Abstract
Microencapsulation of islets is a procedure used to immunoisolate islets in order to obviate the need for immunosuppression of islet transplant recipients. Although microencapsulated islets have routinely been transplanted in the peritoneal cavity, the ideal site for their engraftment remains to be determined. The omentum, a highly vascularized tissue, has been proposed as an alternative site for microencapsulated islet transplantation. An added benefit to the omentum is that implanted microcapsules can be easily retrieved for post-transplant evaluation. This chapter describes a collagenase-based procedure for the retrieval of microencapsulated islets following the harvest of omentum pouch site of transplantation.
Keywords: Alginate, Microencapsulation, Islets, Transplantation, Omentum
1. Introduction
The advantages of cell replacement therapy as a treatment option for Type 1 diabetes compared to insulin treatment particularly in controlling diabetic complications are well established [1–3]. The two major β-cell therapy approaches currently in clinical application are pancreas and islet transplantations, albeit islet transplantation remains an experimental procedure [4], whose potential as a treatment option for Type 1 diabetic patients became apparent with the introduction of the Edmonton protocol [5]. However, there is a long-standing interest in islet transplantation because it is a simpler surgical procedure that easily lends itself to routine clinical application, hence the continued development of different strategies to improve the outcomes of islet transplantation [6]. Still, the limited supply of human pancreas and the need for risky immunosuppressive drugs to prevent transplant rejection remain two major barriers to routine clinical islet transplantation. The procedure to immunoisolate islets by encapsulation prior to transplantation emerged as a strategy to overcome these two barriers [3, 7–9]. In turn, the clinical application of the encapsulation technology has been slow because of a variety of issues that remain unresolved, as has been recently reviewed [3, 9].
Two general approaches to encapsulation have been examined, microencapsulation which involves the enclosure of one or two islets in a microcapsule measuring <1 mm in diameter [10] and macroencapsulation that entails the enclosure of large numbers of islets in one chamber often with sizes measuring up to several centimeters [11, 12]. Although several biopolymers, including agarose, polyethylene glycol (PEG) and amalgams of PEG with alginate have been examined for cell encapsulation, alginate has been the most investigated of these biomaterials for this purpose [7–10, 13–18] primarily because of its biocompatibility and physicochemical characteristics [19], which have advanced its use to the level of clinical trials [20–24].
One of the critical factors that remains to be resolved in encapsulated islet treatment is determination of the optimal site of transplantation. Encapsulated islets have been routinely transplanted in the peritoneal cavity, which lacks a vascular supply resulting in the need to use large quantities of microencapsulated islets to achieve variable results [20–27]. In attempts to transplant these encapsulated islets closer to the blood supply alternative sites such as the kidney capsule and the omentum have been examined [28, 29]. In addition, these alternative sites are attractive because of the retrievability of the tissue site of implant. However, there is still a scarcity of validated procedures to retrieve the encapsulated islets from a resected implant site for detailed post-transplant analyses. The purpose of the present study was therefore to develop a procedure for retrieval of intact encapsulated islets after a prolonged period of transplantation in the omentum of diabetic animals.
To develop this procedure, rat islets were isolated using standard collagenase digestion techniques [30, 31] then encapsulated into alginate-polyornithine-alginate (APA) microcapsules [32, 33] and were then implanted into STZ-induced diabetic Lewis rats [34]. The animals were maintained for 90 days and then the procedure described below was used to retrieve and evaluate the encapsulated islets.
In this study, we have shown that islets encapsulated in the perm-selective APA microcapsules can be transplanted in the omentum where they remain viable over a long time even in the face of chronic hyperglycemia, consistent with previous observations in mice transplanted with islets encapsulated in agarose microcapsules by Kobayashi et al. [29]. In the study by Kobayashi et al., extensive histological assessment was performed on the encapsulated islets in-situ in the pouch; however, retrieval of the encapsulated islets from the pouch was not performed. In the present study, we have designed a reliable procedure for successful isolation of encapsulated islets after long-term implantation in the omentum pouch. The retrieved islets were shown to be viable as assessed by histological techniques as well as by their appropriate responsiveness to changes in glucose concentrations in the dynamic perifusion system.
Although the present study was performed in an isograft model, we have recently reported that encapsulated allografts have long-term function in immunocompetent diabetic rats, thus showing that the omentum pouch is a viable alternative site to the peritoneum [35]. An inability to retrieve encapsulated islet grafts after extended periods of transplantation is a significant drawback of the use of the peritoneal cavity as a site of encapsulated islet transplantation. Another major obstacle to clinical application of the microencapsulated islet technology is the death of large proportions of encapsulated islet grafts owing to prolonged hypoxia after transplantation in the avascular peritoneal cavity, resulting in the need to use large quantities of islets to achieve normoglycemia in experimental diabetic animals. Although islets constitute approximately 1 % of the pancreas, they receive about 6–10 % of the blood flow to this gland [36, 37], indicating a disproportionate level of perfusion in which islets receive and consume lots of oxygen. The usual high oxygen requirement of islets is interrupted during the period preceding isolated islet transplant revascularization, and studies have shown that hypoxia has significant deleterious effects on the survival and function of islets [38–40]. In the immediate post-transplant period, isolated islet transplants are forced to depend upon diffusion of oxygen and nutrients through peripheral perfusion from the surrounding tissue within the site of transplantation [39], until the islet transplants are revascularized by angiogenesis, a process that requires 7–10 days [41]. However, when isolated islets are microencapsulated and transplanted in the avascular peritoneal cavity, no revascularization takes place, thus subjecting the islet grafts to extended periods of hypoxia and eventual death.
It has also been shown that the function of encapsulated islet grafts in the peritoneal cavity is limited even when inflammatory responses against purified alginate microcapsules amount to less than 10 %. The graft dysfunction is accompanied by a gradual decrease in glucose-stimulated insulin secretion, and gradual cell necrosis, which appears to be triggered by inadequate nutrition [42]. To address the problem of inadequate supply of nutrients and oxygen to encapsulated islets transplants, a variety of approaches are being assessed by different investigators. In a recent study, investigators examined the effect of a combination of growth hormone releasing (GHRH) agonist and controlled oxygen supply on the function of a bioartificial macrochamber. In this study islets were encapsulated and maintained within an alginate slab configuration adjacent to an oxygen-permeable membrane to create an immune barrier and allow for oxygenation of the islet graft. The minimally invasive implantable chamber was shown to normalize blood glucose in streptozotocin-induced diabetic rodents for up to 3 months after subcutaneous transplantation [43]. In another study, investigators showed that encapsulation of solid calcium peroxide within hydrophobic polydimethylsiloxane resulted in sustained oxygen generation that lasted for more than 6 weeks and was enough to prevent hypoxia-induced cell dysfunction and death in insulin-producing cells [44].
We conclude from the present study that the omentum represents a viable alternative site of transplantation to the peritoneal cavity not only because it is a highly vascularized tissue loaded with angiogenic factors [45] to promote revascularization and enhance the delivery of oxygen and nutrients to the islet grafts, but also because of the technical ease in which encapsulated islets can be removed for post-transplant analyses, as shown in our study.
2. Materials
2.1. Materials for Islet Microencapsulation
1.5 % Alginate (Pronova UP LVM and UP LVG, Novamatrix, Sandvika Norway).
0.1 % Poly-l-ornithine (PLO) (P5061, Sigma-Aldrich).
100 mM CaCl2 solution (C614–10, Fischer Scientific, Waltham, MA, USA).
55 mM Sodium citrate solution (S467–3, Fischer Scientific).
0.9 % Sodium chloride solution (normal saline) (71376–5KG, Sigma-Aldrich).
2.2. Materials for Microcapsule Implantation
General Surgery Basic Kit (World Precision Instruments, Sarasota, FL, USA).
50 mL Conical tubes.
10 % Betadine solution.
70 % Ethanol.
Sterile gauze.
0.9 % Sodium chloride solution (injectable saline).
4–0 Vicryl sutures.
2.3. Materials for Omentum Retrieval
General Surgery Basic Kit (World Precision Instruments, Sarasota, FL, USA).
50 mL Conical tubes.
10 % Betadine solution.
70 % Ethanol.
Sterile gauze.
Collagenase digestion solution: 1 mg/mL Collagenase P from Clostridium histolyticum (Roche, Indianapolis, IN, USA). Add 25 mg of collagenase to 25 mL Hanks’ Balanced Salt Solution (HBSS) (Mediatech Inc., Manassas, VA, USA). Sterile filter and store at −20 °C and bring to 4 °C prior to use.
Wash solution: 10 % Fetal bovine serum (Gibco, Waltham, MA, USA) with 50 U/mL penicillin and 50 μg/mL of streptomycin in HBSS. Add 50 mL FBS and 5 mL Penn/Strep stock to 445 mL of HBSS, adjust pH to 7.4, sterile filter and store at 4 °C.
Islet culture media: 10 % Fetal bovine serum (Gibco, Waltham, MA, USA), 50 U/mL penicillin and 50 μg/mL of streptomycin, 3.3 mM glucose, 2 g/L sodium bicarbonate, 1 mM HEPES in RPMI 1640 (Sigma). To a bottle of dry RPMI powder add 595 mg glucose, 2 g sodium bicarbonate, 238 mg HEPES, then add 100 mL FBS, 10 mL Penn/Strep stock and 890 mL ultrapure water. Adjust pH to 7.4 with either NaOH or HCL as needed, sterile filter and store at 4 °C (see Note 1).
2.4. Live/Dead Staining Reagents
1 mg/mL Propidium iodide working solution: To 20 mL HBSS add 20 mg propidium iodide (PI) (Sigma). Protect from light and store at 4 °C.
Vybrant CFDA SE Cell Tracer Kit (CFDA) (Invitrogen, Waltham, MA, USA; Cat. No. V12883).
HBSS.
4 % Paraformaldehyde (PFA) Solution: Add 1 mL 16 % PFA (Fisher) to 3 mL of phosphate-buffered Saline (Sigma). Store at 4 °C.
Olympus IX71 inverted microscope equipped with standard filters.
10 × 35 mm petri dish with 2 mm grid marks.
2.5. Dithizone Staining Reagents
10 mL Syringe.
Sterile syringe filter (pore size 0.45 μm) (Fisher).
Hanks’ Balanced Salt Solution (HBSS).
5 mL Dimethyl sulfoxide (DMSO) (Sigma).
50 mg Dithizone (Diphenylthiocarbazone) (Sigma).
10 × 35 mm Petri dish with 2 mm grid marks.
2.6. Dynamic Perifusion Reagents
Perifusion solution A: Add 70 g NaCl, 3.76 g KCl, and 21 g NaHCO3 to 1 L ultrapure water and store at 4 °C.
- Perifusion solution B:
- Prepare a 1 M solution of MgCl2 by adding 4.76 g MgCl2 to 50 mL ultrapure water.
- Prepare a 1 M solution of CaCl2 2H2O by adding 7.35 g of CaCl2 2H2O to 50 mL ultrapure water.
- Prepare perifusion solution B by adding 25.6 mL of the 1 M CaCl2·2H2O solution and 11 mL of the 1 M MgCl2 solution to 963.4 mL of ultrapure water and store at 4 °C.
Glucose (Sigma).
Bovine serum albumin (BSA) (Sigma).
10 × 35 mm Petri dish with 2 mm grid marks.
Peristaltic Pump.
Silicone tubing.
Perifusion chambers.
Water bath.
Collection tubes.
3. Methods
3.1. Islet Microencapsulation
Below is a summary of the encapsulation and implantation process; a more detailed description of the process has been described in Opara et al. [10].
Suspend islets in sodium alginate.
Using microfluidic device generate microspheres and allow spheres to cross-link in CaCl2 for 15 min.
Wash with normal saline, and incubate microspheres in 0.1 % PLO for 30 min.
Liquefaction of the alginate core of the microcapsules is achieved by a brief (2 min) incubation in 55 mM sodium citrate solution.
Wash with normal saline.
Coat outer layer of capsule with 1.25 % LVM for 5 min.
Separate excess alginate from microcapsules and cross-link outer layer.
Place microcapsules in 50 mL conical on ice until transplantation.
3.2. Microcapsule Implantation
After animal is appropriately anesthetized, shave and prep the dorsal area of the rodent with 10 % betadine and ethanol.
Create a small midline incision over the greater omentum.
Place sterile 2 × 2 cm sterile gauze around the midline incision and moisten with saline.
Identify and place the greater omentum on the gauze.
Orient the omentum flat along the gauze and moisten with saline.
Using 4–0 vicryl sutures create a purse string suture along the periphery of the omentum.
Place the microcapsules in the center of the omentum and gently tighten the sutures to close the omentum around the capsules.
Secure the purse string pouch and place in the peritoneal cavity.
Close the midline incision.
3.3. Retrieval of Omentum Pouch and Isolation of Microcapsules
Immediately following euthanasia (see Note 2) shave and disinfect the abdomen with 10 % betadine and then with 70 % ethanol.
Make a small midline incision and expose the greater omentum onto sterile gauze.
Identity the omentum pouch (as seen in Fig. 1a) and resect.
Place the resected omentum pouch (as seen in Fig. 1b, c) into a 50 mL conical tube with 25 mL of the Collagenase digestion solution (see Note 3).
- Place the 50 mL conical tube in a shaking water bath for 20 min at 37 °C.
- Every 5 min during the digestion invert the conical tube.
- Refer to Fig. 2 for a visual representation of the omental pouch over the 20 min digestion period.
After the 20-min digestion period move the 50 mL conical tube into a biosafety cabinet and add 10 mL of the cold wash solution.
Shake the tube gently for 30 s.
Place the tube on ice and allow the capsules to settle to the bottom of the conical tube for approximately 2 min.
Aspirate the digestion solution and added wash solution.
Add 50 mL wash solution.
Again allow the capsules to resettle, aspirate the wash solution and repeat the final wash step. At this point the capsules should be free of omental tissue as seen in Fig. 2c.
Culture the retrieved capsules in the islet culture media and culture under standard conditions until islet viability and functionality testing (see Note 4).
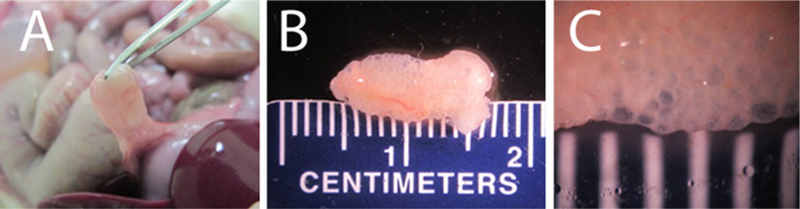
Fig. 1.
Omentum pouch retrieved after 90 days in vivo (a) Omentum immediately before surgical removal of omentum pouch with the microcapsules. (b) Omentum pouch immediately following surgical removal. (c) Omentum pouch with microcapsules magnified, spacing between the white lines is 1 cm
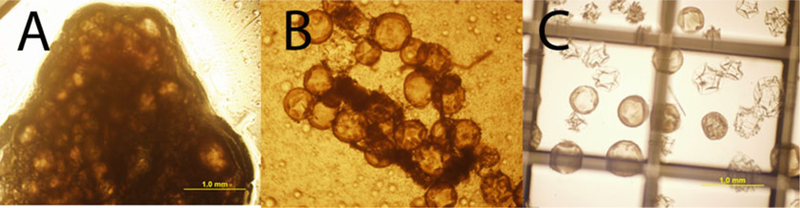
Fig. 2.
Digestion of omentum pouch. (c) Omentum pouch and microcapsules prior to collagenase P digestion. (b) Dispersed omental tissue and microcapsules following 10 min of digestion at 37 °C. (c) Free microcapsules after 20-min digestion and wash in HBSS with 10 % FBS
3.4. Live/Dead Staining of Retrieved Islets
- Prepare the CFDA working solution.
- Bring components A and B of the Vybrant CFDA SE Cell Tracer Kit to room temperature.
- Dissolve the contents of one vial (component A) in 90 μL of the DMSO provided within the kit (Component B).
- Wrap the vial in aluminum foil and initial and date, the stock solution is to be stored at −20 °C, and is good for up to 6 months.
Add 2.5 μL of CFDA stock solution to 1 mL of HBSS.
Vortex to mix.
Collect approximately 50–100 retrieved encapsulated islets.
Pipette supernatant and discard.
Add 200 μL of CFDA working solution.
Resuspend capsules and incubate for 15 min at 37 °C.
Following 15 min, pipette off CFDA working solution and discard.
Add 200 μL of wash solution and incubate for 30 min at 37 °C.
Pipette supernatant and discard.
Add 200 μL of PI working solution.
Incubate for 2 min at room temperature.
Remove PI solution and wash three times with HBSS.
Add 200 μL of 4 % PFA working solution.
Incubate for 10 min on ice.
Remove PFA solution and wash three times with HBSS.
Transfer retrieved islets to a 10 × 35 mm petri dish with 2 mm grid marks.
Examine at least 50 islets under the fluorescent microscope (islets should appear as shown in Fig. 3a–d).
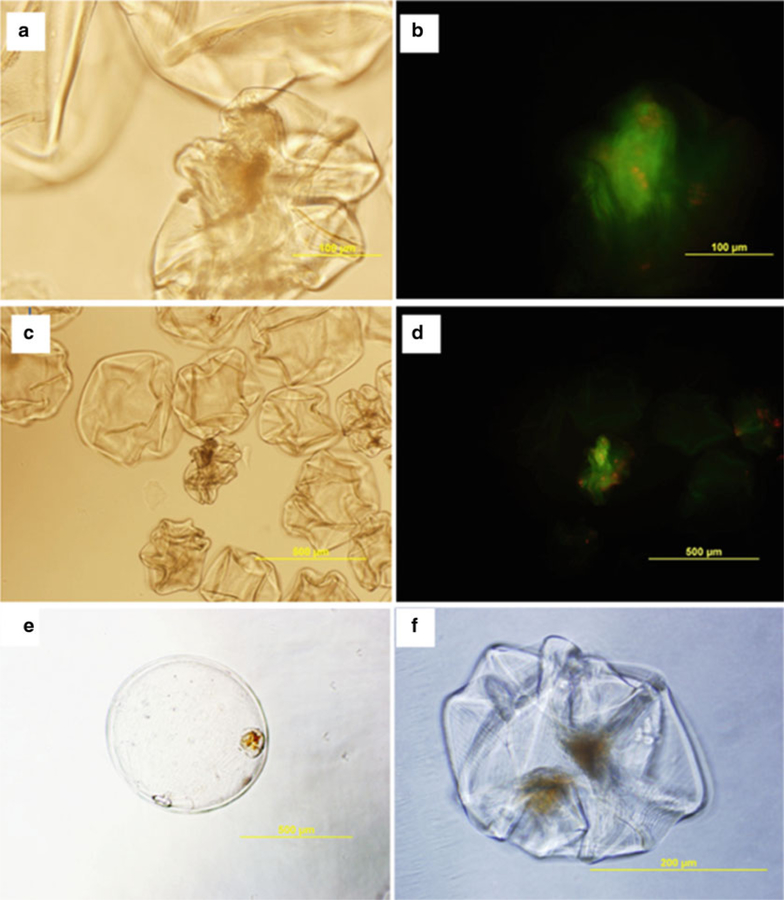
Fig. 3.
Live/dead and dithizone staining of retrieved encapsulated islets. While a portion of retrieved microcapsules retained their spherical shape over time, throughout the isolation process the majority of retrieved capsules collapsed as shown above. However as indicated by the live/dead staining majority of the encapsulated islets were viable. (a) Phase contrast image of encapsulated islets (10× objective) (b) 10× objective of image of encapsulated islets stained with CFDA (green) and Propidium Iodide (red). (c) Phase contrast image of encapsulated islets (4× objective) (d) Encapsulated islets stained with CFDA (green) and Propidium Iodide (red) (4× objective). Additionally, after retrieving islets from the omentum islets were stained with a 2 mg/mL Dithizone solution in HBSS and 25 % DMSO for 2 min, and washed in PBS buffer prior to imaging. (e) 4× objective (f) 10× objective
3.5. Dithizone Staining of Retrieved Islets
- Prepare dithizone working solution.
- Add 50 mg of DTZ to a to 10 mL of DMSO and protect from light.
- Mix on stir plate for 15 min.
- Add 50 mL HBSS.
- Mix on stir plate for 15 min.
- Prior to use, filter solution through a syringe filter (0.45 μm) into a new glass bottle.
Place sample of retrieved islets into a 10 × 35 mm petri dish with 2 mm grid marks with 1 mL of HBSS.
Add 1 mL of dithizone working solution into the dish.
Inspect capsules; islets should appear as in Fig. 3e, f (see Note 5).
3.6. Dynamic Perifusion of Islets and Measurement of Insulin Secretion
- Prepare low-glucose solution.
- Place 100 mL of solution A in a 1000 mL beaker.
- Add 100 mL of solution B.
- Add 800 mL distilled water.
- Add 600 mg glucose to beaker and allow to dissolve.
- Gas the solution with 95 % air/5 % CO2 for 30 min.
- Add 2 g BSA to solution and allow to dissolve.
- Adjust pH, if necessary, to 7.4.
- Prepare high-glucose solution.
- Add 200 mL of the low glucose solution into a 250 mL beaker.
- Add 480 mg glucose.
Bring both high and low glucose solutions to 37 °C and continue to gently gas solution with 95 % air/5 % CO2.
Prime both the tubing and perifusion chamber with low glucose solution (see Note 6).
Place retrieved capsules into a 10 × 35 mm petri dish with 2 mm grid marks and using either a p1000 or p200 pipetter, handpick 60 islets into each perifusion chamber.
Seal the perifusion chambers and place in a water bath at 37 °C.
Set the peristaltic pump at a flow rate of 1.4 mL/min.
Pre-perifuse islets with the low glucose solution for 1 h prior to collecting samples.
After the 1-h period, collect the effluent every 5 min for a 20-min period, keep collected effluent on ice until the end of the assay.
After the 20-min period, begin perifusion with the high-glucose solution and collect samples every 2 min for 30 min.
After the 30-min period, repeat the perifusion with the low-glucose solution and collect samples every 5 min for 20 min.
Cap and store the collected samples at −20 °C until insulin content can be determined through either radioimmunoassay or ELISA (see Note 7).
Examples of functionality testing for islets prior to implantation and following retrieval are shown in Fig. 4.
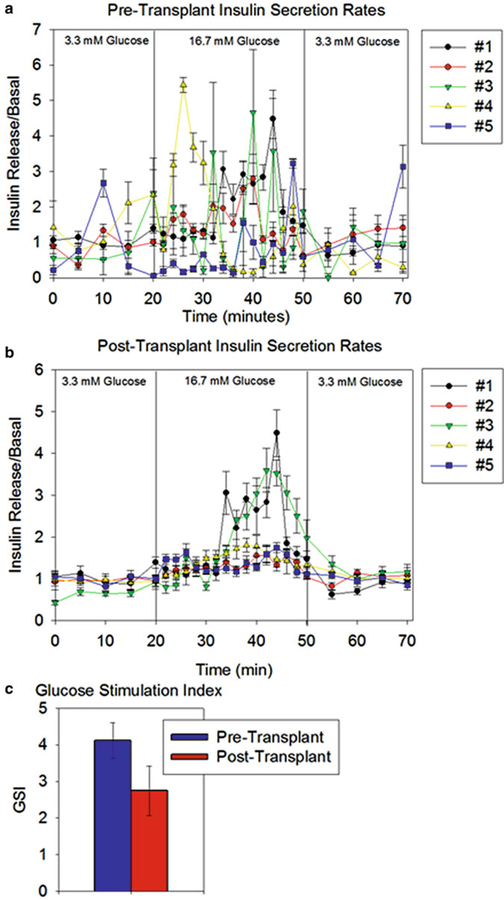
Fig. 4.
Islet Function before and after transplantation. (a) Insulin secretion by encapsulated islets prior to implantation. (b) Insulin secretion by retrieved encapsulated islets 90 days after implantation. (c) Glucose stimulation index (GSI) for encapsulated islets prior to transplantation and following retrieval. The GSI is defined as a ratio of the maximum amount of insulin secreted during the high glucose period over the average amount of insulin secreted over low glucose period. The GSI for retrieved islets was not significantly different from that for the pre-transplant group (2.75 ± 0.68 vs. 4.11 ± 0.48 respectively, p = 0.13). Error bars represent mean ± SEM, N = 5
4. Notes
The culture media described here is specifically for rodent islets, as described in Pareta et al. [37] and is not recommended for either human or porcine islets.
The methods described here were performed according to the animal protocol approved by the Wake Forest University Health Sciences Institutional Animals Care and Use Committee (IACUC). For this procedure STZ-induced diabetic Lewis rats were implanted with 1000 microencapsulated islets within an omental pouch for 90 days prior to retrieval. For more information on this experimental setup please refer to Pareta et al. [37]. For more information on the protocol for islet microencapsulation, please refer to Chapter 16.
Keep the omentum and digestion solution on ice to prevent damage to the retrieved islets prior to digestion.
We recommend to immediately test retrieved capsules following retrieval for the most accurate results. If immediate testing is not possible we recommend culturing the retrieved islets at a low density approximately 100 encapsulated islets/cm2.
Based on our observations, retrieved islets may not stain as deeply red as freshly isolated islets.
Perifusion chambers and tubing setups can vary; for the perifusion results shown here, a plastic flow-through perifusion chambers obtained from Kiyatec Inc. (Greenville, SC, USA) was used with a Masterflex peristaltic pump and Masterflex L/S 16 tubing available from Cole-Parmer (Vernon Hills, IL, USA). We also recommend using commercially available perifusion chambers from Biorep Technologies Inc. (Miami, FL, USA).
7. For the examples provided for this protocol, perifusate samples were analyzed via radioimmunoassay as previously described [46].
Acknowledgements
The authors would like to acknowledge financial support from the National Institutes of Health (RO1 DK080897) and the Vila Rosenfeld Estate, Greenville NC for the work in Dr. Opara’s laboratory at the Wake Forest Institute for Regenerative Medicine.
Also, research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under award # T32EB014836. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.White SA, Shaw JA, Sutherland DER (2009) Pancreas transplantation. Lancet 373(9677):1808–1817 [DOI] [PubMed] [Google Scholar]
- 2.Hills CE, Brunskill NJ (2009) Cellular and physiological effects of C-peptide. Clin Sci (Lond) 116(7):565–574 [DOI] [PubMed] [Google Scholar]
- 3.Opara EC, Mirmalek-Sani SH, Khanna O et al. (2010) Design of a bioartificial pancreas. J Investig Med 58(7):831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berney T, Ricordi C (1999) Islet transplantation. Cell Transplant 8:461–464 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AMJ, Lakey JR, Ryan EA et al. (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 6.Barton FB, Rickels MR, Alejandro R et al. (2012) Improvements in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35(7):1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim F, Sun A (1980) Microencapsulated islets as bioartificial pancreas. Science 210(4472):908–910 [DOI] [PubMed] [Google Scholar]
- 8.Prokop A (2001) Bioartificial pancreas: materials, devices function, and limitations. Diabetes Technol Ther 3(3):431–449 [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan ES, Vegas A, Anderson DG et al. (2011) Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev 32(6):827–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opara EC, McQuilling JP, Farney AC (2013) Microencapsulation of islets for use in a bioartificial pancreas In: Basu J, Ludlow JW (eds) Organ regeneration: methods and protocols. Humana Press, New York, pp 261–266 [Google Scholar]
- 11.Jain K, Yang H, Cai BR et al. (1995) Retrievable, replaceable, macroencaspulated pancreatic islet xenografts. Long-term engraftment without immunosuppression. Transplantation 59(3):319–324 [PubMed] [Google Scholar]
- 12.Muthyala S, Raj VRR, Mohanty M et al. (2011) The reversal of diabetes in rat model using mouse insulin producing cells – a combination approach of tissue engineering and macroencapsulation. Acta Biomater 7(5):2153–2162 [DOI] [PubMed] [Google Scholar]
- 13.Opara EC, Kendall WF (2002) Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther 2:503–511 [DOI] [PubMed] [Google Scholar]
- 14.Weber LM, He J, Bradley B et al. (2006) PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell micro-environments. Acta Biomater 2(1):1–8 [DOI] [PubMed] [Google Scholar]
- 15.Capretto L, Mazzitelli S, Luca G et al. (2010) Preparation and characterization of polysaccharidic microbeads by a microfluidic technique: application to the encapsulation of Sertoli cells. Acta Biomater 6(2):429–435 [DOI] [PubMed] [Google Scholar]
- 16.Rokstad AM, Brekke OL, Steinkjer B et al. (2011) Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater 7(6):2566–2578 [DOI] [PubMed] [Google Scholar]
- 17.Hall KK, Gattas-Asfura KM, Stabler CL (2011) Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater 7(2):614–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam SK, Bilodeau S, Dusseault J et al. (2011) Biocompatibility and physicochemical characteristics of alginate-polycation microcapsules. Acta Biomater 7(4):1683–1692 [DOI] [PubMed] [Google Scholar]
- 20.Soon-Shiong P, Heintz RE, Merideth N et al. (1994) Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343(8903):950–951 [DOI] [PubMed] [Google Scholar]
- 21.Calafiore R, Basta G, Luca G et al. (2006) Microencapsulated pancreatic islet allograft into non-immunosuppressed patients with Type 1 diabetes. Diabetes Care 29(1):137–138 [DOI] [PubMed] [Google Scholar]
- 22.Elliott RB, Escobar L, Tan PL et al. (2007) Live encapsulated porcine islets from type 1 diabetic patient 9.5 yr. after xenotransplantation. Xenotransplantation 14(2):157–161 [DOI] [PubMed] [Google Scholar]
- 23.Tuch BE, Keogh GW, Williams LJ et al. (2009) Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32(10):1887–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Living Cell Technologies. Clinical Trials 2012. Update. http://www.lctglobal.com/
- 25.Sun Y, Ma X, Zhou D et al. (1996) Normalization of diabetes in spontaneously diabetic Cynomolgus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest 98(6):1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Adcock J, Kuhtreiber W et al. (2008) Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 85(3):331–337 [DOI] [PubMed] [Google Scholar]
- 27.Cui H, Tucker-Burden C, Cauffiel SM et al. (2009) Long-term metabolic control of auto-immune diabetes in spontaneously diabetic non-obese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation 88(2):160–169 [DOI] [PubMed] [Google Scholar]
- 28.Dufrane D, Goebbels RM, Saliez A et al. (2006) Six month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81(9):1345–1353 [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T, Aomatsu Y, Iwata H et al. (2006) Survival of microencapsulated islets at 400 days post transplantation in the omental pouch of NOD mice. Cell Transplant 15(4):359–365 [DOI] [PubMed] [Google Scholar]
- 30.Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets from the pancreas. Diabetes 16(1):35–39 [DOI] [PubMed] [Google Scholar]
- 31.Field J, Farney A, Sutherland DE (1996) Improved islet isolation from rat pancreas using 35% bovine serum albumin in combination with Dextran gradient separation. Transplantation 61(10):1554–1556 [DOI] [PubMed] [Google Scholar]
- 32.Tendulkar S, Mirmalek-Sani SH, Childers C et al. (2012) A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices 14(3):461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darrabie MD, Kendall WF, Opara EC (2005) Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials 26(34):6846–6852 [DOI] [PubMed] [Google Scholar]
- 34.McQuilling JP, Arenas-Herrera J, Childers C et al. (2011) New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch. Transplant Proc 43(9):3262–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pareta R, McQuilling JP, Sivanandane S et al. (2014) Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas 43:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lifson N, Lassa CV, Dixit PK (1985) Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol 249(1):E43–E48 [DOI] [PubMed] [Google Scholar]
- 37.Jansson L, Hellerström C (1983) Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia 25(1):45–50 [DOI] [PubMed] [Google Scholar]
- 38.Dionne KE, Colton CK, Yarmush ML (1993) Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42(1):12–21 [DOI] [PubMed] [Google Scholar]
- 39.Davalli AM, Scaglia L, Zangen DH et al. (1996) Vulnerability of transplanted islets in the immediate post transplantation period; dynamic changes in structure and function. Diabetes 45(9):1161–1167 [DOI] [PubMed] [Google Scholar]
- 40.Mendoza V, Klein D, Ichii H et al. (2005) Protection of islets in culture by delivery of oxygen binding neuroglobin via protein transduction. Transplant Proc 37(1):237–240 [DOI] [PubMed] [Google Scholar]
- 41.Jones GL, Juszczak MT, Hughes SJ et al. (2007) Time course and quantification of pancreatic islet revascularization following intra-portal transplantation. Cell Transplant 16(5): 505–516 [DOI] [PubMed] [Google Scholar]
- 42.De Vos P, Van Straaten JFM, Nieuwenhuizen AG et al. (1999) Why do microencapsulated islet grafts fail in the absence of fibrotic over-growth? Diabetes 48(7):1381–1388 [DOI] [PubMed] [Google Scholar]
- 43.Ludwig B, Rotem A, Schmid J et al. (2012) Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone agonist. Proc Natl Acad Sci U S A 109(13):5022–5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedraza E, Coronel MM, Fraker CA et al. (2012) Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated oxygen generating biomaterials. Proc Natl Acad Sci U S A 109(11):4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldsmith HS, Griffith AL, Kupferman A et al. (1984) Lipid angiogenic factor from omentum. JAMA 252(15):2034–2036 [PubMed] [Google Scholar]
- 46.Herbert V, Lau KS, Gottlieb CW et al. (1965) Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25(10):1375–1384 [DOI] [PubMed] [Google Scholar]