Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is characterized by its genetic variation and limited cross protection among heterologous strains. Even though several viral structural proteins have been regarded as inducers of neutralizing antibodies (NAs) against PRRSV, the mechanism underlying limited cross-neutralization among heterologous strains is still controversial. In the present study, examinations of NA cross reaction between a highly pathogenic PRRSV (HP-PRRSV) strain, JXwn06, and a low pathogenic PRRSV (LP-PRRSV) strain, HB-1/3.9, were conducted with viral neutralization assays in MARC-145 cells. None of the JXwn06-hyperimmuned pigs’ sera could neutralize HB-1/3.9 in vitro and vice versa. To address the genetic variation between these two viruses that are associated with limited cross-neutralization, chimeric viruses with coding regions swapped between these two strains were constructed. Viral neutralization assays indicated that variations in nonstructural protein 2 (nsp2) and structural proteins together contribute to weak cross-neutralization activity between JXwn06 and HB-1/3.9. Furthermore, we substituted the nsp2-, glycoprotein2 (GP2)-, GP3-, and GP4-coding regions together, or nsp2-, GP5-, and membrane (M) protein-coding regions simultaneously between these two viruses to construct chimeric viruses to test cross-neutralization reactivity with hyperimmunized sera induced by their parental viruses. The results indicated that the swapped nsp2 and GP5-M viruses increased the neutralization reactivity with the donor strain antisera in MARC-145 cells. Taken together, these results show that variations in nsp2 and GP5-M correlate with the limited neutralization reactivity between the heterologous strains HP-PRRSV JXwn06 and LP-PRRSV HB-1/3.9.
Electronic supplementary material
The online version of this article (10.1007/s12250-019-00149-6) contains supplementary material, which is available to authorized users.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), Neutralizing antibody (NA), Non-structural protein 2 (nsp2), Structural proteins (SPs)
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is a significant animal disease characterized by late term reproductive failure in pregnant sows and respiratory distress in all-age pigs. It has been impacting the global swine industry since it was first identified in North American and Europe in the late 1980s (Wensvoort et al.1991; Collins et al.1992; Han et al.2017). PRRS virus (PRRSV), the etiological agent of PRRS, belongs to the newly classified genus Porartevirus in the family Arteriviridae in the order Nidovirales (Kuhn et al.2016). It is an enveloped RNA virus with an approximately 15 kb single-stranded, positive-sense genome. The genome of PRRSV contains two small stretches of untranslated regions (UTRs) at each end of the genome and at least 12 overlapping open reading frames (ORFs) (Li et al.2015; Lunney et al.2016). ORF1a and ORF1b occupy two-thirds of the viral genome and encode two replicase polyproteins—pp1a and pp1ab—which are processed into 16 nonstructural proteins (nsps), including nsp1α, nsp1β, nsp2, nsp2TF, nsp2N, nsp3, nsp4, nsp5, nsp6, nsp7α, nsp7β, and nsp8, and nsp9–12. ORF2a, ORF2b, ORFs3–7, and ORF5a encode the structural proteins, including GP2a, E, GP3, GP4, GP5a, GP5, M, and the nucleocapsid protein (N).
A typical feature of the adaptive immune response in PRRSV-infected pigs is delayed appearance of neutralizing antibodies (Labarque et al.2000). In previous studies, the GP5 has been suspected to be the major neutralizing target since the discovery of PRRSV. Meanwhile, a linear neutralizing epitope composed of amino acids 37–45 (aa 37–45) and a non-neutralizing epitope (aa 27–30) were both identified in the GP5 protein (Ostrowski et al.2002). Furthermore, aa 32–34, aa 38–39, and aa 57–59 within the N-terminal ectodomain of GP5 were reported to be associated with NA induction and neutralization sensitivity of the virus (Kim et al.2013). A residue of 70 aa in the M protein was demonstrated to play an important role in regulating neutralization susceptibility to NAs in porcine serum (Fan et al.2016). However, immunizing pigs with polypeptides of the GP5-M ectodomain could raise specific antibodies, providing partial protection without a detectable NA response. At the same time, M-GP5 ectodomain-specific antibodies purified from PRRSV-neutralizing serum can bind to the virus without neutralization capability (Li and Murtaugh 2012). Therefore, it appears that antibody against the GP5/M ectodomain epitope alone may not be sufficient to completely neutralize the virus. Besides, GP2, GP3, and GP4 are considered able to form a multi-protein complex that plays a vital role in viral infectivity and receptor binding (Wissink et al.2005; Das et al.2010). A growing number of studies illustrated that GP2 and GP3 are the targets of NAs and that GP4 contains a viral-neutralizing epitope and GP4-specific NA, which might be a driving force in PRRSV evolution (Vanhee et al.2010; Costers et al.2010a, b). Interestedly, Leng et al. (2017) has reported that ORF1a contains a neutralization region.
Because of the conflicting data from various studies, the mechanism of antibody-mediated PRRSV neutralization is still unclear. In the present study, we initially prepared antisera with high titer NAs against JXwn06 and HB-1/3.9 and observed no cross-neutralization activity between the two strains. Subsequently, we used full-length PRRSV infectious clones with RvJXwn and RvHB-1/3.9 as backbones to construct a series of chimeric viruses by individually exchanging the corresponding regions within the genomes. The rescued viruses were then analyzed for their growth kinetics in vitro and their reactivity to sera from animals immunized with either parental virus to better understand the neutralizing antibody target region of PRRSV.
Materials and Methods
Cells and Viruses
MARC-145 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories Inc, South Logan, UT, USA), and maintained at 37 °C with 5% CO2. Three PRRSV strains, JXwn06 (GenBank accession No. EF641008.1), HB-1/3.9 (GenBank accession No. EU360130.1), and JXwn06-81c (GenBank accession No. HQ233604.1), which is an attenuated virus obtained from JXwn06 through serial passaging on MARC-145 cells, were used in this study (Gao et al.2004; Liu et al.2011).
Preparation of Antisera
Antisera against JXwn06 were produced in four PRRSV-naive pigs using the following immunization procedure: the pigs were initially immunized with JXwn06-81c virus at a dose of 2 × 105 median tissue culture infectious dose (TCID50)/2 mL and were boosted three times intranasally and intramuscularly with inoculations of 2 × 105 TCID50/2 mL of JXwn06 at 56 days post-inoculation (DPI), 126 DPI, and 161 DPI, respectively. All of the pigs were euthanized to collect their sera at 182 DPI. Antisera against HB-1/3.9 was prepared using the same procedure with HB-1/3.9 virus used as the immunizing antigen. The titers of PRRSV N protein antibodies were measured using a commercial IDEXX Herdchek PRRS 3XR ELISA kit (IDEXX, Westbrook, ME, USA).
Construction of PRRSV Chimeric Full-Length cDNA Clones
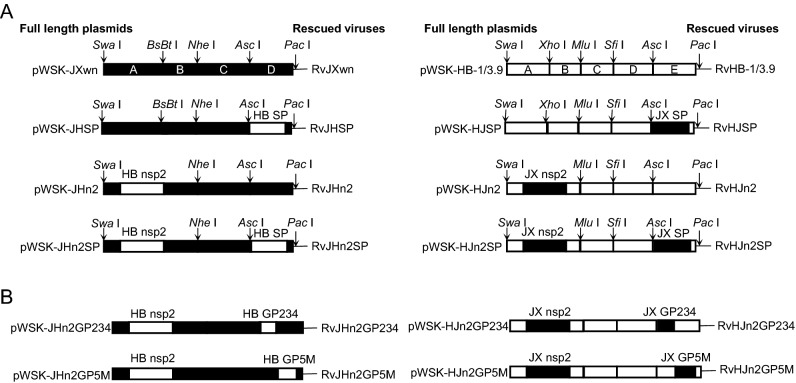
PRRSV full-length infectious cDNA clone plasmids pWSK-JXwn, pWSK-HB-1/3.9, pWSK-JHSP, and pWSK-HJSP have been previously constructed in our laboratory (Li Y et al.2014). Strategies used to construct the PRRSV chimeric full-length cDNA clones are illustrated in Fig. 1. The nsp2-coding region was swapped between pWSK-JXwn and pWSK-HB-1/3.9 using the unique restriction enzymes Swa I and Nhe I or Mlu I (New England Biolabs, Ipswich, MA, USA). Briefly, the nsp2-coding region, which was amplified from one full-length plasmid, and the regions flanking nsp2, which were amplified from the other full-length plasmid, were connected by fusion PCR using the primers shown in Supplementary Table S1. Further, a new fragment A + B of pWSK-JXwn, containing the nsp2-coding region of HB-1/3.9 and the restriction enzyme site pairs Swa I/Nhe I, and a new fragment A + B of pWSK-HB-1/3.9, containing the nsp2-coding region of JXwn06 and the restriction enzyme site pairs Swa I/Mlu I, were generated. Subsequently, the new fragments were ligated to their parental plasmids using the respective restriction enzymes to construct pWSK-JHn2 and pWSK-HJn2.
Fig. 1.
Construction strategy for the full-length cDNA clones. A Full-length infectious clones with exchanged SPs, nsp2, and nsp2 + SPs-coding regions. B Full-length infectious clones with exchanged nsp2 + GP234 and nsp2 + GP5M. These boxes represent the genomic fragments of parental backbone viruses RvJXwn (black) or RvHB-1/3.9 (white). Restriction enzyme sites used for cloning are shown above the bars. Designations of each full-length plasmid and each rescued virus are shown on the left and right side, respectively.
To swap the structural proteins-coding regions between pWSK-JHn2 and pWSK-HJn2, we followed a method similar to that described above (Fig. 1A and 1B). The chimeric plasmids, with pWSK-JXwn as the backbone, contained the nsp2- and SPs-, GP234-, or GP5M-coding regions from pWSK-HB-1/3.9 and were individually named pWSK-JHn2SP, pWSK-JHn2GP234, and pWSK-JHn2GP5M. Correspondingly, pWSK-HJn2SP, pWSK-HJn2GP234, and pWSK-HJn2GP5M, with pWSK-HB-1/3.9 as a backbone, were also constructed.
Recovery and Identification of Chimeric Viruses
To rescue the chimeric viruses, MARC-145 cells, seeded at approximately 80% confluency in 6-well plates, were transfected with 2.5 μg of the recombinant plasmids with LTX (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Cells were observed daily and virus was harvested at 72 h post transfection. To confirm the rescued viruses, indirect immunofluorescence assay (IFA) was carried out using the PRRSV N-specific monoclonal antibody (McAb) SDOW17 (Rural Technologies, Inc., Brookings, SD, USA). Finally, the RNAs of chimeric viruses passaged three times were extracted, reversed transcribed, and subjected to PCR and sequencing.
Growth Kinetics of the Rescued Viruses in MARC-145 Cells
Confluent monolayers of MARC-145 cells seeded in 24-well plates were individually infected with the chimeric viruses and their parental viruses at a multiplicity of infection (MOI) of 0.1. The infectious titers of the samples harvested at 0, 12, 24, 36, 48, 60, and 72 h post-infection (hpi) were determined by IFA in MARC-145 cells and quantified as TCID50/mL. All tests were independently repeated three times.
Serum Virus Neutralization (SVN) Assay in MARC-145 Cells
Sera were heat inactivated at 56 °C for 30 min, and then serum virus neutralization assays were conducted as previously described (Wu et al.2001). In brief, serum samples diluted twofold with DMEM media were incubated with an equal volume of the respective virus at a titer of 2 × 103 TCID50/mL at 37 °C. After 1 h of incubation, 100 μL of the mixture was transferred to a 96-well cell culture plate with confluent MARC-145 cells and incubated for another 1 h at 37 °C and 5% CO2. The well contents were removed, and then the cells were washed three times with PBS, followed by the addition of fresh DMEM media with 2% FBS to each well. After 24 h, the plates were fixed in ice-free ethanol, and PRRSV-positive cells were detected by IFA with anti-N protein antibody SDOW17. The titers of NA to PRRSV were calculated using the Reed-Muench method as described previously (Labarque et al.2000).
Statistical Analysis
Data were expressed as means ± standard deviations (SD). The significance of the variability among the groups was determined by two-way ANOVA using GraphPad Prism software (version 5.0). Differences were considered statistically significant at P < 0.05.
Results
Preparation of PRRSV Antibodies from Hyperimmunized Animals
Four specific pathogen-free large white pigs were used to prepare the anti-JXwn06 sera, another two pigs were used for HB-1/3.9. Excepting JXwn06-2, all of the pigs in the JXwn06 or HB-1/3.9 inoculated groups seroconverted at 3 weeks post-inoculation (Fig. 2A and 2C). The levels of PRRSV NA titers in the peripheral blood of the immunized animals against the challenge strain were monitored by virus neutralization assay in MARC-145 cells at different times post-infection. NA was detected at 42 DPI, and NA titers gradually increased in each of the immunized animals post immunization (Fig. 2B and 2D). Twenty-one days after the second boost, NA titers reached their peak, ranging from 1:32 to 1:98 for most of the animals. Based on this data, 182 DPI, which corresponded to 21 days after the third boost, was set as the end of the hyperimmunization process and the time when animals were anesthetized with Zoletil (Virbac, France). All sera were individually collected by artery intubation and then stored at − 80 °C for further use.
Fig. 2.
Kinetics of PRRSV N protein and neutralizing antibodies in sera of inoculated pigs. Pigs were vaccinated with JXwn06-81c or HB-1/3.9 and boosted at 56, 126, and 161 DPI using JXwn06 or HB-1/3.9, respectively. Serum samples were collected at the indicated time points. A and C PRRSV N protein antibody kinetics of the immunized pigs. Antibodies were detected using an IDEXX Herdchek PRRS 3XR ELISA kit, and the antibody level was expressed as a sample value/positive value (S/P) ratio. A ratio of 0.4 was regarded as seroconversion. B and D Levels of neutralizing antibodies (NAs) against the homologous strain in the sera of immunized pigs at the indicated time points were determined by serum virus neutralization assay in MARC-145 cells. Data are shown as the means ± standard deviations (error bars) from three independent trials.
Limited Cross-Neutralization Between JXwn06 and HB-1/3.9
To test the NA cross reaction between JXwn06 and HB-1/3.9, neutralization assays were performed in MARC-145 cells with specific viruses and collected sera. The overall NA titers are summarized in Fig. 3. All sera reacted with their homologous strains, except for anti-JXwn06 serum-4, which showed a low affinity for JXwn06 with the lowest NA titer (1:15). The worse health situation of pig with JXwn06-4 inoculation shown as smallest size and lightest weight may be related to the lowest level of NA (data not shown). Regarding the NA cross reaction, all of the pig hyperimmunized sera against JXwn06 reacted with the HB-1/3.9 strain at cross NA titers ranging from 1:3 to 1:1, which was considered to have no neutralization activity (Lopez et al.2007). Meanwhile, anti-HB-1/3.9 serum-1 did not neutralize JXwn06 with a cross NA titer of 1:8. Interestingly, anti-HB-1/3.9 serum-2 could reacted with JXwn06; however, a significant difference was observed between the NA titer of HB-1/3.9 (1:89) and JXwn06 (1:31). These results indicated that the NA cross reaction between JXwn06 and HB-1/3.9 was limited.
Fig. 3.
Neutralization antibody cross reaction as determined by SVN assay between JXwn06 and HB-1/3.9 using hyperimmuned pig JXwn06 and HB-1/3.9 antisera. Data are shown as the means ± standard deviations (error bars) from three independent experiments. Asterisk (*) and pound (#) indicate significant difference in NA titers between RvJXwn and RvHB-1/3.9 in the anti-JXwn06 sera and anti-HB-1/3.9 sera, respectively (***P < 0.001; ### P < 0.001).
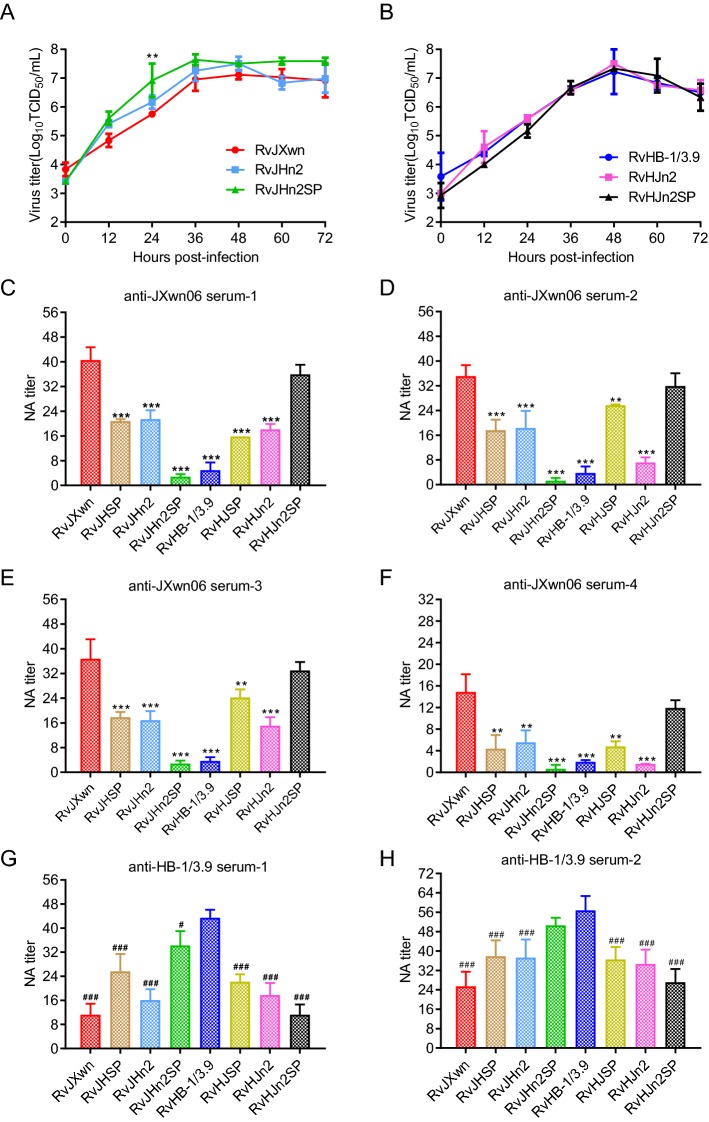
Both Nsp2 and SPs Play Important Roles in Virus Neutralization In Vitro
Recently, nsp2 was described as a virion-associated SP that existed in or on viral particles in multiple isoforms (Kappes et al.2013), and the study of Leng et al. (2017) showed that ORF1a contains a neutralization region. These data drove speculation that nsp2 is associated with neutralization activity. Based on these studies, chimeric viruses with nsp2 and SP-coding regions exchanged between RvJXwn and RvHB-1/3.9 were rescued and used for neutralization tests. Multi-step growth curves in MARC-145 cells showed that all of the chimeric viruses had growth kinetics similar to their respective parental viruses (Fig. 4A and 4B). Overall NA titers with various pig sera are summarized in Fig. 4C–4H. As for the four anti-JXwn06 sera (Fig. 4C–4F), NA titers against RvJHn2 (1:21, 1:18, 1:17, and 1:6) were lower than those against RvJXwn (1:41, 1:35, 1:37, and 1:15), but were comparable to those against RvHJSP (1:16, 1:26, 1:24, and 1:5). The NA titers against RvHJn2 (1:18, 1:7, 1:15, and 1:2) were almost equivalent to those against RvJHSP (1:21, 1:18, 1:18, and 1:4). Strikingly, when nsp2 and SPs were exchanged, the NA titers against RvHJn2SP (1:36, 1:32, 1:33, and 1:12) were consistent with those against RvJXwn. The above results were reverse-verified with the two anti-HB-1/3.9 sera (Fig. 4G and 4H).
Fig. 4.
Growth kinetics of rescued viruses in MARC-145 cells and their reactivities to the antisera. A Growth kinetics of RvJXwn, RvJHn2, and RvJHn2SP in MARC-145 cells. Asterisk (*) indicates a significant difference in the virus titers between RvJXwn and RvJHn2SP (**P < 0.01). B Growth kinetics of RvHB-1/3.9, RvHJn2, and RvHJn2SP in MARC-145 cells. C–F NA titers against indicated viruses in anti-JXwn06 serum-1/2/3/4. Asterisk (*) indicates a significant difference in NA titers between RvJXwn and the indicated chimeric viruses in the four anti-JXwn06 sera. (***P < 0.001). G and H NA titers against indicated viruses in anti-HB-1/3.9 serum-1/2. Pound sign (#) indicates a significant difference in NA titers between RvHB-1/3.9 and the indicated chimeric viruses in the two anti-HB-1/3.9 sera. (# P < 0.05; ### P < 0.001). Data are shown as the means ± standard deviations (error bars) from three independent trials.
The targets of PRRSV NAs have been extensively studied, and several epitopes on the M, GP5, GP2, GP3, and GP4 proteins have been shown to express neutralization activity (Vanhee et al.2011; Costers et al.2010a, b; Zhou et al.2012). Based on this, we hypothesized that differences in the SPs of JXwn06 and HB-1/3.9 led to a weak NA cross reaction. Surprisingly, neutralization assay in MARC-145 cells indicated that (Fig. 4C–4F) RvJHSP retained the ability to be neutralized by the four anti-JXwn06 sera with NA titers of 1:21, 1:18, 1:18, and 1:4, whereas RvHJSP (1:16, 1:26, 1:24, and 1:5) could be partly neutralized by anti-JXwn06 sera. In corresponding studies of neutralization assay with the two anti-HB-1/3.9 sera, the NA titers against RvJHSP (1:26 and 1:38) were higher than the NA titers against RvJXwn (1:11 and 1:25), but less than the NA titers against HB-1/3.9 (1:43 and 1:57). Consistent with this, the NA titers against RvHJSP (1:22 and 1:37) were lower than the NA titers against RvHB-1/3.9, but higher than the NA titers against RvJXwn using HB-1/3.9-inoculated pig sera (Fig. 4G and 4H). Thus, the results showed that RvJHSP and RvHJSP did not neutralize as well as their donor strains did, indicating that differences in SP-coding regions between JXwn06 and HB-1/3.9 only partially contributed to the weak NA cross reaction and that other likely NA targets may be present.
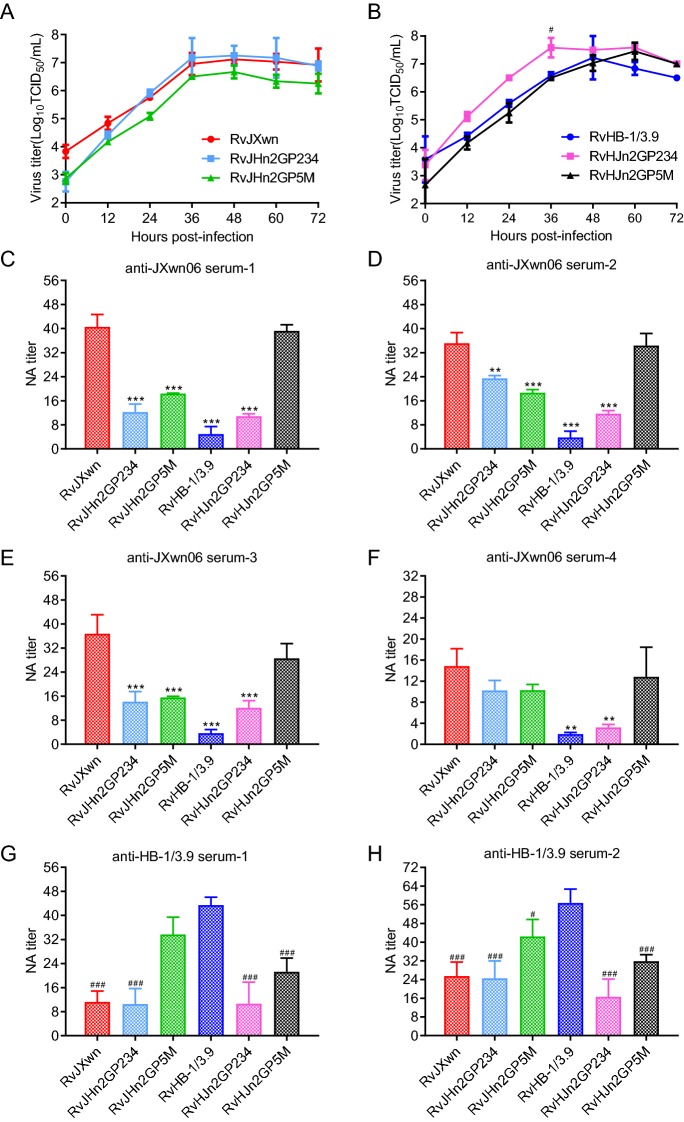
Nsp2 and GP5-M Together Contribute to the Weak Cross-Neutralization Reaction between Heterologous Strains
Subsequently, to further delineate the SP(s) that are involved in the weak cross-reactivity together with nsp2, chimeric viruses with swapped n2 + GP234 or n2 + GP5M-coding regions between RvJXwn and RvHB-1/3.9 were rescued and identified with IFA along with the sequencing of the third-passage viruses (data not shown). The growth properties of the rescued viruses in MARC-145 cells demonstrated that RvJHn2GP234 and RvJHn2GP5M had similar growth kinetics as their parental viruses (Fig. 5A). Similarly, RvHJn2GP234 and RvHJn2GP5M had growth kinetics similar to their backbone virus, with the exception that RvHJn2GP234 had faster growth kinetics than RvHB-1/3.9 at 36 hpi (P < 0.1) (Fig. 5B). The results of the multi-step growth assays suggested that the exchange of n2 + GP234 and n2 + GP5M-coding regions between JXwn06 and HB-1/3.9 does not affect the replication efficiency of each virus. Neutralization assays in MARC-145 cells with anti-JXwn06 serum-1/2/3 (Fig. 5C–5F) revealed the NA titers of both RvJHn2GP234 (1:12, 1:23, and 1:14) and RvJHn2GP5M (1:18, 1:19, and 1:16) to be remarkable lower than that of their parental virus RvJXwn (1:41, 1:35, and 1:37) (P < 0.01), as well as the similar neutralization ability of anti-JXwn06 serum-4 to those rescued viruses. Moreover, the NA titers against RvHJn2GP234 (1:11, 1:12, 1:12, and 1:3) were lower than those against RvJXwn in the sera of the JXwn06-inoculated pigs. Strikingly, no significant difference was observed between the NA titer against RvHJn2GP5M (1:39, 1:34, 1:29, and 1:13) and RvJXwn in the sera of JXwn06-inoculated pigs.
Fig. 5.
In vitro growth properties of the chimeric viruses with exchanged nsp2 + GP234 or nsp2 + GP5M-coding regions, along with their reactivities to the antisera. A Growth kinetics of RvJXwn, RvJHn2GP234, and RvJHn2GP5M in MARC-145 cells. B Growth kinetics of RvHB-1/3.9, RvHJn2GP234 and RvHJn2GP5M in MARC-145 cells. Pound sign (#) indicates a significant difference in the virus titers between RvHB-1/3.9 and RvHJn2GP234 (# P < 0.05). C–F NA titers against indicated viruses in anti-JXwn06 serum-1/2/3/4. Asterisk (*) indicates a significant difference in NA titers between RvJXwn and indicated chimeric viruses in the four anti-JXwn06 sera (***P < 0.001). G and H NA titers against indicated viruses in anti-HB-1/3.9 serum-1/2. Pound sign (#) indicates a significant difference in NA titers between RvHB-1/3.9 and the indicated chimeric viruses in the two anti-HB-1/3.9 sera (#P < 0.05; ### P < 0.001). Data are shown as the means ± standard deviations (error bars) from three independent trials.
Correspondingly, neutralization assays in MARC-145 cells with the two anti-HB-1/3.9 sera (Fig. 5G–5H) revealed that the NA titers of both RvHJn2GP234 (1:11 and 1:17) and RvHJn2GP5M (1:21 and 1:32) were remarkable lower than that of their parental virus RvHB-1/3.9 (1:43 and 1:57) (P < 0.01). Moreover, the NA titers against RvJHn2GP234 (1:11 and 1:24) were lower than those against RvHB-1/3.9 in the sera of the HB-1/3.9-inoculated pigs. Strikingly, no significant difference was observed between the NA titer against RvJHn2GP5M (1:34 and 1:42) and RvHB-1/3.9 in the sera of the HB-1/3.9-inoculated pigs. We therefore concluded that both nsp2 and the GP5-M heterodimer contributed to the observed neutralization activity in MARC-145 cells.
Discussion
A characteristic feature of PRRSV infection is that it usually takes longer (specially periods equal to or higher than four weeks post infection) than other viruses do to establish a detectable level of anti-PRRSV NAs in the infected animals (Lopez and Osorio 2004). In addition, the PRRSV induced neutralizing response of pigs is weak, especially for the heterologous strains, and significantly varies from animal to animal (Lopez and Osorio 2004; Li X et al.2014). To identify the relation between variation in viral proteins and cross-neutralization reactivity among heterologous strains, a serial chimeric viruses with swapped genes or fragments from two heterologous parental strains were constructed to test the neutralization reactivity with PRRSV antiserum immunized with the parental virus.
In previous studies, the exploration of NA targets of PRRSV has primarily focused on viral structural proteins, and several epitopes on the GP2, GP3, GP4, GP5, and membrane (M) protein have been shown to harbor neutralization activity (Costers et al.2010a, b; Vanhee et al.2011; Zhou et al.2012). Tian et al. (2017) demonstrated that chimeric PRRSVs with the full-length sequences of structural genes (ORFs3–6) shuffled via DNA shuffling had an improved heterologous cross-neutralization ability, revealing the important role of structural proteins in cross-neutralization of PRRSV. However, in this study, there is no cross-neutralization reaction between HP-PRRSV strain JXwn06 and LP-PRRSV strain HB-1/3.9 (Fig. 3). Strikingly, in the JXwn06-inoculated pigs’ sera, the NA titer against RvHJSP, which retained the whole SPs-coding regions of JXwn06, was significantly different from that of RvJXwn (Fig. 4C–4F).
Concurrently, same results were obtained with anti-HB-1/3.9 sera (Fig. 4G, 4H). Recent studies have described that multiple isoforms of nsp2 exist in or on viral particles (Kappes et al.2013). Leng et al. (2017) have reported that ORF1a contains a neutralization region. Thus, the contribution of nsp2 to neutralization reactivity was further tested. The neutralization assays revealed that the chimeric viruses carrying swapped n2 + SP-coding regions between RvJXwn and RvHB-1/3.9 could be better neutralized by virus-induced antisera that provided nsp2 + SP. It demonstrated that nsp2 together with SPs might be the target of NAs (Fig. 4C–4H). Thus, there is at least one neutralizing epitope in nsp2 or in the spatial structure formed by nsp2 and SPs together.
Among the structural proteins, GP2, GP3 and GP4 form a multi-protein complex (Das et al.2010) and GP5 and M constitute a heterodimer (Wissink et al.2005). In order to further delineate which SP(s) together with nsp2 are responsible for the weak cross-reactivity, chimeric viruses with swapped n2 + GP234 or n2 + GP5M-coding regions between RvJXwn and RvHB-1/3.9 were constructed and rescued, followed by neutralization assays. As shown in Fig. 5C–5F, the replacement of nsp2 and GP5-M coding regions from RvJXwn to RvHB-1/3.9 induced a significant increase in the NA reactivity of JXwn06 antisera against RvHJn2GP5M. Further, the neutralizing potency of HB-1/3.9 antisera against HB-1/3.9 and RvJHn2GP5M was similar (Fig. 5G and 5H). Notably, in Fig. 5C and 5E, the NA titers of JXwn06 antisera against RvJHn2GP5M were higher than that of RvJHn2GP234 and RvHJn2GP234 (no statistically significant difference), which may be due to the slightly higher replication efficiency of RvJHn2GP234 than RvJHn2GP5M (Fig. 5A). Similarly, in Fig. 5G and 5H, the neutralizing potency of HB-1/3.9 antisera against RvHJn2GP5M was slightly higher than that of RvHJn2GP234 and RvJHn2GP234 (no statistically significant difference); this may be due to the higher replication efficiency of RvHJn2GP234 as compared to RvHJn2GP5M. However, the NA titer of anti-JXwn06 serum-2 against RvJHn2GP234 was higher than that against RvJHn2GP5M (Fig. 5D, with no statistically significant difference), which might be caused by different immune responses to PRRSV infection in different pigs. Li and Murtaugh (2012) constructed recombinant polypeptides of GP5-M ectodomain, and found that specific antibodies in the immunized pigs could provide partial protection without a detectable NA response; meanwhile, the purified specific antibodies could bind to the virus without neutralizing ability. These complicated results also suggest that nsp2 and GP5-M may interact with each other to form a neutralizing antibody epitope with a spatial structure; however, it requires further research.
Pulmonary alveolar macrophage (PAM) is the target cell of PRRSV in vivo (Duan et al.1997). In the model for PRRSV infection on macrophages (Shi et al.2015), the interaction between the M protein and heparin sulfate (HS) is regarded as the first step of infection, followed by the binding of GP5 with macrophage’s sialoadhesin (CD169). The interaction between GP2a-GP3-GP4 heterotrimer and CD163, the macrophage scavenger receptor protein, is associated with the internalization and uncoating processes (Das et al.2010). Besides, CD151 (Shanmukhappa et al.2007) and vimentin (Wang et al.2011) are also linked with the viral entry course. The blockage of any of these four molecules (CD163, CD169, CD151, and vimentin) will hamper viral infection (Shi et al.2015). We performed neutralization assay on PAMs and demonstrated that nsp2 and GP5-M contribute partially to the weak NA cross reaction between JXwn06 and HB-1/3.9 (data not shown); unfortunately, various results were obtained from different tests or using PAMs from different piglets. These complex results may be caused by heterogeneity of PAMs from different piglets. Moreover, the phagocytic function of PAMs resulting in endocytosis of antigen–antibody complexes and antibody-dependent enhancement mediated by non-neutralizing antibody (Gu et al.2015) when mixed with neutralizing antibody in the antisera, together pose obstacles in performing neutralization assays on PAMs. Therefore, further investigation should be done in the future to resolve the current concerns.
In conclusion, the neutralization assay using antisera against HP-PRRSV JXwn06 and LP-PRRSV HB-1/3.9 indicated no cross-neutralization reaction between anti-JXwn06 sera and anti-HB-1/3.9 sera. Moreover, swapping nsp2 with GP5-M contributed to increased neutralization reactivity of the donor strain in MARC-145 cells, by constructing a series of chimeric viruses followed neutralization assays. This study provides important information that might be helpful for further understanding the mechanism of PRRSV neutralization reactivity on cells and developing new generation PRRSV vaccines that aim to provide better heterologous protection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Major Program of National Natural Science Foundation of China (31490603, 31572549) and the National Key Technology R & D Program of China (2015BAD12B01-2).
Author Contributions
JS, XG, LZ, XNG, JH and HY contributed to the study design. JS, BH and XZ performed the experiments in the study. XG, LZ and JS analyzed the data. JS, LZ and XG wrote the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
The animal experiments in this study were approved by The Laboratory Animal Ethical Committee of China Agricultural University. All institutional and national guidelines for the care and use of animals were followed.
References
- Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Et A. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Costers S, Lefebvre DJ, Van Doorsselaere J, Vanhee M, Delputte PL, Nauwynck HJ. GP4 of porcine reproductive and respiratory syndrome virus contains a neutralizing epitope that is susceptible to immunoselection in vitro. Arch Virol. 2010;155:371–378. doi: 10.1007/s00705-009-0582-7. [DOI] [PubMed] [Google Scholar]
- Costers S, Vanhee M, Van Breedam W, Van Doorsselaere J, Geldhof M, Nauwynck HJ. GP4-specific neutralizing antibodies might be a driving force in PRRSV evolution. Virus Res. 2010;154:104–113. doi: 10.1016/j.virusres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Das PB, Dinh PX, Ansari IH, de Lima M, Osorio FA, Pattnaik AK. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J Virol. 2010;84:1731–1740. doi: 10.1128/JVI.01774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Nauwynck HJ, Pensaert MB. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Vet Microbiol. 1997;56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- Fan B, Liu X, Bai J, Zhang T, Zhang Q, Jiang P. Influence of the amino acid residues at 70 in M protein of porcine reproductive and respiratory syndrome virus on viral neutralization susceptibility to the serum antibody. Virol J. 2016;13:51. doi: 10.1186/s12985-016-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZQ, Guo X, Yang HC. Genomic characterization of two Chinese isolates of porcine respiratory and reproductive syndrome virus. Arch Virol. 2004;149:1341–1351. doi: 10.1007/s00705-004-0292-0. [DOI] [PubMed] [Google Scholar]
- Gu W, Guo L, Yu H, Niu J, Huang M, Luo X, Li R, Tian Z, Feng L, Wang Y. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. J Gen Virol. 2015;96:1712–1722. doi: 10.1099/vir.0.000118. [DOI] [PubMed] [Google Scholar]
- Han J, Zhou L, Ge X, Guo X, Yang H. Pathogenesis and control of the Chinese highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2017;209:30–47. doi: 10.1016/j.vetmic.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Kappes MA, Miller CL, Faaberg KS. Highly divergent strains of porcine reproductive and respiratory syndrome virus incorporate multiple isoforms of nonstructural protein 2 into virions. J Virol. 2013;87:13456–13465. doi: 10.1128/JVI.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WI, Kim JJ, Cha SH, Wu WH, Cooper V, Evans R, Choi EJ, Yoon KJ. Significance of genetic variation of PRRSV ORF5 in virus neutralization and molecular determinants corresponding to cross neutralization among PRRS viruses. Vet Microbiol. 2013;162:10–22. doi: 10.1016/j.vetmic.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Kuhn JH, Lauck M, Bailey AL, Shchetinin AM, Vishnevskaya TV, Bao Y, Ng TF, LeBreton M, Schneider BS, Gillis A, Tamoufe U, Diffo JD, Takuo JM, Kondov NO, Coffey LL, Wolfe ND, Delwart E, Clawson AN, Postnikova E, Bollinger L, Lackemeyer MG, Radoshitzky SR, Palacios G, Wada J, Shevtsova ZV, Jahrling PB, Lapin BA, Deriabin PG, Dunowska M, Alkhovsky SV, Rogers J, Friedrich TC, O’Connor DH, Goldberg TL. Reorganization and expansion of the nidoviral family Arteriviridae. Arch Virol. 2016;161:755–768. doi: 10.1007/s00705-015-2672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarque GG, Nauwynck HJ, Van Reeth K, Pensaert MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000;81:1327–1334. doi: 10.1099/0022-1317-81-5-1327. [DOI] [PubMed] [Google Scholar]
- Leng C, Zhang W, Zhang H, Kan Y, Yao L, Zhai H, Li M, Li Z, Liu C, An T, Peng J, Wang Q, Leng Y, Cai X, Tian Z, Tong G. ORF1a of highly pathogenic PRRS attenuated vaccine virus plays a key role in neutralizing antibody induction in piglets and virus neutralization in vitro. Virol J. 2017;14:159. doi: 10.1186/s12985-017-0825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Murtaugh MP. Dissociation of porcine reproductive and respiratory syndrome virus neutralization from antibodies specific to major envelope protein surface epitopes. Virology. 2012;433:367–376. doi: 10.1016/j.virol.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Li X, Galliher-Beckley A, Pappan L, Trible B, Kerrigan M, Beck A, Hesse R, Blecha F, Nietfeld JC, Rowland RR, Shi J. Comparison of host immune responses to homologous and heterologous type II porcine reproductive and respiratory syndrome virus (PRRSV) challenge in vaccinated and unvaccinated pigs. Biomed Res Int. 2014;2014:416727. doi: 10.1155/2014/416727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014;10:e1004216. doi: 10.1371/journal.ppat.1004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tas A, Sun Z, Snijder EJ, Fang Y. Proteolytic processing of the porcine reproductive and respiratory syndrome virus replicase. Virus Res. 2015;202:48–59. doi: 10.1016/j.virusres.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhou R, Zhang J, Zhou L, Jiang Q, Guo X, Ge X, Yang H. Recombination analyses between two strains of porcine reproductive and respiratory syndrome virus in vivo. Virus Res. 2011;155:473–486. doi: 10.1016/j.virusres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lopez OJ, Oliveira MF, Garcia EA, Kwon BJ, Doster A, Osorio FA. Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin Vaccine Immunol. 2007;14:269–275. doi: 10.1128/CVI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JK, Fang Y, Ladinig A, Chen N, Li Y, Rowland B, Renukaradhya GJ. Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci. 2016;4:129–154. doi: 10.1146/annurev-animal-022114-111025. [DOI] [PubMed] [Google Scholar]
- Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002;76:4241–4250. doi: 10.1128/JVI.76.9.4241-4250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmukhappa K, Kim JK, Kapil S. Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection. Virol J. 2007;4:62. doi: 10.1186/1743-422X-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Liu Y, Ding Y, Zhang Y, Zhang J. PRRSV receptors and their roles in virus infection. Arch Microbiol. 2015;197:503–512. doi: 10.1007/s00203-015-1088-1. [DOI] [PubMed] [Google Scholar]
- Tian D, Cao D, Lynn Heffron C, Yugo DM, Rogers AJ, Overend C, Matzinger SR, Subramaniam S, Opriessnig T, LeRoith T, Meng XJ. Enhancing heterologous protection in pigs vaccinated with chimeric porcine reproductive and respiratory syndrome virus containing the full-length sequences of shuffled structural genes of multiple heterologous strains. Vaccine. 2017;35:2427–2434. doi: 10.1016/j.vaccine.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Vanhee M, Costers S, Van Breedam W, Geldhof MF, Van Doorsselaere J, Nauwynck HJ. A variable region in GP4 of European-type porcine reproductive and respiratory syndrome virus induces neutralizing antibodies against homologous but not heterologous virus strains. Viral Immunol. 2010;23:403–413. doi: 10.1089/vim.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhee M, Van Breedam W, Costers S, Geldhof M, Noppe Y, Nauwynck H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine. 2011;29:4794–4804. doi: 10.1016/j.vaccine.2011.04.071. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang L, Ma X, Gao J, Xiao Y, Zhou E. The role of vimentin during PRRSV infection of MARC-145 cells. Bing Du Xue Bao. 2011;27:456–461. [PubMed] [Google Scholar]
- Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, Et A. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wissink EH, Kroese MV, van Wijk HA, Rijsewijk FA, Meulenberg JJ, Rottier PJ. Envelope protein requirements for the assembly of infectious virions of porcine reproductive and respiratory syndrome virus. J Virol. 2005;79:12495–12506. doi: 10.1128/JVI.79.19.12495-12506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WH, Fang Y, Farwell R, Steffen-Bien M, Rowland RR, Christopher-Hennings J, Nelson EA. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology. 2001;287:183–191. doi: 10.1006/viro.2001.1034. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ni YY, Pineyro P, Sanford BJ, Cossaboom CM, Dryman BA, Huang YW, Cao DJ, Meng XJ. DNA shuffling of the GP3 genes of porcine reproductive and respiratory syndrome virus (PRRSV) produces a chimeric virus with an improved cross-neutralizing ability against a heterologous PRRSV strain. Virology. 2012;434:96–109. doi: 10.1016/j.virol.2012.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.