Abstract
Populations of cells exhibit variations in biochemical activity, resulting from many factors including random stochastic variability in protein production, metabolic and cell-cycle states, regulatory mechanisms, and external signaling. The development of methods for the analysis of single cells has allowed for the measurement and understanding of this inherent heterogeneity, yet methods for measuring protein activities on the single-cell scale lag behind their genetic analysis counterparts and typically report on expression rather than activity. This paper presents an approach to measure protein tyrosine phosphatase (PTP) activity in individual cells using self-assembled monolayers for matrix-assisted laser desorption/ionization mass spectrometry (SAMDI-MS). Using flow cytometry, individual cells are first sorted into a well plate containing lysis buffer and a phosphopeptide substrate. After lysis and incubation—during which the PTP enzymes act on the peptide substrate—the reaction substrate and product are immobilized onto arrays of self-assembled monolayers, which are then analyzed using mass spectrometry. PTP activities from thousands of individual cells were measured and their distributions analyzed. This work demonstrates a general method for measuring enzyme activities in lysates derived from individual cells and will contribute to the understanding of cellular heterogeneity in a variety of contexts.
Graphical Abstract
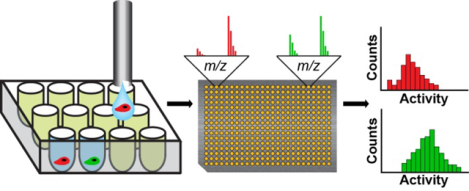
It is now clear that individual cells within a population can show significant variability in signaling activities. This variability can give rise to subpopulations of cells that can display distinct phenotypes or responses to stimuli and drugs.1,2 Increasing interest in the role of heterogeneity in cells has motivated the development of technologies capable of measuring and characterizing activities with single cell sensitivity. Significant progress has been made toward these efforts, particularly in methods for characterizing the genome and transcriptome of individual cells—in part because of the ability to amplify DNA and RNA by PCR—and have resulted in methods that are now well-developed with single-cell sensitivity.3–5 In contrast, analysis of proteins with single-cell sensitivity remains limited, since proteins cannot be amplified in a similar manner.
Several recent advances in single-cell proteomics have enabled studies of the distribution of protein levels, including methods using flow cytometry,6–9 mass cytometry,10 Simoa® immunoassays,11 and single-cell western blotting.12 While these methods can be used to quantitate levels of proteins having specific post-translational modifications, they do not directly measure enzyme activity. Imaging methods have been employed for assaying enzyme activity in single cells, typically relying on fluorogenic substrates and/or products, as well as fluorescent proteins that participate in fluorescence-resonance energy transfer (FRET).13–16 These methods can also provide spatio-temporal information on protein activities, but they lack generality as the development of reagents for a new activity can be difficult and time-consuming.13 Finally, capillary electrophoresis and laser-induced fluorescence (CE-LIF) enables enzyme activity measurements based on conversion of substrates to products with excellent limits of detection.17–19 Yet CE does not have the throughput of methods that use microwell plates.
In this paper, we describe a label-free, high-throughput assay to measure enzyme activity in single cells that uses self-assembled monolayers for MALDI mass spectrometry (SAMDI-MS).20,21 SAMDI-MS employs arrays of self-assembled monolayers (SAMs) of alkanethiolates on gold that present functional groups against a background of tri(ethylene glycol) groups, to enable selective immobilization of substrates and products, which can then be analyzed by matrix-assisted laser desorption-ionization mass spectrometry to quantitate enzyme activity. We have used this method to analyze lysates for a broad range of enzyme activities, including phosphatases, kinases, deacetylases, and acetyltransferases.22–25 In the present study, we demonstrate the measurement of protein tyrosine phosphatase (PTPs) activity at the single-cell level. We measured PTP activities from thousands of individual cells, and demonstrated that SAMDI can detect subtle differences in activity profiles of different cell types.
MATERIALS AND METHODS
Materials.
Armadillo PCR Plates, 384-well, were purchased from Thermo Fisher Scientific. Low-volume, 384-well plates were purchased from Corning. Horseradish Peroxidase, and the luminescent HRP substrate, SuperSignal™ West Femto Maximum Sensitivity Substrate, were also purchased from Thermo Fisher Scientific. PhosSTOP Inhibitor Tablets, Roche cOmplete™ mini EDTA-free Protease Inhibitor Tablets, tris(2-carboxyethyl)phosphine (TCEP), hexadecyl phosphonic acid (HDPA), ethylenediaminetetraacetic acid (EDTA), and 2,4,6-tri-hydroxyacetophenone (THAP) were purchased from Sigma-Aldrich. The phosphopeptide (Ac-IpYERC-NH2) was synthesized using Fmoc solid phase as previously described.26
Preparation of SAM Surfaces.
Steel plates (8×12.3 cm) were cleaned using hexanes, ethanol, and MilliQ water. An electron beam evaporator was used to deposit titanium (5 nm, 0.02 nm s−1) followed by gold (30 nm, 0.05 nm s−1) in a 384-array format using an aluminum mask. To form the SAMs, the plates were soaked in an ethanol solution containing a 1:4 ratio of an asymmetric disulfide terminated with a maleimide group and a tri(ethylene glycol) group, and a symmetric disulfide terminated with tri(ethylene glycol) groups. The solution has a final concentration of 0.5 mM disulfide and 10% terminal maleimide groups. This is expected to produce monolayers with 10% of the alkanethiolates terminated with maleimide groups, based on previous work by our group.27 In any event, the resulting maleimide density is sufficient for immobilizing the peptide substrates and products for quantitative analysis by SAMDI-MS. Plates were typically soaked for a minimum of 48 hours in 4 °C before use.
Cell Culture.
All cell lines (MDA-MB-231 and HEK293) were obtained from ATCC. All cells were cultured in high glucose DMEM (HG-DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). The cells were cultured in a humidified incubator at 37 °C and 5% CO2.
Single-Cell SAMDI Assay for PTP Activity.
The lysis buffer used in the assay was composed of: 25 mM HEPES, 50 mM NaCl, 2.5 mM EDTA, and 0.01 mg/mL bovine serum albumin (BSA), adjusted to pH 7.2, with 5% glycerol, 0.5% Triton X-100, 100 μM TCEP, and protease inhibitor cocktail (1 tablet per 10 mL, Roche cOmplete™, mini, EDTA-free Protease Inhibitor Tablets). Peptide substrate Ac-IpYERC-NH2 was added to reach a final concentration of 40 μM. The solution was dispensed into a 384-well Armadillo plate, 1 μL per well, using a Multidrop Combi Reagent Dispenser (Thermo Fisher Scientific). Cell cultures were dissociated using TrypLE Express and resuspended in a DPBS solution containing 600 μg/mL HRP. A BD FACSARIA IV flow cytometer was used to sort the cells into the well plate using forward and side scattering. Cell lysis and subsequent dephosphorylation reactions took place in the well plate, which was incubated for 4 hours at 37 °C while shaking at 500 RPM. Following the incubation period, 3 μL of 1.5× PhosSTOP inhibitor cocktail (3 tablets per 20 mL water) was added to each well to reach a final concentration of approximately 1×. Half of the reaction mixture in each well (2 μL) was transferred to a Corning low-volume plate. To the latter plate, 10 μL SuperSignal ELISA Femto Substrate working solution was added, and luminescence was measured using a Synergy HI plate reader (Biotek). The initial well plate was flash frozen and lyophilized overnight in order to concentrate the reaction mixtures prior to immobilization. The contents of the plate were resuspended in 2 μL Tris buffer, pH 7.9, with 50 μM TCEP. To prepare the SAM array for immobilization, the steel plate was removed from the SAM solution, rinsed with ethanol, soaked in an ethanol solution of 10 mM HDPA for 10 minutes, then rinsed with ethanol and dried under nitrogen. 1 μL mixture from each well was transferred to an individual gold island. The array was incubated for 1 hour at 37 °C in a humidity chamber, rinsed first with 1× Alconox detergent (10 g Alconox detergent per 1 L water), followed by rinsing with water, then ethanol, and finally dried with nitrogen. Matrix was prepared by dissolving THAP (10 mg/mL) and ammonium citrate dibasic (5 mg/mL) in a 1:1 solution of water and acetonitrile, with 0.1% phosphoric acid. The matrix was applied to each gold island and allowed to dry for approximately 20 minutes, following which the array was analyzed using an AB Sciex 5800 MALDI TOF/TOF instrument in positive reflector mode. Each experiment was repeated a minimum of three times. For experiments with homogenous lysate, the peptide substrate was added directly to a prepared lysate of MDA-MB-231 cells with an approximate concentration of 1 cell/μL, and the mixture was immediately distributed into a portion of the well plate using the Multidrop Combi (the remaining wells already contained sorted cells). The rest of the experiment proceeded as described above.
Data Analysis.
The mass spectra were analyzed to calculate the area under the curve (AUC) for the substrate and product peaks, which were calculated by summing the background-subtracted AUC of [M + H]+, [M + Na]+, and [M + K]+ adducts, using custom software. The relative activity was then calculated using the following equation: Activity = AUCProduct/(AUCProduct + AUCSubstrate). Next, the mass spectrometry data was cross-referenced with the luminescence data to eliminate those wells that did not contain a cell due to sorting error. Wells were eliminated by first filtering out wells with luminescence values that were lower than a threshold, which was calculated as the luminescence midway between the median values for 0 cells and 1 cell, on a log scale. Next, from the remaining wells, those with the largest and smallest 10% of luminescence values were eliminated to remove potential outliers. This selection should not be affected by size, since the volume of the droplet containing the HRP is ~1000 times larger than the average cell analyzed in this study. This data analysis and the Gaussian and gamma distribution fits of histograms were performed using MATLAB (MathWorks). Single-cell data was fit to a gamma distribution, while control and lysate data were fit to a Gaussian distribution. When comparing relative mean activities between cell types, background was subtracted from the means. Variances of single-cell distributions are calculated from the gamma distribution fit parameter: V=kθ2, where k=shape and θ=scale. All experiments were performed with at least three replicates.
Statistical Analysis.
Normality was assessed using the Shapiro-Wilk test. Means were compared with the Wilcox-rank-sum test. Equality of variance was evaluated with the Levene test.
Cell Staining.
CellTracker™ Green CMFDA and CellTracker™ Red CMPTX, purchased from Thermo Fisher Scientific, were used to stain MDA-MB-231 and HEK293 cells, respectively. Each dye was dissolved in 100 μL of Dulbecco’s phosphate-buffered saline (DPBS) with 10.7% dimethyl sulfoxide (DMSO). Each cell tracker solution was diluted in serum-free and antibiotic free HG-DMEM (25 μL dye solution per 5 mL media), which was then added to a flask of confluent, adherent cells. The cells were incubated for 45 minutes at 37 °C and 5% CO2, then the media was replaced with fresh HG-DMEM containing 10% FBS and 1% P/S. The cells were incubated for an additional 45 minutes, at which point they were dissociated using TrypLE Express (Thermo Fisher Scientific), and the two cell populations were mixed into one suspension to be sorted.
RESULTS
Single-Cell SAMDI Assay.
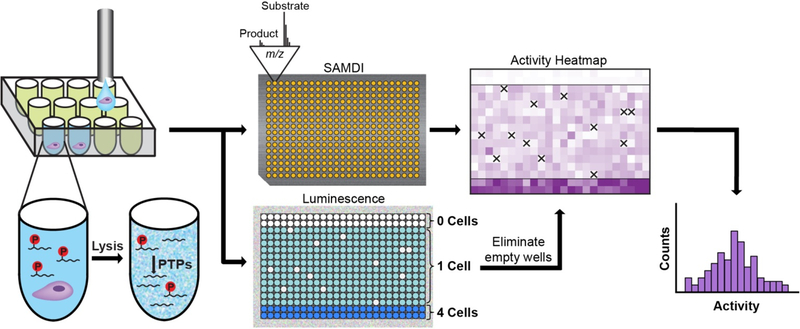
Our approach is illustrated in Fig. 1 and begins by loading a low-volume, 384-well plate with lysis buffer (1 μL) containing a phosphopeptide substrate (Ac-IpYERC-NH2, 40 μM) in each well. Individual cells are then distributed into each well with a flow cytometer. The use of standard microtiter plate formats and integration with flow cytometry is well-suited for the automated analysis of a population of cells. Once in the wells, cells undergo lysis, whereupon the phosphatase enzymes are released and can act on the peptide substrate. After 4 hours of incubation, reactions are quenched by the addition of a phosphatase inhibitor cocktail (3 μL). At this point, the reactions are split; 2 μL from each well is transferred to a second well plate and used to verify deposition of a cell in the well, while the remaining volume is used for measurement of enzyme activity as described next.
Figure 1.
Overview of the single-cell SAMDI assay. A suspension of individual cells, prepared in a buffer containing HRP, is sorted into a low-volume, 384-well plate by flow cytometry. Each well already contains lysis buffer and a phosphopeptide substrate (Ac-IpYERC-NH2). Once deposited, the cells are lysed, and active PTP enzymes can act on the substrate. Following an incubation period, the contents of the well plate are split into two pools. One pool is transferred onto an array of 384 gold islands—each presenting a maleimide-functionalized monolayer—to immobilize both substrate and product present in solution. The array is analyzed using SAMDI mass spectrometry, which quantitates PTP activity in each well by reporting the amount of substrate and product and therefore the yield. To the second pool, a luminescent HRP substrate is added, and the resulting luminescence signal is used to eliminate any wells that did not receive a cell due to error by flow cytometry (which are indicated by the ‘X’s in the heatmap). SAMDI measurements from the remaining wells reveal the distribution of single-cell activities.
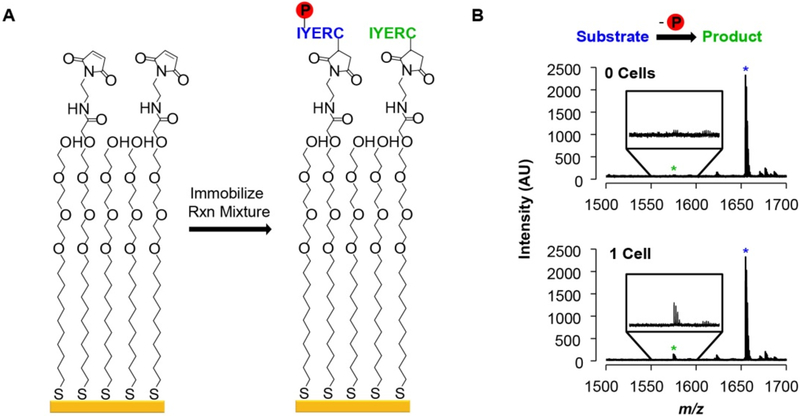
The half of the reaction volume used for enzyme activity analysis is first concentrated by lyophilization overnight and then resuspended in buffer in order to facilitate immobilization of substrate and product. The solutions are then transferred to a metal plate having a 384-spot array of self-assembled monolayers on gold islands (Figure S1). A fraction (10%) of the alkanethiolates in the monolayer present maleimide groups (Fig. 2A), allowing for immobilization of substrates and products by reaction of their terminal cysteine residue with the maleimide group. The remaining alkanethiolates in the monolayer are terminated with the tri(ethylene glycol) groups, which prevent non-specific adsorption of other peptides, proteins, and molecules present in the lysate that would interfere with immobilization and complicate analysis of the mass spectra. The array is then analyzed with SAMDI-MS to quantitate the conversion of substrate to product in each well. In the mass spectra, dephosphorylation of the substrate by phosphatases results in a peak with mass 80 Da lower than the substrate, as shown in the bottom mass spectrum from a single-cell measurement in Fig. 2B (also Figure S2). Integration of the area under the curves corresponding to substrate and product peaks then gives the percent dephosphorylation in each well (see Methods for details). Negative control wells receiving no cells were also assayed to measure the ‘background’ dephosphorylation signal. With phosphopeptide substrates, the intensity of the mass spectrum in this mass range corresponds to a small (few percent) of product (Fig. 2B, top spectrum), and is due to the baseline noise in the spectrum, a higher ionization efficiency of the product compared to the phosphorylated peptide substrate, and a minor amount of product resulting from the synthesis.
Figure 2.
PTP activity measurements using SAMDI. (A) The phosphopeptide substrate and the corresponding dephosphorylated product that are present in a reaction mixture are captured onto a maleimide-presenting self-assembled monolayer. (B) Representative SAMDI spectra from wells containing 0 cells (top) and 1 cell (bottom). Blue star: Adduct peak of substrate. Green star: Adduct peak of product.
The other half of each reaction solution is used to account for the instances in which the cell sorter failed to deposit a cell in a target well. We identified those wells and therefore could eliminate these data from the analysis. We added horseradish peroxidase (HRP) to the cell suspension prior to the cell sorting process.28 Consequently, the droplets that were delivered to the wells (which contained a single cell) had HRP, while wells that did not receive a droplet had no HRP. After the quenching step described above, the second pool of reaction mixtures is transferred to a new well plate, into which a luminescent HRP substrate is then loaded. After incubation for 1 minute, the luminescence is measured in a plate reader, and only wells in which luminescence is observed are used for subsequent analysis (see Figure S3 and Methods for additional details).
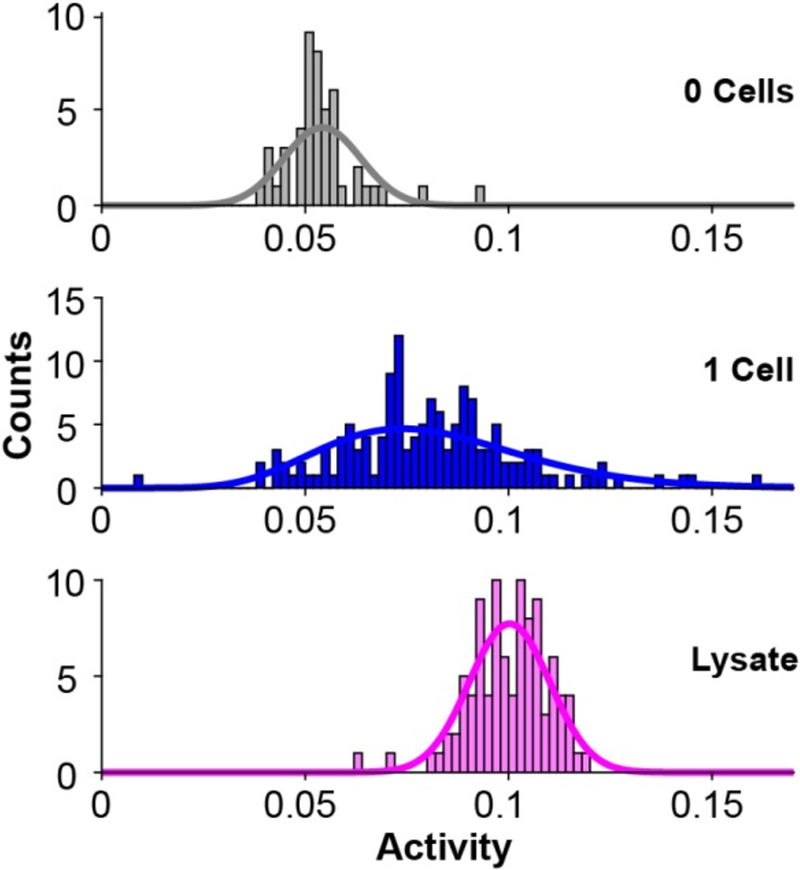
Fig. 3 shows an example of the implementation of this assay, where we sorted 143 individual MDA-MB-231 breast cancer cells into wells and measured their activities as described above. A histogram of single-cell PTP activities shows that the assay has sufficient sensitivity to measure activities in individual cells, and reveals a broad distribution of activities within the population of cells. The PTP activities are described well by a gamma distribution. This is consistent with theoretical frameworks of gene expression and experimental observations of protein concentrations in cells that show gamma distributions of steady state protein concentrations arise from the stochastic nature of the underlying biochemical reactions such as rates of mRNA degradation and number of proteins produced per mRNA.29,30 Thus, the distribution of PTP activity likely reflects a gamma distribution of steady state PTP concentration in the cell population. To determine the extent to which the distribution in activities reflects the technical error in our measurement, we prepared a homogeneous lysate (at a concentration corresponding to 1 cell/μL) and distributed this lysate into 96 wells within the well plate. In this way, the phosphatase activity added to each well should be identical (apart from technical error of the liquid handler), and any variance observed in phosphatase activity represents the technical error (Fig. 3, bottom histogram). We observed that the mean variance in lysate measurements was 27% of the mean variance observed in single-cell measurements. This suggests that the technical variance in the single-cell experiments is approximately a quarter of the total observed variance, and therefore that biological variability is responsible for the majority of the observed activity distribution. We note that steps can be taken to further reduce the technical error: using automated liquid handlers capable of distributing reagents with greater precision, reducing evaporation by controlling the humidity of the assay environment, and optimizing matrix formulations, which can impact signal-to-noise in the mass spectrum.
Figure 3.
Histograms showing the distribution of PTP activities in individual cells from a population, with corresponding fits to a gamma distribution. A control experiment where no cells were added to the wells (48 wells, gray) reveals a background level of measured activity. An experiment to measure activities in individual cells reveals a broad distribution (143 single cells, blue), and a control experiment that measures activities in an identical homogenous lysate (96 wells, pink) reveals the technical variance in the assay, and demonstrates that a significant fraction of the distribution in the analysis of a cell population is due to variation in PTP activity.
Detection of Phosphatase Activity in a Mixed Cell Population.
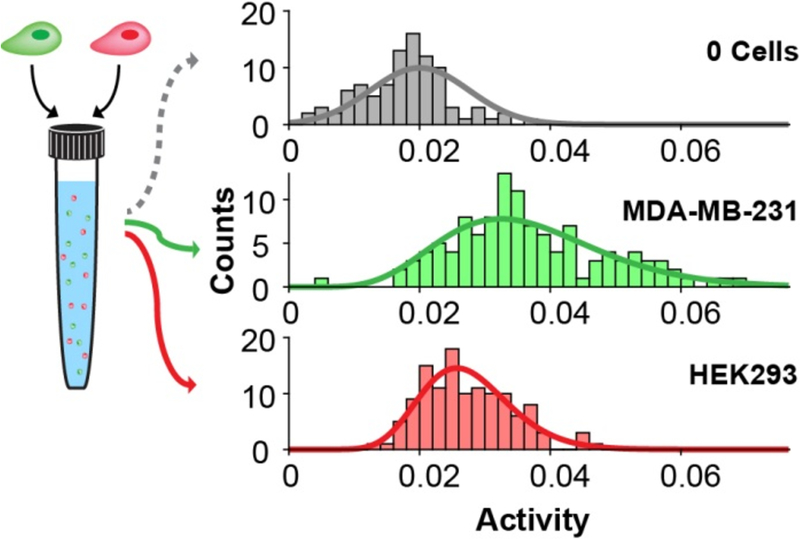
We next demonstrated the utility of the assay for analyzing and comparing the distributions of phosphatase activities in different cell types. We first labeled MDA-MB-231, human breast adenocarcinoma cells, with a green dye (CellTracker™ Green CMFDA) and labeled HEK293, human embryonic kidney cells, with a red dye (CellTracker™ Red CMPTX). We prepared a mixed suspension with approximately equal amounts of both cell types and then performed the assay as described above. In this way, the fluorescence-activated cell sorting (FACS) could place individual cells in each well and also record which cell type was present in each well (Figure S4). Fig. 4 shows the distributions for each of the two cell types. Both are described well by gamma distributions, and it is clear that the MDA-MB-231 cells have higher mean PTP activity than do the HEK293 cell (by 148%, P < 0.002 for all replicates). Further, the MDA-MB-231 cells had significantly broader distributions in activity with a mean variance that is 354% greater than that for the HEK293 cells (P < 0.001 for all replicates). This experiment demonstrates that the SAMDI assay can reveal differences in the variation in enzyme activity between two cell populations. It also shows that the use of a flow cytometer to distribute the cells allows each cell to be characterized for a second property that is reported by fluorescence; here, we used fluorescence to identify the cell type but other examples could measure expression levels of proteins in the cell.
Figure 4.
SAMDI analysis of the distribution of activities in a mixed population of cells. MDA-MB-231 cells (labeled with CellTracker™ Green CMFDA) and HEK293 cells (labeled with CellTracker™ Red CMPTX) were mixed into a single suspension. The top histogram shows the distribution of measurements of the background (0 cells) and a fit to a normal distribution (gray line). The green and red histograms show the distributions of single-cell activities measured from MDA-MB231 and HEK293 cells, respectively, sorted with fluorescence gating, and the lines represent gamma distribution fits.
DISCUSSION
This work demonstrates a label-free assay that has the sensitivity required to measure enzyme activity in a lysate derived from a single cell, and therefore that enables studies of the distributions of activities in a population of cells. The most significant aspect of this work is that it provides a strategy to characterize enzyme activity in individual cells. Most studies of cellular heterogeneity have measured the levels of mRNA transcripts in cells, with more recent work measuring the levels of proteins. But the measurement of enzyme activity has proven significantly more challenging, in part for the lack of general assay formats that have sufficient sensitivity. Our method relies on lysing individual cells in wells of a 384-well plate in the presence of a substrate that can be modified by the enzyme, and selective chemistries that immobilize the substrate and product to a monolayer for analysis by SAMDI mass spectrometry. This method offers several benefits; because the lysate is generated in the presence of the substrate, degradation of the lysate is much less of a concern than is the case with conventional isolation of lysates; the use of mass spectrometry avoids the challenges of developing labeled assay formats for enzyme targets and offers a general strategy for single-cell analysis; and the use of a FACS instrument to sort cells into the wells allows a simultaneous fluorescence-based measurement on the cells, which enables a correlation of enzyme activity with other cellular characteristics such as protein expression.
In this work, we analyzed approximately 200 individual cells per plate in a typical experiment (using the remaining wells for controls or other conditions), and over 3000 single cells in total. The sorting, enzyme reaction, immobilization, SAMDI, and analysis steps take approximately 6 hours, not including the optional overnight lyophilization step, and can be performed on thousands of cells in parallel. SAMDI-MS can accommodate tens of thousands of assays per day and is compatible with the higher density 1,536 well plates. Current sorters can distribute approximately a few thousand cells per hour. A single MALDI instrument can acquire and analyze hundreds of thousands of spectra per day. Comparatively, CE methods for measuring enzyme activity, while highly sensitive, are typically restricted to analysis of dozens of individual cells, though optimized CE methods can allow for the analysis of 3–4 cells/minute.31 Flow cytometry, which measures the presence and/or abundance of proteins, can achieve measurements of up to tens of thousands of cells per second,32 while mass cytometry can achieve ~1000 cells/second, though these methods require reagents for protein detection.10 Single-cell western blotting can analyze approximately 2,000 single cells in 4 hours.12 Single-cell RNA-seq approaches can reach a throughput of thousands of cells, and can profile several thousand transcripts per cell in a day,33,34 while whole genome amplification coupled with next generation sequencing has been employed to analyze copy number variation in hundreds of cells.35–37
While we focused on measuring PTPs in this work, the label-free assay is compatible with nearly the full range of activities, though the method does require a substrate that has high activity for the enzyme and that can be immobilized to a monolayer. The focus of this work on PTPs is timely since mutations and altered regulation within the phosphatase family are associated with many diseases, yet methods for investigating their activity lag behind their kinase counterparts.38–42 We have recently demonstrated that SAMDI-MS offers a reliable approach to assay the phosphatase enzymes.22,43 We do note, however, that the peptide substrates are not specific to a single cellular phosphatase, but are active for a number of enzymes. Therefore, we measure the cumulative activity from multiple PTPs within the cell. Examining the activity of a specific enzyme would require enzyme-specific substrates (which are available for many enzymes, but not PTPs). Alternatively, several substrates can be used simultaneously to create a pattern that reveals the identity of the enzyme. This approach can be enhanced further by incorporating enzyme-specific inhibitors into the assay, as we have demonstrated in studies identifying the contributions of specific deacetylases in lysates.24,44,45 In addition, this (as with any) assay must have sufficient sensitivity and may not be suitable for enzymes present at low copy numbers, or present in an organelle that becomes significantly diluted in the lysate. Approaches that concentrate the lysate or limit the volume of the lysis buffer—using, for example, a microfluidic device—offer a route to extend the application to low abundance enzymes.
This assay represents a new approach to measuring enzyme activity with single-cell resolution and will enable studies of cellular heterogeneity at the level of protein function. The assay utilizes SAMDI-MS, which provides a label-free, purification-free analysis of enzyme activity in cell lysates, and can be performed in high throughput. Along with the current methods for studying single-cell genomics and transcriptomics, this assay offers a new tool that will advance our understanding of both intracellular dynamics and the heterogeneity of activities in both healthy and diseased cell populations.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM105538 and the National Cancer Institute of the National Institutes of Health under award number U54CA199091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
Images of SAMDI plates, examples of single-cell SAMDI mass spectra, luminescence threshold data, FACS data.
M.M. is Founder and Chairman of SAMDI Tech Inc., which uses SAMDI-MS to perform high throughput screening and assay chemistry to clients in the pharmaceutical industry.
REFERENCES
- (1).Zhang L; Vertes A Single-cell mass spectrometry approaches to explore cellular heterogeneity. Angew. Chem. Int. Ed 2018, 57, 4466–4477. [DOI] [PubMed] [Google Scholar]
- (2).Altschuler SJ; Wu LF Cellular heterogeneity: do differences make a difference? Cell 2010, 141, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lawson DA; Bhakta NR; Kessenbrock K; Prummel KD; Yu Y; Takai K; Zhou A; Eyob H; Balakrishnan S; Wang CY; Yaswen P; Goga A; Werb Z Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chung W; Eum HH; Lee HO; Lee KM; Lee HB; Kim KT; Ryu HS; Kim S; Lee JE; Park YH; Kan Z; Han W; Park WY Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lee MCW; Lopez-Diaz FJ; Khan SY; Tariq MA; Dayn Y; Vaske CJ; Radenbaugh AJ; Kim HJ; Emerson BM; Pourmand N Single-cell analyses of transcriptional heterogeneity during drug tolerance transition in cancer cells by RNA sequencing. Proc. Natl. Acad. Sci 2014, 111, E4726–E4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Heath JR; Ribas A; Mischel PS Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 2016, 15, 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wu M; Singh AK Single-cell protein analysis. Curr. Opin. Biotechnol 2012, 23, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Stanford SM et al. High-throughput screen using a single-cell tyrosine phosphatase assay reveals biologically active inhibitors of tyrosine phosphatase CD45. Proc. Natl. Acad. Sci 2012, 109, 13972–13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Schulz KR; Danna EA; Krutzik PO; Nolan GP Single-cell phospho-protein analysis by flow cytometry. Curr. Protoc. Immunol 2012, 96, 8.17.1–8.17.20. [DOI] [PubMed] [Google Scholar]
- (10).Bendall SC; Simonds EF; Qiu P; Amir ED; Krutzik PO; Finck R; Bruggner RV; Melamed R; Trejo A; Ornatsky OI; Balderas RS; Plevritis SK, Sachs K; Pe’er D; Tanner SD; Nolan GP Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011, 332, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rissin DM; Kan CW; Campbell TG, Howes SC; Fournier DR; Song L; Piech T; Patel PP; Chang L; Rivnak AJ; Ferrel EP; Randall JD; Provuncher GK, Walt DR; Duffy DC Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol 2010, 28, 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hughes AL; Spelke DP; Xu Z; Kang CC; Schaffer DV; Herr AE Single-cell western blotting. Nat. Methods 2014, 11, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Allbritton NL; Kovarik ML Measuring enzyme activity in single cells. Trends Biotechnol. 2011, 29, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ni Q; Titov DV; Zhang J Analyzing protein kinase dynamics in living cells with FRET reporters. Methods 2006, 40, 279–286. [DOI] [PubMed] [Google Scholar]
- (15).Ouyang M et al. Simultaneous visualization of pro-tumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer. Cancer Res. 2010, 70, 2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kiyokawa E; Hara S; Nakamura T; Matsuda M Fluorescence (Förster) resonance energy transfer imaging of oncogene activity in living cells. Cancer Sci. 2006, 97, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Phillips RM; Bair E; Lawrence DS; Sims CE; Allbritton NL Measurement of protein tyrosine phosphatase activity in single cells by capillary electrophoresis. Anal. Chem 2013, 85, 6136–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Mainz ER; Serafin DS; Nguyen TT; Sims CE; Allbritton NL Single cell chemical cytometry of Akt activity in rheumatoid arthritis and normal fibroblast-like synoviocytes in response to tumor necrosis factor α. Anal. Chem 2016, 88, 7786–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shoemaker GK; Lorleau J; Lau LH; Gillmor CS; Palcic MM Multiple sampling in single-cell enzyme assays using CE-laser-induced fluorescence to monitor reaction progress. Anal. Chem 2005, 77, 3132–3137. [DOI] [PubMed] [Google Scholar]
- (20).Mrksich M Mass spectrometry of self-assembled monolayers: a new tool for molecular surface science. ACS Nano 2008, 2, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Diagne AB; Li S; Perkowski GA; Mrksich M; Thomson RJ SAMDI mass spectrometry-enabled high-throughput optimization of a traceless petasis reaction. ACS Comb. Sci 2015, 17, 658–662. [DOI] [PubMed] [Google Scholar]
- (22).Berns EJ; Cabezas MD; Mrksich M Cellular assays with a molecular endpoint measured by SAMDI mass spectrometry. Small 2016, 12, 3811–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Min DH; Su J; Mrksich M Profiling kinase activities by using a peptide chip and mass spectrometry. Angew. Chem. Int. Ed 2004, 43, 5973–5977. [DOI] [PubMed] [Google Scholar]
- (24).Kuo HY; DeLuca TA; Miller WM; Mrksich M Profiling deacetylase activities in cell lysates with peptide arrays and SAMDI mass spectrometry. Anal. Chem 2013, 85, 10635–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kornacki JR; Stuparu AD; Mrksich M Acetyltransferase p300/CBP associated factor (PCAF) regulates crosstalk-dependent acetylation of histone H3 by distal site recognition. ACS Chem. Biol 2015, 10, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kilian KA; Mrksich M Angew. Directing stem cell fate by controlling the affinity and density of ligand-receptor interactions at the biomaterials interface. Chem. Int. Ed. Engl 2012, 51, 4891–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Houseman BT; Gawalt ES; Mrksich M Maleimide-functionalized self-assembled monolayers for the preparation of peptide and carbohydrate biochips. Langmuir 2003, 19, 1522–1531. [Google Scholar]
- (28).Rodrigues OR; Monard S A rapid method to verify single-cell deposition setup for cell sorters. Cytometry A 2016, 89, 594–600. [DOI] [PubMed] [Google Scholar]
- (29).Friedman N; Cai L; Xie XS Linking stochastic dynamics to population distribution: an analytical framework of gene expression. Phys. Rev. Lett 2006, 97. [DOI] [PubMed] [Google Scholar]
- (30).Taniguchi Y; Choi PJ; Li GW; Chen H; Babu M; Hearn J; Emili A; Xie XS Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 2010, 329, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dickinson AJ; Armistead PM; Allbritton NL Automated Capillary Electrophoresis System for Fast Single-Cell Analysis. Anal. Chem 2013, 85, 4797–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Edwards BS; Sklar LA Flow cytometry: impact on early drug discovery. J. Biomol. Screen 2015, 20, 689–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Papalexi E; Satija R Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol 2018, 18, 35–45. [DOI] [PubMed] [Google Scholar]
- (34).Hwang B; Lee JH; Bang D Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med 2018, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Shapiro E; Biezuner T; Linnarsson S Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet 2013, 14, 618–630. [DOI] [PubMed] [Google Scholar]
- (36).Navin N; Kendall J; Troge J; Andrews P; Rodgers L; McIndoo J; Cook K; Stepansky A; Levy D; Esposito D; Muthuswamy L; Krasnitz A; McCombie WR; Hicks J; Wigler M Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Baslan T; Kendall J; Rodgers L; Cox H; Riggs M; Stepansky A; Troge J; Ravi K; Esposito D; Lakshmi B; Wigler M; Navin N; Hicks J Genome-wide copy number analysis of single cells. Nature Protoc. 2012, 7, 1024–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lazo JS; Sharlow ER Drugging undruggable molecular cancer targets. Annu. Rev. Pharmacol. Toxicol 2015, 56, 23–40. [DOI] [PubMed] [Google Scholar]
- (39).Zhang ZY Drugging the undruggable: therapeutic potential of targeting protein tyrosine phosphatases. Acc. Chem. Res 2017, 50, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ostman A; Hellberg C; Böhmer FD Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer 2006, 6, 307–320. [DOI] [PubMed] [Google Scholar]
- (41).Aceto N Sausgruber N; Brinkhaus H; Gaidatzis D; Martiny-Baron G; Mazzarol G; Confalonieri S; Quarto M; Hu G; Balwierz PJ; Pachkov M; Elledge SJ; van Nimwegen E; Stadler MB; Bentires-Alj M Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med 2012, 18, 529–537. [DOI] [PubMed] [Google Scholar]
- (42).Motiwala T; Jacob ST Role of protein tyrosine phosphatases in cancer. Prog. Nucleic Acid Res. Mol. Biol 2006, 81, 297–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Szymczak LC; Huang CF; Berns EJ; Mrksich M Combining SAMDI mass spectrometry and peptide arrays to profile phosphatase activities. Methods Enzymol. 2018, 607, 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gurard-Levin ZA; Kim J; Mrksich M Combining mass spectrometry and peptide arrays to profile the specificities of histone deacetylases. ChemBioChem 2009, 10, 2159–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gurard-Levin ZA; Kilian KA; Kim J; Bähr K; Mrksich M Peptide arrays identify isoform-selective substrates for profiling endogenous lysine deacetylase activity. ACS Chem. Bio 2010, 5, 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.