Abstract
Objective
To determine the appropriateness of using MCV/MCH as screening test for beta-thalassemia trait in the present population and also to find the most appropriate cutoff for optimum sensitivity of these indices.
Methods
It was an analytical, observational and cross-sectional study. Complete blood count followed by high-performance liquid chromatography (HPLC) was performed. The MCV and MCH levels were noted in cases and controls.
Results
Thalassemia trait was found in 66 out of 1300 antenatal women with anemia. The MCV and MCH were significantly low in cases (p = 0.0001). MCV had a better AUC (0.650) than MCH (0.635). The most suitable cutoff value of MCV was calculated as 72 fl (sensitivity—63.7%, specificity—68.3%, PPV—9.7%, LR—2.0) and that for MCH was 24 pg (sensitivity—63.6%, specificity—59.4%, PPV—7.7%, LR—1.5) using Youden’s index. When MCH (cutoff of 28 pg) and MCV were combined (cutoff of 74 fl), the sensitivity and specificity were 95% and 16%, respectively.
Conclusion
The sensitivity and specificity of MCV and MCH alone had low detection rate when used in combination had high sensitivity but the specificity was low; therefore, HPLC should be the preferred screening test for beta-thalassemia in Indian women.
Keywords: RBC indices, Microcytic anemia, Carrier testing, Beta-thalassemia trait
Introduction
Anemia is a major health disease in the world, affecting 24.8% of the world population which corresponds to 1.62 billion people [1]. Almost 10% of the world’s thalassemia patients are born in India every year. The average rate of thalassemia carrier is 3.3% in India, which differs from 1 to 17% in different regions of the country [2]. In India, the high cost of treatment creates economic burden to the family; therefore, screening for the beta-thalassemia is a viable option. Pre-marital screening is one of the successful methods to diagnose thalassemia in early age, but pre-marital screening in a country like India is not possible due to social reasons; thus, screening antenatal women is the more feasible and acceptable alternative [3]. The various methods available for mass population screening are red cell indices, naked eye single tube red cell osmotic fragility test (NESTROFT), hemoglobin A2 estimation by HPLC (high-performance liquid chromatography) [4]. Hemoglobin A2 estimation is the gold standard for the diagnosis of beta-thalassemia trait, but its availability in the present health care setup is limited. RBC indices have been proposed as effective screening method for identification of beta-thalassemia, but it is difficult for clinicians to calculate the different indices, and also, it has not been found to be a reliable method to differentiate between beta-thalassemia and iron deficiency anemia [4]. NESTROFT is used in many health care setups throughout the country, but it has been shown to give false positive results in case with iron deficiency anemia [4]. As iron deficiency anemia is the most common cause of anemia in this part of the world, use of NESTROFT or various red cell indices compromises the sensitivity of the test. Recent studies have used mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) to screen beta-thalassemia trait, but its use in antenatal women in South Asia, particularly India where there is high incidence of iron deficiency anemia (IDA), is still not clear. The previous study in a similar setting concluded that the sensitivity and specificity of NESTROFT was 78.48% and 94.14%, respectively. These were much better than those of RBC count (> 5 million/mL3), MCV (< 80 fL) or MCH (< 27 pg) [4–6].
Therefore, the aim of the study was to determine the appropriateness of using MCV/MCH as screening test for beta-thalassemia trait in the present population and also to find the most appropriate cutoff for optimum sensitivity of these indices.
Materials and Methods
The study was carried out in women attending the ANC OPD in the Department of Obstetrics and Gynecology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, in collaboration with Department of Pathology. It was an analytical, observational and cross-sectional study from November 2015 to October 2017, performed after approval from the institutional, ethical and research committee. Antenatal anemic women of any gestation attending ANC OPD were enrolled after consents. Diagnosed case of hemoglobinopathy or those who received blood transfusion in the past 1 month were excluded. For calculation of sample size, the average prevalence was considered to be around 3.3% [3]. Assuming this value to be the reference and allowing a margin of error of 1%, the minimum required sample size for our study at 5% level of significance was obtained as 1226 subjects. A round figure of 1300 sample size was taken.
The demographic and clinical details were noted as part of routine antenatal care and recorded on the antenatal card and proforma. Complete blood count was done by collecting blood sample in EDTA tube. Hemoglobin and red blood cell indices (MCH and MCV) were measured using automated blood cell counter (Sysmex KX-21) on the same day. Antenatal women having hemoglobin less than 11 gm% were labeled anemic. In accordance with ICMR classification of anemia, the hemoglobin level between 10 and 10.9 gm% was taken as mild anemia, hemoglobin level between 7 and 9.9 gm% was considered as moderate anemia, severe anemia was hemoglobin less than 7 gm%, whereas very severe anemia was taken as hemoglobin less than 4 gm%. RBC indices of all anemic women were noted. All women were tested for hemoglobin variants by high-performance liquid chromatography (HPLC) using BIO-RAD Variant II hemoglobin testing system. All antenatal women with HbA2 level more than 3.5% were thalassemia trait positive and were considered as cases, and those with HbA2 at or below 3.5% were considered negative and were taken as controls.
Statistical Analysis
Statistical analysis was performed with SPSS v.16. Mann–Whitney U test was used to compare the values; multivariate analysis and receiver operating curve were constructed for MCV and MCH to find out the impact of each variable to be used as screening test. The main outcome was sensitivity, specificity, PPV and NPV of both MCH and MCV for detection of beta-thalassemia trait in antenatal women taking HPLC as the gold standard. The optimal cutoff point for MCV and MCH was determined using Youden’s index (J). The 95% confidence interval was calculated. In this study, p value < 0.05 was considered statistically significant.
MCV and MCH were also evaluated in combination to find out the most sensitive cutoff. The OAPR (odds of being affected given a positive result) ratio was also calculated in order to find out whether doing RBC indices was practically viable compared to doing HPLC in all antenatal cases.
Results
In this study, 1300 anemic antenatal women were screened for the detection of beta-thalassemia. The MCV, MCH, HPLC, serum ferritin and Hb levels of all women were measured to detect beta-thalassemia. The mean age of the women in the study group was 25 years, and the average period of gestation was 31 weeks. Almost half of the respondents aged between 23 and 27 years (48%) (Table 1). The parity of the women ranged from para zero to para five. The maximum number of women was para one both in cases and in controls. The gestational age ranged from 20 to 40 weeks. The mean gestational age was 31 ± 4.3 weeks. There was no significant difference in age (p = 0.040), parity (p = 0.157) and gestational age (p = 0.839) between cases and controls.
Table 1.
Age distribution of cases and controls
| Beta-thalassemia | Total (%) | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | |||||
| Count (n) | % of total | Count (n) | % of total | |||
| Age group | ||||||
| 18–22 years | 15 | 1.2 | 267 | 20.5 | 282 | 21.7% |
| 23–27 years | 41 | 3.2 | 620 | 47.7 | 661 | 50.8% |
| 28–32 years | 8 | 0.6 | 331 | 25.5 | 339 | 26.1% |
| 33–39 years | 2 | 0.2 | 16 | 1.2 | 18 | 1.4% |
| Total | 66 | 5.1 | 1234 | 94.9 | 1300 | 100% |
The hemoglobin level of the women ranged from 5 to 11 gm% with the mean hemoglobin level of 8.6 ± 1.5 gm%. The maximum number of women had mild anemia (hemoglobin range 9–11 gm%) both in cases and in controls (Table 2). There was significant difference in hemoglobin levels among cases and controls (p = 0.001). The mean MCV was 62 fl in cases (55–114 fl) and 77.8 fl in controls (55–114 fl) (Table 3). The mean MCH was 23.5 pg (18.1–33.2 pg) in cases, whereas it was 25.4 pg (18.9–33 pg) in controls (Table 4). There was significant difference between the MCV and MCH values in cases and controls (p < 0.0001). There was no significant difference in HCT, RDW, serum ferritin value among cases and controls.
Table 2.
Hemoglobin level distribution of cases and controls
| Beta-thalassemia | Total (%) (1300) | |||||
|---|---|---|---|---|---|---|
| Cases (66) | Controls (1234) | |||||
| Count (n) | % of total | Count (n) | % of total | |||
| Hemoglobin (gm%) | ||||||
| < 5 | 1 | 0.1 | 17 | 1.3 | 18 | 1.4% |
| 5–7 | 10 | 0.8 | 282 | 21.7 | 292 | 22.5% |
| 7–9 | 23 | 1.8 | 362 | 27.8 | 385 | 29.6% |
| 9–11 | 32 | 2.5 | 573 | 44.1 | 605 | 46.5% |
| Total | 66 | 5.1 | 1234 | 94.9 | 1300 | 100% |
Table 3.
Comparison of MCV values in cases and controls
| Beta-thalassemia | Total (%) | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | |||||
| Count (n) | % of total | Count (n) | % of total | |||
| Less than 62 fl | 14 | 1.07 | 177 | 13.6 | 191 | 14.7% |
| MCV (fl) | ||||||
| 62–72 fl | 28 | 2.1 | 225 | 17.3 | 253 | 19.4% |
| 72–82 fl | 13 | 1.0 | 393 | 30.2 | 406 | 31.2% |
| 82–92 fl | 7 | 0.5 | 268 | 20.6 | 275 | 21.2% |
| More than 92 fl | 4 | 0.3 | 171 | 13.2 | 175 | 13.5% |
| Total | 66 | 5.1 | 1234 | 94.9 | 1300 | 100% |
Table 4.
Comparison of MCH values in cases and controls
| Beta-thalassemia | Total (%) | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | |||||
| Count (n) | % of total | Count (n) | % of total | |||
| MCH (pg) | ||||||
| Less than 24 | 42 | 3.2 | 559 | 43 | 601 | 46.2% |
| 24–27 | 9 | 0.7 | 229 | 17.6 | 238 | 18.3% |
| 27–31 | 14 | 1.1 | 306 | 23.5 | 320 | 24.6% |
| More than 31 | 1 | 0.1 | 140 | 10.8 | 141 | 10.8% |
| Total | 66 | 5.1 | 1234 | 94.9% | 1300 | 100% |
The critical value of MCV and MCH, when used individually as screening test for beta-thalassemia trait, was derived by constructing the ROC curve. The area under curve for MCV was 0.650 (95% cf 0.586–0.713) (Fig. 1). Using Youden’s index, 72 fl was the most suitable cutoff for MCV. At this cutoff, the sensitivity and specificity were 63.67% and 68.31%, respectively. The positive predictive value was 9.7%, whereas the negative predictive value was 97.23%. The ROC curve of MCH showed ROC as 0.635(95% cf 0.569–0.701) (Fig. 2). Using Youden’s index, the cutoff of MCH was 24 pg. At this cutoff, the sensitivity and specificity of MCH were 63.63% and 59.40%, respectively. The positive predictive value was 7.7%, whereas the negative predictive value was 96.82%.
Fig. 1.
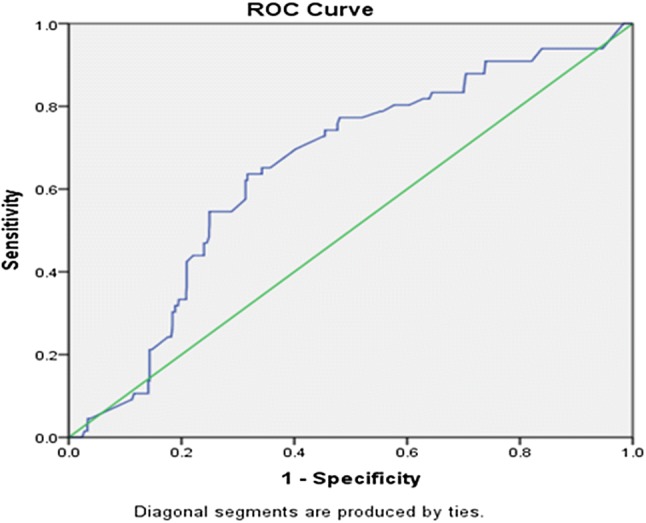
The ROC curve shows the area under curve (AUC) of MCV as 0.650(95% cf 0.586–0.713)
Fig. 2.
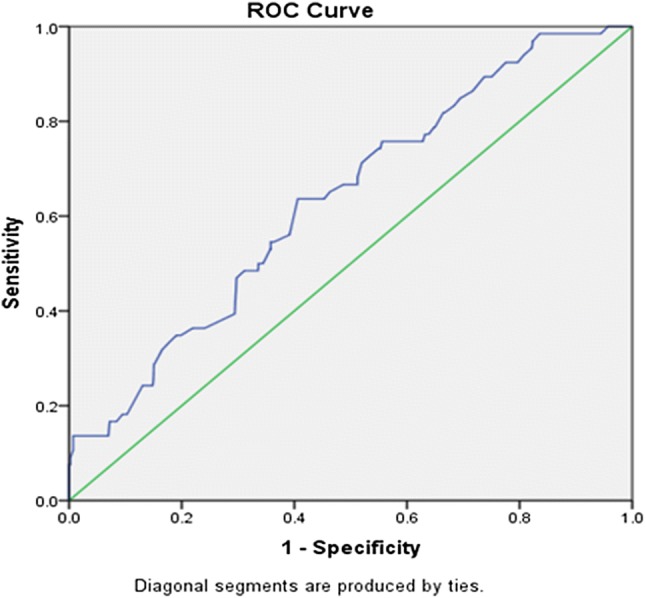
The ROC curve shows the area under curve (AUC) of MCH as 0.635 (95% cf 0.569–0.701)
At a higher cutoff of 80 fl for MCV, the sensitivity was 78.7%, and NPV was 97.5%, whereas at cutoff of 27 pg for MCH, the sensitivity was 77.2%, specificity was 36.1% and NPV was 96.7% (Table 5). When MCV and MCH were considered in combination, MCV at 80 fl and MCH at 27 pg cutoff provided 89.4% sensitivity and 16.4% specificity. It was found that using above cutoffs enabled 1105 out of 1300 women as screen positive and thus, the HPLC of these 1105 women would give 59 positive cases, i.e., the OAPR was 1:19, but at the same time it would miss 7 out of 66 cases. With cutoff of MCV as 74 pg and MCH as 28 fl, the sensitivity was 94% and the OAPR was 1:17. When HPLC was used as screening test for all anemic antenatal women, the sensitivity would be 100% and the OAPR would be 1:20.
Table 5.
Cutoff value, sensitivity, specificity, PPV and NPV of MCH and MCV
| Cutoff | Sensitivity (%) | Specificity | PPV (%) | NPV | |
|---|---|---|---|---|---|
| MCV (low cutoff) | 72 fl | 63.7 | 68.3% | 9.7 | 97.2% |
| MCV (high cutoff) | 80 fl | 78.8 | 44.3% | 7.0 | 97.5% |
| MCH (low cutoff) | 24 pg | 63.6 | 59.4% | 7.7 | 96.8% |
| MCH (high cutoff) | 27 pg | 77.3 | 36.1% | 6.1 | 96.7% |
| MCV and MCH | 80 fl and 27 pg | 89.4 | 16.4% | 5.8 | 98.0% |
| MCV and MCH | 74 fl and 28 pg | 94 | 21.2 | 5.6 | 98.2 |
Discussion
In the present study, thalassemia trait was found to be prevalent among antenatal women with anemia. Both MCV and MCH were significantly low in cases, MCV was a better index compared to MCH. The most suitable cutoff value of MCV and MCH was calculated using Youden’s index. When both indices were used in combination, the sensitivity soared, but the specificity took the hit. The OAPR ratio was also calculated in order to find out whether doing RBC indices was practically viable compared to performing HPLC in all antenatal cases.
Anemia is common in antenatal women in Indian population. Nearly half of the 66 thalassemia trait cases had mild anemia, implying that thalassemia trait cases mostly had mild anemia and were asymptomatic. Similar study was done by Tunkyi et al.[7] in South African urban population, where two thousand pregnant women were studied; the majority of subjects were mildly anemic. In the present study, 66 out of 1300 (5.07%) antenatal women with anemia were identified to have beta-thalassemia trait. Beta-thalassemia carrier rate varies from 1 to 17% with an average of 3.3% carrier rate in India [2].
The ROC curve was constructed, and the area under curve (AUC) of MCV was found to be more than that of MCH signifying that the MCV was a better variable than MCH in beta-thalassemia screening. Using the Youden’s index, the cutoff for MCV became 72 fl and that of MCH was 24 pg, but their sensitivity and specificity individually were low (63.67% and 68.31% for MCV and 63% and 59% for MCH). Therefore, neither of them was found to be good as screening test when used alone. In a study by Bencaiova et al. [8], they found the 95.3% sensitivity and 85.4% specificity taking MCV cutoff of 75 fl. Another study by Prenpanus et al. [9] showed 95% sensitivity and 82% specificity taking 26.5 pg as cutoff of MCH.
Betabi et al. [10] did a study on population from Iran and concluded that due to the high prevalence of IDA (iron deficiency anemia) among women, the combination of IDA and thalassemia minor can cause a misdiagnosis by these indices; therefore, it suggested that physicians should not rely entirely on the results of these indices when they encounter women suspected of having associated IDA. Madan et al. did a study on Indian population and deduced that the mean MCV and MCH were significantly lower in the patients of beta-thalassemia trait (BTT) with IDA than in those without IDA (p < 0.0001). The mean HbA2 was elevated in both (> or = 3.5%), and there was no significant difference between the two groups [2]. Previous work of RBC indices in beta-thalassemia screening has shown that the cutoff of 80 fl for MCV and 27 for MCH is the most appropriate. When we applied it in our study population, it resulted in screening of 19 women to detect one thalassemia trait but at the same time missing 10% cases (89.4% detection rate), whereas using HPLC we would get one thalassemia trait if we screen 20 cases with anemia and we would not miss any case, i.e., 100% sensitivity. With MCV cutoff of 74 pg and MCH at 28 fl, the sensitivity was 94% and the OAPR was 1:17.
Gosh et al. [11] in their study on the Indian population have also suggested that the typical beta-thalassemia carriers may have a normal MCV and/or MCH sometimes, and these individuals may be missed while screening for beta-thalassemia. The method of choice for the hemoglobin analysis is automated cation-exchange HPLC. This gives an accurate estimate of HbA2.
The use of HPLC is more appropriate as screening test in all antenatal anemic women in South Asian region where due to associated iron deficiency anemia, the sensitivity and specificity of RBC indices as screening test are low. If the facility for HPLC does not exist, the new cutoff of 74 pg for MCV and 28 fl for MCH should be used.
Mayura Balliyan
has done her MBBS and MS from the prestigious Lady Hardinge Medical College. She has keen interest in high-risk pregnancy and fetal medicine. This article is a part of her M.D. thesis dissertation.
Author Contributions
Mayura Balliyan conducted the study, co-analyzed the data, and drafted and revised the manuscript. Manisha Kumar was involved in the concept and planning of the study, conducted the study, co-analyzed the data and drafted the manuscript. Anita Nangia conducted the study and drafted the manuscript. Nupur Parakh conducted the study and revised the manuscript.
Compliance with Ethical Standards
Conflict of interest
There is no potential conflict of interest among authors.
Human Participants and Informed Consent
The research involved human participants. Informed consent was taken before the study.
Ethical Standard
Ethical clearance was taken before the study.
Footnotes
Mayura Baliyan is a Resident at Department of Obstetrics and Gynecology, LHMC, New Delhi, India. Manisha Kumar is a Professor (OBGYN) at Department of Obstetrics and Gynecology, LHMC, New Delhi, India. Anita Nangia is a Professor at Department of Pathology, LHMC, New Delhi, India. Nupur Parakh is a Senior Medical Officer at KSCH, New Delhi, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaur K. Anemia ‘a silent killer’ among women in India: present scenario. Eur J Zool Res. 2014;3(1):32–36. [Google Scholar]
- 2.Madan N, Sharma S, Sood SK, et al. Frequency of beta thalassemia trait and other hemoglobinopathies in Northern and Western India. Indian J Hum Genet. 2010;16:16–25. doi: 10.4103/0971-6866.64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamhankar PM, Agarwal S, Arya V, et al. Prevention of homozygous beta thalassemia by premarital screening and prenatal diagnosis in India. Prenat Diagn. 2009;29(1):83–88. doi: 10.1002/pd.2176. [DOI] [PubMed] [Google Scholar]
- 4.Mendiratta SL, Bajaj S, Popli S, et al. Screening of women in the antenatal period for thalassemia carrier status: comparison of NESTROFT, red cell indices, and HPLC analysis. J Fetal Med. 2015;2(I):21–25. doi: 10.1007/s40556-015-0036-0. [DOI] [Google Scholar]
- 5.Mohapatra R, Warang P, Ghosh K, et al. Hemoglobinopathy screening by osmotic fragility test based on flow cytometer or naked eye. Cytom B Clin Cytom. 2016;90(3):279–284. doi: 10.1002/cytob.21205. [DOI] [PubMed] [Google Scholar]
- 6.Piplani S, Manan R, Lalit M, et al. NESTROFT—a valuable, cost effective screening test for beta thalassemia trait in North Indian Punjabi Population. J Clin Diagn Res. 2013;7(12):2784. doi: 10.7860/JCDR/2013/6834.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tunkyi K, Moodley J. Prevalence of anaemia in pregnancy in a regional health facility in South Africa. S Afr Med J. 2015;106(1):101–104. doi: 10.7196/SAMJ.2016.v106i1.9860. [DOI] [PubMed] [Google Scholar]
- 8.Pranpanus S, Sirichotiyakul S, Srisupundit K, et al. Sensitivity and specificity of mean corpuscular hemoglobin (MCH): for screening alpha-thalassemia-1 trait and beta-thalassemia trait. J Med Assoc Thai. 2009;92(6):739–743. [PubMed] [Google Scholar]
- 9.Batebi A, Pourreza A, Esmailian R. Discrimination of beta-thalassemia minor and iron deficiency anemia by screening test for red blood cell indices. Turk J Med Sci. 2012;42(2):275–280. [Google Scholar]
- 10.Ghosh K, Colah R, Manglani M, et al. Guidelines for screening, diagnosis and management of hemoglobinopathies. Indian J Hum Genet. 2014;20(2):101–119. doi: 10.4103/0971-6866.142841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera R, Singh T, Khuana N, et al. HPLC in characterization of hemoglobin profile in thalassemia syndromes and hemoglobinopathies: a clinicohematological correlation. Indian J Hematol Blood Transfus. 2015;31(1):110–115. doi: 10.1007/s12288-014-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


