Abstract
Overexpression of the MDM2 oncogene and mutations in the p53 tumor suppressor commonly occur in hepatocellular carcinoma (HCC) and are associated with increased mortality due to this disease. Inhibiting MDM2 has been demonstrated to be a valid approach for the treatment of HCC. However, most of the MDM2 inhibitors evaluated to date have been designed to block the MDM2 and p53 binding, and have limited efficacy against tumors with mutant or deficient p53. In the present study, we developed a novel MDM2 inhibitor (termed SP141) that has direct effects on MDM2 and exerts anti-HCC activity independent of the p53 status of the cancer cells. We demonstrate that SP141 inhibits cell growth and prevents cell migration and invasion, independent of p53. Mechanistically, SP141 directly binds the MDM2 protein and promotes MDM2 degradation. The inhibition of MDM2 by SP141 also increases the sensitivity of HCC cells to sorafenib. In addition, in orthotopic and patient-derived xenograft models, SP141 inhibits MDM2 expression and suppresses tumor growth and metastasis, without any host toxicity. Furthermore, the inhibition of MDM2 by SP141 is essential for its anti-HCC activities. These results provide support for the further development of SP141 as a lead candidate for the treatment of HCC.
Keywords: CRISPR/Cas9, Hepatocellular carcinoma, MDM2, p53-independent, Patient-derived xenograft
Introduction
Hepatocellular carcinoma (HCC) is the major histological form of primary liver cancer, and causes significant morbidity and mortality worldwide.1, 2 The five-year survival rate for patients with locally advanced or metastatic HCC remains low,2 largely because HCC is relatively resistant to conventional chemotherapy.3, 4 Sorafenib, an oral multikinase inhibitor of the vascular endothelial growth factor receptor, provides limited clinical benefit for patients diagnosed when the cancer has already become regionally advanced or distantly metastatic.5, 6 The clinical impact of the combination of sorafenib plus conventional therapies is still under investigation. Therefore, there is a need to develop novel therapies to improve the outcomes for patients with advanced HCC.
A number of recent studies investigating the initiation and progression of HCC, as well as its response to chemotherapy, have provided new insights into HCC prevention and therapy.5, 6 Several studies have demonstrated that the MDM2 oncogene is amplified and overexpressed in HCC and plays a crucial role in HCC development and progression.7, 8, 9 The overexpression of MDM2 has been linked to increased metastasis, decreased chemosensitivity and more aggressive behavior of HCC.10
The MDM2 oncoprotein is an E3 ubiquitin ligase that targets the p53 tumor suppressor for degradation and represses the p53-mediated transactivation of its target genes, thereby acting as a major negative regulator of p53.11, 12, 13 MDM2 has also been found to exert its oncogenic activity in a p53-independent manner, promoting cell growth and invasion and decreasing cell apoptosis and chemosensitization.13, 14, 15 MDM2 knockdown inhibits cell growth, induces apoptosis, and sensitizes cancer cells to radiotherapy and chemotherapy, even in the absence of wild type p53, validating MDM2 as a promising therapeutic target for human cancers.13, 16, 17, 18
There has been increasing interest in developing MDM2 inhibitors that directly target MDM2 itself rather than the MDM2-p53 interaction, because MDM2-p53 inhibitors, such as nutlin-3,19 RITA,20 and MI-219,21 are only effective against tumors containing wild-type p53, and do not improve the outcomes of cancers with mutant p53. Genetic alterations of p53 are common in human HCC, and these cancers are more aggressive, more likely to metastasize, and largely unresponsive to this type of MDM2 inhibitor.22, 23 Therefore, it is desirable to discover and develop novel MDM2 inhibitors that have direct effects on MDM2 and exert their anticancer activity independent of the p53 status of the cancer cells.
We have recently performed a computer-aided, structure-based rational drug design and high-throughput screening and discovered a novel small molecule MDM2 inhibitor, termed SP141.24, 25 The present study was designed to evaluate the anti-HCC activity of SP141, its potential to chemosensitize HCC cells to other anticancer agents, and to determine the underlying mechanism(s) of action of SP141 in various in vitro and in vivo models of HCC with different backgrounds of p53 (wildtype, mutant, or null). We believe that our results demonstrate the therapeutic potential of targeting MDM2 for HCC and provide a basis for the further development of SP141 for HCC.
Methods
Chemicals, antibodies, plasmids, cell lines, and reagents
SP141 was synthesized and purified by our laboratories, and the structure was confirmed by UV, IR, MS and NMR spectroscopy.24, 25 The purity of the compound was determined to be greater than 99%. Sorafenib was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All chemicals and solvents were of analytical grade. The antibodies against human MDM2 (Ab-2) and p21 (Ab-1) were obtained from EMD Chemicals (Gibbstown, NJ). The antibodies against human p53 (DO-1), ZEB-1 (H120), and Twist (Twist2C1a) were sourced from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against human vimentin (V9), ubiquitin (6C1), and β-actin (AC-15) were from Sigma (St. Louis, MO). The antibodies against human E-cadherin (36/E-Cadherin), N-cadherin (32/N-Cadherin), and β-catenin (14/Beta-Catenin) were purchased from BD Biosciences (San Jose, CA). The goat anti-mouse IgG (H + L) and goat anti-rabbit IgG (H + L) antibodies were from Bio-Rad (Hercules, CA). The wild-type MDM2 and mutant MDM2 (C464A without E3 ligase activity) expression vectors were kindly provided by Dr. J. Chen (Moffitt Cancer Center, Tampa, FL). The other plasmids used in this study were generated in our laboratory.
The immortalized normal human hepatocytes (CL48 and LO2) and HCC cell lines (HepG2, Hep3B, Huh7, SMMC-7721, MHCC97H,26 MHCCLM3,27 and PLC/PRF/5) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The HepG2, Hep3B, and Huh7 cells were obtained from the American Type Culture Collection (Rockville, MD). The MHCC97-H and MHCC-LM3 were established as reported previously. The CL48, LO2, SMMC-7721, and PLC/PRF/5 cell lines were kindly provided by Dr. Y. Yen (City of Hope, Duarte, CA).
Assays for the in vitro anti-HCC activity of SP141
The assays used to examine the effects of SP141 on cell viability (MTT assay), colony formation, cell migration (wound healing assay), and cell invasion (transwell invasion assay) were performed as reported previously.24, 25, 28 In brief, HCC cells were seeded in 96-well plates (3–4 × 103 cells/well) for 24 h and treated with various concentrations of SP141 (0, 0.01, 0.05, 0.1, 0.2, and 0.5 μM) for another 72 h for the MTT assay. To determine the effects of SP141 on colony formation, the cells (1000 cells/well) in 6-well plates were exposed to SP141 (0, 0.2, or 0.5 μM) for 24 h, and then maintained in fresh medium for another 10 days. For the wound healing assay, confluent monolayers of HCC cell lines were scratched using a pipette tip and exposed to SP141 (0, 0.05, or 0.1 μM). Five different fields of each wound were then monitored and photographed at 0, 12, 24, and 48 h with a phase-contrast Olympus microscope (Olympus America Inc., PA, USA). To evaluate the effects of SP141 on cell invasion, the HCC cells (1–2 × 104 cells/well) in the upper well of a Boyden chamber were treated with SP141 (0, 0.05, or 0.1 μM) for 24 h, followed by staining with Mayer's Hematoxylin and Eosin solution. The positively-stained areas were photographed and analyzed.
Western blotting and ubiquitination assays
For the Western blot analyses, the HCC cells and tumor tissues were lysed in NP-40 buffer with a protease inhibitor mixture (Sigma, St. Louis, MO). The lysates were centrifuged and the supernatants were collected and subjected to Western blot analyses.24, 25 The ubiquitination of MDM2 was examined using the previously reported methods. In brief, HCC cells were co-transfected with ubiquitin and MDM2 plasmids and exposed to SP141 for 24 h. The cell lysates were immunoprecipitated with an anti-MDM2 antibody and the MDM2 ubiquitination was examined with an anti-ubiquitin antibody.24, 25
Immunofluorescence
The immunofluorescent detection of MDM2 was performed as described previously.24, 25 Briefly, HCC cells were grown on sterile coverslips in 12-well plates (1–2 × 104 cells/well) and exposed to SP141 at 0.5 μM for 24 h. The cells were then fixed in 4% formalin and incubated with an anti-MDM2 antibody at 4 °C overnight. The cells were subsequently incubated with Alexa Fluor 488 (anti-mouse), counterstained with DAPI, and imaged under a fluorescence microscope (Olympus America Inc. Irving, TX).
Cellular thermal shift assay
The ability of SP141 to bind MDM2 in HCC cells was assessed using the cellular thermal shift assay (CETSA).29 In brief, HepG2 and Huh7 cells were seeded into 60-mm dishes at a density of 6 × 105 cells/dish and exposed to DMSO or 0.5 μM SP141 for 2 h. The cells were then harvested and resuspended in PBS and the cell suspensions were equally aliquoted into 12 PCR tubes. These were heated for 3 min at 42, 44, 46, 48, 50, 52, 54, 56, 58, 60, 62, or 64 °C. After three freeze-thaw cycles, the cells were lysed and the supernatants were subjected to Western blotting.
CRISPR/Cas9-mediated MDM2 knockout in HCC cells
CRISPR/Cas9 vectors containing sgRNA targeting MDM2 were designed and constructed to confirm the importance of MDM2 in HCC growth and to assess the impact of MDM2 knockout on the response of cells to SP141. A plasmid constructed with a protospacer sequence targeting GFP was used as the control vector. All of the specific target sequences were amplified and cloned into lenti-CRISPR vectors and verified by DNA sequencing.30 The primer sequence of sgRNA-MDM2 was 5′-GTTGGGCCCTTCGTGAGAAT-3′. The CRISPR/cas9 vectors were transiently transfected into HCC cells, then the cells were subjected to puromycin antibiotic selection for 24 h. The puromycin-resistant cells were maintained and expanded in fresh culture medium. The protein and mRNA expression levels of MDM2 in the transfected cells were examined by Western blotting and real-time quantitative PCR. The cleavage of MDM2 at the target loci in HCC cells was detected by the T7EN1 assay using the following primers: MDM2-clev-forward, 5′-TGCTAGCATTCCTGTGACTGAG-3′; MDM2-clev-reverse, 5′-AAAGCCCTCTTCAGCTTGTGT-3′.
Development of the HCC orthotopic tumor model, in vivo imaging, and histopathological studies
The animal protocols were approved by the Institutional Animal Use and Care Committee of the University of Houston. Male athymic nude mice (nu/nu, 4–6 weeks) were obtained from Charles River Laboratories International, Inc. (Wilmington, MA). HepG2-GFP cells were orthotopically implanted into the left liver lobe of nude mice. Tumor growth was monitored, and tumors were photographed once a week using an IVIS Lumina XR in vivo imaging system (Caliper, Mountain View, CA). After 10 days, nude mice with HepG2-GFP orthotopic tumors were randomly divided into control and treatment groups (10 mice/group). The mice in the treatment group were administered SP141 in PEG400:ethanol:saline (57.1:14.3:28.6, v/v/v) by intraperitoneal injection at 40 mg/kg/d, 5 d/wk for 20 days. The control group received the vehicle only. At the termination of the experiments, all mice were examined for tumor metastasis to the peritoneum and mesentery. The tumors were removed and processed for immunohistochemistry and Western blotting, and various tissues (lungs, brain, heart, liver, spleen, and kidneys) were removed and fixed for Hematoxylin and Eosin (H&E) staining.
The immunohistochemical staining and H&E staining were performed as described previously.24, 25 Briefly, at the end of experiment, tumors and various tissues were removed from the nude mice bearing HepG2 orthotopic tumors, fixed in 10% formalin, and embedded in paraffin. The tumor and tissue sections (5 μm thick) were then prepared, deparaffinized in xylenes, rehydrated, and washed with PBS. The immunohistochemical staining for MDM2 was performed using biotinylated anti-human antibodies against MDM2 (diluted 1:50 in 5% BSA in PBS). Then, the sections were counterstained with hematoxylin, mounted, and analyzed. For H&E staining, tissue sections were stained in Mayer's Hematoxylin for 10 min and then stained with Eosin for 1 min. Finally, all of the sections were analyzed and imaged under a phase-contrast Olympus microscope (Olympus America Inc., Central Valley, PA).
Establishment of HCC patient-derived xenograft (PDX) models
Fresh tumor tissues from patients who underwent curative resection were placed in ice-chilled high-glucose DMEM with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin and rapidly processed for engraftment. After the removal of necrotic tissue, the tumor specimens were partitioned into 2 × 1 × 1 mm3 sections with a No. 10 scalpel blade under aseptic conditions and were washed three times in ice-cold PBS. Pieces of tumor tissues were subcutaneously transplanted with Matrigel (BD Biosciences) into the right flanks of male non-obese, diabetic, severe combined immunodeficient (NOD/SCID) mice (F0) (4–5 weeks old) using a No. 20 trocar.31 Tumor growth was recorded three to four times per week by measuring the length (L) and width (W) of the tumor with calipers. The tumor volume (TV, mm3) was calculated as TV = 0.5 × L × W2. The HMP431 (MDM2high) and HMP421 (MDM2low) PDX xenograft tumors were transplanted into a final cohort of mice (F4) that were treated with the compound. The mice in the treatment group were administered SP141 in PEG400:ethanol:saline (57.1:14.3:28.6, v/v/v) by intraperitoneal injection at a dose of 40 mg/kg/d, 5 d/wk for 4 weeks. The control group received the vehicle only. At the termination of the experiments, the tumors were removed and processed for immunohistochemical studies.
Statistical analysis
All of the quantitative data were analyzed using the Prism software program version 6 (Graph Pad software Inc., San Diego, CA) and were reported as the means ± SEM from at least three independent experiments. The significance of differences from comparisons of multiple groups was analyzed using a one-way ANOVA or two-way ANOVA when there were one or two variables, respectively. Differences were considered statistically significant at P ≤ 0.05.
Results
MDM2 is overexpressed in HCC cell lines and promotes HCC cell growth and invasion: further proof using CRISPR/Cas9 genome editing
Although it has previously been shown that MDM2 is overexpressed in clinical specimens of HCC,7, 8, 9 we wanted to confirm that our in vitro models recapitulated this overexpression of MDM2 and its association with malignant characteristics. We first examined the MDM2 protein and mRNA expression levels in the CL48 (p53 wild-type (wt)) and LO2 (p53wt) normal human hepatocyte lines and in six HCC cell lines (HepG2 (p53 wt), Hep3B (p53 null), Huh7 (p53 mutant (mt)), MHCC97H (p53 mt), MHCCLM3 (p53 wt), and PLC/PRF/5 (p53 mt)). The data indicated that both the mRNA and protein levels of MDM2 in HCC cells were higher than those in normal hepatocytes (data not shown). To demonstrate the importance of MDM2 in the growth and invasion of HCC cells, we established MDM2 knockout (KO) HepG2, Huh7 and MHCCLM3 cell lines using CRIPSR/Cas9 genome editing. The mRNA and protein expression of MDM2 in the MDM2 KO cell lines and their corresponding parental cell lines are shown in Figs. S1A and S1B. The CRISPR/Cas9-mediated MDM2 KO reduced the HCC cell growth and invasion (Figs. S1C and S1D), regardless of the p53 status of the cells, confirming that MDM2 has a critical role in HCC tumor growth and metastasis.
SP141 inhibits HCC cell growth and metastasis in vitro, independent of p53
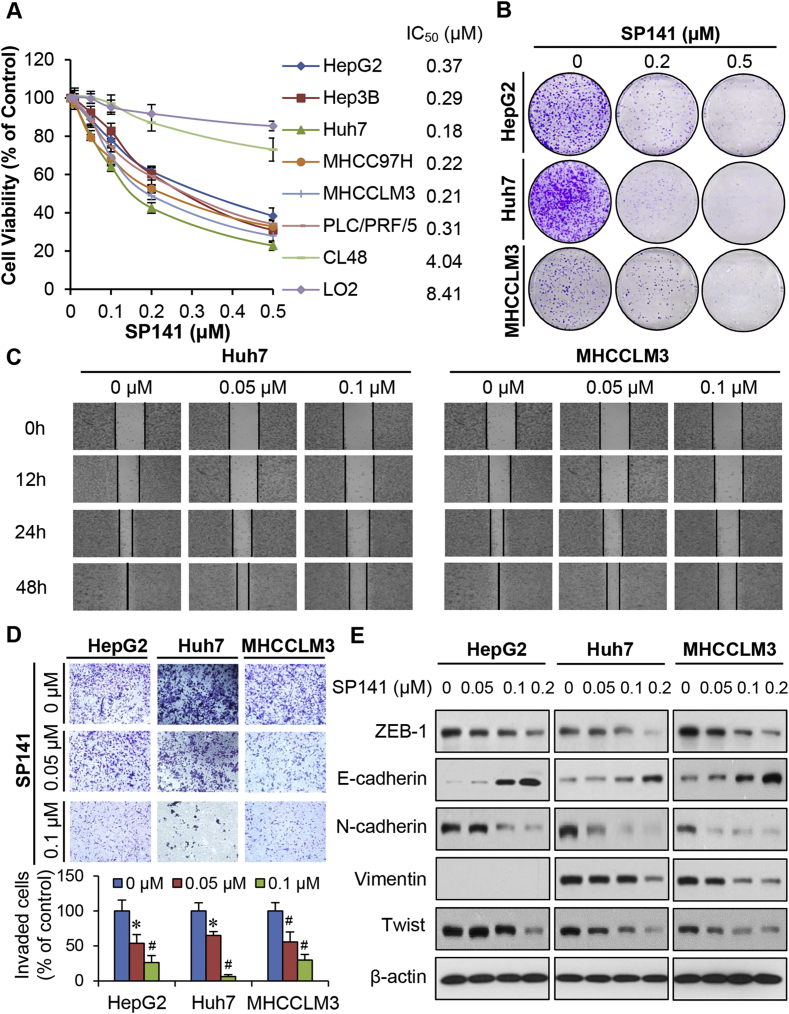
SP141 was examined for its effects on cell viability in the normal human hepatocyte and HCC cell lines mentioned above. As shown in Fig. 1A, all HCC cell lines were sensitive to SP141 treatment, and had submicromolar IC50 values (0.15–0.37 μM), regardless of their p53 status. The normal hepatocytes were much significantly less sensitive to the compound (4.04–8.41 μM), indicating that SP141 exhibits selective cytotoxicity to cancer cells. Similarly, SP141 significantly inhibited the colony formation of HepG2, Huh7, and MHCCLM3 cells in a concentration-dependent and p53-independent manner (Fig. 1B).
Figure 1.
In vitro activity of SP141 in human HCC cells. (A) Non-malignant human hepatocytes (LO2 and CL48) and HCC cells were exposed to various concentrations (0, 0.01, 0.05, 0.1, 0.2, and 0.5 μM) of SP141 for 72 h, followed by measurement of the cell viability via MTT assays. HCC cells were treated with various concentrations of SP141 for 24 h or 48 h, then examined for their (B) clonogenic survival; (C) migration ability; (D) invasion ability; and (E) expression of EMT-related proteins. All assays were performed in triplicate and repeated three times. (*P < 0.05 and #P < 0.01).
MDM2 overexpression is correlated with metastasis in HCC.32 SP141 was further assessed for its effects on cell migration and invasion in HCC cell lines with high metastatic potential.27 As shown in Fig. 1C, in comparison to the control (treated with vehicle) Huh7 and MHCCLM3 cells, low concentrations (0.05 and 0.1 μM) of SP141 effectively prevented the migration of cells into wounded areas. These concentrations of this compound also inhibited the invasion of HepG2, Huh7, and MHCCLM3 cells in a p53-independent manner (Fig. 1D). Considering that SP141 prevented HCC cell migration and invasion, we examined its effects on markers of the epithelial–mesenchymal transition (EMT), a process involved in cell migration and invasion, as well as metastasis.33 We found that SP141 decreased the protein levels of ZEB-1, N-cadherin, vimentin, and Twist, and increased the protein level of E-cadherin in all three HCC cell lines, with significant effects observed beginning at 0.05 μM (Fig. 1E).
SP141 induces MDM2 degradation, regardless of the p53 status
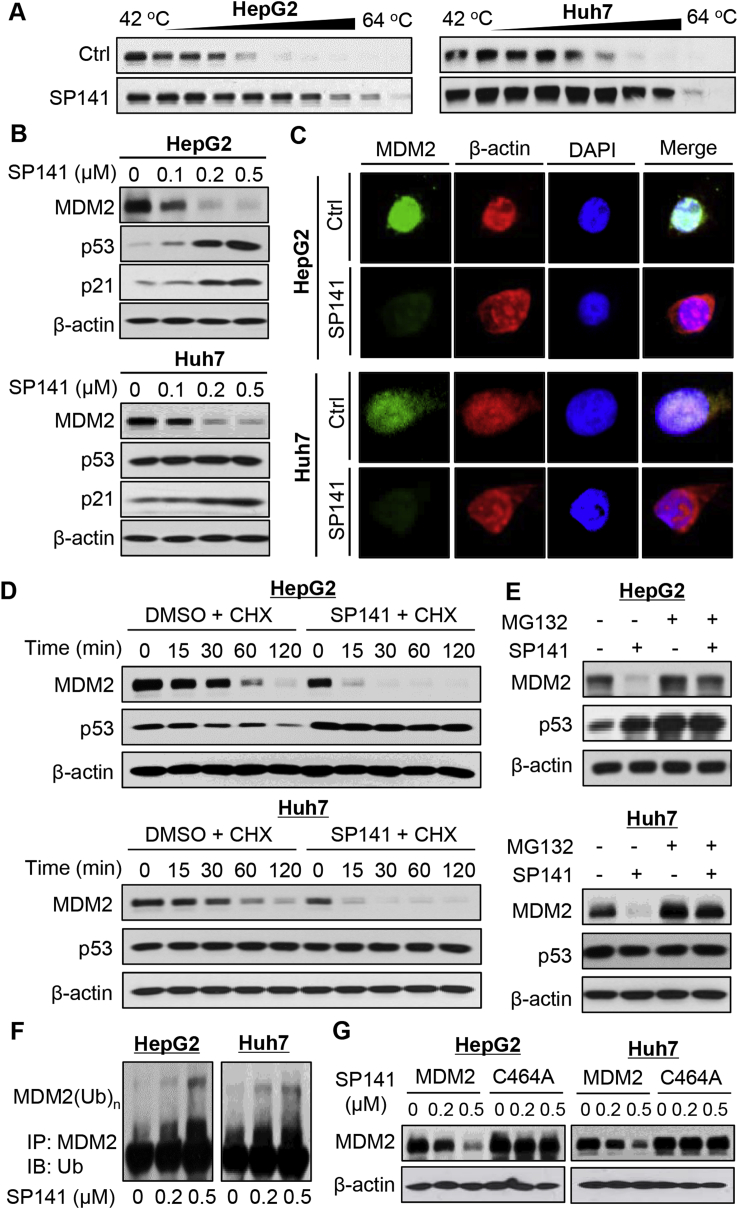
SP141 has previously been demonstrated to specifically bind the MDM2 protein and induce MDM2 degradation.12 This compound effectively bound the MDM2 protein in HepG2 and Huh7 cells, as demonstrated using the CETSA assay (Fig. 2A). We further examined the effects of SP141 on MDM2 and related proteins in HepG2 and Huh7 cells. SP141 treatment significantly reduced the MDM2 expression and increased the protein expression of wild-type p53 and p21 in a concentration-dependent manner (Fig. 2B). However, there were no effects on mutant p53. The SP141-induced MDM2 degradation was confirmed by an immunofluorescence study in both HepG2 and Huh7 cells (Fig. 2C). SP141 was then examined for its effects on MDM2 protein turnover in these cells. As shown in Fig. 2D, SP141 shortened the protein half-life of MDM2 and extended the half-life of wild-type p53. As expected, the compound did not affect mutant p53. We also found that MG-132, a proteasome inhibitor, reduced the inhibitory effects of SP141 on MDM2 in both HepG2 and Huh7 cells (Fig. 2E), suggesting that SP141 inhibits MDM2 by promoting its proteasomal degradation.
Figure 2.
SP141 induces MDM2 degradation in human HCC cells. (A) HepG2 and Huh7 cells were exposed to 1 μM of SP141 for 2 h, followed by cellular thermal shift assays. (B) HCC cells were exposed to various concentrations of SP141 for 24 h. The protein expression levels of MDM2, p53, and p21 were determined. (C) The HepG2 and Huh7 cells were exposed to vehicle or SP141 (0.5 μM) for 24 h, followed by immunofluorescence detection of MDM2. (D) The cells were treated with or without SP141 for 24 h, followed by exposure to a protein synthesis inhibitor, cycloheximide (CHX, 15 μg/mL). The protein levels of MDM2 and p53 were detected at the indicated times after exposure to CHX. (E) The HepG2 and Huh7 cells were treated with or without SP141 (0.5 μM) for 24 h. They were then exposed to MG-132 (25 μM), a proteasome inhibitor, for an additional 6 h. The protein levels of MDM2 and p53 were detected by Western blotting. (F) The cells were co-transfected with MDM2 and ubiquitin plasmids, followed by treatment with SP141 (0, 0.2 and 0.5 μM) for 24 h. Then the cell lysates were subjected to immunoprecipitation with an anti-MDM2 antibody. The ubiquitinated MDM2 was detected using an anti-ubiquitin antibody. (G) The cells were transfected with a wild-type MDM2 plasmid or a mutant MDM2 plasmid (C464A) without E3 ligase activity, followed by exposure to SP141 (0, 0.2 and 0.5 μM) for 24 h. The MDM2 protein levels were detected. All assays were performed in triplicate and repeated three times.
We and other investigators have demonstrated that MDM2 degradation mainly depends on its auto-ubiquitination.12 Thus, we investigated the effects of SP141 on MDM2 ubiquitination. As shown in Fig. 2F, SP141 induced MDM2 ubiquitination in a concentration-dependent manner in both HepG2 and Huh7 cells. It was further observed that SP141 only promoted the degradation of wild-type MDM2, with no significant effect on a MDM2 mutant (C464A) lacking ubiquitin E3 ligase activity (Fig. 2G). This indicated that SP141 induces MDM2 protein degradation by promoting its auto-ubiquitination.
CRISPR/Cas9-mediated MDM2 knockout blocks SP141's anticancer activity
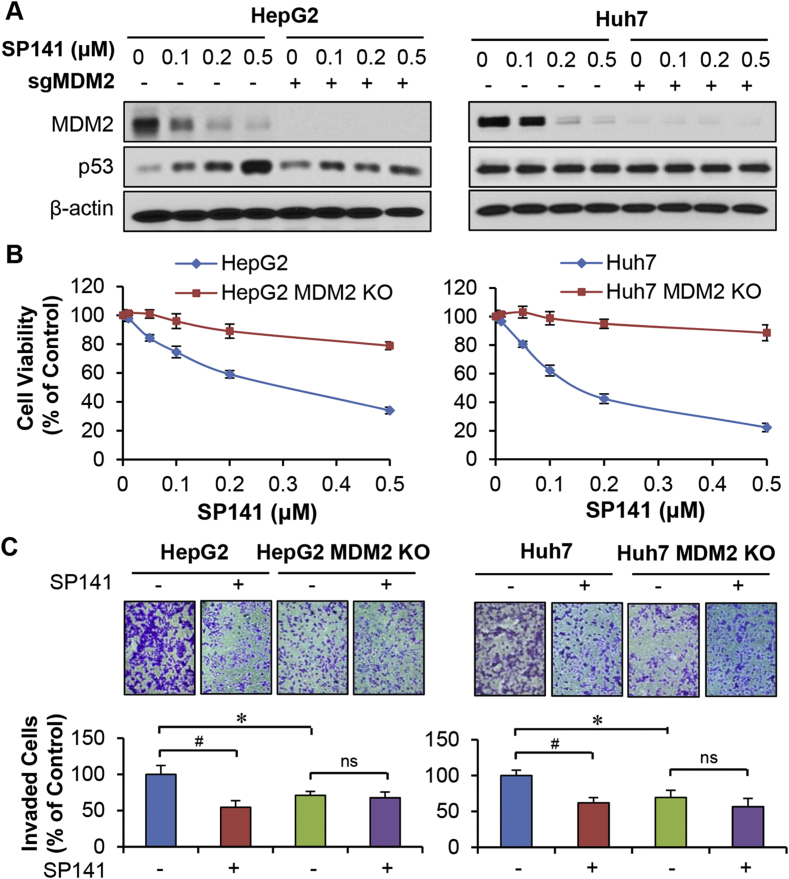
To demonstrate that the effects of SP141 on MDM2 are critical to its anticancer activity, we investigated its effects in the MDM2 knockout (KO) HepG2 and Huh7 cell lines described above that were generated using the CRISPR/Cas9 system. As shown in Fig. 3A, MDM2 KO reduced the effects of SP141 on wild-type p53 activation. We further observed that MDM2 KO decreased SP141's effects on cell growth (Fig. 3B) and invasion (Fig. 3C), confirming the critical role of MDM2 in the anti-HCC activity of SP141.
Figure 3.
CRISPR/Cas9-mediated MDM2 knockout blocks SP141's activity in HCC cells. HepG2 and Huh7 cell lines with or without CRISPR/Cas9-mediated MDM2 knockout (sgMDM2) were exposed to various concentrations of SP141 for (A) 24 h for analyses of protein expression or (B) 72 h for cell viability measurement via MTT assays. (C) These cells were then treated with or without SP141 (0.05 μM) for 24 h, followed by measurement of the cell invasion via transwell cell invasion assays. All assays were performed in triplicate and repeated three times. (*P < 0.05 and #P < 0.01).
SP141 sensitizes HCC cells to sorafenib treatment
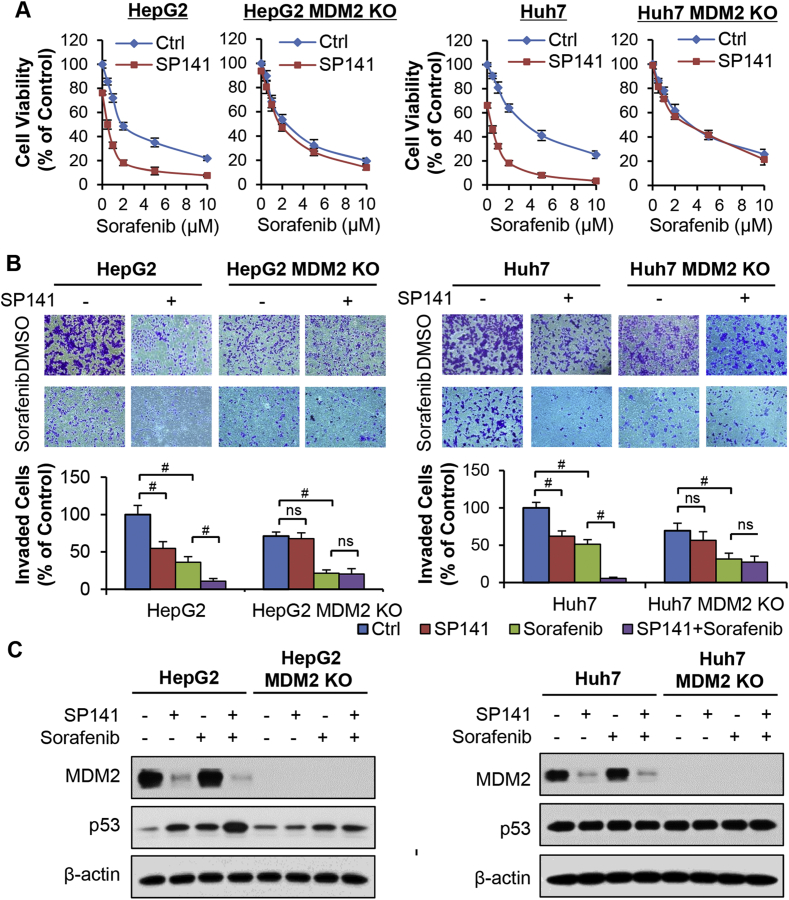
Considering that MDM2 plays an important role in the chemoresistance of HCC,10 SP141 was evaluated for its potential to sensitize HepG2 and Huh7 cells (and their corresponding MDM2 KO cells) to sorafenib treatment. As shown in Fig. 4A and B, treatment with a sublethal concentration of SP141 (0.1 μM) effectively increased the sensitivity of the parental HepG2 and Huh7 cells to sorafenib and significantly enhanced the inhibitory effects of sorafenib on the growth and invasion of both of these cell lines. However, treatment with SP141 did not affect the sorafenib sensitivity of the MDM2 KO cell lines.
Figure 4.
SP141 sensitizes hepatocellular carcinoma cells to sorafenib. (A) HepG2 and Huh7 cells with or without sgMDM2 were treated with SP141 (0.1 μM) and various concentrations (0, 0.5, 1, 2, 5 and 10 μM) of sorafenib for 72 h to evaluate the cell viability via MTT assays. (B) The cells with or without sgMDM2 were treated with SP141 (0.1 μM) and sorafenib (2 μM) for 24 h to measure the cell invasion via transwell invasion assays. (C) The cells with or without sgMDM2 were treated with SP141 (0.1 μM) and various concentrations of sorafenib for 24 h prior to analyses of the protein expression. All assays were performed in triplicate and repeated three times. (*P < 0.05 and #P < 0.01).
We also examined the expression of MDM2-related proteins in the parental and MDM2 KO HCC cells that were treated with or without SP141 and/or sorafenib. As shown in Fig. 4C, sorafenib treatment did not have any effects on the expression of MDM2 and p53 in the parental cells. However, the combination of SP141 plus sorafenib significantly decreased MDM2 and increased p53 expression additively. In the CRISPR/Cas9-induced MDM2 KO cells, the effects of SP141 on p53 activation were greatly reduced (Fig. 4C). In addition, MDM2 KO also attenuated the chemosensitization of the HCC cells to sorafenib by SP141.
SP141 inhibits tumor growth and metastasis in an orthotopic HepG2 model
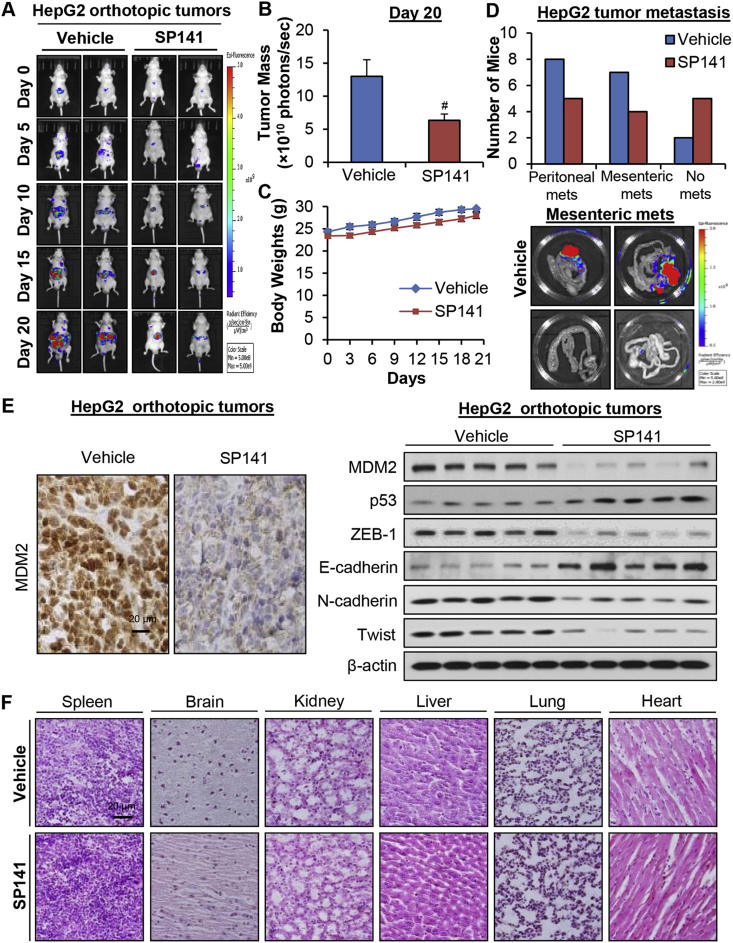
We further assessed the in vivo efficacy of SP141 in an orthotopic HepG2 tumor model. As shown in Fig. 5A, nude mice bearing orthotopic HepG2 tumors were treated with SP141 at 40 mg/kg/day, 5 days/week for 20 days, resulting in 51.3% inhibition of tumor growth compared to the vehicle-treated mice (Fig. 5B). However, no remarkable differences were observed in the average body weights in the control and treatment groups, suggesting that SP141 did not induce significant systemic toxicity (Fig. 5C). At the end of the in vivo studies, we examined the effects of SP141 on HepG2 tumor metastasis to various organs. Necropsies showed that 8 and 7 out of 12 control mice developed metastatic lesions in the peritoneum and mesentery, respectively (Fig. 5D). However, SP141 treatment decreased the incidence of peritoneal dissemination and mesenteric metastasis to 5/10 and 4/10, respectively (Fig. 5D).
Figure 5.
SP141 suppresses tumor growth and metastasis in an orthotopic model of HCC. HepG2-GFP cells were implanted orthotopically into the left liver lobe of nude mice. Nude mice bearing HepG2-GFP orthotopic tumors were administered SP141 by i.p. injection at a dose of 40 mg/kg/d, 5 d/wk for 20 days. (A) Tumor size was monitored once every 5 days by fluorescence imaging via an IVIS in vivo imaging system. (B) On Day 20, the average tumor mass (determined by the detected photons/sec) of the SP141-treated mice was compared with that of the control mice. (C) The mice were monitored for changes in body weight as a surrogate marker for toxicity. (D) At the termination of the experiments, the numbers of mice with metastasis to the peritoneum and mesentery were counted. Representative images showing mesenteric metastasis are presented. (E) The tumors were excised and analyzed for the expression of proteins of interest by immunohistochemistry (scale bar, 20 μm) and Western blotting assays (each lane represents a different tumor sample). (F) Representative H&E-stained tissue and organ samples are presented (scale bar, 20 μm). (*P < 0.05 and #P < 0.01).
Next, we examined the effects of SP141 on MDM2 in the orthotopic HepG2 tumors by immunohistochemical staining and Western blotting (Fig. 5E). Our findings indicated that the expression level of MDM2 was significantly reduced by this compound in vivo. Consistent with the in vitro results, SP141 increased the expression levels of wild-type p53 and E-cadherin and decreased the expression levels of ZEB-1, N-cadherin, and Twist in the orthotopic HepG2 tumors in vivo (Fig. 5E). Of note, the histopathological examinations of various tissues (liver, lung, kidneys, spleen, heart, and brain) from both control and SP141-treated mice indicated that SP141 did not cause damage to any of these organs, consistent with the lack of overt toxicity in the mice (Fig. 5F).
SP141 inhibits tumor growth in HCC PDX models, and these effects are dependent on MDM2 expression
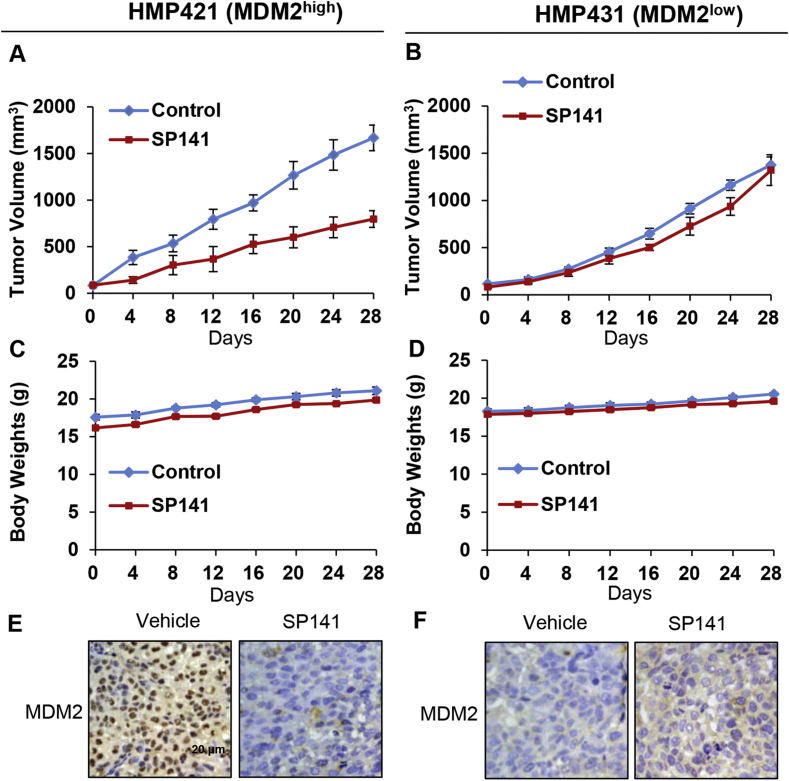
Clinically-relevant patient-derived xenograft (PDX) models have been widely used in translational research and have provided improved predictive value for the clinical outcomes compared to conventional models.31 To determine whether SP141 is effective against HCC PDX tumors, the compound was administered to NOD/SCID mice bearing HCC PDX tumors with different MDM2 expression. As shown in Fig. 6A, SP141 inhibited the growth of the HMP412 (MDM2high) xenograft tumors by about 52.2% (P < 0.01) on Day 28. However, in the HMP431 (MDM2low) xenograft model, SP141 treatment at the same dose did not significantly affect the tumor growth compared with untreated mice (Fig. 6B). No significant differences in the body weights of the mice in the different treatment groups were observed, again suggesting that SP141 was not overtly toxic at the effective dose (Fig. 6C and D).
Figure 6.
Low MDM2 expression blocks the inhibitory effects of SP141 in HCC PDX models. HMP412 (MDM2high) and HMP431 (MDM2low) tumor tissues from patients were implanted into the right flanks of male NOD/SCID mice. After the tumors had grown through three subsequent cycles of excision, fragmentation, and transplantation in new mice, SP141 was administered by i.p. injection at a dose of 40 mg/kg/d, 5 d/wk for 4 weeks. The tumor size was monitored every 4 days in both the HMP412 (MDM2high) (A) and HMP431 (MDM2low) (B) models. The mice were monitored for changes in body weight as a surrogate marker for toxicity in the HMP412 (MDM2high) (C) and HMP431 (MDM2low) (D) models. At the termination of the experiments, the HMP412 (E) and HMP431 (F) tumors were removed and analyzed for the protein expression of MDM2 by immunohistochemistry (scale bar, 20 μm). Representative images are shown.
We further assessed the effects of SP141 on MDM2 in the two PDX models. As expected, the MDM2 levels were dramatically decreased in the SP141-treated HMP412 (high MDM2 expression) tumors compared with the controls (Fig. 6E), but this was not observed in the HMP431 model with low MDM2 expression (Fig. 6F), indicating the critical role of MDM2 in SP141's anticancer activity.
Discussion
MDM2 is overexpressed and activated in various human cancers, and plays pivotal roles in cancer development and progression, acting via pathways both dependent on and independent of p53.13, 14, 15 Given the critical involvement of MDM2 in these processes, it has been the target of numerous strategies intended to treat or prevent cancer. In the present study, we have demonstrated that SP141, which directly binds MDM2 and promotes MDM2 degradation, inhibits cancer cell growth and invasion, induces apoptosis, and sensitizes HCC cells to sorafenib, regardless of their p53 status. Mechanistically, SP141 increases MDM2 self-ubiquitination and promotes its proteasomal degradation. This is distinct from the mechanisms of action of the previously reported MDM2 inhibitors and various inhibitors currently under development, and SP141 is effective even against tumors and cells lacking wild type p53.
Our results in both orthotopic and PDX models demonstrated that SP141 treatment led to significant anti-tumor and anti-metastatic effects. Moreover, SP141 treatment did not lead to any significant loss of body weight or organ damage, suggesting that SP141 was safe at the effective doses. We also demonstrated that the potency and selectivity of SP141 may be due to its inhibition of MDM2, because the various models with different MDM2 expression levels clearly demonstrated that SP141's anticancer activity was dependent on its inhibition of MDM2. The MDM2 CRISPR/Cas9-mediated KO cells and tumors with low endogenous MDM2 expression were resistant to SP141, displaying decreased sensitivity in vitro and attenuated effects in vivo.
There are several reasons why SP141 is particularly effective against human HCC. First, MDM2 overexpression and p53 mutation occur frequently and simultaneously in human HCC.7, 8, 9, 10, 22, 23 Second, MDM2 is associated with the progression, metastasis, and response to chemotherapy in HCC, regardless of the p53 status.7, 8, 9 Third, SP141's anticancer activity depends on MDM2 inhibition, and does not require functional p53.
In summary, SP141 is a specific MDM2 inhibitor that exhibited potent anti-HCC activity alone and in combination with sorafenib in clinically-relevant models of HCC. The inhibition of MDM2 by SP141 resulted in the inhibition of HCC tumor growth and metastasis and the sensitization of HCC cells to chemotherapy with sorafenib, regardless of the p53 status of the cancer cells/tumors. Our results provide additional evidence that MDM2 is a valid target for HCC therapy, and provide a novel candidate for further development as a treatment for HCC. The present results also provide a better understanding of the mechanisms of action of SP141, suggesting new strategies for targeting MDM2 for cancer therapy.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
W.W. and R.Z. were partially supported by National Institutes of Health (NIH)/National Cancer Institute grants (R01 CA186662 and R01CA214019). W.W. and R.Z. were also supported by American Cancer Society (ACS) grant RSG-15-009-01-CDD. R.Z. was also supported by funds for Robert L. Boblitt Endowed Professor in Drug Discovery and research funds from College of Pharmacy and University of Houston. J.C., J.F., and X-R. Y. were supported by grants from the National Natural Science Foundation of China (No. 81272389, 81472674, 81502486). The content of this report is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.06.001.
Contributor Information
Wei Wang, Email: wwang4@central.uh.edu.
Xin-Rong Yang, Email: yang.xinrong@zs-hospital.sh.cn.
Ruiwen Zhang, Email: rzhang27@central.uh.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Yang J.D., Roberts L.R. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag H.B. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito A.F., Abrantes A.M., Tralhão J.G., Botelho M.F. Targeting hepatocellular carcinoma: what did we discover so far? Oncol Rev. 2016;10:302. doi: 10.4081/oncol.2016.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng X., Franklin D.A., Dong J., Zhang Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014;74:7161–7167. doi: 10.1158/0008-5472.CAN-14-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M.F., Zhang Z.Y., Fu J., Yang Y.F., Yun J.P. Correlation between expression of p53, p21/WAF1, and MDM2 proteins and their prognostic significance in primary hepatocellular carcinoma. J Transl Med. 2009;7:110. doi: 10.1186/1479-5876-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santarius T., Shipley J., Brewer D., Stratton M.R., Cooper C.S. A census of amplified and overexpressed human cancer genes. Nat Rev Canc. 2010;10:59–64. doi: 10.1038/nrc2771. [DOI] [PubMed] [Google Scholar]
- 10.Rayburn E., Zhang R., He J., Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets. 2005;5:27–42. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 11.Barak Y., Gottlieb E., Juven-Gershon T., Oren M. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994;8:1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- 12.Fang S., Jensen J.P., Ludwig R.L., Vousden K.H., Weissman A.M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 13.Nag S., Qin J., Srivenugopal K.S. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27:254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohlman S., Manfredi J.J. p53-independent effects of Mdm2. Subcell Biochem. 2014;85:235–246. doi: 10.1007/978-94-017-9211-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouska A., Eischen C.M. Murine double minute 2: p53-independent roads lead to genome instability or death. Trends Biochem Sci. 2009;34:279–286. doi: 10.1016/j.tibs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Li M., Wang H., Agrawal S., Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayburn E.R., Ezell S.J., Zhang R. Recent advances in validating MDM2 as a cancer target. Anti Cancer Agents Med Chem. 2009;9:882–903. doi: 10.2174/187152009789124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nag S., Zhang X., Srivenugopal K.S., Wang M.H., Wang W., Zhang R. Targeting MDM2-p53 interaction for cancer therapy: are we there yet? Curr Med Chem. 2014;21:553–574. doi: 10.2174/09298673113206660325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassilev L.T., Vu B.T., Graves B. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 20.Issaeva N., Bozko P., Enge M. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 21.Shangary S., Qin D., McEachern D. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain S.P., Schwank J., Staib F., Wang X.W., Harris C.C. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- 23.Staib F., Hussain S.P., Hofseth L.J., Wang X.W., Harris C.C. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Qin J.J., Voruganti S. Identification of a new class of MDM2 inhibitor that inhibits growth of orthotopic pancreatic tumors in mice. Gastroenterology. 2014;147:893–902. doi: 10.1053/j.gastro.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W., Qin J.J., Voruganti S. The pyrido[b]indole MDM2 inhibitor SP-141 exerts potent therapeutic effects in breast cancer models. Nat Commun. 2014;5:5086. doi: 10.1038/ncomms6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Tang Z.Y., Ye S.L. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Tang Y., Ye L. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129:43–51. doi: 10.1007/s00432-002-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Cheng J., Qin J.J. RYBP expression is associated with better survival of patients with hepatocellular carcinoma (HCC) and responsiveness to chemotherapy of HCC cells in vitro and in vivo. Oncotarget. 2014;5:11604–11619. doi: 10.18632/oncotarget.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J.J., Wang W., Vorugant S., Wang H., Zhang W.D., Zhang R. Identification of a new class of natural product MDM2 inhibitor: in vitro and in vivo anti-breast cancer activities and target validation. Oncotarget. 2015;6:2623–2640. doi: 10.18632/oncotarget.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolle D., Fabre M., Simon-Coma M. Patient-derived mouse xenografts from pediatric liver cancer predict tumor recurrence and advise clinical management. Hepatology. 2016;64:1121–1135. doi: 10.1002/hep.28621. [DOI] [PubMed] [Google Scholar]
- 32.Ranjan A., Bera K., Iwakuma T. Murine double minute 2, a potential p53-independent regulator of liver cancer metastasis. Hepatoma Res. 2016;2:114–121. doi: 10.20517/2394-5079.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung K.T., Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39. doi: 10.1002/1878-0261.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.