Abstract
Objective
Examination of the current trends and future perspectives of the cell-based therapies in neurosurgery.
Methods
A PubMed/MEDLINE-based systematic review has been performed combining the main Medical Subject Headings (MeSH) regarding the cell- and tissue-based therapies with the “Brain”, “Spinal Cord”, “Spine” and “Skull” MeSH terms. Only articles in English published in the last 10 years and pertinent to neurosurgery have been selected.
Results
A total of 1,173 relevant articles have been chosen. Somatic cells and gene-modification technologies have undergone the greatest development. Immunotherapies and gene therapies have been tested for the cure of glioblastoma, stem cells mainly for brain and spinal cord traumatic injuries. Stem cells have also found a rationale in the treatment of the cranial and spinal bony defects, and of the intervertebral disc degeneration, as well.
Most of the completed or ongoing trials concerning the cell-based therapies in neurosurgery are on phase 2. Future perspectives involve the need to overcome issues related to immunogenicity, oncogenicity and routes for administration. Refinement and improvement of vector design and delivery are required within the gene therapies.
Conclusion
The last decade has been characterised by a progressive evolution of neurosurgery from a purely mechanical phase to a new biological one. This trend has followed the rapid and parallel development of translational medicine and nanotechnologies.
The introduction of new technologies, the optimisation of the already existing ones, and the reduction of costs are among the main challenges of the foreseeable future.
Keywords: Neuroscience, Immunology, Biotechnology, Molecular biology, Cancer research, Regenerative medicine, Oncology, Evidence-based medicine, Clinical research, CAR T-Cell therapy, Cell- and tissue-based therapy, Genetic therapy, Glioblastoma, Immunotherapy, Neurosurgery, Stem cells
Neuroscience; Immunology; Biotechnology; Molecular biology; Cancer research; Regenerative medicine; Oncology; Evidence-based medicine; Clinical research; CAR T-Cell therapy; Cell- and tissue-based therapy; Genetic therapy; Glioblastoma; Immunotherapy; Neurosurgery; Stem cells
1. Introduction
The cell-based approach consists in a therapeutic act carried out by means of transplantation, transfusion or manipulation of cells ultimately aimed to treat or to alter the course of human diseases [1]. It intrinsically involves two main arms: translational medicine on one hand, and development of commercial products for clinical use on the other. The cell-based approach is the backbone of regenerative medicine, and in the last few years, it has led the way to the so-called “cell-based therapies” or “cytotherapies”, which represent the most recent phase of the biotechnological revolution in medicine.
Concurrently with the rapid development of applied biotechnology in both diagnostic and therapeutic fields, neurosurgery has seen a dramatic and parallel transition from an old era intended as purely "mechanical" to a new "biological" one. The most tangible aspect of this phenomenon is represented by the latest World Health Organization's classification of brain tumors, which comprehends a biomolecular connotation aimed at differentiating primitive neoplasms in terms of diagnosis, prognosis and responsiveness to therapy [2].
The same transition is also valid for the goals achieved by translational medicine and concerning efficacy and safety of a series of genetic therapies or immunotherapies for malignant brain tumors tested by an equally large number of clinical trials, most of which have already reached phase 2.
The above goes far beyond the mechanical, physical or chemical approach of “conventional” surgery, radiotherapy and chemotherapy respectively.
Once again, advances in translational medicine and nanotechnologies have allowed for new and revolutionary approaches for neurological diseases, which were historically considered incurable: e.g. use of stem cells for the cure of a spinal cord injury sequelae.
For these reasons, nowadays, but more and more in the near future, neurosurgery ought to consider cell-based therapies among the possible treatment options for a wide range of pathologies affecting the central nervous system (CNS), as well as the spine.
The aim of the present study is a comprehensive review of the literature focused on the rationale and the application fields, as well as the ongoing trends and future perspectives of cell-based therapies in neurosurgery, which are at the basis of the so-called “cell-based approach”.
2. Materials and methods
An online literature search has been performed based upon the PubMed/MEDLINE platform. The MeSH (Medical Subject Headings) database has been used.
The MeSH terms “Cell- and Tissue-Based Therapy”, “Tissue Engineering”, “Regenerative Medicine”, “Guided Tissue Regeneration”, “Cell Engineering”, “Immunotherapy, Active”, “Immunotherapy, Adoptive”, “Stem Cells”, and “Genetic Therapy” have been checked. For each MeSH term, our research has been restricted to specific subheadings, mainly focusing on classification criteria and clinical employment of cell therapies. The aforementioned terms have been combined with further MeSH terms: “Brain”, “Spinal Cord”, “Spine”, and “Skull”. On the basis of their relevance, the articles have been furtherly divided into “neoplastic”, “traumatic”, “vascular” and “neurodegenerative” pathological fields. Only articles in English, published in the last 10 years, and pertinent to neurosurgery have been selected. According to the best match and relevance inferred by the titles and abstracts, an additional sorting has been carried out.
Table 1 reports the literature search strategy used with Mesh Database within Pubmed/MEDLINE platform.
Table 1.
Literature search strategy used with Mesh database within Pubmed/MEDLINE platform.
| MeSH terms | Subheadings |
|---|---|
| Cell- and Tissue-Based Therapy | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
| Tissue Engineering | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
| Regenerative Medicine | Methods/Standards/Trends |
| Guided Tissue Regeneration | Classification/Methods/Standards/Therapeutic use/Trends |
| Cell Engineering | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
| Immunotherapy, Active | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
| Immunotherapy, Adoptive | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
| Stem Cells | Classification/Surgery/Therapy/Transplantation |
| Genetic Therapy | Classification/Methods/Standards/Therapeutic use/Therapy/Trends |
MeSH: Medical Subject Headings.
3. Results
3.1. Literature volume on cellular therapies
The search has retrieved a total of 1,173 articles. The search for “Immunotherapy, Active” has brought forth only articles regarding checkpoint inhibitors and vaccines, which basically consist in chemotherapy and immunomodulation employed in the treatment of brain tumors. Active immunotherapies have been excluded from this study because not involving injection, grafting or implantation of a cellular product into the patient [1].
Fig. 1 reports the flow-chart showing the results of the PubMed/MEDLINE literature search according to the main MeSH terms.
Fig. 1.
Flow-chart showing the results of the PubMed/MEDLINE literature search according to the main MeSH terms.
3.2. Classification of cellular therapies
The cell-based therapies can be classified according to different criteria: the medical specialty (e.g. the neurological, cardiovascular or hematological one); the donor-host relation (e.g. autologous or allogeneic therapies); the type of the advanced-therapy medicinal product (e.g. somatic cell, genetic therapy or tissue engineered products) [3]; and lastly, the underlying technology at the basis of their transplantation, transfusion or manipulation [4].
The rapid growth of these new technologies and their constant refinement over the years has led to such dramatic increase and enrichment of cell therapies that a technology-based classification has become essential to facilitate the comprehension of the basic approaches to the transplantation or manipulation processes of the “therapeutic” cells.
The technology-based classification involves a subdivision into somatic cell, cell immortalization, gene modification, genome editing, cell plasticity, and three-dimensional technologies, as well as, combinations among them [4].
3.2.1. Somatic cell technologies
In summary, somatic cell technologies use autologous, allogenic or xenogenic [5] cells that are purified, propagated and/or differentiated before being administered to patients for therapeutic purposes. No further technological input is brought into play within this huge group of cell-based therapies which involves both the common blood transfusion products, and the more up-to-date stem cells. A non- negligible limitation in the usage of somatic cells, and even of stem cells relies on their susceptibility to genetic and phenotypic modifications with a subsequent potential loss of biological activity as a consequence of extensive tissue culture expansion [6]. Somatic cell technologies involve specific forms of immunotherapies on one hand, and stem cell-based therapies on the other.
3.2.1.1. Immunotherapies
Immunotherapy of CNS tumors includes three well-defined strands: the checkpoint inhibitors, the vaccines and the cellular therapies. Checkpoint inhibitors and vaccines are considered active immunotherapies, whereas engineered T cells, natural killer (NK) cells, and natural killer T (NKT) cells are fundamental for adoptive immunotherapies.
Within the field of adoptive immunotherapies, an integrated approach consists in the therapeutic depletion of regulatory T cells (Tregs), basing upon the assumption that, both the thymus-derived and the inducible ones, they are involved in the immunotolerance of glioblastoma [7, 8].
Nevertheless, Tregs-depletion can be qualified as an immunomodulating approach rather than an adoptive immunotherapy.
3.2.1.1.1. Adoptive immunotherapies in malignant brain tumors
Table 2 summarizes the clinical trials on the adoptive immunotherapies for malignant brain gliomas [9].
Table 2.
Clinical trials on adoptive immunotherapies for malignant brain gliomas.
| # | Identifier | Title | # of Pts. | Interventions | Study Phase | Status | Locations |
|---|---|---|---|---|---|---|---|
| 1 | NCT03392545 | Combination of Immunization and Radiotherapy for Recurrent GBM (InSituVac1) | 30 | Combined immune adjuvants and radiation | 1 | Recruiting | CH |
| 2 | NCT03389230 | Memory-Enriched T Cells in Treating Patients With Recurrent or Refractory Grade III-IV Glioma | 36 | HER2(EQ)BBζ/CD19t + Tcm | 1 | Recruiting | U.S. |
| 3 | NCT03347097 | Tumor-infiltrating T Lymphocyte (TIL) Adoptive Therapy for Patients With Glioblastoma Multiforme | 40 | TIL | 1 | Recruiting | CH |
| 4 | NCT03344250 | Phase I EGFR BATs in Newly Diagnosed Glioblastoma | 18 | EGFR BATs with SOC RT and TMZ | 1 | Recruiting | U.S. |
| 5 | NCT03170141 | Immunogene-modified T (IgT) Cells Against Glioblastoma Multiforme | 20 | Antigen-specific IgT cells | 1 | Enrolling by invitation | CH |
| 6 | NCT02937844 | Pilot Study of Autologous Chimeric Switch Receptor Modified T Cells in Recurrent Glioblastoma Multiforme | 20 | Anti-PD-L1 CSR T cells | 1 | Recruiting | CH |
| 7 | NCT02799238 | Autologuos Lymphoid Effector Cells Specific Against Tumour (ALECSAT) as Add on to Standard of Care in Patients With Glioblastoma | 62 | ALECSAT | 2 | Active, not recruiting | SW |
| 8 | NCT02208362 | Genetically Modified T-cells in Treating Patients With Recurrent or Refractory Malignant Glioma | 92 | IL13Rα2-specific, hinge-optimized, 41BB-costimulatory CAR/truncated CD19-expressing Autologous T lymphocytes, Vaccine Therapy | 1 | Recruiting | U.S. |
| 9 | NCT02060955 | Randomized Phase 2 Study to Investigate Efficacy of ALECSAT in Patients With GBM Measured Compared to Avastin/Irinotecan | 25 | ALECSAT | 2 | Completed | DE |
| 10 | NCT01588769 | A Phase I Study to Investigate Tolerability and Efficacy of ALECSAT Administered to Glioblastoma Multiforme Patients | 23 | Anti-EGFRvIII CAR transduced PBL | 2 | Completed | DE |
| 11 | NCT01454596 | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients With Malignant Gliomas Expressing EGFRvIII | 18 | Anti-EGFRvIII CAR transduced PBL | 2 | Completed | U.S. |
| 12 | NCT01290692 | Study To Test the Safety and Efficacy of TVI-Brain-1 As A Treatment for Recurrent Grade IV Glioma | 86 | TVI-Brain-1 | 2 | Completed | U.S. |
| 13 | NCT01144247 | Cellular Immunotherapy Study for Brain Cancer | 10 | alloreactive CTL | 1 | Completed | U.S. |
| 14 | NCT01082926 | Phase I Study of Cellular Immunotherapy for Recurrent/Refractory Malignant Glioma Using Intratumoral Infusions of GRm13Z40-2, An Allogeneic CD8+ Cytolitic T-Cell Line Genetically Modified to Express the IL 13-Zetakine and HyTK and to be Resistant to Glucocorticoids, in Combination With Interleukin-2 | 6 | therapeutic allogeneic lymphocytes - aldesleukin | 1 | Completed | U.S. |
| 15 | NCT00730613 | Cellular Adoptive Immunotherapy Using Genetically Modified T-Lymphocytes in Treating Patients With Recurrent or Refractory High-Grade Malignant Glioma | 3 | Biological: therapeutic autologous lymphocytes | 1 | Completed | U.S., |
| 16 | NCT00331526 | Cellular Adoptive Immunotherapy in Treating Patients With Glioblastoma Multiforme | 83 | aldesleukin | 2 | Completed | U.S. |
| 17 | NCT00004024 | Biological Therapy Following Surgery and Radiation Therapy in Treating Patients With Primary or Recurrent Astrocytoma or Oligodendroglioma | 60 | aldesleukin, autologous tumor cell vaccine, muromonab-CD3, sargramostim, therapeutic autologous lymphocytes | 2 | Completed | U.S. |
ALECSAT: Autologuos Lymphoid Effector Cells Specific Against Tumour; CAR: chimeric antigen receptor; CTL: cytotoxic T-lymphocytes; EGFR BATs: EGFR Bi-armed Activated T-cells; EGFRvIII: epidermal growth factor receptor variant III; GBM: glioblastoma; GRm13Z40-2: allogeneic CD8+ cytotoxic T-Cell genetically modified to express the IL 13-Zetakine and hygromycin B thymidine kinase and to be resistant to glucocorticoids, in combination with Interleukin-2; HER2(EQ)BBζ/CD19t + Tcm: preparation of genetically modified autologous central memory enriched T-cells (Tcm) expressing a chimeric antigen receptor consisting of an anti-human epidermal growth factor 2 (HER2) variable fragment that is linked to the signaling domain of the T-cell antigen receptor complex zeta chain (BBζ), and truncated cluster of differentiation (CD)19; IgT: Immunogene-modified T; IL-13Rα2: interleukin-13 receptor α2; PBL: peripheral blood lymphocytes; PD-L1 CSR: programmed death Ligand 1 chimeric switch receptor; RT: Radiotherapy; SOC: Standard of Care; TIL: Tumor-infiltrating T-Lymphocyte; TMZ: temozolomide; TVI-Brain-1: personalized combination of T-cell vaccine immunotherapy and activated T-cell therapy (TVAX Biomedical, Inc., Olathe, Kansas, USA); CH: China; U.S.: United States; SW: Sweden; DE: Denmark.
3.2.1.1.1.1. Engineered and activated T cells
Engineered T cells therapies involve T-cell receptor (TCR) transgenic T cells therapy and chimeric antigen receptor (CAR) T cells therapy. “Activated” T cells are the final result of the in vitro immunization process at the basis of “Autologous Lymphoid Effector Cells Specific Against Tumor cells” (ALECSAT) technology.
3.2.1.1.1.1.1. TCR transgenic T cells
In 95% of human T cells, TCR consists of an alpha (α) and a beta (β) chain and bind the major histocompatibility complex (MHC) molecules expressed on the cell surface.
TCR transgenic T cells therapy implicates the isolation of α- and β-chains, their manipulation and insertion into retroviruses or lentiviruses by means of which their cloning can be achieved, and ultimately, the infection of the patient's T cells. TCR transgenic T cells are theoretically able to enhance the immune response against the tumor. Nevertheless, the mispairing between endogenous α/β and transgenic α/β TCR chains has been the main limiting factor for this approach to the point that no clinical trials have been implemented for brain tumors yet.
3.2.1.1.1.1.2. CAR T cells
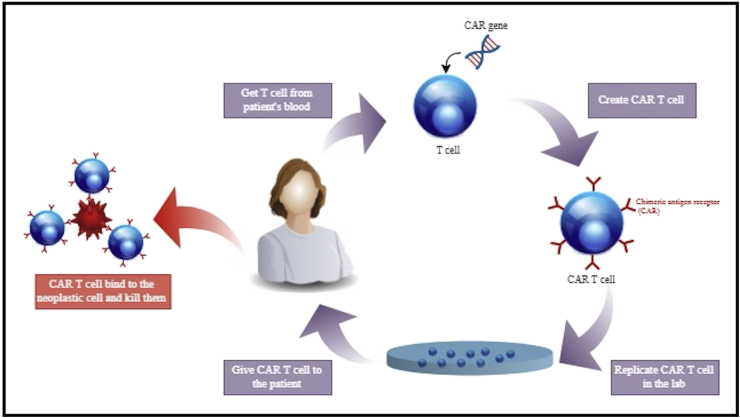
CAR T cells therapy involves leukocyte apheresis, interleukin 2 or anti-CD3 antibodies-induced activation-proliferation of T cells, gamma-retrovirus or lentivirus-mediated transfection of chimeric antigen receptor (CAR) genes, and finally, reintroduction of the engineered CAR-T cells [10]. The chimeric nature of CAR is due to the combination of both antigen-binding and T-cell activating functions into the same single receptor (Fig. 2). Redirected CAR-T cells have the ability to target a specific protein expressed onto the neoplastic cells’ membranes, which are therefore selectively destroyed [11]. The specificity of these cells to a given tumor depends on the types of transfected CAR genes. For glioblastoma, tested CAR genes have been the epidermal growth factor receptor (EGFR) variant III [12, 13], the interleukin-13 receptor a2 (IL-13Ra2) [14], CD133 [14], the human epidermal growth factor receptor 2 (HER2) [15,16], and the erythropoietin-producing hepatocellular carcinoma A2 (EphA2) [17]. An initial leukocyte apheresis upregulates the number of cytokines production promoting a dramatic expansion of the engineered CAR-T cells after their delivery [18]. These engineered smart bullets behave as drugs capable of being selectively activated by the interaction with the tumor, of proliferating, and of implementing their cytotoxic action, overcoming the issue of immunological tolerance. CAR-T cells transplant may be autologous or allogenic.
Fig. 2.
Generation of CAR-T Cells for Brain Tumor: T cells are taken from patient's blood and engineered aiming to include a viral vector expressing the chimeric antigen receptor (CAR). After the expansion in culture, CAR-T cells are infused into the same patient. Into the body, the engineered T cells selectively bind neoplastic cells inducing apoptosis.
3.2.1.1.1.1.3. Autologous Lymphoid Effector Cells Specific Against Tumor cells (ALECSAT)
As opposed to TCR transgenic and CAR T cells therapies, Autologous Lymphoid Effector Cells Specific Against Tumor cells (ALECSAT) therapy is a form of epigenetic cancer immunotherapy, namely, not involving DNA alterations. It was introduced and popularized by CytoVac (Cytovac A/S, Hørsholm, Denmark) for the treatment of prostate cancer and glioblastoma, but also for pancreatic cancer. Basically, ALECSAT therapy is based on the injection of autologous NK and CD8+ cytotoxic T lymphocytes (CTL), both playing as effector cells, previously activated and amplified ex vivo from the patient's blood sample.
ALECSAT immunization protocol lasts 26 days, and it is divided into several distinct phases. In a first step, lymphocytes and monocytes are isolated from the patient's peripheral blood sample. Monocytes are cultured and differentiated into dendritic cells (DCs). A subsequent mature DCs-lymphocytes co-culture generates autologous activated T helper (Th) cells. By means of 5-aza-2′-deoxycytidine, a DNA-demethylation agent, CD4+ Th cells are induced to express cancer/testis antigens (CTA), thus becoming CTA-expressing and activated Th (CTA-Th) cells. CTA-Th cells are then added to non-activated lymphocytes, ultimately obtaining activated and expanded CD8+ CTL effectors. Furthermore, the tumor cells which don't provide an antigen are targeted by activated NK cells [19].
ALECSAT immunotherapy has the advantage of allowing the population of secondary lymphoid organs for a long-lasting effect, also aiming at a wide variety of tumor antigens, and reducing the possibilities of tumor escape at the same time.
3.2.1.1.1.2. NK cells
Despite their well-known strong cytotoxic activity against malignant tumors, NK cells have been reported to be the least abundant immune cells of all in glioblastoma, generally accounting for no more than 2% [20]. The reason seems to be attributable to the high representativeness of the major histocompatibility complex (MHC) class I molecules, and of the human leukocyte-antigen (HLA) ligand type A on glioma cells, both of which are able to interact firstly with inhibitory NK cell and killer immunoglobulin-like receptors (KIRs), and ultimately they can inhibit the task of the NK cells [21]. Different NK cell-based approaches are theoretically possible. The first and most straightforward one involves the use of allogenic NK cells, which cannot be inactivated, not recognizing as own MHC class I molecules and HLA type A ligand expressed by glioma cells. As opposed to other allogenic transplants, infusion of immune cells is associated with a greater ability of avoiding rejection, this aspect being the basis for allogenic immunotherapies [22]. A second possible approach comprehends KIRs antibody-mediated blocking, which dramatically increases the NK cells oncolytic effect. A third feasible way involves antibody-dependent cellular cytotoxicity and is based on the use of antibodies against EGFR.
The fragment crystallizable (FC) region of the antibody also binds the CD16 (FcγIIIA) activating receptor and finally causes cancer cell apoptosis. Further important activating receptors potentially expressed by NK cells are KIR2DS2 (2DS2: two domains, short cytoplasmic tail 2) and NKG2D (G2D: Group 2D). Interestingly, KIR2DS2+ genotype has been associated with a greater cytotoxicity together with inhibition of angiogenesis; sorting, expansion, and autologous transplantation of these types of NK cells have been tested as adoptive immunotherapy against glioblastoma [23]. Immunoligands able to bind NKG2D receptor have a rationale, as well. Yvon and colleagues have reported the potential implications of cord blood NK cells retrovirally transduced to express a dominant negative form of transforming growth factor (TGF)-β receptor II (DNRII) [24]. DNRII makes these cells immune from the detrimental effects of TGF-β produced by microenvironment. NKs’ exosomes [25] and a novel CAR NK cell line targeting EGFR variant III [26] are both object of great interest. Glioblastoma NK cell immunotherapy has been combined with the use of mAb9.2.27 antibody, which is able to inhibit angiogenesis through the secretion of interferon (IFN)-γ and the tumor necrosis factor (TNF)-α [27].
Fig. 3 reports an overview of the main molecular mechanisms involved in NK cell-based immunotherapy for glioblastoma.
Fig. 3.
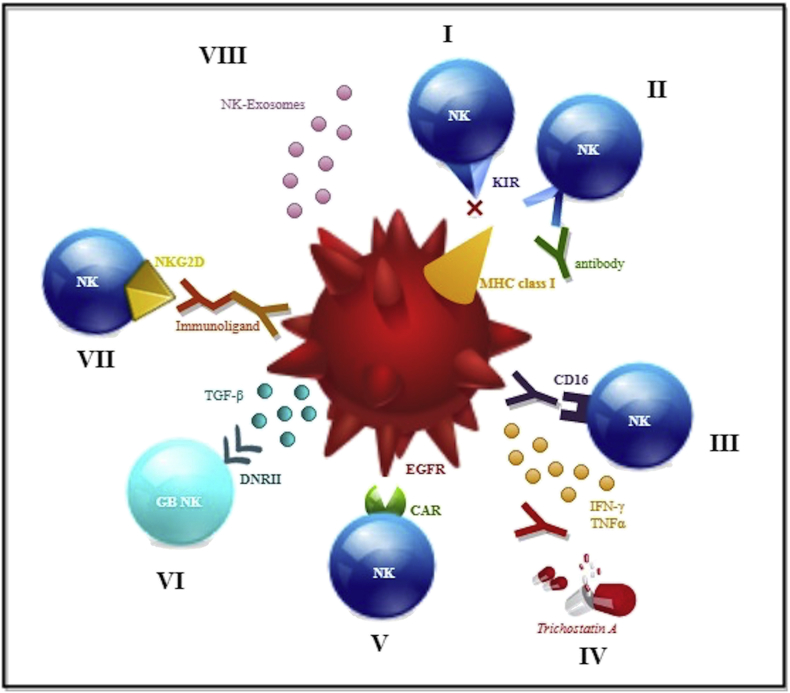
Main Molecular Mechanisms involved in NK Cell-Based Immunotherapy for Glioblastoma: I: Allogenic NKs, expressing different KIRs, cannot bind the MHC class I receptor on neoplastic cells; II: Antibodies against KIR block the interaction with MCH class I on neoplastic cells; III: Antibodies against the neoplastic cells' EGFR induce a CD16-mediated immune response; IV: Drugs, as trichostatin A, sensitize NK cells against neoplastic cells. Antibodies as mAb9.2.27 induce IFN-γ and TNF-α secretion that, in turn, inhibit angiogenesis; V: novel CAR NK cell line selectively targets EGFR variant III expressed onto neoplastic cells, ultimately inducing apoptosis; VI: Cord blood NK cells express dominant negative TGF-β receptor II (DNRII) resulting insensitive to the immunosuppressant action of TGF-β; VII: Immunoligands mediate the binding to tumor-specific antigens by means of the recognition of NKG2D receptor onto NK cells,; VIII: NKs' exosomes promote apoptosis of the neoplastic cells.
3.2.1.1.1.3. NKT cells
The rationale at the basis of NKT cells relies on their potential oncolytic activity against solid tumors, which has proven to be valid also for glioblastoma. The latter, however, tends to escape from NKT cells by means of a higher expression of micro RNA-92a associated with an equally high representativeness of an immune tolerant IL-6+ IL-10 + NKT cell phenotype [28]. A dramatic enhancement of NKT cell cytotoxic activity against glioblastoma has been demonstrated as the result of their expansion in culture using autologous mature DCs loaded with the NKT ligand α-galactosyl ceramide. They may also be used to induce active immunization [29].
Several factors have been reported limiting the effectiveness of immunotherapy on brain tumors. Among others, the lack of tumor antigen expression onto glioblastoma stem cells is responsible for loss of tumor immunological phenotype, and this same factor plays a fundamental role in the escape mechanisms from vaccines and T-cell therapy. At a molecular level, aberrant nitric oxide synthase 2 (NOS2) is gaining growing interest as a potential therapeutic target [30, 31, 32, 33].
3.2.1.2. Stem cell-based therapies
Stem cells are today the expression of somatic cell technology. Contrary to what was believed until the end of the 19th century, Ramón y Cajal in 1913 demonstrated for the first time that neurons can also regenerate after injury.
Stem cell-based therapy offers potential treatment for developmental, traumatic, oncological and degenerative neurological diseases for which currently, there are very few cures.
Stem cells are immature undifferentiated cells with a self-renewal capacity, which practically means they are able to divide giving birth to two daughter cells, the first of which maintains the tissue- specific stem cell heritage, while the other differentiates into an any other specialised cell of a given tissue. Every tissue hosts a population of stem cells able to grow, regenerate, repair or regulate the tissue itself. Based upon their origin, stem cells may be embryonic, fetal or adult ones. Embryonic stem cells (h-ESCs) derive from a blastocyst inner cell mass, whereas fetal stem cells come from fetal blood and tissues, and adult stem cells are present in every differentiated tissue. h-ESCs are characterised by atypical cell cycle regulation, which is at the basis of their indefinite potential of propagation in culture, specific set of markers, lack of contact inhibition, and extreme ability to differentiate. They also have been reported to form teratocarcinomas in nude mice. Fetal stem cells originate blood cells, but also tissues and organs. Umbilical cord blood, amnion/placenta, umbilical cord veins and umbilical cord matrix cells are precious sources of fetal stem cells. Umbilical cord fetal stem cells have the great advantage of being readily available, inexpensive, multipotent and immune from ethical issues involving both embryonic and fetal stem cells, and hindering their widespread implementation [34, 35, 36]. Adult stem cells were identified for the first time in the hematopoietic system, and later on in adult CNS. They were found to possess unexpected plasticity, and extensive regenerative capability.
A large part of the reasons for their employment derives from the fact that they are a source of autologous cells for transplantation, thus unburdened by immunological complications.
Due to their differentiation potential, i.e. plasticity, stem cells may be classified as totipotent, pluripotent or multipotent ones. Theoretically, the unique true totipotent cell is the zygote with its progeny. All types of cells, including embryonic and extra-embryonic tissues, originate from a totipotent cell. Pluripotent cells are also known as h-ESCs because they come from the blastocyst inner cell mass. Totipotent cells may stem from all the three germ layers, giving birth to ectodermal, mesodermal and endodermal tissues, but not embryonic or extra-embryonic ones. Multipotent cells are present in each of the three germ layers ever since embryonic life. These cells are capable of generating a wide spectrum of cell lines all belonging to the same tissue where they reside. This class of cells is responsible for tissue repair after damage and auto-regeneration. Classically, four main types of human multipotent cells are known: mesenchymal stem cells (h-MSCs), neural stem cells (h-NSCs), bone marrow stromal cells, and olfactory ensheathing cells. Interestingly, multipotent cells are present also in adult age.
Regarding CNS, the line of self-renewing stem cells is usually referred to as neural precursor cells or neural stem cells (h-NSCs), and they are capable of differentiating into neurons, astrocytes, and oligodendrocytes, responsible for the maintenance of homeostasis and regenerative processes [37, 38]. h-NSCs have been isolated from the olfactory bulb and the hippocampus, two sites with clear evidence of neuronal turnover. These specific cell types are thought to maintain restricted neural differentiation capabilities or in any case, committed to specific subpopulation lineages [39, 40, 41, 42, 43, 44, 45]. Specific biomarkers reflecting the processes of embryogenesis and adult neurogenesis define both h-ESCs and adult h-NSCs [46].
However, the most interesting and recent class of staminal cells are undoubtedly the human induced pluripotent staminal cells (h-iPSCs). These cells derived from genetically reprogrammed adult somatic cells are believed to carry the same potential of pluripotent cells and are theoretically unlimited in number, as they can be derived from any adult somatic tissue [47, 48, 49]. Oncogenicity is the main concern related to h-iPSCs [49].
3.2.1.2.1. Actual and potential applications of stem cells-based therapies
3.2.1.2.1.1. Brain tumors
It is known that gliomas are characterised by increased resistance to therapeutic approaches. Treatment failure is probably due to the presence of glioma stem cells (GSCs) leading to high recurrence rates and poor survival. GSCs are considered a relevant target for glioma therapy, and GSCs elimination is crucial in treating glioblastoma [50].
The main application field of stem cells in neuroncology is represented by malignant gliomas for which an impelling need for the development of new and more effective therapies exists.
Neural, but also mesenchymal stem cells have been reported to hold a tremendous potential in surrounding glioblastomas, also blocking the spreading of the tumor toward the neighboring and distal sites along the white matter pathways [51]. Furthermore, thanks to their high tropism for the adult brain pathology, as well as their extensive migration capability, these cells may act as selective deliverers of drugs in the sites of the tumor invasion [51, 52, 53, 54]. Engineering of neural or precursor stem cells through a retrovirus-mediated transfer of specific genes into the genome of neural stem cells has led to think of these cells as highly selective antagonists against a glioblastoma invasion [55, 56, 57, 58, 59, 60, 61]. Transgenes may codify for interleukins, lysosomal enzymes or enzymes able to convert prodrugs in physiologically active molecules, which ultimately act as an antiblastic therapy, enhancing the cytotoxic power of conventional chemotherapy on cancer cells, as well. Adenoviruses have been utilised instead to introduce a family of genes codifying for some tumor necrosis factor (TNF)-related apoptosis-inducing ligands into the neural or precursor stem cell DNA capable of causing a selective apoptosis of glioblastoma cells [62, 63, 64, 65, 66]. hNSCs have been extensively used also as carriers of the oncolytic herpes simplex virus, due to the fact that this approach has great potential against recurrent and disseminated gliobalstomas [67, 68, 69, 70, 71, 72]. Last but far from least, neural or precursor stem cells seem to be capable of inherent antitumoral activity against the growth of glioblastoma [55]. This activity is independent from whatever cellular modification, and although the fine mechanisms are still largely unknown, Staflin and colleagues report that hNSCs may elaborate certain factors, such as the transforming growth one (TGF-β), therefore acting as antagonists toward tumor growth [73].
3.2.1.2.1.2. Traumatic brain injury
Mammalian adults’ brain is site of proven neurogenesis in response to ageing [74, 75] or mechanical injury [76, 77, 78, 79]. Throughout our entire lifetime, neurogenesis occurs physiologically at the ventricular-subventricular zone of the lateral ventricle, of the subgranular zone of the hippocampal dentate gyrus, and of the olfactory system. There is plenty of evidence confirming that post-traumatic neurogenesis and brain remodelling attributable to the persistence of hNSCs into adulthood also occurs at cortical level, both proximal to the injury site than distal, and it is mainly sustained by new astrocytes [75, 79, 80, 81]. This has opened the way to replacement of damaged neurons by transplantation of neural stem cells; this approach has been thought as a possible alternative in the treatment of traumatic brain injury sequelae [78, 82, 83, 84, 85, 86, 87, 88, 89, 90].
In summary, the effects of spontaneous endogenous post-traumatic hNSCs activation may be potentially enhanced by an autologous transplant of the same cell population isolated from brain tissue, drawn during brain surgery and preventively treated ex-vivo.
3.2.1.2.1.3. Spinal cord injury
Endogenous and spontaneous neurogenesis supported by astrocytes and oligodendrocytes has been demonstrated after acute spinal cord injury [91]. In animal models, proliferation in the ependymal and periependymal canal originates precursor cells that differentiate toward glial lineages [91]. An essential fact regarding the cell-based approach to spinal cord injury consists in a well-known existing disproportion between the functional restoration and the neuron regeneration/replacement degree. This means that the role played by the transplanted stem cell on the spinal cord injury site is largely beyond simple numeric replacement of damaged neurons at the gray matter level. Just like in the brain, the spinal cord also has a recognised regenerative capacity, although several barriers tend to hinder self-regeneration and functional recovery. Apart from neuron replacement, stem cells act in different ways in order to promote functional recovery. They affect the posttraumatic cord microenvironment through the secretion of a set of bioactive molecules, acting both paracrinely and autocrinely to suppress local immune response, enhance angiogenesis, and inhibit scarring and cell death. In addition, they are responsible for axon remyelination, sprouting and for addressing them toward their targets, as well as for the formation of functional bridges [5, 92, 93, 94, 95]. Important aspects to be defined in this field are the best type of cells to select for transplant, their dosage, the administration route of choice, and the ideal timing for therapy. Unlike traumatic brain injury, a very broad spectrum of autogenic and allogenic stem cells has been employed for the cell-based approach to spinal cord injury, with the obvious advantages of very low or none immunogenicity. Bone marrow and adipose-derived mesenchymal stem cells (MSCs), hematopoietic stem cells, neural precursor cells, olfactory ensheathing, and embryonic cord blood stem cells are examples of this procedure [96, 97, 98, 99, 100]. Even xenogeneic ovine bone marrow MSCs have been reported to be capable of surviving and engrafting into the spinal cord of an injured rat showing transdifferentiation into a neuroglial phenotype able to support functional recovery [5]. However, a series of non-negligible evidence supports the employment of h-ESCs as the best candidates for a cell-based approach to spinal cord injury. The main reasons rely on their indefinite replication potential while maintaining their genetic stability, their pluripotency, and very low immunogenicity. After transplantation, these cells seem to survive for weeks without forming teratomas, differentiating into neurons, oligodendrocytes, and astrocytes, and ultimately, inducing functional improvement [101, 102]. At present the therapeutic dosage of stem cells still remains empiric and widely undetermined due to the fact that the most evidences are based upon animal models.
The administration route of therapeutic stem cells onto injury sites represents a further and to date debated issue. Although several delivery models have been tested, as e.g. direct, intrathecal lumbar, intra-arterial and intravenous injections, the best way to release the cells at the target sites is yet to be determined. As far as the timing is concerned, an acutely injured spinal cord establishes a hostile environment for stem cells, just as fibrosis and cystic changes, characterizing the chronic phase, can limit the effects of the transplanted cells dramatically [103]. Since a spontaneous functional amelioration is potentially obtainable after incomplete cord injury, the current tendency is to transplant the stem cells during the chronic and stabilized phase in order to avoid a false interpretation of their therapeutic effect [104, 105, 106].
3.2.1.2.1.4. Cranial and spinal skeletal defects
Bone marrow MSCs and human adipose-derived stromal cells (h-ASCs) have been reported to have an impressive osteogenic potential [107]. The methodological approaches for the handling of adipose tissue or lipoaspirate, isolation and expansion of hASCs, as well their immunophenotypic characterization and cryopreservation, pointing out advantages and limitations of several techniques able to affect the hASCs quality have recently reviewed [108]. h-ASCs, in particular, have provided an evidence for regeneration of vertebral bone defects when genetically modified to overexpress the recombinant human bone morphogenetic protein (BMP-6) [109].
Recently, h-iPSCs and h-ESCs have also been suggested as candidates to repair skeletal defects [110, 111]. All of these cell lines have been tested for skull and vertebral body restoration combined with pro-osteogenic biomimetic scaffolds, which are able to support cellular viability, as well as to guide cell differentiation for functional engraftment. Relevant results have been achieved for h-iPSCs and h-ESCs in rats on critical-sized calvarial defects >4 mm, and using hydroxyapatite-coated poly-l-lactide-co-glycolide scaffolds engineered to release BMP-2 (PLGA/HA + BMP-2) [112,113]. Not surprisingly, BMP-2 has proved to be a powerful inductor of osteogenesis and incidence of teratomas has been negligible [111]. Acellular bovine alloperiosteum scaffold has also been tested successfully for the same purposes [114]. This set of evidence suggests that local surrounding niches may play a paramount role in guiding the outcome of implanted pluripotent cells.
3.2.1.2.1.5. Intervertebral disc degeneration
Several studies have documented a self-renewal capacity of the intervertebral disc, which is supported by the presence of stem cells within the disc itself [115, 116, 117].
5-bromo-2′-deoxyuridine (BrdU) positive cells have been found in both the anulus fibrosus [115, 118, 119] and the nucleus pulpous (NP) [115, 120] indicating slow but continuous cell proliferation. Although self-regeneration affects especially the outer part of the anulus fibrosus, NP is considered a niche of stem cells. Tie2, GD2 and CD24 are the main cell surface markers of NP stem cells, whereas the hypoxia-inducible factor 1-alpha (HIF-1α) and the tonicity-responsive enhancer binding protein (TonEBP/NFAT5) are considered as functional markers [121]. NP-derived stem cells have been reported to carry a proven osteogenic and chondrogenic capacity, but not an adipogenic differentiation one [122, 123]. The cell-based approach for intervertebral disc degeneration finds its rationale in these aspects. Two main paths are theoretically possible for the cure of a degenerated intervertebral disc: the first involves the enhancement of the intrinsic regenerative capacity of native NP-derived stem cells, while the second comprehends the transplantation of autologous, allogenic or even xenogeneic stem cells. Currently, the second approach has been more investigated by far.
Probably more than in other stem cells, the NP-derived ones are often associated with a specific phenotype characterized by a paramount role of Notch signaling pathways, which also play as functional markers for the identification of these cells within the NP [121]. Notch1 expression pattern has been reported to be very similar to the BrdU positive cells one [115], and its expression is also induced by the hypoxic microenvironment physiologically present at the avascular disc level [124]. This evidence has led to think of Notch1 as a potential target for endogenous stem cell-based repair for a damaged intervertebral disc.
Conversely autologous NP-derived cells represent a detrimental source for stem cell transplant due to their reduced availability in aged discs [116, 117]. Several cell sources have been proposed as an alternative, including chondrocytes and MSCs derived from bone marrow or adipose tissue, which have highlighted advantages and disadvantages [125]. Before the transplant, MSCs can be induced into NP-stem cells in different ways: e.g. treatment with TGF-β, insulin-like growth factor-1, fibroblast growth factor-2, and platelet-derived growth factor [126, 127] or co-culturing with mature NP cells, ultimately resulting in an up-regulation of NP marker genes [128], or also notochordal cell conditioned medium [129]. Regardless of how induction takes place, MSCs are also able to increase matrix production of endogenous degenerative cells, probably because of a paracrine mechanism [121].
3.2.2. Cell immortalization technologies
Cell immortalization technologies are based on a transgenic retroviral-induced modification of a given cell line aimed to clone it exponentially, and therefore to exploit its potential therapeutic effects. This transgene modification may obtain its effects by acting at a transcriptional or post-transcriptional level, and the genetically-induced clonation may occur both in vitro and in vivo [130].
Undoubtedly, the most important example in this field is the c-mycERTAM technology, which basically consists in a retroviral delivery of the c-mycERTAM transgene aimed at making immortal the neural stem cell line CTX derived from fetal cortical brain [130, 131, 132]. To date, the clinical applications of these cells regard stroke management. Conceptually, immortalization technologies differ from a somatic cell one, because the former involves genetic manipulation, which goes beyond simple purification, propagation and/or induced-differentiation. They also diverge from ex vivo/in vivo gene modification technologies, whose object is to modify the target cells qualitatively, often without affecting their numeric representativeness, contrary to what happens in merely quantitative cloning.
Cell immortalization technologies have been employed basically for research purposes in the neurosurgical field. These techniques have allowed to expand our knowledge about the naïve genomic aspects and the in vitro responses to the therapy of some intracranial tumors, and to study them in detail [133, 134, 135, 136].
3.2.3. Gene modification technologies
Gene modification technologies represent an expression of gene-based therapies focused on curing a series of human diseases like cancer, but also neurological, cardiovascular, infective and genetic disorders. Retrovirus, lentivirus and adenovirus have been reported as the most advantageous vectors capable of introducing genetic material into the cellular genome. The virus-mediated insertion of foreign DNA into a target cell ultimately creates chimeric products which are able to perform a tailored interaction with their targets therefore fulfilling the therapeutic action. Although viral transduction techniques have been the most frequently employed gene carriers, plasmid transfection techniques involving liposome nanoparticle-mediated delivery of circular DNA, have also been recently reported [137, 138, 139, 140]. Gene modification technologies have been widely employed in hematology [141, 142, 143], oncology [14, 144, 145, 146], and more recently in neurology, as well [147, 148, 149].
These techniques are aimed at the transfer of a single gene copy codifying a therapeutic enzyme or growth factor into the genome of the target cell.
Two main types of carriers have been used for this purpose: viruses and lipid nanoparticles, both providing advantages and drawbacks. While adenoviruses, herpes simplex virus (HSV) and various retroviruses have gained a primary role in gene therapy for brain tumors, the adeno-associated virus (AAV) has been reserved to non-tumor applications. The main reason for this difference in the use of the aforementioned viruses lies in their dimensions and shapes, and therefore in the specific quantity of genetic material that can be integrated. HSV, lentivirus and adenovirus may range between 100 and 200 nm compared to AAV, which is no larger than 20 nm. This means that lentivirus and HSV have the largest carrying capacity regarding non-viral DNA, and generally, they are considered more suitable as gene-carriers when the intended genetic material is greater than 5 kB [150]. HSV can contain up to 40 kB [151]. Nevertheless, the following limitation must be taken into account: the larger the size of the virus, the more restricted the spread within the tissue will be, and so will the oncolytic effects for large or disseminated tumors. HSV has a very rapid gene expression, but similarly to adenovirus, it may be pathogenic for the human brain being able to trigger explosive immuno-mediated responses. Lipid nanoparticles-mediated transduction of DNA is burdened by a lower immunogenicity; however, at the same time, it seems to be associated with a lower capability of delivering genetic material to the target cell.
By means of the insertion of transgene codifying for one or more ligand peptides, neuroncological gene therapy ultimately makes neoplastic cells susceptible to a given cell-killing drug. This kind of therapy is also referred as suicide gene therapy, which has been employed in a wide spectrum of human cancers, and glioblastoma among them.
Table 3 reports a comparison between viral vectors versus lipid nanoparticles focused on dimension, carrying and spreading capacity, oncolytic effect, and pathogenicity.
Table 3.
Comparison between viral vectors versus lipid nanoparticles focused on dimension, carrying and spreading capacity, oncolytic effect, and pathogenicity.
| Characteristics | Carrier |
||||
|---|---|---|---|---|---|
| Viruses |
Nanoparticles |
||||
| adenoviruses | HSVs | Retroviruses | AAVs | Lipid nanoparticles | |
| Dimension | 100–200 nm | 20 nm | |||
| Carrying capacity | + | ++ | +/- | - | +/- |
| Dimension of carried genetic material | >5Kb | >40 KB | 10-15 Kb | <<5 kb | +/- |
| Spreading capacity | +/- | - - | + | + | - |
| Oncolytic effect in disseminated tumors | +/- | - | +/- | - - | +/- |
| Pathogenicity vs. Host | ++ | ++ | +/- | +/- | - - |
HSVs: herpes simplex viruses; AAVs: adeno-associated viruses; “++”: very high; “+”: high; “+/-”: medium; “-”: low; “- -”: very low.
Table 4 summarizes the clinical trials on gene therapies for malignant brain gliomas [9].
Table 4.
Clinical trials on gene therapies for malignant brain gliomas.
| # | Identifier | Title | # of Pts. | Main Interventions | Study Phase | Status | State |
|---|---|---|---|---|---|---|---|
| 1 | NCT03657576 | Trial of C134 in Patients With Recurrent GBM | 24 | C134 | 1 | Not yet recruiting | U.S. |
| 2 | NCT03603405 | HSV-tk and XRT and Chemotherapy for Newly Diagnosed GBM | 62 | ADV/HSV-tk | 1–2 | Recruiting | U.S. |
| 3 | NCT03596086 | HSV-tk + Valacyclovir + SBRT + Chemotherapy for Recurrent GBM | 36 | ADV/HSV-tk | 1–2 | Recruiting | U.S. |
| 4 | NCT03576612 | GMCI, Nivolumab, and Radiation Therapy in Treating Patients With Newly Diagnosed High-Grade Gliomas | 52 | AdV-tk | 1 | Recruiting | U.S. |
| 5 | NCT03491683 | INO-5401 and INO-9012 Delivered by Electroporation (EP) in Combination With Cemiplimab (REGN2810) in Newly-Diagnosed Glioblastoma (GBM) | 52 | INO-5401 | 1–2 | Active, not recruiting | U.S. |
| 6 | NCT03383978 | Intracranial Injection of NK-92/5.28.z (HER2.taNK) Cells in Patients With Recurrent HER2-positive Glioblastoma (Quilt 3.C001) | 30 | NK-92/5.28.z (HER2.taNK) | 1 | Recruiting | GE |
| 7 | NCT03283631 | Intracerebral EGFR-vIII CAR-T Cells for Recurrent GBM | 24 | EGFRvIII-CARs | 1 | Suspended | U.S. |
| 8 | NCT02844062 | Pilot Study of Autologous Anti-EGFRvIII CAR T Cells in Recurrent Glioblastoma Multiforme | 20 | anti-EGFRvIII CAR T cells | 1 | Recruiting | CH |
| 9 | NCT02664363 | EGFRvIII CAR T Cells for Newly-Diagnosed WHO Grade IV Malignant Glioma | 3 | EGFR vIII CAR T cells | 1 | Active, not recruiting | U.S. |
| 10 | NCT02340156 | Phase II Study of Combined Temozolomide and SGT-53 for Treatment of Recurrent Glioblastoma | 26 | SGT-53 | 2 | Recruiting | U.S. - TA |
| 11 | NCT02062827 | Genetically Engineered HSV-1 Phase 1 Study for the Treatment of Recurrent Malignant Glioma | 36 | M032 (NSC 733972) | 1 | Recruiting | U.S. |
| 12 | NCT02031965 | Oncolytic HSV-1716 in Treating Younger Patients With Refractory or Recurrent High Grade Glioma That Can Be Removed By Surgery | 2 | oncolytic HSV-1716 | 1 | Terminated | U.S. |
| 13 | NCT01811992 | Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma | 19 | Dose Escalation of Ad-hCMV-TK and Ad-hCMV-Flt3L | 1 | Active, not recruiting | U.S. |
| 14 | NCT01454596 | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients With Malignant Gliomas Expressing EGFRvIII | 18 | Anti-EGFRvIII CAR transduced PBL | 1–2 | Completed | U.S. |
| 15 | NCT01269424 | BG & TMZ Therapy of Glioblastoma Multiforme | 10 | MGMTP140K-encoding retroviral vector | 1 | Terminated | U.S. |
| 16 | NCT01205334 | Administration of CMV-Specific Cytotoxic T Cells in Patients With Glioblastoma Multiforme | 2 | Autologous CMV-specific CTL | 1–2 | Terminated | U.S. |
| 17 | NCT01172964 | A Pilot Feasibility Study of Oral 5-Fluorocytosine and Genetically-Modified Neural Stem Cells Expressing E.Coli Cytosine Deaminase for Treatment of Recurrent High Grade Gliomas | 15 | flucytosine | 1 | Completed | U.S. |
| 18 | NCT01156584 | A Study of a Retroviral Replicating Vector Combined With a Prodrug Administered to Patients With Recurrent Malignant Glioma | 54 | Toca 511 vector | 1 | Completed | U.S. |
| 19 | NCT01109095 | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM | 16 | HER.CAR CMV-specific CTLs | 1 | Completed | U.S. |
| 20 | NCT00870181 | ADV-TK Improves Outcome of Recurrent High-Grade Glioma | 47 | ADV-TK/GCV | 2 | Completed | CH |
| 21 | NCT00751270 | Phase 1b Study of AdV-tk + Valacyclovir Combined With Radiation Therapy for Malignant Gliomas | 15 | AdV-tk | 1 | Completed | U.S. |
| 22 | NCT00634231 | A Phase I Study of AdV-tk + Prodrug Therapy in Combination With Radiation Therapy for Pediatric Brain Tumors | 12 | AdV-tk | 1 | Active, not recruiting | U.S. |
| 23 | NCT00589875 | Phase 2a Study of AdV-tk With Standard Radiation Therapy for Malignant Glioma (BrTK02) | 52 | AdV-tk | 1 | Completed | U.S. |
| 24 | NCT00272870 | Treatment of Newly Diagnosed Brain Tumors With Chemotherapy and Radiation Using Cells Modified for Chemoprotection and an Experimental Drug to Decrease the Tumor Cell Resistance to Chemotherapy | 1 | MGMT P140K | 1 | Terminated | U.S. |
| 25 | NCT00004080 | Gene Therapy in Treating Patients With Recurrent or Progressive Brain Tumors | 21–42 | recombinant adenovirus-p53 SCH-58500 | 1 | Completed | U.S. |
| 26 | NCT00002824 | Gene Therapy in Treating Patients With Primary Brain Tumors | 18 | gene therapy | 1 | Completed | U.S. |
| 27 | NCT00004041 | Gene Therapy in Treating Patients With Recurrent Malignant Gliomas | 30 | Ad5CMV-p53 gene | 1 | Completed | U.S. |
| 28 | NCT00005796 | Combination Chemotherapy Plus Gene Therapy in Treating Patients With CNS Tumors | 10 | gene therapy | 1 | Completed | U.S. |
| 29 | NCT00031083 | Dose Escalation Study to Determine the Safety of IFN-Beta Gene Transfer in the Treatment of Grade III & Grade IV Gliomas | 35 | Interferon-beta | 1 | Suspended | U.S. |
Ad5CMV-p53 gene: a defective type-5 adenoviral vector contains a cytomegalovirus (CMV) promoter and expressing wtp53 gene; Ad-hCMV-Flt3L: a human serotype 5, replication-defective adenoviral vector contains a cytomegalovirus promoter and expressing the soluble, immune-mediated stimulatory gene human fms-like tyrosine kinase 3 ligand (Flt3L); Ad-hCMV-TK: a human serotype 5, replication-defective adenoviral vector contains a cytomegalovirus (CMV) promoter and expressing the herpes simplex virus thymidine kinase (HSV-TK) gene; ADV: adenovirus; ADV-TK/GCV: adenoviral vector carrying the herpes simplex virus thymidine kinase gene in combination with the prodrug ganciclovir; AdV-tk: adenoviruses (Ad) carrying the thymidine kinase (HSVtk) gene; Anti-EGFRvIII CAR transduced PBL: an anti-epidermal growth factor receptor variant III (EGFRvIII) chimeric T cell receptor (CAR) gene tranduced with peripheral blood lymphocytes (PBL); BG: 06-benzylguanine; CAR T: chimeric antigen receptor T-cell; CMV: cytomegalovirus; CNS: central nervous system; EGFRvIII: epidermal growth factor receptor variant III; GBM: glioblastoma; GMCI: gene mediated cytotoxic immunotherapy; HER.CAR CMV-specific CTLs: human cytomegalovirus (CMV)-specific; chimeric T cell receptor (CAR) gene; HER2.taNK: human epidermal growth factor 2 (HER2) target-activated natural killer cells (taNK cells); HER2: human epidermal growth factor receptor type II (HER); HSV-tk: herpes simplex virus thymidine kinase gene; M032 (NSC 733972): a second-generation oncolytic herpes simplex virus (oHSV); MGMT P140K: P140K mutant of human O(6)-methylguanine-DNA-methyltransferase (MGMT); SBRT: stereotactic body radiation therapy; SCH-58500: a weakened adenovirus that carries the p53 gene; SGT-53: nanocomplex designed for systemic, tumor-targeting delivery of the wt p53 gene; TMZ: temozolomide; WHO: World Health Organization; XRT: radiation therapy; U.S.: United States; GE: Germany; CH: China; TA: Taiwan.
3.2.3.1. Suicide gene therapies in glioblastoma
3.2.3.1.1. HSV-TK/nucleoside analogue gene therapy
A further approach for glioblastomas consists in the insertion of herpes simplex virus (HSV) thymidine kinase (TK) into the tumor cell genome.
TK is a key enzyme for DNA synthesis and cell division, and at the same time, it is also the target of antiviral drugs as acyclovir, ganciclovir, and valacyclovir which are nucleoside analogues. The introduction of a HSV-TK gene into the tumor cells makes them able to express HSV-TK, which is, in turn, attacked by antiviral drugs [152]. A non-negligible number of phase 1 and 2 trials have already established the safety of TK gene therapy and ganciclovir treatment [153, 154, 155, 156, 157, 158]. With the goal to enhance the local immune response against glioblastoma, immune modulatory genes as interleukin 2 gene have also been delivered in association with TK gene alongside HSV-TK gene therapy [158]. The effects of TK gene-Flt3L gene co-delivery, with the latter acting as DCs stimulator, are under investigation [159].
3.2.3.1.2. 5-FC/CD gene therapy
Similarly to what has already been described for HSV-TK/nucleoside analogue suicide gene therapy, 5-FC/CD therapy involves viral transduction of the cytosine deaminase (CD) gene into glioblastoma cells followed by administration of 5-fluorocytosine (5-FC). CD converts the antifungal drug 5-FC into fluorouracil, an antitumor drug with an oncolytic effect on tumor cells. Phase 1 to 3 trials have demonstrated safety and effectiveness of this kind of approach for glioblastomas and anaplastic astrocytomas [160, 161] (www.clinicaltrials.gov: NCT01985256, NCT01156584 and NCT01470794). Uracil phosphoribosyltransferase (UPRT) is an absent enzyme in mammalian cells, which converts 5-fluorouracil (5-FU) into 5-fluorouridine 5′-monophosphate. A co-expression of CD-UPRT genes has been reported to have a prolonged survival in animal glioblastoma model [162].
3.2.3.1.3. Adenovirus-interferon β therapy
A phase 1 trial has tested safety and efficacy of a stereotactic injection of human IFN-β (hIFN-β)-expressing adenovirus vector in patients with malignant gliomas, therefore concluding that also this “TK-independent” approach induces tumor cell apoptosis with tolerable collateral effects [163].
3.2.3.1.4. Adenovirus-mediated P53 therapy
Adenovirus-mediated p53 gene transfer into glioblastoma cells has been shown to have an oncolytic effect in a phase 1 trial [164]. All except one [165] of the completed or still ongoing trials concerning brain cancer gene therapy involve intracerebral injections of the chimeric cells achieved via open surgery or stereotaxis, because this route of administration has the great advantage of targeting the affected brain areas directly while bypassing the blood-brain barrier [153, 154, 155, 156, 157, 158, 164, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179]. Intraventricular release may be a valid option in case of disseminated tumors.
3.2.4. Genome editing technologies
Unlike gene modification technologies, those applied to genome editing comprehend a broader DNA manipulation through nucleases, which are able to modify, regulate or mark genomic loci, ultimately, producing therapeutic effects. A wide spectrum of nucleases has been detected for these purposes [149].
Differently from gene therapies, whose encoding products by transgenes are specific enzymes or growth factors that act directly as therapeutic tools, genome editing technologies involve wider genome manipulation of the target cells, finally operating a complete knockout, activation or even modification of the given gene. Targeted gene editing has already witnessed extensive use of zinc finger nucleases (ZFNs), meganucleases and transcription activator-like effector nucleases (TALENs). ZFNs are enzymes harboring a DNA-binding and a DNA-cleavage domain which allows them to perform highly selective editing of the target gene [180]. As most nucleases, also ZFNs are intended for ex vivo therapy using patients’ stem cells. TALENs are a further example of nucleases having a binding and a DNA-cleavage domain just like ZFNs. Engineered TALENs are delivered to target cells via plasmids able to transfect the cells and introduce exogenous DNA, which knocks out or modifies the target gene [180]. The most recent nuclease adopted for genome editing purposes is a clustered, regularly interspaced, short palindromic repeat (CRISPR)/Cas9 system. This bacterial immune system derived nucleases uses complementary guide RNAs to target specific sequences, and thus, it has been reported to be more intuitive if compared with other previously employed nucleases in terms of rational design of sequence targeting [181]. The CRISPR/Cas9 system works by targeting genetic material operated by CRISPR sequences and the degradation consisting in a double-stranded break of the same material by recruitment of the Cas9 endonuclease [182, 183].
3.2.5. Cell plasticity technologies
Cell plasticity technologies are aimed to induce a functional transdifferentiation of the cell, which in turn, is used to restore a damaged tissue. Cell plasticity technologies have been improved for the last ten years after the discovery of h-iPSCs [47, 48, 49].
Despite potential risks of tumorigenesis and cancer related to uncontrolled growth, as well as uncertainties related to the safety profile of transdifferentiated cells [184], this approach appears to be a valuable one considering the theoretically unlimited obtainable cell supply, and especially the full immune matching with the receiving patient in case of autologous transdifferentiation.
Cellular plasticity techniques are based on the assumption that a somatic cell, and therefore differentiated in a specific phenotype, under particular induced conditions can differentiate into a completely different phenotype, previously believed to be beyond its own transformation capacity. Transdifferentiation based upon the concept of plasticity can be induced in different ways. The highest and most recent expression of cellular transdifferentiation potential is represented by h-iPSCs [47, 48, 49]. The principal aspects that make this technology one of the most promising and exciting in the near future are the unlimited number of transdifferentiated cells potentially obtainable and their theoretically zero immunogenicity.
3.2.6. Three-dimensional technologies
Three-dimensional (3D) technologies have been proposed in order to overcome the two main limitations regarding tissue engineering, which are basically structural and immunological issues. 3D technologies arise from the full integration between the cellular somatic technologies and the use of biocompatible materials, the scaffolds first. In this context, new scaffolds play a role that goes far beyond their simple mechanical support. While first-generation biomaterials were employed only as inert mechanical substrates for cell growth, decellularized second-generation scaffolds combine their three-dimensional configuration with a physiologic module of mechanical strength. The so-called third-generation or smart scaffolds are even able to encapsulate transplanted cells to protect them from rejection caused by the host's immune system [4, 185, 186, 187]. Today, three-dimensional technologies represent a huge research field in regenerative medicine, as well as one of the most important translational challenges of the 21st century.
4. Discussion
4.1. Overview on the ongoing trends
The last decade has witnessed progressive evolution in neurosurgery mainly consisting in a transition from an era considered "mechanical" because of the kind of technology in use to a new "biological" one instead [30, 31, 33, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215].
This trend has occurred concurrently with the rapid and parallel development of applied biotechnology in both diagnostic and therapeutic fields. The most tangible aspect of this phenomenon is represented by the recent advances in brain tumors genomics and proteomics, which have led to a completely different classification [2] from describing merely histopathological morphological aspects to establishing a biomolecular definition capable of differentiating primitive tumors in terms of diagnosis, prognosis and therapy. A similar concept is also true for the success obtained by translational medicine, which have initiated an innumerable series of clinical trials, many already in phase 2, aimed at testing efficacy and safety of a series of gene therapies or immunotherapies for primary malignant CNS tumors. The aforementioned evolution goes far beyond the mechanical, physical or chemical approach involved in surgery, radiotherapy and chemotherapy respectively. Advances in translational medicine and nanotechnologies have also introduced new and revolutionary approaches to neurological diseases historically considered incurable: e.g. the use of stem cells in spinal cord injuries.
Nevertheless, to date, most cell therapies are still being tested in terms of both safety and efficacy with the only exception of the hematological field where transplantation of hematopoietic stem cells is already a proved and evidence-based treatment option for a wide spectrum of blood related disorders [216, 217].
That much said, nowadays and in the foreseeable future, neurosurgery ought to include the cell-based approach among the treatment options for a wide range of its pathologies.
4.2. Future perspectives
At present, surgery represents the only management able to guarantee long survival and a good outcome for several patients with complex and challenging neurosurgical pathologies, especially as far as oncologic and neurovascular fields are concerned [198, 201, 204, 208].
Promising cell-based therapies seem to have started to modify this scenario dramatically.
Despite recent and countless steps forward which have been taken, implementation of cell therapies in neurosurgical clinical practice is still limited today by the need to overcome several technical issues. Introduction of new technologies, optimization of the presently existing ones, and reduction of the costs are among the main future translational challenges.
4.2.1. Somatic cell technologies
Among somatic cell technologies, at present, the stem cell-based approach to brain and spinal cord trauma is influenced by the availability of such cells, the potential for engraftment, immunogenicity, and important ethical problems related to employment of embryonic or fetal stem cells. The recent advent of iPSs [47, 48, 49], and growing evidence regarding the possibility of using xenogeneic stem cells to induce neuroglial transdifferentiation [5] could partially overcome certain issues, e.g. availability and ethical aspects, but concerns still exist about their immunogenicity and oncogenicity. A further key aspect is connected to the stem cell administration route onto target sites, especially for brain tumors. Refinement of transdifferentiation-inducing techniques, and further clinical trials aimed at assessing a safety and efficacy profile, as well as an optimal dosage will be necessary to improve this kind of cellular therapy.
In the adoptive immunotherapies field, ALECSAT has recently aroused interest. Nevertheless, at the moment, no results are available for 2 completed trials out of the existing 3, of phase 1 and 2.
4.2.2. Gene modification technologies
The future of gene therapies instead will be focused in particular on the refinement and improvement of vector design and delivery, but also on the target gene promotors. To date, no vector is considered perfect. Although the advent of lipid nanoparticles has solved most immunogenicity issues, their potential spreading in the nervous system remains unknown, but apparently quite limited. An emerging need for an in-vivo real time imaging of the vector diffusion within the CNS also exists, and could be the object for further and new research fields to develop. Furthermore, an even higher specificity of the target gene promotors will be required to improve selectivity and specificity for such therapies. Vector delivery still represents a non- negligible problem to overcome when dealing with large or disseminated brain tumors, especially taking into account that current gene therapies find their principal indication in these cancer forms as second-line or adjuvant treatments. Vector design is strictly related to its potential for delivery, its immunogenicity notwithstanding. Indeed, despite their viral origin, immunogenic as such, viral vectors, small sized AAVs firsts, are still those with the highest spreading capacity, although they often have a very small transport capability. Last but far from least, the concept of minimal invasive and tailored biological era contrasts dramatically with the anachronistic need for intraventricular direct release, which still exists for most of these therapies. As a matter of fact, improvement in the administration route is also necessary.
4.2.3. Three-dimensional technologies
3D technologies are focusing more and more frequently on the thixotropic, thermo-responsive, growth factor-encapsulating and in situ self-assembling properties of biomaterials and scaffolds, and very soon organ-like structures or organoids will possibly be available for transplants [4, 185, 186, 187].
As far back as 1999, an impressive editorial by Winn and Howard reported that in the following 100 years, neurosurgery “would have evolved to non-invasively image and assessed the microenvironment (cellular level) of the central nervous system. Furthermore, the era of biological surgery would have been characterized by the location of neurosurgical operating rooms at the outpatient setting. The patient, having been diagnosed by microscreening techniques to have an early abnormality such as a tumor, would then had immunological therapy specific for the tumor type and CNS location to eradicate the abnormality. Then, through a process of disinhibition of native stem cells, regrowth of the diseased area of the central nervous system would have occurred” [218].
In conclusion, what Winn and Howard anticipated 20 years ago corresponds perfectly to the direction cellular therapies are nowadays conducting us to.
5. Conclusion
In the last decade, the rapid development of translational research and nanotechnologies in regenerative medicine has given rise to a still ongoing trend in neurosurgery characterized by its progressive evolution from a purely mechanical era to a new biological one.
Somatic cell technologies, which are at the basis of both immunologic and stem cell-based therapies, and gene-modification technologies have witnessed progress.
Immunotherapies and gene therapies have been widely tested for the cure of malignant brain tumors, especially for glioblastoma, in a non- negligible series of clinical trials, which have already reached phase 2.
Stem-cell based therapies have found a rationale in particular for sequelae of brain and spinal cord injuries, but also as potential treatment for skeletal, cranial and spinal defects, as well as for intervertebral disc degeneration.
The advent of new technologies, optimization of the already existing ones, and reduction of costs are among the main challenges for the future.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We want to thank Eng. Giorgia Di Giusto for her valuable technical support in the data collection and analysis. We also want to thank Angela Elia for preparing figures.
References
- 1.Lefrère J.-J., Berche P. Doctor Brown-Sequard’s therapy. Ann. Endocrinol. 2010;71:69–75. doi: 10.1016/j.ando.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Hanna E., Rémuzat C., Auquier P., Toumi M. Advanced therapy medicinal products: current and future perspectives. J. Market Acc. Health Pol. 2016;4 doi: 10.3402/jmahp.v4.31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mount N.M., Ward S.J., Kefalas P., Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015;370:20150017. doi: 10.1098/rstb.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzzi S., Crovace A.M., Lacitignola L., Valentini V., Francioso E., Rossi G., Invernici G., Galzio R.J., Crovace A. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. Surg. Neurol. Int. 2018;9:19. doi: 10.4103/sni.sni_369_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villa A., Navarro-Galve B., Bueno C., Franco S., Blasco M.A., Martinez-Serrano A. Long-term molecular and cellular stability of human neural stem cell lines. Exp. Cell Res. 2004;294:559–570. doi: 10.1016/j.yexcr.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright D.A., Sengupta S., Han Y., Lesniak M.S. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright D.A., Dey M., Chang A., Lesniak M.S. Targeting Tregs in malignant brain cancer: overcoming IDO. Front. Immunol. 2013;4:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2019. https://clinicaltrials.gov
- 10.Han E.Q., Li X., Wang C., Li T., Han S. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J. Hematol. Oncol. 2013;6:47. doi: 10.1186/1756-8722-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava S., Riddell S.R. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwatra M.M. A rational approach to target the epidermal growth factor receptor in glioblastoma. Curr. Cancer Drug Targets. 2017;17:290–296. doi: 10.2174/1568009616666161227091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padfield E., Ellis H.P., Kurian K.M. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front. Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., Kurien A., Priceman S.J., Wang X., Harshbarger T.L., D’Apuzzo M., Ressler J.A., Jensen M.C., Barish M.E., Chen M., Portnow J., Forman S.J., Badie B. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed N., Salsman V.S., Kew Y., Shaffer D., Powell S., Zhang Y.J., Grossman R.G., Heslop H.E., Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res.: An Official Journal of the American Association for Cancer Research. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde M., Corder A., Chow K.K.H., Mukherjee M., Ashoori A., Kew Y., Zhang Y.J., Baskin D.S., Merchant F.A., Brawley V.S., Byrd T.T., Krebs S., Wu M.F., Liu H., Heslop H.E., Gottschalk S., Gottachalk S., Yvon E., Ahmed N. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow K.K.H., Naik S., Kakarla S., Brawley V.S., Shaffer D.R., Yi Z., Rainusso N., Wu M.-F., Liu H., Kew Y., Grossman R.G., Powell S., Lee D., Ahmed N., Gottschalk S. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muranski P., Boni A., Wrzesinski C., Citrin D.E., Rosenberg S.A., Childs R., Restifo N.P. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat. Clin. Pract. Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger A., Werlenius K., Hallner A., Thorén F.B., Farahmand D., Tisell M., Smits A., Rydenhag B., Jakola A.S., Carén H. Determinants for effective ALECSAT immunotherapy treatment on autologous patient-derived glioblastoma stem cells. Neoplasia (New York, N.Y.) 2018;20:25–31. doi: 10.1016/j.neo.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kmiecik J., Poli A., Brons N.H.C., Waha A., Eide G.E., Enger P.Ø., Zimmer J., Chekenya M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Golán I., Rodríguez de la Fuente L., Costoya J.A. NK cell-based glioblastoma immunotherapy. Cancers. 2018;10 doi: 10.3390/cancers10120522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karantalis V., Schulman I.H., Balkan W., Hare J.M. Allogeneic cell therapy: a new paradigm in therapeutics. Circ. Res. 2015;116:12–15. doi: 10.1161/CIRCRESAHA.114.305495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gras Navarro A., Kmiecik J., Leiss L., Zelkowski M., Engelsen A., Bruserud Ø., Zimmer J., Enger P.Ø., Chekenya M. NK cells with KIR2DS2 immunogenotype have a functional activation advantage to efficiently kill glioblastoma and prolong animal survival. J. Immunol. 2014;193:6192–6206. doi: 10.4049/jimmunol.1400859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yvon E.S., Burga R., Powell A., Cruz C.R., Fernandes R., Barese C., Nguyen T., Abdel-Baki M.S., Bollard C.M. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19:408–418. doi: 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L., Oh J.M., Gangadaran P., Kalimuthu S., Baek S.H., Jeong S.Y., Lee S.-W., Lee J., Ahn B.-C. Targeting and therapy of glioblastoma in a mouse model using exosomes derived from natural killer cells. Front. Immunol. 2018;9:824. doi: 10.3389/fimmu.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Murakami T., Nakazawa T., Natsume A., Nishimura F., Nakamura M., Matsuda R., Omoto K., Tanaka Y., Shida Y., Park Y.-S., Motoyama Y., Nakagawa I., Yamada S., Tamura K., Takeshima Y., Takamura Y., Wakabayashi T., Nakase H. Novel human NK cell line carrying CAR targeting EGFRvIII induces antitumor effects in glioblastoma cells. Anticancer Res. 2018;38:5049–5056. doi: 10.21873/anticanres.12824. [DOI] [PubMed] [Google Scholar]
- 27.Kmiecik J., Gras Navarro A., Poli A., Planagumà J.P., Zimmer J., Chekenya M. Combining NK cells and mAb9.2.27 to combat NG2-dependent and anti-inflammatory signals in glioblastoma. OncoImmunology. 2014;3 doi: 10.4161/onci.27185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang B., Wu W., Wei X., Li Y., Ren G., Fan W. Activation of glioma cells generates immune tolerant NKT cells. J. Biol. Chem. 2014;289:34595–34600. doi: 10.1074/jbc.M114.614503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhodapkar K.M., Cirignano B., Chamian F., Zagzag D., Miller D.C., Finlay J.L., Steinman R.M. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int. J. Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 30.Palumbo P., Lombardi F., Siragusa G., Dehcordi S.R., Luzzi S., Cimini A., Cifone M.G., Cinque B. Involvement of NOS2 activity on human glioma cell growth, clonogenic potential, and neurosphere generation. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19092801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raysi Dehcordi S., Ricci A., Di Vitantonio H., De Paulis D., Luzzi S., Palumbo P., Cinque B., Tempesta D., Coletti G., Cipolloni G., Cifone M.G., Galzio R. Stemness marker detection in the periphery of glioblastoma and ability of glioblastoma to generate glioma stem cells: clinical correlations. World Neurosurg. 2017;105:895–905. doi: 10.1016/j.wneu.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo P., Miconi G., Cinque B., Lombardi F., La Torre C., Dehcordi S.R., Galzio R., Cimini A., Giordano A., Cifone M.G. NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression. Oncotarget. 2017;8:25582–25598. doi: 10.18632/oncotarget.16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palumbo P., Lombardi F., Augello F.R., Giusti I., Luzzi S., Dolo V., Cifone M.G., Cinque B. NOS2 inhibitor 1400W induces autophagic flux and influences extracellular vesicle profile in human glioblastoma U87MG cell line. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss M.L., Medicetty S., Bledsoe A.R., Rachakatla R.S., Choi M., Merchav S., Luo Y., Rao M.S., Velagaleti G., Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells (Dayton, Ohio) 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 35.Weiss M.L., Anderson C., Medicetty S., Seshareddy K.B., Weiss R.J., VanderWerff I., Troyer D., McIntosh K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells (Dayton, Ohio) 2008;26:2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 36.Weiss M.L., Troyer D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay R. Stem cells in the central nervous system. Science (New York, N.Y.) 1997;276:66–71. doi: 10.1126/science.276.5309.66. http://www.ncbi.nlm.nih.gov/pubmed/9082987 [DOI] [PubMed] [Google Scholar]
- 38.Gage F.H. Mammalian neural stem cells. Science (New York, N.Y.) 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. http://www.ncbi.nlm.nih.gov/pubmed/10688783 [DOI] [PubMed] [Google Scholar]
- 39.Ourednik V., Ourednik J., Park K.I., Snyder E.Y. Neural stem cells -- a versatile tool for cell replacement and gene therapy in the central nervous system. Clin. Genet. 1999;56:267–278. doi: 10.1034/j.1399-0004.1999.560403.x. http://www.ncbi.nlm.nih.gov/pubmed/10636444 [DOI] [PubMed] [Google Scholar]
- 40.Zheng T., Marshall G.P., Ii, Chen K.A., Laywell E.D. Transplantation of neural stem/progenitor cells into developing and adult CNS. Methods Mol. Biol. 2009;482:185–197. doi: 10.1007/978-1-59745-060-7_12. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich J., Kempermann G. Role of endogenous neural stem cells in neurological disease and brain repair. Adv. Exp. Med. Biol. 2006;557:191–220. doi: 10.1007/0-387-30128-3_12. [DOI] [PubMed] [Google Scholar]
- 42.Sharp J., Keirstead H.S. Stem cell-based cell replacement strategies for the central nervous system. Neurosci. Lett. 2009;456:107–111. doi: 10.1016/j.neulet.2008.04.106. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong R.J., Svendsen C.N. Neural stem cells: from cell biology to cell replacement. Cell Transplant. 2000;9:139–152. doi: 10.1177/096368970000900202. http://www.ncbi.nlm.nih.gov/pubmed/10811389 [DOI] [PubMed] [Google Scholar]
- 44.Frisén J., Johansson C.B., Lothian C., Lendahl U. Central nervous system stem cells in the embryo and adult. Cell. Mol. Life Sci.: CMLS. 1998;54:935–945. doi: 10.1007/s000180050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trujillo C.A., Schwindt T.T., Martins A.H., Alves J.M., Mello L.E., Ulrich H. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry Part A: The Journal of the International Society for Analytical Cytology. 2009;75:38–53. doi: 10.1002/cyto.a.20666. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. BioMed Res. Int. 2015;2015:727542. doi: 10.1155/2015/727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, N.Y.). 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Miconi G., Palumbo P., Dehcordi S.R., La Torre C., Lombardi F., Evtoski Z., Cimini A.M., Galzio R., Cifone M.G., Cinque B. Immunophenotypic characterization of human glioblastoma stem cells: correlation with clinical outcome. J. Cell. Biochem. 2015;116:864–876. doi: 10.1002/jcb.25043. [DOI] [PubMed] [Google Scholar]
- 51.Aboody K.S., Brown A., Rainov N.G., Bower K.A., Liu S., Yang W., Small J.E., Herrlinger U., Ourednik V., Black P.M., Breakefield X.O., Snyder E.Y. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown A.B., Yang W., Schmidt N.O., Carroll R., Leishear K.K., Rainov N.G., Black P.M., Breakefield X.O., Aboody K.S. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum. Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 53.Kim S.-K., Kim S.U., Park I.H., Bang J.H., Aboody K.S., Wang K.-C., Cho B.-K., Kim M., Menon L.G., Black P.M., Carroll R.S. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin. Cancer Res.: An Official Journal of the American Association for Cancer Research. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 54.Oh M.C., Lim D.A. Novel treatment strategies for malignant gliomas using neural stem cells. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2009;6:458–464. doi: 10.1016/j.nurt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benedetti S., Pirola B., Pollo B., Magrassi L., Bruzzone M.G., Rigamonti D., Galli R., Selleri S., Di Meco F., De Fraja C., Vescovi A., Cattaneo E., Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 56.Lorico A., Mercapide J., Solodushko V., Soloduschko V., Alexeyev M., Fodstad O., Rappa G. Primary neural stem/progenitor cells expressing endostatin or cytochrome P450 for gene therapy of glioblastoma. Cancer Gene Ther. 2008;15:605–615. doi: 10.1038/cgt.2008.23. [DOI] [PubMed] [Google Scholar]
- 57.Benedetti S., Bruzzone M.G., Pollo B., DiMeco F., Magrassi L., Pirola B., Cirenei N., Colombo M.P., Finocchiaro G. Eradication of rat malignant gliomas by retroviral-mediated, in vivo delivery of the interleukin 4 gene. Cancer Res. 1999;59:645–652. http://www.ncbi.nlm.nih.gov/pubmed/9973213 [PubMed] [Google Scholar]
- 58.Li S., Tokuyama T., Yamamoto J., Koide M., Yokota N., Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 59.Burns M.J., Weiss W. Targeted therapy of brain tumors utilizing neural stem and progenitor cells. Front. Biosci.: A Journal and Virtual Library. 2003;8:e228–e234. doi: 10.2741/953. http://www.ncbi.nlm.nih.gov/pubmed/12456351 [DOI] [PubMed] [Google Scholar]
- 60.Colleoni F., Torrente Y. The new challenge of stem cell: brain tumour therapy. Cancer Lett. 2008;272:1–11. doi: 10.1016/j.canlet.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 61.Portnow J., Synold T.W., Badie B., Tirughana R., Lacey S.F., D’Apuzzo M., Metz M.Z., Najbauer J., Bedell V., Vo T., Gutova M., Frankel P., Chen M., Aboody K.S. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin. Cancer Res.: An Official Journal of the American Association for Cancer Research. 2017;23:2951–2960. doi: 10.1158/1078-0432.CCR-16-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ehtesham M., Kabos P., Gutierrez M.A.R., Chung N.H.C., Griffith T.S., Black K.L., Yu J.S. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. http://www.ncbi.nlm.nih.gov/pubmed/12499252 [PubMed] [Google Scholar]
- 63.Shah K., Bureau E., Kim D.-E., Yang K., Tang Y., Weissleder R., Breakefield X.O. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann. Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 64.Yang B., Wu X., Mao Y., Bao W., Gao L., Zhou P., Xie R., Zhou L., Zhu J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. discussion 624. [DOI] [PubMed] [Google Scholar]
- 65.Rowinsky E.K. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J. Clin. Oncol.: Official Journal of the American Society of Clinical Oncology. 2005;23:9394–9407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 66.Mitsiades C.S., Poulaki V., Mitsiades N. The role of apoptosis-inducing receptors of the tumor necrosis factor family in thyroid cancer. J. Endocrinol. 2003;178:205–216. doi: 10.1677/joe.0.1780205. http://www.ncbi.nlm.nih.gov/pubmed/12904168 [DOI] [PubMed] [Google Scholar]
- 67.Herrlinger U., Woiciechowski C., Sena-Esteves M., Aboody K.S., Jacobs A.H., Rainov N.G., Snyder E.Y., Breakefield X.O. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2000;1:347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs A., Tjuvajev J.G., Dubrovin M., Akhurst T., Balatoni J., Beattie B., Joshi R., Finn R., Larson S.M., Herrlinger U., Pechan P.A., Chiocca E.A., Breakefield X.O., Blasberg R.G. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001;61:2983–2995. http://www.ncbi.nlm.nih.gov/pubmed/11306477 [PubMed] [Google Scholar]
- 69.Jia W.W., McDermott M., Goldie J., Cynader M., Tan J., Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type 1. J. Natl. Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. http://www.ncbi.nlm.nih.gov/pubmed/8040888 [DOI] [PubMed] [Google Scholar]
- 70.Nanda D., Vogels R., Havenga M., Avezaat C.J., Bout A., Smitt P.S. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61:8743–8750. http://www.ncbi.nlm.nih.gov/pubmed/11751394 [PubMed] [Google Scholar]
- 71.Ichikawa T., Saeki Y., Tamiya T., Chiocca E.A., Ohmoto T. Herpes simplex type-1 virus-based vectors for gene therapy. Nihon Rinsho. Japanese J. Clin. Med. 2000;58:822–827. http://www.ncbi.nlm.nih.gov/pubmed/10774201 [PubMed] [Google Scholar]
- 72.Todo T. Brain tumor therapy using replication--competent virus vectors. Nihon Rinsho. Japanese J. Clin. Med. 2005;63(Suppl 9):504–509. http://www.ncbi.nlm.nih.gov/pubmed/16201572 [PubMed] [Google Scholar]
- 73.Staflin K., Honeth G., Kalliomäki S., Kjellman C., Edvardsen K., Lindvall M. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 2004;64:5347–5354. doi: 10.1158/0008-5472.CAN-03-1246. [DOI] [PubMed] [Google Scholar]
- 74.Apple D.M., Solano-Fonseca R., Kokovay E. Neurogenesis in the aging brain. Biochem. Pharmacol. 2017;141:77–85. doi: 10.1016/j.bcp.2017.06.116. [DOI] [PubMed] [Google Scholar]
- 75.Kempermann G., van Praag H., Gage F.H. Activity-dependent regulation of neuronal plasticity and self repair. Prog. Brain Res. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. http://www.ncbi.nlm.nih.gov/pubmed/11142036 [DOI] [PubMed] [Google Scholar]
- 76.Wang X., Seekaew P., Gao X., Chen J. Traumatic brain injury stimulates neural stem cell proliferation via mammalian target of rapamycin signaling pathway activation. ENeuro. 2016;3 doi: 10.1523/ENEURO.0162-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong S., Washington P.M., Kim A., Yang C.-P., Yu T.-S., Kernie S.G. Apolipoprotein E regulates injury-induced activation of hippocampal neural stem and progenitor cells. J. Neurotrauma. 2016;33:362–374. doi: 10.1089/neu.2014.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rolfe A., Sun D. 2015. Stem Cell Therapy in Brain Trauma: Implications for Repair and Regeneration of Injured Brain in Experimental TBI Models.http://www.ncbi.nlm.nih.gov/pubmed/26269908 [PubMed] [Google Scholar]
- 79.Kernie S.G., Parent J.M. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol. Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kernie S.G., Erwin T.M., Parada L.F. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J. Neurosci. Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- 81.Burns K.A., Murphy B., Danzer S.C., Kuan C.-Y. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57:1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J., Wu X., Zhang H.L. Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr. Drug Targets. 2005;6:97–110. doi: 10.2174/1389450053345055. http://www.ncbi.nlm.nih.gov/pubmed/15720217 [DOI] [PubMed] [Google Scholar]
- 83.Kulbatski I., Mothe A.J., Nomura H., Tator C.H. Endogenous and exogenous CNS derived stem/progenitor cell approaches for neurotrauma. Curr. Drug Targets. 2005;6:111–126. doi: 10.2174/1389450053345037. http://www.ncbi.nlm.nih.gov/pubmed/15720218 [DOI] [PubMed] [Google Scholar]
- 84.Liu C.Y., Westerlund U., Svensson M., Moe M.C., Varghese M., Berg-Johnsen J., Apuzzo M.L.J., Tirrell D.A., Langmoen I.A. Artificial niches for human adult neural stem cells: possibility for autologous transplantation therapy. J. Hematother. Stem Cell Res. 2003;12:689–699. doi: 10.1089/15258160360732713. [DOI] [PubMed] [Google Scholar]
- 85.Ader M., Schachner M., Bartsch U. Integration and differentiation of neural stem cells after transplantation into the dysmyelinated central nervous system of adult mice. Eur. J. Neurosci. 2004;20:1205–1210. doi: 10.1111/j.1460-9568.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- 86.Shetty A.K., Hattiangady B. Grafted subventricular zone neural stem cells display robust engraftment and similar differentiation properties and form new neurogenic niches in the young and aged Hippocampus. Stem Cell. Transl. Med. 2016;5:1204–1215. doi: 10.5966/sctm.2015-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pang A.-L., Xiong L.-L., Xia Q.-J., Liu F., Wang Y.-C., Liu F., Zhang P., Meng B.-L., Tan S., Wang T.-H. Neural stem cell transplantation is associated with inhibition of apoptosis, Bcl-xL upregulation, and recovery of neurological function in a rat model of traumatic brain injury. Cell Transplant. 2017;26:1262–1275. doi: 10.1177/0963689717715168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma H., Yu B., Kong L., Zhang Y., Shi Y. Transplantation of neural stem cells enhances expression of synaptic protein and promotes functional recovery in a rat model of traumatic brain injury. Mol. Med. Rep. 2011;4:849–856. doi: 10.3892/mmr.2011.510. [DOI] [PubMed] [Google Scholar]
- 89.Beretta S., Cunningham K.M., Haus D.L., Gold E.M., Perez H., López-Velázquez L., Cummings B.J. Effects of human ES-derived neural stem cell transplantation and kindling in a rat model of traumatic brain injury. Cell Transplant. 2017;26:1247–1261. doi: 10.1177/0963689717714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed A.I., Gajavelli S., Spurlock M.S., Chieng L.O., Bullock M.R. Stem cells for therapy in TBI. J. R. Army Med. Corps. 2016;162:98–102. doi: 10.1136/jramc-2015-000475. [DOI] [PubMed] [Google Scholar]
- 91.Yang H., Lu P., McKay H.M., Bernot T., Keirstead H., Steward O., Gage F.H., Edgerton V.R., Tuszynski M.H. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joyce N., Annett G., Wirthlin L., Olson S., Bauer G., Nolta J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 94.Meirelles L. da S., Nardi N.B. Methodology, biology and clinical applications of mesenchymal stem cells. Front. Biosci. (Landmark Edition) 2009;14:4281–4298. doi: 10.2741/3528. http://www.ncbi.nlm.nih.gov/pubmed/19273350 [DOI] [PubMed] [Google Scholar]
- 95.Ryan J.M., Barry F., Murphy J.M., Mahon B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falnikar A., Li K., Lepore A.C. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 2015;1619:91–103. doi: 10.1016/j.brainres.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Granger N., Blamires H., Franklin R.J.M., Jeffery N.D. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain: J. Neurol. 2012;135:3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J.-H., Chung W.-H., Kang E.-H., Chung D.-J., Choi C.-B., Chang H.-S., Lee J.-H., Hwang S.-H., Han H., Choe B.-Y., Kim H.-Y. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J. Neurol. Sci. 2011;300:86–96. doi: 10.1016/j.jns.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 99.Jung D.-I., Ha J., Kang B.-T., Kim J.-W., Quan F.-S., Lee J.-H., Woo E.-J., Park H.-M. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 2009;285:67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 100.McMahill B.G., Borjesson D.L., Sieber-Blum M., Nolta J.A., Sturges B.K. Stem cells in canine spinal cord injury--promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev. 2015;11:180–193. doi: 10.1007/s12015-014-9553-9. [DOI] [PubMed] [Google Scholar]
- 101.McDonald J.W., Liu X.Z., Qu Y., Liu S., Mickey S.K., Turetsky D., Gottlieb D.I., Choi D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 102.Cummings B.J., Uchida N., Tamaki S.J., Salazar D.L., Hooshmand M., Summers R., Gage F.H., Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gabel B.C., Curtis E.I., Marsala M., Ciacci J.D. A review of stem cell therapy for spinal cord injury: large animal models and the frontier in humans. World Neurosurg. 2017;98:438–443. doi: 10.1016/j.wneu.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 104.Dai G., Liu X., Zhang Z., Yang Z., Dai Y., Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 105.El-Kheir W.A., Gabr H., Awad M.R., Ghannam O., Barakat Y., Farghali H.A.M.A., El Maadawi Z.M., Ewes I., Sabaawy H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 106.Mendonça M.V.P., Larocca T.F., de Freitas Souza B.S., Villarreal C.F., Silva L.F.M., Matos A.C., Novaes M.A., Bahia C.M.P., de Oliveira Melo Martinez A.C., Kaneto C.M., Furtado S.B.C., Sampaio G.P., Soares M.B.P., dos Santos R.R. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rapp S.J., Jones D.C., Gerety P., Taylor J.A. Repairing critical-sized rat calvarial defects with progenitor cell-seeded acellular periosteum: a novel biomimetic scaffold. Surgery. 2012;152:595–604. doi: 10.1016/j.surg.2012.07.019. 605.e1; discussion 604-5. [DOI] [PubMed] [Google Scholar]
- 108.Palumbo P., Lombardi F., Siragusa G., Cifone M.G., Cinque B., Giuliani M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheyn D., Kallai I., Tawackoli W., Cohn Yakubovich D., Oh A., Su S., Da X., Lavi A., Kimelman-Bleich N., Zilberman Y., Li N., Bae H., Gazit Z., Pelled G., Gazit D. Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Mol. Pharm. 2011;8:1592–1601. doi: 10.1021/mp200226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ko J.-Y., Park S., Im G.-I. Osteogenesis from human induced pluripotent stem cells: an in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014;23:1788–1797. doi: 10.1089/scd.2014.0043. [DOI] [PubMed] [Google Scholar]
- 111.Levi B., Hyun J.S., Montoro D.T., Lo D.D., Chan C.K.F., Hu S., Sun N., Lee M., Grova M., Connolly A.J., Wu J.C., Gurtner G.C., Weissman I.L., Wan D.C., Longaker M.T. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc. Natl. Acad. Sci. U.S.A. 2012;109:20379–20384. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim S., Kim S.-S., Lee S.-H., Eun Ahn S., Gwak S.-J., Song J.-H., Kim B.-S., Chung H.-M. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–1053. doi: 10.1016/j.biomaterials.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Svanholm H., Nielsen B., Starklint H. Keratin patterns in prostatic hyperplasia and adenocarcinoma. APMIS Suppl. 1988;4:100–108. http://www.ncbi.nlm.nih.gov/pubmed/2465010 [PubMed] [Google Scholar]
- 114.James A.W., Levi B., Nelson E.R., Peng M., Commons G.W., Lee M., Wu B., Longaker M.T. Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo. Stem Cells Dev. 2011;20:427–439. doi: 10.1089/scd.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henriksson H., Thornemo M., Karlsson C., Hägg O., Junevik K., Lindahl A., Brisby H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 116.Sakai D., Nakamura Y., Nakai T., Mishima T., Kato S., Grad S., Alini M., V Risbud M., Chan D., Cheah K.S.E., Yamamura K., Masuda K., Okano H., Ando K., Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yasen M., Fei Q., Hutton W.C., Zhang J., Dong J., Jiang X., Zhang F. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim. Biophys. Sin. 2013;45:368–376. doi: 10.1093/abbs/gmt019. [DOI] [PubMed] [Google Scholar]
- 118.Melrose J., Smith S.M., Fuller E.S., Young A.A., Roughley P.J., Dart A., Little C.B. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur. Spine J.: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2007;16:2193–2205. doi: 10.1007/s00586-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.SMITH J.W., WALMSLEY R. Experimental incision of the intervertebral disc. J. Bone Jt. Surg. 1951;33-B:612–625. doi: 10.1302/0301-620X.33B4.612. http://www.ncbi.nlm.nih.gov/pubmed/14880589 [DOI] [PubMed] [Google Scholar]
- 120.Korecki C.L., Costi J.J., Iatridis J.C. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine. 2008;33:235–241. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi H., Johnson Z.I., V Risbud M. Understanding nucleus pulposus cell phenotype: a prerequisite for stem cell based therapies to treat intervertebral disc degeneration. Curr. Stem Cell Res. Ther. 2015;10:307–316. doi: 10.2174/1574888x10666150113112149. http://www.ncbi.nlm.nih.gov/pubmed/25584906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.V Risbud M., Guttapalli A., Tsai T.-T., Lee J.Y., Danielson K.G., Vaccaro A.R., Albert T.J., Gazit Z., Gazit D., Shapiro I.M. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 123.Blanco J.F., Graciani I.F., Sanchez-Guijo F.M., Muntión S., Hernandez-Campo P., Santamaria C., Carrancio S., Barbado M.-V., Cruz G., Gutierrez-Cosío S., Herrero C., San Miguel J.F., Briñon J.G., del Cañizo M.-C. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine. 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 124.Hiyama A., Skubutyte R., Markova D., Anderson D.G., Yadla S., Sakai D., Mochida J., Albert T.J., Shapiro I.M., V Risbud M. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum. 2011;63:1355–1364. doi: 10.1002/art.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kregar Velikonja N., Urban J., Fröhlich M., Neidlinger-Wilke C., Kletsas D., Potocar U., Turner S., Roberts S. Cell sources for nucleus pulposus regeneration. Eur. Spine J.: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;23(Suppl 3):S364–S374. doi: 10.1007/s00586-013-3106-9. [DOI] [PubMed] [Google Scholar]
- 126.Steck E., Bertram H., Abel R., Chen B., Winter A., Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells (Dayton, Ohio) 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 127.Ehlicke F., Freimark D., Heil B., Dorresteijn A., Czermak P. Intervertebral disc regeneration: influence of growth factors on differentiation of human mesenchymal stem cells (hMSC) Int. J. Artif. Organs. 2010;33:244–252. http://www.ncbi.nlm.nih.gov/pubmed/20458694 [PubMed] [Google Scholar]
- 128.Richardson S.M., V Walker R., Parker S., Rhodes N.P., Hunt J.A., Freemont A.J., Hoyland J.A. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells (Dayton, Ohio) 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 129.Korecki C.L., Taboas J.M., Tuan R.S., Iatridis J.C. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res. Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stevanato L., Corteling R.L., Stroemer P., Hope A., Heward J., Miljan E.A., Sinden J.D. c-MycERTAM transgene silencing in a genetically modified human neural stem cell line implanted into MCAo rodent brain. BMC Neurosci. 2009;10:86. doi: 10.1186/1471-2202-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pollock K., Stroemer P., Patel S., Stevanato L., Hope A., Miljan E., Dong Z., Hodges H., Price J., Sinden J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 132.Maurer J., Fuchs S., Jäger R., Kurz B., Sommer L., Schorle H. Establishment and controlled differentiation of neural crest stem cell lines using conditional transgenesis. Differentiation. 2007;75:580–591. doi: 10.1111/j.1432-0436.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 133.Spiegl-Kreinecker S., Lötsch D., Neumayer K., Kastler L., Gojo J., Pirker C., Pichler J., Weis S., Kumar R., Webersinke G., Gruber A., Berger W. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018 doi: 10.1093/neuonc/noy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song D., Zhang F., Reid R.R., Ye J., Wei Q., Liao J., Zou Y., Fan J., Ma C., Hu X., Qu X., Chen L., Li L., Yu Y., Yu X., Zhang Z., Zhao C., Zeng Z., Zhang R., Yan S., Wu T., Wu X., Shu Y., Lei J., Li Y., Zhang W., Wang J., Lee M.J., Wolf J.M., Huang D., He T.-C. BMP9 induces osteogenesis and adipogenesis in the immortalized human cranial suture progenitors from the patent sutures of craniosynostosis patients. J. Cell Mol. Med. 2017;21:2782–2795. doi: 10.1111/jcmm.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ostrow K.L., Donaldson K., Blakeley J., Belzberg A., Hoke A. Immortalized human Schwann cell lines derived from tumors of schwannomatosis patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janson C., Romanova L., Hansen E., Hubel A., Lam C. Immortalization and functional characterization of rat arachnoid cell lines. Neuroscience. 2011;177:23–34. doi: 10.1016/j.neuroscience.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 137.Guo P., Yu C., Wang Q., Zhang R., Meng X., Feng Y. Liposome lipid-based formulation has the least influence on rAAV transduction compared to other transfection agents. Mol. Ther. - Meth. Clin. Dev. 2018;9:367–375. doi: 10.1016/j.omtm.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zylberberg C., Gaskill K., Pasley S., Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24:441–452. doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- 139.Del Pozo-Rodríguez A., Solinís M.Á., Rodríguez-Gascón A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm.: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V. 2016;109:184–193. doi: 10.1016/j.ejpb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 140.Wang K., Kievit F.M., Zhang M. Nanoparticles for cancer gene therapy: recent advances, challenges, and strategies. Pharmacol. Res. 2016;114:56–66. doi: 10.1016/j.phrs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 141.Boura J.S., Vance M., Yin W., Madeira C., Lobato da Silva C., Porada C.D., Almeida-Porada G. Evaluation of gene delivery strategies to efficiently overexpress functional HLA-G on human bone marrow stromal cells. Mol. Ther. - Meth. Clin. Dev. 2014;2014 doi: 10.1038/mtm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qasim W., Thrasher A.J. Progress and prospects for engineered T cell therapies. Br. J. Haematol. 2014;166:818–829. doi: 10.1111/bjh.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., Steinberg S.M., Stroncek D., Tschernia N., Yuan C., Zhang H., Zhang L., Rosenberg S.A., Wayne A.S., Mackall C.L. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abrate A., Buono R., Canu T., Esposito A., Del Maschio A., Lucianò R., Bettiga A., Colciago G., Guazzoni G., Benigni F., Hedlund P., Altaner C., Montorsi F., Cavarretta I.T.R. Mesenchymal stem cells expressing therapeutic genes induce autochthonous prostate tumour regression. Eur. J. Cancer (Oxford, England: 1990) 2014;50:2478–2488. doi: 10.1016/j.ejca.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 145.Zeltsman M., Dozier J., McGee E., Ngai D., Adusumilli P.S. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl. Res.: J. Lab. Clin. Med. 2017;187:1–10. doi: 10.1016/j.trsl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Magee M.S., Snook A.E. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov. Med. 2014;18:265–271. http://www.ncbi.nlm.nih.gov/pubmed/25425467 [PMC free article] [PubMed] [Google Scholar]
- 147.Choong C.-J., Baba K., Mochizuki H. Gene therapy for neurological disorders. Expert Opin. Biol. Ther. 2016;16:143–159. doi: 10.1517/14712598.2016.1114096. [DOI] [PubMed] [Google Scholar]
- 148.Simonato M., Bennett J., Boulis N.M., Castro M.G., Fink D.J., Goins W.F., Gray S.J., Lowenstein P.R., Vandenberghe L.H., Wilson T.J., Wolfe J.H., Glorioso J.C. Progress in gene therapy for neurological disorders. Nat. Rev. Neurol. 2013;9:277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McMahon M.A., Cleveland D.W. Gene therapy: gene-editing therapy for neurological disease. Nat. Rev. Neurol. 2017;13:7–9. doi: 10.1038/nrneurol.2016.190. [DOI] [PubMed] [Google Scholar]
- 150.Hirsch M.L., Wolf S.J., Samulski R.J. Delivering transgenic DNA exceeding the carrying capacity of AAV vectors. Methods Mol. Biol. 2016;1382:21–39. doi: 10.1007/978-1-4939-3271-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Latchman D.S. Gene delivery and gene therapy with herpes simplex virus-based vectors. Gene. 2001;264:1–9. doi: 10.1016/s0378-1119(01)00322-5. http://www.ncbi.nlm.nih.gov/pubmed/11245972 [DOI] [PubMed] [Google Scholar]
- 152.Black M.E., Newcomb T.G., Wilson H.M., Loeb L.A. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. http://www.ncbi.nlm.nih.gov/pubmed/8622970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Izquierdo M., Martín V., de Felipe P., Izquierdo J.M., Pérez-Higueras A., Cortés M.L., Paz J.F., Isla A., Blázquez M.G. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther. 1996;3:491–495. http://www.ncbi.nlm.nih.gov/pubmed/8789798 [PubMed] [Google Scholar]
- 154.Prados M.D., McDermott M., Chang S.M., Wilson C.B., Fick J., Culver K.W., Van Gilder J., Keles G.E., Spence A., Berger M. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J. Neuro Oncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. http://www.ncbi.nlm.nih.gov/pubmed/14682377 [DOI] [PubMed] [Google Scholar]
- 155.Trask T.W., Trask R.P., Aguilar-Cordova E., Shine H.D., Wyde P.R., Goodman J.C., Hamilton W.J., Rojas-Martinez A., Chen S.H., Woo S.L., Grossman R.G. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 156.Smitt P.S., Driesse M., Wolbers J., Kros M., Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2003;7:851–858. doi: 10.1016/s1525-0016(03)00100-x. http://www.ncbi.nlm.nih.gov/pubmed/12788659 [DOI] [PubMed] [Google Scholar]
- 157.Alavi J.B., Eck S.L. Gene therapy for high grade gliomas. Expert Opin. Biol. Ther. 2001;1:239–252. doi: 10.1517/14712598.1.2.239. [DOI] [PubMed] [Google Scholar]
- 158.Palù G., Cavaggioni A., Calvi P., Franchin E., Pizzato M., Boschetto R., Parolin C., Chilosi M., Ferrini S., Zanusso A., Colombo F. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther. 1999;6:330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- 159.Watowich S.S., Liu Y.-J. Mechanisms regulating dendritic cell specification and development. Immunol. Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ostertag D., Amundson K.K., Lopez Espinoza F., Martin B., Buckley T., Galvão da Silva A.P., Lin A.H., Valenta D.T., Perez O.D., Ibañez C.E., Chen C.-I., Pettersson P.L., Burnett R., Daublebsky V., Hlavaty J., Gunzburg W., Kasahara N., Gruber H.E., Jolly D.J., Robbins J.M. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14:145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mitchell L.A., Lopez Espinoza F., Mendoza D., Kato Y., Inagaki A., Hiraoka K., Kasahara N., Gruber H.E., Jolly D.J., Robbins J.M. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19:930–939. doi: 10.1093/neuonc/nox037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adachi Y., Tamiya T., Ichikawa T., Terada K., Ono Y., Matsumoto K., Furuta T., Hamada H., Ohmoto T. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene with 5-fluorocytosine. Hum. Gene Ther. 2000;11:77–89. doi: 10.1089/10430340050016175. [DOI] [PubMed] [Google Scholar]
- 163.Chiocca E.A., Smith K.M., McKinney B., Palmer C.A., Rosenfeld S., Lillehei K., Hamilton A., DeMasters B.K., Judy K., Kirn D. A phase I trial of Ad.hIFN-β gene therapy for glioma. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 164.Lang F.F., Bruner J.M., Fuller G.N., Aldape K., Prados M.D., Chang S., Berger M.S., McDermott M.W., Kunwar S.M., Junck L.R., Chandler W., Zwiebel J.A., Kaplan R.S., Yung W.K.A. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J. Clin. Oncol.: Official Journal of the American Society of Clinical Oncology. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 165.Freeman A.I., Zakay-Rones Z., Gomori J.M., Linetsky E., Rasooly L., Greenbaum E., Rozenman-Yair S., Panet A., Libson E., Irving C.S., Galun E., Siegal T. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Klatzmann D., Valéry C.A., Bensimon G., Marro B., Boyer O., Mokhtari K., Diquet B., Salzmann J.L., Philippon J. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum. Gene Ther. 1998;9:2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 167.Immonen A., Vapalahti M., Tyynelä K., Hurskainen H., Sandmair A., Vanninen R., Langford G., Murray N., Ylä-Herttuala S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 168.Germano I.M., Fable J., Gultekin S.H., Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J. Neuro Oncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. http://www.ncbi.nlm.nih.gov/pubmed/14682378 [DOI] [PubMed] [Google Scholar]
- 169.Sandmair A.M., Loimas S., Puranen P., Immonen A., Kossila M., Puranen M., Hurskainen H., Tyynelä K., Turunen M., Vanninen R., Lehtolainen P., Paljärvi L., Johansson R., Vapalahti M., Ylä-Herttuala S. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 170.Wheeler L.A., Manzanera A.G., Bell S.D., Cavaliere R., McGregor J.M., Grecula J.C., Newton H.B., Lo S.S., Badie B., Portnow J., Teh B.S., Trask T.W., Baskin D.S., New P.Z., Aguilar L.K., Aguilar-Cordova E., Chiocca E.A. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18:1137–1145. doi: 10.1093/neuonc/now002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ram Z., Culver K.W., Oshiro E.M., Viola J.J., DeVroom H.L., Otto E., Long Z., Chiang Y., McGarrity G.J., Muul L.M., Katz D., Blaese R.M., Oldfield E.H. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat. Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. http://www.ncbi.nlm.nih.gov/pubmed/9396605 [DOI] [PubMed] [Google Scholar]
- 172.Papanastassiou V., Rampling R., Fraser M., Petty R., Hadley D., Nicoll J., Harland J., Mabbs R., Brown M. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 173.Chiocca E.A., Abbed K.M., Tatter S., Louis D.N., Hochberg F.H., Barker F., Kracher J., Grossman S.A., Fisher J.D., Carson K., Rosenblum M., Mikkelsen T., Olson J., Markert J., Rosenfeld S., Nabors L.B., Brem S., Phuphanich S., Freeman S., Kaplan R., Zwiebel J. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 174.Forsyth P., Roldán G., George D., Wallace C., Palmer C.A., Morris D., Cairncross G., Matthews M.V., Markert J., Gillespie Y., Coffey M., Thompson B., Hamilton M. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol. Ther.: The Journal of the American Society of Gene Therapy. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 175.Markert J.M., Medlock M.D., Rabkin S.D., Gillespie G.Y., Todo T., Hunter W.D., Palmer C.A., Feigenbaum F., Tornatore C., Tufaro F., Martuza R.L. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 176.Rampling R., Cruickshank G., Papanastassiou V., Nicoll J., Hadley D., Brennan D., Petty R., MacLean A., Harland J., McKie E., Mabbs R., Brown M. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. http://www.ncbi.nlm.nih.gov/pubmed/10845724 [DOI] [PubMed] [Google Scholar]
- 177.Rainov N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 178.Chiocca E.A., Aguilar L.K., Bell S.D., Kaur B., Hardcastle J., Cavaliere R., McGregor J., Lo S., Ray-Chaudhuri A., Chakravarti A., Grecula J., Newton H., Harris K.S., Grossman R.G., Trask T.W., Baskin D.S., Monterroso C., Manzanera A.G., Aguilar-Cordova E., New P.Z. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J. Clin. Oncol.: Official Journal of the American Society of Clinical Oncology. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shand N., Weber F., Mariani L., Bernstein M., Gianella-Borradori A., Long Z., Sorensen A.G., Barbier N. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum. Gene Ther. 1999;10:2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 180.Wood A.J., Lo T.-W., Zeitler B., Pickle C.S., Ralston E.J., Lee A.H., Amora R., Miller J.C., Leung E., Meng X., Zhang L., Rebar E.J., Gregory P.D., Urnov F.D., Meyer B.J. Targeted genome editing across species using ZFNs and TALENs. Science (New York, N.Y.). 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hitti F.L., Gonzalez-Alegre P., Lucas T.H. Gene therapy for neurologic disease: a neurosurgical review. World Neurosurg. 2018 doi: 10.1016/j.wneu.2018.09.097. [DOI] [PubMed] [Google Scholar]
- 182.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science (New York, N.Y.). 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 183.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ben-David U., Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 185.Smith B.D., Grande D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015;11:213–222. doi: 10.1038/nrrheum.2015.27. [DOI] [PubMed] [Google Scholar]
- 186.Ringe J., Sittinger M. Regenerative medicine: selecting the right biological scaffold for tissue engineering. Nat. Rev. Rheumatol. 2014;10:388–389. doi: 10.1038/nrrheum.2014.79. [DOI] [PubMed] [Google Scholar]
- 187.Pedersen R.A., Mascetti V., Mendjan S. Synthetic organs for regenerative medicine. Cell Stem Cell. 2012;10:646–647. doi: 10.1016/j.stem.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 188.Gallieni M., Del Maestro M., Luzzi S., Trovarelli D., Ricci A., Galzio R. Endoscope-assisted microneurosurgery for intracranial aneurysms: operative technique, reliability, and feasibility based on 14 Years of personal experience. Acta Neurochir. Suppl. 2018;129:19–24. doi: 10.1007/978-3-319-73739-3_3. [DOI] [PubMed] [Google Scholar]
- 189.Ciappetta P., Occhiogrosso G., Luzzi S., D’Urso P.I., Garribba A.P. Jugular tubercle and vertebral artery/posterior inferior cerebellar artery anatomic relationship: a 3-dimensional angiography computed tomography anthropometric study. Neurosurgery. 2009;64:429–436. doi: 10.1227/01.NEU.0000337573.17073.9F. discussion 436. [DOI] [PubMed] [Google Scholar]
- 190.Millimaggi D.F., di Norcia V., Luzzi S., Alfiero T., Galzio R.J., Ricci A. Minimally invasive transforaminal lumbar interbody fusion with percutaneous bilateral pedicle screw fixation for lumbosacral spine degenerative diseases. A retrospective database of 40 consecutive cases and literature review. Turkish Neurosurg. 2018;28:454–461. doi: 10.5137/1019-5149.JTN.19479-16.0. [DOI] [PubMed] [Google Scholar]
- 191.Luzzi S., Del Maestro M., Bongetta D., Zoia C., V Giordano A., Trovarelli D., Raysi Dehcordi S., Galzio R.J. Onyx embolization before the surgical treatment of grade III Spetzler-Martin brain arteriovenous malformations: single-center experience and technical nuances. World Neurosurg. 2018;116:e340–e353. doi: 10.1016/j.wneu.2018.04.203. [DOI] [PubMed] [Google Scholar]
- 192.Del Maestro M., Luzzi S., Gallieni M., Trovarelli D., Giordano A.V., Gallucci M., Ricci A., Galzio R. Surgical treatment of arteriovenous malformations: role of preoperative staged embolization. Acta Neurochir. Suppl. 2018;129:109–113. doi: 10.1007/978-3-319-73739-3_16. [DOI] [PubMed] [Google Scholar]
- 193.Luzzi S., Del Maestro M., Trovarelli D., De Paulis D., Dechordi S.R., Di Vitantonio H., Di Norcia V., Millimaggi D.F., Ricci A., Galzio R.J. Endoscope-assisted microneurosurgery for neurovascular compression syndromes: basic principles, methodology, and technical notes. Asian J. Neurosurg. 2019;14:193–200. doi: 10.4103/ajns.AJNS_279_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Luzzi S., Del Maestro M., Elia A., Vincitorio F., Di Perna G., Zenga F., Garbossa D., Elbabaa S., Galzio R. Morphometric and radiomorphometric study of the correlation between the foramen magnum region and the anterior and posterolateral approaches to ventral intradural lesions. Turkish Neurosurg. 2019 doi: 10.5137/1019-5149.JTN.26052-19.2. [DOI] [PubMed] [Google Scholar]
- 195.Zoia C., Bongetta D., Dorelli G., Luzzi S., Del Maestro M., Galzio R.J. Transnasal endoscopic removal of a retrochiasmatic cavernoma: a case report and review of literature. Surg. Neurol. Int. 2019;10:76. doi: 10.25259/SNI-132-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luzzi S., Zoia C., Rampini A.D., Elia A., Del Maestro M., Carnevale S., Morbini P., Galzio R. Lateral transorbital neuroendoscopic approach for intraconal meningioma of the orbital apex: technical nuances and literature review. World Neurosurg. 2019;131:10–17. doi: 10.1016/j.wneu.2019.07.152. [DOI] [PubMed] [Google Scholar]
- 197.Ricci A., Di Vitantonio H., De Paulis D., Del Maestro M., Raysi S.D., Murrone D., Luzzi S., Galzio R.J. Cortical aneurysms of the middle cerebral artery: a review of the literature. Surg. Neurol. Int. 2017;8:117. doi: 10.4103/sni.sni_50_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Luzzi S., Gallieni M., Del Maestro M., Trovarelli D., Ricci A., Galzio R. Giant and very large intracranial aneurysms: surgical strategies and special issues. Acta Neurochir. Suppl. 2018;129:25–31. doi: 10.1007/978-3-319-73739-3_4. [DOI] [PubMed] [Google Scholar]
- 199.De Tommasi A., Cascardi P., De Tommasi C., Luzzi S., Ciappetta P. Emergency surgery in a severe penetrating skull base injury by a screwdriver: case report and literature review. World J. Emerg. Surg. 2006;1:1–4. doi: 10.1186/1749-7922-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Tommasi A., Luzzi S., D’Urso P.I., De Tommasi C., Resta N., Ciappetta P. Molecular genetic analysis in a case of ganglioglioma: identification of a new mutation. Neurosurgery. 2008;63:976–980. doi: 10.1227/01.NEU.0000327699.93146.CD. discussion 980. [DOI] [PubMed] [Google Scholar]
- 201.Luzzi S., Elia A., Del Maestro M., Elbabaa S.K., Carnevale S., Guerrini F., Caulo M., Morbini P., Galzio R. Dysembryoplastic neuroepithelial tumors: what you need to know. World Neurosurg. 2019;127:255–265. doi: 10.1016/j.wneu.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 202.Ciappetta P., D’urso P.I., Luzzi S., Ingravallo G., Cimmino A., Resta L. Cystic dilation of the ventriculus terminalis in adults. J. Neurosurg. Spine. 2008;8:92–99. doi: 10.3171/SPI-08/01/092. [DOI] [PubMed] [Google Scholar]
- 203.De Tommasi A., Occhiogrosso G., De Tommasi C., Luzzi S., Cimmino A., Ciappetta P. A polycystic variant of a primary intracranial leptomeningeal astrocytoma: case report and literature review. World J. Surg. Oncol. 2007;5:72. doi: 10.1186/1477-7819-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luzzi S., Elia A., Del Maestro M., Morotti A., Elbabaa S.K., Cavallini A., Galzio R. Indication, timing, and surgical treatment of spontaneous intracerebral hemorrhage: systematic review and proposal of a management algorithm. World Neurosurg. 2019;124:e769–e778. doi: 10.1016/j.wneu.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 205.Bellantoni G., Guerrini F., Del Maestro M., Galzio R., Luzzi S. Simple schwannomatosis or an incomplete Coffin-Siris? Report of a particular case. ENeurologicalSci. 2019;14:31–33. doi: 10.1016/j.ensci.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.De Tommasi A., De Tommasi C., Lauta E., Luzzi S., Cimmino A., Ciappetta P. Pre-operative subarachnoid haemorrhage in a patient with spinal tumour. Cephalalgia: An International Journal of Headache. 2006;26:887–890. doi: 10.1111/j.1468-2982.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- 207.Luzzi S. Re: microsurgical resection of a large intraventricular trigonal tumor: 3-dimensional operative video [comment] Oper Neurosurg (Hagerstown) 2018;15:E92–E93. doi: 10.1093/ons/opy068. [DOI] [PubMed] [Google Scholar]
- 208.Zoia C., Bongetta D., Guerrini F., Alicino C., Cattalani A., Bianchini S., Galzio R.J., Luzzi S. Outcome of elderly patients undergoing intracranial meningioma resection: a single center experience. J. Neurosurg. Sci. 2018 doi: 10.23736/S0390-5616.18.04333-3. [DOI] [PubMed] [Google Scholar]
- 209.Guastamacchia E., Triggiani V., Tafaro E., De Tommasi A., De Tommasi C., Luzzi S., Sabbà C., Resta F., Terreni M.R., Losa M. Evolution of a prolactin-secreting pituitary microadenoma into a fatal carcinoma: a case report. Minerva Endocrinol. 2007;32:231–236. [PubMed] [Google Scholar]
- 210.Bongetta D., Zoia C., Luzzi S., Del Maestro M., Peri A., Bichisao G., Sportiello D., Canavero I., Pietrabissa A., Galzio R.J. Neurosurgical issues of bariatric surgery: a systematic review of the literature and principles of diagnosis and treatment. Clin. Neurol. Neurosurg. 2019;176:34–40. doi: 10.1016/j.clineuro.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 211.Ciappetta P., Luzzi S., De Blasi R., D’Urso P.I. Extracranial aneurysms of the posterior inferior cerebellar artery. Literature review and report of a new case. J. Neurosurg. Sci. 2009;53:147–151. http://www.ncbi.nlm.nih.gov/pubmed/20220739 [PubMed] [Google Scholar]
- 212.Pisano P., Guerrini F., Custodi V., Del Maestro M., Galzio R., Luzzi S. Tonic-clonic seizures as a possible complication for cerebrospinal fluid leakage after intradural spinal surgery, a case report. Interdis. Neurosurg. 2020;19:100576. [Google Scholar]
- 213.Luzzi S., Del Maestro M., Galzio R. Letter to the Editor. Preoperative embolization of brain arteriovenous malformations. J. Neurosurg. 2019 doi: 10.3171/2019.6.JNS191541. [DOI] [PubMed] [Google Scholar]
- 214.Spena G., Roca E., Guerrini F., Panciani P.P., Stanzani L., Salmaggi A., Luzzi S., Fontanella M. Risk factors for intraoperative stimulation-related seizures during awake surgery: an analysis of 109 consecutive patients. J. Neuro Oncol. 2019 doi: 10.1007/s11060-019-03295-9. [DOI] [PubMed] [Google Scholar]
- 215.Luzzi S., Giotta Lucifero A., Del Maestro M., Marfia G., Navone S.E., Baldoncini M., Nuñez M., Campero A., Elbabaa S.K., Galzio R. Anterolateral approach for retrostyloid superior parapharyngeal space schwannomas involving the Jugular foramen area: a 20-year experience. World Neurosurg. 2019 doi: 10.1016/j.wneu.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 216.Becker A.J., McCulloch E.A., Till J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. http://www.ncbi.nlm.nih.gov/pubmed/13970094 [DOI] [PubMed] [Google Scholar]
- 217.Weissman I.L., Shizuru J.A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Winn H.R., Howard M.A. The next 100 years of neurosurgery. Lancet (London, England) 1999;354 Suppl:SIV36. doi: 10.1016/s0140-6736(99)90379-3. http://www.ncbi.nlm.nih.gov/pubmed/10691448 [DOI] [PubMed] [Google Scholar]