Abstract
The use of antibodies as targeting molecules or cell penetrating tools has emerged at the forefront of pharmaceutical research. Antibody-directed therapies in the form of antibody-drug conjugates, immune modulators, and antibody-directed enzyme prodrug therapy have been most extensively utilized as hematological, rheumatological, and oncological therapies, but recent developments are identifying additional applications of antibody-mediated delivery systems. A novel application of this technology is for the treatment of glycogen storage disorders (GSDs) via an antibody-enzyme fusion (AEF) platform to penetrate cells and deliver an enzyme to the cytoplasm, nucleus, and/or other organelles. Exciting developments are currently underway for AEFs in treatment of the GSDs Pompe disease and Lafora disease. Antibody-based therapies are quickly becoming an integral part of modern disease therapeutics.
Keywords: Antibody-Drug Conjugate, Antibody-Enzyme Fusion, Antibody-Directed Therapies, Lafora Disease, Pompe Disease, glycogen
Impact of Antibodies on Pharmaceuticals
Inadequate therapeutic delivery due to insufficient cellular uptake is a major limitation in drug development, and especially in enzyme replacement therapy (ERT, see Glossary) [1]. Antibodies possess unique biochemical properties (Box 1) that can be manipulated and utilized as therapeutics or as ideal agents to deliver therapeutic payloads to the desired tissue (Figure 1). They have been particularly advantageous for the targeted delivery of cytotoxic drugs to tumor cells and reducing systemic toxicity in normal tissues. With the advancement of antibody engineering technologies and defined antibody mechanisms of action, many classes of novel antibody or antibody-derived molecules have recently been generated [1]. Their role as a means of cell-specific delivery of chemotherapeutics is now expanding to include delivery of enzymes deficient in multiple diseases including muscular dystrophies and myopathies, neurodegenerative diseases, and glycogen storage diseases (GSDs).
Box 1: Biochemistry of Antibodies and their Fragments.
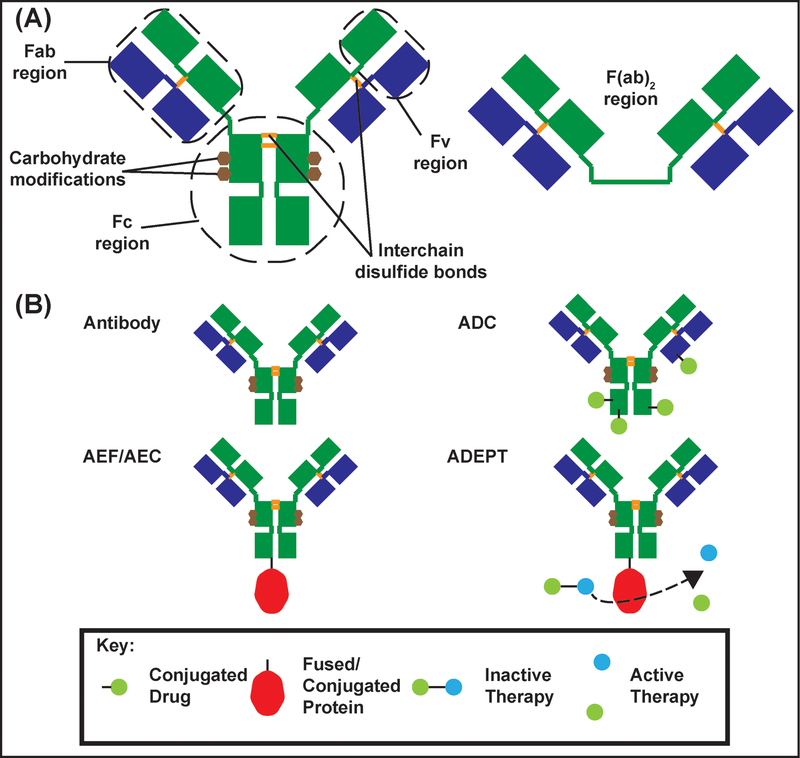
Antibodies are members of the immunoglobulin (Ig) protein family that are biologically relevant in the immune system. In humans, antibodies are produced in B-cell lymphocytes as part of the humoral immune response. Antibody varieties (IgG, IgA, IgM, IgD, IgE) have different functions, locations, and multiplicities within the body. Ig monomers are characteristically Y shaped comprising two heavy chains (green, Figure IA) and two light chains (blue, Figure IA) linked by covalent and non-covalent interactions. The crystallizable fragment (Fc) determines the antibody class and is responsible for effector functions: neutralization, phagocytosis, or complement activation. Antibodies have two antigen binding fragments (Fab) linked to the Fc fragment by flexible hinge regions (Figure IA). The Fab fragments contain unique variable fragments (Fv) that recognize specific epitopes on a target [128]. IgGs are equipped for recognizing specific pathogen antigens whereas the decavalent IgM antibodies (see antibody valency) can recognize large cell surface motifs and branching patterns in large carbohydrates like glycogen [129].
Antibodies and their fragments have become invaluable tools in research, diagnostics, and disease treatment due to their capability to recognize specific epitopes and mediate immune functions. Specifically, mAbs and their fragments have emerged as an important class of therapeutics in the treatment of many oncological and rheumatological disorders. More recently, mAbs have been developed for treatment of hyperlipidemia, asthma, and hematological disorders [2]. Antibody-mediated therapies are increasingly being utilized in clinics to treat multiple disorders [2]. To date, over 50 full length mAbs or antibody fragments have been approved by the United States FDA for clinical applications [2,130] and over 570 mAbs or antibody fragments are in various stages of clinical trials [130]. Smaller antibody fragments are important because of inherent transport limitations of full-length antibodies through certain microenvironments, especially the brain [38,131]. Furthermore, antibodies can undergo extensive protein engineering to yield products with features that allow specific antigen binding and transportation of therapeutic cargo [132] (Figure IB). This engineering gives rise to antibody drug conjugates (ADCs), antibody-enzyme fusions/antibody-enzyme conjugates (AEFs/AECs), and antibody-directed enzyme prodrug therapies (ADEPTs).
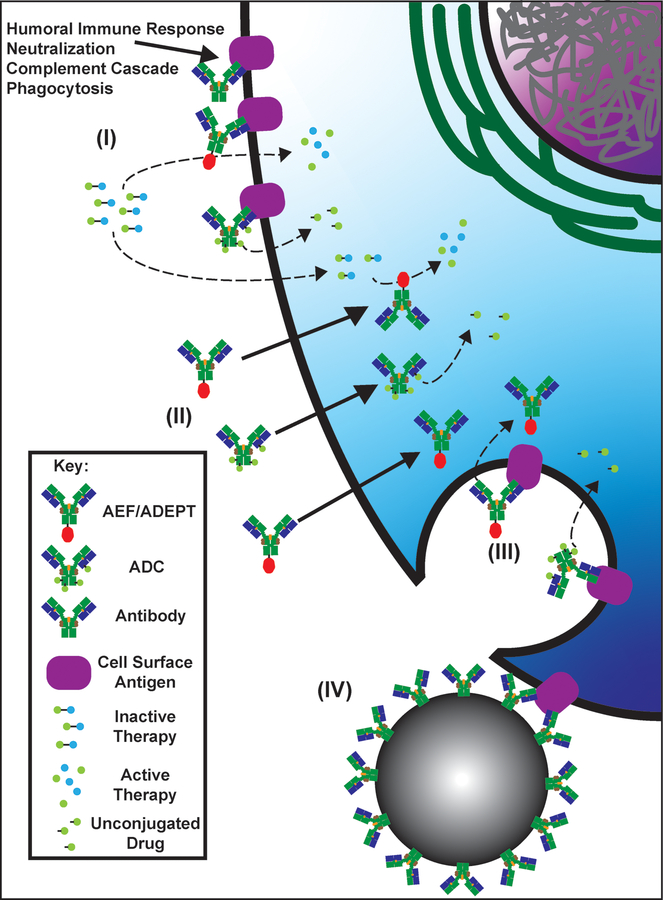
Figure 1: Methods of Antibody Therapy Cellular Delivery.
(I) Modes of extracellular delivery. Antibody therapies bind to extracellular targets or cell surface targets and execute their functions utilizing their attached therapies (enzyme activity, release of conjugated drugs, activation of prodrugs) or initiating immune activation. (II) Nontraditional mechanisms to transport antibodies into cells. These mechanisms transport antibodies and their attached cargo across the plasma membrane directly into the cytoplasm. For example, ENT2 transports the 3E10 antibody, Fc, or Fv into the cytoplasm by a largely undefined mechanism. (III) Antibody binding to cell surface antigens can lead to receptor mediated endocytosis that promotes delivery of antibody therapies to intracellular targets. (IV) Therapeutic nanoparticles can be coated with antibodies to target the nanoparticles to specific tissues or cell types. ADC: antibody-drug conjugate; AEF/ADEPT: antibody-enzyme fusion/antibody-directed enzyme prodrug therapy.
Antibodies and their fragments are currently being tested as delivery agents for several different classes of therapy (Box 1 Figure I; reviewed in [2]). Herein, we review multiple modes of antibody-mediated delivery systems including antibody-drug conjugates (ADCs), antibody-directed enzyme prodrug therapy (ADEPT), and antibody-protein fusions and we analyze the recent advancements utilizing these agents as therapeutics with a focus on antibody-enzyme fusions (AEFs) for GSDs. AEFs have the potential to revolutionize enzyme replacement therapy (ERT), especially for Pompe disease and Lafora disease (LD).
Antibody-Drug Conjugates
ADCs utilize the specificity of an antibody to deliver a conjugated therapeutic that is typically too toxic to be given systemically (Box 1 Figure IB). ADCs act by antibody-directed binding of a specific cell surface antigen [3]. Upon binding, the entire antigen-ADC complex is internalized through receptor-mediated endocytosis (Figure 1III) [4]. Once internalized, the drug is cleaved from the ADC in the lysosome and is then free to act within the cell.
ADCs are a rapidly growing and effective class of anti-cancer therapeutics [5]. They combine the targeting capabilities of monoclonal antibodies (mAbs) with the cytotoxic potential of small molecules to enable tumor specific drug delivery. As a result, they have the capability to overcome the severe side effects and narrow therapeutic window of traditional cancer chemotherapies. In 2000, Gemtuzumab ozogamicin was the first United States Food and Drug Administration (FDA) approved ADC. It is a humanized IgG4 monoclonal antibody directed against the CD-33 transmembrane receptor conjugated to a DNA damaging, calicheamicin cytotoxin derivative, ozogamicin. It received accelerated approval as a single agent for the treatment of first relapse patients with CD-33 positive acute myeloid leukemia [6]. However, the drug was withdrawn from the market in 2010 after a subsequent, post-approval phase III clinical trial raised concerns about safety and clinical benefit [7]. Gemtuzumab ozogamicin was reapproved in 2017 after additional studies using smaller doses, a different dosing schedule, and in combination with other chemotherapies, which increased the benefits of the ADC [8,9]. Currently, there are three additional FDA approved ADCs, each for the treatment of various cancers. Brentuximab vedotin is approved for the treatment of Hodgkin lymphoma and anaplastic large cell lymphoma. The antibody targets cancer antigen CD-30 and is conjugated with anti-mitotic monomethyl auristatin E [10]. Ado-trastuzumab emtansine is approved for metastatic breast cancer. The ADC targets human epidermal growth factor receptor 2 with mAb trastuzumab, and delivers the cytotoxic agent maytansine, which depolymerizes microtubules [11]. Inotuzumab ozogamicin is approved for B-cell precursor acute lymphoblastic leukemia [12]. It consists of a humanized mAb that targets CD-22 linked to ozogamicin. Over 170 ADCs are currently being investigated in multiple stages of clinical development (https://adcreview.com/adc-university/adc-drugmap/). In addition to the approved ADCs previously described, antibodies conjugated to a variety of molecules such as therapeutic enzymes (Box 1 Figure IB) [13], fluorescent or radiolabeled chelator molecules for targeted imaging [14], viruses [15], and nanoparticles (Figure 1IV) [16] are currently in preclinical development.
Despite showing impressive efficacy and safety in clinical trials, ADCs still exhibit a multitude of undesirable effects. Typically, drugs are conjugated to the desired antibody via maleimide or random amine coupling, which results in a heterogeneous mixture of the antibody to drug ratio. The ADC properties vary depending on the configuration of each conjugate resulting in variable drug pharmacokinetics and non-specific binding that both contribute to off target effects [3]. While recent antibody engineering has facilitated the production of fully humanized antibodies instead of murine antibodies, immunogenicity remains an issue that needs further development [5]. Furthermore, humanized antibodies may activate antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity that may narrow the therapeutic window [17]. Optimization of antibodies, linkers, and conjugation chemistries is being done to further reduce systemic toxicity and improve stability, pharmacokinetics, and potency [17].
Antibody-Protein Fusions
In contrast to the heterogeneity of chemically-conjugated ADCs, antibody-protein fusions can be genetically encoded to produce a single product (Box 1 Figure IB) [18]. Genetic linkages eliminate the need for chemical conjugation, resulting in homogenous constructs. Like ADCs, antibody-protein fusions harness the capabilities of the antibody as an effective means of specific epitope targeting. The antibody guides the protein to a target site, where it is internalized via one of multiple mechanisms that is dependent on the specific antibody (Figure 1). While multiple immunoglobulin (Ig) types have been tested as therapeutics, monoclonal IgG has been the most frequently utilized for targeted therapeutic delivery [18]. Antibody fusion constructs can be generated by genetic fusions of the protein of interest to either the complete immunoglobulin or any of its fragments. Complete Ig and Fc fusions usually display slower biodistribution and longer half-life in the blood stream due to their larger size [19]. Conversely, fusions using smaller fragments of the Ig such as the engineered single chain variable fragment (scFv) or antibody binding fragment (Fab) often have faster blood clearance [19]. They also often display improved tumor penetration [20]. Both Fab and scFv fragments have been engineered to form dimers, trimers, or tetramers that improve retention and internalization in comparison to the parental IgG [21]. The antibody-protein fusion design allows for the fusion and delivery of a versatile repertoire of therapeutic payloads such as cytokines, growth factors, enzymes, or ligand binding regions of a receptor. These antibody fusions have been extensively researched and developed and are currently utilized as therapies in multiple classes of diseases such as cancer, rheumatological disorders, and hematologic diseases.
Immunomodulatory Antibody-Protein Fusions
Immunological dysfunction is frequently involved in the initiation and perpetuation of human diseases. Therefore, the modulation of cytokines and immune-receptor signaling is a major target of pharmaceutical R&D investments. Currently, the majority of FDA approved immunomodulating antibody fusions employ the IgG1 Fc fragment. The Fc fragment is typically fused with a portion of either a cell surface receptor or an extracellular ligand that is involved in multiple proinflammatory signaling pathways. The receptor portion competes with the endogenous cell receptors for the natural ligand and thereby inhibits downstream signaling by acting as a ligand sink. Delivery using Fc fusions has been able to overcome dose limiting toxicities of unmodified cytokines, which can initiate widespread innate and adaptive immune functions. Due to their internalization by FcRn receptors, Fc fragments are also able to enhance the pharmacokinetic properties of the fusion construct by increasing transport efficiency and half-life compared to the unconjugated therapeutic [22].
There are currently five immunomodulating antibody fusions on the market that utilize an IgG1 Fc fragment. For the treatment of rheumatoid arthritis (RA), Fc-IgG1 fusion to tumor necrosis factor alpha receptor (TNFαR), Etanercept [23], and Fc-IgG1 attached to Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4), Abatacept [24], have been FDA approved. Both drugs provide improved signs and symptoms, functional status, and quality of life for patients with RA with or without disease-modifying antirheumatic drugs such as methotrexate. Alefacept is a fusion of Fc-IgG1 with the extracellular domain of lymphocyte function associated antigen-3 (LFA-3) from CD2+ T cells and has been approved for treatment of plaque psoriasis [25]. Rilonacept is comprised of Fc-IgG1 combined with both interleukin-1 receptor (IL-1R) and IL-1R accessory protein (IL-1RAcP) to inhibit IL-1 signaling. It is approved for cryopyrin-associated periodic syndrome [26]. Romiplostim, an Fc-IgG1 fused with thrombopoietin to modulate platelet production, is approved for the autoimmune disease immune thrombocytopenic purpura [27].
Immunotherapy using antibody-protein fusions has also been heavily investigated in cancer treatments. Antibodies specific to a tumor associated antigen represent an ideal vehicle for the targeted delivery of therapeutic cytokine payloads to the tumor microenvironment, reducing cytokine induced systemic toxicities [28]. A fusion protein of VEGFR-1 and VEGFR-2 to Fc-IgG1, called Aflibercept, has been approved for metastatic colorectal cancer as well as macular degeneration by reducing new blood vessel growth [29]. A humanized anti-GD2 monoclonal antibody linked to human interleukin-2 (IL-2), hu14.18-IL2, has completed an open label, phase II, single group clinical trial and holds promise for patients with late stage melanoma ()i [30]. and children with recurrent or refractory neuroblastoma ()ii [31]. It is also currently active in a randomized, open label, phase III trial for stage III+ melanoma ()iii. Cytokine fusions using IL-12 [32] and TNFα [33] have also been implemented in clinical testing while antibody fusions with IL-21, interferon-α (IFNα), IFNβ, and IFNγ have also shown antitumor activity in preclinical settings [33].
Central Nervous System Penetration with Antibody-Protein Fusions
Transport across the blood brain barrier (BBB) has been a significant hurdle for the development of large molecule therapeutics such as biologics [34]. Due to the complex physiology and structure of the BBB and the tight junctions on endothelial cells lining the BBB, delivery of large molecular weight therapeutic proteins to the central nervous system (CNS) via systemic administration is generally not possible without targeting assistance [35]. One of the many strategies to overcome this obstacle is to utilize receptor-mediated endocytosis and transcytosis pathways via receptors endogenously expressed at the brain capillary endothelium. The neonatal Fc receptor, a low-density lipoprotein receptor related protein, the transferrin receptor (TfR), and the insulin receptor have been explored for this purpose [34]. Recombinant antibodies have been designed against these receptors to form fusion proteins that cross the BBB to enable delivery of immunoglobulins, peptides and proteins into the brain. This class of antibody-protein fusions are known as receptor-mediated transcytosis (RMT)-targeted therapies because they facilitate CNS delivery of a large biologic through the BBB via endogenous transport mechanisms [36–38].
The use of RMT-targeted antibody-protein fusions to effectively and efficiently deliver therapeutics to the brain has been assessed in treatment of numerous diseases [36,38]. For the treatment of Alzheimer’s disease, a scFv anti-Aβ amyloid antibody was fused to the heavy chain carboxyl terminus of a chimeric mAb against the mouse TfR [37]. Intravenous administration of the fusion protein showed rapid uptake into the mouse brain and demonstrated a 40% reduction in Aβ. Another study illustrated the utility of a bispecific antibody that binds to both TfR and to the amyloid precursor protein cleavage enzyme, β-secretase (BACE1) in a mouse model of Alzheimer’s disease [36]. When compared to monospecific anti-BACE1 antibody, the bispecific antibody accumulated in the mouse brain and led to a greater reduction in brain Aβ after a single systemic dose. The study also indicated that an anti-TfR antibody possessing lower binding affinity for TfR displayed increased brain uptake and broader distribution in brain parenchyma than an anti-TfR antibody with higher binding affinity. They postulated that this result was likely due to the faster dissociation of the antibody from TfR, allowing more molecules to be available for transcytosis across the BBB. More recently, an anti-TfR IgG fusion with arylsulfatase A (ASA), an enzyme deficient in metachromatic leukodystrophy, was successfully delivered to the brain in a mouse model of the disease. The study showed that the anti-TfR fusion did not impact ASA biochemical activity and displayed an excellent safety profile over treatment of up to 12 months [39]. Many other compounds that do not naturally cross the BBB including glial derived neurotrophic factor [40], iduronate-2-sulfatase [41,42], and erythropoietin [43] were successfully delivered to the brain when fused to antibodies targeting brain endothelial receptors. These therapies have been investigated preclinically to alleviate Parkinsonian symptoms, target brain glycosaminoglycans, and for treatment of multiple brain disorders, respectively. Several additional targets for BBB RMT have been discovered offering the potential for multiple new antibody-mediated therapies targeted to the CNS to be developed [44].
Antibody-Enzyme Fusions (AEFs)
AEFs are antibodies fused with therapeutic enzymes. Like other antibody-protein fusions, the antibody is used to target specific extracellular receptors and allow therapeutic enzymes to target specific cells or cross biological barriers (BBB, cell membrane, etc.), and reduce potential side-effects. The classes of diseases where AEFs have been extensively studied are in cancer, lysosomal disorders, and glycogen storage disorders.
AEFs have been used for targeted cancer therapy in two ways – in an antibody-directed enzyme prodrug therapy (ADEPT) system or as an antibody fused to a cytotoxic enzyme as a direct therapeutic. In the direct approach, antibodies have been combined with ribonucleases (RNases) [45–48], or pro-apoptotic enzymes such as granzyme B [49,50], death-associated protein kinase [51], and caspases [52,53]. AEFs involving antibody-RNase fusions have been the most extensively studied. Although RNases can enter cells through the chemical modification of RNase cell binding properties [54], greater specificity can be achieved by linking the RNase to an antibody [55]. Once inside the cell, the antibody-RNase moiety causes cell death by cleaving RNA and by causing cytotoxicity through mechanisms such as inhibiting Ca2+-activated K+ channels [56]. and caspase-mediated induction of apoptosis [57]. A fusion protein composed of human pancreatic RNase and an antibody to the transferrin receptor was the first to demonstrate cytotoxicity in several human tumor cell lines [58]. Since then, tumor suppressor effects of human pancreatic RNase fused with numerous antibodies such as Anti-ErbB2 [46,48], anti-nucloelin [47], or anti-CD30 [45] have been tested in multiple preclinical cancer models. In addition to human pancreatic RNase, the RNases angiogenin [59], onconase [60], and barnase [61] have also been investigated.
Instead of the enzyme fused to the antibody acting as a direct therapeutic, the ADEPT approach utilizes the systemic delivery of an AEF with specific targeting capabilities, followed by an infusion of an inactive prodrug (Figure 1) [62]. ADEPT has mostly been investigated and utilized in cancer therapeutics to overcome the systemic toxicities associated with nonspecific cytotoxic drug delivery [62,63]. The only ADEPTs to enter clinical trials to date are for carcinoembryonic antigen positive (CEA+) colon carcinoma [62]. CEA is a cellular marker frequently upregulated on the surface of many colon carcinomas. The latest study was a single dose, phase I clinical trial that utilized the AEF MFECP1, which utilizes bacterial carboxypeptidase G2 (CPG2) fused with MFE-23, an scFv directed to CEA, in late stage CEA+ cancer patients [64]. The AEF administration is followed by administration of a bis-iodo phenol (BIP) mustard prodrug. CPG2 converts the BIP prodrug to its active form by releasing glutamate residues from its C-terminus. The study confirmed the safety and feasibility of ADEPT as an approach, though overwhelmingly positive results have yet to be achieved [64].
ERT currently exists for the treatment of several lysosomal storage disorders [65]; however, ERT in general has been ineffective in the treatment of neurological symptoms due to constraints on CNS delivery imposed by the BBB. For instance, ERT with intravenous idursulfase is FDA approved for the treatment of the lysosomal storage disease Hunter syndrome (mucopolysaccharidosis type II) [66]. Despite significant symptom improvement with ERT, patients continue to suffer from cognitive impairment [66,67], which affects two-thirds of patients [68]. JR-141, a fusion protein consisting of anti-human TfR antibody and intact human iduronate-2-sulfatase, has been investigated. It has shown efficacy in preclinical models [69] and currently undergoing single group, phase II/III testing for Hunter syndrome patients ()iv. A similar approach using iduronate-2-sulfatase fused to anti-human insulin receptor mAb (AGT-182) has been developed and implemented in a single group, phase I clinical trial for Hunter syndrome patients ()v [70,71]. A protein fusion platform involving anti-human insulin receptor and alpha-L-iduronidase has completed a two stage, phase I/II, open label trial for Hurler syndrome patients (mucopolysaccharidosis I) (AGT-181 )vi [72]. Furthermore, antibody-enzyme fusions involving anti-human insulin receptor and α-N-acetylglucosaminidase were successful in normalizing deficient enzyme levels in mucopolysaccharidosis IIIB fibroblasts [73]. Cumulatively, AEFs have potential for advancing ERT treatment for numerous metabolic diseases with enzyme deficiencies.
Autoantibodies as an Intracellular Delivery Vehicle
Multiple immunoglobulins from autoimmune diseases can penetrate living cells and localize to different cellular compartments, hence their potential as intracellular delivery vehicles is being explored [74–76]. Antinuclear autoantibodies (ANAs) are autoantibodies common in systemic lupus erythematosus (SLE) and possess cell penetrating abilities [77]. Although many ANAs are pathogenic [74,78], some naturally occurring or engineered variants are non-pathogenic and could provide novel methods of delivering therapeutic cargo into cells.
The 3E10 Anti-Nuclear Antibody
The 3E10 ANA penetrates cells and localizes to the cell nucleus without pathogenic effects [79]. It is a naturally occurring, anti-double stranded DNA (dsDNA), autoantibody isolated from a mouse model of SLE [80]. Cell penetration of 3E10 utilizes the equilibrative nucleotide transporter 2 (ENT2, SLC29A2), a key receptor in the nucleoside salvage pathway [79,81]. In addition to utilizing ENT2, the presence of both extracellular DNA and 3E10 DNA binding ability are required for efficient cell penetration [82]. However, the full mechanism of this transport is not well understood. 3E10 and its fragments have yielded positive results as both therapies and co-therapies for different cancers by inhibiting DNA repair pathways [83–86]. This effect is due to the antibody’s nuclear localization and binding affinity to both dsDNA and RAD51, a DNA repair protein.
Engineered 3E10 derivatives as part of a bispecific antibody (bsAb) have yielded positive results in preclinical studies. These antibodies are typically comprised of engineered F(ab)2 fragments (Box 1 Figure IA) that fuse two different Fab fragments together allowing recognition of two different antigens [87]. In one study, a bispecific antibody was generated with 3E10 and 3G5, an antibody that targets the E3 ubiquitin ligase Mdm2. Mdm2 promotes the ubiquitination and degradation of the tumor suppressor protein p53.The 3E10–3G5 bsAb achieved cellular penetration and inhibited Mdm2-directed p53 ubiquitination to increase p53 levels and promote apoptosis in MC-7 human ovarian cancer cells and several human melanoma cancer cells [88,89].
The full-length 3E10 antibody, its Fab, and its scFv fragments can each be used to mediate intracellular cargo delivery [90,91]. Early studies demonstrated that 3E10 could transport large payloads up to 155 kDa (alkaline phosphatase) into COS-7 and CHO cells while maintaining enzyme activity after transport [79]. Similarly, a 3E10-catalase conjugate protected multiple cell lines against H2O2 toxicity, a feat neither catalase nor 3E10 could achieve alone [13].
3E10 Antibody-Protein Fusions
Since its discovery, 3E10 has shown promising potential not only as a therapeutic antibody but also as an antibody-fusion molecule to assist in the delivery of therapeutic proteins [92–97]. 3E10 scFv fusion with p53 or FOXP3 were successful in penetrating and killing tumor cells in vitro and were the first to demonstrate effective intracellular delivery of full-length p53 and FOXP3 proteins in BALB/c mice [93,94]. These studies also demonstrated that the Fv portion of 3E10 was required for successful transport of these proteins into cells. The scFv portion of 3E10 fused to microdystrophin was successful in penetrating multiple cell lines, demonstrating potential as a possible therapy for dystrophin-deficient muscular dystrophies [95]. More recently, a humanized Fab fragment of 3E10 fused to myotubularin was utilized in a mouse model of X-linked myotubular myopathy, a fatal congenital muscle disease caused by deficiency of the lipid phosphatase myotubularin [97]. The 3E10-myotubularin fusion entered muscle cells, the myotubularin enzyme was active, and the muscle pathology was reversed. These examples provide ample data for the utility of 3E10-based antibody-fusion proteins for improved ERT.
Antibody-Enzyme Fusion Therapy for Glycogen Storage Diseases
Glycogen storage diseases are a group of inherited metabolic disorders resulting from absence or deficiency in one of the enzymes responsible for glycogen metabolism. Aberrant glycogen accumulation in multiple tissues is the pathologic hallmark of many of these diseases. Although ERT has been established for Pompe’s disease (GSD type II or acid maltase deficiency), it has not been successfully employed for other GSDs. The current standard of care for most GSDs involves dietary management to modulate normoglycemia and medications to assist in symptom management. As disease sequalae can be alleviated by removal of the aberrant glycogen deposits [98–101], effective cellular delivery of enzymes for glycogen degradation is much needed in improving treatment of these GSDs.
Pompe Disease
Pompe disease (Box 2) is a GSD caused by acid α-glucosidase (GAA) deficiency (Box 2 Figure IIA) [102]. It has an estimated incidence of approximately 1:60,000 [103–105]. ERT using a recombinant human GAA (rhGAA) analog, alglucosidase alpha is currently the standard of care for Pompe patients [106,107]. Alglucosidase alpha is a 110 kDa precursor protein containing mannose-6-phosphate (M6P) groups that enable the enzyme to be internalized by cells via the M6P receptor (M6PR) that traffics compounds to the lysosome. Once inside the lysosome, alglucosidase alpha is cleaved to yield 76 kDa and 70 kDa mature isoforms. A multicenter, open label clinical trial of alglucosidase alfa showed that ERT in infantile forms of Pompe disease significantly improved survival, cardiomyopathy and motor skills ()vii [108]. Therapeutic benefit has also been shown for late onset Pompe patients using randomized, double-blind, placebo-controlled phase III studies ()viii [109]. Despite these encouraging results that have drastically changed the course of Pompe disease, skeletal muscle exhibited only a minimal response to treatment and alglucosidase alpha does not completely halt disease progression [110,111]. The reduced response in skeletal muscle may be attributed to low M6PR concentration [112], resulting in inadequate uptake of alglucosidase alpha in these tissues. As a result, long term survivors continue to suffer from progressive muscle weakness, hearing loss, arrhythmias, dysphagia, and osteopenia [113,114]. Delivery of rhGAA to muscles could be improved by using adeno-associated virus (AAV) delivery of gene encoding an anti-CD63-GAA fusion, which would allow for lysosomal GAA delivery while circumventing the need for the M6PR [115]. Furthermore, alglucosidase alpha only targets lysosomal glycogen accumulations due to its dependence on the M6PR mediated cellular uptake and low pH requirement. Therefore, it does not alleviate the cytoplasmic glycogen burden [116] that builds up as a result of the rupture and shearing of lysosomes and exacerbates muscle pathophysiology in later stages of both infantile and late onset Pompe disease (Box 2 Figure IIB) [117,118]. Given these deficiencies, new therapeutic approaches are still needed.
Box 2: Pathogenesis of Pompe Disease.
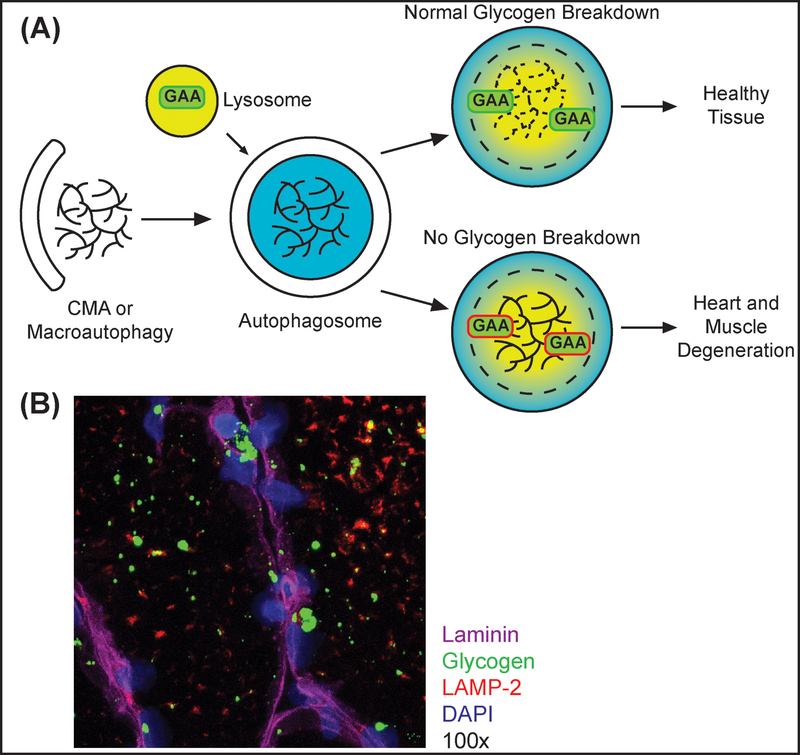
Pompe Disease is caused by mutations in the gene encoding acid, alpha-glucosidase (GAA). The inability to break down lysosomal glycogen leads to intra-lysosomal glycogen accumulation in all tissues, thus Pompe disease is also classified as a lysosomal storage disorder (Figure IIA) [133]. Glycogen is engulfed in autophagosomes via chaperone-mediated autophagy (CMA) or macroautophagy. Autophagosomes fuse with lysosomes containing GAA. In Pompe cells, mutant GAA cannot degrade glycogen leading to build-up of pathogenic, glycogen filled vacuoles. As patients progress, they begin presenting with increased cytoplasmic glycogen, and the inability of lysosomes to fully mature leads to build-up of other lysosomal substrates, which exacerbates disease pathology [133]. Diagnosis is made by quantifying GAA activity from a biopsy with genetic testing to confirm [102]. The classical manifestations of Pompe disease are progressive myopathy and hypertrophic cardiomyopathy. Figure IB shows an immunofluorescent microscopic image of skeletal muscle from a Pompe disease patient. LAMP-2 highlights the location of lysosomes. Glycogen is seen both in the cytoplasm and in the lysosomes. Image courtesy of Dr. Nadine Aziz from Valerion Therapeutics. The disease is classified into infantile, juvenile, and adult onset forms based on the appearance of clinical symptoms [134]. The wide range of disease onset and broad spectrum of symptom severity is dependent on the amount of residual enzyme activity [103]. Respiratory failure is the main cause of mortality in late stage patients [134].
Alternative approaches to ERT are being investigated to improve treatment for Pompe patients including small molecule chaperones, gene therapy, and substrate reduction therapy (SRT). AT2220 is a small molecule chaperone therapy that facilitates normal folding, stabilizing GAA, and improves its function despite amino acid mutations. [135]. AT2220 also increases efficiency of rhGAA ERT, thus, AT2220 may be effective as co-therapy with alglucosidase alpha [106,136]. Open label, non-randomized phase I/II clinical trials using intra-diaphragmatic delivery of AAV mediated GAA gene therapy (rAAV1-hGAA) showed rAAV1-hGAA was safe and led to a modest improvement in ventilatory function in children with end stage disease on ventilator dependence despite ERT ()x [136]. A double-blind, randomized phase I clinical trial involving AAV9 injected intramuscularly in the tibialis anterior is currently underway to assess its ability to improve motor function in late-onset Pompe patients ()xi. SRT by inhibiting glycogen synthase by short hairpin RNA or antisense oligonucleotides, decreased lysosomal glycogen accumulation in skeletal muscle of Pompe mouse models [137].
The recent development of an AEF utilizing the 3E10 Fab fragment fused with the 110 kDA rhGAA (VAL-1221) can overcome these obstacles. VAL-1221 utilizes both the M6PR pathway and the ENT2 pathway for internalization and stabilization of GAA, maintaining its activity at both neutral and low lysosomal pH [107,119]. As a result, it can clear both cytoplasmic and lysosomal glycogen [107]. Blocking M6PR in Pompe patient fibroblasts eliminated uptake of lysosomal but not cytoplasmic GAA [107], suggesting that VAL-1221 utilizes both M6PR-dependent and independent uptake pathways. Thus, VAL-1221 has greater potential for ameliorating skeletal muscle pathophysiology due to enrichment of ENT2 in skeletal muscle cells [120]. Overall, preclinical studies demonstrated that VAL-1221 was at least equally as effective as alglucosidase alpha for targeting lysosomal glycogen inclusions and had much greater potential than alglucosidase alpha for clearing cytoplasmic glycogen in mouse models of Pompe disease [107]. Positive results from a phase I/II study were recently announced for VAL-1221 for late stage Pompe patients who had previously been treated for at least one year with alglucosidase alpha ()ix [119]. Patients treated with VAL-1221 displayed an improved six-minute walk test compared to a control cohort receiving alglucosidase alpha and no serious adverse events were associated with VAL-1221[119].
Lafora Disease
Lafora Disease (LD) (Box 3) is a rare, invariably fatal neurodegenerative epilepsy and GSD [121]. LD is caused by deficiency in one of two proteins: the glycogen phosphatase laforin or the E3-ubiquitin ligase malin (Box 3 Figure IIIA) [121], which leads to the formation of pathogenic polyglucosan aggregates known as Lafora Bodies (LBs) in the brain and other tissues (Box 3 Figure IIIB). At present, LD treatments remain palliative at best [122]. Anti-epileptic drugs and physical therapy may reduce frequency of epileptic episodes and maintain functional status in early stages of disease, but patients invariably become bed-ridden over the course of about 10 years before death [121]. Genetic methods in mouse models that block glycogen synthesis showed that LB accumulations in the brain are the main cause of the clinical manifestations associated with LD [98,99,101]. These results demonstrated that the removal or inhibition of LBs could be an effective therapeutic strategy for LD.
Box 3: Pathogenesis of Lafora Disease.
Lafora Disease (LD) is autosomal recessive GSD that manifests as a severe epileptic disease [120–122]. Onset is in adolescence, in apparently healthy teenagers, with headaches and insidious decline in cognitive function along with epileptic episodes. Initial response to antiepileptic drugs is lost within three years and a constant myoclonus with atypical absence begins. The young person develops dementia, seizes with increased frequency, becomes bedridden, and death comes after a protracted decade of unceasing myoclonus in the form of status epilepticus or aspiration pneumonitis [122,138]. While LD is a devastating disease, genetic and biochemical work over the last 25 years has set the foundation for an effective LD treatment.
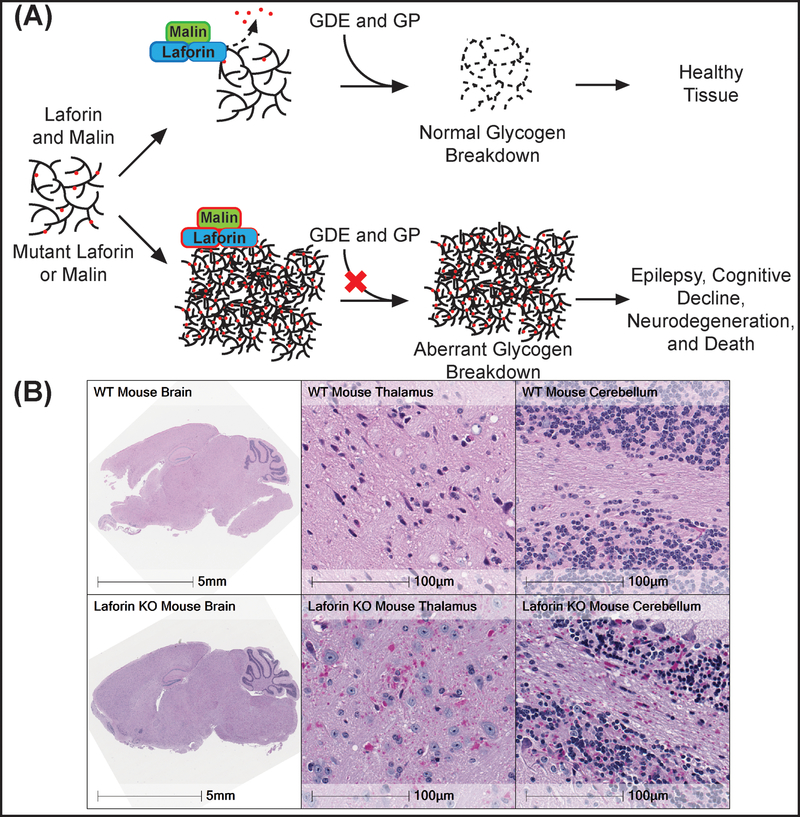
LD is caused by mutations in either the Epilepsy, Progressive Myoclonus 2A (EPM2A) gene, which encodes the glycogen phosphatase laforin, or EPM2B, which encodes the E3 ubiquitin ligase malin [138,139]. Both proteins are regulators of glycogen architecture, which is critical for glycogen catabolism [120,140]. Glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE) break down the sugar polymer. Mutations in EPM2A or EPM2B results in aberrant, glycogen-like aggregates called Lafora bodies (LBs) found in the cytoplasm of cells from nearly all tissues in LD patients and LD mouse models (red circles, Figure IIIA). The LBs contain longer glucose chains than normal glycogen, aberrant branching, and increased phosphorylation (Figure IIIA). While glycogen is water-soluble, these aberrant architectural qualities make LBs water-insoluble and inaccessible to normal glycogen degrading enzymes such as glycogen phosphorylase and debranching enzyme. Thus, LD cells synthesize a glucose cache that they cannot degrade. Although found ubiquitously, LBs exert the most severe effects in the brain by affecting both neurons and astrocytes (Figure IIIB) [141,142], as visualized by Periodic-acid Schiff (PAS) stained brain sections from wild type and Laforin knockout mice (Figure IIIB). The images of the thalami and cerebella are at 40x of the full brain image. Image adapted with permission from [124].
Intracerebroventricular (ICV) administration of VAL-1221 or another 3E10 Fab-based AEF utilizing pancreatic α-amylase (VAL-0417) demonstrated that both agents penetrated cells and dramatically reduced glycogen levels in brains of malin and laforin knock-out mice, two models of LD [123–125]. Furthermore, peripheral delivery of these AEFs via intramuscular or tail vein injection also resulted in decreased LB load in peripheral tissues [126]. While these studies demonstrated the potential for these AEFs in LD treatment, they provided no evidence that either VAL-1221 or VAL-0417 can cross the BBB. Therefore, delivery using methods that specifically target the CNS, rather than systemic administration, is needed for these AEFs. While ICV administration is invasive, it has previously been shown to be a safe mode of drug delivery in life-threatening diseases such as Batten disease (cerliponase alfa) [126,127].
Other Metabolic Disorders
Given their ability to target aberrant glycogen deposits, VAL-1221 and VAL-0417 may have potential applications in other GSDs that accumulate excess glycogen, such as Cori, McArdle, or Danon disease and polyglucosan body diseases such as Andersen disease, familial Wolff-Parkinson-White Syndrome or polyglucosan body myopathy-2. Although substantial preclinical studies are still needed, the success of recent developments in AEF technology provides hope that disease-modifying treatments may soon be available for patients afflicted with a GSD.
Concluding Remarks
With rapid advancements in protein characterization and engineering technologies, antibody therapeutics have risen to prominence as unique tools for precision medicine. While active research in this field is ongoing and there are many questions left to be answered (see Outstanding Questions), important strides have been made in the understanding of membrane transport mechanisms and antibody biochemistry through the development of antibody therapeutics. Antibodies have been dissected into minimal fragments, rebuilt by fusing them to a wide range of biologicals, and engineered into a repertoire of therapeutics for treatment of numerous intractable diseases. Antibody therapies enhance the delivery specificity and cellular penetration of multiple therapeutics and their clinical efficacy is steadily improving due to innovative breakthroughs. As new molecular strategies with enhanced affinity, stability, and expression efficiency emerge, it is foreseeable that antibody fusion technology will have increasing importance in creating novel therapeutics for multiple diseases (see Clinician’s Corner). GSDs provide a particularly interesting target for antibody-mediated therapies because both the replacement of deficient enzymes and degradation of aberrant polyglucosans require transport of large cargo into cells. The advances in AEF technology for Lafora disease and Pompe disease illustrate the exciting potential for enhanced therapeutic delivery of these complex molecules to more effectively combat pathophysiological changes in these debilitating diseases.
Outstanding Questions.
What is the biophysical mechanism by which ENT2 allows 3E10 to transport large biological therapies into cells? Although mechanisms such as receptor-mediated endocytosis and membrane shuttling pathways have been proposed, there has been insufficient evidence for or against these processes. These answers would also improve our understanding of biological membrane transport and possibly further improve antibody-mediated cellular delivery.
Could VAL-1221 or VAL-0417 be modified to also bind a BBB endothelial receptor that allows penetration into the CNS? Peripheral delivery would reduce patient expenses, improve safety, and increase ease of drug administration compared to ICV administration. However, would cellular uptake by ENT2 in the periphery remove too much of the therapy from the blood before it had a chance to cross the BBB?
How will regulatory bodies handle the approval process for the ever-expanding field of antibody-mediated therapeutics? Will AEFs that utilize separately approved antibody fragments and enzymes be able to achieve accelerated approval by regulatory bodies? How will the cost of these therapies be managed to prevent extreme expenses incurred by patients? As more antibody vehicles and therapeutic enzymes are developed, research into the feasibility of these approval and billing policies will be essential to improving development and distribution of life-saving therapies.
What will be the clinical manifestations of long-term survivors of Pompe disease and Lafora disease? As effective therapies are developed that prolongs patient survival, additional unforeseen symptoms are bound to emerge as patients develop into later stages of the disease.
Highlights.
Recent advancements in antibody-mediated enzyme therapies provide improvements to traditional therapies due to their ability to penetrate cell membranes and their capability to target specific cellular antigens.
Newly developed antibody-enzyme fusions (AEFs) provide a wide range of functionality and specificity. Beyond targeting a protein to a specific site, AEFs can be used as antibody-directed enzyme prodrug therapy, receptor-mediated transcytosis-targeted therapies, or in cell-penetrating enzyme-replacement therapy.
Pathogenic glycogen aggregates are a novel target for AEFs that provide unique avenues of therapy for the treatment of glycogen storage diseases such as Pompe disease and Lafora disease.
Acknowledgements
We want to thank Dr. Nadine Aziz of Valerion Therapeutics for acquiring and allowing us to use the immunofluorescence image in Figure IIB and for assisting with edits. We thank members of the M.S.G. lab for their support and assistance in critically reviewing this manuscript. M.S.G is funded in part through a sponsored project from Valerion Therapeutics. Additional funding to M.S.G is provided by NIH grants R01 NS070899, P01 NS097197, and an Epilepsy Foundation New Therapy Commercialization Grant.
Glossary
- Acid α-glucosidase (GAA)
a lysosomal enzyme responsible for the cleavage of α−1,4-and α−1,6-glycosidic bonds within glycogen.
- Antibody-Dependent Cellular Cytotoxicity (ADCC)
A type of antibody mediated cell death caused by Fc receptor signaling that leads to a cell being targeted by non-specific cytotoxic cells (i.e. natural killer cells).
- Antibody Valency
A characteristic of antibodies and their fragments that describes how many antigens they are capable of binding. A bivalent antibody (IgG) possesses two binding sites for its antigen and can bind two antigens, which may or may not be on the same molecule or structure. Monovalent antibodies do not exist naturally in humans but monovalency is found in other antibody-like structures such as antibody fragments, nanobodies, Fc receptors, and major histocompatibility complexes.
- Antinuclear Antibodies (ANA)
Antibodies produced in autoimmune disease patients that recognize nuclear antigens (DNA, nuclear membrane proteins, chromatin proteins, etc.) and often have cell penetrating abilities.
- Blood Brain Barrier (BBB)
a highly selective, semi-permeable, physiological barrier between the blood and the CNS parenchyma. It tightly regulates movement of ions, molecules, and cells between the blood and the brain, critical for CNS homeostasis.
- Complement-Dependent Cytotoxicity
An antibody driven cell death caused by activation of the complement cascade. This response may be desirable (i.e. in cancer therapy) or undesirable (i.e. killing of healthy cells as a side effect).
- Cytokine
Any number of substances, ranging from small molecules to proteins, that are secreted by cells of the immune system in response to immunogenic signals and lead to effects on other cells. This mechanism expands the immune response cascade and is tightly regulated to prevent cytokine toxicity.
- Enzyme Replacement Therapy (ERT)
A method of treating genetic disorders wherein a deficient enzyme is generated by recombinant methods and transfused into the patient to restore normal function.
- Glycogen
A branched polymer of glucose comprised of α−1,4 and α−1,6 glyosidic linkages. It is the main form of glucose storage in animals.
- Monoclonal antibodies (mAbs)
A homogenous population of antibodies that bind to a single epitope.
- Polyglucosans
Aberrant carbohydrate structures comprised α−1,4 and α−1,6 glyosidic linkages. Polyglucosans can be pathogenic if they have abnormal structure or localization within cells as is the case with Lafora bodies.
- Substrate Reduction Therapy (SRT)
A method of treating genetic disorders by blocking production of a substrate to prevent the pathogenic action of a mutant enzyme. This action prevents the uncontrolled build-up of the enzyme product.
- Transferrin receptor (TfR)
A transmembrane glycoprotein composed of two disulfide bonded sub-units involved in iron uptake in vertebrates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Resources:
References
- 1.Rehman K et al. (2016) Delivery of Therapeutic Proteins: Challenges and Strategies. Curr. Drug Targets 17, 1172–88 [DOI] [PubMed] [Google Scholar]
- 2.Elgundi Z et al. (2017) The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev 122, 2–19 [DOI] [PubMed] [Google Scholar]
- 3.Lu J et al. (2016) Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci 17, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie M et al. (2013) Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 5, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panowski S et al. (2014) Site-specific antibody drug conjugates for cancer therapy. MAbs 6, 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimberidou A-M et al. (2005) The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br. J. Haematol 132, 398–409 [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf SH et al. (2013) A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 121, 4854–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hills RK et al. (2014) Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet. Oncol 15, 986–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hitzler J and Estey E (2019) Gemtuzumab ozogamicin in acute myeloid leukemia: act 2, with perhaps more to come. Haematologica 104, 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senter PD and Sievers EL (2012) The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol 30, 631–637 [DOI] [PubMed] [Google Scholar]
- 11.Lewis Phillips GD et al. (2008) Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res 68, 9280–9290 [DOI] [PubMed] [Google Scholar]
- 12.Al-Salama ZT (2018) Inotuzumab Ozogamicin: A Review in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukaemia. Target. Oncol 13, 525–532 [DOI] [PubMed] [Google Scholar]
- 13.Weisbart RH et al. (2000) Novel protein transfection of primary rat cortical neurons using an antibody that penetrates living cells. J. Immunol 164, 6020–6026 [DOI] [PubMed] [Google Scholar]
- 14.Ahn SH et al. (2019) Linear Desferrichrome-Linked Silicon–Rhodamine Antibody Conjugate Enables Targeted Multimodal Imaging of HER2 in Vitro and in Vivo. Mol. Pharm DOI: 10.1021/acs.molpharmaceut.8b01278 [DOI] [PMC free article] [PubMed]
- 15.Stubenrauch K et al. (2001) Conjugation of an antibody Fv fragment to a virus coat protein: cell-specific targeting of recombinant polyoma-virus-like particles. Biochem. J 356, 867–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z et al. (2016) A lupus anti-DNA autoantibody mediates autocatalytic, targeted delivery of nanoparticles to tumors. Oncotarget 7, 59965–59975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaghy H (2016) Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 8, 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X et al. (2013) Fusion protein linkers: property, design and functionality. Adv. Drug Deliv. Rev 65, 1357–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahtis K et al. (2001) Biodistribution properties of (111)indium-labeled C-functionalized trans-cyclohexyl diethylenetriaminepentaacetic acid humanized 3S193 diabody and F(ab’)(2) constructs in a breast carcinoma xenograft model. Clin. Cancer Res 7, 1061–1072 [PubMed] [Google Scholar]
- 20.Graff CP and Wittrup KD (2003) Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res 63, 1288–1296 [PubMed] [Google Scholar]
- 21.Albrecht H et al. (2004) Production of soluble ScFvs with C-terminal-free thiol for site-specific conjugation or stable dimeric ScFvs on demand. Bioconjug. Chem 15, 16–26 [DOI] [PubMed] [Google Scholar]
- 22.Dumont JA et al. (2006) Monomeric Fc fusions: impact on pharmacokinetic and biological activity of protein therapeutics. BioDrugs 20, 151–60 [DOI] [PubMed] [Google Scholar]
- 23.Ducharme E and Weinberg JM (2008) Etanercept. Expert Opin. Biol. Ther 8, 491–502 [DOI] [PubMed] [Google Scholar]
- 24.Genovese MC et al. (2005) Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med 353, 1114–23 [DOI] [PubMed] [Google Scholar]
- 25.Strober BE and Menon K (2007) Alefacept for the treatment of psoriasis and other dermatologic diseases. Dermatol. Ther 20, 270–276 [DOI] [PubMed] [Google Scholar]
- 26.McDermott MF (2009) Rilonacept in the treatment of chronic inflammatory disorders. Drugs of Today 45, 423–430 [DOI] [PubMed] [Google Scholar]
- 27.Molineux G (2011) The development of romiplostim for patients with immune thrombocytopenia. Ann. N. Y. Acad. Sci 1222, 55–63 [DOI] [PubMed] [Google Scholar]
- 28.Tisoncik JR et al. (2012) Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev 76, 16–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart MW (2011) Aflibercept (VEGF-TRAP): The Next Anti-VEGF Drug. Inflamm. Allergy - Drug Targets 10, 497–508 [DOI] [PubMed] [Google Scholar]
- 30.Albertini MR et al. (2012) Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol. Immunother 61, 2261–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shusterman S et al. (2010) Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J. Clin Oncol 28, 4969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudman SM et al. (2011) A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin. Cancer Res 17, 1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitaleri G et al. (2013) Phase I/II study of the tumour-targeting human monoclonal antibody–cytokine fusion protein L19-TNF in patients with advanced solid tumours. J. Cancer Res. Clin. Oncol 139, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar NN et al. (2018) Passive Immunotherapies for Central Nervous System Disorders: Current Delivery Challenges and New Approaches. Bioconjugate Chem 29, 3937–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizzo ME et al. (2018) Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol 5963, 445–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu YJ et al. (2011) Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med 3, 84ra44. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q-H et al. (2011) Receptor-mediated abeta amyloid antibody targeting to Alzheimer’s disease mouse brain. Mol. Pharm 8, 280–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardridge WM (2008) Re-Engineering Biopharmaceuticals for Delivery to Brain with Molecular Trojan Horses. Bioconjug. Chem 19, 1327–1338 [DOI] [PubMed] [Google Scholar]
- 39.Hui EK-W et al. (2019) Preclinical studies of a brain penetrating IgG Trojan horse-arylsulfatase fusion protein in the metachromatic leukodystrophy mouse. Mol. Genet. Metab 126, S77 [Google Scholar]
- 40.Fu A et al. (2010) Intravenous treatment of experimental Parkinson’s disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res 1352, 208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu JZ et al. (2010) Genetic engineering of a bifunctional IgG fusion protein with iduronate-2-sulfatase. Bioconjug. Chem 21, 151–6 [DOI] [PubMed] [Google Scholar]
- 42.Henry AG et al. (2019) Improved brain uptake and efficacy of iduronate 2-sulfatase with the enzyme transport vehicle. Mol. Genet. Metab 126, S72 [Google Scholar]
- 43.Boado RJ et al. (2010) Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J. Pharmacol. Exp. Ther 333, 961–9 [DOI] [PubMed] [Google Scholar]
- 44.Zuchero YJY et al. (2016) Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 89, P70–82 [DOI] [PubMed] [Google Scholar]
- 45.Braschoss S et al. (2007) New anti-CD30 human pancreatic ribonuclease-based immunotoxin reveals strong and specific cytotoxicity in vivo. Leuk Lymphoma 48, 1179–86 [DOI] [PubMed] [Google Scholar]
- 46.D’Avino C et al. (2014) Effects of a second-generation human anti-ErbB2 ImmunoRNase on trastuzumab-resistant tumors and cardiac cells. Protein Eng Des Sel 27, 83–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Avino C et al. (2016) A novel fully human anti-NCL immunoRNase for triple-negative breast cancer therapy. Oncotarget 7, 87016–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Lorenzo C et al. (2002). A new Rnase-based immunoconjugate selectively cytotoxic for ErbB2-overexpressing cells FEBS Lett 516, 208–12 [DOI] [PubMed] [Google Scholar]
- 49.Niesen J et al. (2016) A novel fully-human cytolytic fusion protein based on granzyme B shows in vitro cytotoxicity and ex vivo binding to solid tumors overexpressing the epidermal growth factor receptor. Cancer Lett 374, 229–40 [DOI] [PubMed] [Google Scholar]
- 50.Schiffer S et al. (2013) Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical Hodgkin lymphoma cells in a murine model. Blood Cancer J 3, e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linienthal N et al. (2016) A Novel Recombinant Anti-CD22 Immunokinase Delivers Proapoptotic Activity of Death-Associated Protein Kinase (DAPK) and mediates Cytotoxicity in Neoplastic B Cells. Mol Cancer Ther 15, 971–84 [DOI] [PubMed] [Google Scholar]
- 52.Wang LF et al. (2009) A caspase-6 and anti-HER2 antibody chimeric tumor-targeted proapoptotic molecule decreased metastasis of human osteosarcoma. Cancer Invest 27, 774–80 [DOI] [PubMed] [Google Scholar]
- 53.Xu YM et al. (2004) A caspase-6 and anti-human epidermal growth factor receptor-2 (HER2) antibody chimeric molecule suppresses the growth of HER2-overexpressing tumors. J Immunol 173, 61–7 [DOI] [PubMed] [Google Scholar]
- 54.Futami J and Yamada H (2008) Design of cytotoxic ribonucleases by cationization to enhance intracellular protein delivery. Curr Pharm Biotechnol 9, 180–4 [DOI] [PubMed] [Google Scholar]
- 55.Erickson HA et al. (2006) Cytotoxicity of human RNase-based immunotoxins requires cytosolic access and resistance to ribonuclease inhibition. Protein Eng Des Sel 19, 37–45 [DOI] [PubMed] [Google Scholar]
- 56.Ilinskaya ON et al. (2008) RNase-induced apoptosis: fate of calcium-activated potassium channels. Biochimie 90, 717–25 [DOI] [PubMed] [Google Scholar]
- 57.Spalletti-Cernia D et al. (2003) Antineoplastic ribonucleases selectively kill thyroid carcinoma cells via caspase-mediated induction of apoptosis. J Clin Endocrinol Metab 88, 2900–7 [DOI] [PubMed] [Google Scholar]
- 58.Zewe M et al. (1997) Cloning and cytotoxicity of a human pancreatic RNase immunofusion. Immunotechnology 3, 127–36 [DOI] [PubMed] [Google Scholar]
- 59.Gresch G et al. (2018) Elimination of different leukaemia subtypes using novel CD89-specific human cytolytic fusion proteins. Br J Haematol 183, 313–7 [DOI] [PubMed] [Google Scholar]
- 60.Weber T et al. (2015) A Humanized Anti-CD22-Onconase Antibody-Drug Conjugate Mediates Highly Potent Destruction of Targeted Tumor Cells. J Immunol Res 2015, 561814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edelweiss E et al. (2008) Barnase as a new therapeutic agent triggering apoptosis in human cancer cells. PLOS One 3, e2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma SK and Bagshawe KD (2017) Antibody Directed Enzyme Prodrug Therapy (ADEPT): Trials and tribulations. Adv Drug Deliv Rev 118, 2–7 [DOI] [PubMed] [Google Scholar]
- 63.Andrady C et al. (2011) Antibody–enzyme fusion proteins for cancer therapy. Immunotherapy 3, 193–211 [DOI] [PubMed] [Google Scholar]
- 64.Mayer A et al. (2006) A Phase I Study of Single Administration of Antibody-Directed Enzyme Prodrug Therapy with the Recombinant Anti Ĉarcinoembryonic Antigen Antibody-Enzyme Fusion Protein MFECP1and a Bis-Iodo Phenol Mustard Prodrug. Clin. Cancer Res 11, 814–825 [DOI] [PubMed] [Google Scholar]
- 65.Thomas R and Kermode AR (2019) Enzyme enhancement therapeutics for lysosomal storage diseases: Current status and perspective. Mol. Genet. Metab 126, 83–97 [DOI] [PubMed] [Google Scholar]
- 66.Whiteman DA and Kimura A (2017) Development of idursulfase therapy for mucopolysaccharidosis type II (Hunter syndrome): the past, the present and the future. Drug Des. Devel. Ther 11, 2467–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muenzer J et al. (2016) A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med 18, 73–81 [DOI] [PubMed] [Google Scholar]
- 68.Tylki-Szymańska A (2014) Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr. Endocrinol. Rev 12 Suppl 1, 107–13 [PubMed] [Google Scholar]
- 69.Sonoda H et al. (2018) A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol Ther 26, 1366–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boado RJ et al. (2014) Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng 111, 2317–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu JZ et al. (2011) Expression in CHO cells and pharmacokinetics and brain uptake in the Rhesus monkey of an IgG-iduronate-2-sulfatase fusion protein. Biotechnol. Bioeng 108, 1954–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giugliani R et al. (2018) Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1–2 trial. Orphanet J Rare Dis 13, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boado RJ et al. (2016) Insulin Receptor Antibody-alpha-N-Acetylglucosaminidase Fusion Protein Penetrates the Primate Blood-Brain Barrier and Reduces Glycosoaminoglycans in Sanfilippo Type B Fibroblasts. Mol Pharm 13, 1385–92 [DOI] [PubMed] [Google Scholar]
- 74.Alarcon-Segovia D et al. (1978) Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature 271, 67–9 [DOI] [PubMed] [Google Scholar]
- 75.Okudaira K et al. (1987) Monoclonal murine anti-DNA antibody interacts with living mononuclear cells. Arthritis Rheum 30, 669–78 [DOI] [PubMed] [Google Scholar]
- 76.Dalmau J et al. (1991) Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology 41, 1757–64 [DOI] [PubMed] [Google Scholar]
- 77.Holman HR and Kunkei HG (1957) Affinity between the Lupus Erythematosus Serum Factor and Cell Nuclei and Nucleoprotein. Science (80-. ) 126, 162–163 [DOI] [PubMed] [Google Scholar]
- 78.Alarcon-Segovia D et al. (1979) Antibody penetration into living cells I. Intranuclear Immunoglobulin in Peripheral Blood Mononuclear Cells in Mixed Connective Tissue Disease and Systemic Lupus Eruthematosus. Clin. Exp. Immunol 35, 364–375 [PMC free article] [PubMed] [Google Scholar]
- 79.Zack DJ et al. (1996) Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J. Immunol 157, 2082–2088 [PubMed] [Google Scholar]
- 80.Weisbart R et al. (1990) A conserved anti-DNA antibody idiotype associated with nephritis in murine and human systemic lupus erythematosus. J. Immunol 144, 2653–2658 [PubMed] [Google Scholar]
- 81.Hansen JE et al. (2007) Intranuclear protein transduction through a nucleoside salvage pathway. J. Biol. Chem 282, 20790–20793 [DOI] [PubMed] [Google Scholar]
- 82.Weisbart RH et al. (2015) DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci. Rep 5, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansen JE et al. (2012) Targeting Cancer with a Lupus Autoantibody. Sci. Transl. Med 4, 157ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turchick A et al. (2017) A cell-penetrating antibody inhibits human RAD51 via direct binding. Nucleic Acids Res 45, 11782–11799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noble PW et al. (2015) Optimizing a Lupus Autoantibody for Targeted Cancer Therapy. Cancer Res 75, 2285–2291 [DOI] [PubMed] [Google Scholar]
- 86.Turchick A et al. (2019) Synthetic lethality of a cell-penetrating anti-RAD51 antibody in PTEN-deficient melanoma and glioma cells. Oncotarget 10, 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brinkmann U and Kontermann RE (2017) The making of bispecific antibodies. MAbs 9, 182–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weisbart RH et al. (2012) A Cell-Penetrating Bispecific Antibody for Therapeutic Regulation of Intracellular Targets. Mol. Cancer Ther 11, 2169–2173 [DOI] [PubMed] [Google Scholar]
- 89.Chan G et al. (2016) Combining intracellular antibodies to restore function of mutated p53 in cancer. Int. J. Cancer 138, 182–186 [DOI] [PubMed] [Google Scholar]
- 90.Hansen JE et al. (2005) Antibody mediated transduction of therapeutic proteins into living cells. Sci. World J 5, 782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weisbart RH et al. (1998) An autoantibody is modified for use as a delivery system to target the cell nucleus: Therapeutic implications. J. Autoimmun 11, 539–546 [DOI] [PubMed] [Google Scholar]
- 92.Weisbart RH et al. (2004) Antibody-mediated transduction of p53 selectively kills cancer cells. Int. J. Oncol 25, 1867–73 [PubMed] [Google Scholar]
- 93.Hansen JE et al. (2007) Antibody-mediated p53 protein therapy prevents liver metastasis in vivo. Cancer Res 67, 1769–1774 [DOI] [PubMed] [Google Scholar]
- 94.Heinze E et al. (2009) Antibody-mediated FOXP3 protein therapy induces apoptosis in cancer cells in vitro and inhibits metastasis in vivo. Int. J. Oncol 35, 167–173 [DOI] [PubMed] [Google Scholar]
- 95.Weisbart RH et al. (2005) An intracellular delivery vehicle for protein transduction of micro-dystrophin. J. Drug Target 13, 81–87 [DOI] [PubMed] [Google Scholar]
- 96.Hansen JE et al. (2006) Antibody-mediated Hsp70 protein therapy. Brain Res 1088, 187–196 [DOI] [PubMed] [Google Scholar]
- 97.Lawlor MW et al. (2013) Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopothy. Hum. Mol. Genet 22, 1525–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duran J et al. (2014) Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum Mol Genet 23, 3147–3156 [DOI] [PubMed] [Google Scholar]
- 99.Turnbull J et al. (2011) PTG depletion removes lafora bodies and rescues the fatal epilepsy of lafora disease. PLoS Genet 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Douillard-Guilloux G et al. (2010) Restoration of muscle functionality by genetic suppression of glycogen synthesis in a murine model of Pompe disease. Hum. Mol. Genet 19, 684–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turnbull J et al. (2014) PTG protein depletion rescues malin‐deficient Lafora disease in mouse. Ann. Neurol 75, 442–446 [DOI] [PubMed] [Google Scholar]
- 102.van der Ploeg AT and Reuser AJJ (2008) Pompe’s disease. Lancet (London, England) 372, 1342–53 [DOI] [PubMed] [Google Scholar]
- 103.Martiniuk F et al. (1998) Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am. J. Med. Genet 79, 69–72 [DOI] [PubMed] [Google Scholar]
- 104.Ausems MG et al. (1999) Glycogen storage disease type II: birth prevalence agrees with predicted genotype frequency. Community Genet 2, 91–6 [DOI] [PubMed] [Google Scholar]
- 105.Ausems MG et al. (1999) Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur. J. Hum. Genet 7, 713–6 [DOI] [PubMed] [Google Scholar]
- 106.Xu S et al. (2019) Improved efficacy of a next-generation ERT in murine Pompe disease. JCI Insight 4, e125358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yi H et al. (2017) Antibody-mediated enzyme replacement therapy targeting both lysosomal and cytoplasmic glycogen in Pompe disease. J. Mol. Med 95, 513–521 [DOI] [PubMed] [Google Scholar]
- 108.Nicolino M et al. (2009) Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med 11, 210–9 [DOI] [PubMed] [Google Scholar]
- 109.Winkel LPF et al. (2004) Enzyme replacement therapy in late-onset Pompe’s disease: a three-year follow-up. Ann. Neurol 55, 495–502 [DOI] [PubMed] [Google Scholar]
- 110.Thurberg BL et al. (2006) Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab. Invest 86, 1208–20 [DOI] [PubMed] [Google Scholar]
- 111.Prater SN et al. (2013) Skeletal muscle pathology of infantile Pompe disease during long-term enzyme replacement therapy. Orphanet J. Rare Dis 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Funk B et al. (1992) Expression of the insulin-like growth factor-II/mannose-6-phosphate receptor in multiple human tissues during fetal life and early infancy. J. Clin. Endocrinol. Metab 75, 424–31 [DOI] [PubMed] [Google Scholar]
- 113.Prater SN et al. (2012) The emerging phenotype of long-term survivors with infantile Pompe disease. Genet. Med 14, 800–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kishnani PS et al. (2007) Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 68, 99–109 [DOI] [PubMed] [Google Scholar]
- 115.Baik A et al. (2018) Next-generation antibody-guided enzyme replacement therapy in Pompe disease mice. Mol. Genet. Metab 123, S21 [Google Scholar]
- 116.Van Hove JL et al. (1996) High-level production of recombinant human lysosomal acid alpha-glucosidase in Chinese hamster ovary cells which targets to heart muscle and corrects glycogen accumulation in fibroblasts from patients with Pompe disease. Proc. Natl. Acad. Sci. U. S. A 93, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Griffin JL (1984) Infantile acid maltase deficiency. I. Muscle fiber destruction after lysosomal rupture. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol 45, 23–36 [DOI] [PubMed] [Google Scholar]
- 118.Lewandowska E et al. (2008) Pathology of skeletal muscle cells in adult-onset glycogenosis type II (Pompe disease): ultrastructural study. Folia Neuropathol 46, 123–33 [PubMed] [Google Scholar]
- 119.Kishnani P et al. (2019) Safety and efficacy of VAL-1221, a novel fusion protein targeting cytoplasmic glycogen, in patients with late-onset Pompe disease. Mol. Genet. Metab 126, S85–S86 [Google Scholar]
- 120.Crawford CR et al. (1998) Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. J. Biol. Chem 273, 5288–93 [DOI] [PubMed] [Google Scholar]
- 121.Gentry MS et al. (2018) Lafora disease offers a unique window into neuronal glycogen metabolism. J. Biol. Chem 293, 7117–7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sullivan MA et al. (2017) Pathogenesis of lafora disease: Transition of soluble glycogen to insoluble polyglucosan. Int. J. Mol. Sci 18, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brewer MK et al. (2019) The 4th International Lafora Epilepsy Workshop: Shifting paradigms, paths to treatment, and hope for patients. Epilepsy Behav 90, 284–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brewer MK et al. (2019) Targeting pathogenic Lafora bodies in Lafora disease using an antibody-enzyme fusion. Cell Metabolism In Press [DOI] [PMC free article] [PubMed]
- 125.Austin GL et al. (2019) Central Nervous System Delivery and Biodistribution Analysis of an Antibody–Enzyme Fusion for the Treatment of Lafora Disease. Mol Pharm XXXX, XXX-XXX [DOI] [PMC free article] [PubMed]
- 126.Slavc I et al. (2018) Best practices for the use of intracerebroventricular drug delivery devices. Mol. Genet. Metab 124, 184–188 [DOI] [PubMed] [Google Scholar]
- 127.Cherukuri A et al. (2018) Immunogenicity to cerliponase alfa intracerebroventricular enzyme replacement therapy for CLN2 disease: Results from a Phase 1/2 study. Clin. Immunol 197, 68–76 [DOI] [PubMed] [Google Scholar]
- 128.Saper CB (2009) A Guide to the Perplexed on the Specificity of Antibodies. J. Histochem. Cytochem 57, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirase H et al. (2019) Glycogen distribution in mouse hippocampus. J. Neurosci. Res 00, 1–10 [DOI] [PubMed] [Google Scholar]
- 130.Kaplon H and Reichert JM (2019) Antibodies to watch in 2019. MAbs 11, 219–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wolak D et al. (2015) Probing the extracellular diffusion of antibodies in brain using in vivo integrative optical imaging and ex vivo fluorescence imaging. J Cont Rel 197, 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holliger P and Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat. Biotechnol 23, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 133.Puertollano R and Raben N (2018) Pompe disease: how to solve many problems with one solution. Ann. Transl. Med 6, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raben N et al. (2008) Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum. Mol. Genet 17, 3897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khanna R et al. (2012) The Pharmacological Chaperone AT2220 Increases Recombinant Human Acid a-Glucosidase Uptake and Glycogen Reduction in a Mouse Model of Pompe Disease. PLoS One 7, e40776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puzzo F et al. (2017) Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci. Transl. Med 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clayton NP et al. (2014) Antisense Oligonucleotide-mediated Suppression of Muscle Glycogen Synthase 1 Synthesis as an Approach for Substrate Reduction Therapy of Pompe Disease. Mol. Ther. Nucleic Acids 3, e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Serratosa JM et al. (1999) A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum. Mol. Genet 8, 345–52 [DOI] [PubMed] [Google Scholar]
- 139.Chan EM et al. (2003) Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat. Genet 35, 125–127 [DOI] [PubMed] [Google Scholar]
- 140.Romá-Mateo C et al. (2012) Deciphering the role of malin in the lafora progressive myoclonus epilepsy. IUBMB Life 64, 801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Augé E et al. (2018) Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia 66, 2094–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rubio-Villena C et al. (2018) Astrocytes: New players in progressive myoclonus epilepsy of Lafora type. Hum. Mol. Genet 27, 1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]