Abstract
The impact of infections and inflammation during pregnancy on the developing fetal brain remains incompletely defined with important clinical and research gaps. Though the classic infectious TORCH pathogens [i.e. Toxoplasma gondii, rubella virus, cytomegalovirus (CMV), herpes simplex virus] are known to be directly teratogenic, emerging evidence suggests that these infections represent the most extreme end of a much larger spectrum of injury. We present the accumulating evidence that prenatal exposure to a wide variety of viral and bacterial infections – or simply inflammation – may subtly alter fetal brain development, leading to neuropsychiatric consequences for the child later in life. The link between influenza infections in pregnant women and an increased risk for development of schizophrenia in their children was first described more than 30 years ago. Since then, evidence suggests that a range of infections during pregnancy may also increase risk for autism spectrum disorder and depression in the child. Subsequent studies in animal models demonstrated that both pregnancy infections and inflammation can result in direct injury to neurons and neural progenitor cells or indirect injury through activation of microglia and astrocytes, which can trigger cytokine production and oxidative stress. Infectious exposures can also alter placental serotonin production, which can perturb neurotransmitter signaling in the developing brain. Clinically, detection of these subtle injuries to the fetal brain is difficult. As the neuropsychiatric impact of perinatal infections or inflammation may not be known for decades after birth, our construct for defining teratogenic infections in pregnancy (e.g. TORCH) based on congenital anomalies is insufficient to capture the full adverse impact on the child. We discuss the clinical implications of this body of evidence and how we might place greater emphasis on prevention of prenatal infections. For example, increasing uptake of the seasonal influenza vaccine is a key strategy to reduce perinatal infections and the risk for fetal brain injury. An important research gap exists in understanding how antibiotic therapy during pregnancy impacts the fetal inflammatory load and how to avoid inflammation-mediated injury to the fetal brain. In summary, we discuss the current evidence and mechanisms linking infections and inflammation with the increased lifelong risk of neuropsychiatric disorders in the child, and how we might improve prenatal care to protect the fetal brain.
Keywords: pregnancy, infection, inflammation, fetus, brain, schizophrenia, depression, autism, influenza virus, urinary tract infection, TORCH, microglia, neuronal injury, seasonality of birth hypothesis
Condensation
This review summarizes new evidence for how infections during pregnancy can alter fetal brain development and predispose the child to mental illness decades after birth.
Introduction
The impact of infection and inflammation on the developing fetal brain is poorly understood but is thought to increase the lifetime risk for some types of mental illness. The severe infectious teratogens known by the acronym TORCH [e.g. Toxoplasma gondii, rubella virus, cytomegalovirus, herpes simplex virus] have commanded a focal point in obstetrics due to their potential to cause catastrophic structural anomalies in the fetal brain including anencephaly, ventriculomegaly, deafness, and ocular injury.1–5 However, evidence that other perinatal infections may increase the lifetime risk of schizophrenia for the fetus has accumulated for more than half a century.6 By the 1960s, several studies found a slight increase in the incidence of schizophrenia among children and adults that had been born during the winter months in both northern and southern hemispheres, suggesting a link with viral infections more prevalent during the winter.6–8 These observations led to a “seasonality of birth” hypothesis suggesting that some proportion of adult schizophrenia was caused by virus-induced fetal brain injury.9
Subsequent studies in humans and mouse models linked prenatal exposure to single pathogens, complex infections, and inflammatory disorders with changes in fetal brain development leading to a wide spectrum of cognitive deficits and neuropsychiatric disorders including autism spectrum disorder (ASD).10,11 Recently, the concerning finding that maternal hospitalization with any infection in pregnancy, including urinary tract infections, increased risk of ASD and depression in the exposed offspring suggests that the fetal brain may be more vulnerable than previously thought to a wide variety of infections.11 Overall, it appears that a broad category of infectious and inflammatory events in pregnancy can result in an increased risk of neuropsychiatric disease for exposed children. This evidence requires a reconception of infectious risks during pregnancy beyond those imparted by TORCH pathogens. In this review, we aim to highlight what is currently known about the fetal infectious and inflammatory origins of mental illness. We also discuss the clinical and research implications of how we might reconsider infection prevention and treatment with an emphasis on protecting the fetal brain.
Infectious Prenatal Origins of Schizophrenia, Autism Spectrum Disorder, Bipolar Disorder and Depression
Schizophrenia
The earliest studies of psychiatric disease after exposure to infection in utero focused on schizophrenia. This disorder is typically first diagnosed in early adult life and has been associated with events occurring early in brain development; accordingly, many studies have focused on pregnancy complications and the role of infectious exposures.12 Evidence for the fetal origins of schizophrenia risk include: numerous studies of in utero infection across trimesters,13 an archival cohort study of gestational starvation during the so-called “Dutch Hunger Winter” of Nazi occupation,14 data from the famine years in China’s Anhui Province,15 and studies on the effect of smoking16 and limited maternal weight gain.17 In the 1960s and 1970s, multiple studies found an increased incidence of schizophrenia among adults born during the winter months, suggesting an association with fetal exposure to maternal viral infections; these and other studies culminated in a “seasonality of birth” hypothesis for the etiology of schizophrenia.6–9,18–21
The 1957 influenza pandemic offered an opportunity to study the long-term mental health outcomes of adults who were likely to have been prenatally exposed to influenza. In a study of Finnish adults, there was a markedly higher risk of hospitalization for schizophrenia in adults who were fetuses in the second trimester during the peak of the 1957 influenza epidemic compared to adults who were born in the 6 years prior to the epidemic.22 This “second trimester” effect was observed independently across several greater Helsinki psychiatric hospitals and occurred in both men and women. Subsequent studies focused on serologic testing as a method to link schizophrenia with perinatal exposure to a variety of microbes.23–27 Overall, these studies strongly implicated perinatal infections and complications as risk factors for schizophrenia, but were limited by insufficient power and were mainly exploratory in nature. Significant variability in study exposures and subjects has made systematic reviews of this body of work difficult to interpret, but the preponderance of evidence suggests that prenatal infection and inflammation play important roles in some proportion of schizophrenia.28
Autism
Several systematic and meta-analytic reviews provide converging evidence that infections during pregnancy elevate the risk for ASD in the offspring.29–31 A meta-analysis of 15 studies with more than 40,000 ASD cases demonstrated an increased risk for ASD after prenatal exposure to infection (OR = 1.13, 95% confidence interval (CI): 1.03–1.23)), particularly when the mother was hospitalized for the infection (OR = 1.30, 95% CI: 1.14–1.50).31 The largest of these studies in the meta-analysis could not determine whether the timing of infection during pregnancy was important, but was likely underpowered to detect trimester effects.34 Prenatal fever has also been associated with development of ASD in the Norwegian Mother and Child Cohort Study (114,500 pregnant women). In this study, a second trimester prenatal fever was associated with a 1.40 adjusted odds ratio [aOR; 95% confidence interval (CI) 1.1–1.8]; multiple fevers were associated with an even higher risk of ASD (aoR 3.1, 95% CI 1.3–7.6 with 3 or more fevers). Animal models of both viral and bacterial infections in rodents and rhesus macaques support these findings; maternal infections have been associated with ASD-like phenotypes in the offspring with reduced socialization, atypical vocalizations, and repetitive behaviors.35–45 Both maternal and immune system dysfunction have emerged as central mechanisms that tie together many of the proposed environmental and pregnancy risk factors for ASD.32 For example, there is a clear linkage between the inflammatory response and both environmental toxicants46–48 and obesity.49,50 Meta-analyses also consistently demonstrate small, but significant and precise associations of family history of autoimmune disorders and ASD in offspring.51,52
Further, sexually dimorphic differences in the differential expression of innate immune genes in the brain are implicated in the strong male bias for ASD.53–55 Overall, the evidence supports a role for prenatal infections and other sources of maternal-fetal immune activation in the fetal origins of ASD.
Bipolar Disorder and Depression
The link between exposure to prenatal infections and development of bipolar disorder and depression is less clear. While there have been several studies to determine whether maternal infections during pregnancy increased the risk of bipolar disorder in the child, the results have been mixed and suffered from insufficient power and lack of correction for multiple hypothesis testing.56–58 In at least one study, maternal influenza infection was not linked with development of classical bipolar disorder in the child, but instead was associated with bipolar disorder with psychotic features.59 A recent study similarly found no increased risk for bipolar after maternal infection.11 Reflecting this uncertainty, a systematic review of risk of bipolar disorder after perinatal infection determined that results were mixed and more research was needed.60
There have been comparatively few studies examining the possible increased risk for depression after prenatal exposure to inflammation or infection and the results have also been mixed.11,58,61–70 However, many of these studies have relied on maternal self-report of infection during pregnancy or have studied depression outcomes of adults born during epidemics. Recent evidence from a population-based cohort in Sweden demonstrated increased risk of depression after fetal exposure to any type of hospitalized maternal infection (Hazard Ratio=1.24; 95%CI: 0.88–1.73) including urinary tract infections.11 Separate observational data from the Swedish death registry demonstrated an increased risk of suicide starting at age 21 years among adults who had been exposed to a maternal infection during a hospitalization in utero.11 In addition, multiple studies in mouse models have found that fetal mice exposed to maternal immune activation may demonstrate depression-like behaviors.71–77 Overall, the evidence that prenatal infections underlie the fetal origins of depression is emerging and warrants more investigation.
Mechanisms of Fetal Brain injury
Many bacteria, viruses and parasites can cause direct or indirect injury to the fetal brain resulting in mild and severe neurodevelopmental injuries (Figure 1). The classical TORCH infections are known to cause direct injury to fetal brain cells by crossing the placenta and concentrating within the fetal compartment. These pathogens can cause varying degrees of injury to the cortical white matter, eye, and ear78, resulting in a broad spectrum of pathology, from mild hearing deficit to severe neurodevelopmental delay.79 However, many infectious diseases can also injure the fetal central nervous system indirectly by potentiating the fetal inflammatory response resulting in activation of astrocytes and microglia causing cytokine release, apoptosis, attenuation of growth, and direct cellular damage (see CMV example, Figure 2).78 Placental inflammation is a key feature associated with fetal brain injury; inflammatory mediators or cells in the placenta can be transferred to the fetus, which can ultimately injure the fetal brain either through release of fetal cytokines, neurotransmitters or excitotoxic metabolites (Figures 2 and 3). To understand the pathogenesis of subtle fetal brain injuries that contribute to the future risk of mental illness, we review the linkage between perinatal infections, placental inflammation, activation of astrocytes and microglia in the fetal brain, genetic predisposition and epigenetic modifications.
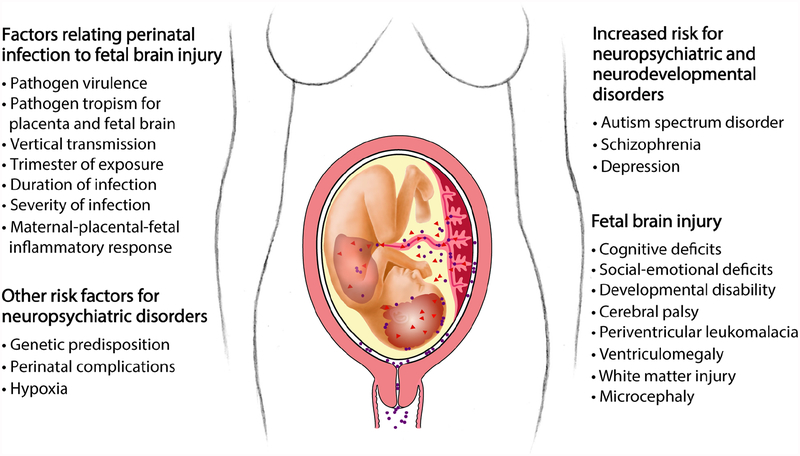
Figure 1.
Factors linking perinatal infections with mild and severe fetal brain injury. Several factors are thought to influence the severity and extent of a maternal infection leading to mild or severe fetal brain injury. Mild fetal brain injuries may not be detected clinically at birth and may only manifest later in life as a neurodevelopmental or neuropsychiatric disorder.
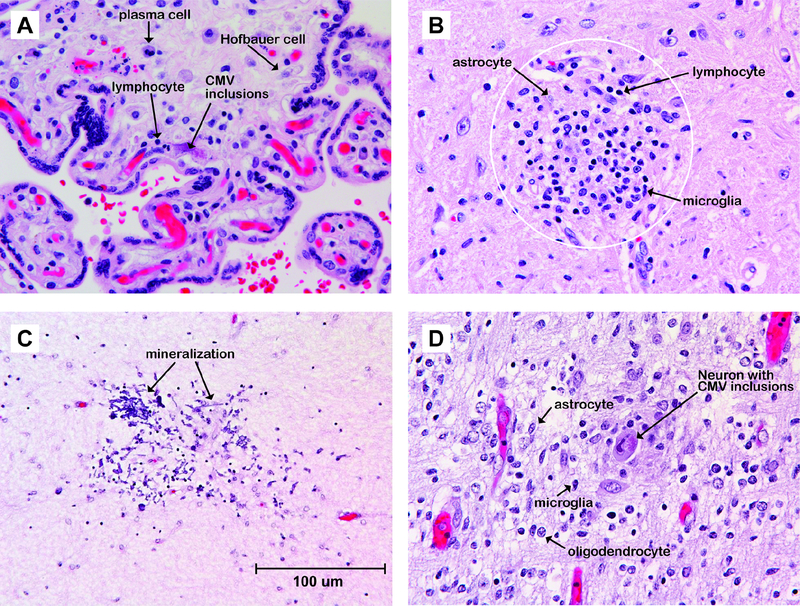
Figure 2.
Photomicrographs of the placenta and fetal or neonatal brain infected with CMV. In the placenta (A), there is hyperplasia of fetal macrophages (Hofbauer cells) and infiltration with lymphocytes and plasma cells. Inclusions are shown, which are pathognomonic for CMV infection. (B) In the brainstem of a 4 month-old infant born at 26 weeks gestation with a prenatal CMV infection, a microglial nodule (within the white circle) is shown with most cells reflecting lymphocytes, activated microglia and reactive astrocytes. (C) In the white matter of a 25-day old neonate born at 24 weeks gestation with a CMV prenatal infection, a focus of remote necrosis and dystrophic mineralization (refractile dark purple deposits) is shown. (D) In the fetal brain of a 23-week fetus, the acute phase of a CMV infection is shown with a hypercellular focus containing a mixture of activated microglial cells, reactive astrocytes, and a presumed neuron with pathognomonic CMV cytoplasmic and nuclear inclusions. A measurement bar representing 100 um is shown in panel C, which is applicable to all panels.
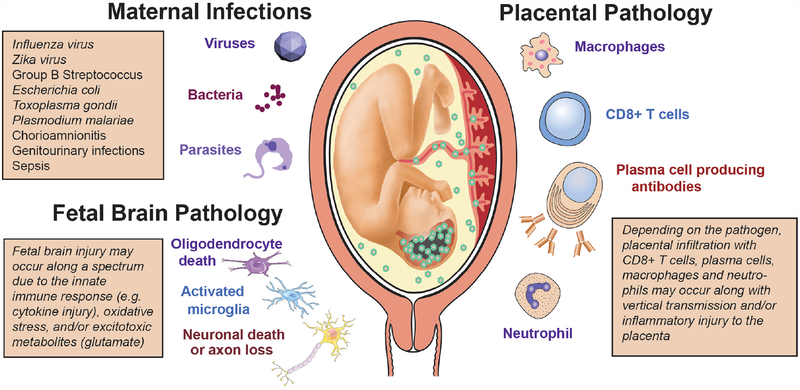
Figure 3.
Perinatal infections, placental immune response and cellular targets in the fetal brain. A spectrum of maternal infections induced by viruses, bacteria and parasites have been implicated in the development of placental pathology and fetal brain injury. Infiltration of the placenta by immune cells, notably maternal CD8+ T cells and plasma cells, has been strongly linked to fetal brain injury. Neutrophilic infiltration of the placenta is classically associated with bacterial infections, like Group B Streptococcus, which can cause meningitis and fetal brain injury. The cellular response in the fetal brain typically associated with perinatal infectious or inflammatory injury reflects activation of microglia and astrocytes with neuronal loss and oligodendrocyte dysfunction. The pathogens listed are associated with fetal brain injury and in some instances with development of mental illness in the child.
Placental inflammation
Among the mechanisms implicated in fetal brain injury, evidence strongly indicates that the immunologic milieu of the placenta plays an important role in neurodevelopment. Placental mediation of immune activation was suggested by a study finding a higher concordance of schizophrenia among monochorionic twins sharing one placenta compared to dichorionic twins, each with its own placenta.80 A recent study demonstrated that many perinatal complications including infections can upregulate transcriptional programs in the placenta involved in oxidative stress response, synaptic function and cellular metabolism.81 Suggestively, these same genetic loci are critical for normal neurodevelopment, and are also independently upregulated in patients with schizophrenia. The genetic risk for schizophrenia appears to be mediated through these perinatal complications such that a diagnosis of schizophrenia was most likely when a patient with a high genetic risk also experienced a perinatal complication; this effect was more pronounced in males. Taken together, these findings suggest that pregnancy complications and presumably inflammation may alter placental regulation of transcriptional programs, which can increase risk for development of schizophrenia.81
Both adaptive and innate immune responses in the placenta have been linked with the fetal origins of mental illness. CD8+ T cell infiltration of the placenta has emerged as a key immunological event following viral infection that can have destructive effects on the placental villous architecture and the chorioamniotic membranes.82 Following lipopolysaccharide-induced intrauterine inflammation in a mouse model, CD8+ T cells accumulated at the maternal-fetal interface; treatment with an anti-inflammatory led to reduced CD8+ T cell infiltration and improved fetal neurobehavioral outcomes.83 Depletion of CD8+ T cells in the same model of intrauterine inflammation was also associated with improved fetal neurologic outcomes and increased cortical neuron density.84 Less is known about the contribution of innate immune responses to the fetal origins of mental illness and the specific role of inflammatory cytokines,32,85,86 but there is some evidence that TGF-β1 and granulocyte colony-stimulating factor may cross the placenta to enter the fetal circulation.87–90 Emerging evidence suggests that IL-17A and IL-2 also play important roles in fetal brain injury.35,91–93 The best support for a role for cytokines in the biology of neuropsychiatric conditions comes from studies of children and adults diagnosed with ASD, in whom interleukin-6 (IL-6) is elevated in the peripheral blood.94–101 IL-6 can cross the placenta94,95 and administration of IL-6 can cause behavioral abnormalities in prenatally exposed mice in the absence of maternal inflammation, which is preventable by IL-6 inhibition.101,102 Activation of both innate and adaptive immune responses in the placenta and periphery are associated with adverse neuropsychiatric outcomes.
Serotonergic dysregulation
The placenta is known to secrete neurotransmitters, which are linked with normal fetal brain development and abnormal neurodevelopment. In mice, maternal inflammation changes placental serotonin secretion which results in concentration of serotonin in the fetal forebrain, decreased serotonergic receptor expression and blunted serotonergic axon outgrowth.103 Fascinatingly, this process appears to occur in the absence of increased levels of inflammatory cytokines within the fetal brain.103–105 Other work has demonstrated a connection between elevated levels of serotonin and altered oligodendrocyte development and myelination.106 Maternal inflammation has also been found in animal studies to change dopaminergic and GABAergic activity in the fetal brain, which correlates with observations from human studies in people with schizophrenia and ASD.107–112 Lastly, maternal immune activation may also change development of cholinergic neurons in the fetal basal forebrain.113 The connection between maternal infections or inflammation, placental neurotransmitter secretion, and fetal brain development is an active area of investigation.
Activated microglia, astrocytes and oligodendrocytes
Perinatal inflammation can activate fetal microglia and astrocytes to trigger cytokine release, which can injure neurons and oligodendrocytes.114 Histopathological studies of the brains of individuals with ASD have found microglial activation and an abnormal morphology and distribution of microglia.99,115–118 Further, in vivo imaging has demonstrated increased microglial activity in patients with ASD119 and other work has demonstrated possible abnormal microglia-neuron interactions.118 In numerous animal studies, maternal inflammation induces microglial activation113,120–122in the fetal brain, although these findings have not been universally replicated.123–125 In vitro studies have demonstrated increased neurotoxic cytokine release from activated microglia, which may damage or kill neurons and glia.113 There have been findings of microglial activation in schizophrenia126–132, though again with substantial inconsistencies, and some work has examined the role of microglia in bipolar disorder and depression.133–135
Astrocyte-associated pathologies are associated with exposure to pregnancy infections and development of ASD through effects on mitochondrial dysfunction, glutamate regulation and neuronal architecture.99,114,136–139 For example, increased expression of mitochondrial potassium channels within astrocytes has been found in people with ASD; in the fetal mouse brain, expression of these channels is also upregulated by a perinatal influenza infection.140–142 Astrocyte dysfunction is also under investigation in depression143,144 and schizophrenia.144 Some organisms like Toxoplasma gondii may increase the risk for schizophrenia through astrocyte activation and dysregulation of kynurenic acid metabolism.145–147 Aberrant astrocyte activation is associated with the development of neuropsychiatric disorders and fetal exposure to obstetrical infections.
Inflammatory cytokines from activated microglia and astrocytes may alter the development of fetal oligodendrocytes148 which has been implicated in the pathology of schizophrenia, depression, ASD and bipolar disorder.149–156 Oligodendrocytes are the myelinating cells of the central nervous system. Evidence suggests that oligodendrocyte precursor dysfunction and hypomyelination may play important roles in ASD pathophysiology.157,158 Several recent and interesting studies are also implicating deficits in myelination and white matter integrity in the pathogenesis of schizophrenia and brain “disconnectivity”.159 Damaged oligodendrocytes and precursors from antenatal exposure to maternal immune activation may also be more susceptible to hypoxic insults over the life course and this combination may increase risk of multiple psychiatric illnesses.160 Interestingly, genes and transcription factors associated with oligodendrocyte myelination function have been found to be downregulated in the brains of adults with schizophrenia and bipolar disorder.161,162 In summary, there is a body of evidence to link obstetrical infections or inflammation with activation of innate immune cells in the fetal brain, which contribute to abnormal oligodendrocyte development and may increase risk for development of a spectrum of neuropsychiatric disorders in the child.
Inflammation, genetic susceptibility and epigenetics
The link between perinatal infection and fetal brain injury reflects a complex spectrum of exposure severity (e.g. pathogen virulence, maternal-fetal immune response) and genetic susceptibility that can alter brain development and predispose to ASD and schizophrenia (Figure 1).163 Maternal immune activation can also alter fetal brain transcription through epigenetic changes even in the apparent absence of fetal inflammation.164 In a mouse model, inflammation that is insufficient to trigger preterm birth was associated with decreased dendritic counts and altered protein expression in the fetal brain165, along with epigenetic changes in the mouse adolescent brain.166 Indirect evidence from one study involving nearly 3,000 children with ASD found that interactions between maternal infection and the presence of a genetic predisposition in the child led to increased ASD symptom severity.167 Schizophrenia has also been associated with epigenetic modifications168–171; epigenetics is the heritable change in gene expression that is not defined by the underlying DNA sequence, which is often accomplished through DNA methylation or histone modifications.172 Perinatal inflammation has been associated with genome-wide methylation changes in the fetal brain173,174 and epigenetic changes in the striatum and hypothalamus thought to increase risk for schizophrenia.166 Inflammation-gene interactions have been found to induce psychosis-like behavior in mice175,176; the interaction between maternal inflammation and gene variants associated with neuropsychiatric disorders (e.g., DISC1, Nurr1) are also linked with a greater risk for psychosis-like behavior in mice than either inflammation or genetic mutation alone.92 In a recent study of five independent cohorts of humans with diverse ancestries, perinatal complications were observed to increase the risk of schizophrenia 5-fold among fetuses with an increased genetic risk.81 In this study, a polygenic risk profile score was constructed based on genome-wide association data from the Psychiatric Genetic Consortium datasets; this polygenic risk score was then overlaid upon the occurrence of obstetrical complications through medical records and personal interviews. When the polygenic risk scores were grouped into quintiles based on levels and then stratified into groups with and without obstetrical complications, the odds ratio for schizophrenia increased with higher polygenic risk scores only in the group with obstetrical complications. An individual having the highest polygenic risk score with an obstetrical complication had an OR of 8.4 (95% CI: 3.8–18.5, p=3 × 10−8). Interestingly, the genes mapping to the loci with the strongest link to schizophrenia also had significantly higher gene expression in the placenta. In summary, evidence from human studies and animal models implicate an interaction between inflammation, perinatal complications and epigenetic changes in the fetal brain that can increase the risk for schizophrenia and ASD.
Clinical Recommendations
As data accumulate on the connection between perinatal inflammation and neuropsychiatric disease, preventing infections during pregnancy assumes greater importance (Box 1). While some perinatal infections are unexpected (e.g. chorioamnionitis), many can be prevented through vaccination including influenza, measles and chicken pox. Influenza vaccination of pregnant women is a best practice for promoting health of the mother and protecting the fetal brain. Influenza infection during pregnancy is associated with serious immediate risks (i.e. maternal mortality, preterm birth),177,178 as well as possible long-term risks of neuropsychiatric disease in the child. Maternal vaccination also partially protects the infant through passive immunity.179–183 The World Health Organization not only recommends that pregnant women receive the influenza vaccine, but that they have highest priority among vulnerable groups.184
Box 1. Clinical and research recommendations.
- Emerging infections
- Strengthen public health surveillance for birth defects and long-term adverse outcomes to better determine whether an emerging infectious disease might be teratogenic or result in subtle fetal brain injuries that could predispose to mental illness.
- Prioritize pregnant women as a high-risk group for efforts to develop acceptable and safe vaccines for use in pregnancy across a spectrum of emerging infections that may be dangerous for pregnancy.
- Enroll pregnant women in clinical trials to study new vaccines that are anticipated to provide them with benefit (e.g. Zika virus vaccine) at the same time as other study participants and collect information about potential adverse outcomes in pregnancy.
- Influenza virus infection
- Improve uptake of the seasonal influenza vaccine in pregnant women and encourage administration as early as possible once the vaccine is available, including during the first trimester to prevent maternal influenza infections.
- Educate pregnant women during “influenza season” to notify their provider right away if they have a fever, in order to expedite administration of antiviral therapeutics and supportive care.
- Preterm labor and intra-amniotic infection
- Perform amniocentesis in women presenting with early preterm labor to better evaluate the risk for amniotic fluid infection and need for antimicrobial therapy.
- Urinary tract infection
- Screen women at risk for genitourinary infections with a urine culture once per trimester. Higher risk individuals include women taking immunosuppressive medications or with autoimmune disease (e.g. systemic lupus erythematosus), sickle cell disease, urinary retention, anatomical urinary tract abnormalities, recurrent urinary tract infections or diabetes.
Despite the well-established efficacy of the vaccine for maternal and neonatal protection from influenza infection, global vaccination rates among pregnant women remain low. In the United States, approximately half of pregnant women are estimated to receive the seasonal influenza vaccine.185,186 Limited data exists outside of the United States187, but recent European data suggested that approximately 25% of pregnant women were vaccinated.188 Lastly, despite evidence that inactivated influenza vaccine is safe to administer in the first trimester, some countries have national policies recommending vaccination only in the second and third trimesters.189–196 These policies leave pregnant women vulnerable to influenza infection in the first trimester, which is a critical period of fetal neurodevelopment.
Although many pathogens have yet to be studied for the risk that they could impart to the developing fetal brain, any severe maternal infection may increase the risk for neuropsychiatric disease in the fetus that may not manifest for many years after birth. Rubeola virus (measles), Zika virus, and malaria represent both new and ancient potential infectious threats to the developing fetal brain. Currently, the United States is in the midst of one of the most significant outbreaks of the measles virus since virtual eradication of measles in the U.S. in 2000.197 Measles infection during pregnancy is linked to preterm labor, preterm birth, and stillbirth.198–201 While pregnant women cannot receive the MMR vaccine, obstetrical providers can encourage their patients to fully vaccinate their children to promote beneficial herd immunity. Pregnant women in Zika and malaria-endemic zones should protect themselves from mosquitos using bed nets, protective clothing and mosquito repellant.202–204 The World Health Organization recommends intermittent preventative therapy with sulfadoxine-pyrimethamine for pregnant women living in regions with middle and high malaria transmission.205 An important part of prenatal care is discussing the fetal risks due to infections that may be acquired during travel that can result in teratogenesis or a severe maternal illness..
Further Research Directions
The studies exploring a fetal origin for mental illness have raised many questions (Box 2). Recent work has suggested that urinary tract infections (UTI) in hospitalized women may increase the risk for autism or depression to a similar degree as infections typically considered more severe (e.g. influenza infection, chorioamnionitis).11 UTIs are the most common infection in reproductive aged women, occur more frequently during pregnancy and can be associated with serious maternal and fetal morbidity and mortality.206,207 Interestingly, there is some evidence linking UTIs with a systemic inflammatory response and preeclampsia.208 Other work has demonstrated that infants born to mothers with a UTI during pregnancy had elevated levels of several pro-inflammatory cytokines.209 Maternal UTIs have also been linked to development of cerebral palsy.210 These studies are suggestive and future work should attempt to correlate UTI-associated local and systemic inflammatory responses with inflammation in the placenta, amniotic fluid and fetus. Animal models have typically studied the link between a systemic or uterine infection with fetal brain injury; new studies could determine whether chronic inflammation resulting from a UTI is sufficient to induce fetal brain injury and activate microglia.
Box 2. Future research directions.
- Epidemiology and policy
- What are the barriers to investigating the link between pregnancy infections, complications, fetal brain injury, birth defects and a long-term increased risk of mental illness for the child?
- What are the barriers to improving seasonal influenza vaccine uptake in pregnant women around the world?
- How might improved prenatal care in low income countries reduce long-term burden of psychiatric disease?
- Pathobiology and the maternal-fetal immune response
- What are the risks posed by emerging infectious diseases to the long-term mental health of the child when an infectious exposure occurs during pregnancy?
- Can emerging infectious diseases penetrate placental defenses?
- What are the placental and fetal immune correlates of fetal brain injury that predispose to a long-term risk of mental illness?
- Is there a gestational age window of greatest susceptibility to fetal brain injury?
- Is there a differential risk for fetal brain injury depending upon fetal sex?
- Antimicrobial therapeutics
- What is the relationship between the use of antibiotics and the fetal inflammatory response? Is this relationship dependent upon the class and type of antibiotic used? How does antibiotic administration timing in relation to infection onset alter inflammatory response?
- Preterm labor and intra-amniotic infection
- Can amniocentesis or vaginal/cervical point of care tests be used to better identify pregnancies with an intra-amniotic infection that might benefit from antibiotics?
- In the context of an intra-amniotic infection, can fetal brain injury and the long-term risk of mental illness in the child be mitigated by the use of anti-inflammatory therapies in conjunction with antibiotics?
- Urinary tract infection
- Does a maternal urinary tract infection result in a regional inflammatory response that imparts a higher risk for subtle fetal brain injury and long-term risk of mental illness?
- Does screening pregnant women at high-risk for recurrent urinary tract infections mitigate the long-term increased risk of mental illness for the fetus?
Questions have also emerged on the pro and anti-inflammatory roles of antibiotics in treating bacterial infections in pregnant women. The duration and extent of the infection coupled with the choice of antimicrobial therapy may play a role in the maternal immune response and possible subsequent neurodevelopmental abnormalities in offspring. Indeed, maternal immune activation may be induced by certain antibiotics, enhancing an inflammatory response detrimental to neurological development via lipopolysaccharide and other pathogen-associated molecular patterns (PAMPs).211 PAMPs have been studied in limited settings but early evidence suggests a possible link to worsened fetal outcomes. In a mouse pregnancy model, treatment of maternal Streptococcus pneumoniae bloodstream infection with ampicillin, known to be bacteriolytic and to induce release of bacterial cell wall components, resulted in abnormal fetal neuronal development.211 Yet, treating the same maternal infection with clindamycin, a non-bacteriolytic protein synthesis inhibitor, had no effect on the fetal brain.211 There are few experimental and epidemiological studies exploring the effect of antimicrobial treatment of systemic or local maternal infections (e.g. UTI) on brain development, but some evidence suggests that dampening pathogen-induced inflammation during pregnancy may mitigate neurodevelopmental abnormalities in offspring.212–215 The alternative, namely not treating a bacterial infection with antibiotics, is simply not an option as this could lead to bacterial dissemination and sepsis with even worse outcomes for the mother and fetus. Overall, investigation of the role of anti-inflammatory drugs with and without antibiotic therapy coupled with fetal outcome remains a significant research gap.
Large birth cohorts with long-term follow-up of the children are essential to investigating the relationship between perinatal infections and risk for neuropsychiatric disorders in the children. With better powered studies, it may be possible to clarify how the gestational timing of the inflammatory insult alters fetal neurodevelopment and whether this risk is modified by fetal sex.81,216–218 Further, it is possible that some portion of more subtle pathologies like Attention Deficit Hyperactivity Disorder may have a fetal origin associated with exposure to inflammation.219,220 Future studies are important to define the role of placental secretion of neurotransmitters and cytokines in mediating fetal injury.102,163 Lastly, a nascent body of work is exploring how the maternal gut microbiome may interact with maternal inflammation to alter the intrauterine environment.221,222
Conclusions
The classic TORCH paradigm was coined to create a mnemonic to aid in the recall of a select number of pathogens (i.e. Treponema pallidum, rubella virus, cytomegalovirus) thought to induce birth defects. However, a growing body of evidence suggests that focusing only on TORCH pathogens as a threat to the fetal brain is insufficient to capture the widening spectrum of pathogens and inflammatory conditions associated with neurocognitive deficits or psychiatric disorders in the child. As fetal brain development continues up to and beyond birth, the brain may be the single most vulnerable fetal organ to infectious and environmental insults over the course of the entire pregnancy.223 The nature of how fetal exposure to infections or maternal immune activation might synergistically increase the risk of these disorders with other risk factors (e.g. genetic) remains understudied. Finally, the clinical emphasis on preventing infections and inflammation in pregnancy to protect the fetal brain has not matched the gravity of the accumulating scientific evidence. Obstetrical providers should ensure that pregnant women receive the influenza vaccine, including in the first trimester, as a safe strategy to protect both the mother from severe disease, as well as the fetal brain. Determining additional interventions to lower the risk of neuropsychiatric disorders in the fetus will require both human cohorts and animal studies to correlate the complex biological events linking perinatal infections with fetal brain injury.
Acknowledgments
We would like to acknowledge Jessie Brown for technical assistance with preparation of the figures.
This work was supported by the National Institutes of Health Grant #AI33976 (L.R. and K.A.W.), #HD097608 (I.B.), R01AG052494 (I.U.M., C.W.), R01DK100644 (I.U.M., C.W.), P20DK119840 (I.U.M.), and T32 GM008244 from the National Institute of General Medical Sciences (B.J.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Contributor Information
Benjamin J.S. AL-HADDAD, Seattle, Washington, USA; Department of Pediatrics, University of Washington.
Elizabeth OLER, Seattle, Washington, USA; Department of Obstetrics & Gynecology, University of Washington.
Blair ARMISTEAD, Seattle, WA; Department of Global Health, University of Washington; Center for Global Infectious Disease Research, Seattle Children’s Research Institute.
Nada A. ELSAYED, Baltimore, MD; Integrated Research Center for Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine.
Daniel R. WEINBERGER, Baltimore, MD; Lieber Institute for Brain Development, Departments of Psychiatry, Neurology, Neuroscience, and the McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University School of Medicine.
Raphael BERNIER, Seattle, WASHINGTON; Department of Psychiatry and Behavioral Sciences, University of Washington.
Irina BURD, Baltimore, MD; Integrated Research Center for Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine; Department of Neurology, Johns Hopkins University School of Medicine.
Raj KAPUR, Seattle, Washington, USA; Department of Pediatrics, University of Washington, Seattle Children’s Hospital.
Bo JACOBSSON, Gothenburg, SWEDEN; Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg; Region Västra Götaland, Sahlgrenska University Hospital; Department of Genetics and Bioinformatics, Domain of Health Data and Digitalization, Institute of Public Health, Oslo, Norway.
Caihong WANG, St. Louis, Missouri; Dept. of Obstetrics and Gynecology, Center for Reproductive Health Sciences, Washington University School of Medicine.
Indira MYSOREKAR, St. Louis, Missouri; Depts. of Obstetrics and Gynecology, Pathology and Immunology; Center for Reproductive Health Sciences, Washington University School of Medicine.
Lakshmi RAJAGOPAL, Seattle, WA; Center for Innate Immunity and Immune Disease, Department of Pediatrics, University of Washington; Center for Global Infectious Disease Research, Seattle Children’s Research Institute.
Kristina M. ADAMS WALDORF, Seattle, WA; Department of Obstetrics & Gynecology and Global Health, Center for Innate Immunity and Immune Disease, Center for Emerging and Reemerging Infectious Diseases, University of Washington; Sahlgrenska Academy, University of Gothenburg, Sweden.
References
- 1.McAlister Gregg N, Banatvala R by JE. Congenital cataract following German measles in the mother. Rev Med Virol. 2001;11(5):277–285. doi: 10.1002/rmv.327 [DOI] [PubMed] [Google Scholar]
- 2.Yazigi A, De Pecoulas AE, Vauloup-Fellous C, Grangeot-Keros L, Ayoubi J-M, Picone O. Fetal and neonatal abnormalities due to congenital rubella syndrome: a review of literature. J Matern Neonatal Med. 2017;30(3):274–278. doi: 10.3109/14767058.2016.1169526 [DOI] [PubMed] [Google Scholar]
- 3.Cluver C, Meyer R, Odendaal H, Geerts L. Congenital rubella with agenesis of the inferior cerebellar vermis and total anomalous pulmonary venous drainage. Ultrasound Obstet Gynecol. 2013;42(2):235–237. doi: 10.1002/uog.12399 [DOI] [PubMed] [Google Scholar]
- 4.Parisot S, Droulle P, Feldmann M, Pinaud P, Marchal C. Unusual encephaloclastic lesions with paraventricular calcification in congenital rubella. Pediatr Radiol. 1991;21(3):229–230. [DOI] [PubMed] [Google Scholar]
- 5.Andrade JQ, Bunduki V, Curti SP, Figueiredo CA, de Oliveira MI, Zugaib M. Rubella in pregnancy: Intrauterine transmission and perinatal outcome during a Brazilian epidemic. J Clin Virol. 2006;35(3):285–291. doi: 10.1016/j.jcv.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Barry H III, Barry H Jr. Season of Birth: An Epidemiological Study in Psychiatry. Arch Gen Psychiatry. 1961;5(3):292–300. doi: 10.1001/archpsyc.1961.01710150074012 [DOI] [PubMed] [Google Scholar]
- 7.Hare EH, Price JS, Slater E. Mental Disorder and Season of Birth. Nature. 1973;241(5390):480. doi: 10.1038/241480a0 [DOI] [PubMed] [Google Scholar]
- 8.Parker G, Neilson M. Mental Disorder and Season of Birth—A Southern Hemisphere Study. Br J Psychiatry. 1976;129(4):355–361. doi:DOI: 10.1192/bjp.129.4.355 [DOI] [PubMed] [Google Scholar]
- 9.Torrey EF, Peterson MR. The Viral Hypothesis of Schizophrenia. Schizophr Bull. 1976;2(1):136–146. doi: 10.1093/schbul/2.1.136 [DOI] [PubMed] [Google Scholar]
- 10.Atladóttir HÓ, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y [DOI] [PubMed] [Google Scholar]
- 11.al-Haddad BJS, Jacobsson B, Chabra S, et al. Long-term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA Psychiatry. 2019. doi: 10.1001/jamapsychiatry.2019.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727. [DOI] [PubMed] [Google Scholar]
- 13.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry. 1992;49(12):983–988. [DOI] [PubMed] [Google Scholar]
- 15.St Clair D, Xu M, Wang P, et al. Rates of Adult Schizophrenia Following Prenatal Exposure to the Chinese Famine of 1959–1961. JAMA. 2005;294(5):557–562. doi: 10.1001/jama.294.5.557 [DOI] [PubMed] [Google Scholar]
- 16.Niemela S, Sourander A, Surcel HM, et al. Prenatal Nicotine Exposure and Risk of Schizophrenia Among Offspring in a National Birth Cohort. Am J Psychiatry. 2016;173(8):799–806. doi: 10.1176/appi.ajp.2016.15060800 [DOI] [PubMed] [Google Scholar]
- 17.Abel KM, Wicks S, Susser ES, et al. Birth Weight, Schizophrenia, and Adult Mental Disorder: Is Risk Confined to the Smallest Babies?Birth Weight, Schizophrenia, and Mental Disorder. Arch Gen Psychiatry. 2010;67(9):923–930. doi: 10.1001/archgenpsychiatry.2010.100 [DOI] [PubMed] [Google Scholar]
- 18.Wrede G, Mednick SA, Huttunen MO, Nilsson CG. Pregnancy and delivery complications in the births of an unselected series of Finnish children with schizophrenic mothers. Acta Psychiatr Scand. 1980;62(4):369–381. doi: 10.1111/j.1600-0447.1980.tb00623.x [DOI] [PubMed] [Google Scholar]
- 19.Machón RA, Mednick SA, Schulsinger F. The Interaction of Seasonality, Place of Birth, Genetic Risk and Subsequent Schizophrenia in a High Risk Sample. Br J Psychiatry. 1983;143(4):383–388. doi:DOI: 10.1192/bjp.143.4.383 [DOI] [PubMed] [Google Scholar]
- 20.Watson CG, Kucala T, Tilleskjor C, Jacobs L. Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch Gen Psychiatry. 1984;41(1):85–90. [DOI] [PubMed] [Google Scholar]
- 21.Tochigi M, Okazaki Y, Kato N, Sasaki T. What causes seasonality of birth in schizophrenia? Neurosci Res. 2004;48(1):1–11. doi: 10.1016/j.neures.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 22.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–192. [DOI] [PubMed] [Google Scholar]
- 23.Brown AS, Schaefer CA, Wyatt RJ, et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26(2):287–295. [DOI] [PubMed] [Google Scholar]
- 24.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1097/01.ogx.0000151642.60544.d2 [DOI] [PubMed] [Google Scholar]
- 25.Brown AS, Schaefer CA, Quesenberry CP, Liu L, Babulas VP, Susser ES. Maternal Exposure to Toxoplasmosis and Risk of Schizophrenia in Adult Offspring. Am J Psychiatry. 2005;162(4):767–773. doi: 10.1176/appi.ajp.162.4.767 [DOI] [PubMed] [Google Scholar]
- 26.Brown AS, Schaefer CA, Quesenberry CP Jr., Shen L, Susser ES. No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am J Psychiatry. 2006;163(12):2178–2180. doi: 10.1176/ajp.2006.163.12.2178 [DOI] [PubMed] [Google Scholar]
- 27.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal Exposure to Maternal Genital and Reproductive Infections and Adult Schizophrenia. Am J Psychiatry. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927 [DOI] [PubMed] [Google Scholar]
- 28.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43(2):239–257. doi: 10.1017/S0033291712000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abib RT, Gaman A, Dargél AA, et al. Intracellular Pathogen Infections and Immune Response in Autism. Neuroimmunomodulation. 2018;25(5–6):271–279. doi: 10.1159/000491821 [DOI] [PubMed] [Google Scholar]
- 30.Lyall K, Croen L, Daniels J, et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health. 2017;38(1):81–102. doi: 10.1146/annurev-publhealth-031816-044318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang HY, Xu LL, Shao L, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, Behav Immun. 2016;58:165–172. doi: 10.1016/j.bbi.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275–1297. doi: 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, Behav Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi GB, Yim YS, Wong H, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science (80- ). 2016;351(6276):933–939. doi: 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández de Cossío L, Guzmán A, van der Veldt S, Luheshi GN. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun. 2017;63:88–98. doi: 10.1016/j.bbi.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 37.Machado CJ, Whitaker AM, Smith SEP, Patterson PH, Bauman MD. Maternal Immune Activation in Nonhuman Primates Alters Social Attention in Juvenile Offspring. Biol Psychiatry. 2015;77(9):823–832. doi: 10.1016/j.biopsych.2014.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. doi: 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin Yim Y, Park A, Berrios J, et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017;549(7673):482–487. doi: 10.1038/nature23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baharnoori M, Bhardwaj SK, Srivastava LK. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull. 2012;38(3):444–456. doi: 10.1093/schbul/sbq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011;(48):2473. doi: 10.3791/2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pendyala G, Chou S, Jung Y, et al. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology. 2017;15:15. doi: 10.1038/npp.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Careaga M, Murai T, Bauman MD. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry. 2017;81(5):391–401. doi: 10.1016/j.biopsych.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–341. doi: 10.1016/j.biopsych.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–5104. [DOI] [PubMed] [Google Scholar]
- 47.Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RPF, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–1155. doi: 10.1289/ehp.1002986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37(1):95–110. doi: 10.1002/pd.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, Zhong X, Jiang L, et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav Brain Res. 2016;296:61–69. doi: 10.1016/j.bbr.2015.08.035 [DOI] [PubMed] [Google Scholar]
- 52.Wu S, Ding Y, Wu F, et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:322–332. doi: 10.1016/j.neubiorev.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 53.Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. 2016;7:10717. doi: 10.1038/ncomms10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta S, Ellis SE, Ashar FN, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748. doi: 10.1038/ncomms6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown AS, Susser ES, Lin SP, Gorman JM. Affective disorders in Holland after prenatal exposure to the 1957 A2 influenza epidemic. Biol Psychiatry. 1995;38(4):270–273. doi: 10.1016/0006-3223(95)00241-8 [DOI] [PubMed] [Google Scholar]
- 57.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational Influenza and Bipolar Disorder in Adult Offspring. JAMA Psychiatry. 2013;70(7):677–685. doi: 10.1001/jamapsychiatry.2013.896 [DOI] [PubMed] [Google Scholar]
- 58.Machón R, Mednick S, Huttunen M. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1997;54(4):322–328. [DOI] [PubMed] [Google Scholar]
- 59.Canetta SE, Bao Y, Co MDT, et al. Serological Documentation of Maternal Influenza Exposure and Bipolar Disorder in Adult Offspring. Am J Psychiatry. 2014;171(5):557–563. doi: 10.1176/appi.ajp.2013.13070943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barichello T, Badawy M, Pitcher MR, et al. Exposure to Perinatal Infections and Bipolar Disorder: A Systematic Review. Curr Mol Med. 2016;16(2):106–118. [DOI] [PubMed] [Google Scholar]
- 61.Simanek AM, Meier HCS. Association Between Prenatal Exposure to Maternal Infection and Offspring Mood Disorders: A Review of the Literature. Curr Probl Pediatr Adolesc Health Care. 2015;45(11):325–364. doi: 10.1016/j.cppeds.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 62.Du Preez A, Leveson J, Zunszain PA, Pariante CM. Inflammatory insults and mental health consequences: does timing matter when it comes to depression? Psychol Med. 2016;46(10):2041–2057. doi: 10.1017/S0033291716000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown AS, Susser ES, Lin SP, Gorman JM. Affective disorders in Holland after prenatal exposure to the 1957 A2 influenza epidemic. Biol Psychiatry. 1995;38(4):270–273. doi: 10.1016/0006-3223(95)00241-8 [DOI] [PubMed] [Google Scholar]
- 64.Takei N, O’Callaghan E, Sham PC, Glover G, Murray RM. Does prenatal influenza divert susceptible females from later affective psychosis to schizophrenia? Acta Psychiatr Scand. 1993;88(5):328–336. doi: 10.1111/j.1600-0447.1993.tb03468.x [DOI] [PubMed] [Google Scholar]
- 65.Mino Y, Oshima I, Okagami K. Mood disorders and influenza epidemics in Japan. Psychiatry Clin Neurosci. 2000;54(1):59–65. doi: 10.1046/j.1440-1819.2000.00638.x [DOI] [PubMed] [Google Scholar]
- 66.Cannon M, Cotter D, Coffey VP, et al. Prenatal Exposure to the 1957 Influenza Epidemic and Adult Schizophrenia: A Follow-Up Study. Br J Psychiatry. 1996;168(3):368–371. doi:DOI: 10.1192/bjp.168.3.368 [DOI] [PubMed] [Google Scholar]
- 67.Morgan V, Castle D, Page A, et al. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: no evidence of a major effect. Schizophr Res. 1997;26(1):25–39. doi: 10.1016/S0920-9964(97)00033-9 [DOI] [PubMed] [Google Scholar]
- 68.Pang D, Syed S, Fine P, Jones PB. No Association between Prenatal Viral Infection and Depression in Later Life—A Long-Term Cohort Study of 6152 Subjects. Can J Psychiatry. 2009;54(8):565–570. doi: 10.1177/070674370905400809 [DOI] [PubMed] [Google Scholar]
- 69.Murphy SK, Fineberg AM, Maxwell SD, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 2017;257:102–110. doi: 10.1016/j.psychres.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lydholm CN, Köhler-Forsberg O, Nordentoft M, et al. Parental Infections Before, During, and After Pregnancy as Risk Factors for Mental Disorders in Childhood and Adolescence: A Nationwide Danish Study. Biol Psychiatry. 2019;85(4):317–325. doi: 10.1016/j.biopsych.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 71.Ronovsky M, Berger S, Zambon A, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun. 2017;63:127–136. doi: 10.1016/j.bbi.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 72.Majidi-Zolbanin J, Doosti M-H, Kosari-Nasab M, Salari A-A. Prenatal maternal immune activation increases anxiety- and depressive-like behaviors in offspring with experimental autoimmune encephalomyelitis. Neuroscience. 2015;294:69–81. doi: 10.1016/j.neuroscience.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 73.Reisinger SN, Kong E, Khan D, et al. Maternal immune activation epigenetically regulates hippocampal serotonin transporter levels. Neurobiol Stress. 2016;4:34–43. doi: 10.1016/j.ynstr.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan D, Fernando P, Cicvaric A, et al. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry Psychiatry. 2014;4:e363. doi: 10.1038/tp.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arad M, Piontkewitz Y, Albelda N, Shaashua L, Weiner I. Immune activation in lactating dams alters sucklings’ brain cytokines and produces non-overlapping behavioral deficits in adult female and male offspring: A novel neurodevelopmental model of sex-specific psychopathology. Brain, Behav Immun. 2017;08:8. doi: 10.1016/j.bbi.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 76.Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065 [DOI] [PubMed] [Google Scholar]
- 77.Ronovsky M, Berger S, Molz B, Berger A, Pollak DD. Animal Models of Maternal Immune Activation in Depression Research. Curr Neuropharmacol. 2016;14(7):688–704. doi: 10.2174/1570159X14666151215095359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of Congenital Cytomegalovirus Infection: Disease Mechanisms and Prospects for Intervention. Clin Microbiol Rev. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yinon Y, Farine D, Yudin MH. No. 240-Cytomegalovirus Infection in Pregnancy. J Obs Gynaecol Can. 2018;40(2):134–141. doi: 10.1016/j.jogc.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 80.Davis JO, Phelps JA, Bracha HS. Prenatal development of monozygotic twins and concordance for schizophrenia. Schizophr Bull. 1995;21(3):357–366. [DOI] [PubMed] [Google Scholar]
- 81.Ursini G, Punzi G, Chen Q, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;(24):792–801. doi: 10.1038/s41591-018-0021-y [DOI] [PubMed] [Google Scholar]
- 82.Kim CJ, Romero R, Chaemsaithong P, Kim J-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53–S69. doi: 10.1016/j.ajog.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lei J, Rosenzweig JM, Mishra MK, et al. Maternal dendrimer-based therapy for inflammation-induced preterm birth and perinatal brain injury. Sci Rep. 2017;7(1):6106. doi: 10.1038/s41598-017-06113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lei J, Xie L, Zhao H, et al. Maternal CD8+ T-cell depletion alleviates intrauterine inflammation-induced perinatal brain injury. Am J Reprod Immunol. 2018;79(5):e12798. doi: 10.1111/aji.12798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdallah MW, Larsen N, Grove J, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2013;14. doi: 10.3109/15622975.2011.639803 [DOI] [PubMed] [Google Scholar]
- 86.Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35. doi: 10.1016/j.clp.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science (80- ). 1994;264(5167):1936 LP–1938. doi: 10.1126/science.8009224 [DOI] [PubMed] [Google Scholar]
- 88.Lennard SN, Stewart F, Allen WR. Transforming growth factor β1 expression in the endometrium of the mare during placentation. Mol Reprod Dev. 1995;42(2):131–140. doi: 10.1002/mrd.1080420202 [DOI] [PubMed] [Google Scholar]
- 89.Calhoun DA, Gersting JA, Lunøe M, Du Y, Christensen RD. Transfer of Recombinant Human Granulocyte Colony Stimulating Factor (rhG-CSF) from the Maternal to the Fetal Circulation is not Dependent Upon a Functional G-CSF-Receptor. Placenta. 2001;22(6):609–612. doi: 10.1053/plac.2001.0682 [DOI] [PubMed] [Google Scholar]
- 90.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35(5):959–972. doi: 10.1093/schbul/sbn022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lawrence SM, Wynn JL. Chorioamnionitis, IL-17A, and fetal origins of neurologic disease. Am J Reprod Immunol. 2018;79(5):e12803–e12803. doi: 10.1111/aji.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyer U Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75(4):307–315. doi: 10.1016/j.biopsych.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 93.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci. 2007;1107. doi: 10.1196/annals.1381.013 [DOI] [PubMed] [Google Scholar]
- 94.Bell MJ, Hallenbeck JM, Gallo V. Determining the Fetal Inflammatory Response in an Experimental Model of Intrauterine Inflammation in Rats. Pediatr Res. 2004;56:541. [DOI] [PubMed] [Google Scholar]
- 95.Liverman CS, Kaftan HA, Cui L, et al. Altered expression of pro-inflammatory and developmental genes in the fetal brain in a mouse model of maternal infection. Neurosci Lett. 2006;399(3):220–225. doi: 10.1016/j.neulet.2006.01.064 [DOI] [PubMed] [Google Scholar]
- 96.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25. doi: 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Chauhan A, Sheikh AM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207. doi: 10.1016/j.jneuroim.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2014;20:440. [DOI] [PubMed] [Google Scholar]
- 99.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57. doi: 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- 100.Wei H, Zou H, Sheikh AM, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, Behav Immun. 2017;62:11–23. doi: 10.1016/j.bbi.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goeden N, Velasquez J, Arnold KA, et al. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J Neurosci. 2016;36(22):6041–6049. doi: 10.1523/JNEUROSCI.2534-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–350. doi: 10.1038/nature09972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muller CL, Anacker AM, Rogers TD, et al. Impact of Maternal Serotonin Transporter Genotype on Placental Serotonin, Fetal Forebrain Serotonin, and Neurodevelopment. Neuropsychopharmacology. 2017;42(2):427–436. doi: 10.1038/npp.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fan L-W, Bhatt A, Tien L-T, et al. Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro. J Neurochem. 2015;133(4):532–543. doi: 10.1111/jnc.12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59(6):546–554. doi: 10.1016/j.biopsych.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 108.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28(10):1778–1789. doi: 10.1038/sj.npp.1300248 [DOI] [PubMed] [Google Scholar]
- 109.Bitanihirwe BKY, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late Prenatal Immune Activation in Mice Leads to Behavioral and Neurochemical Abnormalities Relevant to the Negative Symptoms of Schizophrenia. Neuropsychopharmacology. 2010;35:2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vuillermot S, Joodmardi E, Perlmann T, Ove Ögren S, Feldon J, Meyer U. Prenatal Immune Activation Interacts with Genetic <em>Nurr1</em> Deficiency in the Development of Attentional Impairments. J Neurosci. 2012;32(2):436 LP–451. doi: 10.1523/JNEUROSCI.4831-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643. [DOI] [PubMed] [Google Scholar]
- 112.Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–226. doi: 10.1016/j.pharmthera.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 113.Pratt L, Ni L, Ponzio NM, Jonakait GM. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr Res. 2013;74(4):393–401. doi: 10.1038/pr.2013.126 [DOI] [PubMed] [Google Scholar]
- 114.Zeidán-Chuliá F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JCF. The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev. 2014;38:160–172. doi: 10.1016/j.neubiorev.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 115.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal Fluid and Serum Markers of Inflammation in Autism. Pediatr Neurol. 2005;33(3):195–201. doi: 10.1016/j.pediatrneurol.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 116.Morgan JT, Chana G, Pardo CA, et al. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol Psychiatry. 2010;68(4):368–376. doi: 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- 117.Tetreault NA, Hakeem AY, Jiang S, et al. Microglia in the Cerebral Cortex in Autism. J Autism Dev Disord. 2012;42(12):2569–2584. doi: 10.1007/s10803-012-1513-0 [DOI] [PubMed] [Google Scholar]
- 118.Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 2012;1456:72–81. doi: 10.1016/j.brainres.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 119.Suzuki K, Sugihara G, Ouchi Y, et al. Microglial Activation in Young Adults With Autism Spectrum DisorderMicroglia in Young Adults With ASD. JAMA Psychiatry. 2013;70(1):49–58. doi: 10.1001/jamapsychiatry.2013.272 [DOI] [PubMed] [Google Scholar]
- 120.Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, Wolf RJ. Microglial activation in a neuroinflammational animal model of schizophrenia — a pilot study. Schizophr Res. 2011;131(1):96–100. doi: 10.1016/j.schres.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 121.Van den Eynde K, Missault S, Fransen E, et al. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res. 2014;258:179–186. doi: 10.1016/j.bbr.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 122.Zhu F, Zheng Y, Liu Y, Zhang X, Zhao J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic–polyribocytidilic acid. Psychiatry Res. 2014;219(3):680–686. doi: 10.1016/j.psychres.2014.06.046 [DOI] [PubMed] [Google Scholar]
- 123.Missault S, Van den Eynde K, Vanden Berghe W, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain, Behav Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 124.Smolders S, Smolders SM, Swinnen N, et al. Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo. Front Cell Neurosci. 2015;9:301. doi: 10.3389/fncel.2015.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain, Behav Immun. 2016;55:25–38. doi: 10.1016/j.bbi.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 126.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 127.Takahashi Y, Yu Z, Sakai M, Tomita H. Linking Activation of Microglia and Peripheral Monocytic Cells to the Pathophysiology of Psychiatric Disorders. Front Cell Neurosci. 2016;10:144. doi: 10.3389/fncel.2016.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39(6):376–385. doi: 10.1503/jpn.130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garey L When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010;217(4):324–333. doi: 10.1111/j.1469-7580.2010.01231.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prata J, Santos SG, Almeida MI, Coelho R, Barbosa MA. Bridging Autism Spectrum Disorders and Schizophrenia through inflammation and biomarkers-pre-clinical and clinical investigations. J Neuroinflammation. 2017;14(1):179. doi: 10.1186/s12974-017-0938-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hui CW, St-Pierre A, El Hajj H, et al. Prenatal Immune Challenge in Mice Leads to Partly Sex-Dependent Behavioral, Microglial, and Molecular Abnormalities Associated with Schizophrenia. Front Mol Neurosci. 2018;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Birnbaum R, Weinberger DR. A Genetics Perspective on the Role of the (Neuro)Immune System in Schizophrenia. Schizophr Res. March 2019. doi: 10.1016/j.schres.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinto JV, Passos IC, Librenza-Garcia D, et al. Neuron-glia Interaction as a Possible Pathophysiological Mechanism of Bipolar Disorder. Curr Neuropharmacol. 2018;16(5):519–532. doi: 10.2174/1570159X15666170828170921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yirmiya R, Rimmerman N, Reshef R. Depression as a Microglial Disease. Trends Neurosci. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 135.Czéh B, Nagy SA. Clinical Findings Documenting Cellular and Molecular Abnormalities of Glia in Depressive Disorders. Front Mol Neurosci. 2018;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. The Cerebellum. 2005;4(3):206–210. doi: 10.1080/14734220500208846 [DOI] [PubMed] [Google Scholar]
- 137.Verkhratsky A, Butt AM. Glial Physiology and Pathophysiology. 1st ed. Wiley-Blackwell; 2013. [Google Scholar]
- 138.Choudhury PR, Lahiri S, Rajamma U. Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol Biochem Behav. 2012;100(4):841–849. doi: 10.1016/j.pbb.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 139.Rahn KA, Slusher BS, Kaplin AI. Glutamate in CNS neurodegeneration and cognition and its regulation by GCPII inhibition. Curr Med Chem. 2012;19(9):1335–1345. doi: 10.2174/092986712799462649 [DOI] [PubMed] [Google Scholar]
- 140.Fatemi SH, Folsom TD, Reutiman TJ, Lee S. Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse. 2008;62(7):501–507. doi: 10.1002/syn.20519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fatemi SH, Folsom TD, Reutiman TJ, Sidwell RW. Viral regulation of aquaporin 4, connexin 43, microcephalin and nucleolin. Schizophr Res. 2008;98(1):163–177. doi: 10.1016/j.schres.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J, Li Z, Feng M, et al. Opening of Astrocytic Mitochondrial ATP-Sensitive Potassium Channels Upregulates Electrical Coupling between Hippocampal Astrocytes in Rat Brain Slices. PLoS One. 2013;8(2):e56605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14(11):1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verkhratsky A, Parpura V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis. 2016;85:254–261. doi: 10.1016/j.nbd.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull. 2007;33(3):652–653. doi: 10.1093/schbul/sbm030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson EH, Hunter CA. The role of astrocytes in the immunopathogenesis of toxoplasmic encephalitis. Int J Parasitol. 2004;34(5):543–548. doi: 10.1016/j.ijpara.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 147.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55(1):78–92. doi: 10.1002/glia.20432 [DOI] [PubMed] [Google Scholar]
- 148.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–112. doi: 10.1016/j.schres.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 149.Segal D, Koschnick JR, Slegers LHA, Hof PR. Oligodendrocyte pathophysiology: a new view of schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):503–511. doi: 10.1017/S146114570600722X [DOI] [PubMed] [Google Scholar]
- 150.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6(3):219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93(1):13–24. doi: 10.1016/j.pneurobio.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Konradi C, Sillivan SE, Clay HB. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol Dis. 2012;45(1):37–47. doi: 10.1016/j.nbd.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mauney SA, Pietersen CY, Sonntag K-C, Woo T-UW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res. 2015;169(1–3):374–380. doi: 10.1016/j.schres.2015.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M. Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res Int. 2015;2015:492367. doi: 10.1155/2015/492367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. doi: 10.1016/j.siny.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 156.van Tilborg E, Achterberg EJM, van Kammen CM, et al. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia. 2018;66(1):78–93. doi: 10.1002/glia.23216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carmody DP, Lewis M. Regional white matter development in children with autism spectrum disorders. Dev Psychobiol. 2010;52(8):755–763. doi: 10.1002/dev.20471 [DOI] [PubMed] [Google Scholar]
- 158.Graciarena M, Seiffe A, Nait-Oumesmar B, Depino AM. Hypomyelination and Oligodendroglial Alterations in a Mouse Model of Autism Spectrum Disorder. Front Cell Neurosci. 2019;12:517. doi: 10.3389/fncel.2018.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cassoli JS, Guest PC, Malchow B, Schmitt A, Falkai P, Martins-de-Souza D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. NPJ Schizophr. 2015;1:15034. doi: 10.1038/npjschz.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maas DA, Vallès A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry. 2017;7(7):e1171–e1171. doi: 10.1038/tp.2017.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Keshavarz M Glial cells as key elements in the pathophysiology and treatment of bipolar disorder. Acta Neuropsychiatr. 2017;29(3):140–152. doi:DOI: 10.1017/neu.2016.56 [DOI] [PubMed] [Google Scholar]
- 162.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4 [DOI] [PubMed] [Google Scholar]
- 163.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353(6301):772–777. doi: 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26(4):623–634. doi: 10.1016/j.bbi.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29(6):663–671. doi: 10.1016/j.ijdevneu.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Basil P, Li Q, Dempster EL, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4(9):e434–e434. doi: 10.1038/tp.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mazina V, Gerdts J, Trinh S, et al. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr. 2015;36(2):61–67. doi: 10.1097/DBP.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38(1):138–166. doi: 10.1038/npp.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nestler EJ, Peña CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic Basis of Mental Illness. Neuroscientist. 2016;22(5):447–463. doi: 10.1177/1073858415608147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jaffe AE, Gao Y, Deep-Soboslay A, et al. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 2016;19(1):40–47. doi: 10.1038/nn.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pidsley R, Viana J, Hannon E, et al. Methylomic profiling of human brain tissue supports a neurodevelopmental origin for schizophrenia. Genome Biol. 2014;15(10):483. doi: 10.1186/s13059-014-0483-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.National Institutes of Health. A Scientific Illustration of How Epigenetic Mechanisms Can Affect Health. https://commonfund.nih.gov/epigenomics/figure. Published 2018 Accessed June 3, 2019.
- 173.Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015;10(12):1143–1155. doi: 10.1080/15592294.2015.1114202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Richetto J, Massart R, Weber-Stadlbauer U, Szyf M, Riva MA, Meyer U. Genome-wide DNA Methylation Changes in a Mouse Model of Infection-Mediated Neurodevelopmental Disorders. Biol Psychiatry. 2017;81(3):265–276. doi: 10.1016/j.biopsych.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 175.Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci. 2013;33(18):7654–7666. doi: 10.1523/JNEUROSCI.0091-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abazyan B, Nomura J, Kannan G, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68(12):1172–1181. doi: 10.1016/j.biopsych.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444 [DOI] [PubMed] [Google Scholar]
- 178.Pfitscher LC, Cecatti JG, Pacagnella RC, et al. Severe maternal morbidity due to respiratory disease and impact of 2009 H1N1 influenza A pandemic in Brazil: results from a national multicenter cross-sectional study. BMC Infect Dis. 2016;16(220). doi: 10.1186/s12879-016-1525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nunes MC, Madhi SA. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: A systematic review and meta-analysis. Hum Vaccines Immunother ISSN. 2017;14(3):758–766. doi: 10.1080/21645515.2017.1345385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shakib JH, Korgenski K, Presson AP, et al. Influenza in Infants Born to Women Vaccinated During Pregnancy. Pediatrics. 2016;137(6). doi: 10.1542/peds.2015-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marshall H, Mcmillan M, Andrews RM, Macartney K, Edwards K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum Vaccin Immunother. 2016;12(4):848–856. doi: 10.1080/21645515.2015.1127485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of Maternal Influenza Immunization in Mothers and Infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 183.Madhi SA, Cutland CL, Kuwanda L, et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N Engl J Med. 2014;371(10):918–931. doi: 10.1056/NEJMoa1401480 [DOI] [PubMed] [Google Scholar]
- 184.Weekly epidemiological record. Vaccines against Influenza WHO Position Paper – November 2012; 2012. doi: 10.1111/j.1750-2659.2012.00345.x [DOI]
- 185.Kahn KE, Black CL, Ding H, et al. Influenza and Tdap Vaccination Coverage Among Pregnant Women - United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67(38):1055–1059. doi: 10.15585/mmwr.mm6738a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Centers for Disease Control and Prevention NC for I and RD (NCIRD). Flu Vaccination Coverage Among Pregnant Women – United States, 2015–16 Flu Season | FluVaxView | Seasonal Influenza (Flu) | CDC.
- 187.Yuet C, Yuen S, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – A systematic review. Vaccine. 2014;32:4602–4613. doi: 10.1016/j.vaccine.2014.06.067 [DOI] [PubMed] [Google Scholar]
- 188.European Centre for Disease Prevention and Control. Seasonal Influenza Vaccination and Antiviral Use in EU/EEA Member States. Stockholm; 2018. [Google Scholar]
- 189.Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al. First Trimester Influenza Vaccination and Risks for Major Structural Birth Defects in Offspring. J Pediatr. 2017;187:234–239.e4. doi: 10.1016/j.jpeds.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sperling RS, Riley LE, Group on behalf of TI and EIEW. Influenza Vaccination, Pregnancy Safety, and Risk of Early Pregnancy Loss. Obstet Gynecol. 2018;131(5). [DOI] [PubMed] [Google Scholar]
- 191.Sheffield JS, Greer LG, Rogers VL, et al. Effect of Influenza Vaccination in the First Trimester of Pregnancy. Obstet Gynecol. 2012;120(3). [DOI] [PubMed] [Google Scholar]
- 192.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201(6):547–552. doi: 10.1016/j.ajog.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 193.Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol. 2012;207(3):S38–S46. doi: 10.1016/j.ajog.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 194.Chambers CD, Johnson DL, Xu R, et al. Safety of the 2010–11, 2011–12, 2012–13, and 2013–14 seasonal influenza vaccines in pregnancy: Birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine. 2016;34(37):4443–4449. doi: 10.1016/j.vaccine.2016.06.054 [DOI] [PubMed] [Google Scholar]
- 195.Macias AE, Precioso AR, Falsey AR, Initiative GI. The Global Influenza Initiative recommendations for the vaccination of pregnant women against seasonal influenza. Influenza Other Respi Viruses. 2015;9 Suppl 1(Suppl 1):31–37. doi: 10.1111/irv.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8(1):44–52. doi: 10.1016/S1473-3099(07)70311-0 [DOI] [PubMed] [Google Scholar]
- 197.Centers for Disease Control. Measles | For Healthcare Professionals | CDC.
- 198.Rasmussen SA, Jamieson DJ. What Obstetric Health Care Providers Need to Know About Measles and Pregnancy. Obstet Gynecol. 2015;126(1):163–170. doi: 10.1097/AOG.0000000000000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Manikkavasagan G, Ramsay M. The rationale for the use of measles post-exposure prophylaxis in pregnant women: A review The rationale for the use of measles post-exposure prophylaxis in pregnant women: A review. J Obstet Gynaecol (Lahore). 2009;29(7):572–575. doi: 10.1080/01443610903104478 [DOI] [PubMed] [Google Scholar]
- 200.Atmar RL, Englund JA, Hammill H. Complications of Measles during Pregnancy. Clin Infect Dis. 1992;14(1):217–226. [DOI] [PubMed] [Google Scholar]
- 201.Mclean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recomm Reports. 2013;62(4):1–34. doi: 10.2307/24832555 [DOI] [PubMed] [Google Scholar]
- 202.World Health Organization. Malaria in pregnant women. https://www.who.int/malaria/areas/high_risk_groups/pregnancy/en/. Published 2017 Accessed April 22, 2019.
- 203.Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases (NCEZID)-Division of Vector-Borne Diseases (DVBD). Build a Zika Prevention Kit. https://www.cdc.gov/zika/prevention/prevention-kit.html. Published 2019 AccessedApril 22, 2019.
- 204.Centers for Disease Control. Zika Travel Information | Travelers’ Health | CDC.
- 205.WHO Global Malaria Programme, WHO Department of Reproductive Health and Research. WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP). Geneva; 2014. [Google Scholar]
- 206.Szweda H, Jozwik M. Urinary tract infections during pregnancy - an updated overview. Dev period Med. 2016;20(4):263–272. [PubMed] [Google Scholar]
- 207.Millar LK, Cox SM. URINARY TRACT INFECTIONS COMPLICATING PREGNANCY. Infect Dis Clin North Am. 1997;11(1):13–26. doi: 10.1016/S0891-5520(05)70339-1 [DOI] [PubMed] [Google Scholar]
- 208.Easter SR, Cantonwine DE, Zera CA, Lim K-H, Parry SI, McElrath TF. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol. 2016;214(3):387.e1–387.e7. doi: 10.1016/j.ajog.2015.09.101 [DOI] [PubMed] [Google Scholar]
- 209.Fichorova RN, Beatty N, Sassi RRS, et al. Systemic inflammation in the extremely low gestational age newborn following maternal genitourinary infections. Am J Reprod Immunol. 2015;73(2):162–174. doi: 10.1111/aji.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ahlin K, Himmelmann K, Hagberg G, et al. Cerebral palsy and perinatal infection in children born at term. Obstet Gynecol. 2013;122(1):41–49. doi: 10.1097/AOG.0b013e318297f37f [DOI] [PubMed] [Google Scholar]
- 211.Humann J, Mann B, Gao G, et al. Bacterial Peptidoglycan Traverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe. 2016;19(3):388–399. doi: 10.1016/j.chom.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lanté F, Meunier J, Guiramand J, et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008;18(6):602–609. doi: 10.1002/hipo.20421 [DOI] [PubMed] [Google Scholar]
- 213.De Felice M, Melis M, Aroni S, et al. The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci Ther. 2018;0(0). doi: 10.1111/cns.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ma M, Ren Q, Yang J, et al. Key role of soluble epoxide hydrolase in the neurodevelopmental disorders of offspring after maternal immune activation. Proc Natl Acad Sci. 2019;116(14):7083 LP–7088. doi: 10.1073/pnas.1819234116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang Q, Liu C. Protective effects of quercetin against brain injury in a rat model of lipopolysaccharide-induced fetal brain injury. Int J Dev Neurosci. 2018;71:175–180. doi: 10.1016/j.ijdevneu.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 216.Kalmady SV, Venkatasubramanian G, Shivakumar V, et al. Relationship between Interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naive schizophrenia: evidence for differential susceptibility? PLoS ONE [Electronic Resour. 2014;9(5):e96021. doi: 10.1371/journal.pone.0096021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xuan ICY, Hampson DR. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One. 2014;9(8):e104433–e104433. doi: 10.1371/journal.pone.0104433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155(7):2635–2646. doi: 10.1210/en.2014-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mann JR, McDermott S. Are Maternal Genitourinary Infection and Pre-Eclampsia Associated With ADHD in School-Aged Children? J Atten Disord. 2010;15(8):667–673. doi: 10.1177/1087054710370566 [DOI] [PubMed] [Google Scholar]
- 220.Werenberg Dreier J, Nybo Andersen A-M, Hvolby A, Garne E, Kragh Andersen P, Berg-Beckhoff G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry. 2016;57(4):540–548. doi: 10.1111/jcpp.12480 [DOI] [PubMed] [Google Scholar]
- 221.Kim S, Kim H, Yim YS, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017. doi: 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Osokine I, Erlebacher A. Inflammation and Autism: From Maternal Gut to Fetal Brain. Trends Mol Med. 2017;23(12):1070–1071. doi: 10.1016/j.molmed.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stiles J, Jernigan TL. The Basics of Brain Development. Neuropsychol Rev. 2010;20(4):327–348. doi: 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]