy for peripheral nerve regeneration.
Fig. 5.
Morphologic appearance and morphometric assessments of regenerated nerves in each group. (A-E) The characteristic electron micrographs of regenerated axons in the middle part of the scaffold in the (A) autograft group, (B) MG group, (C) MG+MF group, (D) MG+SCs group, and (E) MG+SCs+MF group at 12 weeks postoperation. (J-K) The sciatic functional index in each group. (F) The cross-sectional area of regenerated nerves in the scaffold. (G) Quantitative analysis of myelinated axons in the scaffold. (H) The diameter of myelinated axons in the scaffold. (I) The G-ratios in the scaffold. (J) The operative left footprints at 12 weeks postoperatively. i) Autograft group. ii) MG group, iii) MG+MF group. iv) MG+SCs group. v) MG+SCs+MF group. (K) The statistical data of the sciatic functional index. (L) The hind paw withdrawal latency time. *p<0.05 compared to MG group; #p<0.05 compared to MG+MF group; &p<0.05 compared to MG+SCs group; all results are presented as the mean ± standard eror of the mean. Abbreviations: MG, the rats were bridged with the magnetic scaffold; MG+MF, the rats were bridged with the magnetic scaffold and under MF exposure after surgery; MG+SCs, the rats were bridged with the Schwann cells-loaded magnetic scaffold; MG+SCs+MF, the rats were bridged with the Schwann cells-loaded magnetic scaffold and under MF after surgery; MF, magnetic field; SCs, Schwann cells. IT, intermediary toe spread; PL, print length; TS, toe spread. Reproduced from Ref. [42] with permission of Dove Medical Press.
6.3. Biodegradable photoactive biomaterials for the treatment of neurological disorders
The interest of using photoactive materials for the treatment of neurological diseases arises from the potential of modulating cell behavior and activity through direct low-intensity pulsed light stimulation [186] and via the material mediated photothermal therapy [104, 207]. In fact, despite less common and mostly performed in experimental settings, there is transcranial light therapy and peripheral nerve light stimulation studies available, which have shown its effect to enhance regional cerebral blood flow, increase neuron activity, and modulate mitochondrial function [186]. Also, studies have shown that the excitation of nerve cells by direct light illumination is largely because of the transient photothermal effect, represented by the generated thermal gradients surrounding the nerve tissue (6°C-10°C), resulting in direct or indirect activation of transmembrane thermo-sensitive ion channels to regenerate action potential [99]. Given the penetration depth of therapeutic light is limited, Kuo et al. also performed deep brain light stimulation in a rat using a fiber-coupled laser stimulator at wavelength of 840 nm with the pulse repetition rate of 130 Hz [208]. Experimental results showed the favorable effect of light stimulation on decreasing the glutamate concentration while increasing the dopamine production in the striatum, which demonstrated its potential application in the treatment of dopamine related diseases such as Parkinson's disease. In addition, hybrid optoelectronic systems consisting of an electronic circuit, an optical fiber, and a photoelectrical converter, have been developed for electrical stimulation of hippocampal living neurons [209]. In the new system, an optical fiber instead of conventional metallic wires was utilized, placing between the electronic circuit and stimulated neurons to provide galvanic isolation from external electric fields and prevent electromagnetic noise. The resultant optoelectronic system was shown to be effective for stimulating the electrophysiological activities of living neurons in the hippocampus, possessing potential to restore regions of brain activity after brain damage in traumatic brain injury or neurodegenerative diseases. Of note, the progress that made in the development of biodegradable optical fibers, such as the PLLA [210] or citrate-based material derived optical fiber [136], open up new avenue to explore the next generation of deep tissue light delivery systems to modulate neuron activity. In fact, in one most recent study, Fu et al. has proceeded to demonstrate the feasibility of using PLLA degradable optical fiber in applications such as intracranial light delivery, deep brain fluorescence sensing and optogenetic interrogation in mice [38], representing as a big step ahead towards the development of fully biocompatible and biodegradable photonic systems for neuroscience uses.
Compared with electrical stimulation and chemical stimulation through therapeutic drugs, direct light stimulation offers improved spatio-temporal resolution. Moreover, the emergence of optogenetics, which combines light stimulation with genetic modification of target cells with light-responsive ion channels, enabled researchers to selectively excite or inhibit a subpopulation of neurons in neural tissues [101], but the imperative targeted gene modification of cell before light stimulation makes the clinical translation more challenging [101, 207]. To address this problem, non-genetic optical stimulation technique taking advantage of photothermal agents as transducers that absorb light and convert it into heat, has been designed to modulate neuron activity with higher specificity. For example, Yoo et al. developed a near-infrared activatable system which could inhibit the neuron activity by binding the gold photothermal nanorods onto cell membrane [207]. More importantly, such an inhibitory effect can be reversed by removing the light source and the degree of inhibition could be tuned by changing the laser intensity, indicating its potential application in the anti-epilepsy therapy by selectively suppressing the hyperactive neurons. In addition, light sensitive conjugated polymers, such as poly(3-hexylthiophene) (P3HT) in a form of thin films could also serve as a platform to optically modulate the membrane depolarization and hyperpolarization mediated by a thermal effect, with potential applications in neurological disease treatment [28]. Although the existing photothermal agents including metal nanoparticles, carbon nanomaterials, and organic photothermal agent, are not degradable, they have been successfully incorporated into matrices of soft materials, including polymer and gels which can be remotely controlled by light irradiation [211], representing tremendous opportunities for future exploration of a wide range of light activatable systems towards the treatment of neurological diseases.
6.4. Biodegradable multi-functional biomaterials for the treatment of neurological disorders
As mentioned above, the functional biomaterials enable multimodality of imaging, allows physical stimulated cell function and drug releasing, and permits further surface modification of targeted ligand for therapeutic purposes. Further, by combining different functional biomaterials, multifunctional systems, which is greatly desired for the optimal management of various neurological diseases, can also be developed to address the multi-dimension of CNS disorders. To this end, the most common way is compositing of different functional biomaterials. For example, encapsulation of magnetic nanoparticles with intrinsic fluorescent citrate-based materials [120] enables drug delivery with magnetic and fluorescent dual-imaging capability. A gold coating on magnetic nanoparticles can provide a number of additional advantages, such as near-infrared absorption for heat conversion and a facile surface for further functionalization to generate core-shell multimodal nanoparticles [206]. Similarly, other core-shell nanoparticles using biodegradable polymeric materials as the core structure to deliver therapeutic drugs while generating gold nanoshells combined with magnetic nanoparticles, have been developed as a multi-functional platform for simultaneous diagnosis through MRI, in combination with dual therapeutic modality of chemotherapy and NIR photothermal therapy [204]. In addition, the development of multifunctional biomaterials, which could possess multiple interesting properties, such as the citrate-based biodegradable materials, offers unique opportunity to establish such a multitask platform. The conductive BPLPAT mentioned above, could serve as a good example, as it enables dual fluorescent and photoacoustic imaging while presented with conductivity allowing electrical stimulation by itself, after incorporating AT oligmer into citrate-based material framework (Fig. 6) [36]. Notably, citrate-based materials possessing numerous pendant carboxyl groups provide further designability to modify the materials with biology moieties [212], such as the targeted ligands to direct selective binding to the disease lesion, and the bioactive parts that exerting neuroprotective and neuropromotive potential. In fact, elastic yet strong citrate-based materials CUPE has been made into nerve conduit, which was further modified using folic acid, the small molecule was recently found to promote the neurotrophin production and stimulate neuronal differentiation [213].
Fig. 6.
Citrate-based fluorescence/photoacoustic dual-imaging enabled biodegradable electroactive polymers. (A) i) Schematic and chemical structure of BPLPAT pre-polymers. ii) Schematic of cross-linked BPLPAT structure. iii) Schematic of functionalities of crosslinked BPLPATs. (B) Fluorescence imaging of PC12 cells uptaken with BPLP and BPLPAT nanoparticles. (C) i) Ultrasound images, ii) photoacoustic images, and iii) superimposed ultrasound and photoacoustic images of BPLP and BPLPAT scaffolds under a ~11 mm thick layer of chicken breast tissue. iv) Ultrasound images, v) photoacoustic images, and vi) superimposed ultrasound and photoacoustic images of BPLP and BPLPAT scaffolds under two layers (total ~23 mm thick) of chicken breast tissue. (D) Schematic of electrical stimulation of PC-12 cells on cross-linked BPLPAT materials. E) SEM images of PC-12 cells on BPLP and BPLPAT films without electrical stimulation (control) and with electrical stimulation. Reproduced from Ref. [36] with permission of John Wiley and Sons.
Interestingly, increasing evidence points out that some elements releasing from materials could affect neuron activities, which potentially adds intrinsic biological regulatory capability into functional biomaterials, making them very promising for the treatment of neurological diseases. For example, magnesium has been found to induce neural depolarization [214]. In particular, citrate, one key building block for citrate-based materials, is considered to exert regulatory function in CNS probably by chelation with Ca2+ and Mg2+, playing a pivotal role as a regulator for glutamate receptors [215], Although the exact physiological role of citrate in CNS remains unclear, a beneficial effect of citric acid on reducing brain oxidative stress and preventing neuronal injury of rats with acute exposure of malathion, a neurotoxic agent [216], has been reported. Similarly, citric acid was shown to attenuate the endotoxin-induced oxidative stress of the brain in another animal study, presented by inhibited lipid peroxidation and decreased inflammatory factor TNF-α production, strongly suggesting the innate neuroprotection nature of citrate molecule [217]. More importantly, by incorporating native anti-oxidant agent ascorbic acid into polymer network [218], a citrate-based materials poly (1,8-octanediol-co-citrate-co-ascorbate) (POCA) with intrinsic anit-oxidant and potential neuroprotective properties, in terms of scavenging of free radicals, iron chelation and the inhibition of lipid peroxidation, has been reported, making it a promising basal biodegradable materials for the treatment of neurological diseases, since oxidative stress and inflammation have been implicated in almost all neurological disorders and their associated secondary injuries [44].
7. Conclusion
This manuscript summarized the recent progress of biodegradable functional biomaterials, specifically biodegradable electroactive biomaterials, biodegradable magnetic biomaterials, and biodegradable photoactive biomaterials for diagnosis and treatment of neurological disorders. Compared to other biodegradable biomaterials, biodegradable functional biomaterials have the unique property of being responsive to external stimulation fields, enabling them with great powers to modulate cellular activities for nerve regeneration, to sense neurotransmitters and biomarkers for disease diagnosis, to perform non-invasive bioimaging for neural stem cell and disease tracking, to facilitate drugs and small molecules to cross BBB for disease targeting, as well as to achieve controlled drug release and hyperthermia treatment for neurological disease therapy. Despite the fact that biodegradable functional biomaterials have shown impressive benefits in understanding and treating neurological disorders in preclinical research, their broad clinical adaptation and application will depend on further exploration and optimization. For instance, for better understanding and optimization of biodegradable functional biomaterials for the management of neurological disorders, we have to uncover how these biomaterials behave in vivo, and this is where more advanced bioimaging and biosensing techniques are of great importance. In addition, advanced nanofabrication techniques and surface modification approaches may endow biodegradable functional biomaterials with more sophisticated nanostructures to improve biocompatibility and cell/material interactions. Furthermore, the combination of biomaterials and stem cell therapies is emerging to revolutionize neurological disorders treatment [62], we believe the introduction of biodegradable functional biomaterials to stem cell therapies would significantly improve the therapy results through providing better controlling of neural stem labeling, transplantation, and differentiation. It is our belief that at this materials-design age, the innovation of functional degradable biomaterials will spur the search of solutions to tackle the challenges in neurological disorder management.
Acknowledgements
The authors would like to acknowledge financial support from the National Institutes of Health Awards (CA182670, EB024829, and AR072731). The authors also thank Mr. Digeng Du for editing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
J.Y. and The Pennsylvania State University have a financial interest in Acuitive Technologies, Inc. and Aleo BME, Inc. These interests have been reviewed by the University’s Institutional and Individual Conflict of Interest Committees and are currently being managed by the University.
References
- [1].Haider B, von Oertzen J, Neurological disorders, Best Pract Res Clin Obstet Gynaecol 27(6) (2013) 867–75. [DOI] [PubMed] [Google Scholar]
- [2].Gooch CL, Pracht E, Borenstein AR, The burden of neurological disease in the United States: A summery report and call for action, Annals of Neurology 81(4) (2017) 479–484. [DOI] [PubMed] [Google Scholar]
- [3].Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS, CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015, Neuro-Oncology 20(suppl_4) (2018) iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kalia LV, Lang AE, Parkinson's disease, The Lancet 386(9996) (2015) 896–912. [DOI] [PubMed] [Google Scholar]
- [5].Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM, Alzheimer's disease, The Lancet 388(10043) (2016) 505–517. [DOI] [PubMed] [Google Scholar]
- [6].Filbin MT, Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS, Nat Rev Neurosci 4(9) (2003) 703–13. [DOI] [PubMed] [Google Scholar]
- [7].Schmidt CE, Leach JB, Neural tissue engineering: strategies for repair and regeneration, Annu Rev Biomed Eng 5 (2003) 293–347. [DOI] [PubMed] [Google Scholar]
- [8].Kanwar JR, Sun X, Punj V, Sriramoju B, Mohan RR, Zhou SF, Chauhan A, Kanwar RK, Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal, Nanomedicine 8(4) (2012) 399–414. [DOI] [PubMed] [Google Scholar]
- [9].Gendelman HE, Anantharam V, Bronich T, Ghaisas S, Jin H, Kanthasamy A, Liu X, McMillan JM, Mosley L, Narasimhan B, Mallapragada SK, Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases, Nanomedicine: NBM 11(3) (2015) 751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Silva GA, Neuroscience nanotechnology: progress, opportunities and challenges, Nat Rev Neurosci 7(1)(2006) 65–74. [DOI] [PubMed] [Google Scholar]
- [11].Gendelman HE, Mosley RL, Boska MD, McMillan J, The promise of nanoneuromedicine, Nanomedicine 9(2) (2014) 171–176. [DOI] [PubMed] [Google Scholar]
- [12].Guo B, Sheng Z, Kenry K, Hu D, Lin X, Xu S, Liu C, Zheng H, Liu B, Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window, Mater. Horiz 4(6) (2017) 1151–1156. [Google Scholar]
- [13].Li W, Chen R, Lv J, Wang H, Liu Y, Peng Y, Qian Z, Fu G, Nie L, In Vivo Photoacoustic Imaging of Brain Injury and Rehabilitation by High-Efficient Near-Infrared Dye Labeled Mesenchymal Stem Cells with Enhanced Brain Barrier Permeability, Adv Sci (Weinh) 5(2) (2018) 1700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amiri H, Saeidi K, Borhani P, Manafirad A, Ghavami M, Zerbi V, Alzheimer's disease: pathophysiology and applications of magnetic nanoparticles as MRI theranostic agents, ACS Chem Neurosci 4(11) (2013) 1417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu P, Ceto S, Wang Y, Graham L, Wu D, Kumamaru H, Staufenberg E, Tuszynski MH, Prolonged human neural stem cell maturation supports recovery in injured rodent CNS, J Clin Invest 127(9) (2017) 3287–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shoemaker LD, Kornblum HI, Neural Stem Cells (NSCs) and Proteomics, Mol Cell Proteomics 15(2)(2016)344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao G, Song Y, Zhang S, Yang L, Xu S, Zhang Y, Xu H, Gao F, Li Z, Cai X, A high-sensitive nano-modified biosensor for dynamic monitoring of glutamate and neural spike covariation from rat cortex to hippocampal sub-regions, J Neurosci Methods 291 (2017) 122–130. [DOI] [PubMed] [Google Scholar]
- [18].Li SS, Lin CW, Wei KC, Huang CY, Hsu PH, Liu HL, Lu YJ, Lin SC, Yang HW, Ma CC, Non-invasive screening for early Alzheimer's disease diagnosis by a sensitively immunomagnetic biosensor, Sci Rep 6 (2016) 25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shen C, New EJ, What has fluorescent sensing told us about copper and brain malfunction?, Metallomics 7(1) (2015) 56–65. [DOI] [PubMed] [Google Scholar]
- [20].Soleymani J, Advanced materials for optical sensing and biosensing of neurotransmitters, Trends Anal Chem 72 (2015) 27–44. [Google Scholar]
- [21].Haris M, Nath K, Cai K, Singh A, Crescenzi R, Kogan F, Verma G, Reddy S, Hariharan H, Melhem ER, Reddy R, Imaging of glutamate neurotransmitter alterations in Alzheimer's disease, NMR Biomed 26(4) (2013) 386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chu Y, Kordower JH, Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease, Neurobiol Dis 25(1)(2007) 134–49. [DOI] [PubMed] [Google Scholar]
- [23].Goldstein DS, Holmes C, Lopez GJ, Wu T, Sharabi Y, Cerebrospinal fluid biomarkers of central dopamine deficiency predict Parkinson's disease, Parkinsonism Relat Disord 50 (2018) 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhong Y, Bellamkonda RV, Biomaterials for the central nervous system, J Royal Soc Interface 5(26) (2008) 957–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li GN, Hoffman-Kim D, Tissue-engineered platforms of axon guidance, Tissue Eng Part B Rev 14(1)(2008)33–51. [DOI] [PubMed] [Google Scholar]
- [26].Bahadur D, Giri J. Biomaterials and magnetism. Sadhana 28 (2003) 639–656. [Google Scholar]
- [27].Duke AR, Jenkins MW, Lu H, McManus JM, Chiel HJ, Jansen ED, Transient and selective suppression of neural activity with infrared light, Sci Rep 3 (2013) 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martino N, Feyen P, Porro M, Bossio C, Zucchetti E, Ghezzi D, Benfenati F, Lanzani G, Antognazza MR, Photothermal cellular stimulation in functional bio-polymer interfaces, Sci Rep 5 (2015) 8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guimard NK, Gomez N, Schmidt CE, Conducting polymers in biomedical engineering, Prog Polym Sci 32(8-9) (2007) 876–921. [Google Scholar]
- [30].Li X, Wei J, Aifantis KE, Fan Y, Feng Q, Cui FZ, Watari F, Current investigations into magnetic nanoparticles for biomedical applications, J Biomed Mater Res A 104(5) (2016) 1285–96. [DOI] [PubMed] [Google Scholar]
- [31].Li W, Luo R, Lin X, Jadhav AD, Zhang Z, Yan L, Chan CY, Chen X, He J, Chen CH, Shi P, Remote modulation of neural activities via near-infrared triggered release of biomolecules, Biomaterials 65 (2015) 76–85. [DOI] [PubMed] [Google Scholar]
- [32].Chiang CS, Shen YS, Liu JJ, Shyu WC, Chen SY, Synergistic Combination of Multistage Magnetic Guidance and Optimized Ligand Density in Targeting a Nanoplatform for Enhanced Cancer Therapy, Adv Healthc Mater 5(16) (2016) 2131–41. [DOI] [PubMed] [Google Scholar]
- [33].Zha Z, Deng Z, Li Y, Li C, Wang J, Wang S, Qu E, Dai Z, Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging, Nanoscale 5(10) (2013) 4462–7. [DOI] [PubMed] [Google Scholar]
- [34].Wadghiri YZ, Li J, Wang J, Hoang DM, Sun Y, Xu H, Tsui W, Li Y, Boutajangout A, Wang A, de Leon M, Wisniewski T, Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer's disease transgenic mice using magnetic resonance microimaging, PLoS One 8(2) (2013) e57097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Z, Wang Y, Wang Z, Zhao J, Gutkind JS, Srivatsan A, Zhang G, Liao HS, Fu X, Jin A, Tong X, Niu G, Chen X. Polymeric Nanovehicle Regulated Spatiotemporal Real-Time Imaging of the Differentiation Dynamics of Transplanted Neural Stem Cells after Traumatic Brain Injury, ACS nano 9(2015) 6683–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shan D, Kothapalli SR, Ravnic DJ, Gerhard E, Kim JP, Guo J, Ma C, Guo J, Gui L, Sun L, Lu D, Yang J, Development of Citrate-Based Dual-Imaging Enabled Biodegradable Electroactive Polymers, Adv Funct Mater 28(34) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Granot D, Nkansah MK, Bennewitz MF, Tang KS, Markakis EA, Shapiro EM, Clinically viable magnetic poly(lactide-co-glycolide) particles for MRI-based cell tracking, Magn Reson Med 71(3) (2014) 1238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fu R, Luo W, Nazempour R, Tan D, Ding H, Zhang K, Yin L, Guan J, Sheng X, Implantable and Biodegradable Poly(L-lactic acid) Fibers for Optical Neural Interfaces, Adv Opt Mater 6(3) (2018). [Google Scholar]
- [39].Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL, Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging, Proc Natl Acad Sci USA 108(46) (2011) 18837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pal RK, Farghaly AA, Wang C, Collinson MM, Kundu SC, Yadavalli VK, Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors, Biosens Bioelectron 81 (2016) 294–302. [DOI] [PubMed] [Google Scholar]
- [41].Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma PX, Conductive PPY/PDLLA conduit for peripheral nerve regeneration, Biomaterials 35(1) (2014) 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu Z, Zhu S, Liu L, Ge J, Huang L, Sun Z, Zeng W, Huang J, Luo Z, A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field, Int J Nanomedicine 12 (2017) 7815–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McGinn MJ, Povlishock JT, Pathophysiology of Traumatic Brain Injury, Neurosurg Clin N Am 27(4) (2016) 397–407. [DOI] [PubMed] [Google Scholar]
- [44].Borgens RB, Liu-Snyder P, Understanding second injury, Q Rev Biol 87(2) (2012) 89–127. [DOI] [PubMed] [Google Scholar]
- [45].Ghajar J, Traumatic brain injury, The Lancet 356(9233) (2000) 923–929. [DOI] [PubMed] [Google Scholar]
- [46].Chakraborty S, Skolnick B, Narayan RK, Neuroprotection Trials in Traumatic Brain Injury, Curr Neurol Neurosci Rep 16(4) (2016). [DOI] [PubMed] [Google Scholar]
- [47].Pardridge WM, The Blood-Brain Barrier: Bottleneck in Brain Drug Development, Neuro RX 2(1) (2005)3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McKinney PA, Brain tumours: incidence, survival, and aetiology, J Neurol Neurosurg Psychiatry 75(suppl 2) (2004) ii12–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS, CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012, Neuro Oncol 17 Suppl 4 (2015) iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thomas AA, Brennan CW, DeAngelis LM, Omuro AM, Emerging therapies for glioblastoma, JAMA Neurol 71(11) (2014) 1437–44. [DOI] [PubMed] [Google Scholar]
- [51].Alexander BM, Cloughesy TF, Aldult Glioblastoma, J Clin Oncol 35(21) (2017) 2402–2409. [DOI] [PubMed] [Google Scholar]
- [52].Vos T, Allen C, Arora M, Barber RM, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015, The Lancet 388(10053) (2016) 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang H, Naghavi M, Allen C, Barber RM, Bhuta Z, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015, The Lancet 388(10053) (2016) 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lane CA, Hardy J, Schott JM, Alzheimer's disease, Eur J Neurol 25(1) (2018) 59–70. [DOI] [PubMed] [Google Scholar]
- [55].Liu G, Men P, Harris PL, Rolston RK, Perry G, Smith MA, Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance, Neurosci Lett 406(3) (2006) 189–93. [DOI] [PubMed] [Google Scholar]
- [56].Moshé SL, Perucca E, Ryvlin P, Tomson T, Epilepsy: new advances, The Lancet 385(9971) (2015)884–898. [DOI] [PubMed] [Google Scholar]
- [57].Massimo M, Curia G, Biagini G, Ragsdale D, Avoli M, Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders, The Lacent Neurology 9(4) (2010) 413–424. [DOI] [PubMed] [Google Scholar]
- [58].Hankey GJ, Stroke, The Lancet 389(10069) (2017) 641–654. [DOI] [PubMed] [Google Scholar]
- [59].Tallantyre EC, Robertson NP, Acute stroke management, J Neurol 262(1) (2015) 239–42. [DOI] [PubMed] [Google Scholar]
- [60].Simats A, Garcia-Berrocoso T, Montaner J, Neuroinflammatory biomarkers: From stroke diagnosis and prognosis to therapy, Biochim biophys acta 1862(3) (2016) 411–24. [DOI] [PubMed] [Google Scholar]
- [61].Wang K, Chen Z, Huang J, Huang L, Luo N, Liang X, Liang M, Xie W, Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects, Clin Exp Pharmacol Physiol 44(8) (2017) 862–871. [DOI] [PubMed] [Google Scholar]
- [62].Zhang B, Yan W, Zhu Y, Yang W, Le W, Chen B, Zhu R, Cheng L, Nanomaterials in Neural-Stem-Cell-Mediated Regenerative Medicine: Imaging and Treatment of Neurological Diseases, Adv Mater 30(17) (2018) e1705694. [DOI] [PubMed] [Google Scholar]
- [63].Guo B, Glavas L, Albertsson AC, Biodegradable and electrically conducting polymers for biomedical applications, Prog Polym Sci 38(9) (2013) 1263–1286. [Google Scholar]
- [64].Adams CF, Rai A, Sneddon G, Yiu HH, Polyak B, Chari DM, Increasing magnetite contents of polymeric magnetic particles dramatically improves labeling of neural stem cell transplant populations, Nanomedicine 11 (2015) 19–29. [DOI] [PubMed] [Google Scholar]
- [65].Shan D, Hsieh JT, Bai X, Yang J, Citrate-Based Fluorescent Biomaterials, Adv Healthc Mater 7(18)(2018) 1800532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S, Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering, J Tissue Eng Regen Med 5(4) (2011) e17–35. [DOI] [PubMed] [Google Scholar]
- [67].Shi G, Zhang Z, Rouabhia M, The regulation of cell functions electrically using biodegradable polypyrrole-polylactide conductors, Biomaterials 29(28) (2008) 3792–8. [DOI] [PubMed] [Google Scholar]
- [68].Wood M, Willits RK, Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth, Bioelectromagnetics 27(4) (2006) 328–31. [DOI] [PubMed] [Google Scholar]
- [69].Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, Kitamura N, Kawano M, Tan-Takeuchi K, Ohtsuka C, Miyawaki A, Takashima A, Ogawa M, Toyama Y, Okano H, Kondo T, Electrical stimulation modulates fate determination of differentiating embryonic stem cells, Stem Cells 25(3) (2007) 562–70. [DOI] [PubMed] [Google Scholar]
- [70].Chan KM, Curran MW, Gordon T, The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice, J Physiol 594(13) (2016) 3553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sirivisoot S, Pareta RA, Webster TJ, A conductive nanostructured polymer electrodeposited on titanium as a controllable, local drug delivery platform, J Biomed Mater Res A 99A(4) (2011) 586–597. [DOI] [PubMed] [Google Scholar]
- [72].Moona JM, Thapliyal N, Hussain KK, Goyal RN, Shim YB, Conducting polymer-based electrochemical biosensors for neurotransmitters: A review, Biosens Bioelectron 102 (2018) 540–552. [DOI] [PubMed] [Google Scholar]
- [73].Manivasagan P, Bui NQ, Bharathiraja S, Moorthy MS, Oh YO, Song K, Seo H, Yoon M, Oh J, Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer, Sci Rep 7 (2017) 43593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Guarino V, Alvarez-Perez MA, Borriello A, Napolitano T, Ambrosio L, Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv Healthc Mater, 2(1) (2013) 218–227. [DOI] [PubMed] [Google Scholar]
- [75].Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, Lu L, Windebank AJ, Yaszemski MJ, The development of electrically conductive polycaprolactone fumarate– polypyrrole composite materials for nerve regeneration, Biomaterials 31(23) (2010) 5916–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Alves-Sampaio A, Garcia-Rama C, Collazos-Castro JE, Biofunctionalized PEDOT-coated microfibers for the treatment of spinal cord injury, Biomaterials 89 (2016) 98–113. [DOI] [PubMed] [Google Scholar]
- [77].Wu Y, Wang L, Guo B, Shao Y, Ma PX, Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering, Biomaterials 87 (2016) 18–31. [DOI] [PubMed] [Google Scholar]
- [78].Klumpp S, Faivre D. Magnetotactic bacteria-Magnetic navigation on the microscale, Eur Phys J Spec Top 225(2016) 2173–2188. [Google Scholar]
- [79].Pauling L, Coryell CD. The magnetic properties and structures of hemoglobin, oxyhemoglobin and carbonmonox yhemoglobin, Chemistry 22(1936) 210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Majidi S, Sehrig FZ, Farkhani SM, Goloujeh MS, Akbarzadeh A, Current methods for synthesis of magnetic nanoparticles, Artif Cells Nanomed Biotechnol 44(2) (2016) 722–34. [DOI] [PubMed] [Google Scholar]
- [81].Gil S, Mano JF, Magnetic composite biomaterials for tissue engineering, Biomater Sci 2(6) (2014) 812–818. [DOI] [PubMed] [Google Scholar]
- [82].Cardoso VF, Francesko A, Ribeiro C, Banobre-Lopez M, Martins P, Lanceros-Mendez S, Advances in Magnetic Nanoparticles for Biomedical Applications, Adv Healthc Mater 7(5) (2018). [DOI] [PubMed] [Google Scholar]
- [83].Zeinali Sehrig F, Majidi S, Nikzamir N, Nikzamir N, Nikzamir M, Akbarzadeh A, Magnetic nanoparticles as potential candidates for biomedical and biological applications, Artif Cells Nanomed Biotechnol 44(3) (2016) 918–27. [DOI] [PubMed] [Google Scholar]
- [84].Canfarotta F, Piletsky SA, Engineered magnetic nanoparticles for biomedical applications, Adv Healthc Mater 3(2) (2014) 160–75. [DOI] [PubMed] [Google Scholar]
- [85].Calero M, Gutierrez L, Salas G, Luengo Y, Lazaro A, Acedo P, Morales MP, Miranda R, Villanueva A, Efficient and safe internalization of magnetic iron oxide nanoparticles: two fundamental requirements for biomedical applications, Nanomedicine 10(4) (2014) 733–43. [DOI] [PubMed] [Google Scholar]
- [86].Ruiz A, Morais PC, Bentes de Azevedo R, Lacava ZGM, Villanueva A, del Puerto Morales M, Magnetic nanoparticles coated with dimercaptosuccinic acid: development, characterization, and application in biomedicine, J Nanopart Res 16(11) (2014). [Google Scholar]
- [87].Ruiz A, Hernandez Y, Cabal C, Gonzalez E, Veintemillas-Verdaguer S, Martinez E, Morales MP, Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol, Nanoscale 5(23) (2013) 11400–8. [DOI] [PubMed] [Google Scholar]
- [88].Sun Q, Cheng D, Yu X, Zhang Z, Dai J, Li H, Liang B, Shuai X, A pH-sensitive polymeric nanovesicle based on biodegradable poly(ethylene glycol)-b-poly(2-(diisopropylamino)ethyl aspartate) as a MRI-visible drug delivery system, J Mater Chem 21(39) (2011). [Google Scholar]
- [89].Kim DH, Vitol EA, Liu J, Balasubramanian S, Gosztola DJ, Cohen EE, Novosad V, Rozhkova EA, Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles, Langmuir 29(24) (2013) 7425–32. [DOI] [PubMed] [Google Scholar]
- [90].Wei Y, Zhang X, Song Y, Han B, Hu X, Wang X, Lin Y, Deng X, Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration, Biomed Mater 6(5) (2011) 055008. [DOI] [PubMed] [Google Scholar]
- [91].Cheng D, Hong G, Wang W, Yuan R, Ai H, Shen J, Liang B, Gao J, Shuai X, Nonclustered magnetite nanoparticle encapsulated biodegradable polymeric micelles with enhanced properties for in vivo tumor imaging, J Mater Chem 21(13) (2011). [Google Scholar]
- [92].Ye F, Barrefelt A, Asem H, Abedi-Valugerdi M, El-Serafi I, Saghafian M, Abu-Salah K, Alrokayan S, Muhammed M, Hassan M, Biodegradable polymeric vesicles containing magnetic nanoparticles, quantum dots and anticancer drugs for drug delivery and imaging, Biomaterials 35(12) (2014)3885–94. [DOI] [PubMed] [Google Scholar]
- [93].Ryu JH, Koo H, Sun IC, Yuk SH, Choi K, Kim K, Kwon IC, Tumor-targeting multifunctional nanoparticles for theragnosis: new paradigm for cancer therapy, Adv Drug Deliv Rev 64(13) (2012) 1447–58. [DOI] [PubMed] [Google Scholar]
- [94].Cayce JM, Friedman RM, Jansen ED, Mahavaden-Jansen A, Roe AW, Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo, Neuroimage 57(1) (2011) 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A, Pulsed laser versus electrical energy for peripheral nerve stimulation, J Neurosci Methods 163(2) (2007) 326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR, Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells, Sci Rep 7(1) (2017) 7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kang H, Zhang K, Wong DSH, Han F, Li B, Bian L, Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization, Biomaterials 178 (2018) 681–696. [DOI] [PubMed] [Google Scholar]
- [98].Farah N, Zoubi A, Matar S, Golan L, Marom A, Butson CR, Brosh I, Shoham S, Holographically patterned activation using photo-absorber induced neural-thermal stimulation, J Neural Eng 10(5)(2013) 056004. [DOI] [PubMed] [Google Scholar]
- [99].Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A, Jansen ED, Biophysical mechanisms of transient optical stimulation of peripheral nerve, Biophys J 93(7) (2007) 2567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F, Infrared light excites cells by changing their electrical capacitance, Nat Commun 3 (2012) 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Repina NA, Rosenbloom A, Mukherjee A, Schaffer DV, Kane RS, At Light Speed: Advances in Optogenetic Systems for Regulating Cell Signaling and Behavior, Annu Rev Chem Biomol Eng 8 (2017) 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Young AT, Cornwell N, Daniele MA, Neuro-Nano Interfaces: Utilizing Nano-Coatings and Nanoparticles to Enable Next-Generation Electrophysiological Recording, Neural Stimulation, and Biochemical Modulation, Adv Funct Mater 28(12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K, Optogenetics in neural systems, Neuron 71(1) (2011) 9–34. [DOI] [PubMed] [Google Scholar]
- [104].Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K, Semiconducting Polymer Nanobioconjugates for Targeted Photothermal Activation of Neurons, J Am Chem Soc 138(29) (2016) 9049–52. [DOI] [PubMed] [Google Scholar]
- [105].Feyen P, Colombo E, Endeman D, Nova M, Laudato L, Martino N, Antognazza MR, Lanzani G, Benfenati F, Ghezzi D, Light-evoked hyperpolarization and silencing of neurons by conjugated polymers, Sci Rep 6 (2016) 22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chu M, Shao Y, Peng J, Dai X, Li H, Wu Q, Shi D, Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles, Biomaterials 34(16) (2013) 4078–88. [DOI] [PubMed] [Google Scholar]
- [107].Chen H, Burnett J, Zhang F, Zhang J, Paholak H, Sun D, Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy, J Mater Chem B 2(7) (2014) 757–765. [DOI] [PubMed] [Google Scholar]
- [108].Janib SM, Moses AS, MacKay JA, Imaging and drug delivery using theranostic nanoparticles, Adv Drug Deliv Rev 62(11) (2010) 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yuan L, Lin W, Zheng K, He L, Huang W, Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging, Chem Soc Rev 42(2) (2013) 622–61. [DOI] [PubMed] [Google Scholar]
- [110].Yu J, Wu C, Zhang X, Ye F, Gallina ME, Rong Y, Wu IC, Sun W, Chan YH, Chiu DT, Stable functionalization of small semiconducting polymer dots via covalent cross-linking and their application for specific cellular imaging, Adv Mater 24(26) (2012) 3498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Song F, Chan WC, Principles of conjugating quantum dots to proteins via carbodiimide chemistry, Nanotechnology 22(49) (2011) 494006. [DOI] [PubMed] [Google Scholar]
- [112].Yang J, Webb AR, Ameer GA, Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering, Adv Mater 16(6) (2004) 511–516. [Google Scholar]
- [113].Guo J, Xie Z, Tran RT, Xie D, Jin D, Bai X, Yang J, Click chemistry plays a dual role in biodegradable polymer design, Adv Mater 26(12) (2014) 1906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tran RT, Yang J, Ameer GA, Citrate-Based Biomaterials and Their Applications in Regenerative Engineering, Annu Rev Mater Res 45 (2015) 277–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Guo J, Kim GB, Shan D, Kim JP, Hu J, Wang W, Hamad FG, Qian G, Rizk EB, Yang J, Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives, Biomaterials 112 (2017) 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ma C, Gerhard E, Lin Q, Xia S, Armstrong AD, Yang J, In vitro cytocompatibility evaluation of poly(octamethylene citrate) monomers toward their use in orthopedic regenerative engineering, Bioact Mater 3(1) (2018) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gyawali D, Kim JP, Yang J, Highly photostable nanogels for fluorescence-based theranostics, Bioact Mater 3(1) (2018) 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gyawali D, Zhou S, Tran RT, Zhang Y, Liu C, Bai X, Yang J, Fluorescence imaging enabled biodegradable photostable polymeric micelles, Adv Healthc Mater 3(2) (2014) 182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yang J, Zhang Y, Gutam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L, Development of aliphatic biodegradable photoluminescent polymers, Proc Natl Acad Sci USA 106(25) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wadajkar AS, Kadapure T, Zhang Y, Cui W, Nguyen KT, Yang J, Dual-imaging enabled cancer-targeting nanoparticles, Adv Healthc Mater 1(4) (2012) 450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang Y, Yang J, Design Strategies for Fluorescent Biodegradable Polymeric Biomaterials, J Mater Chem B 1(2) (2013) 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Xie Z, Zhang Y, Liu L, Weng H, Mason RP, Tang L, Nguyen KT, Hsieh JT, Yang J, Development of intrinsically photoluminescent and photostable polylactones, Adv Mater 26(26) (2014) 4491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hu J, Guo J, Xie Z, Shan D, Gerhard E, Qian G, Yang J, Fluorescence imaging enabled poly(lactide-co-glycolide), Acta Biomater 29 (2016) 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kim JP, Xie Z, Creer M, Liu Z, Yang J, Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of Cystic Fibrosis, Chem Sci 8(1) (2017) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhang Y, Tran RT, Qattan IS, Tsai YT, Tang L, Liu C, Yang J, Fluorescence imaging enabled urethane-doped citrate-based biodegradable elastomers, Biomaterials 34(16) (2013) 4048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Xie Z, Su Y, Kim GB, Selvi E, Ma C, Aragon-Sanabria V, Hsieh JT, Dong C, Yang J, Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery, Small 13(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li J, Tian Y, Shan D, Gong A, Zeng L, Ren W, Xiang L, Gerhard E, Zhao J, Yang J, Wu A, Neuropeptide Y Y1 receptor-mediated biodegradable photoluminescent nanobubbles as ultrasound contrast agents for targeted breast cancer imaging, Biomaterials 116 (2017) 106–117. [DOI] [PubMed] [Google Scholar]
- [128].Chu L, Wang S, Li K, Xi W, Zhao X, Qian J, Biocompatible near-infrared fluorescent nanoparticles for macro and microscopic in vivo functional bioimaging, Biomed Opt Express 5(11) (2014)4076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tong L, Gattass RR, Ashcom JB, He S, Lou J, Shen M, Maxwell I, Mazur E. Subwavelength-diameter silica wires for low-loss optical wave guiding, Nature 426(2003) 816–819 [DOI] [PubMed] [Google Scholar]
- [130].Xin H, Li Y, Liu X, Li B, Escherichia coli-based biophotonic waveguides, Nano Lett 13(7) (2013) 3408–13. [DOI] [PubMed] [Google Scholar]
- [131].Xin H, Li Y, Li B, Bacteria-based branched structures for bionanophotonics, Laser Photonics Rev 9(5)(2015)554–563. [Google Scholar]
- [132].Tao H, Kaplan DL, Omenetto FG, Silk materials--a road to sustainable high technology, Adv Mater 24(21) (2012) 2824–37. [DOI] [PubMed] [Google Scholar]
- [133].Jung W, Paulson B, Choi K, Son JY, Nazari T, Park SH, Kim JH, Oh K, Fabrication and characteristics of thin-film waveguides based on DNA biomaterials, Optical Processes in Organic Materials and Nanostructures II, 2013. [Google Scholar]
- [134].Mironenko AY, Sergeev AA, Nazirov AE, Modin EB, Voznesenskiy SS, Bratskaya SY, H2S optical waveguide gas sensors based on chitosan/Au and chitosan/Ag nanocomposites, Sens Actuators B Chem 225 (2016) 348–353. [Google Scholar]
- [135].Nizamoglu S, Gather MC, Humar M, Choi M, Kim S, Kim KS, Hahn SK, Scarcelli G, Randolph M, Redmond RW, Yun SH, Bioabsorbable polymer optical waveguides for deep-tissue photomedicine, Nat Commun 7 (2016) 10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Shan D, Zhang C, Kalaba S, Mehta N, Kim GB, Liu Z, Yang J, Flexible biodegradable citrate-based polymeric step-index optical fiber, Biomaterials 143 (2017) 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shan D, Gerhard E, Zhang C, Tierney JW, Xie D, Liu Z, Yang J, Polymeric biomaterials for biophotonic applications, Bioact Mater 3(4) (2018) 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kumar N, Kumar R, Nanomedicine for Neurological Disorder, Nanotechnology and Nanomaterials in the Treatment of Life-threatening Diseases 2014, pp. 109–175. [Google Scholar]
- [139].Beard P, Biomedical photoacoustic imaging, Interface Focus 1(4) (2011) 602–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Knox HJ, Hedhli J, Kim TW, Khalili K, Dobrucki LW, Chan J, A bioreducible N-oxide-based probe for photoacoustic imaging of hypoxia, Nat Commun 8(1) (2017) 1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang D, Wu Y, Xia J, Review on photoacoustic imaging of the brain using nanoprobes, Neurophotonics 3(1) (2016) 010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wang S, Lin J, Wang T, Chen X, Huang P, Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications, Theranostics 6(13) (2016) 2394–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Xu H, Li Q, Wang L, He Y, Shi J, Tang B, Fan C, Nanoscale optical probes for cellular imaging, Chem Soc Rev 43(8) (2014) 2650–61. [DOI] [PubMed] [Google Scholar]
- [144].Wang X, Ku G. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent, Opt Lett 29(2004) 730–732. [DOI] [PubMed] [Google Scholar]
- [145].Filonov GS, Krumholz A, Xia J, Yao J, Wang LV, Verkhusha VV, Deep-tissue photoacoustic tomography of a genetically encoded near-infrared fluorescent probe, Angew Chem Int Ed Engl 51(6) (2012) 1448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Liu J, Cai X, Pan HC, Bandla A, Chuan CK, Wang S, Thakor N, Liao LD, Liu B, Molecular Engineering of Photoacoustic Performance by Chalcogenide Variation in Conjugated Polymer Nanoparticles for Brain Vascular Imaging, Small 14(13) (2018) e1703732. [DOI] [PubMed] [Google Scholar]
- [147].Szpak A, Fiejdasz S, Prendota W, Straczek T, Kapusta C, Szmyd J, Nowakowska M, Zapotoczny S, T-T Dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes, J Nanopart Res l6(11) (2014) 2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Figuerola A, Di Corato R, Manna L, Pellegrino T, From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications, Pharmacological research : the official journal of the Italian Pharmacological Society 62(2) (2010) 126–43. [DOI] [PubMed] [Google Scholar]
- [149].Choi JS, Lee JH, Shin TH, Song HT, Kim EY, Cheon J, Self-Confirming “AND” Logic Nanoparticles for Fault-Free MRI, J Am Chem Soc 132 (2010) 11015–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Corato RD, Gazeau F, Visage CL, Fayol D, Levitz P, Lux F, Letourneur D, Luciani N, Tillement O, Wilhelm C, High-Resolution Cellular MRI: Gadolinium and Iron Oxide Nanoparticles for in-Depth Dual-Cell Imaging of Engineered Tissue Constructs, ACS nano 7(9) (2013) 7500–7512. [DOI] [PubMed] [Google Scholar]
- [151].De M, Chou SS, Joshi HM, Dravid VP, Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications, Adv Drug Deliv Rev 63(14-15) (2011) 1282–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Mertens ME, Hermann A, Bühren A, Olde-Damink L, Möckel D, Gremse F, Ehling J, Kiessling F, Lammers T, Iron Oxide-Labeled Collagen Scaffolds for Non-Invasive MR Imaging in Tissue Engineering, Adv Funct Mater 24 (2014) 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lin C, Cai S, Feng J, Positive Contrast Imaging of SPIO Nanoparticles, J Nanomater 2012 (2012) 1–9. [Google Scholar]
- [154].Liu Y, Chen Z, Liu C, Yu D, Lu Z, Zhang N, Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer, Biomaterials 32(22) (2011) 5167–76. [DOI] [PubMed] [Google Scholar]
- [155].Onuki Y, Jacobs I, Artemov D, Kato Y, Noninvasive visualization of in vivo release and intratumoral distribution of surrogate MR contrast agent using the dual MR contrast technique, Biomaterials 31(27) (2010) 7132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Stroh A, Boltze J, Sieland K, Hild K, Gutzeit C, Jung T, Kressel J, Hau S, Reich D, Grune T, Zimmer C, Impact of Magnetic Labeling on Human and Mouse Stem Cells and Their Long-Term Magnetic Resonance Tracking in a Rat Model of Parkinson Disease, Mol Imaging 8(3) (2009). [PubMed] [Google Scholar]
- [157].Pietracupa S, Martin-Bastida A, Piccini P, Iron metabolism and its detection through MRI in parkinsonian disorders: a systematic review, Neurol Sci 38(12) (2017) 2095–2101. [DOI] [PubMed] [Google Scholar]
- [158].Bandmann O, Weiss KH, Kaler SG, Wilson's disease and other neurological copper disorders, The Lancet Neurol 14(1) (2015) 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA, Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review, J Cereb Blood Flow Metab 30(1) (2010) 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS, Multifunctional nanoparticles for brain tumor imaging and therapy, Adv Drug Deliv Rev 66 (2014) 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Abe K, Fujita M, Over 10 years MRI observation of a patient with neuronal intranuclear inclusion disease, BMJ Case Rep 2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Norman AB, Thomas SR, Pratt RG, Lu SY, Norgren RB. Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent, Brain Res 594(1992) 279–283. [DOI] [PubMed] [Google Scholar]
- [163].Appel AA, Anastasio MA, Larson JC, Brey EM, Imaging challenges in biomaterials and tissue engineering, Biomaterials 34(28) (2013) 6615–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zheng C, Zheng M, Gong P, Jia D, Zhang P, Shi B, Sheng Z, Ma Y, Cai L, Indocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imaging, Biomaterials 33(22) (2012) 5603–9. [DOI] [PubMed] [Google Scholar]
- [165].Guo Z, Park S, Yoon J, Shin I, Recent progress in the development of near-infrared fluorescent probes for bioimaging applications, Chem Soc Rev 43(1) (2014) 16–29. [DOI] [PubMed] [Google Scholar]
- [166].Hilderbrand SA, Weissleder R, Near-infrared fluorescence: application to in vivo molecular imaging, Curr Opin Chem Biol 14(1) (2010) 71–9. [DOI] [PubMed] [Google Scholar]
- [167].Ntziachristos V, Ripoll J, Wang LV, Weissleder R, Looking and listening to light: the evolution of whole-body photonic imaging, Nat Biotechnol 23(3) (2005) 313–20. [DOI] [PubMed] [Google Scholar]
- [168].Adams C, Israel LL, Ostrovsky S, Taylor A, Poptani H, Lellouche JP, Chari D, Development of Multifunctional Magnetic Nanoparticles for Genetic Engineering and Tracking of Neural Stem Cells, Adv Healthc Mater 5(7) (2016) 841–9. [DOI] [PubMed] [Google Scholar]
- [169].Jiang L, Zhou Q, Mu K, Xie H, Zhu Y, Zhu W, Zhao Y, Xu H, Yang X, pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats, Biomaterials 34(30) (2013) 7418–28. [DOI] [PubMed] [Google Scholar]
- [170].Qiao Y, Gumin J, MacLellan CJ, Gao F, Bouchard R, Lang FF, Stafford RJ, Melancon MP, Magnetic resonance and photoacoustic imaging of brain tumor mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injection, Nanotechnology 29(16) (2018) 165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Jones BE, From waking to sleeping: neuronal and chemical substrates, Trends Pharmacol Sci 26(11)(2005)578–86. [DOI] [PubMed] [Google Scholar]
- [172].Deiana S, Platt B, Riedel G, The cholinergic system and spatial learning, Behav Brain Res 221(2) (2011) 389–411. [DOI] [PubMed] [Google Scholar]
- [173].Picciotto MR, Higley MJ, Mineur YS, Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior, Neuron 76(1) (2012) 116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Berson A, Barbash S, Shaltiel G, Goll Y, Hanin G, Greenberg DS, Ketzef M, Becker AJ, Friedman A, Soreq H, Cholinergic-associated loss of hnRNP-A/B in Alzheimer's disease impairs cortical splicing and cognitive function in mice, EMBO Mol Med 4(8) (2012) 730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Schliebs R, Basal forebrain cholinergic dysfunction in Alzheimer's disease--interrelationship with beta-amyloid, inflammation and neurotrophin signaling, Neurochem Res 30(6-7) (2005) 895–908. [DOI] [PubMed] [Google Scholar]
- [176].Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW, Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex, PLoS Biol 4(11) (2006) e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yang JL, Sykora P, Wilson III DM, Mattson MP, Bohr VA, The excitatory neurotransmitter glutamate stimulates DNA repair to increase neuronal resiliency, Mech Ageing Dev 132(8-9) (2011)405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Justice JB, Quantitative microdialysis of neurotransmitters, J Neurosci Methods 48(1993) 263–276. [DOI] [PubMed] [Google Scholar]
- [179].Garden GA, Campbell BM, Glial biomarkers in human central nervous system disease, Glia 64(10) (2016) 1755–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/β-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults, Arch Neurol. 64(2007) 343–349. [DOI] [PubMed] [Google Scholar]
- [181].Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers, Lancet Neurol 12(2013) 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Qian CG, Zhu S, Feng PJ, Chen YL, Yu JC, Tang X, Liu Y, Shen QD, Conjugated Polymer Nanoparticles for Fluorescence Imaging and Sensing of Neurotransmitter Dopamine in Living Cells and the Brains of Zebrafish Larvae, ACS Appl Mater Interfaces 7(33) (2015) 18581–9. [DOI] [PubMed] [Google Scholar]
- [183].Kergoat L, Piro B, Simon DT, Pham MC, Noel V, Berggren M, Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites, Adv Mater 26(32) (2014) 5658–64. [DOI] [PubMed] [Google Scholar]
- [184].Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS, Regenerative therapies for central nervous system diseases: a biomaterials approach, Neuropsychopharmacology 39(1) (2014) 169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Haggerty AE, Oudega M, Biomaterials for spinal cord repair, Neurosci Bull 29(4) (2013) 445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Schulz R, Gerloff C, Hummel FC, Non-invasive brain stimulation in neurological diseases, Neuropharmacology 64 (2013) 579–87. [DOI] [PubMed] [Google Scholar]
- [187].Mogul DJ, van Drongelen W, Electrical control of epilepsy, Annu Rev Biomed Eng 16 (2014) 483–504. [DOI] [PubMed] [Google Scholar]
- [188].Li S, Zaninotto AL, Neville IS, Paiva WS, Nunn D, Fregni F, Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence, Neuropsychiatr Dis Treat 11 (2015) 1573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Okun MS, Deep-brain stimulation--entering the era of human neural-network modulation, N Engl J Med 371(15) (2014) 1369–1373. [DOI] [PubMed] [Google Scholar]
- [190].Haastert-Talini K, Grothe C, Electrical stimulation for promoting peripheral nerve regeneration, Int Rev Neurobiol 109 (2013) 111–24. [DOI] [PubMed] [Google Scholar]
- [191].Nguyen HT, Sapp S, Wei C, Chow JK, Nguyen A, Coursen J, Luebben S, Chang E, Ross R, Schmidt CE, Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth, J Biomed Mater Res A 102(8) (2014) 2554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Lee JM, Moon JY, Kim TH, Lee SW, Ahrberg CD, Chung BG, Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation, Sens Actuators B Chem 258 (2018) 1042–1050. [Google Scholar]
- [193].Kotwal A, Schimidt CE, Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials, Biomaterials 22(10) (2001) 1055–1064. [DOI] [PubMed] [Google Scholar]
- [194].Zarrintaj P, Bakhshandeh B, Rezaeian I, Heshmatian B, Ganjali MR, A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering, Sci Rep 7(1) (2017) 17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Tandon B, Magaz A, Balint R, Blaker JJ, Cartmell SH, Electroactive biomaterials: Vehicles for controlled delivery of therapeutic agents for drug delivery and tissue regeneration, Adv Drug Deliv Rev 129 (2018) 148–168. [DOI] [PubMed] [Google Scholar]
- [196].Hardy JG, Mouser DJ, Arroyo-Currás N, Geissler S, Chow JK, Nguy L, Kim JM, Schmidt CE, Biodegradable electroactive polymers for electrochemically-triggered drug delivery, J Mater Chem B 2(39) (2014) 6809–6822. [DOI] [PubMed] [Google Scholar]
- [197].Gupta AK, Gupta M, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials 26(18) (2005) 3995–4021. [DOI] [PubMed] [Google Scholar]
- [198].Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A, Remote control of ion channels and neurons through magnetic-field heating of nanoparticles, Nat Nanotechnol 5(8) (2010) 602–6. [DOI] [PubMed] [Google Scholar]
- [199].Munshi R, Qadri SM, Zhang Q, Rubio IC, del Pino P, Pralle A, Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LF, Sibov TT, Amaro E Jr., Tannus A, Vidoto EL, Martins MJ, Santos RS, Gamarra LF, Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment, Int J Nanomedicine 6 (2011) 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yoo J, Lee E, Kim HY, Youn DH, Jung J, Kim H, Chang Y, Lee W, Shin J, Baek S, Jang W, Jun W, Kim S, Hong J, Park HJ, Lengner CJ, Moh SH, Kwon Y, Kim J, Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson's disease therapy, Nat Nanotechnol 12(10) (2017) 1006–1014. [DOI] [PubMed] [Google Scholar]
- [202].Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW, Park SW, Shin JS, Cheon J, A magnetic switch for the control of cell death signalling in in vitro and in vivo systems, Nat Mater 11(12) (2012) 1038–43. [DOI] [PubMed] [Google Scholar]
- [203].Rahimi M, Wadajkar A, Subramanian K, Yousef M, Cui W, Hsieh JT, Nguyen KT, In vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled drug delivery, Nanomedicine 6(5) (2010) 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Park H, Yang J, Seo S, Kim K, Suh J, Kim D, Haam S, Yoo KH, Multifunctional nanoparticles for photothermally controlled drug delivery and magnetic resonance imaging enhancement, Small 4(2) (2008) 192–6. [DOI] [PubMed] [Google Scholar]
- [205].Zhang G, Gao J, Qian J, Cai D, Zheng K, Yu Z, Wang J, Zhong K, Zhang X, Wu Z, A Multifunctional Magnetic Composite Material as a Drug Delivery System and a Magnetic Resonance Contrast Agent, Part Part Syst Char 31(9) (2014) 976–984. [Google Scholar]
- [206].Shah B, Yin PT, Ghoshal S, Lee KB, Multimodal magnetic core-shell nanoparticles for effective stem-cell differentiation and imaging, Angew Chem Int Ed Engl 52(24) (2013) 6190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Yoo S, Hong S, Choi Y, Park J, Nam Y, Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers, ACS nano 8(8) (2014) 8040–8049. [DOI] [PubMed] [Google Scholar]
- [208].Kuo JR, Lin SS, Liu J, Chen SH, Chio CC, Wang JJ, Liu JM, Deep brain light stimulation effects on glutamate and dopamine concentration, Biomed Opt Express 6(1) (2015) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Mishchenko MA, Gerasimova SA, Lebedeva AV, Lepekhina LS, Pisarchik AN, Kazantsev VB, Optoelectronic system for brain neuronal network stimulation, PloS one 13(6) (2018) e0198396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Fu R, Luo W, Nazempour R, Tan D, Ding H, Zhang K, Yin L, Guan J, Sheng X, Implantable and Biodegradable Poly(l-lactic acid) Fibers for Optical Neural Interfaces, Adv Opt Mater 6(3) (2018) 1700941. [Google Scholar]
- [211].Bisoyi HK, Urbas AM, Li Q, Soft Materials Driven by Photothermal Effect and Their Applications, Adv Opt Mater 6(15) (2018) 1800458. [Google Scholar]
- [212].Ma C, Gerhard E, Lu D, Yang J, Citrate chemistry and biology for biomaterials design, Biomaterials 178 (2018) 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kim GB, Chen Y, Kang W, Guo J, Payne R, Li H, Wei Q, Baker J, Dong C, Zhang S, Wong PK, Rizk EB, Yan J, Yang J, The critical chemical and mechanical regulation of folic acid on neural engineering, Biomaterials 178 (2018) 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Yamanaka R, Tabata S, Shindo Y, Hotta K, Suzuki K, Soga T, Oka K, Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress, Sci Rep 6 (2016) 30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Westergaard N, Waagepetersen HS, Belhage B, Schousboe A, Citrate, a ubiquitous key metabolite with regulatory function in the CNS, Neurochem Res (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [216].Abdel-Salam OM, Youness ER, Mohammed NA, Yassen NN, Khadrawy YA, El-Toukhy SE, Sleem AA, Novel neuroprotective and hepatoprotective effects of citric acid in acute malathion intoxication, Asian Pac J Trop Med 9(12) (2016) 1181–1194. [DOI] [PubMed] [Google Scholar]
- [217].Abdel-Salam OM, Youness ER, Mohammed NA, Morsy SM, Omara EA, Sleem AA, Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice, J Med Food 17(5) (2014)588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].van Lith R, Gregory EK, Yang J, Kibbe MR, Ameer GA, Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues, Biomaterials 35(28) (2014) 8113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]