Summary
ASAP1 is a multi-domain ArfGAP that controls cell migration, spreading, and focal adhesion dynamics. Although its GAP activity contributes to remodeling of the actin cytoskeleton, it does not fully explain all cellular functions of ASAP1. Here we find that ASAP1 regulates actin filament assembly directly through its N-BAR domain and controls stress fiber maintenance. ASAP1 depletion caused defects in stress fiber organization. Conversely, overexpression of ASAP1 enhanced actin remodeling. The BAR-PH fragment was sufficient to affect actin. ASAP1 with the BAR domain replaced with the BAR domain of the related ACAP1 did not affect actin. The BAR-PH tandem of ASAP1 bound and bundled actin filaments directly, whereas the presence of the ArfGAP and the C-terminal linker/SH3 domain reduced binding and bundling of filaments by BAR-PH. Together these data provide evidence that ASAP1 may regulate the actin cytoskeleton through direct interaction of the BAR-PH domain with actin filaments.
Subject Areas: Biological Sciences, Cell Biology, Functional Aspects of Cell Biology
Graphical Abstract
Highlights
-
•
Downregulation of ArfGAP ASAP1 leads to loss of stress fibers
-
•
ASAP1 N-BAR domain organizes actin filaments into bundles
-
•
Actin bundling by the BAR domain is intramolecularly inhibited by C-terminal domains
-
•
Actin remodeling property is not shared with a related ArfGAP ACAP1
Biological Sciences; Cell Biology; Functional Aspects of Cell Biology
Introduction
Assembly, maintenance, and regulation of the actin cytoskeleton are important for normal cell functions, such as cytokinesis, immune function, morphogenesis, and migration (Gavin, 1999, Wang, 1984, Izdebska et al., 2018, Rougerie et al., 2013, Vonna et al., 2007, Wynn et al., 2013, Blanchoin et al., 2014). Actin dynamics are controlled by an array of actin-regulating proteins (Lappalainen, 2016, Pollard, 2016). Among the diverse families of actin-regulating proteins, the BAR domain proteins have emerged as facilitators of actin filament reorganization. ASAP1, a BAR-domain-containing member of the human ArfGAP family, has been established as a regulator of the actin cytoskeleton and focal adhesion (FA) complexes. Recent observations suggest that regions outside of the ArfGAP domain participate in the regulation of the actin structures (Randazzo et al., 2000, Chen et al., 2016).
In humans, 31 genes encode proteins containing an ArfGAP (ADP ribosylation factor GTPase-activating protein) domain, which stimulates the hydrolysis of the GTP nucleotide by the Arf (ADP-ribosylation factor) GTPases (Kahn et al., 2008). The ArfGAP family has been shown to regulate actin-based structures (reviewed in Tanna et al., 2019). ASAP and ACAP family members are unique in that they contain an N-terminal BAR domain. ASAP1 (also known as DEF1, PAG2, AMAP1, centaurin β4, and DDEF1) is composed of N-BAR, PH, ArfGAP, ankyrin and a stretch of eight E/DLPPKP repeats, a proline-rich domain, and SH3 domains. It was first discovered on the basis of GAP activity and for binding Src and later, as a differentiation enhancement factor (Bharti et al., 2007, Brown et al., 1998, King et al., 1999). ASAP1 affects processes in normal cells, such as neurite outgrowth, and in diseases, such as uveal melanoma and colorectal, prostate, breast, and head and neck cancers (Inoue et al., 2008, Mazelova et al., 2009, Wang et al., 2012, Muller et al., 2010, Ehlers et al., 2005, Sato et al., 2014, Lin et al., 2008, Li et al., 2018).
In addition to Src, it binds the oncogenes CrkL, FAK, and cortactin. ASAP1 localizes to the FAs via its interaction with focal adhesion kinase (FAK) and adapter protein CrkL and to the membrane ruffles through CD2AP (Randazzo et al., 2000, Liu et al., 2002, Liu et al., 2005, Oda et al., 2003). ASAP1 has been shown to regulate podosome and invadopodia formation (Yonenaga et al., 2005, Onodera et al., 2005). The BAR domain of ASAP1 induces membrane tubulation, as described for many other BAR domain-containing proteins (Nie et al., 2006). Downregulation of ASAP1 expression in rat fibroblasts inhibits cell migration and impedes cell spreading (Liu et al., 2005), whereas in NIH 3T3 fibroblasts and HeLa cells, knockdown of ASAP1 accelerates cell migration and cell spreading (Chen et al., 2016).
The BAR domain superfamily is composed of four subfamilies—BAR, N-BAR (previously clustered together with BAR), F-BAR, and I-BAR. BAR domain proteins have additional domains—catalytic, lipid-binding, or protein-protein interaction domains—which may also serve as regulatory elements to control the localization and activity of the BAR domain (reviewed in Carman and Dominguez, 2018). N-BAR domain protein BIN1 (amphiphysin II) has been shown to directly organize actin filaments into bundles and stabilize tau-induced actin bundles via its BAR domain, which may affect neuronal functions, such as those dysregulated in Alzheimer disease (Drager et al., 2017, Lasorsa et al., 2018, Calafate et al., 2016). A member of the F-BAR domain family, PACSIN2 (syndapin II), has also been shown to directly bind actin filaments and is important for maintenance of the adherens junctions in endothelial cells (Kostan et al., 2014, Dorland et al., 2016). The I-BAR domain family members (MIM) Missing-in-Metastasis and IRSp53 have been shown to bundle actin in vitro and induce formation of filopodia (Nakagawa, 2003, Yamagishi et al., 2004, Millard et al., 2005, Lee et al., 2007). Members of the BAR superfamily thus stand at the crossroads of cellular signaling, membrane, and cytoskeletal dynamics.
Actin filaments take on multiple forms, one of them being actin stress fibers. There are three major types of stress fibers—ventral stress fibers (VSFs), dorsal stress fibers (DSFs), and transverse arcs (TAs). Stress fibers participate in dynamic processes during cell migration and adhesion to substratum and are assembled via different mechanisms (Hotulainen and Lappalainen, 2006, Tojkander et al., 2011, Tojkander et al., 2012, Tojkander et al., 2015). VSFs and DSFs are anchored to FAs—VSFs anchor at both ends and DSFs anchor at one end only. They also differ in location, orientation, and composition, with VSFs rich in non-muscle myosin 2, and DSFs having little detectable NM2. TAs are prominent in actively migrating and spreading cells and do not associate with FAs, but rather extend to the DSFs. TAs and DSFs may combine to form VSFs (Kovac et al., 2013, Vallenius, 2013).
Recently, we have determined that the BAR-PH region of ASAP1 binds non-muscle myosin 2A (NM2A), ASAP1 and NM2A colocalize at circular dorsal ruffles upon mitogen stimulation, and downregulation of ASAP1 leads to disruption in actin and NM2A colocalization (Chen et al., 2016). The proteomics screen that was used to identify non-muscle myosin 2A also identified actin as a putative binding partner of the BAR-PH region. We have additionally detected irregular F-actin staining in the fibroblasts depleted of ASAP1. The emerging field of the BAR-domain superfamily as actin regulators has led us to hypothesize that ASAP1 could also be directly involved in the assembly or maintenance of actin structures. As ASAP1 contributes to FA assembly and regulates association of NM2A with actin filaments, we focused on the VSFs as a model of bundled actin filaments. We chose mouse NIH 3T3 and primary human foreskin fibroblasts (HFFs), which contain prominent VSFs, as cell models to study the role ASAP1 may play in the regulation of actin structures. Our biochemical and cell biological data indicate that the N-BAR domain of ASAP1 directly binds and bundles actin filaments and regulates dynamic actin structures.
Results
Depletion of ASAP1 Leads to Loss of Ventral Stress Fibers and Perturbs Levels of Filamentous Actin in Fibroblasts
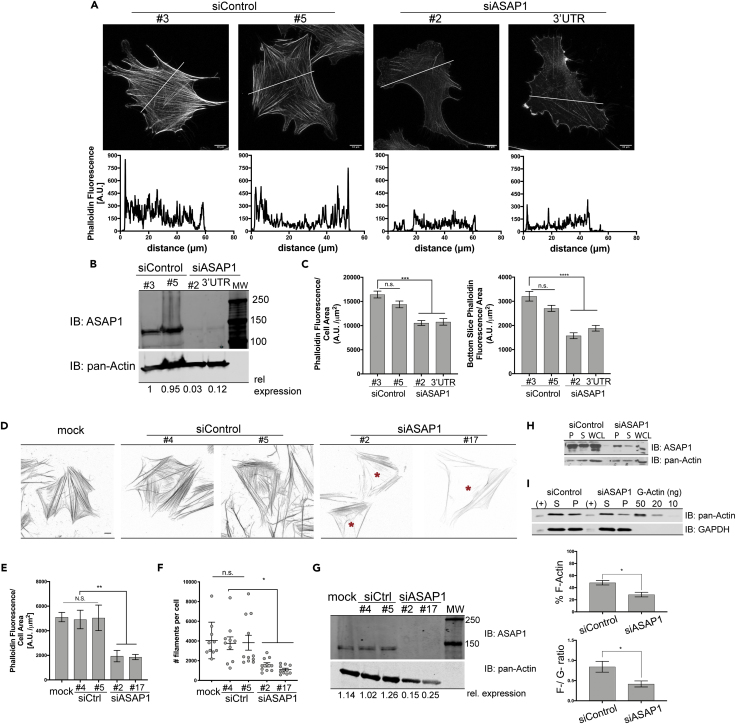
We have previously observed that the knockdown of ASAP1 in NIH 3T3 fibroblasts leads to reduced colocalization of NM2A and F-actin (Chen et al., 2016). In cells with reduced expression of ASAP1, the morphology appeared irregular with presence of thinner, misaligned stress fibers compared with cells with unaltered ASAP1 levels. To further evaluate the effect of ASAP1 on actin stress fibers, we transiently transfected mouse NIH 3T3 fibroblast and primary HFF-1 cells with two independent control and ASAP1 small interfering RNAs (siRNAs), re-plated the cells on fibronectin in serum-free media for 5.5 h, stained with fluorescently labeled phalloidin, and examined single cells using confocal microscopy. Total phalloidin fluorescence intensity, a readout of total filamentous actin content in the cell, as well as the phalloidin intensity of the ventral portion, decreased by 20%–30% in NIH 3T3 cells (Figures 1A–1C) and 30%–40% in HFF-1 cells transfected with ASAP1 siRNA (Figures S1A–S1D). We then extracted and stained stress fibers attached to the fibronectin matrix from the HFF-1 cells to observe changes in VSF organization (Katoh et al., 2000, Eltzner et al., 2015). Cells transfected with ASAP1 siRNA had fewer VSFs than the control or mock-treated cells, as indicated by the red asterisks in Figure 1D. Total phalloidin fluorescence quantification confirmed that the ASAP1-depleted cells had less filamentous actin (Figure 1E) and fewer stress fibers than the control cells (Figure 1F). In addition, we used immunoblotting-based analysis (see In Vivo F-Actin/G-Actin Assay in Materials and Methods) to probe for changes in the ratios of globular and filamentous actin. NIH 3T3 fibroblasts were transfected with control or ASAP1 siRNA for 72 h (ASAP1 expression is shown in Figure 1G), plated in fibronectin-coated plates for 5.5 h, lysed in F-actin stabilization buffer, and subjected to high-speed centrifugation at 100,000 × g for 1 h at 37°C to separate actin filaments (pellet, P) and globular actin (supernatant, S). Consistently with immunofluorescence (IF)-based assays, immunoblotting-based analysis showed 40% decrease in filamentous actin content (% F-actin) of 3T3 cells (48% ± 4% in control cells versus 28% ± 4% in siASAP1 cells) and ratio of filamentous to globular actin (F-/G-ratio) reduced from 0.84 ± 0.13 in control cells to 0.41 ± 0.07 in knockdown cells (Figures 1H and 1I). Thus, ASAP1 is important for maintaining cellular filamentous actin levels and VSF network.
Figure 1.
Reduced Expression of ASAP1 Decreases the Levels of Filamentous Actin and Affects Distribution of Stress Fibers in Primary Foreskin and 3T3 Fibroblasts
(A–C) Effect of ASAP1 on actin in mouse NIH 3T3 fibroblasts. NIH 3T3 fibroblasts were transiently transfected with two independent control or ASAP1 siRNAs; 72 h post-transfection, cells were re-plated on fibronectin-coated coverslips for 5.5 h, fixed, and stained with phalloidin Alexa Fluor 488. Single-cell z stack images were acquired on a Leica SP8 confocal microscope using 63× oil objective. (A) Representative maximum intensity projections (MIPs) of F-actin stained with phalloidin Alexa Fluor 488 control and ASAP1-depleted 3T3 cells are shown. Line scans of fluorescence intensity perpendicular to the visualized stress fibers (white lines in images) of the sum of stacks are presented below the images. Scale bar, 10 μm. (B) ASAP1 levels in siRNA-transfected cells. ASAP1 in cells lysates was determined by quantitative immunoblotting. (C) Cellular content of F-actin. Relative F-actin content in cells was determined by quantifying phalloidin fluorescence, n = 15. Results are presented as total surface fluorescence divided by cell area (left panel) or fluorescence of the bottom cell slice only divided by the area (right panel) with the units being arbitrary fluorescence units (AU)/μm2 and representative of three independent experiments. See also Figure S1.
(D–G) Effect of ASAP1 on actin in primary human foreskin fibroblasts (HFF-1). HFF-1s were mock treated or transiently transfected with two independent control or ASAP1 siRNAs. Post-transfection, cells were re-plated on fibronectin-coated coverslips, followed by stress fiber extraction. Isolated stress fibers were fixed and stained with phalloidin Alexa Fluor 488. (D) Representative MIP images. LUTs (look-up tables) were inverted in Fiji and contrast enhanced equally across all images. Scale bar, 10 μm. Red asterisks indicate lack of substratum-attached stress fibers in siASAP1 cells. (E) Actin content in transfected HFF-1 cells. Filamentous actin content in extracted cells was quantified as total cell fluorescence on sum of stacks per cell area; 10 cells for each condition were analyzed. (F) Estimate of number of stress fibers in transfected HFF-1s. (G) ASAP1 levels in transfected HFF-1s. ASAP1 levels were determined as described for Figure 1B.
(H and I) Effect of ASAP1 knockdown on F-actin content of NIH 3T3 fibroblasts. NIH 3T3 fibroblasts were transfected as previously described and processed with in vivo actin assay kit to assess F-actin and G-actin content by immunoblotting. (H) ASAP1 levels in the siRNA-transfected cells. ASAP1 levels were determined by immunoblot. (I) Relative F- and G-actin content of cells. Quantitative immunoblots of cellular fractions containing F- and G-actin, as indicated, from control and ASAP1 siRNA-treated cells are shown. The percentage of filamentous actin (% F-Actin) and F- to G-actin ratios were calculated and the summary of 5 combined experiments is presented in the bar graphs below the blots. S, supernatant; P, pellet; WCL, whole-cell lysate; (+), positive immunotransfer control G-actin standard.
Quantifications represented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant.
ASAP1 Remodels Actin in Fibroblasts, Binds to and Bundles Actin Filaments In Vitro, and the C Terminus of ASAP1 Modulates N-BAR-Mediated Actin Binding and Bundling Activity
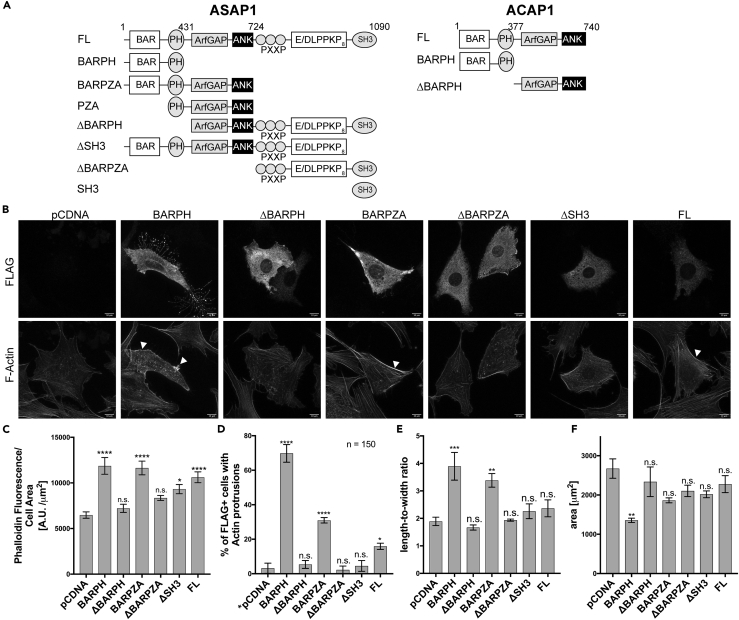
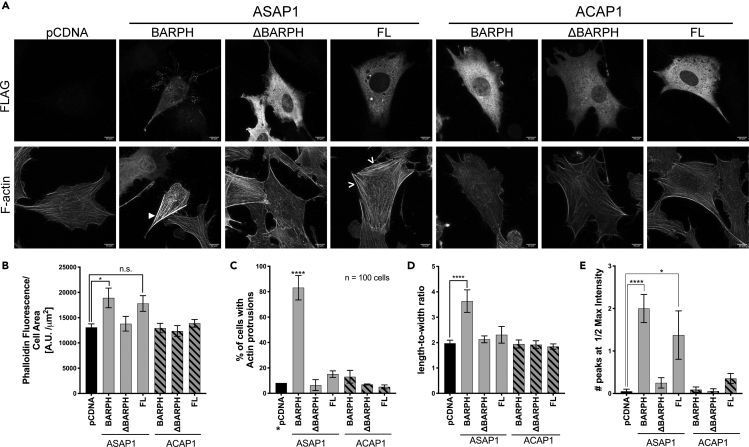
We next examined the effect of overexpression of ASAP1 on the filamentous actin content and stress fibers. We transiently transfected NIH 3T3 cells with FLAG-tagged full-length or domain truncations of ASAP1 (see Figure 2A for domain boundaries and nomenclature), re-plated cells on fibronectin in serum-free media for 5.5 h, stained with fluorescent anti-FLAG antibody and phalloidin, and examined cells using confocal microscopy (representative images shown in Figure 2B). Quantification of total phalloidin fluorescence indicated that only constructs containing the BAR-PH tandem led to the increase of filamentous actin content (Figure 2C). We did not observe any differences in phalloidin fluorescence intensity between BAR-PH, BARPZA (BAR-PH-GAP-Ank), and the full-length ASAP1. We also observed similar phalloidin fluorescence intensity between low-, mid-, and high-full-length ASAP1-expressing cells (Figure S2A). Phalloidin staining also revealed multiple actin microspikes and protrusions in cells expressing ASAP1 BAR-PH (Figure 2D), whereas overexpression of BARPZA and the full-length ASAP1 protein had lower number of cells with actin protrusions (30% and 17%, respectively, compared with 70% of BAR-PH-expressing cells). In addition, we observed that cells expressing the BAR-PH and BARPZA have a collapsed, elongated cell shape (Figure 2E), and BAR-PH-expressing cells have a reduced cell area (Figure 2F).
Figure 2.
Overexpression of the BAR-PH Tandem of ASAP1 Induces Aberrant Actin Protrusions and Increases Filamentous Actin Content in NIH 3T3 Fibroblasts
(A) Domain architecture of ASAP1 and ACAP1. Schematics of the full length and recombinant proteins are shown. BAR, Bin/Amphiphysin/Rvs; PH, Pleckstrin Homology; ArfGAP, Arf GTPase-activating protein; ANK, ankyrin repeat; PXXP, proline-rich motif; E/DLPPKP, a stretch of eight repeats of E/DLPPKP; SH3, Src homology 3 domain.
(B–F) Effect of BAR-PH tandem overexpression on actin in NIH 3T3 fibroblasts. NIH 3T3 cells were transiently transfected with empty pCDNA vector or FLAG-tagged full-length or domain truncations of ASAP1, re-plated after 24 h on fibronectin-coated coverslips, fixed, and stained with anti-FLAG antibody (Alexa Fluor 488) and fluorescently labeled phalloidin (Alexa Fluor 594). Images were acquired as described for Figure 1. (B) Images of cells overexpressing the BAR-PH tandem. Representative MIPs of the FLAG channel (recombinant ASAP1 protein, upper panels) and phalloidin channel (actin, lower panels) are shown. White arrowheads point to areas of thickened cortical actin. Scale bar, 10 μm. (C) F-actin content of cells expressing ASAP1 and fragments of ASAP1. Phalloidin fluorescence was used as a measure of filamentous actin as described in Figure 1. See also Figure S2. (D) Effect of ASAP1 and domain fragments of ASAP1 on actin protrusions. The percentage of FLAG-positive cells with actin protrusions is presented. Random empty vector cells were counted for presence of protrusions as the control. (E) Effect of expression of ASAP1 and domain fragments on cell shape. Length-to-width ratios were determined as described in Figure 1. (F) Effect of overexpression of ASAP1 and domain fragments on cell area. Cell area was determined as described for Figure 1. A representative experiment of three independent experiments is shown. The data are summarized as means ± SEM, for (C, D, and F); 15 cells were analyzed for each condition. The data were analyzed with one-way ANOVA followed by multiple comparisons using the Tukey's post hoc test, in which all conditions were compared to the pCDNA control, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
We have previously observed that ASAP1 loss leads to decrease in number of mature adhesions and stress fibers are known to guide maturation of FAs (Chen et al., 2016, Oakes et al., 2012). Co-staining of BAR-PH- and ΔBAR-PH-expressing 3T3 fibroblasts with paxillin, an FA marker, revealed subcellular localization of ΔBAR-PH, but not BAR-PH, to immature adhesions (Figure S2B).
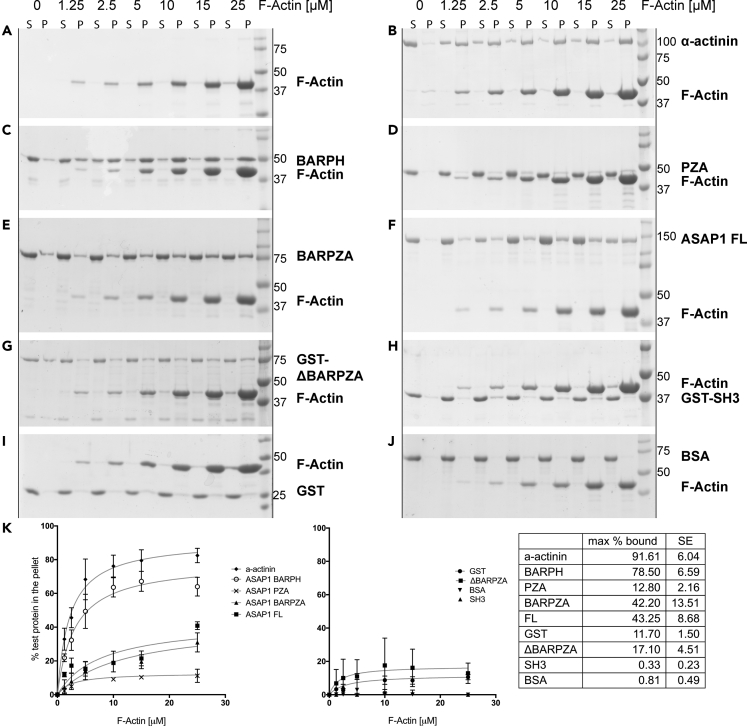
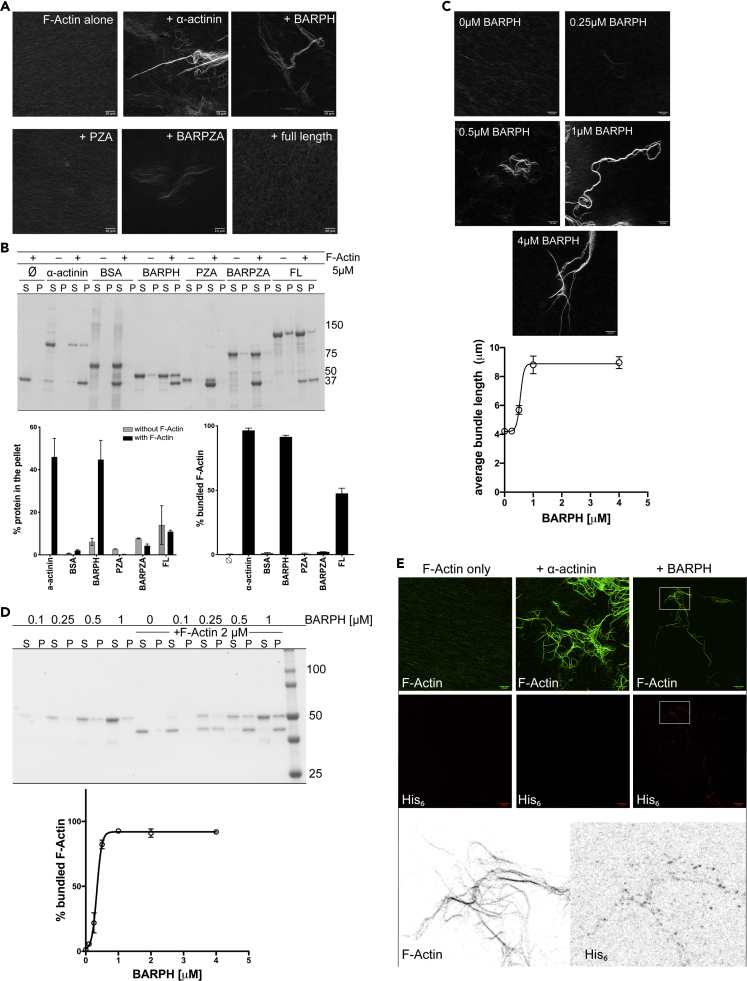
The observation that overexpression of ASAP1 BAR-PH leads to reorganization of actin stress fibers and formation of actin microspikes in cells led us to hypothesize that direct interaction between the BAR-PH tandem of ASAP1 and actin filaments mediates actin remodeling. To determine if ASAP1 binds directly to actin and to dissect the region that binds to filaments, we purified recombinant full-length and fragments of ASAP1 (see Figure S3A for protein purity) and performed binding assays using high-speed actin co-sedimentation. Purified ASAP1 and ASAP1 fragments, as well as negative (BSA) and positive (α-actinin) controls, at 2 μM, were incubated with increasing (0–25 μM) concentrations of F-actin for 30 min at room temperature in F-actin buffer and subjected to high-speed centrifugation at 150,000 × g for 1.5 h at 23°C to pellet actin filaments and proteins bound to filaments. Pellet (actin filaments and bound proteins) and supernatant (unbound proteins and residual G-actin) fractions were resuspended in an equal volume of Laemmli sample buffer and resolved on SDS-PAGE. Pellet fractions were analyzed as a percentage of total (pellet/(supernatant + pellet)) and plotted for all proteins examined (Figure 3K). The majority of actin was in the filamentous form (Figure 3A). We observed that, similar to the positive control α-actinin (Figure 3B), ASAP1 BAR-PH bound to actin filaments in a concentration-dependent manner (Figure 3C). PZA, the fragment that contains the PH domain, but lacks the BAR domain, did not co-sediment with actin filaments, suggesting that the BAR domain is the actin-binding module in the BAR-PH tandem (Figure 3D). ASAP1 BARPZA, and FL, which in addition to the BAR-PH have the GAP and ANK repeats and GAP, ANK, and PXXP, E/DLPPKP, and SH3 domains, respectively, bound less efficiently. Forty-two percent and 43% of BARPZA and FL proteins were found in the pellet, respectively (Figures 3E and 3F). There was no detectable binding of ΔBARPZA, which only contains PXXP, E/DLPPKP, and SH3 domains, to actin (Figure 3G). The isolated SH3 domain, likewise, did not bind actin filaments (GST-SH3) (Figure 3H). Neither GST nor BSA bound to BAR-PH (Figures 3I and 3J). The results from the high-speed actin co-sedimentation assay (summarized in the table) thus indicate that the BAR domain of ASAP1 binds to actin filaments in vitro.
Figure 3.
ASAP1 Binds Directly to Actin Filaments through Its N-BAR Domain
(A–J) Purified recombinant ASAP1 full-length and domain fragments (2 μM) were subjected to high-speed co-sedimentation assay with the indicated concentrations (0–25 μM) of rabbit muscle F-actin. Actin filaments and filament-bound proteins (P, pellet) were separated from residual globular actin and unbound proteins (S, supernatant), resolved on single percentage (10 or 12%) SDS-PAGE, and stained with GelCode Blue. Co-sedimentation with BSA and GST (negative controls) and α-actinin (positive control) was performed in the same fashion. F-actin was titrated into solutions containing (A) no additional proteins, (B) α-actinin, (C) ASAP1 BAR-PH, (D) PZA, (E) BARPZA, (F) full-length ASAP1, (G) GST-ΔBARPZA, (H) GST-SH3, (I) GST, and (J) BSA. Gels are representative of at least three independent experiments.
(K) Summary of quantification of binding for 3 experiments. The extent of binding to actin was calculated as the percentage of total protein in the pellet, e.g., ASAP1 in P/(ASAP1 in P + ASAP1 in S) × 100%, and plotted against the concentration of actin. Data from three independent experiments were combined, and each point on the graph is a mean ± SEM. Table (right) summarizes the estimated % maximum bound of each test protein with standard error (SE) at saturating actin concentration.
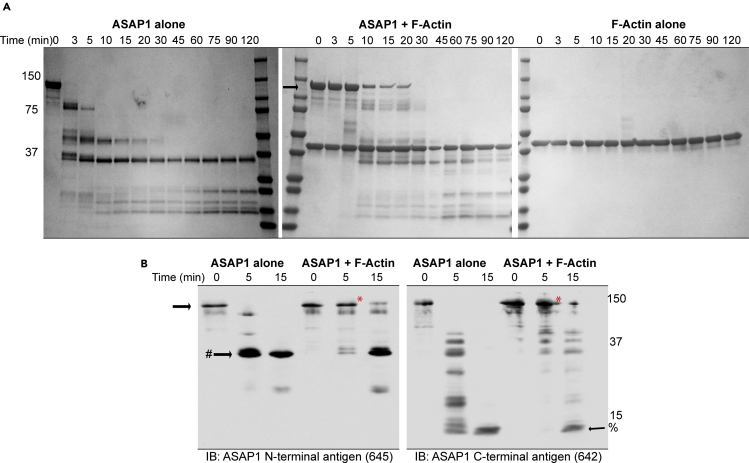
Protease protection was used as a complementary approach to assess binding of ASAP1 to actin filaments. Subtilisin A is a commonly used protease to study protein interactions with filamentous actin, because unlike globular actin, filamentous actin is not susceptible to degradation by subtilisin A (Fievez and Carlier, 1993). We reasoned that interaction with actin filaments will protect ASAP1 from proteolytic degradation by subtilisin A. ASAP1 (1 mg/mL) and F-actin (0.5 mg/mL), incubated together or alone under similar conditions as those used for co-sedimentation, were treated with subtilisin A (0.1 μg/mL) for the duration indicated in Figure 4. A parallel experiment with globular actin (G-actin) in place of F-actin was performed in a similar manner (Figure S3B). Incubation of ASAP1 alone with subtilisin A led to conversion of the protein into lower-molecular-weight products after 3 min (Figure 4A, left gel). When ASAP1 was pre-incubated with F-actin, digestion of the full-length ASAP1 band (black arrow) was slowed, with loss of full-length ASAP1 requiring 20 min (middle gel). F-actin was resistant to proteolysis, as reported in the literature (right gel). G-actin did not affect the rate of ASAP1 proteolysis (Figure S3B). To assess which region is protected from subtilisin A degradation by F-actin binding, 0-, 5-, and 15-min aliquots were subjected to quantitative immunoblotting using antibodies against N- and C-terminal antigens of ASAP1. We confirmed that full-length ASAP1 is protected at 5 min in the presence of F-actin (red asterisk) when compared with ASAP1 alone. Using the antibody to the N terminus, a proteolytic product around 37 kDa was detected at 5 min in the absence of F-actin. In the presence of F-actin, the rate of generation of the 37-kDa product was slowed, with a faint signal at 5 min and a robust signal after 15 min of protease treatment (Figure 4B). In the blot using the antibody to the C terminus of ASAP1, protease products forming a ladder-like signal were detected after 5 min of subtilisin A treatment. Including F-actin with ASAP1 diminished the laddering, even at the longest treatment interval examined, 15 min. These results suggest that both termini of ASAP1 are protected from protease degradation in the presence of actin filaments.
Figure 4.
ASAP1 Binding to F-Actin Assessed by Limited Proteolysis
Recombinant WT ASAP1 FL (1 mg/mL) was incubated with F-actin (0.5 mg/mL) in the F-buffer for 30 min and incubated with subtilisin A (0.1 μg/mL). ASAP1 and F-actin alone were used as controls. Aliquots from the proteolysis reactions were taken at the indicated times.
(A) Examination of total protein. Aliquots removed from the incubation were analyzed by SDS-PAGE followed by staining for proteins with GelCode Blue. Representative gels of ASAP1 alone (left), ASAP1 with F-actin (middle), or F-actin alone (right) are shown. See also Figure S3B.
(B) Protein fragmentation pattern analyzed by immunoblot. Aliquots from 0, 5, and 15 min time points were separated on 12% SDS-PAGE in duplicates, transferred onto the nitrocellulose membrane, and each half was probed with an antibody against the N- (645, left) or C-terminal (642, right) region of ASAP1. Full-length ASAP1 is indicated with black arrows and red asterisks. The major proteolytic products detected with the antibody to the N terminus are indicated with the black arrow with a # sign. The major proteolytic products detected with the antibody to the C terminus are indicated with the black arrow with a % sign.
The in vitro biochemical data indicated that ASAP1 interacts directly with actin filaments and experiments in cells are consistent with the idea that ASAP1 controls stress fibers. Actin structures, such as stress fibers and microspikes, are made up of F-actin bundles, which are cross-linked aligned actin filaments. ASAP1 is a homodimer, so it may be able to bind two actin filaments simultaneously. These results taken together led us to hypothesize that the BAR domain of ASAP1 may bundle actin. We used two independent assays to assess bundling activity of ASAP1—a fluorescence-based actin-bundling assay, wherein actin bundles can be examined by staining with fluorescent phalloidin, and a low-speed co-sedimentation assay, wherein actin filaments cross-linked by a bundling protein sediment at a low speed (10,000–14,000 × g range) (Yamada et al., 2013, Yamada et al., 2016, Lin-Jones and Burnside, 2007). As shown in Figures 5A, 5B, and S4A–S4C, BAR-PH region induced thick actin bundles similar to α-actinin, a known actin-bundling protein, whereas BARPZA and full-length proteins, which have a reduced actin binding, did not bundle F-actin efficiently as determined by fluorescence microscopy and no F-actin bundling was detected by the low-speed co-sedimentation assay. PZA, which does not bind actin filaments, had no actin-bundling activity. We next tested BAR-PH actin bundling at different concentrations of BAR-PH. As shown in Figures 5C and 5D, titration of increasing concentrations of BAR-PH induced longer, more prominent bundles as examined by fluorescence microscopy, with maximum length achieved around 1 μM of BAR-PH. Low-speed co-sedimentation assays with increasing concentration of BAR-PH produced similar results.
Figure 5.
ASAP1 Bundles Actin Filaments In Vitro through Its N-BAR Domain and the C Terminus of ASAP1 Negatively Modulates the Bundling Activity
(A) Effect of ASAP1 on bundling of F-actin assessed by fluorescence microscopy. Rabbit muscle F-actin (3.3 μM) alone or with ASAP1, the indicated truncations of ASAP1, or α-actinin (positive control) (3.3 μM) were mixed in F-actin buffer and spotted on poly-L-lysine-coated coverslips. After 30 min, coverslips were fixed and stained with rhodamine phalloidin. Images were taken on Leica SP8 confocal microscope using 63× oil objective. Representative maximum intensity projection images are shown, scale bar, 10 μm. Data are representative of 5 independent experiments.
(B) Effect of ASAP1 on bundling of F-actin assessed by a low-speed co-sedimentation assay. F-actin (5 μM) alone, ASAP1 or ASAP1 truncations or α-actinin alone (2 μM), or F-actin with the indicated proteins were subjected to low-speed co-sedimentation assay. Equal volumes of the supernatant (S) and resuspended pellet (P) were resolved on gradient SDS-PAGE and stained with GelCode Blue. A gel representative of three experiments is shown in the upper panel. In the lower left panel, the percentage of the indicated proteins that sedimented in the absence and presence of F-actin, calculated from three experiments, is shown. In the right lower panel, the percentage of F-actin that sedimented in the presence of the indicated proteins is shown. The means ± SEM from three experiments are presented. See also Figures S4A–S4C.
(C) Effect of BAR-PH and length of F-actin bundles. F-actin (1 μM) was incubated with the indicated concentrations of BAR-PH and subjected to IF-based bundling assay as in (A). Filament length was approximated using the ridge plugin in Fiji/ImageJ. Results are representative of three independent experiments. Results are summarized as the mean ± SEM, Scale bar, 10 μm.
(D) Bundling efficiency of BAR-PH. The percentage of F-actin bundled at different concentrations of BAR-PH was determined by the sedimentation assay. Increasing concentrations of BAR-PH (0.1–4 μM) were titrated into a solution containing a fixed concentration of F-actin (2 μM) and incubated for 30 min at 23°C. Actin bundles were separated from free filaments by centrifugation at 14,000 × g for 30 min. A representative gel is shown in the upper panel. A summary of the combined results of three experiments is shown in the lower panel. The mean % bundled F-actin ± SEM at each BAR-PH concentration is presented.
(E) Incorporation of BAR-PH into actin bundles. Colocalization of BAR-PH with actin bundles was assessed by immunofluorescence. F-Actin alone (1 μM), with α-actinin (1 μM, tagless), as a positive actin-bundling control, or with ASAP1 BAR-PH (1 μM, His6-tagged) was incubated as in (A) and double-stained for F-actin (phalloidin Alexa Fluor 488) and His6-tag (goat anti-mouse Alexa Fluor 594) and analyzed by microscopy as in (A). A single Z-slice for each condition is shown, brightness and contrast are enhanced equally for presentation purposes, scale bar, 10 μm. As α-actinin does not have a His6 tag, there was no signal in the His6 channel under this condition. Areas shown in white rectangles were enlarged with LUTs inverted to reveal puncta.
We and others have previously observed that the stability of the BAR domain in some BAR domain-containing proteins requires the presence of its PH domain mate (Eberth et al., 2009, Nie et al., 2006), suggesting that the two domains fold together with integrated function. As the PH domain of ASAP1 binds to phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), we tested if PI(4,5)P2 affected the actin-bundling activity of the BAR domain. We performed the fluorescence-based bundling assay as described previously, in the absence or presence of large unilamellar vesicles, containing 2.5% PI(4,5)P2. As shown in Figure S4I, the presence of phospholipids did not produce an appreciable effect on bundle length. We also considered that the PH domain may affect the activity independent of phospholipid binding. As we were unsuccessful in purifying the isolated BAR domain as a recombinant protein, we assessed the effects of the BAR domain on actin filaments using a cellular assay, as was done for Figure 2. As shown in Figures S4D–S4H, overexpression of the BAR domain alone did not produce an effect on total F-actin content, did not lead to stress fiber reorganization, ordid not lead to changes in cell shape, compared with the BAR-PH. Thus the PH domain is required for BAR domain-mediated bundling, although by itself PZA (see Figure 5) or the isolated PH domain (data not shown) was not sufficient to induce bundling. Together these results indicate that BAR-PH of ASAP1 bundles actin, which may regulate actin stress fibers, cortical actin, and actin microspikes.
We next determined if the BAR-PH tandem of ASAP1 is incorporated into bundled actin. F-actin was incubated alone, with ASAP1 BAR-PH, or with α-actinin, spotted on poly-L-lysine-coated coverslips, double-stained with mouse anti-His6 antibody, followed by anti-mouse Alexa Fluor 594 and Phalloidin Alexa Fluor 488 and imaged by confocal microscopy. Single z-slices in Figure 5E indicate actin bundle formation in the presence of α-actinin (upper panel, F-actin), although there was no signal in the His6 channel, as α-actinin has no polyhistidine tag. In the representative slice from BAR-PH/F-actin IF, actin bundle formation is observed (upper panel, F-actin), with punctate signals from anti-His6 staining decorating the bundles (lower panel, His6), indicating incorporation of ASAP1 into the bundles at discrete intervals.
Interaction of actin-binding proteins with actin filaments may be driven by interactions between specific amino acid patches or may be electrostatically driven. If electrostatic, the strength of interaction with actin may depend on the ionic strength (Disanza et al., 2006, Mattila et al., 2007, Lee et al., 2007, Millard et al., 2007, Drager et al., 2017). Therefore, we performed the bundling assay between ASAP1 BAR-PH and F-actin at a 50–150 mM range of KCl concentration. As shown in Figure S4J, full actin-bundling activity of BAR-PH is retained at KCl concentration of 150 mM, suggesting that the binding is not based on purely electrostatic interactions.
The BAR-PH Tandem of a Related Arf GAP, ACAP1, Does Not Affect Actin Stress Fibers
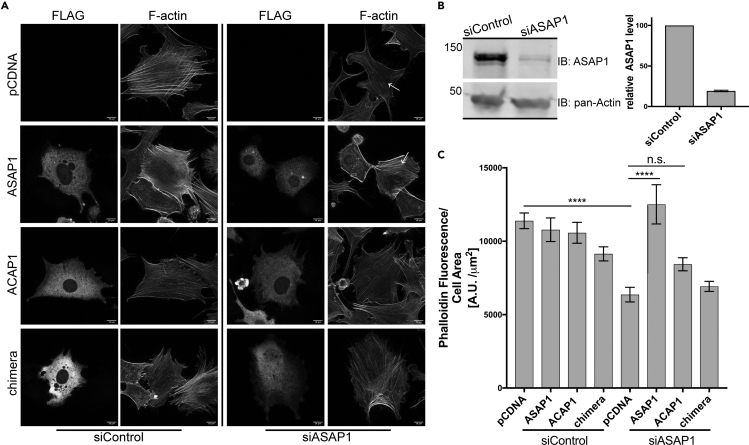
In the human ArfGAP family, ACAPs and ASAPs are the only two subtypes that contain a BAR domain, with each subtype containing three members each (ASAP1–3 and ACAP1–3). To investigate whether the actin-modulating activity of BAR-PH is unique to ASAP1 or is shared between the groups, we overexpressed BAR-PH, ΔBARPH, or full-length ASAP1 and ACAP1 in 3T3 and HFF-1 fibroblasts and compared their effects on cell morphology and actin remodeling. ACAP1 is known to induce membrane protrusions, which we have observed; however, we did not detect actin microspikes or thickening of actin stress fibers in ACAP1 BAR-PH-overexpressing cells (closed white arrowheads) (Figures 6A, 6C, and S5A). Cells overexpressing ACAP1 BAR-PH had cell area and shape similar to that of the empty vector (Figures 6D, S5C, and S5D). Likewise, full-length ACAP1 overexpression did not increase filamentous actin content, compared with the empty vector control (Figures 6B, 6E, and S5B). We then examined whether ACAP1 BAR-PH is able to rescue the loss of filamentous actin caused by ASAP1 knockdown. Overexpression of ACAP1 in siASAP1 cells (knockdown efficiency is shown in Figure 7B) did not lead to restoration of F-actin or reappearance of stress fibers (Figures 7A and S6A). Moreover, overexpression of ACAP1-ASAP1 chimera, where the BAR-PH region of ASAP1 is replaced with that of ACAP1 did not rescue the effects of ASAP1 loss (Figures 7A and 7C). These results indicate that only ASAP1 BAR domain remodels actin structures.
Figure 6.
The ASAP1, but Not ACAP1, BAR-PH Tandem Induces Actin Remodeling and Leads to Cell Area Collapse
NIH 3T3 fibroblasts were transiently transfected with empty vector pCDNA or tagged BAR-PH, ΔBARPH, or full-length ACAP1 and ASAP1. Cells were processed as in Figure 2.
(A) Representative images of transfected cells. MIPs of representative transfected cells stained for FLAG or hemagglutinin tag (upper panel) and F-actin (phalloidin) (bottom panel) are shown. Closed white arrowhead indicates thickening of cortical actin induced by ASAP1 BAR-PH expression; open white arrowheads indicate increased intensity of F-actin staining induced by full-length ASAP1. Scale bar, 10 μm.
(B) Effect of ASAP1 and ACAP1 on F-actin content. F-actin content was estimated from quantification of fluorescence from phalloidin Alexa Fluor 488 as described in Figure 1. The mean ± SEM of total fluorescence (arbitrary units) divided by cell area is presented. See also Figure S5.
(C) Relative effects of ASAP1 and ACAP1 overexpression on the formation of microspikes. The average percentage, mean ± SEM, of cells expressing the indicated proteins that formed microspikes, labeled actin protrusions in the figure axis, is presented. As a control, random empty vector cells (labeled pCDNA in the figure) were counted.
(D) Relative effect of ASAP1 and ACAP1 on cell shape. The length-to-width ratios of cells expressing the indicated proteins were determined, the mean ± SEM is presented.
(E) Relative effect of ASAP1 and ACAP1 on stress fibers. In cells expressing the indicated proteins and labeled with phalloidin Alexa Fluor 488, two lines per cell were drawn perpendicular to the visualized stress fibers. The fluorescence intensity through the line was determined and the number of peaks with intensity greater than half of the maximum intensity peak was determined for 15 cells under each condition. The results are presented as the mean ± SEM. For (B, D, and E), n = 15 cells. *p < 0.05, ****p < 0.0001, n.s., not significant, compared with the empty vector control, one-way ANOVA with Tukey's post hoc tests. Results shown are a representative of at least three independent experiments and are independent of the transfection reagent used.
Figure 7.
ACAP1 Is Unable to Restore Filamentous Actin Depletion Caused by ASAP1 Loss
NIH 3T3 cells were transfected with a control siRNA or siRNA against 3′ UTR region of mouse ASAP1. After 48 h, siControl and siASAP1 cells were transfected with empty vector (pCDNA), full-length ASAP1, ACAP1 or a chimera of the BAR-PH of ACAP1, and the ArfGAP, Ank repeat, proline-rich, E/DLPPKP, and SH3 domains of ASAP1; 24 h post-transfection (72 h total), cells were re-plated on fibronectin-coated coverslips and processed for imaging as before.
(A) Representative images of cells transfected with siRNA and expression plasmids as indicated. White arrows indicate loss of stress fibers in the cell body induced by ASAP1 knockdown (siASAP1 pCDNA) and the rescue of stress fibers following reconstitution of ASAP1 (siASAP1 ASAP1). See also Figure S6.
(B) Relative ASAP1 expression levels in the control and knockdown cells used for rescue. Protein levels were determined by immunoblot of cell lysates.
(C) Relative F-actin content of transfected cells. F-actin content was quantified as described in Figure 1. Data are mean ± SEM of a representative experiment, n = 15 cells, scale bar, 10 μm. ****p < 0.0001, n.s., not significant, one-way ANOVA with Tukey's post hoc tests, no significance was found in any other groups.
Discussion
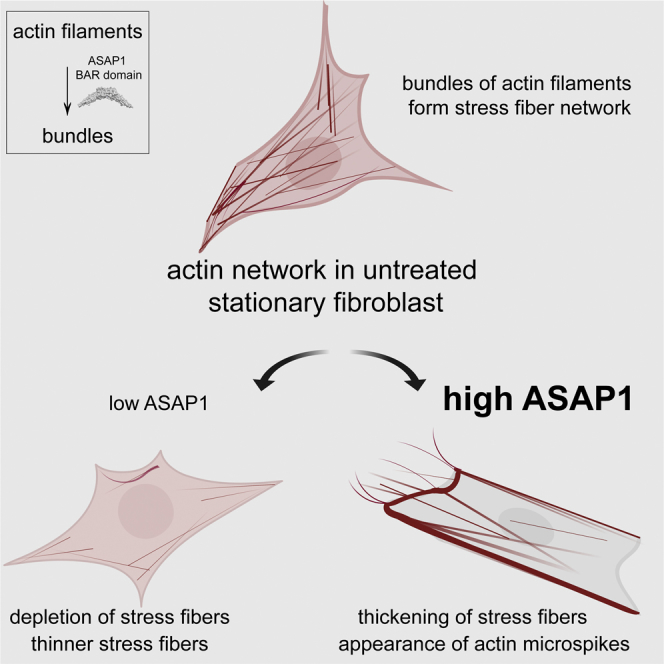
ASAP1 has been previously implicated in modulating the actin cytoskeleton through its regulation of FA complexes. Here we report that (1) downregulation of ASAP1 results in perturbation of F-actin content and diminishes stress fiber network, (2) ASAP1 directly interacts with actin filaments through its N-BAR domain, and (3) the BAR-PH module is sufficient for actin binding and cellular actin remodeling. The effects of ASAP1 on the actin cytoskeleton may contribute, at least in part, to the reported effects of ASAP1 on differentiation, proliferation, invasion, and metastasis (Izdebska et al., 2018, Yang and Lin, 2018, Fife et al., 2014).
Regulation of actin is specific for a subset of BAR-domain-containing proteins. Several members of the N-BAR domain subfamily have been reported to directly bind to actin filaments—BIN1, PICK1, and, as described in this study, ASAP1 (Rocca et al., 2008, Drager et al., 2017), whereas two members, endophilin and oligophrenin-1, have been found to not directly interact with actin through their BAR domains (Kostan et al., 2014, Fauchereau et al., 2003). To evaluate if actin-remodeling properties are conserved within the BAR-containing group of the ArfGAP family, we compared the effect of the overexpression of ACAP1 and ASAP1 on actin in cultured cells. Although both are N-BAR-domain containing ArfGAPs, ACAP1 did not exert the same effect on the actin structures as ASAP1. Expression of the ACAP1-ASAP1 chimera, where the localization signal through the PXXP/SH3 domains is preserved, but the BAR-PH of ASAP1 is replaced with that of ACAP1, did not produce the same cellular effect as the full-length ASAP1. These results support the idea that, among the Arf GAPs, this mechanism for regulating actin is specific for the ASAP subtype, and the difference between the ASAP1 and ACAP1 BAR-PH domains is another example of disparate functions of BAR domains (Millard et al., 2007, Krugmann et al., 2001, Scita et al., 2008, Stanishneva-Konovalova et al., 2016, Kessels and Qualmann, 2015). In our ongoing studies, we are investigating structural determinants of interaction of the BAR domains with F-actin and whether this mechanism for regulating actin is preserved in the entire ASAP subfamily.
Actin-binding activity of ASAP1 BAR-PH is inhibited by the ArfGAP domain. In this study we observed that the presence of the ArfGAP domain reduces binding and bundling activity of BAR-PH and leads to a less pronounced effect on cellular actin. We have previously reported that the BAR domain decreases the catalytic power of the ArfGAP domain toward its GTPase substrate, and a similar observation was made for other BAR domain-containing GAPs, such as RhoGAPs oligophrenin-1, and GRAF1 (Fauchereau et al., 2003, Jian et al., 2009, Eberth et al., 2009). This may suggest a reciprocal inter-domain regulation that coordinates spatiotemporal interaction of the ASAP1 GAP domain with Arf and of the N-BAR domain with actin, NM2A, or the membrane. In our future studies, we will examine how binding of ASAP1 substrates Arf1 and Arf5 contributes to the activity of the BAR domain of ASAP1.
Activity of the BAR domain of ASAP1 may also be regulated via C-terminal auto-inhibition. We observed that the fragments with intact SH3 domain inhibited formation of the actin microspikes and weakened actin binding, suggesting an auto-inhibitory role. Regulation of the BAR domains by their C termini is well documented. I-BAR domain member IRSp53 requires interactions of Esp8, WAVE1 and Mena, and Cdc42 to induce bundling activity and filopodia formation and SH3 domain serves both as an auto-inhibitory module and a localization signal (Misra et al., 2010, Misra et al., 2012, Disanza et al., 2006, Krugmann et al., 2001, Miki et al., 2000, Nakagawa, 2003, Yamagishi et al., 2004, Robens et al., 2010). F-BAR domain member syndapin I (PACSIN1), which contains an SH3 domain, connected by a long linker, is also subject to auto-regulation. The solved crystal structure of auto-inhibited syndapin I shows that the linker acts as a flexible arm allowing the SH3 domain to associate with F-BAR domain (Rao et al., 2010). Interaction of syndapin I SH3 domain with dynamin and WASL (Wiskott-Aldrich syndrome like) family of proteins might release auto-inhibition at sites of membrane remodeling (Roos and Kelly, 1998, Qualmann et al., 1999, Itoh et al., 2005). N-BAR domain member endophilin is also auto-inhibited by its SH3 domain, and interaction with dynamin through its proline-rich domain is required for recruitment of endophilin to the membrane (Meinecke et al., 2013). The C-terminal SH3 domain of ASAP1 connects to the GAP domain and ankyrin motifs through the proline-rich sequences and a stretch of E/DLPPKP repeats of unknown function and structure. This stretch is predicted to be intrinsically disordered and may be a flexible linker arm similar to that in syndapin I, allowing the SH3 domain to associate with the BAR domain. In preliminary experiments, we have found that the SH3 domain of ASAP1 binds to the BAR domain in trans, and future studies are aimed to determine the effect of SH3 on BAR domain-mediated actin bundling and whether SH3 binding partners can regulate the interaction.
In summary, we have tested the hypothesis that ASAP1 ArfGAP directly binds and bundles actin filaments through its N-BAR domain to control the organization of actin stress fibers. Our results indicate that ASAP1 controls cellular levels of filamentous actin, the BAR domain of ASAP1 participates in maintenance and arrangement of VSFs in fibroblasts, and the actin remodeling activity of ASAP1 is modulated by its GAP and SH3 domains. In our future studies, we will address the molecular basis of regulation of ASAP1 actin remodeling activity and the mechanism by which the GAP and the SH3 domains regulate actin bundling mediated by the ASAP1 BAR-PH.
Limitations of the Study
Our in vitro experimental evidence suggests that actin remodeling activity of ASAP1 is auto-inhibited intramolecularly by its C terminus. However, at present, we lack information on the mechanism of this regulation. Further investigation is required into the possible roles that binding partners of ASAP1 may play in controlling its actin remodeling activity. In addition, it is unknown how ASAP1 actin-reorganizing activity contributes to physiological processes, in which ASAP1 is a player, e.g., cell differentiation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Itoro Akpan and Dr. Valarie Barr (National Cancer Institute, Bethesda, MD) for technical assistance with confocal microscopy. We would like to thank Dr. James R. Sellers (National Heart, Lung, and Blood Institute, Bethesda, MD) for providing pre-formed actin filaments and for critical reading of the manuscript. We would also like to thank Dr. Vincent Schram and Lynne Holtzclaw (National Institute of Child Health and Human Development, Bethesda, MD) for feedback on sample preparation and microscopy image analysis. This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (project number BC007365).
Author Contributions
Conceptualization, P.A.R., A.G., and T.V.; Methodology, A.G., T.V., and P.A.R.; Validation, A.G. and T.V.; Investigation, Figures 1A–1G, 2, 3, 5, 6, 7, and S1–S6—A.G., Figures 1H–1I and 4, T.V.; Formal analysis, A.G. and T.V.; Resources, A.G., X.J., and R.L., Visualization, A.G.; Writing – Original Draft, A.G.; Writing – Review and Editing, A.G., T.V., R.L., X.J., and P.A.R., Funding Acquisition, P.A.R.; Supervision, P.A.R.
Declaration of Interests
The authors declare no competing interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.015.
Supplemental Information
References
- Bharti S., Inoue H., Bharti K., Hirsch D.S., Nie Z., Yoon H.Y., Artym V., Yamada K.M., Mueller S.C., Barr V.A., Randazzo P.A. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol. Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- Brown M.T., Andrade J., Radhakrishna H., Donaldson J.G., Cooper J.A., Randazzo P.A. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafate S., Flavin W., Verstreken P., Moechars D. Loss of Bin1 promotes the propagation of tau pathology. Cell Rep. 2016;17:931–940. doi: 10.1016/j.celrep.2016.09.063. [DOI] [PubMed] [Google Scholar]
- Carman P.J., Dominguez R. BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton. Biophys. Rev. 2018;10:1587–1604. doi: 10.1007/s12551-018-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.W., Jian X., Heissler S.M., Le K., Luo R., Jenkins L.M., Nagy A., Moss J., Sellers J.R., Randazzo P.A. The arf GTPase-activating protein, ASAP1, binds nonmuscle myosin 2A to control remodeling of the actomyosin network. J. Biol. Chem. 2016;291:7517–7526. doi: 10.1074/jbc.M115.701292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanza A., Mantoani S., Hertzog M., Gerboth S., Frittoli E., Steffen A., Berhoerster K., Kreienkamp H.-J., Milanesi F., Fiore P.P.D. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8–IRSp53 complex. Nat. Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- Dorland Y.L., Malinova T.S., Van Stalborch A.M., Grieve A.G., Van Geemen D., Jansen N.S., De Kreuk B.J., Nawaz K. The F-BAR protein pacsin2 inhibits asymmetric VE-cadherin internalization from tensile adherens junctions. Nat. Commun. 2016;7:12210. doi: 10.1038/ncomms12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager N.M., Nachman E., Winterhoff M., Bruhmann S., Shah P., Katsinelos T., Boulant S., Teleman A.A., Faix J., Jahn T.R. Bin1 directly remodels actin dynamics through its BAR domain. EMBO Rep. 2017;18:2051–2066. doi: 10.15252/embr.201744137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberth A., Lundmark R., Gremer L., Dvorsky R., Koessmeier K.T., Mcmahon H.T., Ahmadian M.R. A BAR domain-mediated autoinhibitory mechanism for RhoGAPs of the GRAF family. Biochem. J. 2009;417:371–377. doi: 10.1042/BJ20081535. [DOI] [PubMed] [Google Scholar]
- Ehlers J.P., Worley L., Onken M.D., Harbour J.W. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin. Cancer Res. 2005;11:3609–3613. doi: 10.1158/1078-0432.CCR-04-1941. [DOI] [PubMed] [Google Scholar]
- Eltzner B., Wollnik C., Gottschlich C., Huckemann S., Rehfeldt F. The filament sensor for near real-time detection of cytoskeletal fiber structures. PLoS One. 2015;10:e0126346. doi: 10.1371/journal.pone.0126346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchereau F., Herbrand U., Chafey P., Eberth A., Koulakoff A., Vinet M.-C., Ahmadian M.R., Chelly J., Billuart P. The RhoGAP activity of OPHN1, a new F-actin-binding protein, is negatively controlled by its amino-terminal domain. Mol. Cell Neurosci. 2003;23:574–586. doi: 10.1016/s1044-7431(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Fievez S., Carlier M.F. Conformational changes in subdomain-2 of G-actin upon polymerization into F-actin and upon binding myosin subfragment-1. FEBS Lett. 1993;316:186–190. doi: 10.1016/0014-5793(93)81212-i. [DOI] [PubMed] [Google Scholar]
- Fife C.M., Mccarroll J.A., Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014;171:5507–5523. doi: 10.1111/bph.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin R.H. Synergy of cytoskeleton components - cytoskeletal polymers exhibit both structural and functional synergy. Bioscience. 1999;49:641–655. [Google Scholar]
- Hotulainen P., Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Ha V.L., Prekeris R., Randazzo P.A. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol. Biol. Cell. 2008;19:4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Erdmann K.S., Roux A., Habermann B., Werner H., De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-bar proteins. Dev. Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Izdebska M., Zielinska W., Grzanka D., Gagat M. The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. Biomed. Res. Int. 2018;2018:4578373. doi: 10.1155/2018/4578373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian X., Brown P., Schuck P., Gruschus J.M., Balbo A., Hinshaw J.E., Randazzo P.A. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J. Biol. Chem. 2009;284:1652–1663. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R.A., Bruford E., Inoue H., Logsdon J.M., Jr., Nie Z., Premont R.T., Randazzo P.A., Satake M., Theibert A.B., Zapp M.L., Cassel D. Consensus nomenclature for the human ArfGAP domain-containing proteins. J. Cell Biol. 2008;182:1039–1044. doi: 10.1083/jcb.200806041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Kano Y., Fujiwara K. Isolation and in vitro contraction of stress fibers. Methods Enzymol. 2000;325:369–380. doi: 10.1016/s0076-6879(00)25458-x. [DOI] [PubMed] [Google Scholar]
- Kessels M.M., Qualmann B. Different functional modes of BAR domain proteins in formation and plasticity of mammalian postsynapses. J. Cell Sci. 2015;128:3177–3185. doi: 10.1242/jcs.174193. [DOI] [PubMed] [Google Scholar]
- King F.J., Hu E., Harris D.F., Sarraf P., Spiegelman B.M., Roberts T.M. DEF-1, a novel Src SH3 binding protein that promotes adipogenesis in fibroblastic cell lines. Mol. Cell Biol. 1999;19:2330–2337. doi: 10.1128/mcb.19.3.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostan J., Salzer U., Orlova A., Toro I., Hodnik V., Senju Y., Zou J., Schreiner C., Steiner J., Merilainen J. Direct interaction of actin filaments with F-BAR protein pacsin2. EMBO Rep. 2014;15:1154–1162. doi: 10.15252/embr.201439267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac B., Teo J.L., Makela T.P., Vallenius T. Assembly of non-contractile dorsal stress fibers requires alpha-actinin-1 and Rac1 in migrating and spreading cells. J. Cell Sci. 2013;126:263–273. doi: 10.1242/jcs.115063. [DOI] [PubMed] [Google Scholar]
- Krugmann S., Jordens I., Gevaert K., Driessens M., Vandekerckhove J., Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- Lappalainen P. Actin-binding proteins: the long road to understanding the dynamic landscape of cellular actin networks. Mol. Biol. Cell. 2016;27:2519–2522. doi: 10.1091/mbc.E15-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorsa A., Malki I., Cantrelle F.X., Merzougui H., Boll E., Lambert J.C., Landrieu I. Structural basis of tau interaction with BIN1 and regulation by tau phosphorylation. Front. Mol. Neurosci. 2018;11:421. doi: 10.3389/fnmol.2018.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kerff F., Chereau D., Ferron F., Klug A., Dominguez R. Structural basis for the actin-binding function of missing-in-metastasis. Structure. 2007;15:145–155. doi: 10.1016/j.str.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhang D., Yu J., Liu H., Chen Z., Zhong H., Wan Y. CCL18-dependent translocation of AMAP1 is critical for epithelial to mesenchymal transition in breast cancer. J. Cell Physiol. 2018;233:3207–3217. doi: 10.1002/jcp.26164. [DOI] [PubMed] [Google Scholar]
- Lin D., Watahiki A., Bayani J., Zhang F., Liu L., Ling V., Sadar M.D., English J., Fazli L., So A. ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res. 2008;68:4352–4359. doi: 10.1158/0008-5472.CAN-07-5237. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J., Burnside B. Retina-specific protein fascin 2 is an actin cross-linker associated with actin bundles in photoreceptor inner segments and calycal processes. Invest. Ophthalmol. Vis. Sci. 2007;48:1380–1388. doi: 10.1167/iovs.06-0763. [DOI] [PubMed] [Google Scholar]
- Liu Y., Loijens J.C., Martin K.H., Karginov A.V., Parsons J.T. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yerushalmi G.M., Grigera P.R., Parsons J.T. Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J. Biol. Chem. 2005;280:8884–8892. doi: 10.1074/jbc.M412200200. [DOI] [PubMed] [Google Scholar]
- Mattila P.K., Pykalainen A., Saarikangas J., Paavilainen V.O., Vihinen H., Jokitalo E., Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J. Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J., Astuto-Gribble L., Inoue H., Tam B.M., Schonteich E., Prekeris R., Moritz O.L., Randazzo P.A., Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke M., Boucrot E., Camdere G., Hon W.C., Mittal R., Mcmahon H.T. Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J. Biol. Chem. 2013;288:6651–6661. doi: 10.1074/jbc.M112.444869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H., Yamaguchi H., Suetsugu S., Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Millard T.H., Bompard G., Heung M.Y., Dafforn T.R., Scott D.J., Machesky L.M., Fütterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 2005;24:240–250. doi: 10.1038/sj.emboj.7600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard T.H., Dawson J., Machesky L.M. Characterisation of IRTKS, a novel IRSp53/MIM family actin regulator with distinct filament bundling properties. J. Cell Sci. 2007;120:1663–1672. doi: 10.1242/jcs.001776. [DOI] [PubMed] [Google Scholar]
- Misra A., George B., Rajmohan R., Jain N., Wong M.H., Kambadur R., Thanabalu T. Insulin Receptor Substrate protein 53kDa (IRSp53) is a negative regulator of myogenic differentiation. Int. J. Biochem. Cell Biol. 2012;44:928–941. doi: 10.1016/j.biocel.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Misra A., Rajmohan R., Lim R.P.Z., Bhattacharyya S., Thanabalu T. The mammalian Verprolin, WIRE induces filopodia independent of N-WASP through IRSp53. Exp. Cell Res. 2010;316:2810–2824. doi: 10.1016/j.yexcr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Muller T., Stein U., Poletti A., Garzia L., Rothley M., Plaumann D., Thiele W., Bauer M., Galasso A., Schlag P. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene. 2010;29:2393–2403. doi: 10.1038/onc.2010.6. [DOI] [PubMed] [Google Scholar]
- Nakagawa H. IRSp53 is colocalised with WAVE2 at the tips of protruding lamellipodia and filopodia independently of Mena. J. Cell Sci. 2003;116:2577–2583. doi: 10.1242/jcs.00462. [DOI] [PubMed] [Google Scholar]
- Nie Z., Hirsch D.S., Luo R., Jian X., Stauffer S., Cremesti A., Andrade J., Lebowitz J., Marino M., Ahvazi B. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr. Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Oakes P.W., Beckham Y., Stricker J., Gardel M.L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A., Wada I., Miura K., Okawa K., Kadoya T., Kato T., Nishihara H., Maeda M., Tanaka S., Nagashima K. CrkL directs ASAP1 to peripheral focal adhesions. J. Biol. Chem. 2003;278:6456–6460. doi: 10.1074/jbc.M210817200. [DOI] [PubMed] [Google Scholar]
- Onodera Y., Hashimoto S., Hashimoto A., Morishige M., Mazaki Y., Yamada A., Ogawa E., Adachi M., Sakurai T., Manabe T. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 2016;8:a018226. doi: 10.1101/cshperspect.a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B., Roos J., Digregorio P.J., Kelly R.B. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo P.A., Andrade J., Miura K., Brown M.T., Long Y.Q., Stauffer S., Roller P., Cooper J.A. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. U S A. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y., Ma Q., Vahedi-Faridi A., Sundborger A., Pechstein A., Puchkov D., Luo L., Shupliakov O., Saenger W., Haucke V. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc. Natl. Acad. Sci. U S A. 2010;107:8213–8218. doi: 10.1073/pnas.1003478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robens J.M., Yeow-Fong L., Ng E., Hall C., Manser E. Regulation of IRSp53-dependent filopodial dynamics by antagonism between 14-3-3 binding and SH3-mediated localization. Mol. Cell Biol. 2010;30:829–844. doi: 10.1128/MCB.01574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca D.L., Martin S., Jenkins E.L., Hanley J.G. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat. Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J., Kelly R.B. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with <i>Drosophila</i> dynamin. J. Biol. Chem. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Rougerie P., Miskolci V., Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol. Rev. 2013;256:222–239. doi: 10.1111/imr.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Hatanaka K.C., Hatanaka Y., Hatakeyama H., Hashimoto A., Matsuno Y., Fukuda S., Sabe H. High level expression of AMAP1 protein correlates with poor prognosis and survival after surgery of head and neck squamous cell carcinoma patients. Cell Commun. Signal. 2014;12:17. doi: 10.1186/1478-811X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G., Confalonieri S., Lappalainen P., Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18:52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stanishneva-Konovalova T.B., Derkacheva N.I., Polevova S.V., Sokolova O.S. The role of BAR domain proteins in the regulation of membrane dynamics. Acta Nat. 2016;8:60–69. [PMC free article] [PubMed] [Google Scholar]
- Tanna C.E., Goss L.B., Ludwig C.G., Chen P.W. Arf GAPs as regulators of the actin cytoskeleton-an update. Int. J. Mol. Sci. 2019;20:E442. doi: 10.3390/ijms20020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojkander S., Gateva G., Husain A., Krishnan R., Lappalainen P. Generation of contractile actomyosin bundles depends on mechanosensitive actin filament assembly and disassembly. Elife. 2015;4:e06126. doi: 10.7554/eLife.06126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojkander S., Gateva G., Lappalainen P. Actin stress fibers–assembly, dynamics and biological roles. J. Cell Sci. 2012;125:1855–1864. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- Tojkander S., Gateva G., Schevzov G., Hotulainen P., Naumanen P., Martin C., Gunning P.W., Lappalainen P. A molecular pathway for myosin II recruitment to stress fibers. Curr. Biol. 2011;21:539–550. doi: 10.1016/j.cub.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Vallenius T. Actin stress fibre subtypes in mesenchymal-migrating cells. Open Biol. 2013;3:130001. doi: 10.1098/rsob.130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonna L., Wiedemann A., Aepfelbacher M., Sackmann E. Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur. Biophys. J. 2007;36:145–151. doi: 10.1007/s00249-006-0118-y. [DOI] [PubMed] [Google Scholar]
- Wang J., Morita Y., Mazelova J., Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31:4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L. Reorganization of actin filament bundles in living fibroblasts. J. Cell Biol. 1984;99:1478–1485. doi: 10.1083/jcb.99.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Abe T., Satoh A., Okazaki N., Tago S., Kobayashi K., Yoshida Y., Oda Y., Watanabe M., Tomizawa K. Stabilization of actin bundles by a dynamin 1/cortactin ring complex is necessary for growth cone filopodia. J. Neurosci. 2013;33:4514–4526. doi: 10.1523/JNEUROSCI.2762-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Takeda T., Michiue H., Abe T., Takei K. Actin bundling by dynamin 2 and cortactin is implicated in cell migration by stabilizing filopodia in human non-small cell lung carcinoma cells. Int. J. Oncol. 2016;49:877–886. doi: 10.3892/ijo.2016.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi A., Masuda M., Ohki T., Onishi H., Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J. Biol. Chem. 2004;279:14929–14936. doi: 10.1074/jbc.M309408200. [DOI] [PubMed] [Google Scholar]
- Yang X., Lin Y. Functions of nuclear actin-binding proteins in human cancer. Oncol. Lett. 2018;15:2743–2748. doi: 10.3892/ol.2017.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonenaga Y., Mori A., Onodera H., Yasuda S., Oe H., Fujimoto A., Tachibana T., Imamura M. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159–166. doi: 10.1159/000087840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.