Abstract
The 2019 Nobel Prize in Physiology or Medicine was awarded to three physician scientists, Drs. William G. Kaelin, Jr., Peter Ratcliffe and Gregg Semenza, for their groundbreaking work revealing how cells sense and adapt to oxygen availability. Here, we summarize the history of their discoveries.
The 2019 Nobel Prize in Physiology or Medicine was awarded to three physician scientists, Drs. William G. Kaelin, Jr., Peter Ratcliffe and Gregg Semenza, for their groundbreaking work revealing how cells sense and adapt to oxygen availability. We are fortunate to have had the opportunity to work in Dr. William Kaelin's laboratory as postdoctoral trainees (Fig. 1). Here, we provide a brief description of the history and timeline of Dr. Kaelin's research, as well as that by Drs. Ratcliffe and Semenza.
Figure 1.
Dr. William G. Kaelin Jr. and the authors (A) Dr. Kaelin and his trainees. From left to right: Dr. William Kaelin, Dr. Samuel McBrayer, Dr. Qing Zhang, Dr. Kimberley Briggs and Dr. Alan Baik (B) Dr. Kaelin and Dr. Haifeng Yang (C) Dr. Kaelin and Dr. Wenyi Wei (D) Dr. Kaelin and his trainees. From left to right: Dr. Wenyi Wei, Dr. Qin Yan, Dr. William Kim, Dr. Archana Bommi-Reddy, Dr. William Kaelin, Dr. Lianjie Li and Dr. Yoji Andrew Minamishima (E) Dr. Kaelin and the authors at Fenway Park, Boston, MA on August 31, 2015 after the memorial service for Dr. Kaelin's beloved wife, Dr. Carolyn Kaelin, who died of brain cancer. From left to right: Dr. Qin Yan, Dr. Haifeng Yang, Dr. William Kaelin, Dr. Wenyi Wei, and Dr. Qing Zhang.
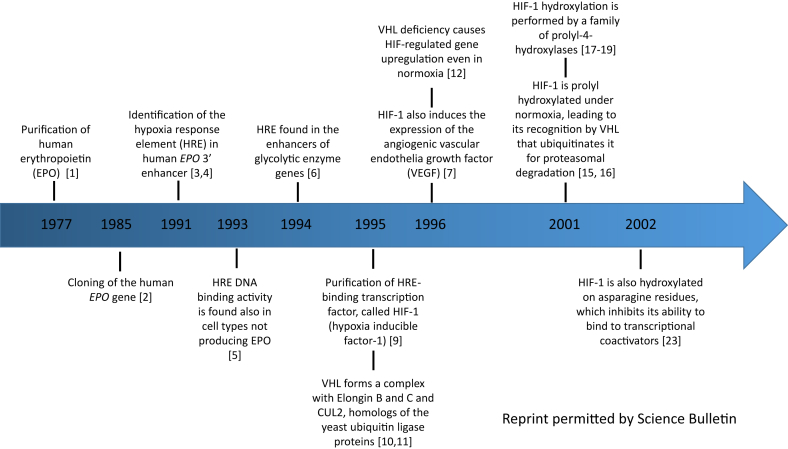
Oxygen is vital for all living organisms. During the course of evolution, animals have developed the ability to adapt to changes in the oxygen concentration on earth. However, it was unclear how animals can sense and adapt to changes in oxygen availability until around thirty years ago. The pioneering work performed by the laboratories of Drs. William Kaelin, Jr., Peter Ratcliffe and Gregg Semenza paved the way to understand the molecular mechanism of oxygen sensing. We herein summarize the major milestones in the history of research on the oxygen sensing pathway (Fig. 2).
Figure 2.
Milestones in the history of oxygen sensing research (Reprint permitted by Science Bulletin).
The journey started with the purification of erythropoietin (EPO), a glycoprotein hormone produced by the fetal liver and then by adult kidneys. The protein was purified in 19771 and the gene was cloned in 1985.2 At that time, it was known that EPO was produced in response to a low blood oxygen concentration. However, it was not known how EPO was regulated by low oxygen. Semenza and his colleagues found that a region located on the 3’ enhancer of EPO, currently known as the Hypoxia Response Element (HRE), was responsible for nuclear factor binding and EPO expression under hypoxia.3 This finding was subsequently confirmed in the same year by Ratcliffe and his colleagues.4 In addition, Ratcliffe and colleagues found that HRE DNA binding can occur in all cell types, including those not involved in EPO production, suggesting that this may be a general oxygen sensing mechanism that can regulate the expression of other genes that may be oxygen responsive,5 including genes encoding glycolytic enzymes and the angiogenic factor, vascular endothelial growth factor (VEGF).6,7
However, the nuclear factor binding to the enhancers of EPO remained elusive. In 1995, Dr. Guang-Liang Wang and Dr. Bing-Hua Jiang, both working as postdoctoral fellows under Semenza, purified and cloned this transcription factor, which they named hypoxia inducible factor-1 (HIF-1).8,9 HIF-1 is composed of two subunits, the oxygen labile HIF-1α and the constitutively expressed HIF-1β (also called ARNT, aryl hydrocarbon receptor nuclear translocator). Despite the fact that the mRNA expression of both subunits typically remains constant under either normoxia or hypoxia, the HIF-1α protein was found to be induced and accumulate under hypoxia, suggesting that some type of post-transcriptional/post-translational modification(s) contribute to the regulation of HIF-1α at the protein level.
Kaelin's group at Harvard made major contributions to deciphering this regulatory mechanism. Based on his clinical experience as an oncologist, Kaelin was aware that most kidney tumors that lose expression of the von Hippel-Lindau (VHL) tumor suppressor are highly vascularized. When he and his group found that VHL forms a complex with Elongin B and C and CUL2, homologs of yeast ubiquitin ligase proteins,10,11 he hypothesized that the absence of VHL could lead to VEGF stabilization and subsequent increased vascularity. Indeed, he soon discovered that VHL deficiency led to upregulation of VEGF and other HIF targets, even under normoxia.12 The E3 ubiquitin ligase activity of the VHL complex was subsequently demonstrated after Joan and Ronald Conaway collaborated with Kaelin to identify another essential protein called Rbx1, which is required for the ubiquitin ligase activity.13,14 It did not take long for both Kaelin and Ratcliffe to ascertain the molecular link between VHL and HIF. Indeed, in two back-to-back landmark papers published in 2001,15,16 they demonstrated that VHL binds HIF-1α under normoxia, but fails to do so under hypoxia, suggesting that VHL recognizes a modified form of HIF-1α.
This modification turned out to be prolyl hydroxylation on the proline 564 residue, which is essential for the recognition of HIF-1α by the VHL complex. Recognition of the modification leads to the ubiquitination and subsequent proteasomal degradation of HIF-1α. This breakthrough demonstrated that oxygen-dependent prolyl hydroxylation is key for mammalian oxygen sensing. However, this led to another question, namely, what was responsible for the HIF-1 oxygen-dependent hydroxylation. Ratcliffe, Kaelin (with the Conaways) and Steve McKnight all found the same answer using completely independent methods: HIF-1α hydroxylation is catalyzed by a family of prolyl-4-hydroxylases (PHDs) that are enzymatically inactive under hypoxia.17, 18, 19
Ratcliffe, Semenza, and others had already shown that HIF-1 C-terminal transactivation domains were also subject to oxygen-dependent regulation, which altered the transcriptional activity of HIF-1 without affecting the protein's stability.20,21 Semenza and colleagues identified and characterized a protein that interacts with HIF-1 and impairs its transcriptional activity, which was accordingly named factor inhibiting HIF-1 (FIH-1).22 Richard Bruick's group subsequently showed that FIH-1 is the asparaginyl hydroxylase of HIF-1, and this hydroxylation inhibits its ability to bind to transcriptional coactivators.23
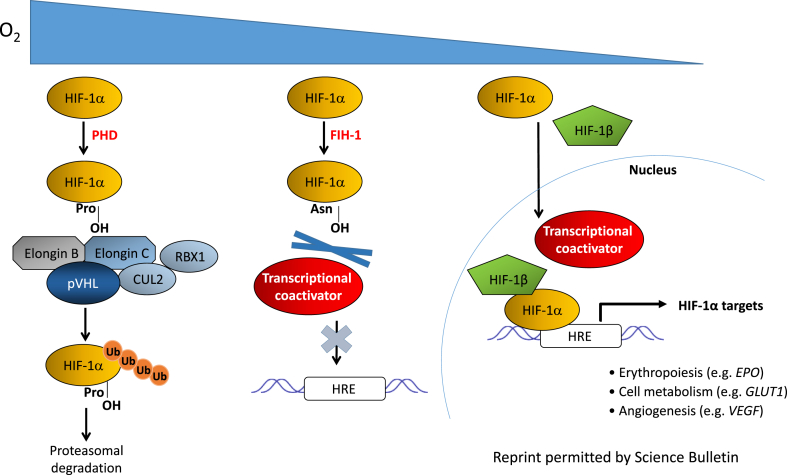
The discovery of HIF prolyl and asparaginyl hydroxylation completed the elegant network of oxygen-modulated regulation of HIF-1 activity. Interestingly, FIH-1 can be enzymatically active at a lower oxygen concentration than PHDs.24 FIH-1 hydroxylates asparagine 803, which is located in the C-terminal transactivation domain of HIF-1α, thereby inhibiting HIF-1α transcriptional activity by preventing its interaction with transcriptional coactivators, including CBP/p30023. As oxygen levels keep increasing, the PHDs become active and prolyl hydroxylate HIF-1α, promoting the interaction of HIF-1α with the VHL E3 ubiquitin ligase complex, which leads to HIF-1α ubiquitination and subsequent proteasomal degradation (Fig. 3).
Figure 3.
A schematic representation of HIF-1α regulation under normoxic and hypoxic conditions (Reprint permitted by Science Bulletin).
In less than a decade, Kaelin, Ratcliffe and Semenza delineated the detailed molecular mechanism by which the oxygen sensing pathway works. It is important to point out that these three outstanding scientists worked independently and solved many puzzles from different perspectives: Kaelin started his line of research from his experiences in oncology and based on biochemical investigations; Ratcliffe's studies started from his role as a nephrologist, and Semenza utilized his expertise in medical genetics. Their Nobel prize-winning work embodies the importance of cross-disciplinary approaches in tackling critical questions in physiology and human diseases.
Importantly, their work opened up the important field of research focused on mammalian oxygen sensing. At present, there are close to 70 enzyme family members that may depend on oxygen for their functions besides prolyl hydroxylases.25 In fact, some of the enzymes involved in epigenetic regulation, including KDM5A (also known as RBP2) histone demethylase, which identified by Kaelin and Yi Zhang,26 were recently shown to be oxygen sensing enzymes.27,28 It remains to be determined how other enzymes would work in oxygen sensing, and if the effects of the various enzymes are synergistic, complementary or whether they lead to feedback inhibition. It is also important to note that there may be other prolyl hydroxylase substrates besides HIF.29,30 For example, HIF-2α inhibitors only showed a partial response in kidney cancer cell lines and patient-derived xenografts,31,32 suggesting that there are other VHL substrates.29 Although research in this field will continue to expand for the foreseeable future, the findings have already been translated to the clinic. For example, a pan-prolyl hydroxylase inhibitor, Roxadustat, has just been approved in China for the treatment of anemia caused by kidney failure.
In summary, Drs. Kaelin, Ratcliffe and Semenza paved the road for understanding oxygen sensing and adaptability, and major efforts are currently underway to develop therapeutic strategies to treat human diseases such as anemia, coronary artery disease, inflammatory bowel diseases and cancer.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We are eternally grateful for the invaluable guidance provided by Dr. William Kaelin of the Dana-Farber Cancer Institute of Harvard Medical School during our memorable postdoctoral training. We also thank the Science Bulletin in China for granting permission for the reprint.33 We apologize to the numerous investigators whose important relevant publications cannot be cited due to space constraints. The research conducted in the authors' laboratories was supported in part by grants from the American Cancer Society Research Scholar Award (to Q. Z), the National Institutes of Health (R01CA211732 to Q.Z. and R01CA237586 and P50CA121974 (to Q.Y.)), the Cancer Prevention & Research Institute of Texas (to Q. Z), and the VHLA award (to H.Y.). The funding sources were not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Qing Zhang, Email: Qing.Zhang@UTSouthwestern.edu.
Qin Yan, Email: qin.yan@yale.edu.
Haifeng Yang, Email: Haifeng.Yang@jefferson.edu.
Wenyi Wei, Email: wwei2@bidmc.harvard.edu.
References
- 1.Miyake T., Kung C.K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 2.Lin F.K. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza G.L., Nejfelt M.K., Chi S.M., Antonarakis S.E. vol. 88. 1991. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene; pp. 5680–5684. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugh C.W., Tan C.C., Jones R.W., Ratcliffe P.J. vol. 88. 1991. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3' to the mouse erythropoietin gene; pp. 10553–10557. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firth J.D., Ebert B.L., Pugh C.W., Ratcliffe P.J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci U S A. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsythe J.A. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 10.Kibel A., Iliopoulos O., DeCaprio J.A., Kaelin W.G., Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 11.Lonergan K.M. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul 2. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliopoulos O., Levy A.P., Jiang C., Kaelin W.G., Jr., Goldberg M.A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamura T. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 14.Kamura T. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivan M. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 16.Jaakkola P. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 17.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 18.Epstein A.C. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 19.Ivan M. vol. 99. 2002. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor; pp. 13459–13464. (Proceedings of the National Academy of Sciences of the United States of America). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang B.H., Zheng J.Z., Leung S.W., Roe R., Semenza G.L. Transactivation and inhibitory domains of hypoxia-inducible factor 1 alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 21.Pugh C.W., O'Rourke J.F., Nagao M., Gleadle J.M., Ratcliffe P.J. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 22.Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1 alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lando D. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flashman E. Evidence for the slow reaction of hypoxia-inducible factor prolyl hydroxylase 2 with oxygen. FEBS J. 2010;277:4089–4099. doi: 10.1111/j.1742-4658.2010.07804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losman J.A., Kaelin W.G., Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013;27:836–852. doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klose R.J. The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty A.A. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363:1217–1222. doi: 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batie M. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science. 2019;363:1222–1226. doi: 10.1126/science.aau5870. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science. 2018;361:290–295. doi: 10.1126/science.aap8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X. Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev. 2014;28:1429–1444. doi: 10.1101/gad.242131.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H. On-target efficacy of a HIF2alpha antagonist in preclinical kidney cancer models. Nature. 2016 doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016 doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zurlo G., Zhang Q. The history of oxygen sensing: 2016 lasker award for basic medical research. Sci Bull. 2016;61:1665–1668. [Google Scholar]