Abstract
Down syndrome (DS), as a typical genomic aneuploidy, is a common cause of various birth defects, among which is congenital heart disease (CHD). 40–60% neonates with DS have some kinds of CHD. However, the molecular pathogenic mechanisms of DS associated CHD are still not fully understood. This review summarizes available studies on DS associated CHD from seven aspects so as to provide a crucial and updated overview of what we known so far in this domain.
Keywords: Down syndrome, Congenital heart disease, Molecular mechanisms
Introduction
Defined as the abnormal copy number of genomic regions, genomic aneuploidy is a common reason of human genetic diseases. Down syndrome (DS), trisomy 21, as a typical genomic aneuploidy, is the most common autosomal abnormality in live infants, with an incidence of 1/700–800.1 Various types of congenital malformations always occur in DS patients, among which is congenital heart disease (CHD).2, 3, 4 40–60% newborns with DS have some kinds of CHD, including 45% atrioventricular septal defects (AVSD), 35% ventricular septal defects (VSD), 8% isolated secundum atrial septal defects (ASD), 7% isolated persistent patent ductus arteriosus (PDA), 4% tetralogy of Fallot (TOF), and 1% other.5 In recent years, the number of DS associated CHD (DS-CHD) present a growing trend6 and complex congenital heart defects have become less common in infants diagnosed with Down syndrome, which could be a result of selective abortion of fetuses with DS, or due to general improvements in antenatal diagnostics of complex congenital heart defects.7 Scientists have obtained the genome of CHD in DS, yet they haven't fully revealed the underlying genomic or gene expression variation that contribute to the phenotype of CHD in DS. Here, we summarize previous research findings so as to provide a crucial and updated overview of what we known so far in this domain and point out the potential research directions in the future based on this knowledge.
Pathogenesis of CHD in DS
DS has complex phenotype and two hypotheses are used for explaining the DS associated CHD. Gene dosage amplification hypothesis claims that an increased dosage of genes on human chromosome 21 (Hsa 21) in DS may increase the level of gene expression.8, 9 Gene mutation hypothesis holds that under the trisomy 21 background, certain locus mutations can result in the occurrence of CHD.10, 11 In 1992, Korenberg et al12 first proposed the concept of DS-CHD critical region (DSCR, 4–5 Mb region from D21S55 (21q22.2) to MX1). Subsequently, the scope of this area gradually narrowed. Recently, this region was narrowed to 0.96 Mb on 21q22.2 and was suggested to interact with another region (highly restricted DSCR, HR-DSCR) to explain the potential relationship between DS and CHD.13 This narrowed DS associated CHD critical region not only provides an effective method to identify specific genes, but also help us to seek for pathogenic gene exactly.
However, this approach is limited by the complex karyotype of such individuals, the heterogeneity of the phenotype and the rarity of the condition. For purpose of studying the molecular mechanism of DS associated CHD, many genes, genomic variations, signal pathways11, 14, 15, 16, 17, 18, 19, 20 have been investigated and additional variables for DS-CHD as well as some environmental exposures and stochastic events.21, 22
Gene dosage and genomic variations in DS-CHD
Genes on chromosome 21
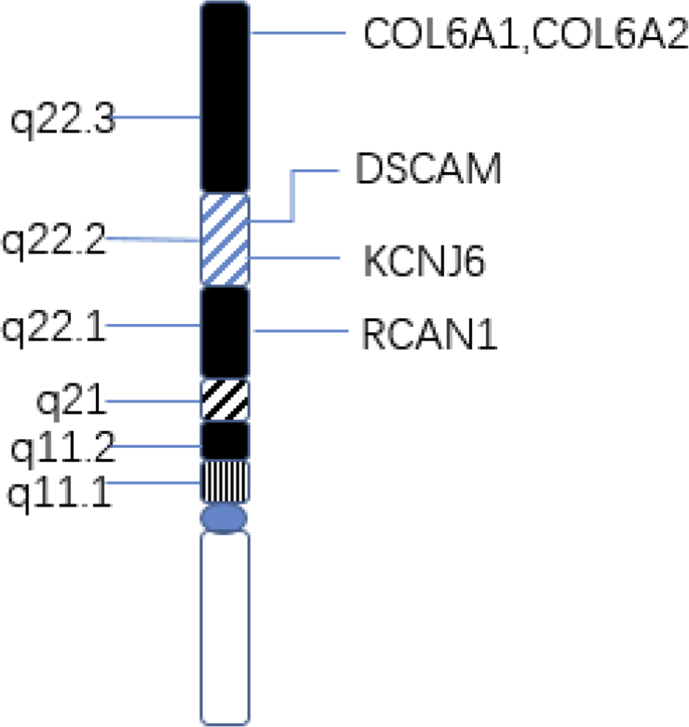
DSCAM, COL6A1, COL6A2, KCNJ6 and RCAN1 have been considered as candidate genes for the increased CHD risk in children with DS (Fig. 1).23, 24, 25
Figure 1.
Critical genes on human chromosome 21 in DS with CHD.
DSCAM, located on 21q22.2, is a member of the immunoglobulin superfamily of cell adhesion molecules (Ig-CAMs) and is involved in the nervous system development.26 Previous studies have suggested that, due to the gene dosage multiplier in trisomy 21, the expression of intercellular mucoprotein increases abnormally before endocardial cushion development, which enhanced the adhesion between cells and affected the fusion of endocardial, causing AVSD.27
Collagen VI is made up of three chains encoded by separate genes, two of which (COL6A1∼2) lie on chromosome 21 (21q22.3).28 Collagen VI is expressed in fetal hearts (5–18 weeks of development) and involved in the formation of the original atrioventricular septum, including the middle and lower part of the atrioventricular septal valve and the membrane.28, 29 Overexpression of type VI collagen (COL6A1, COL6A2) has been suggested to play a critical role in the pathogenesis of AVSD in DS.29 It's an interesting phenomenon that AVSD, VSD, PS and TOF could be observed if both DSCAM and COL6A1 were co-expressed, while only DSCAM was copied, just TOF was seen. It indicates that DSCAM and COL6A1 may have synergistic effect on the overall cardiac defects.24
RCAN1, also known as DSCR1, is located at 21q22.1 and is of great significance in the occurrence of DS-CHD.30 RCAN1 encodes one of the calcineurin inhibitors and negatively regulates calmodulin phosphorylation.31 Calcineurin can dephosphorylate the nuclear factor of active T cell (NFAT), which is the first transcription factor only expressed in cardiac endothelial cells, and its overexpression can promote NFATc1 nuclear translocation, resulting in target gene activation.32 The overexpression of RCAN1 in the trisomy 21 lead to decrease dephosphorylation of NFAT by inhibiting activity of calcineurin during the development of embryonic cardiac tubes, which affects the development of heart valves and septum, bringing out CHD.33, 34
KCNJ6 gene encodes for the Kir3.2/GIRK2 protein subunits of G protein-regulated K+(K G) channels and could activate K+ (K G) channels.35 It has been reported that overexpress Kir3.2 proteins in DS could reduce negative chronotropic effect on sino-atrial node and alter cardiac regulation and arrhythmogenicity.36
Genes on other chromosome
However, not all patients with DS have defects of septum and valve, and such defects are also observed in patients without DS. Other genes, not localized on chromosome 21, may play important roles in the development of these cardiac anomalies. As a matter of fact, the genes CRELD1, CRELD2 and ALK2 seem to increase susceptibility to DS-CHD.11, 37, 38, 39
The CRELD family of proteins have two members: CRELD1 and CRELD2. CRELD1, located on 3p25, is the first found to be involved in the pathogenesis of isolated AVSD (with or without DS).40 It is expressed during endocardiac cushion development and encodes a cell surface protein that acts as cell adhesion molecule. Several studies have found missense mutations in CRELD1 in DS patients with AVSD.37, 41 Nevertheless, a separated study on isolated AVSD (without DS) showed no significant pathogenic mutations in an additional 31 individuals.41 Together these results indicate that mutations in CRELD1 may contribute to the pathogenesis of AVSD in the context of trisomy 21.
Mapping of CRELD2 by FISH shows that it locates on 22q13.38 As the only homologous gene of CRELD1, CRELD2 encode proteins that are highly similar with CRELD1 in structure and function, and the expression of CRELD2 and CRELD2 overlaps spatially and temporally during embryonic development, indicating that they play a similar role in the biological pathway.37, 38
Bone morphogenetic proteins (BMPs) and associated signaling pathways are participated in the regulation of a lot of processes in cardiovascular development.42 Indwelling on 1q, ALK2 gene, encoding the type I receptor for BMPs, plays a vital nonredundant role in early phases of endocardial cushion formation during the period of cardiac morphogenesis. Eliminating ALK2 gene from the endocardium structure of developing mouse embryo may reduce phosphorylation of BMP, which lead to a failure of endocardium cushion to fuse.43 So far, although only one literature has reported ALK mutation (p.His286Asp) in a DS patient with primary ASD through large-scale candidate gene-sequencing screen,39 these data remind that ALK2-mediated reduction of BMP signaling pathway may generate CHD in DS background.
MicroRNAs
MicroRNAs (miRNAs) have a size of ∼20 nt and their main action are the negative regulation of gene expression. The major mode of action of these miRNAs is to interact with the 3‘-untranslated region of their target messenger RNAs (mRNAs), hence induce mRNAs degradation or inhibit their translation.
It has been reported that DS was linked to five miRNAs, including miR-99a, let-7c, miR-125b-2, miR-155 and miR-802,44 all of which were identified on human chromosome 21 and over-expressed in cardiac tissue of patients with trisomy 21.44 Some researchers45 observed that overexpression of the cluster could decrease their targets in fetal heart tissue of Down syndrome, suggesting that the miR- 99a/let-7c cluster may contribute to CHD in Down syndrome. By bioinformatics analysis, Wang L et al46 speculate AUTS2, and KIAA2022 might participate in AVSD in DS patients by interacting with miR-518a, miR-518e, miR-518f, miR-528a, and miR-96. These data need to be further verified by in vivo and in vitro experiments.
CNVs and SNP
The genetic architecture of the CHD risk for DS seems to be complex, which also include copy number variations (CNVs) and single nucleotide polymorphisms (SNP) on chr21 and probably in the rest of the genome.17 The observation that AVSD is the most frequent CHD in Caucasian patients with DS,47 while VSD is the preponderant CHD in Asian48 suggests that the presence of SNP showing population-related variability probably leads to the different predisposition to specific CHDs in the various ethnicities. However, other experimental results showed that CNVs didn't appear to increase the risk of DS associated AVSD.49, 50 As is AVSD in the euploid population, DS associated AVSD is similarly heterogeneous.
Folate/homocysteine metabolism
Folate is an essential substance which methylates DNA and proteins and converts homocysteine into methionine by donating methyl groups for purine and pyrimidine synthesis. A population-based case-control study22 suggested that short of maternal folic acid supplementation was associated with septal defects in newborn with DS. However the mechanism is not fully understood. Three aspects can be considered: folate transport and absorption, folate and homocysteine metabolism. Folate is absorbed by the small intestine in the form of monoglutamic acid by reduced folate carrier (SLC19A1). It has been reported51 that SNP rs1051266 (c.80A > G) might alter folate uptake and became a risk factor for DS-CHD. Enzymes involved in the metabolic pathway of folate and homocysteine include MTHFR (Methylenetetrahydrofolate Reductase), MTR (Methionine Synthase), MTRR (Methionine Synthase Reductase) and CBS (Cystathionine β-synthase). Up to now, only a few case-control researches assessed these genes as CHD risk factors in DS patients.21 Locke et al51 and Brandalize et al52 found SNPs of MTHFR (c.677C > T and c.1298A > C) in mothers of DS individuals increased the risk of having a baby with DS-AVSD, while Božovi' c et al53 got the contradictory conclusion that no association between the presence of either MTHFR 677C > T or MTHFR 1298A > C polymorphisms in the risk of having a child with DS-CHD. Recently, using meta-analysis in some big databases and Clinical Trials, Zhang Y et al54 found that MTHFR C677T and A1298C were not associated with CHD (OR, 1.05; 95% CI, 0.89–1.25), while MTHFR C677T was connected with Down syndrome (OR, 1.65; 95% CI, 1.39–1.95). Those results are inconsistent, however, disrupting of the genes may be involved in folate/homocysteine metabolism still could influence the development of embryonic heart in DS individuals.
Nongenetic factor
Besides above factors, several studies55, 56 have demonstrated that perturbation of tissue of extracardiac origin, known as dorsal mesocardial protrusion (DMP), represents another major determinant causing CHD in DS. The cluster of extracardiac mesenchymal cells arises from the posterior segment of the second heart field (SHF) in the splanchnic mesoderm, and grows toward the atrial surface of the primitive atrioventricular canal, in particular toward the inferior dorsal endocardial cushion, to close the primary atrial foramen and form the atrioventricular junction.57, 58 In trisomy 21 background, disturbing the development of DMP may lead to AVSD.55
Pathway signal
Recent studies have also suggested the potential contribution of VEGF-A, Sonic Hedgehog (Shh) signaling, the cross-presentation of particulate exogenous antigens (phagosomes) and the methionine salvage pathway, calcineurin/NFAT and folate pathways to the pathogenicity of DS-CHD.11, 15, 31, 51, 59
Acting in the VEGF-A pathway, six missense variants (COL6A1, COL6A2, CRELD1, FBLN2, FRZB and GATA5) were found to be damaged among individuals with DS and complete AVSD,11 suggesting that rare variants in the VEGF-A pathway might generate the genetic backbone of AVSD in DS.
Hedgehog signaling is an evolutionarily conserved signaling pathway that plays an important role in embryonic development and adult tissue homeostasis. The DMP development requires cilia-based Sonic (Shh) signaling, and cilia are structurally present in the SHF.60, 61 Because of abnormal expression of Shh signaling, decreasing DMP proliferation could be observed in mice, which inhibited the fusion of the atrioventricular cushions with the inferior margin of the septum primum and resulted in AVSD.58, 60
Using the individualized pathway aberrance score (iPAS) method, Chen YQ et al59 analyzed 10 human DS patients and 5 control samples and predicted the cross-presentation of particulate exogenous antigens (phagosomes) and the methionine salvage pathway could be good indicators of DS-CHD. However, the specific molecular mechanism needs to be further studied.
Calcineurin/NFAT and folate pathways are no longer detailed since we have described earlier in this article.
Concluding remarks
In this review, we summarized the possible molecular mechanism of DS associated CHD from seven aspects. Gene dosage imbalance hypothesis and gene mutation hypothesis are the main pathogenesis. Genes related to DS with CHD include DSCAM, COL6A1∼2, KCNJ6, RCAN1, CRELD1, CRELD2 and ALK2. Among these genes, DSCAM, COL6A1∼2, KCNJ6 and RCAN1 located on chr 21 cause abnormal heart development through different mechanisms by gene dosage effect, while CRELD1, CRELD2 and ALK2 lied on other chromosome result in CHD due to gene locus mutation. MicroRNA, miR-518a, miR-518e, miR-518f, miR-528a, and miR-96, might participate in AVSD in DS patients by negatively regulating AUTS2 and KIAA2022 gene expression. Overexpression of the miR- 99a/let-7c cluster subsequently decreased their targets in fetal DS heart tissue. SNP and CNVs probably contributes to the different predisposition to specific CHDs in the various ethnicities. Lack of maternal folic acid supplementation is associated with septal defects in infants with DS although the mechanism is not fully understood. On note, disturbing the development of DMP could generate the defect of endocardial cushion in trisomy 21. Finally, the involved signal pathways include VEGF-A, Sonic Hedgehog (Shh) signaling, calcineurin/NFAT, and folate pathway. They play indispensable roles in the development of DS-CHD by those genes above mentioned. In addition to the factors mentioned above, there may be other new mechanisms that have not yet been discovered, and we believe that the CHD phenotype in DS patients is a combination of multiple factors which interact each other synergistically or antagonistically and participate in the occurrence of DS-CHD.
Future research
Our comprehending of the molecular pathogenesis of DS associated CHD is significantly insufficiency. Many important future research goals need to be achieved. Functional of all HSA21 genes related to CHD should be analyzed in timing and cellular specificity of expression. Exploring of coding and non-coding DNA on other chromosome that tend to occur DS-CHD will also be important. Finally, relevant non-coding RNAs (miRNAs, lncRNA) and their targets need to be verified. Now all the research that we're doing on DS-CHD is like the blind man feeling the elephant, one day we will know all the truth.
Conflict of interest
There is no conflict of interest regarding the publication of this article.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Weijerman M.E., van Furth A.M., Vonk N.A., van Wouwe J.P., Broers C.J., Gemke R.J. Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: a national study. J Pediatr. 2008;152(1):15–19. doi: 10.1016/j.jpeds.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Freeman S.B., Bean L.H., Allen E.G. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. 2008;10(3):173–180. doi: 10.1097/GIM.0b013e3181634867. [DOI] [PubMed] [Google Scholar]
- 3.Morris J.K., Garne E., Wellesley D. Major congenital anomalies in babies born with Down syndrome: a EUROCAT population-based registry study. Am J Med Genet. 2014;164A(12):2979–2986. doi: 10.1002/ajmg.a.36780. [DOI] [PubMed] [Google Scholar]
- 4.Stoll C., Dott B., Alembik Y., Roth M.P. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet. 2015;58(12):674–680. doi: 10.1016/j.ejmg.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Freeman S.B., Taft L.F., Dooley K.J. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80(3):213–217. [PubMed] [Google Scholar]
- 6.Pfitzer C., Helm P.C., Rosenthal L.M., Berger F., UMM B., Schmitt K.R. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur J Pediatr. 2018;177(1):107–115. doi: 10.1007/s00431-017-3041-6. [DOI] [PubMed] [Google Scholar]
- 7.Bergström S., Carr H., Petersson G. Trends in congenital heart defects in infants with down syndrome. Pediatrics. 2016;138(1) doi: 10.1542/peds.2016-0123. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis S.E., Lyle R., Dermitzakis E.T., Reymond A., Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 9.Sinet P.M., Théophile D., Rahmani Z. Mapping of the Down syndrome phenotype on chromosome 21 at the molecular level. Biomed Pharmacother. 1994;48(5–6):247–252. doi: 10.1016/0753-3322(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 10.Letourneau A., Santoni F.A., Bonilla X. Domains of genome-wide gene expression dysregulation in Down's syndrome. Nature. 2014;508(7496):345–350. doi: 10.1038/nature13200. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman C., Locke A.E., Feingold E. An excess of deleterious variants in VEGF-A pathway genes in Down-syndrome-associated atrioventricular septal defects. Am J Hum Genet. 2012;91(4):646–659. doi: 10.1016/j.ajhg.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korenberg J.R., Bradley C., Disteche C.M. Down syndrome: molecular mapping of the congenital heart disease and duodenal stenosis. Am J Hum Genet. 1992;50(2):294–302. [PMC free article] [PubMed] [Google Scholar]
- 13.Pelleri M.C., Gennari E., Locatelli C. Genotype-phenotype correlation for congenital heart disease in Down syndrome through analysis of partial trisomy 21 cases. Genomics. 2017;109(5–6):391–400. doi: 10.1016/j.ygeno.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Park J., Oh Y., Chung K.C. Two key genes closely implicated with the neuropathological characteristics in Down syndrome: DYRK1A and RCAN1. BMB Rep. 2009;42(1):6–15. doi: 10.5483/bmbrep.2009.42.1.006. [DOI] [PubMed] [Google Scholar]
- 15.Ripoll C., Rivals I., Ait Y.E. Molecular signatures of cardiac defects in Down syndrome lymphoblastoid cell lines suggest altered ciliome and Hedgehog pathways. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahed A.C., Gelb B.D., Seidman J.G., Seidman C.E. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112(4):707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sailani M.R., Makrythanasis P., Valsesia A. The complex SNP and CNV genetic architecture of the increased risk of congenital heart defects in Down syndrome. Genome Res. 2013;23(9):1410–1421. doi: 10.1101/gr.147991.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izzo A., Manco R., Bonfiglio F. NRIP1/RIP140 siRNA-mediated attenuation counteracts mitochondrial dysfunction in Down syndrome. Hum Mol Genet. 2014;23(16):4406–4419. doi: 10.1093/hmg/ddu157. [DOI] [PubMed] [Google Scholar]
- 19.Smith T., Rajakaruna C., Caputo M., Emanueli C. MicroRNAs in congenital heart disease. Ann Transl Med. 2015;3(21):333. doi: 10.3978/j.issn.2305-5839.2015.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q.J., Liu Z.P. Histone methylations in heart development, congenital and adult heart diseases. Epigenomics. 2015;7(2):321–330. doi: 10.2217/epi.14.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppedè F. The genetics of folate metabolism and maternal risk of birth of a child with Down syndrome and associated congenital heart defects. Front Genet. 2015;6:223. doi: 10.3389/fgene.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bean L.J., Allen E.G., Tinker S.W. Lack of maternal folic acid supplementation is associated with heart defects in Down syndrome: a report from the National Down Syndrome Project. Birth Defects Res A Clin Mol Teratol. 2011;91(10):885–893. doi: 10.1002/bdra.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaki R., Kosaki K., Matsushima K., Mitsui N., Matsumoto N., Ohashi H. Refining chromosomal region critical for Down syndrome-related heart defects with a case of cryptic 21q22.2 duplication. Congenital Anom (Kyoto) 2005;45(2):62–64. doi: 10.1111/j.1741-4520.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 24.Grossman T.R., Gamliel A., Wessells R.J. Over-expression of DSCAM and COL6A2 cooperatively generates congenital heart defects. PLoS Genet. 2011;7(11) doi: 10.1371/journal.pgen.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlevy L., Bennett M., Slender A. Down's syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse. Cardiovasc Res. 2010;88(2):287–295. doi: 10.1093/cvr/cvq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwala K.L., Nakamura S., Tsutsumi Y., Yamakawa K. Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res Mol Brain Res. 2000;79(1–2):118–126. doi: 10.1016/s0169-328x(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 27.Barlow G.M., Chen X.N., Shi Z.Y. Down syndrome congenital heart disease: a narrowed region and a candidate gene. Genet Med. 2001;3(2):91–101. doi: 10.1097/00125817-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Davies G.E., Howard C.M., Gorman L.M. Polymorphisms and linkage disequilibrium in the COL6A1 and COL6A2 gene cluster: novel DNA polymorphisms in the region of a candidate gene for congenital heart defects in Down's syndrome. Hum Genet. 1993;90(5):521–525. doi: 10.1007/BF00217452. [DOI] [PubMed] [Google Scholar]
- 29.Gittenberger-de G.A.C., Bartram U., Oosthoek P.W. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1109–1116. doi: 10.1002/ar.a.10126. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes J.J., Pritchard M.A., Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997;44(3):358–361. doi: 10.1006/geno.1997.4866. [DOI] [PubMed] [Google Scholar]
- 31.Fuentes J.J., Genescà L., Kingsbury T.J. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9(11):1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 32.de la Pompa J.L., Timmerman L.A., Takimoto H. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392(6672):182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 33.Casas C., Martínez S., Pritchard M.A. Dscr1, a novel endogenous inhibitor of calcineurin signaling, is expressed in the primitive ventricle of the heart and during neurogenesis. Mech Dev. 2001;101(1–2):289–292. doi: 10.1016/s0925-4773(00)00583-9. [DOI] [PubMed] [Google Scholar]
- 34.Lange A.W., Molkentin J.D., Yutzey K.E. DSCR1 gene expression is dependent on NFATc1 during cardiac valve formation and colocalizes with anomalous organ development in trisomy 16 mice. Dev Biol. 2004;266(2):346–360. doi: 10.1016/j.ydbio.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Isomoto S., Kondo C., Takahashi N. A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun. 1996;218(1):286–291. doi: 10.1006/bbrc.1996.0050. [DOI] [PubMed] [Google Scholar]
- 36.Lignon J.M., Bichler Z., Hivert B. Altered heart rate control in transgenic mice carrying the KCNJ6 gene of the human chromosome 21. Physiol Genom. 2008;33(2):230–239. doi: 10.1152/physiolgenomics.00143.2007. [DOI] [PubMed] [Google Scholar]
- 37.Maslen C.L., Babcock D., Robinson S.W. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome. Am J Med Genet. 2006;140(22):2501–2505. doi: 10.1002/ajmg.a.31494. [DOI] [PubMed] [Google Scholar]
- 38.Maslen C.L., Babcock D., Redig J.K., Kapeli K., Akkari Y.M., Olson S.B. CRELD2: gene mapping, alternate splicing, and comparative genomic identification of the promoter region. Gene. 2006;382:111–120. doi: 10.1016/j.gene.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Joziasse I.C., Smith K.A., Chocron S. ALK2 mutation in a patient with Down's syndrome and a congenital heart defect. Eur J Hum Genet. 2011;19(4):389–393. doi: 10.1038/ejhg.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson S.W., Morris C.D., Goldmuntz E. Missense mutations in CRELD1 are associated with cardiac atrioventricular septal defects. Am J Hum Genet. 2003;72(4):1047–1052. doi: 10.1086/374319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asim A., Agarwal S., Panigrahi I., Sarangi A.N., Muthuswamy S., Kapoor A. CRELD1 gene variants and atrioventricular septal defects in Down syndrome. Gene. 2018;641:180–185. doi: 10.1016/j.gene.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 42.van Wijk B., Moorman A.F., van den Hoff M.J. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74(2):244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Sridurongrit S., Dudas M. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286(1):299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latronico M.V., Catalucci D., Condorelli G. MicroRNA and cardiac pathologies. Physiol Genom. 2008;34(3):239–242. doi: 10.1152/physiolgenomics.90254.2008. [DOI] [PubMed] [Google Scholar]
- 45.Coppola A., Romito A., Borel C. Cardiomyogenesis is controlled by the miR-99a/let-7c cluster and epigenetic modifications. Stem Cell Res. 2014;12(2):323–337. doi: 10.1016/j.scr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Li Z., Song X., Liu L., Su G., Cui Y. Bioinformatic analysis of genes and MicroRNAs associated with atrioventricular septal defect in down syndrome patients. Int Heart J. 2016;57(4):490–495. doi: 10.1536/ihj.15-319. [DOI] [PubMed] [Google Scholar]
- 47.Park S.C., Mathews R.A., Zuberbuhler J.R., Rowe R.D., Neches W.H., Lenox C.C. Down syndrome with congenital heart malformation. Am J Dis Child. 1977;131(1):29–33. doi: 10.1001/archpedi.1977.02120140031003. [DOI] [PubMed] [Google Scholar]
- 48.Lo N.S., Leung P.M., Lau K.C., Yeung C.Y. Congenital cardiovascular malformations in Chinese children with Down's syndrome. Chin Med J (Engl). 1989;102(5):382–386. [PubMed] [Google Scholar]
- 49.Rambo-Martin B.L., Mulle J.G., Cutler D.J. Analysis of copy number variants on chromosome 21 in down syndrome-associated congenital heart defects. G3 (Bethesda) 2018;8(1):105–111. doi: 10.1534/g3.117.300366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramachandran D., Mulle J.G., Locke A.E. Contribution of copy-number variation to Down syndrome-associated atrioventricular septal defects. Genet Med. 2015;17(7):554–560. doi: 10.1038/gim.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Locke A.E., Dooley K.J., Tinker S.W. Variation in folate pathway genes contributes to risk of congenital heart defects among individuals with Down syndrome. Genet Epidemiol. 2010;34(6):613–623. doi: 10.1002/gepi.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandalize A.P., Bandinelli E., dos S.P.A., Roisenberg I., Schüler-Faccini L. Evaluation of C677T and A1298C polymorphisms of the MTHFR gene as maternal risk factors for Down syndrome and congenital heart defects. Am J Med Genet. 2009;149A(10):2080–2087. doi: 10.1002/ajmg.a.32989. [DOI] [PubMed] [Google Scholar]
- 53.Božović I.B., Vraneković J., Cizmarević N.S., Mahulja-Stamenković V., Prpić I., Brajenović-Milić B. MTHFR C677T and A1298C polymorphisms as a risk factor for congenital heart defects in Down syndrome. Pediatr Int. 2011;53(4):546–550. doi: 10.1111/j.1442-200X.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., He X., Xiong X. The association between maternal methylenetetrahydrofolate reductase C677T and A1298C polymorphism and birth defects and adverse pregnancy outcomes. Prenat Diagn. 2019;39(1):3–9. doi: 10.1002/pd.5396. [DOI] [PubMed] [Google Scholar]
- 55.Blom N.A., Ottenkamp J., Wenink A.G., Gittenberger-de G.A.C. Deficiency of the vestibular spine in atrioventricular septal defects in human fetuses with down syndrome. Am J Cardiol. 2003;91(2):180–184. doi: 10.1016/s0002-9149(02)03106-5. [DOI] [PubMed] [Google Scholar]
- 56.Gittenberger-de G.A.C., Calkoen E.E., Poelmann R.E., Bartelings M.M., Jongbloed M.R. Morphogenesis and molecular considerations on congenital cardiac septal defects. Ann Med. 2014;46(8):640–652. doi: 10.3109/07853890.2014.959557. [DOI] [PubMed] [Google Scholar]
- 57.Snarr B.S., Wirrig E.E., Phelps A.L., Trusk T.C., Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dynam. 2007;236(5):1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briggs L.E., Kakarla J., Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84(1):117–130. doi: 10.1016/j.diff.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y.Q., Li T., Guo W.Y., Su F.J., Zhang Y.X. Identification of altered pathways in Down syndrome-associated congenital heart defects using an individualized pathway aberrance score. Genet Mol Res. 2016;15(2) doi: 10.4238/gmr.15027601. [DOI] [PubMed] [Google Scholar]
- 60.Goddeeris M.M., Rho S., Petiet A. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135(10):1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burnicka-Turek O., Steimle J.D., Huang W. Cilia gene mutations cause atrioventricular septal defects by multiple mechanisms. Hum Mol Genet. 2016;25(14):3011–3028. doi: 10.1093/hmg/ddw155. [DOI] [PMC free article] [PubMed] [Google Scholar]