Abstract
Background
Patient-reported outcomes (PROs) are important when evaluating treatment benefits in rheumatoid arthritis (RA). We compared upadacitinib, an oral, selective JAK-1 inhibitor, with placebo to assess clinically meaningful improvements in PROs in patients with RA who have had inadequate responses to biologic disease-modifying antirheumatic drugs (bDMARD-IR).
Methods
PRO responses between upadacitinib 15 mg or 30 mg and placebo were evaluated at week 12 from the SELECT-BEYOND trial. Improvement was determined by measuring Patient Global Assessment of Disease Activity (PtGA), pain, Health Assessment Questionnaire Disability Index (HAQ-DI), Short Form-36 Health Survey (SF-36), duration and severity of morning (AM) stiffness, and Insomnia Severity Index (ISI). Least squares mean changes and percentage of patients reporting improvements ≥ minimum clinically important differences (MCID) and scores greater than or equal to normative values were determined. The number needed to treat (NNT) to achieve clinically meaningful improvements was calculated.
Results
In 498 patients, both upadacitinib doses resulted in statistically significant changes from baseline versus placebo in PtGA, pain, HAQ-DI, SF-36 Physical Component Summary (PCS), 7 of 8 SF-36 domains (15 mg), 6 of 8 SF-36 domains (30 mg), and AM stiffness duration and severity. Compared with placebo, more upadacitinib-treated patients reported improvements ≥ MCID in PtGA, pain, HAQ-DI, SF-36 PCS, 7 of 8 SF-36 domains (15 mg), 5 of 8 SF-36 domains (30 mg), AM stiffness duration and severity, and ISI (30 mg) and scores ≥ normative values in HAQ-DI and SF-36 domains. Across most PROs, NNTs to achieve MCID with upadacitinib ranged from 4 to 7 patients.
Conclusions
In bDMARD-IR RA patients, upadacitinib (15 mg or 30 mg) improved multiple aspects of quality of life, and more patients reached clinically meaningful improvements approaching normative values compared with placebo.
Trial registration
The trial is registered with ClinicalTrials.gov (NCT02706847), registered 6 March 2016.
Keywords: Rheumatoid arthritis, Patient-reported outcome measures, JAK inhibitor, Quality of life, Treatment outcomes, Upadacitinib, Fatigue, HAQ, Pain, SF-36, MCID
Background
Rheumatoid arthritis (RA) is a chronic, inflammatory, and destructive disease of the synovial joints that is associated with substantial clinical burden, reduced health-related quality of life (HRQOL), and shortened life expectancy [1, 2]. Most patients with RA experience impaired physical functioning, chronic pain, fatigue, and morning (AM) stiffness, which affect their HRQOL [2–8]. In addition, patients with RA have reported that sleep disturbances are a key determinant of their well-being [3, 9–11]. These reports are supported by cross-sectional studies, which have demonstrated that sleep disturbance correlates with greater pain, disease activity, and fatigue in RA [12–15]. Thus, understanding the patient’s perspective of how a therapy impacts multiple aspects of HRQOL is crucial when evaluating the efficacy of treatments for RA [16–18].
Despite several treatment options, a significant proportion of patients with RA become refractory, or do not adequately respond, to available therapies [19–21]. Several classes of drugs are available to treat inflammation and thereby provide relief from symptoms associated with RA. Current treatment options include conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as methotrexate (MTX), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs) [2, 22]. Janus kinase (JAK) inhibitors are a new class of tsDMARDs approved for use in RA [2, 22]. Upadacitinib, a potent JAK inhibitor with preferential activity against JAK1, has been evaluated across RA populations (MTX-naive, MTX-inadequate responders [MTX-IR], csDMARD-inadequate responders [csDMARD-IR], and bDMARD-inadequate responders [bDMARD-IR]) both as monotherapy and in combination with csDMARDs [23–26].
SELECT-BEYOND is a phase 3, randomized controlled trial (RCT) of upadacitinib 15 mg or 30 mg once daily in patients with RA who are bDMARD-IR and receiving background csDMARDs. The RCT demonstrated that significantly more upadacitinib-treated patients had American College of Rheumatology 20% improvement (ACR20) responses and lower disease activity than placebo [25]. The objectives of the following analyses are to compare response rates and numbers needed to treat (NNTs) with upadacitinib versus placebo for PROs and assess the achievement of clinically meaningful improvements and normative values in patients with treatment-refractory RA.
Methods
Study design and participants
The full study design of SELECT-BEYOND, a phase 3 RCT (NCT02706847), has been published previously [24, 25]. Patients aged 18 years and older with moderate to severe RA for ≥ 3 months were randomized 1:1:1 to receive oral upadacitinib 15 or 30 mg once daily, or placebo, for 12 weeks. Patients were excluded if they had prior exposure to a JAK inhibitor. The study was approved by independent ethics committees or institutional review boards at each study site and conducted in accordance with ethical principles outlined in the current Declaration of Helsinki and consistent with International Conference on Harmonisation Good Clinical Practice and Good Epidemiology Practices, along with all applicable local regulatory requirements. All patient data were deidentified and complied with patient confidentiality requirements.
Patient-reported outcome assessment
Several clinically relevant PROs and assessments were used to evaluate the potential impact of upadacitinib on patient HRQOL and disease burden. These include Patient Global Assessment of Disease Activity (PtGA); pain on a visual analog scale (VAS); Health Assessment Questionnaire Disability Index (HAQ-DI) [27]; HRQOL using the Short Form 36 Health Survey (SF-36), which includes 8 domains scored 0–100 (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health) and 2 aggregate Physical (PCS) and Mental Component Summary (MCS) scores (range of 0–100) [28, 29] with higher scores indicating better HRQOL; AM stiffness severity measured by a numeric rating scale ranging from 0 to 10 with higher scores indicating greater severity, duration of AM stiffness measured in minutes [8, 30, 31]; the Insomnia Severity Index (ISI) to identify and grade insomnia severity (scores range from 0 to 28 where higher scores indicate increased insomnia) [32–34]; and the Euro Qol 5-Dimension 5-Level Questionnaire (EQ-5D-5L), which includes a general health status index measured by a 0–100 VAS and consists of 5 health states [35].
Clinically meaningful responses for each PRO were defined as changes from baseline greater than or equal to minimal clinically important difference (MCID) or greater than or equal to normative values. MCIDs were defined as ≥ 10-point decreases for PtGA [30], ≥ 10-point decreases for pain VAS [30], ≥ 0.22-point decreases for HAQ-DI [30], ≥ 2.5-point increases in SF-36 PCS and MCS [36], ≥ 5-point increases in SF-36 domains [36], ≥ 8.4-point decreases for ISI (moderate improvement) [32], and ≥ 0.05-cm increases for EQ-5D-5L [35]. Owing to the lack of a predefined MCID for AM stiffness in the literature, the minimum important difference (MID) was defined as a reduction of ≥ 1 point for severity and one half standard deviation of the mean baseline values for duration of AM stiffness [8, 30, 31]. Normative values for PROs were obtained from the literature and defined as the following: ≤ 20 for PtGA [37], 0.25 for HAQ-DI [38], 50 for SF-36 PCS and MCS [39], 0–7 for ISI [33], and 0.915 for EQ-5D-5L [40]. Normative values are not available for pain VAS or AM stiffness [8, 30, 31, 41]. The eight SF-36 domains were compared with age- and gender-matched (A/G) normative US population values as a benchmark [42].
Statistical analyses
All patients included in the intention-to-treat population of the BEYOND RCT were eligible for this post hoc analysis. Mean changes were calculated as changes in least squares mean (LSM) from baseline to week 12 for upadacitinib versus placebo based on a mixed effect for repeated measures model. Responses for each PRO were estimated at weeks 1, 4, and 12. Non-responder imputation (NRI) was used to impute missing responses. Between-group differences in responses were assessed using chi-square tests. Time to response was assessed by the Kaplan-Meier analysis and was compared using log-rank test. “Spydergrams” were used to compare SF-36 domain responses [42]. In this study, NNTs were measured as the number of patients needed to achieve one additional responder, defined as the reciprocal of the response rate difference between treatment and placebo groups, and were calculated for each PRO at week 12.
Results
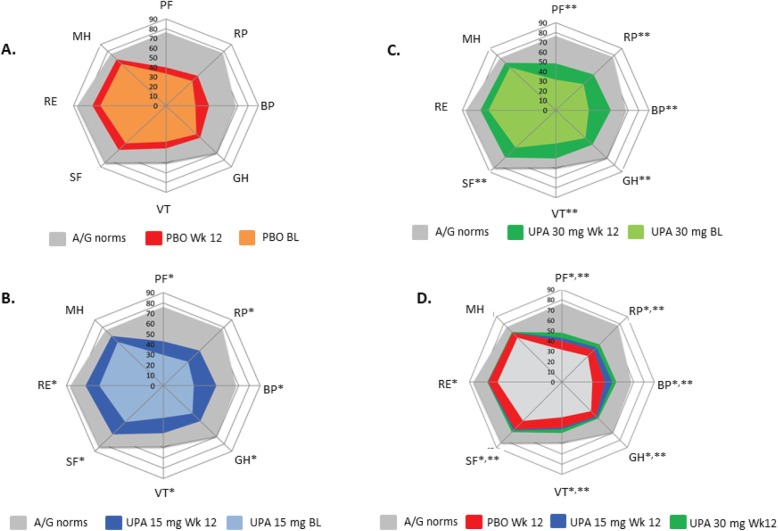
Patient disposition and demographic information have been published [24]. Disease characteristics and baseline PRO values across treatment groups were well-balanced (Tables 1 and 2). Decrements in PROs from normative values at baseline indicate that patients in this RCT reported substantial impairments in HRQOL. SF-36 mean baseline domain scores were approximately 25–50 points lower than in the A/G normative US population (Fig. 1).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Placebo (n = 169) | Upadacitinib 15 mg (n = 164) | Upadacitinib 30 mg (n = 165) |
|---|---|---|---|
| Age (y), mean ± SD | 57.6 ± 11.4 | 56.3 ± 11.3 | 57.3 ± 11.6 |
| Female, n (%) | 143 (84.6) | 137 (83.5) | 138 (83.6) |
| Race, n (%) | |||
| White | 143 (84.6) | 142 (86.6) | 148 (89.7) |
| Black | 21 (12.4) | 17 (10.4) | 10 (6.1) |
| Asian | 5 (3.0) | 2 (1.2) | 2 (1.2) |
| Other | 0 (0) | 3 (1.8) | 5 (3.0) |
| BMI (kg/m2), mean ± SD | 29.7 ± 7.4 | 31.2 ± 7.3 | 29.7 ± 6.2 |
| Duration of RA (y), mean ± SD | 14.5 ± 9.2 | 12.4 ± 9.4 | 12.7 ± 9.7 |
| Failed ≥ 1 anti-TNF, n (%) | 152 (89.9) | 146 (89.0) | 151 (92.1) |
| Failed ≥ 1 bDMARD due to lack of efficacy, n (%) | 159 (94.1) | 146 (89.0) | 139 (84.8) |
bDMARD biologic disease-modifying antirheumatic drug, RA rheumatoid arthritis, SD standard deviation, TNF tumor necrosis factor, y years
Table 2.
Baseline values and LSM changes from baseline at week 12
| PRO | Baseline value, mean ± SD | LSM change from baseline (95% CI) | ||||
|---|---|---|---|---|---|---|
| Placebo | Upadacitinib 15 mg | Upadacitinib 30 mg | Placebo | Upadacitinib 15 mg | Upadacitinib 30 mg | |
| PtGA | 66.3 ± 22.7 (n = 166) | 67.2 ± 19.6 (n = 163) | 64.7 ± 21.1 (n = 163) | − 10.03 (− 14.22, − 5.84) (n = 145) | − 26.04* (− 30.16, − 21.93) (n = 156) | − 29.27* (− 33.43, − 25.12) (n = 147) |
| Pain VAS | 68.9 ± 21.0 (n = 166) | 68.2 ± 19.8 (n = 163) | 65.3 ± 20.7 (n = 161) | − 10.38 (− 14.60, − 6.16) (n = 145) | − 25.91* (− 30.05, − 21.76) (n = 156) | − 30.92* (− 35.12, − 26.72) (n = 146) |
| HAQ-DI | 1.6 ± 0.6 (n = 166) | 1.7 ± 0.6 (n = 163) | 1.6 ± 0.6 (n = 161) | − 0.16 (− 0.25, − 0.08) (n = 145) | − 0.41* (− 0.50, − 0.33) (n = 156) | − 0.44* (− 0.52, − 0.35) (n = 146) |
| SF-36 PCS | 31.6 ± 7.2 (n = 166) | 30.6 ± 7.8 (n = 163) | 31.5 ± 7.3 (n = 162) | 2.39 (1.14, 3.64) (n = 145) | 5.83* (4.60, 7.05) (n = 156) | 7.02* (5.78, 8.25) (n = 147) |
| SF-36 MCS | 45.9 ± 12.6 (n = 166) | 44.0 ± 11.7 (n = 163) | 45.9 ± 12.3 (n = 162) | 3.01 (1.65, 4.37) (n = 145) | 4.54 (3.22, 5.87) (n = 156) | 3.37 (2.03, 4.72) (n = 147) |
| SF-36 PF | 32.0 ± 8.9 (n = 166) | 30.6 ± 9.3 (n = 163) | 31.2 ± 8.1 (n = 162) | 1.54 (0.25, 2.84) (n = 145) | 4.56* (3.29, 5.83) (n = 156) | 6.15* (4.87, 7.43) (n = 147) |
| SF-36 RP | 34.2 ± 8.0 (n = 166) | 33.0 ± 8.8 (n = 163) | 34.9 ± 7.6 (n = 162) | 2.15 (0.95, 3.35) (n = 145) | 5.10* (3.93, 6.27) (n = 156) | 5.17* (3.99, 6.36) (n = 147) |
| SF-36 BP | 33.2 (6.4) (n = 166) | 33.1 (6.4) (n = 163) | 34.5 (6.9) (n = 162) | 4.71 (3.38, 6.04) (n = 145) | 8.12* (6.82, 9.42) (n = 156) | 9.09* (7.78, 10.41) (n = 147) |
| SF-36 GH | 38.9 ± 9.7 (n = 166) | 37.1 ± 8.2 (n = 163) | 38.1 ± 9.1 (n = 162) | 2.17 (1.01, 3.34) (n = 145) | 4.31** (3.17, 5.45) (n = 156) | 4.25*** (3.10, 5.41) (n = 147) |
| SF-36 VT | 40.6 ± 9.3 (n = 166) | 37.7 ± 10.0 (n = 163) | 39.2 ± 9.4 (n = 162) | 3.10 (1.69, 4.51) (n = 145) | 6.28* (4.90, 7.65) (n = 156) | 6.86* (5.47, 8.26) (n = 147) |
| SF-36 SF | 39.5 ± 10.9 (n = 166) | 37.3 ± 10.4 (n = 163) | 39.1 ± 10.8 (n = 162) | 3.45 (2.10, 4.81) (n = 145) | 5.87*** (4.55, 7.19) (n = 156) | 5.35*** (4.01, 6.69) (n = 147) |
| SF-36 RE |
41.0 ± 12.6 (n = 166) |
39.2 ± 12.7 (n = 163) | 41.5 ± 12.8 (n = 162) | 2.66 (1.24, 4.07) (n = 145) | 4.72*** (3.34, 6.10) (n = 156) | 3.78 (2.38, 5.17) (n = 147) |
| SF-36 MH |
43.6 ± 12.5 (n = 166) |
42.9 ± 11.3 (n = 163) | 44.2 ± 11.4 (n = 162) | 2.96 (1.63, 4.29) (n = 145) | 3.83 (2.53, 5.12) (n = 156) | 3.53 (2.21, 4.84) (n = 147) |
| AM stiffness duration, minutes |
138.4 ± 178.6 (n = 169) |
140.4 ± 189.7 (n = 164) |
184.5 ± 284.9 (n = 165) |
− 15.07 (− 43.30, 13.16) (n = 147) | − 81.47* (− 109.52, − 53.42) (n = 157) | − 79.13* (− 107.26, − 51.00) (n = 148) |
| AM stiffness severity | 6.8 ± 2.3 (n = 169) | 6.8 ± 2.1 (n = 164) | 6.5 ± 2.2 (n = 165) | − 1.57 (− 1.98, − 1.17) (n = 146) | − 2.86* (− 3.26, − 2.46) (n = 157) | − 3.22* (− 3.62, − 2.82) (n = 147) |
| ISI | 12.3 ± 6.9 (n = 153) | 12.3 ± 6.9 (n = 152) | 12.3 ± 6.6 (n = 151) | − 1.69 (− 2.55, − 0.83) (n = 130) | − 2.53 (− 3.36, − 1.70) (n = 142) | − 3.32** (− 4.15, − 2.49) (n = 138) |
| EQ-5D-5L | 49.7 ± 24.9 (n = 166) | 50.7 ± 23.0 (n = 163) | 51.8 ± 21.8 (n = 160) | 7.45 (3.86, 11.04) (n = 145) | 15.03** (11.55, 18.51) (n = 156) | 15.12** (11.55, 18.69) (n = 145) |
AM morning, BP bodily pain, CI confidence interval, EQ-5D-5L Euro Qol 5-Dimension 5-Level Questionnaire, GH general health, HAQ-DI Health Assessment Questionnaire Disability Index, LSM least squares mean, MCS Mental Component Summary, MH mental health, PCS Physical Component Summary, PF physical functioning, PRO patient-reported outcome, PtGA Patient Global Assessment of Disease Activity, RE role-emotional, RP role-physical, SD standard deviation, SF social functioning, SF-36 Short Form-36 Health Survey, VAS visual analog scale, VT vitality
*P ≤ 0.001 for upadacitinib vs placebo
**P < 0.01 for upadacitinib vs placebo
***P < 0.05 for upadacitinib vs placebo
Fig. 1.
BL and week 12 scores across SF-36 domains relative to age- and gender-adjusted (A/G) norms for the general US population. a Placebo. b UPA 15 mg. c UPA 30 mg. d Combined. All scores were based on a scale of 0 to 100, where 0 is the worst and 100 is the best. No further transformations were applied. *P < 0.05 for UPA 15 mg vs PBO. **P < 0.05 for UPA 30 mg vs PBO. BL, baseline; BP, bodily pain; GH, general health; MH, mental health; PBO, placebo; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, Short Form-36 Health Survey; UPA, upadacitinib; VT, vitality; Wk, week
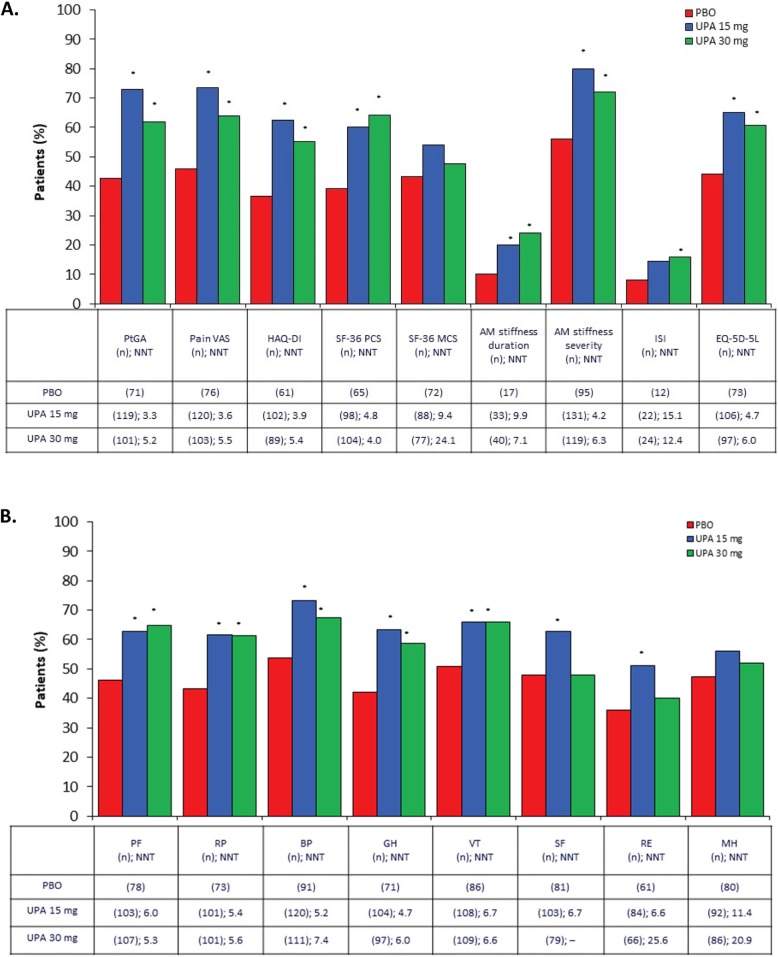
Compared with placebo, statistically significant improvements at week 12 were evident with both upadacitinib 15 mg and 30 mg for PtGA, pain VAS, HAQ-DI, PCS, and AM stiffness (all P ≤ 0.001, Table 2). Duration of AM stiffness was reduced from baseline by 43% and 58% in the upadacitinib 15 mg and 30 mg groups, respectively, versus 11% in the placebo group, and 72% and 80% of patients receiving upadacitinib 15 mg and 30 mg, respectively, versus 52% of patients receiving placebo reported a reduction in severity greater than or equal to MID. MCS baseline values were close to normative values, and although changes from baseline were numerically greater with upadacitinib 15 mg and 30 mg (4.54 and 3.37, respectively) compared with placebo (3.01), they were not statistically significant (P = 0.52). Changes from baseline in SF-36 domain scores with upadacitinib 15 mg exceeded placebo across all eight domains and were statistically significant except in the SF-36 mental health domain. For upadacitinib 30 mg, changes from baseline were statistically significant across all domains except mental health and role-emotional. All mean improvements for upadacitinib were clinically meaningful (Fig. 2). NNTs for upadacitinib 15 mg versus placebo ranged from 3 to 4 for PtGA, pain VAS, and HAQ-DI; 4 to 5 for PCS and AM stiffness severity; and 5 to 7 for seven of eight SF-36 domains. Similar results were reported with upadacitinib 30 mg.
Fig. 2.
Patients reporting improvements ≥ MCID at week 12 across PROs. a Results from multiple patient health-related quality of life assessments. b Results from the SF-36 subdomains. SF-36 domains were rescored from 0 to 100, where 0 is the worst and 100 is the best. No further transformations were applied. *P < 0.05 for UPA vs PBO. AM, morning; BP, bodily pain; EQ-5D-5L, Euro Qol 5-Dimension 5-Level Questionnaire; GH, general health; HAQ-DI, Health Assessment Questionnaire Disability Index; ISI, Insomnia Severity Index; MCID, minimum clinically important difference; MCS, Mental Component Summary; MH, mental health; NNT, number needed to treat; PBO, placebo; PCS, Physical Component Summary; PF, physical functioning; PRO, patient-reported outcome; PtGA, Patient Global Assessment of Disease Activity; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, Short Form-36 Health Survey; UPA, upadacitinib; VAS, visual analog scale; VT, vitality
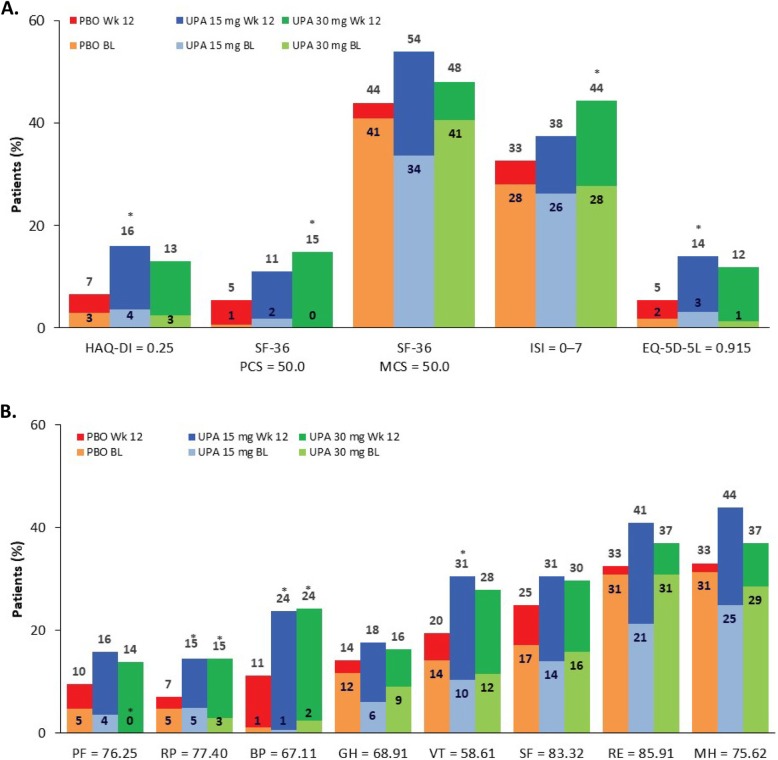
In both upadacitinib groups, median time to response was 2 weeks for pain VAS and HAQ-DI compared with 4 weeks for these scores in the placebo group. Time to response for severity of AM stiffness was 1 week for both upadacitinib cohorts versus 2 weeks for the placebo cohort. At week 12, a significantly greater percentage of patients in the upadacitinib 15 mg group versus the placebo group reported scores greater than or equal to normative values in PtGA (28% vs 15%); HAQ-DI (16% vs 7%); the role-physical (15% vs 7%), bodily pain (24% vs 11%), and vitality (31% vs 20%) domains; and EQ-5D-5L (14% vs 5%) (all P < 0.05). Likewise, upadacitinib 30 mg had significantly more patients report scores greater than or equal to normative values compared with placebo in PtGA (36% vs 15%), PCS (15% vs 5%), role-physical (15% vs 7%) and bodily pain (24% vs 11%) domains, and ISI (44% vs 33%) (all P < 0.05, Fig. 3).
Fig. 3.
Patients reporting scores ≥ normative values. a Select PROs at baseline and week 12. b Short Form 36 (SF-36) domains. SF-36 domains were rescored from 0 to 100, where 0 is the worst and 100 is the best. No further transformations were applied. *P < 0.05 for UPA vs PBO. BL, baseline; BP, bodily pain; EQ-5D-5L, Euro Qol 5-Dimension 5-Level Questionnaire; GH, general health; HAQ-DI, Health Assessment Questionnaire Disability Index; ISI, Insomnia Severity Index; MCS, Mental Component Summary; MH, mental health; PBO, placebo; PCS, Physical Component Summary; PF, physical functioning; PRO, patient-reported outcome; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, Short Form-36 Health Survey; UPA, upadacitinib; VT, vitality; Wk, week
Discussion
Although there are a number of first-line therapies that improve RA outcomes, these same therapies may not be efficacious as second- or third-line therapy [19]. Additionally, not only is it essential to demonstrate that new treatments reduce the signs and symptoms of active RA in patients with refractory disease, but it is also necessary to show that these new treatments improve HRQOL outcomes from the patient’s perspective. We analyzed data collected on several measures during SELECT-BEYOND to gain insight into the benefits of upadacitinib on HRQOL in patients with treatment-refractory RA. Upadacitinib treatment resulted in rapid and clinically meaningful improvements in outcomes of importance to patients with refractory disease: disease activity, pain, physical function, and AM stiffness, even in a difficult to treat population. Upadacitinib 15 mg and 30 mg had an equally effective impact on most of the PROs in this bDMARD-IR RA population. Upadacitinib-treated patients reported a substantial reduction in duration of AM stiffness and a significant decrease in severity as early as week 1. Reduced sleep quality is common among patients with RA and is associated with disease activity, pain, and functional disability [43, 44]. Consistent with this finding, upadacitinib-treated patients with improvements in PtGA, pain VAS, and HAQ-DI scores also reported significant improvement in ISI scores. These clinically relevant improvements in PROs are consistent with the positive efficacy findings reported in this csDMARD-IR population [25, 45] and suggest that upadacitinib may be an important treatment option in patients with active and refractory RA.
Although a number of studies have shown that patients with RA who fail to respond to one anti-TNF agent may benefit from treatment with a second or third anti-TNF agent [46–51], the response is likely to decline as the number of anti-TNF agents increases [52]. Less information is available for the more recently approved JAK inhibitors and interleukin-6 receptor antagonist. In ORAL-STEP [53], a phase 3 RCT of tofacitinib in bDMARD-IR patients, the percentage of tofacitinib-treated (5 mg) patients reporting improvements ≥ MCID was less than in upadacitinib-treated (15 mg) patients in PtGA (65% vs 73%) and pain (69% vs 74%), similar in HAQ-DI (61% vs 63%) and SF-36 MCS (54% each), and greater in SF-36 PCS (68% vs 60%). Different results were observed in RA-BEACON [54], a phase 3 RCT of baricitinib in bDMARD-IR patients, where fewer baricitinib-treated (2 mg) patients reported improvements ≥ MCID than those treated with upadacitinib (15 mg) in SF-36 PCS (49% vs 60%) and MCS (33% vs 54%). In TARGET [55], a phase 3 RCT of sarilumab in bDMARD-IR patients, fewer sarilumab-treated (200 mg) patients reported improvements ≥ MCID than those treated with upadacitinib in PtGA (25% vs 73%), pain (26% vs 74%), HAQ-DI (21% vs 63%), SF-36 PCS (21% vs 60%), and SF-36 MCS (17% vs 54%). Treatment with upadacitinib, baricitinib [54], or sarilumab [55] significantly improved AM stiffness compared with placebo; however, it is difficult to compare results across studies because AM stiffness was assessed differently in these studies. BEYOND reported mean change in duration, RA-Beacon reported median change in duration, and TARGET reported change on a VAS scale. Overall, upadacitinib showed improvements in PROs that were better or comparable to those seen with the IL-6 receptor antagonist or other JAK inhibitors [53–55].
There are several strengths in these analyses. Data were collected during a phase 3 RCT, which ensures patients are closely followed for an extended period and PROs are consistently measured. The randomized and blinded study design mitigates biases that may arise due to differences between treatment groups without knowledge of treatment allocation. The blinded study design also allows for unbiased reporting from a patient’s perspective. The validated PROs used in these analyses evaluate different aspects of the patient’s experience which may shed light on how patients perceive the effects of upadacitinib on a wide range of typical RA-related impediments. Use of MCID and normative values to measure responses makes these data clinically meaningful and interpretable for patients.
There are also limitations in these results. PROs were collected at fixed visits, and therefore, response could not be assessed at time intervals between visits. Because the duration of treatment was relatively short (12 weeks), additional studies are needed to determine if the improvements observed with upadacitinib treatment are maintained long-term. Furthermore, results may not be generalizable to all patients with RA, as clinical trial participants are selected based on specific inclusion/exclusion criteria and may differ from the broader RA patient population in clinical practice. The patients were bDMARD treatment failures, and results could be different in other scenarios. Lastly, the method used to impute missing data (NRI) assumes that missing PRO scores are associated with non-response, which may underestimate the true rate of response.
Conclusion
In conclusion, over 12 weeks, results from the SELECT-BEYOND trial demonstrated that in difficult-to-treat bDMARD-IR patients with active RA, treatment with upadacitinib compared with placebo resulted in significantly more patients with clinically meaningful improvements in PROs and responses that approached normative values. Furthermore, the NNTs to achieve clinically meaningful responses were ≤ 10, which are generally considered favorable [56]. Upadacitinib may be a treatment option for bDMARD-IR patients with RA providing clinically significant relief from symptoms that impair HRQOL.
Acknowledgements
Medical writing assistance was provided by Tonya Goodman of JK Associates, Inc., a member of the Fishawack Group of Companies, Conshohocken, PA, and was funded by AbbVie.
Abbreviations
- ACR20
American College of Rheumatology 20% improvement
- A/G
Age- and gender-matched
- AM
Morning
- bDMARD
Biologic disease-modifying antirheumatic drug
- csDMARD
Conventional synthetic disease modifying antirheumatic drug
- EQ-5D-5L
Euro Qol 5-Dimension 5-Level Questionnaire
- HAQ-DI
Health Assessment Questionnaire Disability Index
- HRQOL
Health-related quality of life
- IR
Inadequate responder
- ISI
Insomnia Severity Index
- JAK
Janus kinase
- LSM
Least squares mean
- MCID
Minimum clinically important differences
- MCS
Mental Component Summary
- MTX
Methotrexate
- NNT
Number needed to treat
- NRI
Non-responder imputation
- PCS
Physical Component Summary
- PRO
Patient-reported outcome
- PtGA
Patient Global Assessment of Disease Activity
- RA
Rheumatoid arthritis
- RCT
Randomized controlled trial
- SF-6D
Short-Form Six Dimension
- SF-36
Short Form-36 Health Survey
- tsDMARD
Targeted synthetic biologic disease-modifying antirheumatic drug
- VAS
Visual analog scale
Authors’ contributions
All authors contributed to the conception and design, acquisition of the data, analysis and interpretation of the data, and drafting of the manuscript. All authors revised the manuscript for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the abstract. All authors participated in the interpretation of data, critical review of the drafts, and development of the publication, and maintained control over the final content.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by independent ethics committees or institutional review boards at each study site and conducted in accordance with ethical principles outlined in the current Declaration of Helsinki and consistent with International Conference on Harmonisation Good Clinical Practice and Good Epidemiology Practices, along with all applicable local regulatory requirements. All patient data were deidentified and complied with patient confidentiality requirements.
Consent for publication
Not applicable.
Competing interests
VS is a consultant for AbbVie, Amgen, AstraZeneca, BMS, Celgene, Genentech, GSK, Janssen, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, and UCB, and is involved in advisory boards for AbbVie, Amgen, AstraZeneca, BMS, Celgene, Genentech, GSK, Janssen, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi, and UCB.
MS is a consultant for AbbVie, BMS, Eli Lilly, JNJ, and UCB, and is a member of the speaker bureau for AbbVie and BMS.
JP is a consultant for AbbVie, Amgen, BMS, Celltrion, GSK, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, Sandoz, and UCB.
NT, AF, SM, AP, and MF are employees of AbbVie and may own AbbVie stock or stock options.
AG is a former employee of AbbVie and may own AbbVie stock or stock options.
YS is an employee of Analysis Group Inc., which received consulting fees from AbbVie for this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vibeke Strand, Email: vstrand@stanford.edu.
Michael Schiff, Email: michael.schiff@me.com.
Namita Tundia, Email: namita.tundia@abbvie.com.
Alan Friedman, Email: alan.friedman@abbvie.com.
Sebastian Meerwein, Email: sebastian.meerwein@abbvie.com.
Aileen Pangan, Email: aileen.pangan@abbvie.com.
Arijit Ganguli, Email: arijit.ganguli@abbvie.com.
Mahesh Fuldeore, Email: mahesh.fuldeore@abbvie.com.
Yan Song, Email: yan.song@analysisgroup.com.
Janet Pope, Email: janet.pope@sjhc.london.on.ca.
References
- 1.Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther. 2014;16:R56. doi: 10.1186/ar4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol. 2006;33:1942–1951. [PubMed] [Google Scholar]
- 4.Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 2010;70:121–145. doi: 10.2165/11531980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Matcham F, Scott IC, Rayner L. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:123–130. doi: 10.1016/j.semarthrit.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson T, Morris M, Calnan M, Richards P, Hewlett S. What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res. 2010;62:640–646. doi: 10.1002/acr.20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokka T, Kautiainen H, Pincus T. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12:R42. doi: 10.1186/ar2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halls S, Dures E, Kirwan J, Pollock J, Baker G, Edmunds A, Hewlett S. Stiffness is more than just duration and severity: a qualitative exploration in people with rheumatoid arthritis. Rheumatology (Oxford) 2015;54(4):615–622. doi: 10.1093/rheumatology/keu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drewes AM, Svendsen L, Taagholt SJ. Sleep in rheumatoid arthritis: a comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol. 1998;37:71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Roehrs T, Diederichs C, Gillis M. Nocturnal sleep, daytime sleepiness, and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med. 2013;14:109–115. doi: 10.1016/j.sleep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Crosby LJ. Factors which contribute to fatigue associated with rheumatoid arthritis. J Adv Nurs. 1991;16:974–981. doi: 10.1111/j.1365-2648.1991.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy S, Creed F, Jayson MI. Psychiatric disorder and illness behaviour in rheumatoid arthritis. Rheumatology. 1988;27:357–363. doi: 10.1093/rheumatology/27.5.357. [DOI] [PubMed] [Google Scholar]
- 13.Frank RG, Beck NC, Parker JC. Depression in rheumatoid arthritis. J Rheumatol. 1988;15:920–925. [PubMed] [Google Scholar]
- 14.Goes ACJ, Reis LAB, Silva MBG. Rheumatoid arthritis and sleep quality. Rev Bras Reumatol Engl Ed. 2017;57(4):294–298. doi: 10.1016/j.rbr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Druce KL, Cordingley L, Short V, Moore S, Hellman B, James B, Lunt M. Quality of life, sleep and rheumatoid arthritis (QUASAR): a protocol for a prospective UK mHealth study to investigate the relationship between sleep and quality of life in adults with rheumatoid arthritis. BMJ Open. 2018;8:e018752. doi: 10.1136/bmjopen-2017-018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings Y, Dixon WG, Hyrich KL, Askling J, Gossec L. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016;18(1):251. doi: 10.1186/s13075-016-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orbai AM, Bingham CO. Patient reported outcomes in rheumatoid arthritis clinical trials. Curr Rheumatol Rep. 2015;17(4):28. doi: 10.1007/s11926-015-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis--progress at OMERACT 7. J Rheumatol. 2005;32(11):2250–2256. [PubMed] [Google Scholar]
- 19.Kearsley-Fleet L, Davies R, De Cock D. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77:1405–1412. doi: 10.1136/annrheumdis-2018-213378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Hair MJH, Jacobs JWG, Schoneveld JLM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatol (Oxford) 2018;57:1135–1144. doi: 10.1093/rheumatology/kex349. [DOI] [PubMed] [Google Scholar]
- 21.Polido-Pereira J, Vieira-Sousa E, Fonseca JE. Rheumatoid arthritis: what is refractory disease and how to manage it? Autoimmun Rev. 2011;10:707–713. doi: 10.1016/j.autrev.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 23.Klünder B, Mohamed MF, Othman AA. Population pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I and II clinical trials. Clin Pharmacokinet. 2018;57(8):977–988. doi: 10.1007/s40262-017-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese MC, Smolen JS, Weinblatt ME, Burmester GR, Meerwein S, Camp HS, Wang L, Othman AA, Khan N, Pangan AL, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–2866. doi: 10.1002/art.39808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, Zhou Y, Mohamed ME, Meerwein S, Pangan AL. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 26.Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, Khan N, Pangan AL, Jungerwirth S, Keystone EC. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12):2867–2877. doi: 10.1002/art.39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruce B, Fries J. The health assessment questionnaire (HAQ) Clin Exp Rheumatol. 2005;23(Suppl 39):S14–S18. [PubMed] [Google Scholar]
- 28.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S383–S412. doi: 10.1002/acr.20541. [DOI] [PubMed] [Google Scholar]
- 30.Strand V, Boers M, Idzerda L, Kirwan JR, Kvien TK, Tugwell PS, Dougados M. It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol. 2011;38(8):1720–1727. doi: 10.3899/jrheum.110392. [DOI] [PubMed] [Google Scholar]
- 31.Orbai AM, Smith KC, Bartlett SJ, De Leon E, Bingham CO. “Stiffness has different meanings, I think, to everyone”: examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(11):1662–1672. doi: 10.1002/acr.22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang KI, Grigg-Damberger M, Andrews N, O'Rourke C, Bena J, Foldvary-Schaefer N. Severity of self-reported insomnia in adults with epilepsy is related to comorbid medical disorders and depressive symptoms. Epilepsy Behav. 2016;60:27–32. doi: 10.1016/j.yebeh.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 35.Marra CA, Woolcott JC, Kopec JA, Shojania K, Offer R, Brazier JE, Esdaile JM, Anis AH. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med. 2005;60(7):1571–1582. doi: 10.1016/j.socscimed.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):260–265. doi: 10.1136/ard.2007.069690. [DOI] [PubMed] [Google Scholar]
- 37.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S14–S36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004;50(3):953–960. doi: 10.1002/art.20048. [DOI] [PubMed] [Google Scholar]
- 39.Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, Koetse W, Purcaru O, Bennett B, Burkhardt H. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomized, placebo-controlled trial. Ann Rheum Dis. 2015;74:843–850. doi: 10.1136/annrheumdis-2013-204632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz A, Kohlmann T, Stobel-Richter Y, Zeuger M, Brahler E. The quality of life questionnaire EQ-5D-5L: psychometric properties and normative values for the general German population. Qual Life Res. 2014;23:443–447. doi: 10.1007/s11136-013-0498-2. [DOI] [PubMed] [Google Scholar]
- 41.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 42.Strand V, Crawford B, Singh J, Choy E, Smolen JS, Khanna D. Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis. 2009;68(12):1800–1804. doi: 10.1136/ard.2009.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austad C, Kvien TK, Olsen IC, Uhlig T. Sleep disturbance in patients with rheumatoid arthritis is related to fatigue, disease activity, and other patient-reported outcomes. Scand J Rheumatol. 2017;46(2):95–103. doi: 10.3109/03009742.2016.1168482. [DOI] [PubMed] [Google Scholar]
- 44.Sariyildiz MA, Batmaz I, Bozkurt M, Bez Y, Cetincakmak MG, Yazmalar L, Ucar D, Celepkolu T. Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res. 2014;6(1):44–52. doi: 10.4021/jocmr1648w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burmester GR, Kremer JM, van den Bosch F, Kivitz A. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 46.Atzeni F, Sarzi-Puttini P, Gorla R, Marchesoni A, Caporali R. Switching rheumatoid arthritis treatments: an update. Autoimmun Rev. 2011;10(7):397–403. doi: 10.1016/j.autrev.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Alivernini S, Laria A, Gremese E, Zoli A, Ferraccioli G. ACR70-disease activity score remission achievement from switches between all the available biological agents in rheumatoid arthritis: a systematic review of the literature. Arthritis Res Ther. 2009;11(6):R163. doi: 10.1186/ar2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scrivo R, Conti F, Spinelli FR, Truglia S, Magrini L, Di Franco M, Ceccarelli F, Valesini G. Switching between TNFalpha antagonists in rheumatoid arthritis: personal experience and review of the literature. Reumatismo. 2009;61(2):107–117. doi: 10.4081/reumatismo.2009.107. [DOI] [PubMed] [Google Scholar]
- 49.Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi: 10.1002/art.22331. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Vollenhoven RF. Switching between biological agents. Clin Exp Rheumatol. 2004;22(5 Suppl 35):S115–S121. [PubMed] [Google Scholar]
- 52.Rendas-Baum R, Wallenstein GV, Koncz T, Kosinski M, Yang M, Bradley J, Zwillich SH. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res Ther. 2011;13(1):R25. doi: 10.1186/ar3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strand V, Burmester GR, Zerbini CA, Mebus CA, Zwillich SH, Gruben D, Wallenstein GV. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res (Hoboken) 2015;67(4):475–483. doi: 10.1002/acr.22453. [DOI] [PubMed] [Google Scholar]
- 54.Smolen JS, Kremer JM, Gaich CL, DeLozier AM, Schlichting DE, Xie L, Stoykov I, Rooney T, Bird P, Sanchez Burson JM, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON) Ann Rheum Dis. 2017;76:694–700. doi: 10.1136/annrheumdis-2016-209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strand V, Reaney M, Chen CI, Proudfoot CW, Guillonneau S, Bauer D, Mangan E, Graham NM, van Hoogstraten H, Lin Y, et al. Sarilumab improves patient-reported outcomes in rheumatoid arthritis patients with inadequate response/intolerance to tumour necrosis factor inhibitors. RMD Open. 2017;3(1):e000416. doi: 10.1136/rmdopen-2016-000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siwek J, Newman DH. Introducing medicine by the numbers: a collaboration of the NNT Group and AFP. Am Fam Physician. 2015;91:434–435. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.