Abstract
The emergence of injectable hydrogels as biomaterials has been a revolutionary breakthrough in the field of on-demand drug delivery and tissue engineering. The promising features of these systems include their biodegradability, biocompatibility, permeability, ease of the surgical implantation, and most importantly exhibit minimally invasiveness. These hydrogels have been explored as sustained and on-demand release carriers for the various bioactive agents, growth factors, live cells, various hydrophobic drugs and as extracellular matrices for tissue engineering. Present review is an attempt to highlight the recent systems explored for on demand drug release and tissue engineering. It also gives an overview of the role of nanotechnology in the advancements of injectable hydrogels. The future prospects and challenges of these hydrogels have also been addressed.
Keywords: Injectable hydrogels, biomaterials, Nanomedicine, Pluronics, Chitosan, Drug delivery, Nanotechnology
1. INTRODUCTION
Injectable hydrogels by definition are the hydrogels which are in the form of sol or liquid at room temperature (20–25° C) and gel at body temperature (37° C). Since 1960 hydrogels have been explored for biomedical applications [1–4] and the recent demand has urged to develop injectable hydrogel systems, which can be synthesized by using various biopolymers, temperature sensitive polymers and loaded with the pharmaceutical excipients or live cells to form a hydrogel depot that undergo sol-gel transition. The excellent features of these hydrogels in comparison to the other forms of the hydrogels include their simple implantation process which limit the surgical procedures and also their ease of synthesis methods have highlighted their potential application as a drug carrier [5–8], seal-ants [9], tissue engineered products [10], surgical glues [11].
The field of regenerative medicine has come up with the novel approaches to improve, restore and replace the damaged tissues of the various parts of the human body. Various combination of therapies are being explored for this translational application. Cell therapies as well as immunomodulation therapies are in high demand. Interestingly hydrogel based scaffolds have taken upper hand in mimicking the extracellular matrix for the structural support resemblance and artificial organs and tissues [12]. The basic features that a synthetic scaffold should fulfill the properties that include the exact mechanical strength, porous structure and the free diffusion of the various nutrients across the cells. The cytocompatibility of these artificial scaffolds takes on the major concern. Hydrogels fulfill these criterion by having all the features with the three dimensional network of polymeric chains and have proven to be promising artificial extra cellular matrices (ECMs) [13]. The synthesis methods of the hydrogels especially the injectable hydrogels which are the most demanding comprise of simple, non-toxic chemical crosslinkers and enzymes for the crosslinking. These hydrogels can be made in desired shape and have the capability to adhere to the surrounding tissues during their formation. Researchers are involved in developing more advanced injectable systems for long-term cell encapsulation and on demand drug release which exhibit high stability when injected in the body [14]. Nanomedicine is making more precise dominant steps in applying nanoformulations for specific applications including drug delivery for on-demand, sustained release, gene delivery, tissue engineering, imaging and sensing applications [15–18]. The various routes of administration like transdermal, vaginal, intranasal, intra-pulmonary and one of the preferred route is parenteral route, which is opted for last many years exhibited many limitations such as frequent administration which results in poor patient compliance [19, 20]. In the last few years, broad and intensive research with the emergence of nanotechnology the invention of nanomedicine as well as to prolong the release of the bioactives for extended time has been suggested. This requirement is being fulfilled by soft injectable hydrogels. There are many biopolymers used for the synthesis of these hydrogels like chitosan, Polyethyleglycol (PEG), Poly (N-isopropylacrylamide) (PNIPAAm), Polylactic acid (PLA), Polyglycolic acid (PGA), Hydroxyethylcellulose (HEC), Pluronics, α, β-glycerophosphate have been extensively explored. The general synthesis methods employed for the synthesis of injectable hydrogels is via physical crosslinking methods using the hydrogen bond formation or ionic interactions. The temperature sensitivity of certain polymers for example PNIPAAm, are exploited in the synthesis method by modulating their properties of swelling and elasticity by the addition of hydrophillic polymers like PEG.[21] Other synthesis methods include the chemical crosslinking methods using the enzyme crosslinking [22], free radical polymerization or high energy radiations as well as condensation reactions [23]. These methods vary to the different type of polymers used and diverse biomedical applications of the synthesized hydrogels [24]. Table 1 highlights few recent noteworthy studies of stimuli responsive injectable hydrogels used for different biomedical applications. Literature showed the use of PEG–PNIPAAm based hydrogel for the parenteral administration. These hydrogels exhibit good gelling mechanical strength and thus have least effects on the initial burst effect of the drug.
Table 1.
Stimuli responsive injectable hydrogels for different biomedical applications.
| Biopolymers | Gel Processing | Stimuli response | Application | References |
|---|---|---|---|---|
| Chitosan-beta glycerophosphate-hydroxyethyl cellulose (CH-GP-HEC) | Sol- gel transition at 37 °C | Temperature responsive | Cartilage Tissue engineering | [26] |
| Dextran sulfate (DS) | Coupling of a cysteine terminated peptide to HA-maleimide | Bio-responsive | On-demand matrix metalloproteinase inhibition | [27] |
| Methacrylate-polylactide (MAPLA), N-isopropylacrylamide (NIPAAm) and 2-hydroxyethyl methacrylate (HEMA) | sol-gel transition | Thermally responsive | Hydrolytic Lability | [28] |
| Heparin-bearing poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactide) (Hep-PCLA) | Temperature-induced sol-to-gel transitions | Thermally responsive | Protein Delivery | [29] |
| Poly(ethylene glycol)-poly(sulfamethazine carbonate urethane) (PEG-PSMCU) copolymers | sol-gel transition | pH and temperature sensitive | Sustained delivery of cationic proteins | [30] |
| Hyaluronic acid methyl cellulose | Sol-gel transition | Pressure sensitive | Spinal cord injury repair | [31] |
| Phenylboronic acid (PBA) and glucose. | Complexation | Glucose responsive | Sensing application for monitoring blood glucose levels. | [32] |
The increased bioavailability by having the on demand drug release these injectable gels are being extensively explored in drug delivery.
The present article gives an overview of the injectable hydrogels explored recently for tissue engineering and drug delivery. The ability of cell encapsulated hydrogels to get implanted into the body to eradicate the bone and cartilage defects for tissue restoration [25]. The addition of the nanofillers like metal nanoparticles, silica nanoparticles, clay, carbon nanotubes (CNT) etc. in the nanocomposite hydrogel matrices increases the mechanical strength of the hydrogels and play a major role in improving the shape, irregular bone defects and the osteoinductive properties.
2. IN SITU FORMING GELS: SYNTHESIS, POLYMERS, ROUTES OF DELIVERY
The general methods for the synthesis of in situ hydrogels involve the use of non-toxic chemical cross linkers as well as physical interactions. The physical interactions include the hydrophobic and ionic interactions which are quite popular. The recently opted techniques are using the supramolecular chemistry [12]. These techniques offer potential benefits like the easy route for delivery, non-invasive techniques for better patient compliance. The other fascinating and potential chemical crosslinking techniques employed for the synthesis are Michael addition utilizing thiols and amine as nucleophile and added to α, β unsaturated carbonyl compounds [33]. Moreover click chemistry [34] is also explored for the development of injectable gels for rapid reactivity in physiological conditions. The other methods for chemical crosslinking is by Schiff base reactions as well as by enzyme interactions [35] and self-assembly [36]. The biopolymers already mentioned above including chitosan and alginate use the electrostatic interactions between them and the polyionic molecules for the formation of the gels [37]. PNIPAAm have the property of phase transition with temperature change and have been extensively exploited for in situ gels.
The most important aspects of the in situ gels system are that these gels can be delivered in a non-invasive manner using diverse route for administrations. Intrathecal [38], buccal, intraperitoneal (i.p.), vaginal [39], ocular [40, 41], nasal [42] and rectal routes of administrations for in situ gels are being exploited for various drugs. The natural polymers used for the synthesis of in situ gel systems, owing to their biocompatible and biodegradable nature include pectin [43], hydroxylpropyl methylcellulose (HPMC) [44] guar gum, xyloglucan, cellulose [45, 46], and sodium alginates [47]. The calcium ions are used for pectin gelation. The property of thermal reversibility exhibited by the biopolymer like xyloglucan is used for i.p. and oral delivery [48]. The metal ions divalent or trivalent are important for alginates gelation. The use of pH responsive features of the polymers are exploited for the buccal adhesion. The time of contact is extended for the antifungal drugs. One of the important pH responsive polymer is Carbopol which shows liquid form at acidic pH and forms gel in alkaline pH [49]. Pluronics and polyols, glycerophosphates are extensively exploited for in situ gels [39, 50].
3. APPLICATION OF INJECTABLE GELS AS ON-DEMAND DRUG RELEASE SYSTEMS
The drug delivery systems that have the ability to allow the remote, and reliable drug release are in high demand. Their ability to imbibe large amount of the drug inside it and deliver the drugs to the target site when in ON-state and do not release any of the amount of the drug in off state and may be frequently converted to ON state is ideal system. Though these systems are being synthesized by novel technologies yet none of the systems is in clinics. The methodologies lack their effective application in vivo because of the absence of the local implanted heat source and their multiple thermal cycles as well as the slow response time and exact drug dosing. Though few successful investigation using radiofrequency – activated microchips have shown very promising on demand drug release. Cheng et al [51] reported an interesting injectable hydrogel which has multiple features like near infrared (NIR) responsiveness as well as on demand degradable supramolecular hydrogel for controlled drug delivery. These stimuli responsive hydrogel based systems have the ability to yield a local high-dose and to overcome the limitation of non-specific distribution in normal tissues for best efficacy. This NIR responsive supramolecular hydrogel consisted of alpha-cyclodextrin (α-CD) and poly (ethylene glycol) (PEG)-modified dendrimers and the encapsulation of the platinum (Pt) nanoparticles (DEPt–PEG). The synthesis methodology of the gels comprised of host- guest inclusion by threading the PEG chains inside the cavities of α-CD. The physical crosslinking by hydrogen bond formation was seen for the formation of hydrogels [52]. It was shown that these gels were able to degrade on the exposure of NIR and showed the potential as a local delivery system for spatiotemporally controlled drug release. The main feature of these gels were their photo thermal responsiveness and the biocompatibility, which make them potential candidate to be used in chemotherapy.
A very recent study carried out by research group demonstrated the use of the in situ gelling hydrogels developed using superparamagnetic iron oxide nanoparticles (SPIONs) and thermoresponsive microgels showed pulsatile release of the model drug (4 kDa fluorescein labeled dextran). These particles have gained recent attention owing to the fact that they are inorganic actuating nanoparticles capable to be used in a non-invasive manner and also exhibit thermo-responsive characteristics. Their tendency to regulate the release of the drug by outer control over the extended period of time have been explored.
The group showed that these injectable gels have ~4-fold enhancement of release when in on state as compared to the off state. Fig. 1 shows the synthesis of these microgels has 90 % decrease in volume when heated from 37 to 43°C. This is the temperature range for the physiological temperature to the highest temperature before which the local tissue damage is observed. The application of the alternating magnetic field (AMF) the generation of heat by SPIONs raises the local temperature of the microgels and hence create free volume within the composites and thus increased drug release occurs through diffusion [53]. Other important studies show the application of the on demand drug release using the injectable hydrogels by exploiting the NIR responsive feature of the hydrogels.
Fig. (1).
Fabrication of nanocomposites and their proposed mechanism of externally AMF-controlled enhanced drug release. “Reprinted (adapted) with permission from [53]. Copyright (2015) American Chemical Society.”
The nanomedicine is actively involved in utilizing the features like spatiotemporal sensitivity of the biological systems. These sensitivity provides platform for developing the novel therapeutics. The various natural and synthetic polymers have been exploited for the localized therapy. For example a recent report suggests that the ultrasound sensitivity of the gels can be used to obtain a digital drug release of the bioactive molecules. These devices result in the constant release of the drug [54]. The ionically crosslinked hydrogels have been disrupted by applying the ultrasound and release of the drug takes place. This study also highlighted that by applying regular ultrasound, stimulated drug release substantially reduce the growth of the tumor as compared to the native sustained release of the drug. The amount of ultrasound used for the disruption is not that high to cause permanent damage to the materials but it is sufficient to release the small molecules or bioactives. This type of systems has direct application to the clinics as they provide approach to directly deliver the drug to the tumor site. These systems have significant advantage in providing outer control on the dose and timing from outside and greatly benefit the implantable systems [54].
The injectable hydrogels have also been exploited for the disease rheumatoid arthritis, this disease treatment poses potential challenges owing to the flexible disease activity consisting of exacerbations of inflammation punctuated by periods of remission. Thus a preferred localized system which is able to release the drug specifically during the period of exacerbation, as well as self-titrating in response to the level of inflammation. A perfect example of self-assembled nanofibrous hydrogel system developed by Vemula et al [55] showing the encapsulation of the specific enzymes and their release which are significantly upregulated in the diseased state including matrix metalloproteinases (MMP-2 and MMP-9) and esterases. The study showed that fibrous gels can withstand the shear force experienced during the joint, when injected into healthy joints of mice and get dis assembled to release the agents. This type of systems provides great platform for the future localized treatment of the proteolytic diseases [55].
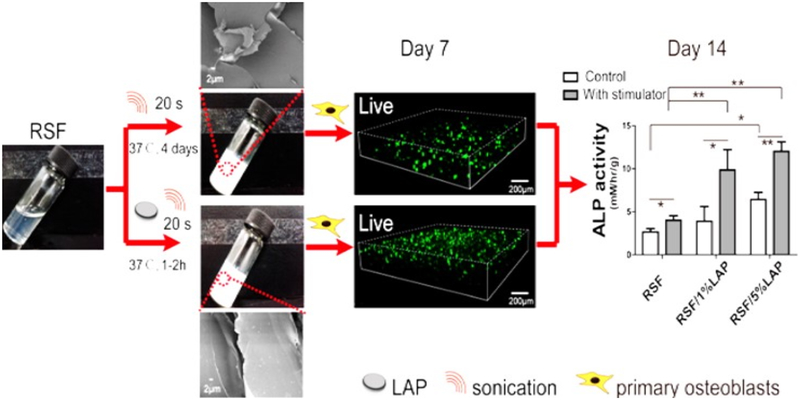
Various natural polymer based materials are being exploited for bone defect repair. For example in a recent study regenerated silk fibroin (RSF) [56] has been explored for bone defects. This group demonstrated the use of laponite nanoplatelet (LAP) which is known to promote the osteoblast growth when incorporated in RSF hydrogel (Fig. 2). An injectable hydrogel of RSF/LAP formed showed cell proliferation and improved osteogenic differentiation.
Fig. (2).
Injectable Silk Fibroin Hydrogel with Laponite Nanoplatelets “Reprinted with permission from [56]. Copyright (2016) American Chemical Society.”
The ability to release the drug by the change in the stimulus have been explored to great extent for clinical applications. Among the various stimuli light has been the major triggering source for activating remote controlled operations and ability to completely control on the release of the cargo. Ultraviolet (UV), near infrared (NIR) light [57] have been explored for the stimuli. Though UV and NIR radiations are accompanied by many drawbacks and certain limitations are associated with them [58, 59]. Such as premature aging by the disruption of the cellular response in the dermis by the application of UV light. The hydrogels in the form of beads have been explored by many groups. A recent study show the visible light induced volume change at body temperature by the hydrogel beads many hundreds micrometer sized and synthesized using alginate templating method. These light sensitive hydrogel beads are successful examples of remotely triggered drug release system. The DEX drug release rate can be controlled by adjusting the intensity of the visible light. These beads possess potential application in transdermal patch. These type of system lay platform for the on-demand sequential as well as sustained release of the drug using light as an external stimuli [60].
The research undergoing in the field of stimulus responsive delivery systems show that light sensitive hydrogels owe a great potential in the transdermal controlled drug delivery. The most important factor to apply them safely is to control the exposure time as well as the power of the light [61]. These systems give an added advantage over the drug delivery systems using subcutaneous injections, which can act as an on demand transdermal patch. NIR as an external response has also been exploited for specifically on demand drug release systems. These systems are generally classified on the basis of the presence of photosensitive groups o-nitrobenzyl and azobenzene [62]. These groups are responsible for triggering the drug release when NIR is applied. Moreover when nanofillers like nanorods based on Carbon nanotubes (CNT) are incorporated in the hydrogel matrix, photo degradation is observed which is responsible for the drug release. The structural variations in these nanostructure occurs like the swelling and deswelling of the gels is responsible for release of the drug.
The main concern behind using the photocontrolled drug delivery systems is to overcome the limitation associated with light source such as the UV light is accompanied by the drawback of not penetrating inside the skin deeply and result in major absorption with the fat tissues [63, 64]. This results to poor reaction and effects the drug release. Similarly NIR is also followed by the limitation to be directly used and also consumption of high energy. The suggested platform to overcome these issues is to create an in situ environment for the UV by using a transducer which can convert the NIR to UV radiations. The high penetration of NIR is a very crucial aspect utilized in drug delivery application [65].
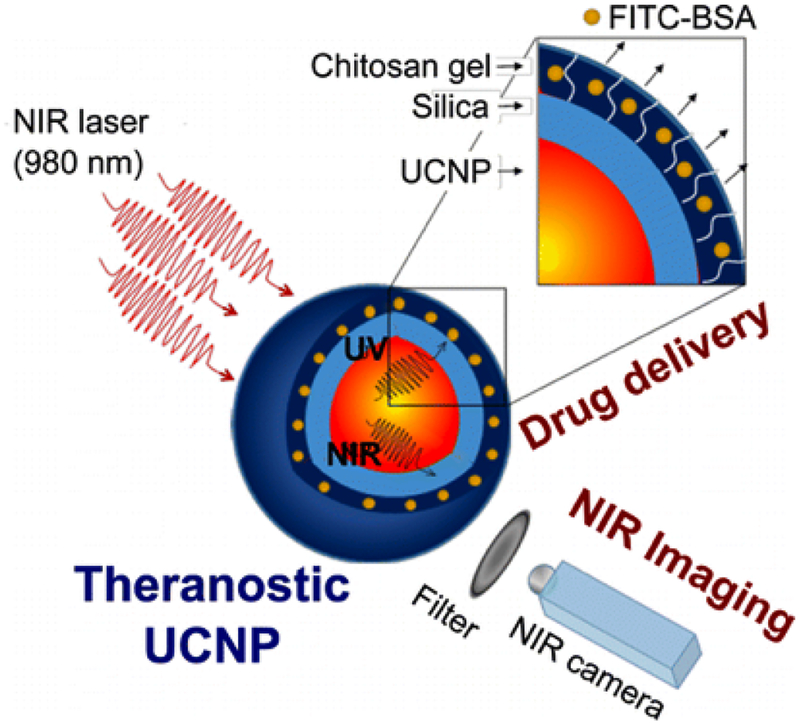
One of the recent studies by Jalani et al. [64] showed the doping of Lanthanide in nanoparticles results in converting them as tranducers having the ability to convert the NIR to shorter wavelength to UV to Vis regions of the spectrum. Present study developed a photocleavable crosslinker (PhL) bearing the succinimidyl group and an acetylene group on respective ends. Lanthanide doped upconverting nanoparticles (UCNP) were capped by using oleate and was used to coat the chitosan hydrogel. This group demonstrated the use of the lanthanide doped LiYF4:Yb3+/Tm3+@SiO2 encapsulated by fluorescent-bovine serum albumin (FITC-BSA) inside the hydrogel and showed the release completely dependent on the light source. It also showed that the upconverted NIR permits the particle tracking under the tissue (Fig. 3). This study showed the potential of these systems in theranostics, and imaging [64].
Fig. (3).
Multifunctional Theranostic Platform for NIR Imaging and On-Demand Macromolecular Delivery. “Reprinted with permission from [64] Copyright (2016) American Chemical Society.
Many studies suggest the use of on demand drug delivery systems using stimuli responsive hydrogels for the treatment of local infections, tumors, and wound healing applications [66, 67].
3.2. Application of Injectable Gels as Tissue Engineering
The emergence of the injectable hydrogels as smart materials is mainly due to their effective application in comparison to the traditional surgeries, which have come up with lowering down of the cost on the healthcare and improving the patient compliance. The development in the field of tissue engineering have come up with novel injectable hydrogel based systems with improved properties covering the ease of their handling and repairing the defects. The injectable hydrogels have come up as biomaterials. The ability of encapsulating various therapeutic agents by injectable hydrogels have been used for drug delivery to various human tissues. For example application of injectable hydrogels for bone tissue engineering and cartilage tissue engineering have taken up an upper hand for replacement of defected organs. Injectable hydrogels have been synthesized using wide range of natural polymers as well as synthetic polymers. Among natural polymers hyaluronic acid [68], alginate [69], decellularized ECM [70], agarose [71], matrigel [72], chitosan and fibrin [73] have been extensively exploited. In this section, we are going to highlight few important and noteworthy injectable hydrogel based systems used for cardiac, bone and cartilage repair.
Hyaluronic acid (HA) is found in many tissues and biological fluids of various bacteria and vertebrates. This natural polymer is known to have high molecular weight and consist of alternating D-glucuonic acid and N-acetyl D-glucosamine. This polymer plays an important role in the functioning of various tissues and cells. It is known for its features like biocompatibility, gel forming ability, ease of modification and hence employed for tissue engineering [75]. The degradation of HA by hyaluronidases is an added advantage to be used for controlled release of the drugs.
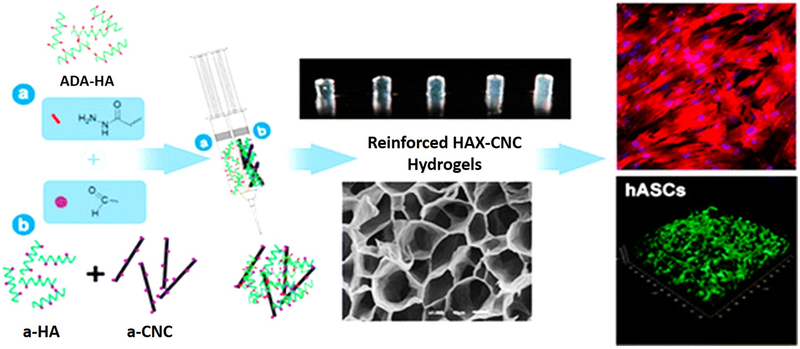
There is an immense work going on for the improvement of the mechanical strength of HA based hydrogels. Many nanofillers are being used for increasing the strength like Carbon nanotubes, or nanocrystals of cellulose. A recent report showed the development of injectable hydrogel of adipic acid dihydrazide-modified HA (ADH-HA) and aldehyde, which was improved by HA (a-HA) and also added content of the aldehyde modified nanocrystal (Fig. 4). These hydrogel showed improved mechanical properties such as compact network and stiffed properties having a storage modulus E′, of 152.4 kPa when nanocrystal concentration is 0.25wt% and this content was responsible for the improvement of the elastic modulus by 135% in comparison to hydrogels without the crystal. A distinct proliferative activity was observed when human adipose derived stem cells (hASCs) were encapsulated in it [74].
Fig. (4).
Injectable hyaluronic acid/cellulose nanocrystals bionanocomposite Hydrogels for tissue engineering Applications. “Reprinted with permission from Reference [74]. Copyright (2015) American Chemical Society.”
The field of tissue engineering and regenerative medicine have emerged with novel technologies, which give innovative approach to the organ transplant as well as repair of the damaged tissues in heart [76]. These approaches utilize the injectable forms of cells to the myocardium. This technology have explored stem cells, which resemble the cardiac cell type, the differentiated cell type or the full cardiac cells [77]. Hydrogels as a biomaterials have been extensively exploited to support the injured heart tissue. Many upcoming research articles pose the absence of any biomaterials for the cell types are grown in situ by tissue engineering for repair of the damaged cardiac tissue. Many injectable hydrogel based systems are compared to those using no biomaterial which possess certain limitation such as low cell retention on the desired site. The additional support delivered by the hydrogel matrix have shown effective regeneration of cardiac tissues [78]. The complete control on the release kinetics by modulating the characteristic features of the hydrogel matrix gives an excellent platform for tissue engineering. Certain growth factors, cell types, proteins, DNA plasmids for the promotion of the anti-apoptosis and angiogenesis are being encapsulated in the injectable hydrogels [79]. Hydrogels are applied for the scaffold engineering owing to the porous structure and moreover the rubbery tissue like morphology support the resemblance and integrity for the tissue formation and act as potential adhesives for the interface between the biomaterial and the tissue. They can serve as scaffolds that provide structural integrity to tissue constructs, control drug and protein delivery to tissues and cultures, and serve as adhesives or barriers between tissue and material surfaces. A number of studies show the application of injectable hydrogels for the stem cell delivery of infarcted myocardium. A very interesting study [80] demonstrated the use of four injectable hydrogels for left ventricle remodeling. Tetronic-fibrinogen (TF) and polyethylene glycol (PEG)-fibrinogen (PF) conjugates were employed in this study. The utilization of multiple properties of the biomaterial like stiffness and highest modulus showed the best neovascularization [80]. HA nanoparticles are also exploited as nanofiller in the hydrogel systems for the encapsulation of stem cell mesenchymal MSCs. These cells showed the existence of the live cell up to 27 days after the encapsulation. The hydrogel mineralization was supported by the combination of the encapsulated MSCs. This type of systems promotes and gives a very potential platform for the in situ forming nanocomposite hydrogels which simultaneously promote the cell delivery as well as mineralization which is the most important condition for craniofacial bone tissue engineering [81].
In the last few decades the musculoskeletal problems are growing at major scale and human healthcare has been affected largely by it. Economically also the cost on the bone and joint care have been recognized by the world health organization (WHO) predominantly [82, 83]. Joint replacement surgeries are on high demand with existing style of living. The large defects are major concern for the restoration of the complete function of the bone. The similar kinds of osteoconductive matrix release the similar kind of the progenitor cells and factors. The use of degradable polymers to design this matrix as a three dimensional scaffold was being opted and now the upsurge is to develop in situ scaffolds. The emergence of in situ forming scaffolds have come up with advantages and thus eliminating the defect site and provides better contact between the scaffold and their surrounding tissues. Methyl acrylates and PMMA are used as bone cements. The injectable hydrogels are being intensively explored for the bone grafts. An interesting study shows the development of hydrogel using 1, 4-butanediol diacrylate and tricalciumphosphate. Strontium chloride and Zinc chloride were added for increasing the osteoblast cell density [84]. The recent research is more focused on the development of nanocomposite hydrogels having organic and inorganic composite materials that have similar properties of bone.
Collagen, one of the known natural polymer is extensively used for the bone tissue engineering [85]. The injectable form of the gelatin gel has provided great platform for filling the bone defects. One of the very recent study highlighted the use of gelatin- water-glycerol gel for broad spectrum carrier. This gel was highly biocompatible in vivo. This gel was injected with the demineralized bone matrix and it showed high osteogenesis performance. The study highlighted the property of degradability as well as hydrophilicity. The study demonstrated the ternary gel as irradiation sterilized and universally applied injectable system [86]. The recent approaches utilizes the degradation of the many hydrogel based materials for the in vivo development of the scaffold materials. The overall motive is to develop such system which have a matching rate with the development of the new scaffold with the degradation of the scaffold. The injectable hydrogel systems are preferred as they can carry the imaging agents along with them and make easy for the imaging the in vivo journey of the carrier and hence reduce the number of animals required for the study. One of the very recent study show the development of in situ HA based gel by the Michael addition and thiol sulfide exchange reactions [87]. The study show the structure–degradation relation very important parameter to decide the development of the new in vitro scaffold models for tissue engineering.
Other techniques used to synthesize injectable hydrogels is by using the ring-opening copolymerization. Interestingly, nanohydroxyapatite when incorporated into the biodegradable triblock copolymer poly (ethylene glycol)–poly(ε-caprolactone) −poly (ethylene glycol) formed injectable nanocomposite and showed potential in orthopedic tissue engineering (Fig. 5). Thus these systems are important platforms for the development of key biomaterials which have the excellence in recapitulating the environment for the in vivo tissue engineering.
Fig. (5).
Thermosensitive hydrogel formation of pure PECE and n-HA/PECE nanocomposites with 20 wt % HA at room temperature (R.T.) and 37 °C. Reprinted with permission from Reference [88]. Copyright (2009) American Chemical Society.”
CONCLUSION AND PERSPECTIVES
From the overall discussion in the present review, we can conclude that the forms of the hydrogels have critical impact on the route of administration as well as the entire characteristics of the hydrogels for different application. The present article gives an overview of the injectable form of the hydrogel for the on demand and sustained release of the drugs. These hydrogels have been innovatively designed for the tissue engineering applications. The most important fact about these systems is their stimuli responsiveness such as they can be designed responsive to light, temperature, electric and magnetic field. The modulation of the dose and release by using external control has a great perspective in drug delivery. Various nanofillers are being introduced to increase the encapsulation efficiency and also the carbon based nanomaterials are being explored for better efficacy of the hydrogel systems. The flexibility to functionalize the hydrogels these systems hold a great potential in diagnostics and imaging. The novel system pave the way for developing hybrid injectable hydrogels with specific bioactives and molecular response. The targeted drug delivery uses the features of the hydrogels to be modified structurally and can provide precise control on the drug release and recognition of molecular agents. The article discuss various stimuli such as light, NIR, UV, magnetic field as well as electric field for on demand drug release systems, There is an immense need to develop such systems using these sources and improving their efficacy and eliminating all the drawbacks associated with them such as heat generation, carcinogenic properties as well as the increased risk of toxicity when these stimuli responsive hydrogels are retained in the body for long term.
The efforts by the physicist, chemist and materials scientist have already layed a platform for the triggered release systems which comes with the synergism in development of the novel material, fabrication of the device and the mechanism behind the stimuli triggering. The overall motive behind these efforts is that these systems should exhibit the features before they are in market or in clinics. The tunability in the on state should have wide window and it could be adjusted as required for therapeutic relevance. The off state should be very stable which shows no leakage for the longevity of the implanted device These systems should be checked for inflammatory response [89]. The main emphasis of the research community working in the field of engineered hydrogels for the delivery of the various growth factors to the endogenous progenitor cells in vivo is on working on the subsequent resorption of the hydrogel matrix and thereby promoting the tissue regeneration. Scaffold degradation, appropriate tracking of the system and sensing diagnostics are the demanding future topics that need to be addressed more critically.
ACKNOWLEDGEMENTS
Authors acknowledge NIH grants namely R01-DA027049, RO1-DA 034547, R01-DA037838, R01-DA-040537, and RO1-DA-042706A.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Vashist A, Gupta Y, Ahmad S. Interpenetrating biopolymer network based hydrogels for an effective drug delivery system. Carbohydrate Polym 2012; 87: 1433–9. [Google Scholar]
- [2].Vashist A, Kaushik A, Vashist A, et al. Recent trends on hydrogel based drug delivery systems for infectious diseases. Biomater Sci 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wichterle O, Lim D. Hydrophilic gels for biological use. Nature 1960; 185: 117–8. [Google Scholar]
- [4].Bae KH, Wang L-S, Kurisawa M. Injectable biodegradable hydrogels: progress and challenges. J Mater Chem 2013; 1: 5371–88. [DOI] [PubMed] [Google Scholar]
- [5].Atta S, Khaliq S, Islam A, et al. Injectable biopolymer based hydrogels for drug delivery applications. Int J Biol Macromol 2015; 80: 240–5. [DOI] [PubMed] [Google Scholar]
- [6].Singh NK, Lee DS. In situ gelling pH-and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J Controlled Release 2014; 193: 214–27. [DOI] [PubMed] [Google Scholar]
- [7].Vashist A, Ahmad S. Hydrogels: smart materials for drug delivery. Oriental J Chem 2013; 29: 861–70. [Google Scholar]
- [8].Vashist A, Shahabuddin S, Gupta YK, Ahmad S. Polyol induced interpenetrating networks: chitosan-methylmethacrylate based biocompatible and pH responsive hydrogels for drug delivery system. J Mater Chem 2013; 1: 168–78. [DOI] [PubMed] [Google Scholar]
- [9].Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine 2011; 36: 1906–12. [DOI] [PubMed] [Google Scholar]
- [10].Vashist A, Ahmad S. Hydrogels in tissue engineering: scope and applications. Curr Pharm Biotechnol 2015; 16: 606–20. [DOI] [PubMed] [Google Scholar]
- [11].Yamada Y, Boo JS, Ozawa R, et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Cranio-Maxillofacial Surg 2003; 31: 27–33. [DOI] [PubMed] [Google Scholar]
- [12].Yang J-A, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci 2014; 39: 1973–86. [Google Scholar]
- [13].Kim BS, Park IK, Hoshiba T, et al. Design of artificial extracellular matrices for tissue engineering. Prog Polym Sci 2011; 36: 238–68. [Google Scholar]
- [14].Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science 2012; 336: 1124–8. [DOI] [PubMed] [Google Scholar]
- [15].Sagar V, Atluri VSR, Pilakka-Kanthikeel S, Nair M. Magnetic nanotherapeutics for dysregulated synaptic plasticity during neuroAIDS and drug abuse. Mol Brain 2016; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jayant R, Atluri V, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int J Nanomed 2015; 10: 1077–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jayant RD, Sosa D, Kaushik A, Atluri V, Vashist A, Tomitaka A, Nair M. Current status of non-viral gene therapy for CNS disorders. Expert Opin Drug Deliv 2016; 13: 1433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nair M, Jayant RD, Kaushik A, Sagar V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ghandehari H. Materials for advanced drug delivery in the 21st century: a focus area for Advanced Drug Delivery Reviews. Adv Drug Deliv Rev 2008; 60: 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexander A, Dwivedi S, Giri TK, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Nanomed Controlled Release 2012; 164: 26–40. [DOI] [PubMed] [Google Scholar]
- [21].Ho E, Lowman A, Marcolongo M. Synthesis and characterization of an injectable hydrogel with tunable mechanical properties for soft tissue repair. Biomacromolecules 2006; 7: 3223–8. [DOI] [PubMed] [Google Scholar]
- [22].Jin R, Teixeira LM, Dijkstra P, Van Blitterswijk C, Karperien M, Feijen J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran–hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010; 31: 3103–13. [DOI] [PubMed] [Google Scholar]
- [23].Schaal JL, Li X, Mastria E, et al. Injectable polypeptide micelles that form radiation crosslinked hydrogels in situ for intratumoral radiotherapy. J Control Release 2016; 228: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels … A review. Saudi Pharm J 2016; 24: 554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kodama N, Nagata M, Tabata Y, Ozeki M, Ninomiya T, Takagi R. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone 2009; 44: 699–707. [DOI] [PubMed] [Google Scholar]
- [26].Naderi-Meshkin H, Andreas K, Matin MM, et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int 2014; 38: 72–84. [DOI] [PubMed] [Google Scholar]
- [27].Purcell BP, Lobb D, Charati MB, et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater 2014; 13: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma Z, Nelson DM, Hong Y, Wagner WR. A thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability. Biomacromolecules 2010; 11: 1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sim HJ, Thambi T, Lee DS. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J Nanomed Mater Chem 2015; 3: 8892–901. [DOI] [PubMed] [Google Scholar]
- [30].Phan VHG, Thambi T, Gil MS, Lee DS. Temperature and pH- sensitive injectable hydrogels based on poly(sulfamethazine carbonate urethane) for sustained delivery of cationic proteins. Polymer 2017; 109: 38–48. [Google Scholar]
- [31].Khaing ZZ, Ehsanipour A, Hofstetter CP, Seidlits SK. Injectable hydrogels for spinal cord repair: a focus on swelling and intraspinal pressure. Cells Tissues Organs 2016; 202: 67–84. [DOI] [PubMed] [Google Scholar]
- [32].Dong Y, Wang W, Veiseh O, et al. Injectable and Glucose- Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016; 32: 8743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Progress in Polymer Science 2006; 31: 487–531. [Google Scholar]
- [34].Nimmo CM, Shoichet MS. Regenerative biomaterials that “click”: Simple, aqueous-based protocols for hydrogel synthesis, surface immobilization, and 3D patterning. Bioconjugate Chem 2011; 22: 2199–209. [DOI] [PubMed] [Google Scholar]
- [35].Kim K, Park S, Yang JA, et al. Injectable hyaluronic acid–tyramine hydrogels for the treatment of rheumatoid arthritis. Acta biomaterialia 2011; 7: 666–74. [DOI] [PubMed] [Google Scholar]
- [36].Tai H, Howard D, Takae S, et al. Photo-cross-linked hydrogels from thermoresponsive PEGMEMA-PPGMA-EGDMA copolymers containing multiple methacrylate groups: mechanical property, swelling, protein release, and cytotoxicity. Biomacromolecules 2009; 10: 2895–903. [DOI] [PubMed] [Google Scholar]
- [37].Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Nanomed Pharm Biopharm 2004; 57: 35–52. [DOI] [PubMed] [Google Scholar]
- [38].Cao SE, Chen SY, Tian JM, et al. Intrathecal delivery of ketorolac loaded in situ gels for prolonged analgesic and anti-Inflammatory activity in vertebral fracture. Tropical J Nanomed Pharm Res 2016; 15: 5–11. [Google Scholar]
- [39].Qiumian Y, Xiaocui Y, Rui L, et al. In vitro Antifungal Activity of Pluronic-based Thermo-Sensitive Itraconazole Gels for Vaginal Administration. J Nanomed Pharm Biomed Sci 2016; 6. [Google Scholar]
- [40].Mandal S, Thimmasetty MK, Prabhushankar G, Geetha M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int J Nanomed Pharm Invest 2012; 2: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Morsi N, Ghorab D, Refai H, Teba H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int J Nanomed Pharm 2016; 506: 57–67. [DOI] [PubMed] [Google Scholar]
- [42].Singh RM, Kumar A, Pathak K. Mucoadhesive in situ nasal gelling drug delivery systems for modulated drug delivery. Expert Opin Drug Deliv 2013; 10: 115–30. [DOI] [PubMed] [Google Scholar]
- [43].Ni Y, Yates KM. In-situ gel formation of pectin. In: ed.êds. Google Patents; 2004. [Google Scholar]
- [44].Pandey P, Cabot PJ, Wallwork B, Panizza BJ, Parekh HS. Formulation, functional evaluation and ex vivo performance of thermoresponsive soluble gels-A platform for therapeutic delivery to mucosal sinus tissue. Eur J Nanomed Pharm Sci 2017; 96: 499–507. [DOI] [PubMed] [Google Scholar]
- [45].Dimic-Misic K, Rantanen J, Maloney TC, Gane PA. Gel structure phase behavior in micro nanofibrillated cellulose containing in situ precipitated calcium carbonate. J Nanomed Applied Polym Sci 2016. [Google Scholar]
- [46].Barse R, Kokare C, Tagalpallewar A. Influence of hydroxypropyl-methylcellulose and poloxamer composite on developed ophthalmic in situ gel: Ex vivo and in vivo characterization. J Nanomed Drug Deliv Sci Technol 2016; 33: 66–74. [Google Scholar]
- [47].Kaur P, Garg T, Rath G, Goyal AK. In situ nasal gel drug delivery: A novel approach for brain targeting through the mucosal membrane. Artificial Cells, Nanomed Biotechnol 2016; 44: 1167–76. [DOI] [PubMed] [Google Scholar]
- [48].Devasani S, Dev A, Rathod SR, Deshmukh G. An overview of in situ gelling systems. Pharm Biol Evaluations 2016; 3: 60–9. [Google Scholar]
- [49].Kouchak M. In Situ Gelling Systems for Drug Delivery. Jundishapur J Nanomed Nat Pharm Prod 2014; 9: e20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sanson N, Rieger J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym Chem 2010; 1: 965–77. [Google Scholar]
- [51].Wang X, Wang C, Zhang Q, Cheng Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem Commun 2016; 52: 978–81. [DOI] [PubMed] [Google Scholar]
- [52].Yu J, Ha W, Sun JN, Shi YP. Supramolecular hybrid hydrogel based on host–guest interaction and its application in drug delivery. ACS Appl Mater Interfaces 2014; 6: 19544–51. [DOI] [PubMed] [Google Scholar]
- [53].Campbell S, Maitland D, Hoare T. Enhanced pulsatile drug release from injectable magnetic hydrogels with embedded thermosensitive microgels. ACS Macro Lett 2015; 4: 312–6. [DOI] [PubMed] [Google Scholar]
- [54].Huebsch N, Kearney CJ, Zhao X, et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Natl Acad Sci 2014; 111: 9762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vemula PK, Boilard E, Syed A, et al. On-demand drug delivery from self-assembled nanofibrous gels: A new approach for treatment of proteolytic disease. J Nanomed Biomed Mater Res 2011; 97: 103–10. [DOI] [PubMed] [Google Scholar]
- [56].Su D, Jiang L, Chen X, Dong J, Shao Z. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl Mater Interfaces 2016; 8: 9619–28. [DOI] [PubMed] [Google Scholar]
- [57].Sagar V, Atluri V, Tomitaka A, et al. Coupling of transient near infrared photonic with magnetic nanoparticle for potential dissipation-free biomedical application in brain. Sci Rep 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peng K, Tomatsu I, Kros A. Light controlled protein release from a supramolecular hydrogel. Chem Commun 2010; 46: 4094–6. [DOI] [PubMed] [Google Scholar]
- [59].Tong R, Hemmati HD, Langer R, Kohane DS. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J Nanomed Am Chem Soc 2012; 134: 8848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim H, Lee H, Seong K-Y, Lee E, Yang SY, Yoon J. Visible light-triggered on-demand drug release from hybrid hydrogels and its application in transdermal patches. Adv Healthcare Mater 2015; 4: 2071–7. [DOI] [PubMed] [Google Scholar]
- [61].Lee E, Lee H, Yoo SI, Yoon J. Photothermally triggered fast responding hydrogels incorporating a hydrophobic moiety for light-controlled microvalves. ACS Appl Mater Interfaces 2014; 6: 16949–55. [DOI] [PubMed] [Google Scholar]
- [62].Coti KK, Belowich ME, Liong M, et al. Mechanised nanoparticles for drug delivery. Nanoscale 2009; 1: 16–39. [DOI] [PubMed] [Google Scholar]
- [63].Szacilowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G. Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev 2005; 105: 2647–94. [DOI] [PubMed] [Google Scholar]
- [64].Jalani G, Naccache R, Rosenzweig DH, Haglund L, Vetrone F, Cerruti M. Photocleavable hydrogel-coated upconverting nanoparticles: a multifunctional theranostic platform for nir imaging and on-demand macromolecular delivery. J Nanomed Am Chem Soc 2016; 138: 1078–83. [DOI] [PubMed] [Google Scholar]
- [65].Yang X, Liu X, Liu Z, Pu F, Ren J, Qu X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv Mater 2012; 24: 2890–5. [DOI] [PubMed] [Google Scholar]
- [66].Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013; 12: 991–1003. [DOI] [PubMed] [Google Scholar]
- [67].Blum AP, Kammeyer JK, Rush AM, Callmann CE, Hahn ME, Gianneschi NC. Stimuli-responsive nanomaterials for biomedical applications. J Nanomed Am Chem Soc 2015; 137: 2140–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yoon SJ, Fang YH, Lim CH, et al. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Nanomed Biomed Mater Res 2009; 91: 163–71. [DOI] [PubMed] [Google Scholar]
- [69].Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly (aldehyde guluronate) hydrogels for bone tissue engineering. J Nanomed Biomed Mater Res 2001; 56: 228–33. [DOI] [PubMed] [Google Scholar]
- [70].Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 2009; 30: 5409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hoemann C, Sun J, Legare A, McKee M, Buschmann M. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage 2005; 13: 318–29. [DOI] [PubMed] [Google Scholar]
- [72].Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng 2005; 11: 1860–6. [DOI] [PubMed] [Google Scholar]
- [73].Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Rev 2008; 14: 199–215. [DOI] [PubMed] [Google Scholar]
- [74].Domingues RMA, Silva M, Gershovich P, et al. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem 2015; 26: 1571–81. [DOI] [PubMed] [Google Scholar]
- [75].Knopf-Marques H, Pravda M, Wolfova L, et al. Hyaluronic acid and its derivatives in coating and delivery systems: applications in tissue engineering, regenerative medicine and immunomodulation. Adv Healthcare Mater 2016. [DOI] [PubMed] [Google Scholar]
- [76].Wobma H, Vunjak-Novakovic G. Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng Rev 2016; 22: 101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kolettis TM, Vilaeti A, Dimos K, Tsitou N, Agathopoulos S. Tissue engineering for post-myocardial infarction ventricular remodeling. Mini-Rev Med Chemy 2011; 11: 263–70. [DOI] [PubMed] [Google Scholar]
- [78].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater 2009; 21: 3307–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hasan A, Khattab A, Islam MA, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci 2015; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Plotkin M, Vaibavi SR, Rufaihah AJ, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials 2014; 35: 1429–38. [DOI] [PubMed] [Google Scholar]
- [81].Watson BM, Vo TN, Engel PS, Mikos AG. Biodegradable, in situ-forming cell-laden hydrogel composites of hydroxyapatite nanoparticles for bone regeneration. Indus Eng Chem Res 2015; 54: 10206–11. [Google Scholar]
- [82].Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bullet World Health Organization 2003; 81: 646–56. [PMC free article] [PubMed] [Google Scholar]
- [83].Mody GM, Woolf AD. The global burden of musculoskeletal disorders. Business Briefing: Eur Pharmacother 2003: 1–5. [Google Scholar]
- [84].Tommasi G, Perni S, Prokopovich P. An injectable hydrogel as bone graft material with added antimicrobial properties. Tissue Engineering. Part A 2016; 22: 862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomaterialia 2008; 4: 1126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhao Y, Han L, Yan J, et al. Irradiation sterilized gelatin–water–glycerol ternary gel as an injectable carrier for bone tissue engineering. Adv Healthcare Mater 2016: n/a-n/a. [DOI] [PubMed] [Google Scholar]
- [87].Ranga A, Lutolf MP, Hilborn J, Ossipov DA. Hyaluronic acid hydrogels formed in situ by transglutaminase-catalyzed reaction. Biomacromolecules 2016; 17: 1553–60. [DOI] [PubMed] [Google Scholar]
- [88].Fu S, Guo G, Gong C, et al. Injectable Biodegradable Thermosensitive Hydrogel Composite for Orthopedic Tissue Engineering. 1. Preparation and Characterization of Nanohydroxyapatite/Poly (ethylene glycol)− Poly (ε-caprolactone)− Poly (ethylene glycol) Hydrogel Nanocomposites. J Nanomed Phys Chem 2009; 113: 16518–25. [DOI] [PubMed] [Google Scholar]
- [89].Timko BP, Kohane DS. Materials to clinical devices: technologies for remotely triggered drug delivery. Clin Therapeut 2012; 34: S25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]