Abstract
Background:
Interstitial fibrosis and tubular atrophy (IFTA) and vascular injury are frequent histologic features of lupus nephritis renal biopsies, but their clinical correlates and prognostic value are not well understood. This cohort study investigated demographic, clinical and laboratory characteristics, and outcomes, associated with IFTA and vascular injury in lupus nephritis.
Methods:
Reports of all renal biopsies performed at an academic medical center (1990-2017) with WHO/ISN/RPS Class II-V lupus nephritis were reviewed. Demographics, clinical variables and labs at biopsy, treatment, and date of death were collected. Additional data from the U.S. Renal Data System (USRDS) provided dates of ESRD and death after ESRD. Multivariable regression analyses identified demographic and clinical factors associated with each histologic finding. Cumulative incidence functions and multivariable Cox proportional hazard models estimated the risk of progression to ESRD and death.
Results:
Within 202 initial biopsies, IFTA was associated with the patient’s SLICC/ACR damage index (without renal domain) and serum creatinine, and vascular injury was associated with serum creatinine in multivariable models. In Cox regression models adjusting for age, sex, race, serum creatinine, calendar year, and biopsy class, moderate/severe IFTA was associated with elevated ESRD (HRSD 5.18, 95% CI 2.53, 10.59) and death (HR 4.19, 95%CI 1.27, 13.81). After adjustment for age, sex and race, moderate/severe vascular injury was associated with ESRD (HRSD 2.13, 95% CI 1.21, 3.75) and but this relationship was not significant after adjustment for serum creatinine and calendar year.
Conclusions:
IFTA is a strong predictor of ESRD and death, even in proliferative nephritis, and a risk factor for poor outcomes independent of class. Vascular injury is a strong predictor of prognosis, but not independent of serum creatinine and class. The prognostic value of these lesions calls for consideration when determining treatment for lupus nephritis.
Keywords: lupus nephritis, histology, biopsy, pathology, interstitial fibrosis, tubular atrophy, vascular injury, hypertension, organ damage
1.1. Introduction
Lupus nephritis is one of the most common manifestations of systemic lupus erythematosus (SLE), affecting approximately 40% of SLE patients1. It is also one of the more severe manifestations, associated with high morbidity and mortality2. Renal biopsy is critical in the diagnosis and management of lupus nephritis, as it defines the nature of renal involvement. In the World Health Organization/International Society of Nephrology/Renal Pathology Society (WHO/ISN/RPS) classifications, based on glomerular pathology, classes III and IV describe proliferative lupus glomerulonephritis which has been associated with a worse prognosis4–8. Treatment guidelines recommend immunosuppressive therapy for these patients3,8,9. In past studies, predictors of poor response to therapy and progression to end-stage renal disease (ESRD) in lupus nephritis have also implicated clinical factors, including elevated double-stranded DNA (dsDNA) and low complement levels despite treatment, hypertension, elevated serum creatinine, proteinuria and medication non-adherence1,10–13.
Although glomerulonephritis is the most common cause of kidney disease in SLE2, emerging evidence also implicates extraglomerular lesions indicative of chronic disease, interstitial fibrosis and tubular atrophy (IFTA)4,5 and vascular injury6,7, in the progression of lupus nephritis. Interstitial fibrosis, defined simply as the accumulation of matrix proteins in the renal interstitum14, and tubular atrophy are often observed together and thus are reported as one lesion. Vascular injury is defined as arterial or arteriolar thickening of the intima with or without necrosis or proliferation8,15. Both IFTA and vascular injury are patterns of injury, but their clinical associations in lupus nephritis have not been well described. As these lesions are not included in the WHO/ISN/RPS classification, it is less clear whether they hold prognostic information beyond that of the class. Yu et al reported that within a group of 313 patients from northern China, scores of tubular atrophy and interstitial fibrosis, respectively, were positively correlated with scores of glomerular sclerosis, but were also confirmed to be independent risk factors for renal outcome16. More recently, moderate to severe tubulointerstitial damage on lupus nephritis biopsy was associated with risk of ESRD in a cohort in New York17. Huang et al studied 79 lupus nephritis renal biopsies and reported worse prognosis among those with vascular injury compared to those without18, and Wu et al also demonstrated the importance of vascular lesions in prognosis19.
The goals of this study were to characterize the demographic and clinical factors associated with IFTA and vascular injury on lupus nephritis biopsy, and to investigate whether these lesions are associated with the progression of lupus nephritis to end-stage renal disease (ESRD) and death. We also aimed to investigate whether these lesions are predictors of ESRD and death among people with proliferative lupus glomerulonephritis, and if either is an independent risk factor for ESRD and death after adjustment for class. Understanding the associations between clinical presentations and these renal biopsy findings may improve treatment decisions for these patients and provide insight into the etiology of these lesions.
1.2. Materials and Methods
1.2.1. Study Population
Biopsies were identified using the Brigham and Women’s Hospital (BWH) Lupus Center Registry of validated SLE cases fulfilling 1997 ACR criteria for SLE Classification20 and followed at the BWH Lupus Center since 197221, as well as a BWH registry of renal biopsies performed since 199622. Biopsy reports of all first renal biopsies for each subject showing Class II-V lupus nephritis were identified and reviewed for this study. We included biopsies from patients ≥18 years of age, obtained between March 1, 1990 and May 31, 2017.
1.2.2. Renal Pathology
We abstracted from the biopsy reports: number of glomeruli assessed, WHO/ISN/RPS Class4–8, IFTA, vascular injury, and global glomerulosclerosis documentation. All biopsies were read in the BWH Department of Renal Pathology using criteria standardized across the department. 70% of biopsies were read by one senior pathologist (HR) and the other 30% by his trainees with him. All biopsies had light, immunofluorescence and electron microscopy for the primary diagnosis, and the lesions were assessed using all four stains routinely used in lupus nephritis biopsies (hematoxylin and eosin (H&E), Periodic acid-Schiff (PAS), trichrome, and silver stain)23,24. The entire cortical area was scored to determine each diagnosis. IFTA was reported as the estimated percentage seen in the cortical area of the biopsy sample25. Absent was defined as <10%, mild 10-24%, moderate 25%-50%, and severe >50% of the surface area8,25,26. In the primary analysis, we combined mild, moderate and severe IFTA to create a dichotomous “any vs. no IFTA” variable. As a secondary outcome, we analyzed none/mild vs. moderate/ severe IFTA.
Vascular injury was assessed as a composite measure between medial and subintimal sclerosis and scored according to subintimal narrowing of the lumen7,27. The extent of vascular injury was described as absent, with the lumen narrowed by <10%, mild 10-24%, moderate 25-50%, or severe, lumen narrowed by >50%8,25,26. In the primary analysis, we combined mild, moderate and severe vascular injury into an overall outcome of “any vascular injury”. As a secondary outcome for vascular injury, we analyzed none/mild vs. moderate/severe vascular injury. Biopsy samples with either proliferative nephritis (either class III or class IV) plus class V were assessed as class III or IV nephritis, respectively. Samples with class II and V were analyzed as class V nephritis.
1.2.3. Demographic and Clinical Data
Data were abstracted from the BWH Lupus Registry and supplemented with data from the Research Patient Data Repository (RPDR), a research repository of all electronic medical records for all BWH patients28. RPDR contains billing codes, clinical notes, and test and imaging results for all patients seen at BWH. We collected the following variables from the RPDR: sociodemographic factors (age at SLE diagnosis, age at biopsy, sex, race/ethnicity), clinical variables (systolic and diastolic blood pressure, medications as below, dates of progression to ESRD and death). We defined hypertension as two or more readings showing systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg within one month of renal biopsy, regardless of antihypertensive medications. We collected the following medications from the medical records for the 6 months prior to and 6 months after the renal biopsy date: hydroxychloroquine, glucocorticoids (mean steroid dose, classified as prednisone ≥20 mg/day or equivalent), immunosuppressants (azathioprine, cyclophosphamide, mycophenolate mofetil, methotrexate, rituximab, cyclosporine or tacrolimus), non-steroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme (ACE) inhibitors, and other anti-hypertensive agents. We collected laboratory values ANA and antiphospholipid antibodies (lupus anticoagulant, anti-cardiolipin, and anti-Beta-2 glycoprotein) at any time prior to the biopsy date. All other laboratory values were collected within the 30 days prior to biopsy, including hemoglobin (continuous), platelet count (continuous), serum creatinine (continuous), serum albumin (continuous), anti-double-stranded DNA, C3, C4, anti-Smith, anti-Ro, anti-La, urine protein/creatinine ratio, ≥25 urinary red blood cells (RBC, present/absent), ≥25 urinary white blood cells (WBC, present/absent). Documentation of antiphospholipid antibodies was not available for 100 individuals. To assess organ damage related to SLE, we used the SLE International Collaborating Clinics (SLICC)/ACR-Damage Index (DI) score, collected by medical record review for all patients who had ≥6 months of clinical information available at the time of renal biopsy29,30. As all individuals had renal involvement, we assessed organ damage with a modified SLICC/ACR-DI without the renal domain (excluding estimated glomerular filtration rate <50%, proteinuria >3.5 g/24 hours, and ESRD).
Dates of death, both inside and outside of our hospital system, were obtained from the National Death Index and linked to the RPDR records. We obtained a data use agreement with the U.S. Renal Data System (USRDS) to enable linking of our patient data with national records on all patients who progressed to ESRD and needed renal replacement therapy31. USRDS is the US national registry of all patients necessitating renal replacement therapy, including nearly all ESRD patients nationwide since 198931. Unique identifiers for all study patients were forwarded in an Institutional Review Board (IRB)-approved protocol to USRDS, who returned linked data with date of ESRD and deaths after ESRD for any patients who developed these conditions, through December 31, 2015. All aspects of this study were approved by the BWH Institutional Review Board.
1.2.4. Statistical Methods
We first conducted bivariable analyses assessing associations between demographic and clinical variables, and histologic findings of IFTA or vascular injury on renal biopsy, employing Fisher’s exact, Chi-square, t-tests or Wilcoxon Rank Sum tests as appropriate. We examined the distribution of glomerular class on biopsy with findings of IFTA or vascular injury by Chi-square test. We then constructed multivariable logistic regression models for clinical and demographic factors associated with IFTA and vascular injury, including age, race, sex, SLE duration, and calendar year of biopsy, and significant covariates from our bivariable analyses. Additionally, we examined the intensification in medication therapy based on biopsy results with findings of IFTA, vascular injury, and glomerulonephritis class by Chi-square test.
We conducted survival analyses examining progression from the date of renal biopsy to ESRD and death among individuals with different glomerulonephritis classes, IFTA, and vascular injury on biopsy. We graphed cumulative incidence functions with competing risk of death32 for the outcome of ESRD (and the outcome of death separately). We stratified these cumulative incidence curves by class of lupus nephritis and presence or absence of concomitant renal biopsy lesions and used Gray’s test for comparing the cumulative incidence function curves. Cumulative incidence functions were censored for death or loss to follow-up (at last visit) over the first ten years post-biopsy.
Multivariable Cox proportional hazards models, accounting for the competing risk of death in models of ESRD33, were used to estimate hazard ratios with 95% confidence intervals (CI) for risk of ESRD and death separately for each of the pathologic lesions. We employed three sequential models: Model A, adjusting for age, sex and race, Model B, additionally adjusting for serum creatinine and calendar year of biopsy, and Model C, adding class as well. The proportional hazards assumption was verified using the cumulative incidence functions. Missing data (as denoted in Table 1) were excluded from each analysis. Analyses were conducted in SAS, v 9.4 (SAS Institute, Cary, NC).
Table 1.
Clinical Factors and Interstitial Fibrosis and Tubular Atrophy (IFTA) and Vascular Injury, Bivariable Associations within 202 Initial Lupus Nephritis Renal Biopsies
| Interstitial Fibrosis and Tubular Atrophy | Vascular Injury | |||||
|---|---|---|---|---|---|---|
| Any IFTA (n= 119) | No IFTA (n= 83) | p* | Any vascular injury (n= 135) | No vascular injury (n=67) | p* | |
| Sociodemographic Factors | ||||||
| Age, years, mean (SD) | 38.2 (13.1) | 33.7 (11.8) | 0.01 | 38.4 (13.0) | 32.3 (11.3) | 0.001 |
| Duration of SLE at biopsy, years, median (IQR) | 5.1 (0.9, 11.9) | 2.1 (0.4, 7.5) | 0.05 | 5.3 (0.9, 11.4) | 1.8 (0.4, 6.3) | 0.02 |
| Female, n (%) | 99 (83.2) | 71 (85.5) | 0.65 | 113 (83.7) | 57 (85.1) | 0.80 |
| Non-African American, n (%) | 73 (62.4) | 55 (67.9) | 0.43 | 79 (60.3) | 49 (73.1) | 0.07 |
| African American, n (%) | 44 (37.6) | 26 (32.1) | 52 (39.7) | 18 (26.9) | ||
| Clinical Factors | ||||||
| SLICC/ACR-DI without renal score, median (IQR)Ѳ | 3 (1, 5) | 1 (0, 3) | 0.002 | 2 (1, 5) | 1.5 (0, 4) | 0.08 |
| Hypertension (SBP ≥140 mm Hg or DBP≥ 90 mm Hg), n (%) | 40 (38.1) | 13 (18.6) | 0.006 | 43 (36.4) | 10 (17.5) | 0.01 |
| Receiving hydroxychloroquine, n (%) | 61 (55.0) | 56 (74.7) | 0.006 | 77 (61.1) | 40 (66.7) | 0.46 |
| Receiving glucocorticoid ≥20 mg/day, n (%)** | 44 (39.6) | 38 (50.7) | 0.14 | 50 (39.7) | 32 (53.3) | 0.08 |
| Receiving immunosuppressive therapy, n (%)*** | 50 (45.1) | 23 (30.7) | 0.05 | 57 (45.2) | 16 (26.7) | 0.02 |
| Receiving antihypertensives, n (%) | 72 (64.9) | 31 (41.3) | 0.002 | 80 (63.5) | 23 (38.3) | 0.001 |
| Receiving NSAIDs, n (%) | 51 (46.0) | 35 (46.7) | 0.92 | 58 (46.0) | 28 (46.7) | 0.94 |
| Laboratory Values | ||||||
| Hemoglobin g/dL, mean (SD) | 10.4 (2.0) | 11.3 (1.7) | 0.002 | 10.7 (1.9) | 11.0 (1.9) | 0.32 |
| Platelet count per 1000 mm3, mean (SD) | 252 (107) | 256 (104) | 0.79 | 251 (109) | 258 (98.6) | 0.66 |
| Serum creatinine, mg/dL, mean (SD) | 1.8 (1.9) | 0.9 (0.6) | <0.0001 | 1.6 (1.9) | 0.9 (0.7) | 0.0001 |
| Serum albumin, g/dL, mean (SD) | 3.0 (0.7) | 3.2 (0.7) | 0.02 | 3.1 (0.7) | 3.1 (0.8) | 0.55 |
| Anti-dsDNA positive, n (%) | 83 (81.4) | 58 (80.6) | 0.89 | 93 (80.2) | 48 (82.8) | 0.68 |
| C3, mg/dL, mean (SD) | 70.6 (35.2) | 65.7 (31.5) | 0.39 | 72.1 (34.6) | 61.5 (31.1) | 0.05 |
| C4, mg/dL, mean (SD) | 13.8 (12.1) | 10.8 (7.7) | 0.09 | 13.1 (11.8) | 11.7 (7.8) | 0.76 |
| Antiphospholipid antibodies positive, n (%) | 22 (34.9) | 14 (34.2) | 0.94 | 22 (30.6) | 14 (43.8) | 0.19 |
| Anti-Ro positive, n (%) | 60 (50.4) | 51 (61.5) | 0.29 | 73 (54.3) | 38 (56.7) | 0.60 |
| Anti-La positive, n (%) | 38 (31.9) | 24 (28.9) | 0.87 | 42 (31.1) | 20 (29.9) | 0.54 |
| Urine protein/creatinine ≥1 g/24 hr, n (%) | 84 (82.4) | 45 (64.3) | 0.007 | 90 (78.3) | 39 (68.4) | 0.16 |
| ≥25 urine WBC per hpf, n (%) | 26 (23.6) | 16 (21.3) | 0.71 | 25 (20.3) | 17 (27.4) | 0.28 |
| ≥25 urine RBC per hpf, n (%) | 55 (50.0) | 28 (37.3) | 0.09 | 58 (47.2) | 25 (40.3) | 0.38 |
Percentages, means and medians reflect total numbers excluding those with missing values. Number of missing values are: Duration of SLE at biopsy= 4, African American/Non-African American= 4, SLICC/ACR-DI = 12, hypertension= 27, pre-biopsy medications= 16, hemoglobin= 17, platelets= 17, serum creatinine= 14, serum albumin= 23, dsDNA= 28, C3= 34, C4= 39, Antiphospholipid antibodies (lupus anticoagulant, anticardiolipin IgM and IgG and/or anti-Beta-2 glycoprotein I antibodies)= 98, urine protein/creatinine ratio= 30, urine WBC/RBCs= 17
Continuous variables evaluated with t-test or Wilcoxon, binary and categorical variables assessed using Chi-square or Fisher’s exact tests as appropriate
Systemic Lupus Erythematosus International Collaborating Clinics Damage Index without renal indices
Prednisone or equivalent of mean ≥20 mg/day over 6 months prior to biopsy
Azathioprine, cyclophosphamide, mycophenolate mofetil, methotrexate, rituximab, cyclosporine or tacrolimus at the time of biopsy
1.3. Results
We included 202 first lupus nephritis biopsies. The mean (SD) patient age was 36 (13) years, 84% were female, 35% were African American, and anti-dsDNA antibody was positive in 81%. Median (IQR) SLE duration prior to biopsy was 3.7 (0.6-9.8) years and mean (SD) serum creatinine was 1.4 (1.6) mg/dL at the time of biopsy. Classes of glomerulonephritis were 11% class II, 26% III, 38% IV, and 25% V. An average of 12% of glomeruli were globally sclerosed. Of the 202 biopsies included, 190 (94%) met suggested adequacy criteria of ≥10 glomeruli present in the sample8. Among those with medication data, 39% were receiving immunosuppressive agents and 44% were receiving high-dose steroids in the 6 months prior to biopsy. Overall, 53 of 202 patients (26%) progressed to ESRD at a mean (SD) of 4.7 (4.3) years after biopsy and 18 (9%) died at mean (SD) 8.5 (5.4) years after biopsy. Thirteen of the 18 total deaths (72%) occurred after progression to ESRD.
1.3.1. Interstitial Fibrosis and Tubular Atrophy
IFTA was identified in 119 lupus nephritis biopsies (59%). Acute interstitial nephritis was also noted in 15 of the IFTA-containing biopsies, but was not analyzed separately as it has not been found to be predictive of outcomes34. Ninety-eight of 202 total (49%) patients had both IFTA and vascular injury. Of those with evidence of IFTA, mild was most common, observed in 77 biopsies (65%) and moderate or severe IFTA was observed in 42 (35%). Patients with IFTA were older than patients with none (38 vs. 34 years, p=0.01) and had a longer duration of SLE prior to biopsy (5 vs.2 years, p=0.05) (Table 1). There were no statistically significant differences in sex, race, anti-Ro or anti-La antibody positivity. There was also no statistically significant difference in the distribution of lupus glomerulonephritis class among those with and without IFTA (Table 2). In bivariable analyses, presence of IFTA on renal biopsy was associated with several laboratory abnormalities, including lower hemoglobin (10.4 vs. 11.3 g/dL, p=0.002), higher serum creatinine (1.8 vs. 0.9 mg/dL, p<0.0001), lower serum albumin (3.0 vs. 3.2 g/dL, p=0.02) and higher prevalence of urine protein/creatinine ratio equivalent to ≥1 g/24 hours (82% vs. 64%, p=0.007). IFTA was also associated with higher median SLICC/ACR-DI without renal domains (3 vs. 1, p=0.002), higher use of immunosuppressive therapy (45 vs. 31%, p=0.05) and higher use of anti-hypertensive medications (65 vs. 41%, p=0.002) in the 6 months prior to biopsy. Presence of IFTA was associated with decreased use of hydroxychloroquine (55 vs. 75%, p=0.006) prior to biopsy.
Table 2.
Presence of Interstitial Fibrosis and Tubular Atrophy (IFTA) and Vascular Injury in relation to WHO/ISN/RPS Lupus Nephritis Class within 202 First Lupus Nephritis Renal Biopsies
| Class Lupus Nephritis | Biopsies with IFTA n=119 of 202 | p* | Biopsies with vascular injury n=135 of 202 | p* |
|---|---|---|---|---|
| Class II, n (%) | 11 (48) | 0.53 | 17 (74) | 0.72 |
| Class III, n (%) | 33 (63) | 34 (65) | ||
| Class IV, n (%) | 47 (62) | 48 (63) | ||
| Class V, n (%) | 28 (55) | 36 (71) | ||
| **Proliferative GN, n (%) | 80 (63) | 0.17 | 82 (64) | 0.27 |
| Non-proliferative GN, n (%) | 39 (53) | 53 (72) |
Chi-squared test of the distribution of IFTA or vascular injury among those with WHO/ISN/RPS class on renal biopsy
Class III/IV glomerulonephritis
Following multivariable adjustment for age, race, sex, SLE duration at biopsy, calendar year of biopsy, urine protein/creatinine ratio and hemoglobin concentration, both serum creatinine and SLICC/ACR-DI without the renal domain remained significantly associated with IFTA. The multivariable OR for IFTA was 2.53 (95% CI 1.16, 5.55) per 1 mg/dL increase in serum creatinine, and 1.19 (95% CI 1.03, 1.38) per unit of the SLICC-DI without renal domain (Table 3). Multivariable regression models revealed that serum creatinine per 1 mg/dL (OR 4.14, 95% CI 2.02, 8.48) and SLICC/ACR-DI without the renal domain per unit (OR 1.34, 95% CI 1.09, 1.65) were also associated with presence of moderate/severe IFTA.
Table 3.
Multivariable Associations between Pre-Biopsy Clinical Factors and IFTA within 202 Initial Lupus Nephritis Biopsies
| Model 1 Outcome: Any IFTA vs. none | Model 2 Outcome: Any IFTA vs. none | Model 2 Outcome: Moderate/Severe IFTA vs. None/Mild IFTA | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age at biopsy, per year | 1.02 (0.99, 1.05) | 1.00 (0.97, 1.04) | 0.97 (0.92, 1.02) |
| Black (vs. non-Black) race | 1.09 (0.56, 2.14) | 0.58 (0.26, 1.29) | 1.81 (0.61,5.36) |
| Female (vs. male) | 0.72 (0.29, 1.83) | 0.59 (0.20, 1.72) | 0.34 (0.08, 1.50) |
| SLE duration, per year | 1.03 (0.98, 1.07) | 1.02 (0.97, 1.07) | 1.06 (0.99, 1.14) |
| Serum creatinine, per mg/dl | - | 2.53 (1.16, 5.55) | 4.14 (2.02, 8.48) |
| Urine protein/creatinine ratio ≥1 g/24 hr, per year | - | 1.99 (0.87, 4.55) | 2.03 (0.44, 9.31) |
| SLICC/ACR DI (without renal domain), per unit | - | 1.19 (1.03, 1.38) | 1.34 (1.09, 1.65) |
| Hemoglobin concentration, per g/dL | - | 0.89 (0.72, 1.08) | 1.08 (0.80, 1.46) |
All models additionally adjusted for calendar year of biopsy
1.3.2. Vascular Injury
Of the 202 initial lupus nephritis biopsies, 135 (67%) exhibited any vascular injury, of which 70 (52%) had moderate or severe vascular injury. Patients with vascular injury were older than patients without (38 vs.32 years, p=0.001) and had a longer duration of SLE (5.3 years vs. 1.8 years, p=0.02) (Table 1). Lupus nephritis class was not statistically associated with presence of vascular injury (Table 2).
In bivariable analysis, presence of vascular injury on biopsy was associated with hypertension (36% vs. 18%, p=0.01), elevated creatinine (1.6 vs. 0.9 mg/dL, p=0.0001), greater use of immunosuppressive therapy (45 vs. 27%, p=0.02) and greater use of anti-hypertensives (64 vs. 38%, p=0.001) in the 6 months prior to biopsy. There were no significant associations between vascular injury and sex, race, antiphospholipid antibodies or platelet count. Following multivariable adjustment for age, sex, race, SLE duration and calendar year of biopsy, serum creatinine and hypertension did not remain associated with the presence of vascular injury (Table 4). We found serum creatinine to be associated with the severity of vascular injury (OR 1.64, 95% CI 1.14, 2.36), rather than the presence of vascular injury, after controlling for age, sex, race, duration of disease and calendar year of biopsy.
Table 4.
Multivariable Associations between Demographic and Clinical Factors and Vascular Injury within 202 Initial Lupus Nephritis Biopsies
| Model 1 Outcome: Vascular injury vs. none | Model 2 Outcome: Vascular injury vs. none | Model 2 Outcome: Moderate/Severe Vascular injury vs. None/Mild Vascular injury | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age at biopsy, per year | 1.04 (1.01, 1.07) | 1.02 (0.98, 1.05) | 1.01 (0.98, 1.05) |
| Black vs. non-Black race | 1.90 (0.96, 3.76) | 1.50 (0.71, 3.16) | 1.24 (0.59, 2.58) |
| Female vs. male | 1.05 (0.44, 2.53) | 1.10 (0.39, 3.11) | 0.91 (0.33, 2.51) |
| SLE duration, per year | 1.02 (0.97, 1.07) | 1.01 (0.97, 1.06) | 1.00 (0.96, 1.05) |
| Serum creatinine, per mg/dl | - | 1.47 (0.91, 2.38) | 1.64 (1.14, 2.36) |
| Hypertension (SBP ≥140 or DBP ≥90 mm Hg), per year | - | 1.95 (0.82, 4.64) | 2.03 (0.94, 4.37) |
All models additionally controlled for calendar year of biopsy.
1.3.3. Medication Intensification
Overall rates of hydroxychloroquine, high-dose steroids, immunosuppressive agents and anti-hypertensive use all increased from the pre- to post-biopsy period (62% to 72% for HCQ, 45% to 61% for high-dose steroids, 39% to 76% for immunosuppressive agents, and 56% to 82% for anti-hypertensives). Only 32% of those with IFTA on biopsy experienced immunosuppressive agent intensification post-biopsy vs. 53% of those without (p<0.01), and only 34% of those with vascular injury on biopsy experienced immunosuppressive agent intensification post-biopsy vs. 55% of those without (p<0.01). Of those with proliferative glomerulonephritis, 51% experienced immunosuppressive agent intensification post-biopsy vs. 23% of those without (p<0.001), and 32% with proliferative glomerulonephritis experienced high-dose steroid intensification vs. 16% of those without (p=0.02).
1.3.4. Risk of Progression to ESRD
We observed that 41 cases of ESRD developed among 119 individuals with IFTA vs. 12 cases among 83 individuals without, and mean (SD) time to ESRD among those with IFTA was 4.5 (4.5) years. We also identified 41 cases of ESRD among 135 people with vascular injury on biopsy vs. 12 cases among 67 people without. Overall mean (SD) time to ESRD among those with vascular injury was 4.3 (4.5) years.
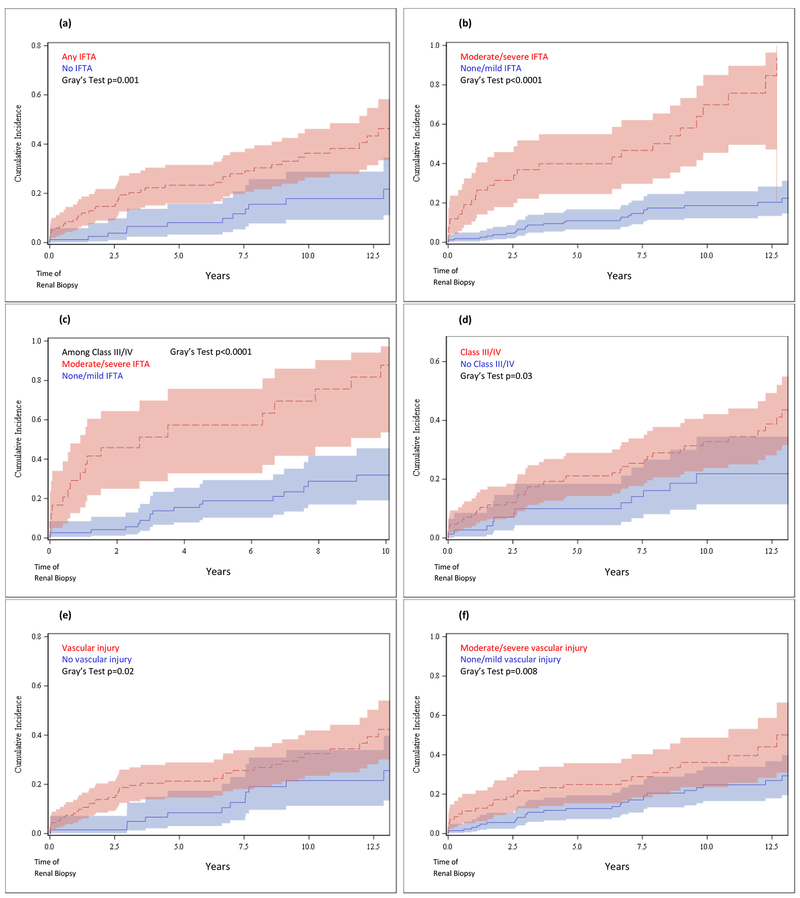
Cumulative incidence curves for development of ESRD (with competing risk of death) from time of biopsy by IFTA are shown in Figures 1a–c. Risk of ESRD was higher among those with IFTA vs. those without (Gray’s test p=0.001) and was also higher among those with moderate/severe IFTA vs. those with none/mild IFTA (p<0.0001), persisting among those with proliferative nephritis (p<0.0001). By ten years post-biopsy, the risk of ESRD among people with proliferative nephritis was as high as 89% (95% CI 55-98%) for those with moderate/severe IFTA, vs. 33% (95% CI 20-46%) for those without. By comparison, cumulative incidence curves for ESRD by class (Figure 1d) displayed an only slightly elevated risk of ESRD among those with proliferative nephritis vs. those without (p=0.03), and 10-year risk of ESRD was 33% (95% CI 24-43%) for those with proliferative nephritis vs. 22% (95% CI 12-34%) for those without.
Figure 1. Cumulative Incidence Functions for ESRD after Biopsy (accounting for Competing Risk of Death*).
*All cumulative incidence functions for ESRD account for competing risk of death.
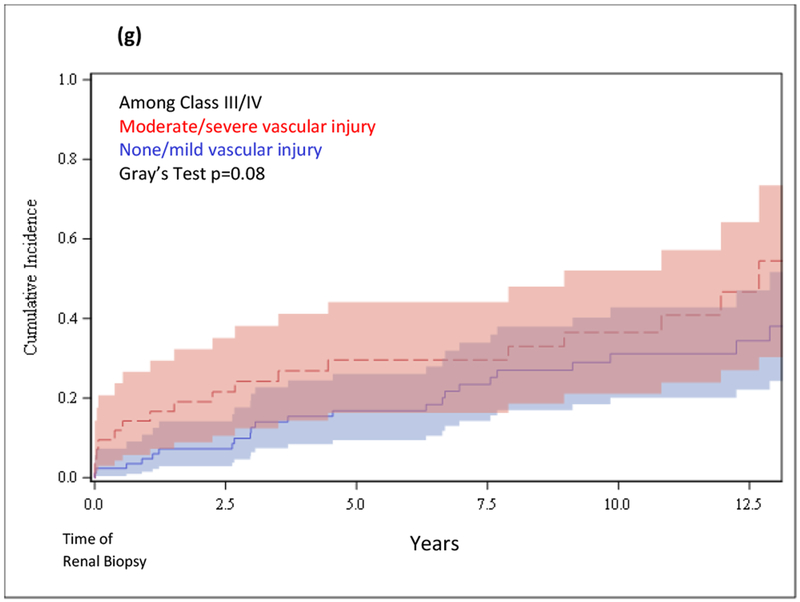
(a) Cumulative incidence function for survival to ESRD after renal biopsy in those with IFTA vs. no IFTA. (b) Cumulative incidence function for survival to ESRD after renal biopsy in those with moderate/severe IFTA vs. none/mild IFTA. (c) Cumulative incidence function for survival to ESRD after renal biopsy among those with Class III/IV nephritis, in those with moderate/severe IFTA vs. none/mild IFTA. (d) Cumulative incidence function for survival to ESRD after renal biopsy in those with Class III/IV nephritis vs. no Class III/IV nephritis. (e) Cumulative incidence function for survival to ESRD after renal biopsy in those with vascular injury vs. no vascular injury. (f) Cumulative incidence function for survival to ESRD after renal biopsy in those with moderate/severe vascular injury vs. none/mild vascular injury. (g) Cumulative incidence function for survival to ESRD after renal biopsy among those with Class III/IV nephritis, in those with moderate/severe vascular injury vs. none/mild vascular injury.
Risk of ESRD was also higher among those with vascular injury vs. those without (p=0.02) (Figure 1e). Upon stratification by severity, we found risk of ESRD to be higher among those with moderate/severe vascular injury vs. none/mild (p=0.008) (Figure 1f), however this disparity was attenuated among those with proliferative nephritis (p=0.08) (Figure 1g).
In subdistribution (SD) Cox models, the risk of ESRD was over 2.6-fold elevated for those with IFTA vs. those without, after adjusting for age, sex and race, and accounting for the competing risk of death (Table 5). After further adjustment for serum creatinine, calendar year of biopsy and lupus nephritis class, the point estimate remained 2.6-fold elevated, and increased to over five-fold elevated for those with moderate/severe IFTA vs. none/mild (HRSD 5.18, 95% CI 2.53, 10.59). Among those with proliferative nephritis, risk of ESRD was over 7-fold elevated for those with moderate/severe IFTA vs. none/mild after adjusting for age, sex and race, and remained significantly elevated upon further adjustment for serum creatinine and calendar year of biopsy (HRSD 6.75, 95% CI 2.90, 15.70).
Table 5.
Multivariable-Adjusted Hazard Ratios* for End-Stage Renal Disease (ESRD) and Death according to Pathologic Findings of Interstitial Fibrosis and Tubular Atrophy (IFTA) and Vascular Injury on Lupus Nephritis Renal Biopsy (n=202)
| ESRD | Death | ||||||
|---|---|---|---|---|---|---|---|
| Pathologic Finding | Model A*: HRSD (95%CI) | Model B**: HRSD (95%CI) | Model C***: HRSD (95%CI) | Model A*: HR(95%CI) | Model B**: HR(95%CI) | Model C***: HR(95%CI) | |
| IFTA | Any IFTA (vs. none) | 2.65 (1.40, 5.01) | 2.73 (1.20, 6.22) | 2.56 (1.11, 5.90) | 5.61 (1.26, 24.92) | 3.61 (0.79, 16.60) | 3.75 (0.82, 17.26) |
| Moderate/severe IFTA (vs. none/mild) | 5.61 (3.30, 9.55) | 4.37 (2.25, 8.46) | 5.18 (2.53, 10.59) | 6.75 (2.43, 18.74) | 4.46 (1.39, 14.29) | 4.19 (1.27, 13.81) | |
| Moderate/severe IFTA (vs. none/mild) among proliferative GN only | 7.78 (3.93, 15.39) | 6.75 (2.90, 15.70) | - | 3.85 (1.07, 13.90) | 1.77 (0.30, 10.37) | - | |
| Vascular Injury | Any vascular injury (vs. none) | 1.79 (0.96, 3.34) | 1.62 (0.74, 3.53) | 1.61 (0.73, 3.55) | 2.04 (0.63, 6.66) | 1.34 (0.39, 4.58) | 1.32 (0.38, 4.54) |
| Moderate/severe vascular injury (vs. none/mild) | 2.13 (1.21, 3.75) | 1.64 (0.88, 3.04) | 1.46 (0.75, 2.84) | 2.68 (0.98, 7.28) | 2.14 (0.70, 6.49) | 2.15 (0.71, 6.49) | |
| Moderate/severe vascular injury (vs. none/mild) among proliferative GN only | 1.58 (0.82, 3.05) | 1.08 (0.49, 2.38) | - | 1.84 (0.52, 6.47) | 1.16 (0.25, 5.38) | - | |
Model A: adjusted for age, sex and race
Model B: Model A + serum creatinine and calendar year of biopsy
Model C: Model B + WHO/ISN/RPS Class
Risk of ESRD was also elevated among those with moderate/severe vascular injury vs. none/mild (HRSD 2.13, 95% CI 1.21, 3.75), but did not persist after further adjustment or stratification among those with proliferative nephritis (Table 5).
1.3.4. Risk of Progression to Death
We observed 16 deaths among those with IFTA vs. 2 deaths among those without, and 14 deaths among those with vascular injury vs. 4 deaths among those without. By comparison, there were 11 deaths among 128 people with proliferative lupus nephritis vs. 7 deaths among 74 people without proliferative lupus nephritis. Mean (SD) time to death among those with IFTA was 8.0 (5.5) years, and mean (SD) time to death among those with vascular injury was 7.8 (4.7) years.
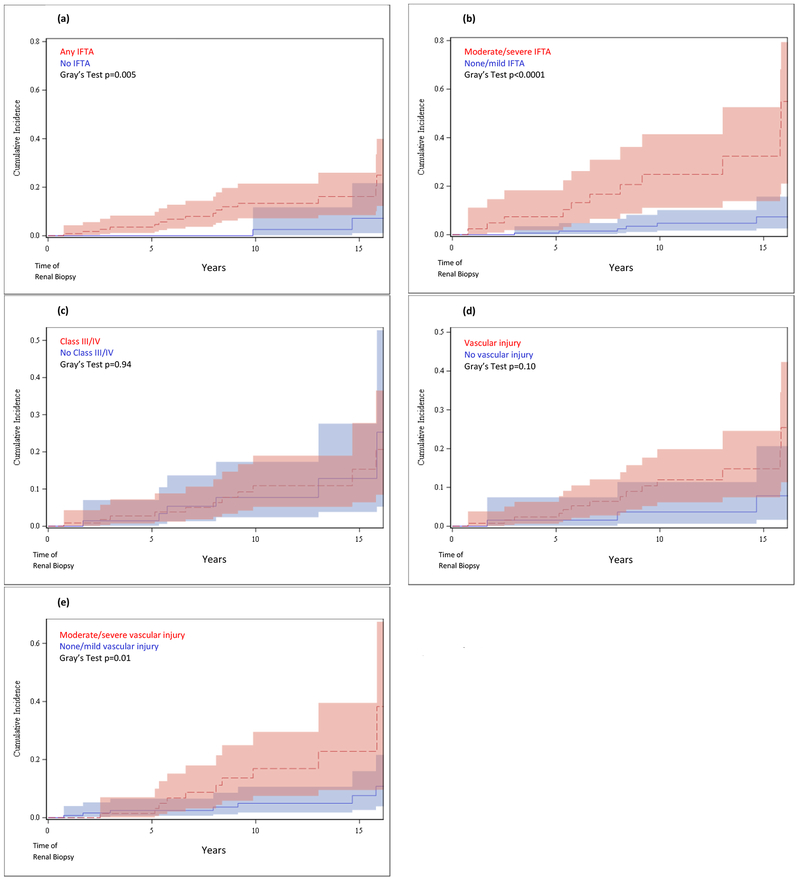
Cumulative incidence curves for risk of death from time of biopsy by IFTA are shown in Figures 2a–b. Although there were few deaths, IFTA was more strongly associated with death (Gray’s test p=0.005 for presence/absence) than was class (p=0.94 for proliferative vs. non-proliferative) (Figure 2c). The risk of death was significantly elevated among those with moderate/severe IFTA vs. none/mild (p<0.0001), and the 10-year risk of death among those with moderate/severe IFTA was 27% (95% CI 13-43%) vs. 5% (95% CI 2-10%) for those with none/mild IFTA.
Figure 2. Cumulative Incidence Functions for Death after Biopsy.
(a) Cumulative incidence function for death after renal biopsy in those with IFTA vs. no IFTA. (b) Cumulative incidence function for death after renal biopsy in those with moderate/severe IFTA vs. none/mild IFTA. (c) Cumulative incidence function for death after renal biopsy in those with Class III/IV nephritis vs. no Class III/IV nephritis. (d) Cumulative incidence function for death after renal biopsy in those with vascular injury vs. no vascular injury. (e) Cumulative incidence function for death after renal biopsy in those with moderate/severe vascular injury vs. none/mild vascular injury.
We found the severity of vascular injury, rather than the presence, to be predictive of death (p=0.01) for moderate/severe vascular injury vs. none/mild, while p=0.10 for any vs. no vascular injury) (Figures 2d–e).
In multivariable proportional hazards models, we found the risk of death was very elevated for those with any IFTA vs. none (HR 5.61, 95% CI 1.26, 24.92) when adjusted for age, sex and race (Table 5). The risk of death remained elevated for those with moderate/severe IFTA vs. none/mild, even among people with proliferative nephritis (HR 3.85, 95% CI 1.07, 13.90). Upon further adjustment for serum creatinine, calendar year and biopsy class, risk of death remained significantly increased for those with moderate/severe IFTA vs. none/mild (HR 4.19, 95% CI 1.27, 13.81).
Among those with vascular injury on biopsy, the hazard ratio for death was elevated, although this was not statistically significant (HR 2.68, 95% CI 0.98, 7.28) (Table 5).
1.4. Discussion
In this large sample of initial lupus nephritis biopsies, IFTA and vascular injury were common findings, and neither was associated with WHO/ISN/RPS class of glomerulonephritis, with SLE-related serologies or with demographic factors. Vascular injury was observed in over half of the biopsies, consistent with the prevalence reported in prior studies18,35 and IFTA and vascular injury commonly occurred together. In bivariable analyses, we found presence of IFTA was associated with higher serum creatinine and more SLE-related damage (SLICC/ACR-DI score without the renal domain) at the time of biopsy, and both remained significant upon multivariable adjustment. SLICC-DI includes irreversible damage from SLE in multiple organ systems, including cerebrovascular accident, myocardial infarction, and osteonecrosis, and thus is representative of longer-standing and more severe SLE. We also found that higher serum creatinine at the time of biopsy was associated with moderate/severe vascular injury.
Past studies have demonstrated associations between elevated systolic or diastolic blood pressures and vascular injury severity on renal biopsies from patients with a range of causes of glomerulonephritis36,37. We saw an association between hypertension and presence of vascular injury in bivariable analyses, which did not persist on multivariable adjustment. Vascular injury, particularly intimal thickening, may be a cause of hypertension rather than a consequence, and the association between the two on initial lupus nephritis renal biopsy is - clinically important as hypertension may signal vascular injury and thus poorer prognosis. The presence of hypertension, elevated serum creatinine and other organ SLE-related damage at a patient’s presentation with lupus nephritis should increase clinical suspicion of the presence of IFTA or vascular injury. We did not find that serologic markers, including anti-double-stranded DNA antibodies, anti-Ro/La antibodies, and low complement, were associated with either IFTA or vascular injury.
We found that immunosuppressant and anti-hypertensive medication use in the 6-month pre-biopsy period were associated with presence of both IFTA and vascular injury on biopsy, likely because these histologic features are associated with a more severe disease phenotype. We also found hydroxychloroquine use at time of biopsy was positively associated with the presence of IFTA (in contrast to a recent report of an inverse association between the two38), but no association with vascular injury. Although this is not a rigorous pharmacoepidemiology study, IFTA and vascular injury on biopsy were not related to medication changes post-biopsy as proliferative glomerulonephritis was.
We observed a significantly higher risk of progression to ESRD and death among patients with IFTA found in lupus nephritis renal biopsy, particularly among those with proliferative glomerulonephritis. We also observed IFTA to be an independent risk factor for both ESRD and death, after adjustment for class. Percentage of global glomerulosclerosis increased proportionally with severity of IFTA on biopsy, as predicted given global glomerulosclerosis is also a surrogate marker of kidney function39,40. Additionally, we observed greater risk of ESRD and death among patients with vascular injury, although adjustment for serum creatinine attenuated these results, suggesting the two are collinear. Taken together with other recent studies with similar findings16–19,34,41, these results provide added evidence that these two histologic findings, IFTA in particular, have important prognostic information in lupus nephritis.
Currently the degree of IFTA does not play a large role in treatment decisions. Our finding of no association with WHO/ISN/RPS class of lupus nephritis contrasts with previous work showing an association between IFTA and class IV lupus nephritis. Thus, IFTA may be a marker of risk of ESRD and death in addition to class16,17,42,43. This adds to the growing body of evidence suggesting IFTA and glomerular damage may occur independent of each other, perhaps by different mechanisms entirely17,44,45. We also found IFTA to be associated with hypertension, as has been demonstrated previously38. The 2003 ISN/RPS Classification and the American College of Rheumatology suggest that biopsies document in the diagnostic line the presence of IFTA and the extent of vascular injury3,8, and a recent consensus report from the Renal Pathology Society includes documentation of IFTA and vascular injury in their suggested basic format of a kidney biopsy report25. Some chronicity indices include components of IFTA, while extraglomerular vascular injury is generally not included8, and even so activity and chronicity indices are not consistently used to supplement biopsy reports9.
Our analysis has several strengths. We included a large number of biopsies and subjects were followed in our healthcare system for up to 25 years. We linked medical records with USRDS to ensure capture of all dates of ESRD, and deaths after ESRD. IFTA, vascular injury, and WHO/ISN/RPS class on renal biopsies were well-documented and consistent within our institution. There were some limitations as well. It is difficult to ascertain the directionality of the observed clinico-pathologic correlations (e.g., association of SLICC-DI with IFTA). Unfortunately, we did have missing medication use data for some subjects prior to biopsy, mainly because they were referred to our institution for their kidney biopsy and there was incomplete documentation of medications at that visit. Additionally, many subjects may not have been receiving lupus nephritis medications as the diagnosis of SLE may have been unsure pre-biopsy. Additionally, complete clinical data were not available for all subjects for antiphospholipid antibody status, limiting our investigations of associations with this clinical feature of lupus. We also were unable to categorize vascular lesions as atherosclerotic versus other as this was not consistently documented. Lastly, we were not able to re-review all biopsies, but relied on previous reports.
Evidence is accumulating that incorporating the histologic findings of IFTA and vascular injury into prognostic algorithms may improve identification of patients at increased risk for progression and poor outcomes in lupus nephritis. Growing understanding of these lesions and their clinical correlates may also lead to their inclusion in the design of lupus nephritis clinical trials, potentially providing insight into novel lupus nephritis therapies targeting them.
Acknowledgments
Funding: Dr. Costenbader’s research is supported by NIH NIAMS R01 057327 and K24 AR066109. Dr. Feldman’s research is supported by NIH NIAMS K23 AR071500.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
References
- 1.Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney international 2016;90:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almaani S, Meara A, Rovin BH. Update on Lupus Nephritis. Clinical journal of the American Society of Nephrology : CJASN 2017;12:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn BH, McMahon M, Wilkinson A, et al. American College of Rheumatology Guidelines for Screening, Case Definition, Treatment and Management of Lupus Nephritis. Arthritis Care Res 2012;64:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appel GB, Silva FG, Pirani CL, Meltzer JI, Estes D. Renal involvement in systemic lupud erythematosus (SLE): a study of 56 patients emphasizing histologic classification. Medicine 1978;57:371–410. [DOI] [PubMed] [Google Scholar]
- 5.Churg JBJ, Glassock RJ. Renal Disease: Classification and Atlas of Glomerular diseases. 2nd ed. New York: Igaku-Shoin; 1995. [Google Scholar]
- 6.Churg JSL. Renal Disease: Classification and Atlas of Glomerular Disease. Tokyo: Igaku-Shoin; 1982. [Google Scholar]
- 7.RT M Kidney Pathology Decennial 1966-1975. East Norwalk, CT: Appleton-Century-Crofts; 1975. [Google Scholar]
- 8.Weening JJ, D’Agati VD, Schwartz MM, et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz GS, D’Agati VD. The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney international 2007;71:491–5. [DOI] [PubMed] [Google Scholar]
- 10.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated With Increased Subsequent Acute Care Utilization Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthritis Care Res 2015;67:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastian HM, Roseman JM, McGwin G Jr., et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002;11:152–60. [DOI] [PubMed] [Google Scholar]
- 12.Huong DL, Papo T, Beaufils H, et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine 1999;78:148–66. [DOI] [PubMed] [Google Scholar]
- 13.Ayodele OE, Okpechi IG, Swanepoel CR. Predictors of poor renal outcome in patients with biopsy-proven lupus nephritis. Nephrology (Carlton, Vic) 2010;15:482–90. [DOI] [PubMed] [Google Scholar]
- 14.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 1996;7:2495–508. [DOI] [PubMed] [Google Scholar]
- 15.Lhotta K, Rumpelt HJ, König P, Mayer G, Kronenberg F. Cigarette smoking and vascular pathology in renal biopsies. Kidney international 2002;61:648–54. [DOI] [PubMed] [Google Scholar]
- 16.Yu F, Wu LH, Tan Y, et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney international 2010;77:820–9. [DOI] [PubMed] [Google Scholar]
- 17.Broder A, Mowrey WB, Khan HN, et al. Tubulointerstitial damage predicts end stage renal disease in lupus nephritis with preserved to moderately impaired renal function: A retrospective cohort study. Semin Arthritis Rheum 2018;47:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Han SS, Qin DD, et al. Renal Interstitial Arteriosclerotic Lesions in Lupus Nephritis Patients: A Cohort Study from China. PloS one 2015;10:e0141547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu LH, Yu F, Tan Y, et al. Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney international 2013;83:715–23. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 21.Merola JF, Bermas B, Lu B, et al. Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus 2014;23:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava A, Palsson R, Kaze AD, et al. The Prognostic Value of Histopathologic Lesions in Native Kidney Biopsy Specimens: Results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018;29:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med 2009;133:233–48. [DOI] [PubMed] [Google Scholar]
- 24.Luciano RL, Moeckel GW. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am J Kidney Dis 2019;73:404–15. [DOI] [PubMed] [Google Scholar]
- 25.Sethi S, Haas M, Markowitz GS, et al. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J Am Soc Nephrol 2016;27:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang A, Gibson IW, Cohen AH, et al. A position paper on standardizing the nonneoplastic kidney biopsy report. Clinical journal of the American Society of Nephrology : CJASN 2012;7:1365–8. [DOI] [PubMed] [Google Scholar]
- 27.Descombes E, Droz D, Drouet L, Grunfeld JP, Lesavre P. Renal vascular lesions in lupus nephritis. Medicine 1997;76:355–68. [DOI] [PubMed] [Google Scholar]
- 28.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annual Symposium proceedings AMIA Symposium 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 29.Bernatsky S, Clarke A, Abrahamowicz M, Neville C, Karp I, Pineau CA. A comparison of prospective and retrospective evaluations of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. J Rheumatol 2005;32:820–3. [PubMed] [Google Scholar]
- 30.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus. Arthritis & Rheumatism 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 31.2017 Researcher’s Guide to the USRDS Database. In: System USRD, ed. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. [Google Scholar]
- 32.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007;13:559–65. [DOI] [PubMed] [Google Scholar]
- 33.Fine JG, RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 34.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res 2011;63:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber C, Herzenberg A, Aghdassi E, et al. Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clinical journal of the American Society of Nephrology : CJASN 2012;7:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saltz M, Sommers SC, Smithwick RH. Clinicopathologic correlations of renal biopsies from essential hypertensive patients. Circulation 1957;16:207–12. [DOI] [PubMed] [Google Scholar]
- 37.Baki AH, Soliman Y, Seif EI. Histopathological Association between Vascular Hypertensive Changes and Different Types of Glomerulopathies. Arab journal of nephrology and transplantation 2014;7:21–6. [PubMed] [Google Scholar]
- 38.Londono Jimenez A, Mowrey WB, Putterman C, Buyon J, Goilav B, Broder A. Brief Report: Tubulointerstitial Damage in Lupus Nephritis: A Comparison of the Factors Associated With Tubulointerstitial Inflammation and Renal Scarring. Arthritis Rheumatol 2018;70:1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney international 2012;82:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney international 1987;31:1153–9. [DOI] [PubMed] [Google Scholar]
- 41.Wilson PC, Kashgarian M, Moeckel G. Interstitial inflammation and interstitial fibrosis and tubular atrophy predict renal survival in lupus nephritis. Clinical Kidney Journal 2017:sfx093-sfx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhingra S, Qureshi R, Abdellatif A, Gaber LW, Truong LD. Tubulointerstitial nephritis in systemic lupus erythematosus: innocent bystander or ominous presage. Histology and histopathology 2014;29:553–65. [DOI] [PubMed] [Google Scholar]
- 43.Pagni F, Galimberti S, Galbiati E, et al. Tubulointerstitial lesions in lupus nephritis: International multicentre study in a large cohort of patients with repeat biopsy. Nephrology 2016;21:35–45. [DOI] [PubMed] [Google Scholar]
- 44.Clark MR, Trotter K, Chang A. The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis. Semin Nephrol 2015;35:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trotter K, Clark MR, Liarski VM. Overview of pathophysiology and treatment of human lupus nephritis. Curr Opin Rheumatol 2016;28:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]