Abstract
Objective:
Sensory over-responsivity (SOR), an atypical negative reaction to sensory stimuli, is highly prevalent in Autism Spectrum Disorders (ASD). Prior work has related SOR to increased brain response in sensory-limbic regions. This study investigated where these atypical responses fall in three fundamental stages of sensory processing: 1) arousal (i.e. initial response); 2) habituation (i.e. change in response over time); and 3) generalization of response to novel stimuli. Different areas of atypical response would require distinct intervention approaches.
Methods:
Functional MRI was used to examine these patterns of neural habituation to two sets of similar mildly aversive auditory and tactile stimuli in 42 high-functioning youth with ASD (21 high-SOR; 21 low-SOR) and 27 age-matched TD youth (age 8-17). The relationship between SOR and change in amygdala-prefrontal functional connectivity across the sensory stimulation was also examined.
Results:
Across repeated sensory stimulation, ASD-high-SOR youth showed reduced ability to maintain habituation in amygdala and relevant sensory cortices, and to maintain inhibition of irrelevant sensory cortices. These results indicate that sensory habituation is a dynamic, time-varying process dependent on sustained regulation across time, which is a particular deficit in ASD-high-SOR youth. However, ASD-low-SOR youth also showed distinct, non-typical neural response patterns including reduced responsiveness to novel but similar stimuli and increases in prefrontal-amygdala regulation across the sensory exposure.
Conclusions:
Results suggest that all children with autism have sensory abnormalities as related to brain function, but whether it is expressed behaviorally depends on top-down regulatory mechanisms. Results are discussed in terms of targeted intervention approaches.
Autism Spectrum Disorders (ASD), estimated to affect at least 1 in 59 children(1), are difficult to characterize neurobiologically due to significant phenotypic heterogeneity among individuals with ASD. A key source of this heterogeneity is sensory processing atypicalities(2), particularly Sensory Over-Responsivity (SOR), which has prevalence rates of 56-70% in individuals with ASD(3). SOR limits individuals’ ability to participate in the community, complete daily living tasks, and interact socially(4-6). SOR is well-characterized behaviorally as avoidance of and/or severe negative responses to sensory stimuli (e.g., noisy environments, scratchy clothing)(7), but until recently, little was known about the neurobiology underlying SOR. Examining the neurobiological underpinnings of heterogeneity in ASD is essential to moving towards a precision medicine approach to treating autism. Neuroimaging studies support the relationship between behavioral heterogeneity in SOR and distinct patterns of brain responses to sensory stimuli, for example, youth with SOR show sensory-limbic over-responsivity to mildly aversive visual, auditory, and tactile stimul(8,9). The mechanisms underlying this over-activation are poorly understood but a recent study(9) suggested that youth with SOR have both slower sensory-limbic habituation and reduced prefrontal regulation of amygdalar responses to sensory stimuli(9). These findings have important implications for intervention: difficulty with habituation contraindicates exposure therapy for SOR. However, additional research is needed to determine whether a) youth with ASD and SOR habituate more slowly or not at all; and b) SOR also relates to difficulties with generalization.
Generalization is key to exposure therapy because it is impossible to conduct graduated exposure on every sensory stimulus and situation (exposure would be less effective if it reduced responses to lawnmowers but not to blenders). Importantly, Green et al.(9) also showed that while ASD individuals without SOR had typical sensory-limbic habituation, they showed atypical increased prefrontal regulation of amygdala activity. Thus, while low-SOR youth showed more typical behavioral sensory responsiveness, their brain responses were distinct from both TD and high-SOR ASD youth. These different profiles could represent compensatory mechanisms and/or atypically high neural demands when processing sensory stimuli despite seemingly typical behavioral responses. A better characterization of the distinct neural profiles of autistic individuals with varied behavioral sensory responses is essential to developing targeted interventions. Therefore, this study examined patterns of responses to mildly aversive sensory stimulation in youth with ASD with high or low behavioral SOR, and in TD youth, in key brain regions of interest (ROIs) identified from prior studies (amygdala, sensory cortices, prefrontal cortex). We focused on neural responses across repeated stimulation using extended initial exposure and a subsequent generalization period where participants were exposed to similar but new stimuli.
Other studies have found habituation, adaptation, and/or inhibitory deficits in both tactile and auditory modalities in ASD. In a tactile discrimination study, adaptation stimuli had a reduced effect on ASD youth, suggesting deficits in habituation and inhibition.(10) Adults with ASD have been found to not reduce their subjective ratings of sounds over time, suggesting habituation difficulty(11). Multiple studies have demonstrated that youth with ASD have atypical pre-pulse inhibition, suggesting reduced sensorimotor gating.(12) Finally, infants at high-risk for autism have reduced auditory-evoked-potential habituation to repeated tones.(13) Taken together, these studies suggest deficits in sensory gating, the process by which an initial stimulus leads to a reduction in response to subsequent stimuli. However, these studies either did not examine how these basic sensory processes related to SOR, or found limited relationships between the two. Responses to a basic pulse or tone may not reflect responses to complex real-world environments. Individuals with ASD have also shown decreased amygdala habituation to higher-level social stimuli(14-16), but no other fMRI studies examined how within-ASD-group differences in SOR relate to neural responses to ecologically valid sensory stimuli across multiple levels (basic sensory processing, emotional arousal, top-down inhibition). We hypothesized that ASD youth with SOR would show deficits in habituation of amygdala and sensory cortices even across longer stimulus presentations than previous examined, as well as deficits in generalization to new but similar stimuli, compared to both TD and low-SOR ASD participants. Additionally, we predicted that ASD youth without SOR would show patterns of habituation and generalization distinct from both TD and ASD youth with SOR, including greater prefrontal down-regulation.
Methods
Participants
Participants were 42 youth with ASD and 27 TD matched controls aged 8.2-18.0 years (M=13.63). Participants had a full-scale IQ within the normal range on the Weschler Abbreviated Scales of Intelligence(17). Groups did not differ significantly in age, motion during fMRI, or Performance IQ. The TD group had significantly higher Verbal and Full-Scale IQ (FSIQ) (Table 1). FSIQ was thus tested as a covariate in all group comparisons. ASD participants had a diagnosis of ASD, confirmed with the Autism Diagnostic Interview–Revised(18) and Autism Diagnostic Observation Schedule(19), and severity assessed with the Vineland and SRS(20) (Table 1). Sixteen ASD participants were taking psychoactive medications (selective serotonin reuptake inhibitors: N=1; psychostimulants: N=7; multiple medications: N=8). There were no significant within-ASD differences between participants with and without medication in any of the ROIs of interest or in connectivity values.
Table 1.
Descriptive statistics.
| High-SOR ASD | Low-SOR ASD | TD | F or χ2 | |
|---|---|---|---|---|
| Age | 13.28 (3.35) | 14.22 (2.32) | 13.53 (2.79) | 0.62 |
| Gender (% male) | 81% (n=17) | 71% (n=15) | 67% (n=18) | 1.22 |
| Handedness (% right-handed) | 95% (n=20) | 95% (n=20) | 89% (n=24) | 0.61 |
| FSIQ | 105.24 (15.20) | 103.62a (15.46) | 112.22a (11.26) | 2.67+ |
| VIQ | 99.10a (17.16) | 101.38 (14.24) | 109.78a (12.36) | 3.68* |
| PIQ | 110.81 (15.62) | 106.19 (18.83) | 111.70 (10.38) | 0.88 |
| Mean Absolute Motion - Exposure | 0.48 (.30) | 0.38 (.22) | 0.42 (.22) | 0.72 |
| Mean Absolute Motion - Gen | 0.49a (.27) | 0.33a (.20) | 0.37 (.25) | 2.49+ |
| Mean Relative motion - Exposure | 0.17 (.13) | 0.15 (.15) | 0.16 (.16) | 0.10 |
| Mean Relative Motion - Gen | 0.15 (.10) | 0.16 (.12) | 0.13 (.05) | 1.00 |
| Volumes Scrubbed - Exposure | 26.71 (19.84) | 24.81 (18.13) | 18.85 (15.55) | 1.28 |
| Volumes Scrubbed - Gen | 21.95a (18.13) | 19.70 (11.10) | 13.46a (10.94) | 2.48+ |
| SensOR tactile count | 6.57 (3.40) | 2.19 (2.14) | 0.59 (0.84) | 42.34*** |
| SensOR auditory count | 7.95 (4.38) | 2.00^ (1.84) | 0.33^ (0.68) | 51.77*** |
| SSP auditory | 6.14 (1.80) | 8.62 (1.53) | 9.89 (0.32) | 48.48*** |
| SSP tactile sensitivity | 24.00 (4.80) | 30.42 (4.18) | 33.96 (1.70) | 44.14*** |
| SOR composite | 1.07 (.63) | −0.19 (.31) | −0.72 (.14) | 124.44*** |
| SSP underresponsivity | 24.05a (6.55) | 26.86b (7.16) | 34.15ab (1.43) | 22.56*** |
| Vineland Adaptive Behavior Composite | 70.95 (12.06) | 84.14 (18.20) | 111.96 (17.21) | 39.99*** |
| SRS total score | 74.64 (8.00) | 62.70 (11.77) | 43.54 (4.55) | 82.19*** |
p<.10,
p<.05,
p<.01;
p<.001.
Denotes the two groups that are significantly different from each other. If no notation in any groups, all three groups are significantly different from each other.
Denotes that these two groups are marginally significant from each other (p<.10) whereas all other groups are significantly different from each other at a minimum of p<.05.
Note: Lower SSP scores indicate higher symptom severity. N=21 High-SOR ASD, 21 Low-SOR ASD, 26 TD except for Generalization analyses where N=21 High-SOR, 20 Low-SOR, 26 TD.
fMRI Sensory Paradigm
Participants were exposed to two consecutive paradigms: an initial “Exposure” phase lasting 8.5-min where they experienced six blocks each of 15-sec auditory, tactile, and “joint” (simultaneous auditory+tactile) conditions. The exposure paradigm was similar to one previously used(9) but longer to allow more time for habituation. The subsequent “Generalization” phase lasted 5.75-min where participants experienced four blocks each of novel but similar 15-sec auditory, tactile, and joint stimuli matched for aversiveness with Exposure stimuli. Auditory stimuli consisted of pulsing pink and violet noise sounds. Tactile stimuli were two different scratchy sponges rubbed on participants’ inner left arms at one stroke/sec. Participants focused on a central fixation cross throughout the task, with 12.5-sec of fixation between trials and 12.5-sec initial and final fixations. Data on these paradigms have not been previously published. Additional details on stimuli and MRI data acquisition in Supplementary Methods.
Behavioral Measures
Child sensory questionnaires were completed by parents (Table 1). An SOR composite score was created by standardizing and averaging auditory and tactile subscales of the SOR measures across all participants. To compare SOR subgroups, ASD was divided into SOR-high and SOR-low by median split on the SOR composite, and compared to the TD group (excluding 1 high-SOR TD). SOR-high vs. low groups did not differ significantly in age, IQ, or overall motion (Table 1). The SOR-high group had significantly more motion during the first half of Generalization (Supplementary Table 1) so motion was tested as a covariate in generalization analyses and included when significant at p<.10.
Short Sensory Profile
(SSP(21)) This parent report measure of sensory dysregulation across modalities is widely used. We used the two auditory sensitivity items from the Auditory/Visual subscale, and the Tactile Sensitivity, and Under-responsive/Seeks Sensation subscales. Higher scores indicate lower impairment. This measure has strong reliability and validity.(22)
Sensory Over-Responsivity (SensOR) Inventory.
(23) This is a parent checklist of sensations that bother their child. The auditory and tactile subscales were used for this study. The number of items parents rate as bothering their child discriminates between children with and without SOR.(23)
MRI Data Acquisition
Scans were acquired on a Siemens Prisma 3-Tesla magnetic resonance imaging scanner. Each functional run involved the acquisition of 706 (Exposure) or 476 (Generalization) multiband EPI volumes (gradient-echo, TR=720ms, TE=37ms, flip angle=52, 104×90 matrix, 208mm FOV, 72 slices, voxel size=2×2×2mm). Prescan-normalize was used after signal inhomogeneities were apparent in the first few scans, and groups were matched on percentage with (75% ASD, 81% TD). Auditory stimuli were presented using magnet-compatible, noise-cancelling headphones. Participants wore earplugs to reduce scanner noise.
fMRI Data Analysis
Analyses were performed using FSL 5.0.10 (www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian Kernel FWHM=5mm), and high-pass temporal filtering (t>0.01 Hz). Functional data were linearly registered to a common stereotaxic space by registering to the MNI152 T1 2mm brain (12 degrees of freedom).
FSL’s fMRI Expert Analysis Tool (FEAT) 6.0 was used for statistical analyses. Fixed-effects models were run separately for each subject, then combined in a higher-level mixed-effects model to investigate within and between-group differences. Single-subject models for all analyses included twelve motion parameters as covariates. Each experimental condition (Auditory, Tactile, or Joint) was modeled with respect to fixation during rest. Higher-level group analyses were carried out using FSL’s FLAME 1&2 (FMRIB’s Local Analysis of Mixed Effects State).(24-26)
Neural habituation.
Change in neural response (“habituation”) to the Joint (auditory+tactile) stimuli across Exposure and Generalization phases in key ROIs was compared between SOR subgroups (SOR-low, SOR-high, TD). ROIs were chosen based on regions shown to be related to SOR in prior studies(8,9) and included right and left amygdala, postcentral gyri, Heschl’s gyrus, and orbitofrontal cortex (OFC), and ventral medial prefrontal cortex (vmPFC). Primary visual cortex (V1) was also examined to determine group differences in inhibition of sensory regions unrelated to the stimuli presented. For each participant, parameter estimates from the six Exposure blocks and four Generalization blocks (vs. fixation) were extracted from the masks. See Supplementary Methods for ROI masks and outlier adjustment details. Repeated-measure ANOVAs examined group differences in ROIs across time; Exposure analyses included three timepoints: EarlyE (blocks 1-2), MiddleE (blocks 3-4), and LateE (blocks 5-6). Generalization analyses also had three timepoints: the LateE block, to examine change from the Exposure to Generalization phase, then EarlyG (blocks 1-2) and LateG (blocks 3-4). ANOVAs included within-subjects factors of block (EarlyE, MiddleE, LateE, or LateE, EarlyG, LateG) and laterality (when relevant), as well as the between-subjects factor of SOR group (SOR-low, SOR-high, TD). FSIQ was included as a covariate if it had an effect at p<0.1.
Functional connectivity.
A psychophysiological interaction (PPI) analysis examined how amygdala-frontal functional connectivity changed from the first to second half of the Joint Exposure phase as a function of SOR. PPI examines the interaction between task and the time series of a seed region (here, right and left amygdala) to identify brain areas where activity is more correlated with the seed region during one part of the task compared to the other (here, the first vs. second half of Joint Exposure). For consistency with habituation analyses, amygdala seeds used were the same as for the habituation analyses (4mm spheres around the peak Exposure coordinates for each group, added together). Because our interest was in prefrontal regulation of the amygdala, we constrained analyses to the frontal lobes using a mask that included frontal pole, frontal gyri, anterior cingulate, and OFC Harvard-Oxford masks. SOR composite score was entered as a regressor to determine frontal regions where change in amygdala connectivity across the Exposure condition related to SOR. Analyses were thresholded at Z>1.7 (p<.05), and cluster-corrected within the frontal lobes at p<.05.
Exposure/Generalization within- and between-group comparisons.
Though not a focus of the current study, whole-brain analyses were run using methods consistent with our prior work(8,9) to allow easy comparisons. Within-group activation maps for each condition (vs. fixation) were thresholded at Z>2.3 (p<.01) and whole-brain cluster-corrected at p<.05 using FSL. Between-group comparison thresholds were Z>1.7 (p<.05), whole-brain cluster-corrected at p<.05; only clusters with peaks of Z>2.3 are reported as significant. FSIQ was covaried in all between-group analyses.
Results
Behavioral Results
Independent-sample t-tests showed that the ASD group had significantly more severe SOR symptoms than the TD group (Table 1). The SOR-high group (by definition) had higher SOR scores than the SOR-low group; there were no group differences in under-responsivity (demographic comparisons in Table 1).
fMRI Results
Neural habituation during Joint condition
The habituation analyses compared ASD-high-SOR, ASD-low-SOR, and TD groups on initial response to the Joint auditory+tactile stimuli, and change over time (habituation) in BOLD responses across: 1)Exposure phase - brain response across early, middle, and late timepoints of the initial six blocks of sensory stimulation; 2)Generalization phase - change in brain response from the late timepoint of Exposure through the first and second halves of sensory stimulation with novel but similar stimuli. Analyses were conducted within key ROIs: sensory cortex and frontal-limbic regions (amygdala, OFC, vmPFC). There were no significant group differences in initial response to the Joint stimuli in any ROI, thus results below focus on group differences in the linear (slope) and quadratic (change in slope) change over time (Figures 1-2). Full ANOVA statistics in Table 2a-b and Supplementary Results.
Figure 1.
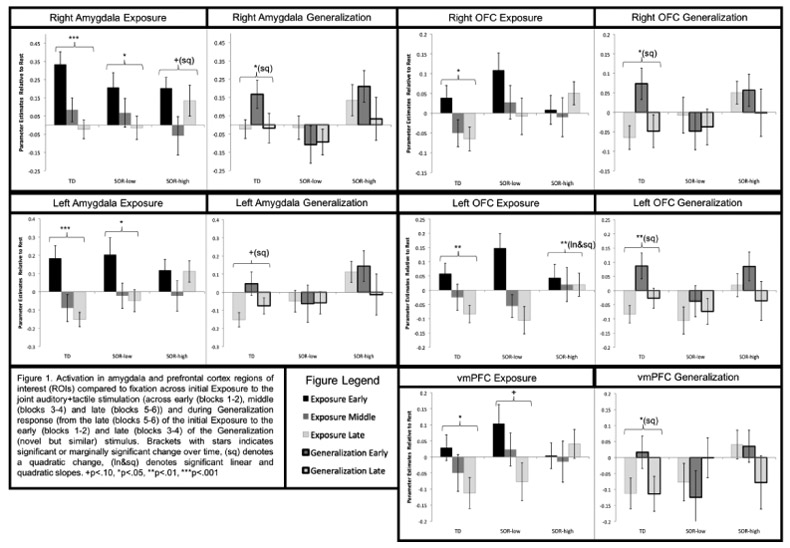
Activation in amygdala and prefrontal cortex regions of interest (ROIs) across initial Exposure to the joint auditory+tactile stimulation (across early (blocks 1-2), middle (blocks 3-4) and late (blocks 5-6)) and during Generalization response (from the late (blocks 5-6) of the initial Exposure to the early (blocks 1-2) and late (blocks 3-4) of the Generalization (novel but similar) stimulus. Brackets with stars indicates significant or marginally significant change over time, (sq) denotes a quadratic change, (ln&sq) denotes significant linear and quadratic slopes. +p<.10, *p<.05, **p<.01, ***p<.001.
Figure 2.
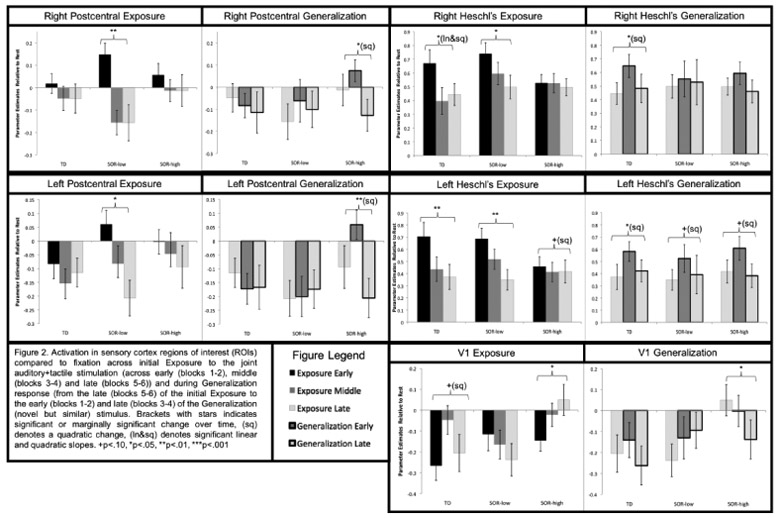
Activation in sensory cortex regions of interest (ROIs) across initial Exposure to the joint auditory+tactile stimulation (across early (blocks 1-2), middle (blocks 3-4) and late (blocks 5-6)) and during Generalization response (from the late (blocks 5-6) of the initial Exposure to the early (blocks 1-2) and late (blocks 3-4) of the Generalization (novel but similar) stimulus. Brackets with stars indicates significant or marginally significant change over time, (sq) denotes a quadratic change, (ln&sq) denotes significant linear and quadratic slopes. +p<.10, *p<.05, **p<.01, ***p<.001.
Table 2a.
Repeated-measures ANOVA predicting changes in amygdala and pre/postcentral gyrus activation across the scan by SOR category.
| Amygdala | Postcentral Gyrus | vmPFC | |||||
|---|---|---|---|---|---|---|---|
| MS | F | MS | F | MS | F | ||
| Exposure | Main effect of time | ||||||
| Linear | 2.86 | 20.19*** | 1.27 | 10.94** | 0.30 | 5.94* | |
| Quadratic | 1.11 | 5.90* | 0.01 | 0.07 | 0.006 | 0.11 | |
| Main effect of SOR | 0.02 | 0.09 | 0.13 | 0.67 | 0.08 | 1.02 | |
| TimeXSOR | |||||||
| Linear | 0.57 | 4.01* | 0.37 | 3.18* | 0.14 | 2.83+ | |
| Quadratic | 0.11 | 0.60 | 0.04 | 0.49 | 0.01 | 0.14 | |
| Main effect of laterality | 0.53 | 12.92** | 0.70 | 24.71*** | -- | -- | |
| SORXlaterality | 0.17 | 4.14* | 0.01 | 0.17 | -- | -- | |
| TimeXlaterality | |||||||
| Linear | 0.01 | 0.08 | 0.004 | 0.25 | -- | -- | |
| Quadratic | 0.001 | 0.04 | 0.02 | 2.07 | -- | -- | |
| TimeXlateralityXSOR | |||||||
| Linear | 0.01 | 0.19 | 0.01 | 0.38 | -- | -- | |
| Quadratic | 0.05 | 2.26 | 0.01 | 1.14 | -- | -- | |
| Generalization | Main effect of time | ||||||
| Linear | 0.05 | 0.25 | 0.004 | 0.02 | 0.00 | 0.00 | |
| Quadratic | 0.78 | 4.04* | 0.31 | 2.48 | 0.002 | 0.03 | |
| Main effect of SOR | 0.90 | 2.98+ | 0.35 | 1.15 | 0.18 | 2.37 | |
| TimeXSOR | |||||||
| Linear | 0.19 | 0.92 | 0.15 | 0.75 | 0.10 | 1.23 | |
| Quadratic | 0.38 | 1.98 | 0.41 | 3.30* | 0.16 | 2.01 | |
| Main effect of laterality | 0.20 | 3.22+ | 0.02 | 0.67 | -- | -- | |
|
SORXlaterality TimeXlaterality |
|||||||
| Linear | 0.02 | 0.40 | 0.00 | 0.01 | -- | -- | |
| Quadratic | 0.00 | 0.01 | 0.002 | 0.17 | -- | -- | |
| TimeXlateralityXSOR | |||||||
| Linear | 0.01 | 0.26 | 0.01 | 0.16 | -- | -- | |
| Quadratic | 0.01 | 0.38 | 0.04 | 3.44* | -- | -- | |
| Main effect of IQ | -- | -- | 0.15 | 0.48 | -- | -- | |
| TimeXIQ | |||||||
| Linear | -- | -- | 0.00 | 0.002 | -- | -- | |
| Quadratic | -- | -- | 0.40 | 3.22+ | -- | -- | |
| Main effect of motion | -- | -- | -- | -- | 0.36 | 4.82* | |
| TimeXMotion | |||||||
| Linear | -- | -- | -- | -- | .001 | .01 | |
| Quadratic | -- | -- | -- | -- | .04 | 0.55 | |
p<.10;
p<.05;
p<.01;
p<.001.
Note: SOR indicates a comparison of the three SOR category groups: ASD-low SOR, ASD-high SOR, and TD-no SOR
Table 2b.
Repeated-measures ANOVA predicting changes in auditory, visual, and orbital frontal cortex activation across the scan by diagnostic status and by SOR category.
| Heschl’s Gyrus | V1 | Orbital Frontal | |||||
|---|---|---|---|---|---|---|---|
| MS | F | MS | F | MS | F | ||
| Exposure | Main effect of time | ||||||
| Linear | 0.00 | 0.001 | 0.07 | 0.55 | 0.67 | 10.63** | |
| Quadratic | 0.16 | 1.55 | 0.25 | 2.45 | 0.10 | 1.95 | |
| Main effect of SOR | 0.01 | 0.01 | 0.39 | 2.88+ | 0.08 | 0.96 | |
| TimeXSOR | |||||||
| Linear | 0.41 | 3.09+ | 0.27 | 2.18 | 0.20 | 3.28* | |
| Quadratic | 0.05 | 0.52 | 0.16 | 1.51 | 0.01 | 0.11 | |
| TimeXSORXIQ | |||||||
| Linear | 0.53 | 3.95* | -- | -- | -- | -- | |
| Quadratic | 0.68 | 0.64 | -- | -- | -- | -- | |
| Main effect of laterality | 0.10 | 1.37 | -- | -- | 0.01 | 0.45 | |
| SORXlaterality | 0.06 | 0.83 | -- | -- | 0.04 | 2.05 | |
| SORXIQXlaterality | 0.04 | 0.56 | -- | -- | |||
| TimeXlaterality | |||||||
| Linear | 0.13 | 6.18* | -- | -- | 0.11 | 14.33*** | |
| Quadratic | 0.00 | 0.00 | -- | -- | 0.00 | 0.001 | |
| TimeXlateralityXSOR | |||||||
| Linear | 0.01 | 0.57 | -- | -- | 0.02 | 1.97 | |
| Quadratic | 0.03 | 1.83 | -- | -- | 0.02 | 2.16 | |
| Main effect of IQ | 3.46 | 4.91* | -- | -- | -- | -- | |
| Generalization | Main effect of time | ||||||
| Linear | 1.66 | 5.17* | 0.03 | 0.21 | 0.00 | 0.006 | |
| Quadratic | 0.09 | 0.47 | 0.89 | 6.97* | 0.23 | 3.61+ | |
| Main effect of SOR | 0.77 | 1.09 | 0.52 | 2.63+ | 0.35 | 3.95* | |
| TimeXSOR | |||||||
| Linear | 0.38 | 1.17 | 0.80 | 5.38** | 0.05 | 0.74 | |
| Quadratic | 0.51 | 2.77+ | 0.004 | 0.03 | 0.11 | 1.66 | |
| Main effect of laterality | 0.004 | 0.08 | -- | -- | 0.03 | 1.91 | |
| SORXlaterality | 0.13 | 2.46+ | -- | -- | 0.03 | 1.80 | |
| TimeXlaterality | |||||||
| Linear | 0.11 | 3.11+ | -- | -- | 0.00 | 0.008 | |
| Quadratic | 0.02 | 1.06 | -- | -- | 0.04 | 2.95+ | |
| TimeXlateralityXSOR | |||||||
| Linear | 0.10 | 2.78+ | -- | -- | 0.01 | 0.80 | |
| Quadratic | 0.01 | 0.27 | -- | -- | 0.01 | 0.69 | |
| Main effect of IQ | 0.11 | 0.20 | 0.01 | 0.07 | -- | -- | |
| TimeXIQ | |||||||
| Linear | 1.28 | 3.98+ | 0.10 | 0.63 | |||
| Quadratic | 0.12 | 0.09 | 0.72 | 5.64* | |||
| Main effect of mean motion | 2.14 | 3.06+ | 1.53 | 7.79** | 0.45 | 4.67* | |
| TimeXMotion | |||||||
| Linear | 0.61 | 1.88 | 0.22 | 1.47 | 0.001 | 0.01 | |
| Quadratic | 0.09 | 0.47 | 0.68 | 5.34* | 0.02 | 0.31 | |
p<.10;
p<.05;
p<.01;
p<.001.
Note: SOR indicates a comparison of the three SOR category groups: ASD-low SOR, ASD-high SOR, and TD-no SOR
Somatosensory cortex.
The groups differed significantly in habituation during both Exposure and Generalization phases. The SOR-low group showed significant decreases in postcentral gyrus across the initial auditory+tactile exposure, whereas TD and SOR-high groups had no significant change. In response to the Generalization stimuli, the SOR-high group showed first increased, then decreased responses; the other two groups showed no significant change. Group differences in the Generalization phase were greater for left than for right postcentral gyrus, driven by the TD and SOR-low groups inhibiting the (irrelevant) left postcentral gyrus.
Auditory cortex.
Activation decreased bilaterally across the Exposure phase for SOR-low and TD groups, but just as seen in somatosensory cortex, the SOR-high group showed no significant habituation. For the Generalization stimuli, all three groups showed an increase, then decrease in left auditory cortex, but only the TD group showed this pattern in right auditory cortex; neither ASD group showed right hemisphere auditory changes.
Primary visual cortex (V1).
Because the SOR-high group activated left somatosensory cortex, a region not expected to respond to the left-arm stimulation, we further investigated atypical inhibition (decreased activity vs. baseline) in extraneous sensory cortices by examining V1. All three groups showed initial inhibition of V1, but this inhibition was sustained for TD and SOR-low groups whereas the SOR-high group had significant V1 increases across the Exposure phase, and overall greater activation than either SOR-low or TD subjects. The TD and SOR-low groups continued to show V1 inhibition throughout the Generalization phase, though the SOR-low group had trend-level increases (still below baseline). The SOR-high group showed significant decreases in V1 activation, from significant activation at the end of the Exposure phase to significant inhibition at the end of the Generalization phase.
Amygdala.
The SOR-low and TD groups showed sustained amygdala decreases across the Exposure phase, whereas the SOR-high group initially decreased and then increased amygdala response. For the Generalization stimulus, only the TD group initially increased then decreased response. Additionally, the SOR-high group had significantly higher amygdala response than the SOR-low group across the Generalization phase: the SOR-high group started high and stayed high, whereas the SOR-low group started low and stayed low, showing no significant amygdala response to the novel stimulus.
Prefrontal cortex.
Bilateral OFC and vmPFC activity decreased significantly across the Exposure period for SOR-low and TD groups only. In response to the Generalization stimulus, all groups showed initial bilateral OFC signal increases and then subsequent decreases. Across the Generalization phase, there was significantly greater OFC activation in the SOR-high compared to the SOR-low group. The vmPFC showed a similar pattern of activation in the Generalization phase, but the change in activation, TimeXSOR interaction, and main effect of SOR were not significant.
Functional connectivity.
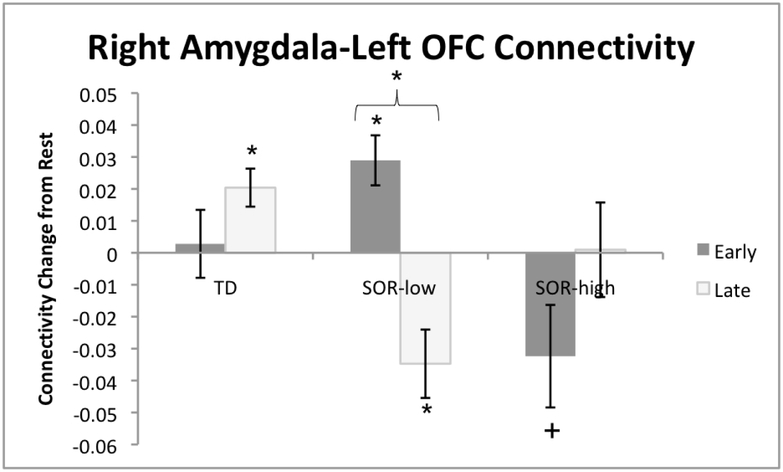
Considering that SOR was related to reduced amygdala habituation, we did a functional connectivity analysis to examine how SOR related to changes in amygdala-prefrontal connectivity from the first to second half of Exposure to the auditory+tactile stimuli. Within the ASD group, SOR was correlated with significant connectivity changes between right amygdala and left OFC. There was no correlation between SOR and change in left amygdala connectivity. To determine the direction of effects, we extracted parameter estimates from the left OFC and conducted a repeated-measures ANOVA with SOR group as a between-groups factor and Time as within-subjects factor (Figure 3; Supplementary Results). There was (by definition) an SOR*Time effect. Post-hoc analysis showed that the SOR-low group switched from positive to negative connectivity across the Exposure phase, whereas the SOR-high group had significant negative connectivity in the first half of Exposure only (the difference with the second half of Exposure did not reach significance). The TD group showed no significant changes in connectivity.
Figure 3.
Results from psychophysiological interaction (PPI) analysis examining how SOR related to changes in amygdala-prefrontal connectivity from the first to second half of Exposure to the auditory+tactile stimuli. Figure illustrates results from bottom-up analysis, using extracted parameter estimates of connectivity with right amygdala from the left orbital frontal cortex (OFC) separately in the first half (Early) and second half (Late) of Exposure. The SOR-low group switched from positive to negative connectivity across the Exposure phase, whereas the SOR-high group initially had significant negative connectivity values which then changed to non-significant by the second half of Exposure, though this change did not reach significance. The TD group showed no significant changes in connectivity. Brackets with stars indicates significant change over time, stars above or below a bar denotes a significant change from baseline. +p<.10, *p<.05.
Whole-brain within- and between-group results.
During the initial Joint (auditory+tactile) Exposure, the ASD group had greater activation in bilateral parietal lobule/postcentral gyrus, left precentral gyrus, left amygdala, right superior temporal gyrus, right OFC, and mPFC. During Joint Generalization, the ASD group had greater activation in right parietal lobule/postcentral gyrus and precentral gyrus. There were no significant TD>ASD differences. Auditory and Tactile conditions are described in the Supplement; see Supplementary Tables 2-7 and Supplementary Figures 1-3.
Discussion
In this study, we demonstrate that children and adolescents with ASD who have low or high SOR evidence atypical and distinct patterns of brain responses to multiple aversive sensory stimuli, and generalization of response to new, similar stimuli. These patterns suggest unique underlying neurobiological mechanisms for sensory processing difficulties in autism:
For individuals with ASD, habituation is a dynamic process involving top-down regulation, which is impaired in high-SOR ASD youth. High-SOR ASD youth lacked habituation across sensory cortices and amygdala, consistent with our prior findings,(9) which are extended here to show that longer sensory exposure does not allow delayed habituation. Rather, amygdala decreases were unsustained, which, along with the finding that the high-SOR ASD group showed initial but unsustained amygdala-prefrontal connectivity, suggests the high-SOR group cannot maintain regulation of sensory responses across extended periods of time. This contrasts with what we observed in the low-SOR group, which switched from positive to negative amygdala-prefrontal connectivity across sensory exposure, suggesting they are engaging regulatory processes, consistent with their significant habituation of sensory and amygdala responses. Strikingly, though we explored amygdala connectivity with the entire frontal lobe, the right amygdala-left OFC connectivity found here to be associated with SOR was the same as previously found in Green et al. (2015).(9) Notably, we further show here that the key SOR-group difference is in their patterns of connectivity change across sensory exposure.
Unlike high-SOR ASD youth, low-SOR youth lacked neural responsiveness to new but similar stimuli, potentially indicating over-regulation, difficulty with discrimination, or hyporesponsivity. The low-SOR group showed no significant change in responses to the new stimuli in any sensory or fronto-amygdala regions of interest. This hyporesponsivity to the new stimuli could be caused by over-regulation in which an initially adaptive inhibition of response becomes over-generalized, inhibiting responses to new information, which is supported by EEG research.(27,28) In contrast, the TD group showed an increase to the new stimulus that rapidly attenuated in most regions. The high-SOR group also had higher responsiveness to the new stimuli in most regions, but –notably- no significant change in responsiveness from the end of the initial exposure period.
Finally, ASD with SOR cannot maintain inhibition of irrelevant sensory cortices, leading to reduced cross-modality segregation of sensory responses. Following auditory and left-arm tactile stimulation, TD and low-SOR ASD participants showed inhibition (decreased activation compared to fixation) in left postcentral gyrus and visual cortex. In contrast, the high-SOR ASD group showed a marked inability to maintain downregulation across sensory exposure and/or with a novel stimulus. This is consistent with the high-SOR pattern of inability to maintain prefrontal-amygdala regulation. Reduced inhibition of irrelevant sensory cortices is also consistent with findings by Keehn et al.(29) showing impaired downregulation of visual cortex during auditory processing; importantly, we further show that SOR-related downregulation abnormalities are dynamic across time. This inability to maintain downregulation across time is consistent with the emerging theory of GABA/Glutamate imbalance.(30-32) Children with ASD show reduced sensorimotor GABA levels, associated with a lower tactile dynamic detection threshold, suggesting abnormalities in feed-forward inhibitory mechanisms.(32)
However, a limitation of this study is the lack of detailed under-responsivity measures. Research on sensory subgroupings within ASD(33) suggests a subgroup with severe mixed hypo- and hyper-responsivity, consistent with our “high-SOR” group having the highest scores on both SOR and under-responsivity. Other subgroups include mild hypo-responsivity, and mild hyper-responsivity,(33) consistent with our “low-SOR” group which shows lower SOR and under-responsivity scores than the high-SOR group, but still higher than typical. Therefore, the high- and low-SOR groups may actually be better characterized as severe and mild sensory groups; future research including more in-depth hypo-responsivity measures and observational sensory measures can better characterize these groups. Given the relatively small sample size of the current study, replication with a larger sample is necessary both to confirm the results and to characterize potentially mixed profiles within the low-SOR group.
Additionally, future research should address the effect of development on SOR and its neural correlates; age-related increases in prefrontal regulation of the amygdala could underlie age-related decreases in SOR seen in some individuals with ASD(34).
Conclusions and Clinical Implications
Taken together, our results indicate that in ASD, sensory habituation is a dynamic, time-varying process dependent on sustained regulation across time, which is specifically impaired in ASD youth with high SOR. However, ASD youth with low SOR also show distinct, non-typical response patterns including increased prefrontal regulation and reduced reactivity to new stimuli. These results validate the need for future research testing personalized interventions for SOR; while our findings indicate that sensory processing is atypical across most ASD youth, different subgroups show distinct profiles that go beyond simple over- or under-responsivity classifications. Accordingly, exposure therapy could be more effective for low-SOR youth, who show ability to downregulate, than for high-SOR youth, who cannot maintain downregulation and may be better served by building top-down coping strategies. However, exposure therapy for low-SOR youth may need to be paired with interventions that enhance the salience of novel cues and prevent over-regulation.
Supplementary Material
Acknowledgments and Funding
This work was supported by grants from the the Simons Foundation Autism Research Initiative (grant number 345389), National Institute of Child Health and Human Development (P50 HD055784), and the National Institute of Mental Health (R01MH100028; K08 MH112871). The authors were also supported by the following training grants/fellowships: a National Research Service Award postdoctoral fellowship to SG (F32 MH105167), a National Institute of Neurological Disorders and Stroke pre/post-doctoral training grant (F99 NS105206) to L.M.H., an NICHD predoctoral fellowship (F31 HD088102) to J.L., a National Institute of Neurological Disorders and Stroke institutional training grant (T32NS048004) and a National Research Service Award predoctoral fellowship (F31MH110140) to K.L., fellowships from the Roche/ARCS Foundation Scholar Award Program in the Life Sciences to K.L. and J.L, and a National Research Service Award predoctoral fellowship (F31HD090937) to T.T.
Drs. Green, and Hernandez, Katherine Lawrence, Janelle Liu, Dr. Tsang, Jillian Yeargin, Kaitlin Cummings, and Drs. Laugeson, Dapretto and Bookheimer report no biomedical financial interests or potential conflicts of interests.
For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project described was supported by Grant Numbers RR12169, RR13642 and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
These research efforts were conducted in part under the auspices of The Help Group-UCLA Autism Research Alliance, which contributed to participant recruitment.
Footnotes
Author Disclosures: Drs. Green, and Hernandez, Katherine Lawrence, Janelle Liu, Dr. Tsang, Jillian Yeargin, Kaitlin Cummings, and Drs. Laugeson, Dapretto and Bookheimer report no biomedical financial interests or potential conflicts of interests.
References
- 1.Baio J Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ [Internet]. 2018. [cited 2018 Aug 16];67 Available from: https://www.cdc.gov/mmwr/volumes/67/ss/ss6706a1.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uljarević M, Baranek G, Vivanti G, Hedley D, Hudry K, Lane A. Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Res Off J Int Soc Autism Res. 2017. May;10(5):703–10. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J Autism Dev Disord. 2008. May 30;39(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 4.Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, Baranek GT. Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Res. 2016. April 1;1316–1327. [DOI] [PubMed] [Google Scholar]
- 5.Glod M, Riby DM, Honey E, Rodgers J. Psychological Correlates of Sensory Processing Patterns in Individuals with Autism Spectrum Disorder: A Systematic Review. Rev J Autism Dev Disord. 2015. April 15;2(2): 199–221. [Google Scholar]
- 6.Pfeiffer B, Kinnealey M, Reed C, Herzberg G. Sensory modulation and affective disorders in children and adolescents with Asperger’s disorder. Am J Occup Ther Off Publ Am Occup Ther Assoc. 2005. June;59(3):335–45. [DOI] [PubMed] [Google Scholar]
- 7.Liss M Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006. March 1; 10(2): 155–72. [DOI] [PubMed] [Google Scholar]
- 8.Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, et al. Overreactive Brain Responses to Sensory Stimuli in Youth With Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013. November;52(11):1158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry [Internet]. 2015. June 10 [cited 2015 Jul 10]; Available from: 10.1001/jamapsychiatry.2015.0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puts NAJ, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RAE. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014. May 1; 111(9): 1803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson RP, Aylward J, White S, Rees G. A striking reduction of simple loudness adaptation in autism. Sci Rep [Internet]. 2015. November 5 [cited 2015 Nov 12];5 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4633623/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng C-H, Chan P-YS, Hsu S-C, Liu C-Y. Meta-analysis of sensorimotor gating in patients with autism spectrum disorders. Psychiatry Res [Internet]. 2017. September 12 [cited 2017 Sep 26];0(0). Available from: http://www.psy-journal.com/article/S0165-1781(17)30450-X/fulltext [DOI] [PubMed] [Google Scholar]
- 13.Guiraud JA, Kushnerenko E, Tomalski P, Davies K, Ribeiro H, Johnson MH, et al. Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport. 2011. November 16;22(16):845–9. [DOI] [PubMed] [Google Scholar]
- 14.Kleinhans N, Johnson L, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166(4):467–475. [DOI] [PubMed] [Google Scholar]
- 15.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala Habituation and Prefrontal Functional Connectivity in Youth With Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013. January;52(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam FI, King JA, Geisler D, Korb FM, Sareng J, Ritschel F, et al. Altered behavioral and amygdala habituation in high-functioning adults with autism spectrum disorder: an fMRI study. Sci Rep. 2017. October 19;7(1):13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harbourt Brace & Company; 1999. [Google Scholar]
- 18.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994. October;24(5):659–85. [DOI] [PubMed] [Google Scholar]
- 19.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Western Psychological Services; 2012. [Google Scholar]
- 20.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003;33(4):427–33. [DOI] [PubMed] [Google Scholar]
- 21.Dunn W The Sensory Profile: User’s Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 22.McIntosh DN, Miller LJ. Evaluation of Sensory Processing In: Dunn W, editor. The Sensory profile: Examiner’s manual. San Antonio, TX: The Psychological Corporation; 1999. p. 59–73. [Google Scholar]
- 23.Schoen SA, Miller LJ, Green KE. Pilot study of the sensory over-responsivity scales: Assessment and inventory. Am J Occup Ther. 2008;62:393–406. [DOI] [PubMed] [Google Scholar]
- 24.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003. October;20(2):1052–63. [DOI] [PubMed] [Google Scholar]
- 25.Woolrich M Robust group analysis using outlier inference. NeuroImage. 2008. June;41(2):286–301. [DOI] [PubMed] [Google Scholar]
- 26.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004. April;21(4):1732–47. [DOI] [PubMed] [Google Scholar]
- 27.Simon DM, Damiano CR, Woynaroski TG, Ibañez LV, Murias M, Stone WL, et al. Neural Correlates of Sensory Hyporesponsiveness in Toddlers at High Risk for Autism Spectrum Disorder. J Autism Dev Disord. 2017. September;47(9):2710–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascio CJ, Gu C, Schauder KB, Key AP, Yoder P. Somatosensory Event-Related Potentials and Association with Tactile Behavioral Responsiveness Patterns in Children with ASD. Brain Topogr. 2015. November 1;28(6):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jao Keehn RJ, Sanchez SS, Stewart CR, Zhao W, Grenesko-Stevens EL, Keehn B, et al. Impaired downregulation of visual cortex during auditory processing is associated with autism symptomatology in children and adolescents with autism spectrum disorder. Autism Res. 2016. May 1;130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takarae Y, Sweeney J. Neural Hyperexcitability in Autism Spectrum Disorders. Brain Sci [Internet]. 2017. October 13 [cited 2018 Feb 14];7(10). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5664056/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee E, Lee J, Kim E. Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biol Psychiatry. 2017. May 15;81(10):838–47. [DOI] [PubMed] [Google Scholar]
- 32.Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2016. September 1;608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ausderau KK, Furlong M, Sideris J, Bulluck J, Little LM, Watson LR, et al. Sensory subtypes in children with autism spectrum disorder: latent profile transition analysis using a national survey of sensory features. J Child Psychol Psychiatry. 2014. August 1;55(8):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern JK. The pattern of sensory processing abnormalities in autism. Autism. 2006. September 1;10(5):480–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.