Abstract
Background.
Definitive surgical and radiation therapy (RT) treatments are evolving rapidly for stage I non-small cell lung cancer (NSCLC). We hypothesized that utilization of definitive therapies increased between 2000 and 2010 and that survival improved for stage I NSCLC patients over the same time period. Secondary objectives were determining trends in patterns of care and predictors of utilization.
Methods.
Population-based, observational, comparative effectiveness study used Surveillance, Epidemiology, and End Results-18 data from 2000 to 2010. The main outcome measure was 2-year risk of death for stage I NSCLC.
Results.
Between 2000 and 2010, 40,589 patients (62%) underwent surgery, 10,048 (15%) received RT, 2,130 (3%) received both surgery and RT, and 11,537 (18%) received neither surgery nor RT. Annually, the odds of receiving either definitive RT or undergoing surgery increased relative to the odds of receiving no treatment (odds ratio [OR] radiation 1.04, 95% confidence interval [CI]: 1.03 to 1.05; OR surgery 1.05, 95% CI: 1.04 to 1.05). Among surgical patients, the proportion of sublobar resections steadily increased from 12.9% to 17.9%. For all patients, the 2-year risk of death decreased by 3.5% each year (hazard ratio [HR] 0.965, 95% CI: 0.962 to 0.969), driven primarily by improved survival for surgical (annualized HR 0.959, 95% CI: 0.954 to 0.964) and RT (annualized HR 0.942, 95% CI: 0.935 to 0.949) patients.
Conclusions.
Between 2000 and 2010, stage I NSCLC patients were more likely to receive definitive treatment with either surgery or RT, leading to a decline in the number of untreated patients. Survival also improved substantially for stage I NSCLC patients, with the largest survival improvements observed in patients undergoing definitive RT.
Despite multiple treatment options, lung cancer remains the leading cause of cancer death in the United States, with an estimated 224,390 diagnoses and 158,080 deaths projected for 2016 [1]. Although roughly 25% of nonsmall cell lung cancer (NSCLC) diagnoses are stage I [2], the number of early-stage NSCLC diagnoses is projected to increase, driven by an aging “baby boomer” population [3] and increased low-dose chest computed tomography screening of high-risk individuals, in light of the mortality benefit shown by the National Lung Screening Trial [4] and the ensuing decision to reimburse for screening by the Centers for Medicare and Medicaid Services [5].
Current data continue to support lobar resection with mediastinal lymph node sampling as the primary curative option for stage I NSCLC [6]. Advances in medical physics and radiation therapy (RT) technology now permit the precise and accurate delivery of ablative doses of radiation, termed stereotactic body RT (SBRT). Currently, SBRT is the recommended treatment for patients medically unfit for surgical procedures [6]. Analogous to these improvements in RT, advances in surgical technology and techniques have enhanced the efficacy of surgical procedures [7, 8]. As examples, robotic-assisted resection has the potential to enhance surgical visibility and maneuverability, thoracoscopic surgery may reduce perioperative morbidity, and anatomic sublobar resection is thought to improve outcomes in select patients [7, 8]. These techniques have improved the risk–benefit ratio of surgery and have made it a more attractive option for both patients and their providers. Although randomized trials are currently under way, there is no category I evidence demonstrating a clear survival advantage for surgery or SBRT [9].
In light of this incomplete understanding, we examined the utilization and relative survival outcomes after surgery and radiation over recent years (2000 to 2010), using the large and population-based [10] Surveillance, Epidemiology, and End Results (SEER)-18 database to determine trends in the utilization and outcomes of definitive therapy for stage I NSCLC. We hypothesized that over time, the odds of receiving either definitive surgery (eg, surgery without radiation) or RT (eg, radiation without surgery) increased relative to the odds of receiving no treatment. We also hypothesized that the overall survival (OS) of patients treated for stage I NSCLC improved over time.
Patients and Methods
Patients
The National Cancer Institute SEER-18 database contains regional research data (1973 to 2011) submitted in November 2013 with the Katrina/Rita population adjustment, collected from 18 American cancer registries, chosen for their data quality and population diversity [10]. Patients coded with their first primary, stage I NSCLC over an 11-year period (2000 to 2010) were extracted for analysis. Pathologic stage was extracted for surgical patients, and clinical stage was used for radiation patients. Death certificate-only and autopsy-only cases were excluded. In addition to patient treatment information and survival outcomes, we also queried the SEER database for information on overall lung cancer incidence, mortality rate, and staging trends to better understand the context of our hypothesis testing.
Definitions
Site-specific surgery codes were used to create categorical variables for whether a cancer-directed operation was performed. Treatment categories included (1) surgery alone, (2) RT alone, (3) both RT and surgery, or (4) neither RT nor surgery. Chemotherapy information was not available in the SEER-18 data nor were RT details such as modality, dose, fractionation, or intent.
Data Analysis
Pearson χ2 tests evaluated the association between patient characteristics, histologic findings, year of diagnosis, and treatment type, and logistic regression was used to calculate unadjusted odds ratios (ORs) of a patient receiving one type of treatment, using no definitive treatment (no surgery or RT) as the reference. We used Kaplan-Meier analyses to generate survival plots, and 2-year OS estimates were generated with Kaplan-Meier analyses. Log-rank tests were then used to compare OS differences. Cox proportional hazards regression methods were used to calculate hazard ratios (HRs; 95% confidence interval [CI]) to estimate risk of overall death. Annual increment of risk was calculated, adjusting for age at diagnosis, sex, race, marital status, histologic findings, and treatment. Finally, a multivariable hazard model was constructed to estimate the risk of death according to each treatment type, adjusting for year, age, sex, race, histologic findings, and marital status.
SEER*Stat version 8.2.1 software was used in extracting data from the SEER database. Analyses were conducted using SAS software version 9.3 (SAS Institute Inc, Cary, NC).
Results
Staging Trends
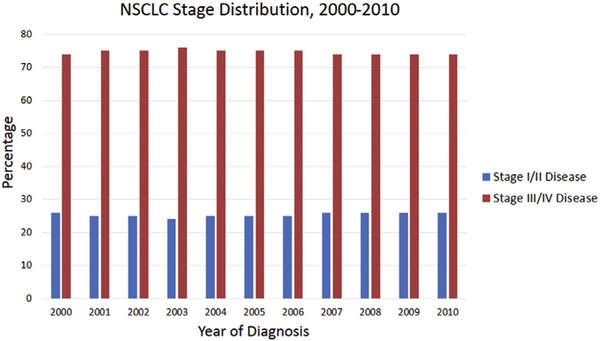
For NSCLC, in our data set, the odds of being diagnosed with early stage (I and II) disease increased slightly (by 0.4% each year), compared with the odds of being diagnosed with late stage (III and IV) disease (p < 0.001). Overall, this translated into a slight shift in staging from a 25% and 75% distribution of early- and late-stage disease at the beginning of our study, to 26% and 74% distribution toward the end of our study (Fig 1; Supplemental Table 1). Within stage I disease, there was a 2.5% annual increase in the odds of being staged with IA as opposed to IB disease (p < 0.001).
Fig 1.
Percent distribution of non-small cell lung cancer (NSCLC) staging as defined by the American Joint Committee on Cancer (AJCC) between the years 2000 and 2010. Histogram divided into early stage (I and II) disease in blue and late stage (III and IV) disease in red for ease of comparison.
Patient and Treatment Details and Trends
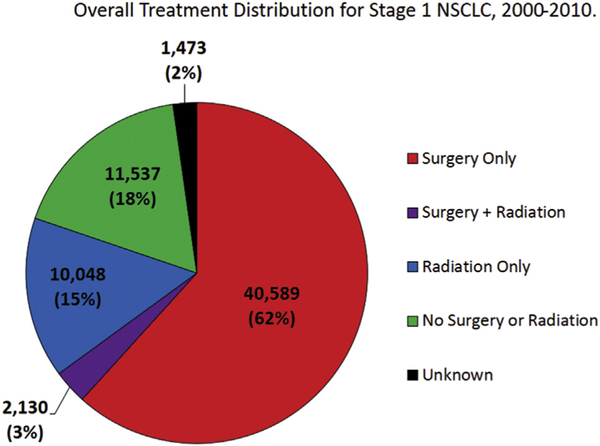
Between January 2000 and December 2010, 65,197 stage I NSCLC patients were registered in the SEER-18 database. Overall, 40,589 patients (62%) underwent surgery, 10,048 (15%) received RT, 2,130 (3%) received both surgery and RT, and the remaining 11,537 patients (18%) received neither definitive therapy. Treatment information for 1,473 patients (2%) was unknown (Fig 2). Among the examined variables, RT patients were more likely to be older, men, Black, and unmarried compared than surgical patients. In addition, patients with squamous cell carcinoma were more likely to receive RT either alone or in combination with surgery than surgery alone. (Table 1).
Fig 2.
Distribution of definitive therapy for stage I non-small cell lung cancer (NSCLC) between 2000 and 2010. The proportion of patients undergoing surgery during this time span are represented in red. Patients treated with combined surgery and radiation therapy (RT) are represented in purple. Patients treated with RT only are represented in blue. Patients receiving neither RT nor surgery are represented in green. Patients with unknown treatment details are represented in black.
Table 1.
Adjusted Odds Ratios of Stage I NSCLC Treatment Groups Compared With No Treatment
| Variable | Surgery Only (n = 42,242) |
Radiation Only (n = 10,048) |
Surgery and Radiation (n = 2,130) |
|---|---|---|---|
| Year of diagnosis | 1.045 (1.038–1.052) | 1.039 (1.030–1.047) | 0.987 (0.972–1.002) |
| Age at diagnosis | |||
| 40–49 y (reference) | 1.000 | 1.000 | 1.000 |
| 50–59 y | 0.816 (0.688–0.969) | 1.243 (0.967–1.598) | 0.595 (0.463–0.764) |
| 60–69 y | 0.614 (0.522–0.723) | 1.479 (1.165–1.878) | 0.386 (0.304–0.489) |
| 70–79 y | 0.363 (0.309–0.426) | 1.321 (1.044–1.673) | 0.182 (0.144–0.231) |
| ≥80 y | 0.119 (0.101–0.140) | 1.045 (0.825–1.325) | 0.048 (0.037–0.064) |
| Sex | |||
| Female (reference) | 1.000 | 1.000 | 1.000 |
| Male | 0.715 (0.683–0.749) | 0.862 (0.814–0.912) | 0.925 (0.839–1.021) |
| Race | |||
| White (reference) | 1.000 | 1.000 | 1.000 |
| Black | 0.479 (0.446–0.515) | 0.822 (0.755–0.895) | 0.620 (0.532–0.723) |
| Other | 0.906 (0.822–0.998) | 0.739 (0.651–0.839) | 0.670 (0.530–0.847) |
| Marital status | |||
| Married (reference) | 1.000 | 1.000 | 1.000 |
| Not married | 0.510 (0.487–0.534) | 0.753 (0.711–0.798) | 0.513 (0.464–0.566) |
| Histologic finding | |||
| Adeno (reference) | 1.000 | 1.000 | 1.000 |
| Squamous | 0.639 (0.601–0.679) | 1.384 (1.286–1.491) | 0.931 (0.829–1.046) |
| Large cell | 0.678 (0.597–0.771) | 1.230 (1.054–1.435) | 1.148 (0.919–1.434) |
| Others | 0.250 (0.236–0.264) | 0.624 (0.582–0.668) | 0.284 (0.251–0.322) |
Values are odds ratio (95% confidence interval).
Adeno = adenocarcinoma; NSCLC = non-small cell lung cancer.
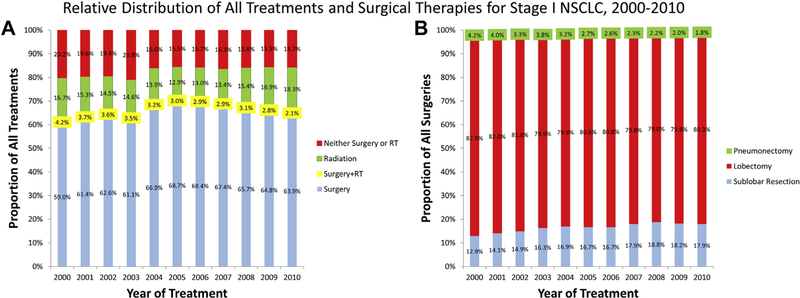
From 2000 to 2010, the proportion of untreated patients decreased from 20.2% to 15.3%. Over this time, the likelihood of a patient receiving definitive surgery increased relative to the likelihood of not being treated (OR 1.045, 95% CI: 1.038 to 1.052; Table 1). In 2000, 58.1% (n = 3523) of patients underwent surgery and this increased to 63.9% (n = 3913) in 2010 (relative proportions shown in Fig 3A; absolute counts numbers are shown in Supplemental Fig 1). Although the predominant surgical modality for these patients remained lobectomy, there was a steady increase in the proportion who underwent sublobar resections, from 12.9% in 2000 to 17.9% in 2010 (Fig 3B). Similarly, the likelihood of a patient receiving definitive RT also increased relative to the likelihood of not being treated (OR 1.038, 95% CI: 1.030 to 1.047; Table 1), with the proportion of RT courses increasing from 17.2% (n = 1043) in 2000 to 18.3% (n = 1121) in 2010. Use of both surgery and RT occurred infrequently (n = 2130), and the odds a patient received both treatments did not significantly change over time (OR 0.987, 95% CI: 0.973 to 1.002; Table 1).
Fig 3.
Annual proportions of (A) all treatments and (B) types of surgery used to treat Stage I non-small cell lung cancer (NSCLC) between 2000 and 2010. For A, types of treatments include surgery (blue), combined surgery and radiation therapy (RT) (yellow), radiation therapy (green), or no treatment (red). Types of surgery in B include pneumonectomy (green), lobectomy (red), or sublobar resection (blue).
Survival
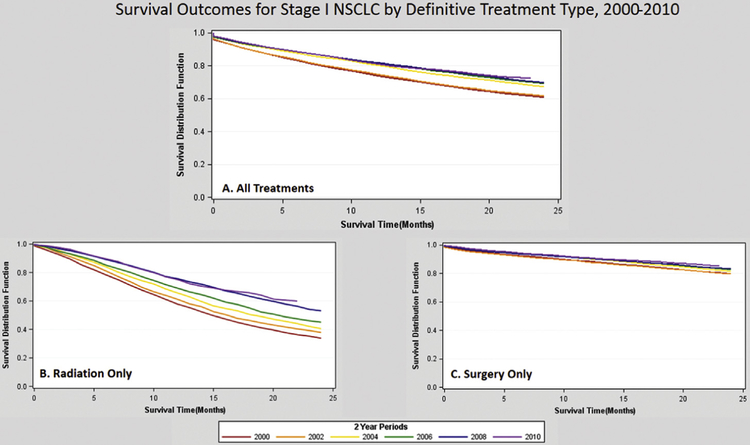
As a whole and regardless of treatment received, 2-year OS improved from 61% (95% CI: 59.4% to 61.8%) in 2000 to 70% (95% CI: 68.4% to 70.7%) in 2009 in patients diagnosed with stage I NSCLC. The increase in 2-year OS for all stage I NSCLC patients corresponded to a 3.5% annual decrease in the risk of death (HR 0.965, 95% CI: 0.962 to 0.969) regardless of treatment modality. Survival improvements in patients who either underwent surgery (HR 0.959, 95% CI: 0.954 to 0.964) or received RT (HR 0.942, 95% CI: 0.935 to 0.949) accounted for gains observed in this cohort, and the gains in RT were significantly greater than the gains in surgical procedures. Patients who did not undergo treatment (HR 0.992, 95% CI: 0.986 to 0.998) or underwent combined RT and surgery (HR 0.980, 95% CI: 0.962 to 0.997) did not experience a statistically significant annual change in survival (Table 2). Kaplan-Meier survival estimates stratified by diagnosis year are presented in 2-year increments from 2000 to 2010 in Figure 4 and Supplemental Figures 2 and 3.
Table 2.
Unadjusted and Adjusted Risk of Death for Stage I NSCLC (N = 65,957)
| Variable | No. | Unadjusted Risk HR (95% CI) |
Adjusted Riska HR (95% CI) |
Adjusted With Interactionb HR (95% CI) |
|---|---|---|---|---|
| Yearly increment | … | 0.962 (0.958–0.965) | 0.965 (0.962–0.969) | NA |
| Age at diagnosisc | … | 1.043 (1.042–1.044) | 1.028 (1.027–1.029) | 1.028 (1.027–1.029) |
| Sex | ||||
| Female (reference) | 32,833 | 1.000 | 1.000 | 1.000 |
| Male | 33,124 | 1.334 (1.307–1.361) | 1.378 (1.348–1.407) | 1.378 (1.348–1.407) |
| Race | ||||
| White (reference) | 56,240 | 1.000 | 1.000 | 1.000 |
| Black | 6,108 | 1.168 (1.129–1.208) | 1.045 (1.010–1.082) | 1.043 (1.008–1.080) |
| Other | 3,609 | 0.794 (0.757–0.834) | 0.799 (0.761–0.839) | 0.800 (0.762–0.840) |
| Marital status | ||||
| Married (reference) | 35,550 | 1.000 | 1.000 | 1.000 |
| Not married | 30,407 | 1.326 (1.300–1.353) | 1.181 (1.156–1.207) | 1.182 (1.157–1.208) |
| Histologic finding | ||||
| Adeno (reference) | 25,674 | 1.000 | 1.000 | 1.000 |
| Squamous | 18,589 | 1.581 (1.542–1.622) | 1.279 (1.247–1.312) | 1.278 (1.246–1.312) |
| Large cell | 2,490 | 1.510 (1.434–1.590) | 1.303 (1.237–1.373) | 1.299 (1.233–1.369) |
| Others | 19,204 | 1.509 (1.472–1.548) | 1.052 (1.026–1.080) | 1.049 (1.022–1.077) |
| Treatment | ||||
| No surgery or radiation (reference) | 11,537 | 1.000 | 1.000 | NA |
| Surgery only | 42,242 | 0.195 (0.190–0.200) | 0.230 (0.224–0.236) | NA |
| Radiation only | 10,048 | 0.690 (0.670–0.711) | 0.680 (0.660–0.700) | NA |
| Surgery and radiation | 2,130 | 0.364 (0.344–0.385) | 0.429 (0.406–0.455) | NA |
| Yearly increment by treatment group | ||||
| No surgery or radiation | … | NA | NA | 0.992 (0.986–0.998) |
| Surgery only | … | NA | NA | 0.959 (0.954–0.964) |
| Radiation only | … | NA | NA | 0.942 (0.935–0.949) |
| Surgery and radiation | … | NA | NA | 0.980 (0.962–0.997) |
Refers to estimated HRs generated in multiple variable model that included year, age, sex, race, marital status, histologic finding, and treatment.
Refers to the aforementioned adjustments in addition to an adjustment for the interaction between year and treatment, which yields an annualized overall survival with the prior year as the reference.
Median 70 years, range: 40 to 105 years.
Adeno = adenocarcinoma; CI = confidence interval; HR = hazard ratio; NA = not applicable; NSCLC = non-small cell lung cancer.
Fig 4.
Survival outcomes for stage I non-small cell lung cancer (NSCLC) by definitive treatment type, 2000 to 2010. Kaplan-Meier survival curves, in months, for the years 2000 (red), 2002 (orange), 2004 (yellow), 2006 (green), 2008 (blue), and 2010 (purple) in (A) all patients, (B) patients receiving radiation therapy only, and (C) patients receiving surgery only.
Crude predictors for decreased survival included older age, male sex, black race, unmarried status, and non-adenocarcinoma histologic finding. On multiple variable adjustment, statistically significant effects on survival were maintained for all variables, although the effect sizes diminished (Table 2). These covariates were then included when modeling the survival effects of the various treatments, using non-treatment as the baseline comparator. Adjusting for age, sex, race, marital status, and histologic findings, RT patients appeared one-third less likely (HR 0.680, 95% CI: 0.660 to 0.700) to die as patients who did not receive treatment, and surgical patients were three-quarters less likely to die (HR 0.230, 95% CI: 0.224 to 0.236). Patients who underwent surgery and received radiation were approximately one-half (HR 0.429, 95% CI: 0.406 to 0.455) as likely to die as untreated patients (Table 2).
Comment
Given rapid and recent advancements in both surgical and radiation techniques, we sought to characterize temporal changes in treatment patterns and survival outcomes for stage I NSCLC. Recent reports have assessed such changes in treatment and outcomes for stage I lung cancer, but they used arbitrary cutoffs-offs in time periods [11, 12]. Our conceptual model of time classified it as a continuous rather than categorical variable, permitting a more granular understanding of how time has affected treatment and outcomes.
From 2000 to 2010, the overall likelihood of survival increased with each passing year regardless of treatment modality, and these improvements were statistically limited to patients who either underwent surgery or received radiation (but not both). Fewer patients were left untreated over time, due to distinct increases in the utilization of both surgery and RT. We anticipated that more patients would be treated with curative intent over time, akin to a trend noted among elderly British [13] and Dutch [12] patients with early stage NSCLC. Indeed, the proportion of patients treated definitively with either surgery or RT increased from 2000 to 2010, likely due to less-invasive surgeries and more-advanced techniques for delivering RT. In terms of RT specifically, temporal evidence from claims [14], survey [15, 16], and institutional data [17–19] suggest that the 2004 debut of SBRT [20] resulted in increased utilization of RT among medically inoperable patients, which may explain the increased likelihood that a patient would receive RT over time. However, we were surprised to find that the magnitude of increase in the likelihood of receiving RT was no different from the increase in the likelihood of undergoing surgery, even despite increasing enthusiasm for SBRT [16] and evidence suggesting its rapid adoption both domestically [21] and internationally [13].
Because we sought to explain the reasons for similar increases in reliance on RT and surgery, it was clear that increasing enthusiasm for sublobar resections led to a decreased relative proportion of lobectomies. Although the increased use of surgery can only be partially explained by increased use in sublobar resection, this trend emerged even as prospective randomized clinical trials [22] and propensity-score matched analyses [23] suggest possibly worse survival for sublobar resection compared with lobectomy. It is possible, although not shown directly by these data, that patients unfit for lobectomy received sublobar resections rather than referrals for RT. Randomized trials comparing sublobar resections with SBRT will be especially important if the trend toward increased use of these less-invasive surgeries continues.
Between 2000 and 2010, radiation patients demonstrated the largest temporal improvement in survival. This difference was notable even when compared with their surgical counterparts, affirming other evidence that advances in radiation technology [15, 24, 25] apply equally to early [26] and advanced-stage [27] patients. SBRT, in specific, permits dose escalation with improved sparing of normal tissue [28–31], resulting in improved local control and survival compared with older techniques of radiation therapy [32, 33]. Although it is possible that the improvements we observe relate exclusively to a temporal increase in healthier patients with fewer comorbidities receiving radiation treatment in later years, we believe that the alternative possibility that there is a true causal impact of improved radiotherapeutic treatment in this setting merits further investigation. Without prospective randomized trials to determine whether a survival advantage exists for surgery or RT in a more generalized patient cohort, SBRT must remain the recommended therapy for elderly or medically inoperable patients [23]. However, it is our hope that drawing attention to the rapid improvements in survival for radiation patients will not only encourage appropriate referrals of elderly and inoperable early-stage NSCLC patients but also focus attention on accrual of operable patients to prospective trials comparing surgery with SBRT [34].
Survival analyses such as ours are commonly used to measure progress in the treatment of malignancy and to determine whether new treatments are efficacious. However, this approach bears several well-known caveats. For example, earlier detection or more accurate characterization of malignancy by improved staging techniques (including positron emission tomography scans) can lead to stage migration, or the so-called “Will-Rogers” phenomenon [35], whereby aggressive diagnoses are “diluted” by more clinically indolent versions of the same disease although earlier stage disease is “purified.” A relative decrease in the proportion of early-stage disease and increase in advanced disease can result in observations of improved survival for all patients without actually altering the course of disease. To address this concern, we examined the stage distribution of stage I NSCLC in our cohort and were reassured to note a relatively constant temporal stage distribution, and, if anything, increases in the proportion of early-stage disease.
Secular trends in systemic therapy, supportive care, or palliative care could also explain the improved survival for patients over time [36]. However, our results demonstrate that survival did not improve appreciably in the cohort of untreated patients, nor did it change for patients who underwent surgery and received RT. Thus, improvements in supportive care can only partially explain OS improvements in the definitively treated cohort, because such improvements would have translated to uniform benefits for patients in all treatment categories. In the surgical patients specifically, decreases in perioperative death could have partially driven improved survival outcomes in this cohort, and in subsequent studies it may be worthwhile to exclude patients who died within 90 days of their operation.
Survival gains in the context of increased cancer incidence (due to increased cancer screening) and an unchanged cancer mortality rate would also suggest that overdiagnosis rather than improved therapy explains survival improvements [37]. For all stages, we found that although mortality rates remained stable, incidence actually decreased [38], which we internally verified with our own analysis (Supplemental Fig 4). This finding at least partially assuages the concern that improvements in OS arose from better detection and staging.
We attempted to minimize hidden biases and the numerous changing variables which can affect the outcomes of such population-based studies by limiting our analysis to a relatively short and recent timeframe. However, some limitations merit comment. Reporting a 2-year OS outcome shortens our patient follow-up in comparison with a more traditional 5-year metric, but it also permits a more immediate understanding of survival trends in a disease that we demonstrate can cause death in <2 years. Furthermore, the most recent SEER data do not track out to 5 years, and so we opted to use 2-year survival as a “surrogate” end point until more long-term data become available. Second, as discussed above, the optimal assessment of anticancer therapy is based on cancer-specific deaths, not survival data [37]. Unfortunately, data about stage-specific deaths are not available in the SEER database, but we are partially reassured by incidence, staging, and mortality findings discussed above. In addition, SEER data contain little information about the specifics of staging, pathologic process, patient comorbidity, treatment intent, or detail [39]. Therefore, we could not examine the use or efficacy of various RT modalities (such as SBRT) or have confidence that all RT patients were treated definitively. Medicare-linked data provide more detail [40], although it is costly to acquire and excludes younger (and healthier) patients, which would further bias our results. Finally, we acknowledge that improved survival of SEER patients cannot be credited solely to use of more-advanced surgery or radiation and that secular trends in supportive care, patient selection, and patient health awareness may partially explain the improved outcomes.
To conclude, utilization of both surgery and RT for stage I NSCLC has increased recently, leaving fewer patients untreated for ostensibly curable disease. Our findings suggest that over time the efficacy of these treatments has improved, although there is some variation with which they are applied. Resource-intensive treatments for cancer must always be used judiciously, and efforts to ensure their appropriate and consistent application among all patients with curable disease should be paramount. Further study is needed to confirm and improve understanding of the factors that lead to treatment selection, modality selection, and the observed improvements in survival.
Supplementary Material
Acknowledgments
This work was funded by a research grant from the Woodworth family. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Presented at the American Society for Clinical Oncology Multidisciplinary Thoracic Symposium, Chicago, IL, Jun 1–5, 2012.
The Supplemental Table and Figures can be viewed in the online version of this article (http://dx.doi.org/10.1016/j.athoracsur.2017.06.065) on http://www.annalsthoracicsurgery.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29–33. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65. [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team,Aberle DR,Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrek Jensen T, Chin J, Ashby L, Hermansen J, Dolph Hutter J. CMS decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Centers for Medicare and Medicaid Services; Available at https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Updated 2015. Accessed October 15, 2015. [Google Scholar]
- 6.Ettinger D, Akerly W, Borghael H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236–71. [DOI] [PubMed] [Google Scholar]
- 7.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2005;82:408–15; discussion 415–6. [DOI] [PubMed] [Google Scholar]
- 8.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691–8; discussion 698–700. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603–11. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3–18. [DOI] [PubMed] [Google Scholar]
- 11.Haque W, Szeja S, Tann A, Kalra S, Teh BS. Changes in treatment patterns and overall survival in patients with early-stage non-small cell lung cancer in the United States after the incorporation of stereotactic ablative radiation therapy: a population-based analysis. Am J Clin Oncol 2016. January 14; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Palma D, Visser O, Lagerwaard F, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non–small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153–9. [DOI] [PubMed] [Google Scholar]
- 13.Riaz S, Linklater K, Page R, Peake M, Moller H, Lüchtenborg M. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax 2012;67: 811–4. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services. Part B physician/supplier national data top 200 level 1 current terminology (HCPCS/CPT) codes 2006–2011. Available at http://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/MedicareFeeforSvcPartsAB/MedicareUtilizationforPartB.html, ed 2006–2011. Accessed January 5, 2016.
- 15.Movsas B, Moughan J, Komaki R, et al. Radiotherapy patterns of care study in lung carcinoma. J Clin Oncol 2003;21:4553–9. [DOI] [PubMed] [Google Scholar]
- 16.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer 2011;117:4566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6. [DOI] [PubMed] [Google Scholar]
- 19.Grills I, Mangona V, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928–35. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute/Radiation Therapy Oncology Group. Stereotactic body radiation therapy in treating patients with inoperable Stage I or Stage II non-small cell lung cancer. Available at https://clinicaltrials.gov/ct2/show/record/NCT00087438. Accessed September 28, 2017.
- 21.Yu JB, Soulos PR, Cramer LD, Decker RH, Kim AW, Gross CP. Diffusion of stereotactic body radiotherapy (SBRT) for early-stage non-small cell lung cancer (NSCLC) in the Medicare population, 2007–2009. J Clin Oncol 2014;32(Suppl): 7575 (abstract). [Google Scholar]
- 22.Ginsberg R, Rubinstein L. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615–22. [DOI] [PubMed] [Google Scholar]
- 23.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia NS, Mamet R, Zornosa C, Niland JC, D’Amico TA, Hayman JA. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer 2012;118:4339–45. [DOI] [PubMed] [Google Scholar]
- 25.Guadagnolo BA, Huo J, Liao KP, Buchholz TA, Das P. Changing trends in radiation therapy technologies in the last year of life for patients diagnosed with metastatic cancer in the United States. Cancer 2012;119:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang LC, Komaki R, Allen P, Guerrero T, Mohan R, Cox JD. Comparison of outcomes for patients with medically inoperable stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:108–16. [DOI] [PubMed] [Google Scholar]
- 27.Chen AB, Neville BA, Sher DJ, Chen K, Schrag D. Survival outcomes after radiation therapy for stage III non-small-cell lung cancer after adoption of computed tomography-based simulation. J Clin Oncol 2011;29:2305–11. [DOI] [PubMed] [Google Scholar]
- 28.Emami B, Purdy JA, Manolis J, et al. Three-dimensional treatment planning for lung cancer. Int J Radiat Oncol Biol Phys 1991;21:217–27. [DOI] [PubMed] [Google Scholar]
- 29.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2002;53:1337–49. [DOI] [PubMed] [Google Scholar]
- 30.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999;44:911–9. [DOI] [PubMed] [Google Scholar]
- 31.Valle LF, Jagsi R, Bobiak SN, et al. Variation in definitive therapy for localized non-small cell lung cancer among National Comprehensive Cancer Network institutions. Int J Radiat Oncol Biol Phys 2016;94:360–7. [DOI] [PubMed] [Google Scholar]
- 32.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94–100. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305–16. [DOI] [PubMed] [Google Scholar]
- 34.University of Texas Southwestern Medical Center. JoLT-ca sublobar resection (SR) versus stereotactic ablative radiotherapy (SAbR) for lung cancer (STABLE-MATES). Available at http://clinicaltrials.gov. NLM identifier: . Accessed November 18, 2015.
- 35.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604–8. [DOI] [PubMed] [Google Scholar]
- 36.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–42. [DOI] [PubMed] [Google Scholar]
- 37.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA 2000;283:2975–8. [DOI] [PubMed] [Google Scholar]
- 38.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer 2014;120:2883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology 2009;23:288–95. [PubMed] [Google Scholar]
- 40.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.