Abstract
Ras gene mutation has been observed in more than 30% of cancers, and 90% of pancreatic, lung and colon cancers. Ras proteins (K-Ras, H-Ras, N-Ras) act as molecular switches which are activated by binding to GTP. They play a role in the cascade of cell process control (proliferation and cell division). In the inactive state, transforming GTP to GDP leads to the activation of GTpase in Ras gene. However, the mutation in Ras leads to the loss of internal GTPase activity and permanent activation of the protein. The activated Ras can promote the cell death or stop cell growth, which are facilitated by Ras-association domain family. Various studies have been conducted to determine the importance of losing RASSF proteins in Ras-induced tumors. This paper examines the role of Ras and RASSF proteins. In general, RASSF proteins can be used as a suitable means for targeting a large group of Ras-induced tumors.
Keywords: Cancer, Colon, H-Ras, K-Ras, Lung, N-Ras, Pancreatic
Introduction
Mutation in Ras gene has been observed in more than 30% of cancers and 90% of pancreatic, lung and colon cancers.1, 2 Ras proteins (K-Ras, H-Ras, N-Ras) function as molecular switches, which are activated by binding to GTP which plays a role in the cascade of cell process control (proliferation and cell division).1 In the inactive state, transforming GTP to GDP leads to activation of GTpase in Ras gene.3 However, the mutation in Ras results in loss of internal GTPase activity and permanent activation of the protein.4 Ras is an oncoprotein which is typically active in the cancers whose activation is associated with human cancers.5 In the present study, RAS mutation and promoter methylation of RASSF1A were reported as non-small cell lung cancer tumors, which showed no general correlation, but tumors with K-Ras mutations and RASSF1A methylation had a lower overall survival than other tumors.6
The same results were reported in another study in relation to Hepatocellular carcinoma tumors with the promoter methylation of NORE1A and Ras activation, suggesting that most Ras-activating tumors and RASSF methylation have more aggressive potency than tumors without RASSF methylation.7 The results indicated that, there is a good correlation between the K-Ras-positive cancer cells and the loss of RASSF proteins. For example, the loss of RASSF2 increases the proliferation and ability to attack the K-Ras-positive lung cancer cells. Other researches have reported similar results for RASSF3 in the lung cancer cell line. These cells are resistant to chemotherapy.1 Other studies have shown that, expression of RASSF6 in a melanoma cell causes mutation in the B-Raf message and reduces the invasiveness of cells.8, 9
Also, the inhibition of RASSF1A in cells induces the apoptosis induced by DNA damage, and is treated with cisplatin.10, 11 These observations indicate that, epigenetic therapy is designed to restore the expression of the RASSF protein, and may be a valid strategy to treat aggressive Ras-positive and RASSF-negative suppressors. This approach may be very appealing, because the Ras direct target has not yet been determined.
RASSF proteins
RASSF family proteins contain 10 members all of which contain Ras-related domain.12 Therefore, RASSF is called Ras-related domain family.13 RASSF1 has an RA domain at the end of C through RASSF6, while RASSF7 has RA domain at the end of N through RASSF10.14 The C-terminal of RASSF proteins has been widely studied, which has been observed to be inactive epigenetically in most cancers; therefore, we will focus on this matter.15
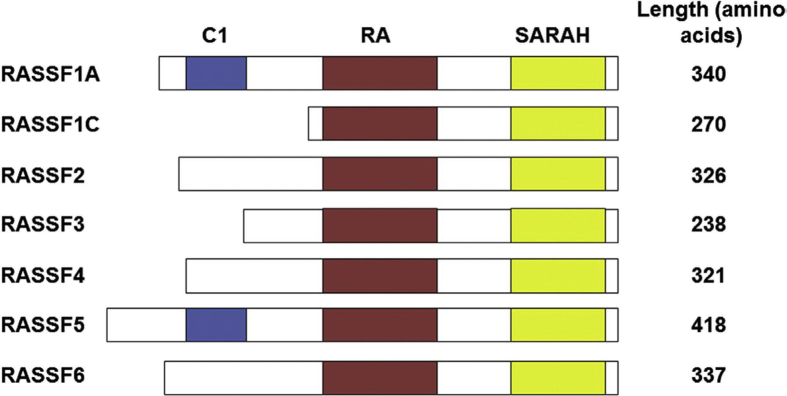
One of the major characteristics of RASSF proteins is that they lack enzymatic activities. On the other hand, they seem to act as scaffold molecules and via forming scaffolds with proteins of apoptosis signaling pathways and senescence, reducing Ras suppressing effect on the growth and survival.1, 8 The structural domains related to C-terminal RASSF have been displayed in (Fig. 1).
Figure 1.
The structure of C-terminal protein of RASSF; C1 finger domain on Ra; Ras-related domain; Salvador/RASSF/Hippo domain, SARAH (All C-terminal RASSF proteins have Salvador/RASSF/Hippo domain, which directly bind to mammalian sterile 20 like (MST) and Hippo signaling pathway).1, 17
Another unique characteristic of RASSF is the high epigenetic deactivation rate in different cancers. Epigenetics refers to the changes in gene expression not caused by changes in DNA sequence.16 In RASSF proteins, CpG islands methylation of promoter areas leads to the degradation of RASSF proteins in cells. The suppression of RASSF proteins can intensify Ras transformation and separates it from apoptosis and senescence pathways.16 Therefore, degrading RASSF protein facilitates Ras transformation. The relationship between C-terminal RASSF proteins and Ras function has been summarized in the following sections.
RASSF1
RASSF1 gene has been found in the selection of hybrids for proteins which interact with DNA repairing protein, group A-complementary protein (XPA).17 This gene has proved to produce two main transcripts: RASSF1A and RASSF1C, both of which have RA domain. There are several other isoforms which have not been considered due to the deficient information.18, 31
It has been shown that RASSF1 proteins can bind to active Ras and activate Ras-dependent apoptosis.13 At first, there was disagreement on the physiological nature of interaction between Ras and RASSF1, but some groups suggested that there is no direct interaction between them.18 However, some studies reported that Ras forms an endogenous complex with RASSF1 with a direct interaction.7 It was observed that RASSF1 was bound to K-Ras and could not bind to non-farnesylated Ras.19 As a result, positive results were not expected in the experiments of recombinant H-Ras protein obtained from bacteria or non-farnesylated Ras mutants of yeast.20
Early studies suggested that RASSF1A gene is reduced in human tumor cells and suppresses tumorigenic phenotype.21 RASSF1A has no enzymatic activity and functions under K-Ras control, like Scaffold proteins.22 This makes K-Ras control the suppressing pathways of several tumors.
The first known biologic property of RASSF1A was that RASSF1A can enhance suppression of G1 and G2/M stages in the cell cycle.23 The suppression of G2/M can be expressed by the severe effect of RASSF1A over-expression on microtubule-associated proteins (MAPS).7, 23 RASSF1A directly binds to MAPS, where these proteins bind to tubulin. Note that MAPS modulates microtubule polymerization.24 RASSF1A is associated with all forms of tubulin including gamma tubulin in spindle poles and can describe RASSF1A capability in suppressing genetic instability caused by K-Ras.25 Advanced technical studies suggest that in interphase cells, RASSF1A is associated with a set of Golgi apparatus microtubules evoking its correct polarity and direction.26 In addition to microtubules, RASSF1A is identified in the locus and protein associated with mitochondria.
In addition to the effect on the microtubules, it is clear that RASSF1A is involved in binding Ras to pro-apoptosis signaling pathways: Bax pathway and Hippo pathway. Bax is a pro-apoptosis protein with Bcl2 or BH domain which is vital for most apoptosis forms in cells. In 2005, two studies revealed that RASSF1A is an intermediary of Bax pathway and activates it.7, 19 K-Ras amplifies RASSF1A and MOAP-1 interaction and stimulates the Bax activation and transfer to mitochondria. RASSSF1A suppression degrades Ras potential for activating Bax in tumor cells.27
RASSF1A binds Ras to other pro-apoptosis signaling pathways (Hippo pathway).28 The most important RASSF proteins are proteins 1–6 which contain SARAH motif in C-terminal. This protein binds to MST1 and MST2 hypokinases.29 MST kinases become phosphorylated and suppress tumor suppressing kinase (LAT) in the kinase cascade. LAT kinases have different functions, but their most important function is co-activating yes-associated protein (YAP) and tafazzin (TAZ).19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 YAP/TAX phosphorylation by LATs stimulates their exit from locus and proteosomal degradation.30 YAP acts as an oncogene and survival factor, whose suppression by Hippo pathways can lead to apoptosis and senescence.31 Hippo pathway has an important role in natural cells' homeostasis which is not modulated in human cancers.32 This lack of modulation leads to YAP activation and pro-growth effects.16 RASSF1A is employed to bind Ras to Hippo pathway, and the Ras interaction with RASSF1A stimulates MST kinase stability and activation.33 Therefore, the loss of RASSF1A causes Ras not to bind to Hippo pathway suppressing apoptosis signaling. However, the situation may be somehow complex.10 In in vivo system, using RASSF1A point mutation prevents MST kinase binding, where cardiomyocytes and cardiac fibroblasts act distinctly relative to RASSF1A/Hippo signaling.34 Therefore, cell characteristics may be associated with the main results of this pathway.
RASSF1A can form a complex with mouse double minute 2 homolog (MDM2) and ligase ubiquitin and degrade p53 and Rb.35 This association with p53 is the reason for the synergetic tumor formation through suppressing RASSf1A/p53 heterozygotes. The role of Ras in this process is not clear yet.35
In addition, RASSF1A has an important role in response to DNA damage and repair. A group found that RASSF1A is involved in MST2 and LAST1 activation and leads to p73 pro-apoptosis protein stabilization.7, 19 Therefore, in RASSF1A-defected cells in which is DNA damaged, apoptosis activation process is suppressed and leads to the survival of mutation carrier cells and cancer.
Other studies suggest that the mechanism which controls DNA repair by RASSF1A involves the modulation of DNA repair proteins acetylation through SIRT1 deacetylase.36 It has been reported that RASSF1A can form complex with HDAC6 deacetylase. Therefore, RASSF1A binds through several deacetylases to K-Ras for controlling acetylation.37 Degradation of RASSF1A may create defect in acetylome. As acetylation is more involved than phosphorylation in transformations after translation, this effect can bear high importance in Ras-induced tumor and the tumor response to acetyl transferase suppressors.1, 34, 35, 36, 37, 38
The studies on the transgenic rats have confirmed RASSF1A suppression effect.36 In these rats, depending on age and carcinogen treatment, automatic suppression of tumor was observed.38 However, the results revealed that in heterozygote rats, more tumors develop compared to homozygote rats, suggesting that the cell may preserve minimum RASSF1A expression for its survival.1, 37 The studies about deleting RASSF1A and p53 showed that there is an increase in the number of rats without RASSF1A and p53 with high automatic tumor during young ages. This signifies that the results of suppressing RASSF1A and Ras activation should be studied in rats.38
The main alternative of RASSF1 is RASSSF1C, which is the shortest form of RASSF1A without N-terminal.11 RASSF1C can form a complex with K-Ras and create apoptotic properties. RASSF1C protein expression is eliminated in some tumor cells. Indeed, in cases where RASSF1A protein expression is preserved, it is no longer expressed. This means that RASSF1C can be modulated after transcription, which in some cells acts as tumor suppressor.38, 39
Contradictory roles have been reported about RASSSF1C. Clearly, after DNA damage, RASSF1C has an important role in the death of ovarian cancer cells and activation of kinase pathway at the jun N-terminal kinase (JKN). Other studies suggested that RASSF1C can have a mild stimulating effect on cancer cells and β-catenin modulation.40, 41, 42, 43, 81 Nevertheless, the physiologic functions of these isoforms are not clear.
RASSF5 (NORE1)
The second member of RASSF family which has been studied well is RASSF5. This member of Ras family produces two main protein isoforms: RASSF5A (NORE1A) and NORE1B (RAPL).43, 44 NORE1A is widely expressed in the tissue, while NORE1B is limited typically limited to the lymphatic structure.45 Indeed, NORE1A binds through GTP-dependent method and second effector to Ras, which is known as Ras-binding protein. RAPL/NORE1B is identified as Rap-binding agent.1 Unlike RASSF1A, NORE1A binds easily to H-Ras. NORE1A, like RASSF1A, can bind to MST kinases and modulate Hippo apoptosis pathway.46 However, delete mutation shows that the Hippo pathway is not required for suppressing the growth and function of NORE1A, and it can stimulate Hippo kinase cascade.46, 48
NORE1A expression grows through epigenetic mechanisms in tumors. On the other hand, its expression diminishes through calpain and ubiquitin at the protein level.1 NORE1A expression severely drops in malignant liver cancer.46 It has also been found that NORE1A suppresses the tumor, where the displacement of inactive NORE1A gene in human leads to inheritance of cancer syndrome.1
Other evidence suggests that NORE1A over-expression has a strong effect on Ras senescence, where its suppression reduces senescence response and increases transformation by Ras.49 NORE1A can interact with MDM2 (p53 negative modulator); this interaction can be used to induce ubiqitation and HIPK1 oncoprotein degradation, suggesting that NORE1A acts as a scaffolding molecule.1, 46, 47, 48, 49 These studies demonstrate that NORE1A/MDM2 interaction modulates Ras and creates another level of Ras/NORE1A/DMD interactions.
Another major component of Ras/NORE1A signaling is β-catenin protein. This is a binding protein and transcription cofactor activated in Wnt signaling pathway.50, 81 Under normal conditions, it phosphorylates β-catenin multi-protein complex and provides the possibility of its binding to SCFβ-TrCP ligase ubiquitous complex, which acts as oncogene in cancer.51 β-TrCp is the diagnosis element for SCF β-TrCPT substrate which can act as tumor suppressor as it influences β-catenin degradation.49, 50, 51 Therefore, in cancers without NORE1A, negative modulation of β-catenin-related Ras is impaired. This indicates that they play a role in RASSF proteins and uncontrollable cell growth.
The studies of human tumor reveal that NORE1A is inactive while RAS is active. However, a study showed that Ras signaling is active and NORE1A promoter methylation occurs in tumors with low survival.52 This suggests that NORE1A has a minor effect on human tumors. Other studies revealed that in rats without NORE1A and Ras, NORE1A suppresses the cancer.53, 54
In another study, other NORE1 isoforms indicated that NORE1B plays an important role in the immunity cells (it is an integral element of immunity system) whose expression in lymphatic elements is higher than in other RASSF proteins. It is also associated with MST1 such NORE1B and NORE1A.53, 54, 55, 56 Its two forms are synergetic and modulate T-cell multiplication negatively (when T-cell antigen receptor is stimulated). In addition, it has shown that NORE1B, along with Ras, modulates T-call signaling, Ras activation, and Ras signaling in immunity cells.56
A study revealed that in liver cancer, 62% of NORE1B promoter is methylated.55, 56 This evidence indicates that in some systems, the loss of NORE1B reflects tumorigenic activity. However, this study found that NORE1B interacts with RASSF1A, and the simultaneous activity of NORE1B and RASSF1A prevents liver cancer.57, 58 Nevertheless, further studies are required to understand NORE1B activity mechanism.
RASSF2
RASSF2 protein is one of the important and vital members of RASSF family which is known as a metastasis suppressor which forms androgen complex by Ras effector domain and K-Ras.59 This complex is specific for K-Ras with a weak interaction with H-Ras. The rate of RASSF2 expression is high in the brain, but it is low in other tissues.60 This protein is deactivated in some cancers by epigenetic changes, where oncogene changes induced by K-Ras are increased. The lack of RASSF2 expression leads to augmented growth, impaired adhesion, and elevated phosphorylated AKT.60, 61
The highest rate of RASSF2 promoter methylation has been observed in prostate tumors, where more than 95% of promoters are methylated.62 This methylation has a high correlation with the reduction of protein expression in prostate cancer.1, 63, 64 Therefore, RASSF2 is used as an effective indicator of prostate cancer as its promoter is methylated and can be diagnosed using urine samples and polymerase chain reaction.
RASSF2 protein has pro-apoptosis activity and acts through binding to prostate apoptosis response protein (PAR-4), which is modulated by K-Ras and transfers PAR-4 to the locus by activating K-Ras promoter.1 PAR-4 interacts with TNF-related apoptosis inducing ligand in locus and induces apoptosis. This protein can modulate both kb and JNK pathway, but the exact mechanism of these effects and its relationship with Ras are not clear yet.65, 66
RASSF3
RASSF3 can bind to activated K-Ras, but the androgen complex between Ras and RASSF3 has not been confirmed yet. The simultaneous activity of K-Ras and RASSF3 induces cell death.67 There has been no evidence in other studies showing RASSF3 methylation or reduction of protein expression in tumors.67, 68, 69 However, RASSF3 has been determined in some colon cancers and lymphoblastic leukemia. In addition, there is evidence on the loss of RASSF3 function in human cancer, but its frequency is lower compared to other members of RASSF family.70
Evidence indicates that RASSF3 inactivation leads to a defect in DNA repair, increases genomic instability, and causes changes in lung tumor.13 This protein (RASSF3) can bind to MST1, but cannot activate it. This protein contributes to apoptosis through P53 and modulates it through binding and degrading MDM2.8, 71
RASSF4 (AD037)
This protein can bind through effector domain to active K-Ras. However, lack of antibody for RASSF4 prevents formation of endogen complex structure between them. RASSF4 induces Ras-dependent apoptosis and in some cancer cells (nasopharyngeal), the expression of this protein diminishes with promoter methylation.72, 73 The reduction of RASSF4 expression is related to cancer stem cells in cells similar to cancer cells.
The findings indicated that RASSF4 expression declines in some tumors, but in the human breast cancer, RASSF4 expression was elevated. RASSF4 has different biologic effects in different cell systems, with this protein causing enhanced YAP expression, intensified cell growth, and prevention of senescence through binding to MST1 and suppression of Hippo signaling in Alveolar rhabdomyosarcoma cancer.8, 73, 74, 75
RASSF6
This protein is the first identified member of RASSF family which can bind to active K-Ras through effective domain and suppress Hippo pathway by binding to MST kinases.76, 77, 78, 79, 80 RASSF6 can induce apoptosis whose suppression can promote the changes in tumor cells' phenotype. In primary cancers, this protein declines epigenetically whose is lower than that of RASSF1A and RASSF5 and is exclusively active in neuroblastoma, pediatric leukemia, melanoma, and melanoma metastasis.1, 9, 81 RASSF6 expression strongly increases in some cancers (ovarian cancer). In some cases, this protein is pro-tumorigenic like Hippo suppression RASSF4.82
RASSF6 induces apoptosis independent of Hippo signaling. As with other members of RASSF family, this protein interacts with MDM2 protein and modulates p53, apoptosis, and cell cycle.83 This protein, like RASSF1A, can form Ras complex along with MOAP-1 protein active Bax. In melanoma cells, this protein can amplify the relationship between MST1 suppression kinase and B-Raf mutation, and suppress the mitogen-activated kinase protein (MAPK) pathway.9, 84 Note that RASSF6 has various mechanisms which can use Ras for tumor suppression.
Conclusion
According to studies on human tumors, RASSF1A is inactive epigenetically. Loss of this protein suppresses some growth pathways, with the reason for developing Ras tumors being reduced RASSF1A expression.
The methylation of NORE1A promoter and Ras activation have been reported in hepatocellular carcinoma tumors. This means that most tumors with Ras activation and RASSF methylation have a greater attackability to tumors without RASSF methylation. These data suggest that there is a relationship between positive K-Ras cancer cells and RASFF protein degradation. For example, the loss of RASSF2 expression increases the multiplication and invasion of positive K-Ras cancer cells of lung, which are resistant to chemotherapy. Other studies revealed that RASSF6 expression in melanoma cell leads to mutation in B-Raf signaling and reduces the invasion of cells. This evidence demonstrate that epigenetic treatment is designed for recovering RASSF protein expression, which is a valid approach to help in the treatment of positive Ras and negative RASSF tumors.
A group of DNA methyl transferase suppressors can be used to prevent DNMTs function. 5-Azacitidine and Decitabine are DNA methyl transferase suppressors, both of which are nucleoside analogues. These suppressors deactivate DNA methyl transferase in DNA-protein complex. These medicines are used to treat Myelodysplastic syndrome and acute myeloid leukemia. However, their effectiveness in the treatment of solid tumors is limited, and their high doses lead to undesired side-effects like bone marrow suppression and nausea.
RASSF1A promoter methylation occurs through DNA methyl-transferase enzyme (DNMT3B). Nanaomaicin A is an antibiotic identified as DNMT3B exclusive suppressor. The treatment with this antibiotic has led to the re-expression of RASSF1A in lung cancer cells as well as melanoma (treatment with Nanaomaicin A leads to the re-expression of RASSF6) and suppresses tumor phenotype. Therefore, Nanaomaicin A degrades melanoma cancer cells, suggesting that it has a considerable potential for re-expression of all RASSF proteins in Ras-induced tumor cells. This treatment has no side-effects and is used in the treatment of most cancers. Nevertheless, there has been no report so far about the clinical effect of Nananmaicin A as an anti-cancer drug.
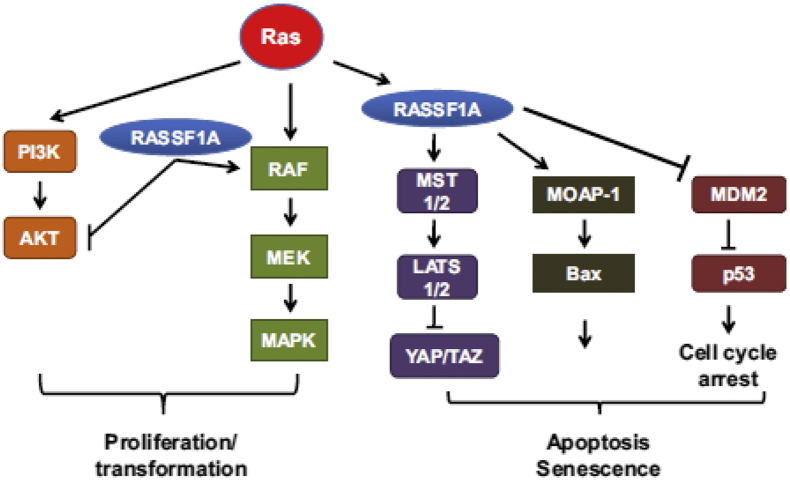
Another clinical aspect related to RASSF protein is using it as a biological marker in human tumor. The reduction of RASSF1A expression is a common event in human cancers which has a widespread relationship with cancer phenotypes. Promoter methylation related to this protein (RASSF1A) can be identified in the mucus, serum, and urine. RASSF2 methylation can be diagnosed in the urine of prostate cancer patients. In general, the study of RASSF proteins' methylation is a non-invasive process which provides information about cancer phenotype, where this information can be used to develop exclusive therapies for cancer-suffering patients. Fig. 2 demonstrates a summary of RASSF protein involvement in Ras signaling pathways.
Figure 2.
Signaling pathway with involvement of RASSF1A. This protein is involved in various signaling pathways which can induce apoptosis through Ras activation. It also acts independently of Ras and suppresses Akt signaling pathway through different mechanisms.1, 38, 84
Further studies are required to determine the importance of losing RASSF proteins in tumors caused by Ras. For example, RASSF1A-free mouse has higher sensitivity to tumors where both effects of losing RASSF1A and activating Ras should be considered. In general, RASSF proteins can be a suitable means for targeting a large set of Ras-induced tumors.
Conflict of interest
None declared.
Acknowledgments
None declared.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Azmi Asfar. Elsevier; 2016. Conquering RAS: From Biology to Cancer Therapy. [Google Scholar]
- 2.Zinatizadeh M.R., Masoumalinejad Z., Parnak F. Prevalence of Mycoplasma hyorhinis contamination in tissues samples from cancer patients: a Brief Report. Mod Med Lab J. 2017;1(3):91–95. [Google Scholar]
- 3.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 4.Donninger H., Vos M.D., Clark G.J. The RASSF1A tumor suppressor. J Cell Sci. 2007;120(18):3163–3172. doi: 10.1242/jcs.010389. [DOI] [PubMed] [Google Scholar]
- 5.Overmeyer J.H., Maltese W.A. Death pathways triggered by activated Ras in cancer cells. Front Biosci. 2011;16(5):1693–1713. doi: 10.2741/3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D.H., Kim J.S., Park J.H. Relationship of Ras association domain family 1 methylation and K-ras mutation in primary non-small cell lung cancer. Cancer Res. 2003;63(19):6206–6211. [PubMed] [Google Scholar]
- 7.Calvisi D.F., Ladu S., Gorden A. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130(4):1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu A., Ishiguro F., Tanaka I. RASSF3 downregulation increases malignant phenotypes of non-small cell lung cancer. Lung Cancer. 2014;83(1):23–29. doi: 10.1016/j.lungcan.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Mezzanotte J.J., Hill V.C., Schmidt M.L. RASSF6 exhibits promoter hypermethylation in metastatic melanoma and inhibits invasion in melanoma cells. Epigenetics. 2014;9(11):1496–1503. doi: 10.4161/15592294.2014.983361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton G., Yee K.S., Scarce S., O'Neill E. ATM regulates a RASSF1A-dependent DNA damage response. Curr Biol. 2009;19(23):2020–2025. doi: 10.1016/j.cub.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C., Tommasi S., Liu L., Yee J.K., Dammann R., Pfeifer G.P. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol. 2007;17(8):700–705. doi: 10.1016/j.cub.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 13.Khokhlatchev A., Rabizadeh S., Xavier R. Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol. 2002;12(4):253–265. doi: 10.1016/s0960-9822(02)00683-8. [DOI] [PubMed] [Google Scholar]
- 14.Donninger H., Calvisi D.F., Barnoud T. NORE1A is a Ras senescence effector that controls the apoptotic/senescent balance of p53 via HIPK2. J Cell Biol. 2015;208(6):777–789. doi: 10.1083/jcb.201408087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matallanas D., Romano D., Yee K. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27(6):962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vos M.D., Ellis C.A., Bell A., Birrer M.J., Clark G.J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J Biol Chem. 2000;275(46):35669–35672. doi: 10.1074/jbc.C000463200. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz-Vega S., Khokhlatchev A., Nedwidek M. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21(9):1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 18.Land H., Parada L.F., Weinberg R.A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 19.Shivakumar L., Minna J., Sakamaki T., Pestell R., White M.A. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol. 2002;22(12):4309–4318. doi: 10.1128/MCB.22.12.4309-4318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallol A., Agathanggelou A., Fenton S.L. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64(12):4112–4116. doi: 10.1158/0008-5472.CAN-04-0267. [DOI] [PubMed] [Google Scholar]
- 21.Vos M.D., Martinez A., Elam C. A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res. 2004;64(12):4244–4250. doi: 10.1158/0008-5472.CAN-04-0339. [DOI] [PubMed] [Google Scholar]
- 22.Arnette C., Efimova N., Zhu X., Clark G.J., Kaverina I. Microtubule segment stabilization by RASSF1A is required for proper microtubule dynamics and Golgi integrity. Mol Biol Cell. 2014;25(6):800–810. doi: 10.1091/mbc.E13-07-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L., Vo A., Liu G., McKeehan W.L. Distinct structural domains within C19ORF5 support association with stabilized microtubules and mitochondrial aggregation and genome destruction. Cancer Res. 2005;65(10):4191–4201. doi: 10.1158/0008-5472.CAN-04-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baksh S., Tommasi S., Fenton S. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18(6):637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Vos M.D., Dallol A., Eckfeld K. The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem. 2006;281(18):4557–4563. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- 26.Praskova M., Khoklatchev A., Ortiz-Vega S., Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381(2):453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey K.F., Zhang X., Thomas D.M. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 29.Del Re D.P., Matsuda T., Zhai P. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Investig. 2010;120(10):3555–3567. doi: 10.1172/JCI43569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romano D., Matallanas D., Weitsman G., Preisinger C., Ng T., Kolch W. Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt. Cancer Res. 2010;70(3):1195–1203. doi: 10.1158/0008-5472.CAN-09-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song M.S., Song S.J., Kim S.Y., Oh H.J., Lim D.S. The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J. 2008;27(13):1863–1874. doi: 10.1038/emboj.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tommasi S., Besaratinia A., Wilczynski S.P., Pfeifer G.P. Loss of RASSF1A enhances p53-mediated tumor predisposition and accelerates progression to aneuploidy. Oncogene. 2011;30(6):690–700. doi: 10.1038/onc.2010.440. [DOI] [PubMed] [Google Scholar]
- 33.Pefani D.E., Latusek R., Pires I. RASSF1A-LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat Cell Biol. 2014;16(10):962–971. doi: 10.1038/ncb3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee K.S., Grochola L., Hamilton G. A RASSF1A polymorphism restricts p53/p73 activation and associates with poor survival and accelerated age of onset of soft tissue sarcoma. Cancer Res. 2012;72(9):2206–2217. doi: 10.1158/0008-5472.CAN-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donninger H., Clark J., Rinaldo F. The RASSF1A tumor suppressor regulates XPA-mediated DNA repair. Mol Cell Biol. 2015;35(1):277–287. doi: 10.1128/MCB.00202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao B., Xie X.J., Huang C. RASSF1A polymorphism A133S is associated with early onset breast cancer in BRCA1/2 mutation carriers. Cancer Res. 2008;68(1):22–25. doi: 10.1158/0008-5472.CAN-07-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schagdarsurengin U., Seidel C., Ulbrich E.J., Kolbl H., Dittmer J., Dammann R. A polymorphism at codon 133 of the tumor suppressor RASSF1A is associated with tumorous alteration of the breast. Int J Oncol. 2005;27(1):185–191. [PubMed] [Google Scholar]
- 38.Oceandy D., Pickard A., Prehar S. Tumor suppressor Ras-association domain family 1 isoform A is a novel regulator of cardiac hypertrophy. Circulation. 2009;120(7):607–616. doi: 10.1161/CIRCULATIONAHA.109.868554. [DOI] [PubMed] [Google Scholar]
- 39.Jung H.Y., Jung J.S., Whang Y.M., Kim Y.H. RASSF1A suppresses cell migration through inactivation of HDAC6 and increase of acetylated alpha-tubulin. Cancer Res Treat. 2013;45(2):134–144. doi: 10.4143/crt.2013.45.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19(6):1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tommasi S., Dammann R., Zhang Z. Tumor susceptibility of RASSF1A knockout mice. Cancer Res. 2005;65(1):92–98. [PubMed] [Google Scholar]
- 42.Ram R.R., Mendiratta S., Bodemann B.O., Torres M.J., Eskiocak U., White M.A. RASSF1A inactivation unleashes a tumor suppressor/oncogene cascade with context-dependent consequences on cell cycle progression. Mol Cell Biol. 2014;34(12):2350–2358. doi: 10.1128/MCB.01506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitagawa D., Kajiho H., Negishi T. Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J. 2006;25(14):3286–3297. doi: 10.1038/sj.emboj.7601212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenzato A., Martino C., Dani N. The cellular apoptosis susceptibility CAS/CSE1L gene protects ovarian cancer cells from death by suppressing RASSF1C. FASEB J. 2012;26(6):2446–2456. doi: 10.1096/fj.11-195982. [DOI] [PubMed] [Google Scholar]
- 45.Estrabaud E., Lassot I., Blot G. RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with betaTrCP. Cancer Res. 2007;67(3):1054–1061. doi: 10.1158/0008-5472.CAN-06-2530. [DOI] [PubMed] [Google Scholar]
- 46.Vavvas D., Li X., Avruch J., Zhang X.F. Identification of Nore1 as a potential ras effector. J Biol Chem. 1998;273(10):5439–5442. doi: 10.1074/jbc.273.10.5439. [DOI] [PubMed] [Google Scholar]
- 47.Wohlgemuth S., Kiel C., Kramer A., Serrano L., Wittinghofer F., Herrmann C. Recognizing and defining true Ras binding domains I: biochemical analysis. J Mol Biol. 2005;348(3):741–758. doi: 10.1016/j.jmb.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 48.Kuznetsov S., Khokhlatchev A.V. The growth and tumor suppressors NORE1A and RASSF1A are targets for calpain-mediated proteolysis. PLoS One. 2008;3(12):e3997. doi: 10.1371/journal.pone.0003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J., Liu W.O., Vos M.D. The t(1;3) breakpoint-spanning genes LSAMP and NORE1 are involved in clear cell renal cell carcinomas. Cancer Cell. 2003;4(5):405–413. doi: 10.1016/s1535-6108(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 50.Aoyama Y., Avruch J., Zhang X.F. Nore1 inhibits tumor cell growth independent of Ras or the MST1/2 kinases. Oncogene. 2004;23(19):3426–3433. doi: 10.1038/sj.onc.1207486. [DOI] [PubMed] [Google Scholar]
- 51.Calvisi D.F., Donninger H., Vos M.D. NORE1A tumor suppressor candidate modulates p21CIP1 via p53. Cancer Res. 2009;69(11):4629–4637. doi: 10.1158/0008-5472.CAN-08-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puca R., Nardinocchi L., Givol D., D'Orazi G. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene. 2010;29(31):4378–4387. doi: 10.1038/onc.2010.183. [DOI] [PubMed] [Google Scholar]
- 53.Lee D., Park S.J., Sung K.S. MDM2 associates with Ras effector NORE1 to induce the degradation of oncoprotein HIPK1. EMBO Rep. 2012;13(2):163–169. doi: 10.1038/embor.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt M.L., Donninger H., Clark G.J. Ras regulates SCF(beta-TrCP) protein activity and specificity via its effector protein NORE1A. J Biol Chem. 2014;289(45):31102–31110. doi: 10.1074/jbc.M114.594283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orford K., Crockett C., Jensen J.P., Weissman A.M., Byers S.W. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272(40):24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 56.Park J., Kang S.I., Lee S.Y. Tumor suppressor ras association domain family 5 (RASSF5/NORE1) mediates death receptor ligand-induced apoptosis. J Biol Chem. 2010;285(45):35029–35038. doi: 10.1074/jbc.M110.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katagiri K., Ohnishi N., Kabashima K. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5(10):1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 58.Zhou D., Medoff B.D., Chen L. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc Natl Acad Sci USA. 2008;105(51):20321–20326. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishiguro K., Avruch J., Landry A. Nore1B regulates TCR signaling via ras and Carma1. Cell Signal. 2006;18(10):1647–1654. doi: 10.1016/j.cellsig.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miertzschke M., Stanley P., Bunney T.D., Rodrigues-Lima F., Hogg N., Katan M. Characterization of interactions of adapter protein RAPL/Nore1B with RAP GTPases and their role in T cell migration. J Biol Chem. 2007;282(42):30629–30642. doi: 10.1074/jbc.M704361200. [DOI] [PubMed] [Google Scholar]
- 61.Macheiner D., Heller G., Kappel S. NORE1B, a candidate tumor suppressor, is epigenetically silenced in human hepatocellular carcinoma. J Hepatol. 2006;45(1):81–89. doi: 10.1016/j.jhep.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Macheiner D., Gauglhofer C., Rodgarkia-Dara C. NORE1B is a putative tumor suppressor in hepatocarcinogenesis and may act via RASSF1A. Cancer Res. 2009;69(1):235–242. doi: 10.1158/0008-5472.CAN-08-2144. [DOI] [PubMed] [Google Scholar]
- 63.Vos M.D., Ellis C.A., Elam C., Ulku A.S., Taylor B.J., Clark G.J. RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem. 2003;278(30):28045–28051. doi: 10.1074/jbc.M300554200. [DOI] [PubMed] [Google Scholar]
- 64.Akino K., Toyota M., Suzuki H. The Ras effector RASSF2 is a novel tumor-suppressor gene in human colorectal cancer. Gastroenterology. 2005;129(1):156–169. doi: 10.1053/j.gastro.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 65.Donninger H., Hesson L., Vos M. The Ras effector RASSF2 controls the PAR-4 tumor suppressor. Mol Cell Biol. 2010;30(11):2608–2620. doi: 10.1128/MCB.00208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne S.R., Serth J., Schostak M. DNA methylation biomarkers of prostate cancer: confirmation of candidates and evidence urine is the most sensitive body fluid for non-invasive detection. The Prostate. 2009;69(12):1257–1269. doi: 10.1002/pros.20967. [DOI] [PubMed] [Google Scholar]
- 67.Perez-Janices N., Blanco-Lugin I., Tunon M.T. EPB41L3, TSP-1 and RASSF2 as new clinically relevant prognostic biomarkers in diffuse gliomas. Oncotarget. 2015;6(1):368–380. doi: 10.18632/oncotarget.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song H., Oh S., Oh H.J., Lim D.S. Role of the tumor suppressor RASSF2 in regulation of MST1 kinase activity. Biochem Biophys Res Commun. 2010;391(1):969–973. doi: 10.1016/j.bbrc.2009.11.175. [DOI] [PubMed] [Google Scholar]
- 69.Cooper W.N., Hesson L.B., Matallanas D. RASSF2 associates with and stabilizes the proapoptotic kinase MST2. Oncogene. 2009;28(33):2988–2998. doi: 10.1038/onc.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imai T., Toyota M., Suzuki H. Epigenetic inactivation of RASSF2 in oral squamous cell carcinoma. Cancer Sci. 2008;99(5):958–966. doi: 10.1111/j.1349-7006.2008.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burghel G.J., Lin W.Y., Whitehouse H. Identification of candidate driver genes in common focal chromosomal aberrations of microsatellite stable colorectal cancer. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Safavi S., Hansson M., Karlsson K., Bilogalv A., Johansson B., Paulsson K. Novel gene targets detected by genomic profiling in a consecutive series of 126 adults with acute lymphoblastic leukemia. Haematologica. 2015;100(1):55–61. doi: 10.3324/haematol.2014.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo H., Liu H., Wei J. Functional single nucleotide polymorphisms of the RASSF3 gene and susceptibility to squamous cell carcinoma of the head and neck. Eur J Cancer. 2014;50(3):582–592. doi: 10.1016/j.ejca.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng H., Liu H., Zhao S., Wu J., Fan J., Liao J. Silencing of RASSF3 by DNA hypermethylation is associated with tumorigenesis in somatotroph adenomas. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kudo T., Ikeda M., Nishikawa M. The RASSF3 candidate tumor suppressor induces apoptosis and G1-S cell-cycle arrest via p53. Cancer Res. 2012;72(11):2901–2911. doi: 10.1158/0008-5472.CAN-12-0572. [DOI] [PubMed] [Google Scholar]
- 76.Eckfeld K., Hesson L., Vos M.D., Bieche I., Latif F., Clark G.J. RASSF4/AD037 is a potential ras effector/tumor suppressor of the RASSF family. Cancer Res. 2004;64(23):8688–8693. doi: 10.1158/0008-5472.CAN-04-2065. [DOI] [PubMed] [Google Scholar]
- 77.Chow L.S., Lo K.W., Kwong J., Wong A.Y., Huang D.P. Aberrant methylation of RASSF4/AD037 in nasopharyngeal carcinoma. Oncol Rep. 2004;12(4):781–787. [PubMed] [Google Scholar]
- 78.Michifuri Y., Hirohashi Y., Torigoe T. Small proline-rich protein-1B is overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal. Biochem Biophys Res Commun. 2013;439(1):96–102. doi: 10.1016/j.bbrc.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 79.Crose L.E., Galindo K.A., Kephart J.G. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J Clin Investig. 2014;124(1):285–296. doi: 10.1172/JCI67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen N.P., Donninger H., Vos M.D. RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene. 2007;26(42):6203–6211. doi: 10.1038/sj.onc.1210440. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda M., Hirabayashi S., Fujiwara N. Ras-association domain family protein 6 induces apoptosis via both caspase-dependent and caspase-independent pathways. Exp Cell Res. 2007;313(7):1484–1495. doi: 10.1016/j.yexcr.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Keramatinia A., Ahadi A., Akbari M.E. Genomic profiling of chronic myelogenous leukemia: basic and clinical approach. J Cancer Prev. 2017;22(2):74–81. doi: 10.15430/JCP.2017.22.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taghavi A., Akbari M.E., Hashemi-Bahremani M. Gene expression profiling of the 8q22-24 position in human breast cancer: TSPYL5, MTDH, ATAD2 and CCNE2 genes are implicated in oncogenesis, while WISP1 and EXT1 genes may predict a risk of metastasis. Oncol Lett. 2016;12(5):3845–3855. doi: 10.3892/ol.2016.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nooshinfar E., Bashash D., Abbasalizadeh M., Safaroghli-Azar A., Sadreazami P., Akbari M.E. The molecular mechanisms of tobacco in cancer pathogenesis. Iran J Cancer Prev. 2017;10(1):e7902. [Google Scholar]