Abstract
Fungal infections, ranging from superficial to life-threatening infections, represent a major public health problem that affects 25% of the worldwide population. In this context, the study of host-pathogen interactions within the host is crucial to advance antifungal therapy. However, since fungal cells are usually outnumbered by host cells, the fungal transcriptome frequently remains uncovered. We compared three different methods to selectively lyse human cells from in vitro mixes, composed of Candida cells and peripheral blood mononuclear cells. In order to prevent transcriptional modification, the mixes were stored in RNAlater. We evaluated the enrichment of fungal cells through cell counting using microscopy and aimed to further enrich fungal nucleic acids by centrifugation and by reducing contaminant nucleic acids from the host. We verified the enrichment of fungal DNA and RNA through qPCR and RT-qPCR respectively and confirmed that the resulting RNA has high integrity scores, suitable for downstream applications. The enrichment method provided here, i.e., lysis with Buffer RLT followed by centrifugation, may contribute to increase the proportion of nucleic acids from fungi in clinical samples, thus promoting more comprehensive analysis of fungal transcriptional profiles. Although we focused on C. albicans, the enrichment may be applicable to other fungal pathogens.
Subject terms: Microbiology, Fungi
Introduction
Superficial fungal infections represent a major public health problem worldwide, affecting 25% of the population. These infections are caused mainly by several species of dermatophytes of which Trichophyton rubrum is the most common1. Though superficial fungal infections are rarely life-threatening, they generally require very long and tedious antifungal treatments2. Mucosal infections such as vulvovaginal candidiasis are also very common, affecting millions of women every year. In fact, it is the second most common type of vaginal infection after bacterial vaginosis3. This infection develops into recurrent vulvovaginal candidiasis in many patients (5–8%) which impacts directly on their social lives and can be fatal in immunocompromised individuals4,5. Systemic, severe and life-threatening fungal infections, have also a significant impact on public health with cryptococcosis, aspergillosis and candidiasis as main diseases, with candidiasis as the most common of them6. They have a high rate of mortality, reaching 46% in candidaemia cases in Europe7. Candidiasis is mainly caused by C. albicans, although other Candida species have emerged during the last years, such as C. glabrata, C. parapsilosis and C. tropicalis, and very recently the multidrug-resistant pathogen C. auris has been involved in several outbreaks8,9.
Given the clinical relevance of fungal infections, there have been efforts to elucidate host-pathogen interactions in recent years, involving candidiasis10–12, aspergillosis13–16 and cryptococcosis17,18. However, most of these studies were focused on the transcriptome of the host. Transcriptomic studies which identify comprehensively differently expressed genes from the fungus are predominantly limited to in vitro experiments in which cells are cultured19 or animal models infected with a high inoculum of the pathogen20, which is usually not representative for the low amount of fungal cells encountered in the host. Although in vitro experiments contribute to improve our knowledge regarding the mechanisms of fungal pathogenicity and the immune response of the host, they reflect within-host interactions only to a limited extent, because these are further influenced by signalling pathway networks involving different metabolites, cell types and other microorganisms that are present in the ecosystem of the host. The use of a high burden of the pathogen in animal studies counterbalances the proportion of the fungus that is present in clinical samples. In fact, RNA content from the fungal pathogen was recently found to constitute only about 0.1% of total RNA21. Therefore, for true host-pathogen interaction studies, carried out on clinical samples, there is a need to enrich fungal RNA to obtain a more comprehensive analysis of transcriptional profiles from the pathogen side. To our knowledge, there are only three studies that have attempted to solve this question and developed different enrichment methods. Amorim-Vaz et al.21 used a large set of Candida-specific probes to selectively enrich fungal mRNAs, in combination with subsequent RNA-seq, Andes et al.22 developed a simple approach to enrich fungal RNA through the lysis of human cells with Triton X-100 and Hebecker et al.23 used buffer RLT (Qiagen) with a homogenizer to lyse murine cells, although none of these studies started from RNAlater.
In this paper, we compared three different methods to enrich fungal nucleic acids from in vitro mixes composed of different amounts of Candida cells and peripheral blood mononuclear cells (PBMCs) that had been stored in RNAlater in order to halt transcription. We evaluated the efficiency of the enrichment through cell counting, quantitative PCR (qPCR) and quantitative reverse transcription PCR (RT-qPCR). Finally, we checked RNA quality of fungal RNA obtained after the enrichment.
Results
Brief outline of the study
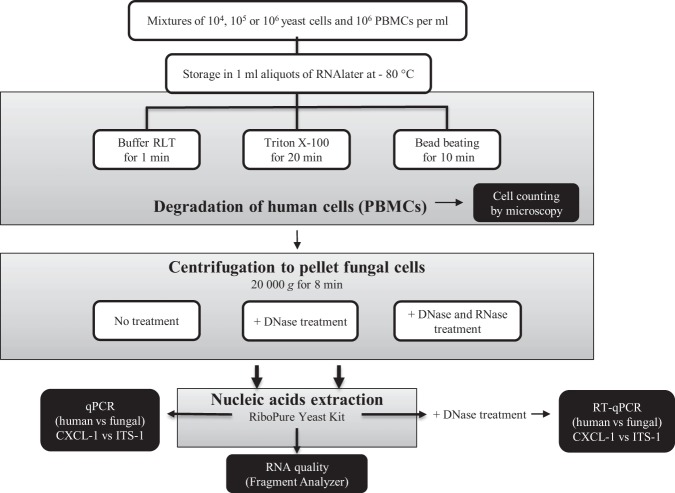
Figure 1 summarizes the plan of the study. Three different methods to enrich fungal cells and further enrich nucleic acids from pathogenic fungi were compared. First, we prepared aliquots of mixes composed of 106 yeast cells/ml and 106 PBMCs/ml, and stored these mixtures in aliquots of 1 ml in RNAlater at −80 °C.
Figure 1.
A schematic overview of the study set up.
For the three different lysis treatments, we determined the number of yeast cells and PBMCs that survived the treatment by means of microscopy.
Subsequently, we also investigated whether fungal nucleic acids could be further enriched by centrifugation to precipitate intact yeast cells and to reduce human DNA and RNA from the lysed PBMCs, and we quantified DNA and RNA by qPCR and RT-qPCR respectively, and analysed RNA integrity with a Fragment Analyzer.
Finally, we determined the efficacy of these enrichment procedures for mixtures containing yeast cells reduced 10- and 100-fold relative to the human counterpart.
Enrichment of fungal cells through lysis of human cells
Unlike fungal cells, human cells do not have a protective cell wall to deal with adverse conditions. This distinction can be used to enrich fungal cells through the differential lysis of human cells, which can potentially constitute the first step to enrich nucleic acids from the pathogenic fungus in a clinical sample. Here, we tested the effect of three different methods, all at room temperature, to lyse human cells: i) chemically with Triton X-100 for 20 min and ii) with Buffer RLT supplemented with 1% β-mercaptoethanol (i.e., 143 mM) for at least 1 min (in house protocol), and iii) mechanically by bead beating with 0.5 mm zirconium beads in saline for 10 min. Results are depicted in Fig. 2. Using microscopy, we observed that the number of yeast cells remained invariable among all three methods considered in the study.
Figure 2.
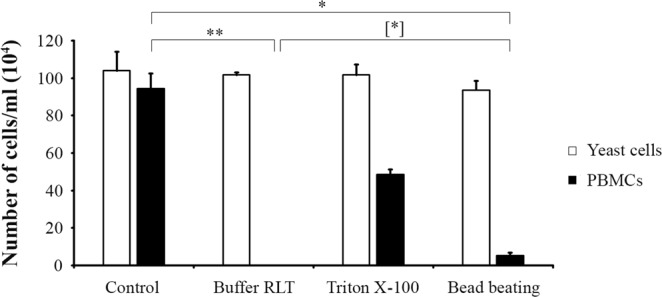
Number of yeast cells and PBMCs per ml determined by a haemocytometer, at magnification of 400x, after different cell lysis methods. Error bars represent the standard deviations of results from six biological replicates. Statistically significant differences are marked with one (p < 0.05) or two (p < 0.005) asterisks (Friedman test) and with one bracketed asterisk (p < 0.05) (Wilcoxon test).
For PBMCs, we observed that Buffer RLT completely lysed human cells (0 cells/ml) and that bead beating lysed most of them (5.3 × 104 residual cells/ml). This reduction was significant as compared to the control (9.4 × 105 cells/ml) (p < 0.05) and in addition, the differences between Buffer RLT and bead beating were significant as well. Triton X-100 did not effectively lyse human cells (4.9 × 105 residual cells/ml). Thus, these results indicate that Buffer RLT and bead beating selectively lyse human cells and therefore, these methods can be used to enrich fungal cells after RNAlater storage.
Treatment with Buffer RLT is the most efficient method to enrich fungal DNA
As we observed that Buffer RLT and bead beating in saline did reduce the number of human cells, while not affecting the number of yeast cells, we next questioned whether DNA from the fungal pathogen would be enriched as well. For this purpose, the mixes were treated with the three methods of cell lysis mentioned above, but followed by a centrifugation step (20 000 g, 8 min, room temperature), aiming at concentrating fungal cells and reducing nucleic acids and cell debris from human cells.
qPCR results of nucleic acids extracted with the RiboPure Yeast Kit are shown in Table 1 (human DNA and fungal DNA), targeting CXCL1 (human gene) and the internal transcribed spacer region 1 (ITS-1) DNA (yeast gene), respectively. Treatment with Buffer RLT followed by centrifugation induced a significant reduction of human DNA (p < 0.05), resulting in a Cq value of 35.9 which corresponds to only 0.09% human DNA as compared to the control (Cq value of 25.3) (Table 1). Enrichment with bead beating also reduced significantly human DNA (Cq value of 27.8) but a paired samples t-test showed that Buffer RLT is significantly more efficient (p < 0.001). Triton X-100 reduced human DNA to 63.8% (Cq value of 26.0), but this was not statistically significant. Additional incorporation of a DNase treatment to remove contaminating human DNA from samples treated with Buffer RLT did not further improve the enrichment, as Cq values of 36.21 and 36.50 were obtained before and after DNase treatment (see Supplementary Table S1). In addition, Table 1 shows that fungal DNA was not affected by any of the enrichment methods considered in this study. We concluded that bead beating and treatment with Buffer RLT enriched fungal cells as well as fungal DNA, by reducing the human DNA content of the mixes, with Buffer RLT treatment being the most efficient method.
Table 1.
Effect of different enrichment methods on PCR-based amplificationa of human DNA, fungal DNA, human RNA and fungal RNA.
| Human DNA | Cq valueb | SD | Log cells/mlc | CV | DNA %c | CV |
|---|---|---|---|---|---|---|
| No enrichment | 25.31 | 0.46 | 5.99 | 0.02 | 100.00 | 0.26 |
| Buffer RLT | 35.89** | 0.59 | 2.96 | 0.06 | 0.09 | 0.32 |
| Triton X-100 | 26.00 | 0.28 | 5.79 | 0.01 | 63.82 | 0.17 |
| Bead beating | 27.84* | 1.62 | 5.27 | 0.09 | 18.93 | 0.66 |
| Fungal DNA | Cq value | SD | Log cells/mlc | CV | DNA %c | CV |
| No enrichment | 23.20 | 0.22 | 6.05 | 0.01 | 100.00 | 0.13 |
| Buffer RLT | 23.50 | 0.47 | 5.97 | 0.02 | 83.08 | 0.25 |
| Triton X-100 | 23.87 | 0.66 | 5.87 | 0.03 | 66.03 | 0.34 |
| Bead beating | 23.60 | 1.62 | 5.94 | 0.07 | 77.91 | 0.63 |
| Human RNA | Cq valueb | SD | Log cells/mlc | CV | RNA %c | CV |
| No enrichment | 30.17 | 0.37 | 6.13 | 0.02 | 100.00 | 0.20 |
| Buffer RLT | 35.00** | 0.61 | 4.85 | 0.03 | 5.20 | 0.31 |
| Triton X-100 | 34.53** | 0.60 | 4.98 | 0.03 | 6.93 | 0.31 |
| Bead beating | 34.25** | 1.05 | 5.05 | 0.06 | 8.20 | 0.48 |
| Fungal RNA | Cq valueb | SD | Log cells/mlc | CV | RNA %c | CV |
| No enrichment | 20.25 | 0.48 | 5.93 | 0.03 | 100.00 | 0.29 |
| Buffer RLT | 20.97 | 0.22 | 5.71 | 0.01 | 59.94 | 0.14 |
| Triton X-100 | 37.70** | 0.77 | 0.52 | 0.46 | 0.00 | 0.42 |
| Bead beating | 34.02** | 0.82 | 1.66 | 0.15 | 0.01 | 0.44 |
aHuman nucleic acids amplified with CXCL-1 primers and fungal nucleic acids amplified with ITS-1 primers.
bCq values are means of six biological replicates composed of mixes of 106 PBMCs and 106 Candida cells. Statistically significant differences are marked with one (p < 0.005) or two asterisks (p < 0.001) (Linear mixed model).
cLog cells/ml, DNA percentage and RNA percentage were calculated by extrapolation of Cq values with a standard curve.
SD: Standard deviation. CV: Coefficient of variation.
Treatment with Buffer RLT additionally enriches fungal RNA
After testing the enrichment of cells and of DNA from the pathogenic fungus with three different methods, and with the addition of a centrifugation step, we evaluated whether RNA was also enriched together with DNA. Therefore, the nucleic acids obtained from the differently treated mixes after extraction by means of the RiboPure Yeast Kit, were further treated with DNase to obtain pure RNA, and quantitative reverse transcription PCR (RT-qPCR) was carried out. We observed that treatment with Buffer RLT, Triton X-100 and bead beating resulted in very low amounts of human RNA, i.e. 5.2, 6.9 and 8.2% respectively (Table 1). Double enzymatic pretreatment prior to the lysis of fungal cells, first with DNase and subsequently with RNase, to remove remaining human nucleic acids, did not further improve the RNA enrichment (Supplementary Table S1). Furthermore, Table 1 shows no significant differences between treatment with Buffer RLT and the untreated control for fungal ITS-1 RNA, whereas fungal RNA drastically decreased with both bead beating and Triton X-100 treatment, i.e. no residual RNA and 0.01% residual RNA, respectively, indicating that not only human but also fungal RNA was lost. These results suggest that only treatment with Buffer RLT can be used to enrich fungal RNA.
Enrichment with Buffer RLT in 1:10 and 1:100 ratios
After having shown that Buffer RLT enrichment can be used to enrich nucleic acids from the fungal pathogen in samples with equal numbers of fungal and human cells, through the lysis of human cells, the enrichment procedure was tested in Candida/PBMC ratios of 1:10 and 1:100. To this purpose, we prepared mixtures of 105 Candida cells/106 PBMCs per ml and 104 Candida cells/106 PMBCs per ml. Cq values after qPCR of nucleic acids extracts for human and fungal DNA and after RT-qPCR of human and fungal RNA are shown in Table 2. DNA from human cells was significantly reduced to 0.65% and 0.67% of the initial number of cells (p < 0.05) for the 105 and 104 yeast cells/ml mixtures, respectively, while DNA from yeasts was (not significantly) reduced to 68.6% and 53.1%. Accordingly, human RNA significantly dropped to 4.9% and 9.3% (p < 0.05), while fungal cDNA was not significantly reduced to 61.5% and 34.7% for the 105 and 104 yeast cells/ml mixtures. Together, these results demonstrate that enrichment with Buffer RLT enriches fungal nucleic acids from mixtures with human cells, also in cases when low proportions of fungal cells are present.
Table 2.
Effect of Buffer RLT treatment on DNA and RNA enrichment from yeast cells for mixtures composed of different ratios of PBMCs and Candida cells, as determined by qPCR-based amplificationa of human DNA and fungal DNA, and by RT-qPCR based amplificationa of human RNA and fungal RNA.
| Human DNA | Cq valueb | SD | Log cells/mlc | CV | DNA %c | CV |
|---|---|---|---|---|---|---|
| 106 PBMCs + 105 Candida cells | ||||||
| No enrichment | 24.39 | 0.67 | 6.08 | 0.03 | 100.00 | 0.34 |
| Buffer RLT | 32.52* | 0.55 | 3.89 | 0.04 | 0.65 | 0.29 |
| 106 PBMCs + 104 Candida cells | ||||||
| No enrichment | 24.87 | 0.13 | 5.95 | 0.01 | 100.00 | 0.08 |
| Buffer RLT | 32.95* | 0.65 | 3.77 | 0.05 | 0.67 | 0.33 |
| Fungal DNA | Cq valueb | SD | Log cells/mlc | CV | DNA %c | CV |
| 106 PBMCs + 105 Candida cells | ||||||
| No enrichment | 23.74 | 0.59 | 5.02 | 0.03 | 100.00 | 0.32 |
| Buffer RLT | 24.31 | 0.58 | 4.85 | 0.03 | 68.56 | 0.32 |
| 106 PBMCs + 104 Candida cells | ||||||
| No enrichment | 27.77 | 0.10 | 3.85 | 0.01 | 100.00 | 0.07 |
| Buffer RLT | 28.72 | 0.25 | 3.57 | 0.07 | 53.05 | 0.45 |
| Human RNA | Cq valueb | SD | Log cells/mlc | CV | RNA %c | CV |
| 106 PBMCs + 105 Candida cells | ||||||
| No enrichment | 30.65 | 0.88 | 6.09 | 0.04 | 100.00 | 0.45 |
| Buffer RLT | 35.15* | 0.98 | 4.78 | 0.06 | 4.91 | 0.48 |
| 106 PBMCs + 104 Candida cells | ||||||
| No enrichment | 30.74 | 1.65 | 4.37 | 0.10 | 100.00 | 0.64 |
| Buffer RLT | 34.57* | 0.71 | 3.34 | 0.06 | 9.30 | 0.36 |
| Fungal RNA | Cq valueb | SD | Log cells/mlc | CV | RNA %c | CV |
| 106 PBMCs + 105 Candida cells | ||||||
| No enrichment | 22.51 | 0.52 | 5.06 | 0.03 | 100.00 | 0.28 |
| Buffer RLT | 23.26 | 0.46 | 4.84 | 0.03 | 61.47 | 0.26 |
| 106 PBMCs + 104 Candida cells | ||||||
| No enrichment | 25.47 | 0.54 | 4.22 | 0.04 | 100.00 | 0.30 |
| Buffer RLT | 27.10 | 0.82 | 3.76 | 0.06 | 34.69 | 0.41 |
aHuman nucleic acids amplified with CXCL-1 primers and fungal nucleic acids amplified with ITS-1 primers.
bCq values are means of six biological replicates. Statistically significant differences are marked with one asterisk (p < 0.05) (paired-samples Wilcoxon test).
cLog cells/ml, DNA percentage and RNA percentage were calculated by extrapolation of Cq values with a standard curve.
SD: Standard deviation. CV: Coefficient of variation.
RNA quality is suitable for transcriptomic analysis
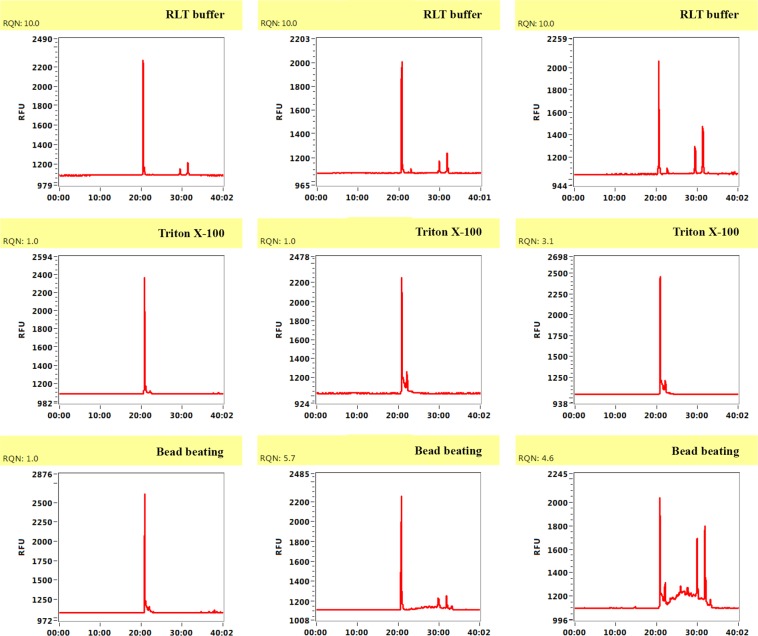
Obtaining high quality RNA is essential to perform comprehensive and reliable transcriptomic analyses. After having shown that enrichment with Buffer RLT is an efficient method to enrich both DNA and RNA from the pathogen fungus from mixes with 10- to 100-fold more human cells. We analysed RNA integrity to check to what extent it can be used for high-quality RNA-demanding experiments such as RNA-seq. Electropherograms of some RNA samples enriched with different methods are shown in Fig. 3. RQN values were around 10 for Buffer RLT enrichment and no degradation was detected. Contrarily, Triton X-100 and bead beating enrichments resulted in poor electropherogram profiles with evident RNA degradation. We concluded that enrichment with Buffer RLT confers high-quality RNA which can be used in transcriptomic analysis.
Figure 3.
RNA integrity of some RNA samples after enrichment with buffer RLT, Triton X-100 and bead beating. Electropherogram profiles were determined with a Fragment Analyzer. Quality for Fragment Analyzer is shown as the RQN value. Reported on a scale of 1 to 10, with higher values indicating a better quality of total RNA. Values above 7 are considered to represent high quality and non-degraded RNA.
Discussion
Host-pathogen interactions in vivo are frequently hampered by the low proportion of microbial cells as compared to host cells, resulting in poor transcriptional profiles from the pathogen side. In this study, we approached this problem by comparing different methods to selectively lyse human cells in order to increase the proportion of fungal cells. This enrichment of fungal cells through differential cell lysis was further complemented with centrifugation to decrease contaminant cell debris and nucleic acids of the human host cells. In our study, we started from mixtures containing 106 Candida cells and 106 PBMCs, that had been stored in RNAlater, a solution that has been shown to be as efficient as snap-frozen methods to keep transcriptomic profiles unmodified24. Its use is increasing during recent years because it allows short-term storage of clinical samples at above-freezing temperatures for RNA extraction purposes. This is especially relevant in clinical settings in which samples cannot be frozen immediately in liquid nitrogen.
The methods compared for differential cell lysis were Buffer RLT treatment, Triton X-100 incubation and bead beating in saline.
Triton X-100 is a non-ionic surfactant widely used to lyse different types of human cells such as red blood cells25, monocytes26 and dendritic cells27.
Bead beating has also been used extensively to lyse different microorganisms including bacteria28,29 and fungi30,31 and less often human cells32. Although bead beating is usually applied in combination with a lysis buffer, we used saline instead to avoid lysis of yeast cells.
Although Buffer RLT is a commercial lysis buffer which has been used in different protocols and kits for the lysis of cells and tissues prior to RNA extraction33–35, its effectiveness as a differential lysis buffer for human and fungal cells has not been studied yet.
Cell lysis was previously determined indirectly by quantification of released haemoglobin25, of released LDH26 and of metabolic activity and efficiency of protein extraction27. Here, we used microscopy and cell counting to directly quantify the number and type of cells that were lysed. We found that yeast cells were resistant to all three lysis methods, as we had expected, since i) the fungal cell wall confers protection against different stress conditions36, ii) the cell mixtures had been stored in RNAlater, which makes cells harder to lyse37, and iii) a combination of both bead beating and an efficient lysis buffer is needed to effectively lyse Candida cells37. We found that Buffer RLT was the most efficient method to lyse human cells. In addition, Buffer RLT was also the fastest method, since pipetting up and down several times was sufficient to lyse 100% of human cells, while Triton X-100 and bead beating required protocols lasting for 20 and 10 min respectively.
The reduced effectiveness in lysis of the PBMCs (<100%) that we obtained with Triton X-100 and bead beating in saline is probably due to the pre-storage in RNAlater, since Triton X-100 is a well-known detergent widely used in cell lysis but its use for samples stored in RNAlater has not been evaluated previously. In fact, there are no studies analysing the efficiency of cell lysis in samples stored in RNAlater apart from our recent study with C. albicans37. Buffer RLT is a more powerful chemical agent (chaotropic) that is not affected by RNAlater.
In addition, using qPCR and RT-qPCR, we demonstrated that after cell lysis with Buffer RLT, both fungal DNA and RNA can be further enriched by means of a centrifugation step that effectively reduced human nucleic acids. However, we observed that pellets of fungal cells were not strongly attached at the bottom of the tube, which may be due to the presence of large amounts of (human) DNA in the supernatant, which may increase viscosity, and interfere with the centrifugation process. Therefore, care should be taken at removal of the supernatants, to avoid removal of the pellet. For this reason, we also recommend to not include more than three samples per run of centrifugation. Using the enrichment with Buffer RLT and centrifugation, only 0.1% human RNA and 5.2% human DNA were left, which represents an important reduction of (human) nucleic acids. In a further effort to completely remove contaminating human nucleic acids, we also added DNase and RNase digestion after Buffer RLT treatment and centrifugation prior to RNA extraction. Andes et al.23, who also used Triton X-100 to enrich fungal cells, had shown that RNase digestion not only decreased human GADPH mRNA, but surprisingly also increased fungal actin mRNA, according to RT-qPCR. However, we did not succeed to reduce human RNA with Triton X-100, and enzymatic digestion did not further increase fungal RNA. Our contradictory results can be explained by the influence of RNAlater which can impair cell lysis and enzymatic treatments.
Using Buffer RLT treatment, followed by centrifugation, fungal DNA and RNA were reduced to - statistically non-significant - 83.1% and 59.9% of the original amount present, as assessed by qPCR and RT-qPCR. In summary, the Buffer RLT enrichment in combination with centrifugation was most efficient to reduce human RNA and can be performed in less than 10 min, further reducing the risk of unwanted transcriptomic profile changes.
Furthermore, we simulated the low proportion of fungal cells that is encountered in the host during colonisation or infection by reducing the number of fungal cells 10- and 100-fold relative to the number of human cells. This strategy has been used in other in vitro experiments38–40, and gives a better approximation of host-pathogen ratios present in vivo. It should be noted that many in vitro studies used a number of pathogen cells higher than that of the host cells, most likely, in order to, for example, increase the signal of luminescence during ROS production assays41, to increase the signal obtained during chromatographic analyses to study metabolite profiles42 or to stimulate a stronger immune response43,44. Most importantly, we obtained similar results to our experiments when using a 1:1 ratio.
Although we aimed to mimic the in vivo situation by mixing PBMCs and yeast cells in different ratios, a limitation of our study may be that these mixtures did not reflect possible interactions which may occur in vivo, such as PBMC stimulation, lysis, invasion of human cells by yeast cells or adherence between human and yeast cells.
Finally, we obtained high-quality RNA after Buffer RLT enrichment, as assessed with Fragment Analyzer, demonstrating that RNA integrity was not compromised during the enrichment process. This facilitates RNA-seq experiments and any other downstream application.
Apart from RNA, we also enriched DNA. Although we did not analyse DNA quality, we assume that Buffer RLT enrichment may be used for genomic studies as well, since DNA is more stable than RNA. Indeed, also genomic studies have to cope with the overwhelming amount of host DNA contamination in clinical samples. For example, a recent study reported about the need of DNA enrichment in metagenomic studies of sputum, and described a microbe enrichment microfluidic device coupled with DNA amplification45. However, although this enrichment is suitable for different bacteria, fungi are not so well recovered because of their larger size.
The enrichment method described here is based on the selective lysis of human cells, taking advantage of the protective properties that the cell wall confers to fungal cells. A comparable protocol has been published using RNAPure instead of Buffer RLT, but without explicitly showing the efficiency46. Andes et al.23 also enriched fungal RNA through the lysis of human cells. However, their protocol requires two incubations during 20 min at 37 °C and as such gene expression prior to RNA extraction may be changed, which would no longer match the expression profile encountered during the infection. Another study used 55.000 Candida-specific probes to match every gene and splicing variant of the fungal transcriptome22. As such, these authors carried out an enrichment after and not prior to RNA extraction. This gives an important advantage since transcriptomic profiles may change during the enrichment process. However, disadvantages of this method include certain cross-hybridization that is inherent to the technique and the cost of the probes. Using RNA-seq in two animal models, they obtained from 0.03% to 0.1% of reads aligned to C. albicans before the enrichment, and fungal reads were enriched to 58–69% with the probes. Since they do not provide how many of the 31–42% of unmapped reads were mapped as human, the provenance of these reads is unknown.
In summary, the clinical relevance of fungal infections has boosted the interest in host-pathogen interactions. Expanding our knowledge in this field will contribute to develop new diagnostic tools, identify potential vaccine candidates and improve the antifungal treatments we have used for more than 30 years and that are becoming increasingly obsolete47. The enrichment described here may enable more comprehensive analyses of transcriptional profiles from fungal pathogens within the host. Furthermore, we should evaluate whether this approach is also applicable for dermatophytes, whose cell walls are even tougher than Candida.
Methods
Culture of Candida albicans
Candida albicans reference strain ATCC 90028 was grown on Sabouraud Glucose Agar plates with Chloramphenicol (50 μg/ml) and transferred to Yeast extract - Peptone Dextrose broth. Cell suspensions were subcultured at 32 °C in static conditions overnight until the logarithmic growth phase was reached. Cells were counted with a microscope by using a haemocytometer (Bürker chamber). Cell suspensions were adjusted with the same broth to 2 × 106, 2 × 105 and 2 × 104 cells/ml and centrifuged at 8000 g for 10 min, whereafter pellets were resuspended in 1-ml aliquots in RNAlater (Invitrogen, Carlsbad, CA) and placed at 4 °C following immediate preparation of yeast cells/PBMCs mixtures.
PBMC isolation
PBMCs were isolated from a buffycoat as described previously48 after informed consent from all participants. Cell pellets were resuspended in RNAlater to a concentration of 2 × 106 cells/ml and divided into 1-ml aliquots and placed at 4 °C following immediate preparation of yeast cells/PBMCs mixtures. We confirm that all methods were carried out in accordance to relevant guidelines and regulations and that all experimental protocols were approved by the ethical committee of the University of Ghent (EC/2016/0192).
Preparation of yeast cells/PBMCs mixtures and RNAlater treatment
Volumes of 500 µl of 1 ml-aliquots containing 2 × 106, 2 × 105 and 2 × 104 yeast cells/ml and 500 µl of 1 ml-aliquots with 2 × 106 PBMCs/ml were mixed together to obtain 1-ml aliquots of 106:106, 105:106 and 104:106 yeasts cells:PBMCs mixtures. These mixtures were stored overnight at 4 °C to enable the RNAlater to penetrate into the cells, and subsequently stored at −80 °C.
Cell lysis of human cells
Mixtures of yeast cells and PBMCs that had been stored at −80 °C, were thawed, centrifuged for 10 min at 20 000 g and resuspended in 1-ml aliquots of i) Triton X-100 (final concentration, 1%), ii) Buffer RLT (Qiagen, Hilden, Germany) supplemented with 1% β-mercaptoethanol and iii) saline. Buffer RLT and Triton X-100 samples were incubated for at least 1 min and 20 min respectively, while saline samples were transferred to prefilled tubes with 0.5 mm zirconium beads and further bead beaten for 10 min in a hands-free vortex genie-2 (MO BIO Laboratories, Carlsbad, CA), fitted with a vortex adapter that allows bead beating in a horizontal position.
Microscopic visualization and cell viability determination
After the different cell lysis treatments, cell suspensions were centrifuged for 10 min at 8000 g to collect intact yeast cells and PBMCs. They were then resuspended in 1 ml of saline prior to cell counting with a microscope. Ten µl of this saline cell suspension was loaded into a haemocytometer. Yeast cells and PBMCs could be observed with light microscopy and were counted in 25 small squares with 0.2 mm sides (together representing a 0.1 µl volume) at a magnification of 400x. The resulting number of cells/ml was calculated as follows: cells/ml = cell number (in 25 small squares) × 1 (dilution) × 104.
Centrifugation conditions for enrichment
Centrifugation at 20 000 g for 8 min at room temperature was used to pellet fungal cells. Supernatants containing cell debris from human cells that had been lysed with the three different methods were discarded very carefully, since fungal pellets were very loose and they can be detached very quickly. No more than three samples were centrifuged per run of centrifugation to minimize the risk of fungal pellets being removed together with cell debris from human cells.
DNase and RNase treatment
Pellets of intact yeast cells were treated to digest contaminating human DNA and RNA. First, pellets were resuspended in 86 µl nuclease-free water, and DNase treatment was performed by addition of 10 µl of 10x DNase I buffer and 4 µl of DNase I (2 U/µl) and by incubation for 30 min at 37 °C. Second, the yeast pellet was processed for RNase treatment by addition of 98 µl of nuclease-free water and 2 µl of RNase A Solution (4 mg/ml). The mixture was then incubated for 1 h at 37 °C.
Nucleic acids extraction
Nucleic acids were isolated using the RiboPure Yeast Kit (Invitrogen) and following the manufacturer’s instruction of the kit. Half of the resulting nucleic acids was used directly for DNA quantification by means of qPCR, and the other half was treated with DNase I to obtain pure RNA. RNA was then analyzed for quality with a NanoDrop spectrophotometer and a Fragment Analyzer (DNF-472 High-Sensitivity Total RNA, Applied Biosystems) and subsequently quantified with RT-qPCR.
qPCR and RT-qPCR analysis
Reverse transcription was performed for 5 min at 25 °C followed by 60 min at 42 °C with random hexamer primers and with 5 µl of total RNA according to the instructions of the manufacturer of the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). qPCR from both cDNA and DNA was performed in duplicate with a LightCycler 480 (Roche). We used CXCL1_F forward (5′-GGA AAG AGA GAC ACA GCT GCA-3′) and CXCL1_R reverse (5′-AGA AGA CTT CTC CTA AGC GAT GC-3′) primers, previously described (Van Belleghem et al.48), targeting the human gene encoding the cytokine CXCL1, and CA_rRNA F (5′-TTT GCT TGA AAG ACG GTA-3′) and CA_rRNA R (5′-TTG AAG ATA TAC GTG GTG G-3′) targeting the ITS-1 gene of C. albicans (adapted from49). PCR master mixes to quantify both human cDNA/DNA and yeast cDNA/DNA were prepared similarly, i.e. Roche LC480 high resolution melting (HRM) mix (Roche, Basel, Switzerland), 0.5 µM primers and 2 µl sample (cDNA or DNA) in a final volume of 10 µl, with exception of MgCl2 which was used at a concentration of 2 mM and 3 mM to amplify human cDNA/DNA and yeast cDNA/DNA respectively. The thermal cycling program consisted of a pre-incubation step for 10 min at 95 °C, amplification for 45 cycles of 30 s at 95 °C, 30 s at 59 °C and 30 s at 72 °C. Results were analyzed with the LightCycler 480 software 1.5 (Roche).
Statistical analysis
For statistical comparisons of cell lysis and PCR data (cq values), independent experiments performed with six replicates were considered. Data were analyzed using the linear mixed model for repeated measures followed by Bonferroni’s multiple testing correction, and with Friedman’s test and Wilcoxon’s test where the assumption of normality was not acceptable, using the IBM SPSS Statistics software v 25.0 (IBM, Armonk, NY, USA).
Supplementary information
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 642095. This work has been supported by the Ghent University Hospital.
Author contributions
A.R. and M.V. conceived and designed the experiments, analysed the data and wrote the paper. A.R. performed the experiments. B.G. and P.C. performed RNA quality analysis. All authors have read and approved the manuscript.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54608-x.
References
- 1.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;4:2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 2.Pires CA, et al. Clinical, epidemiological, and therapeutic profile of dermatophytosis. An Bras Dermatol. 2014;89:259–264. doi: 10.1590/abd1806-4841.20142569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368–1379. doi: 10.1001/jama.291.11.1368. [DOI] [PubMed] [Google Scholar]
- 4.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 5.Aballéa S, et al. Subjective health status and health-related quality of life among women with recurrent vulvovaginal candidosis (RVVC) in Europe and the USA. Health Qual Life Outcomes. 2013;11:169. doi: 10.1186/1477-7525-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koehler P, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Schelenz S, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart SL, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno VM, et al. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. MBio. 2015;6:e00182–15. doi: 10.1128/mBio.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, et al. New signaling pathways govern the host response to C. albicans infection in various niches. Genome Res. 2015;25:679–689. doi: 10.1101/gr.187427.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchner FR, et al. Persistence of Candida albicans in the oral mucosa induces a curbed inflammatory host response that is independent of immunosuppression. Front Immunol. 2019;10:330. doi: 10.3389/fimmu.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, et al. Transcriptome profiles of human lung epithelial cells A549 interacting with Aspergillus fumigatus by RNA-Seq. PLoS One. 2015;10:e0135720. doi: 10.1371/journal.pone.0135720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kale SD, et al. Modulation of immune signaling and metabolism highlights host and fungal transcriptional responses in mouse models of invasive pulmonary aspergillosis. Sci Rep. 2017;7:17096. doi: 10.1038/s41598-017-17000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar J, Cerqueira GC, Wortman JR, Clemons KV, Stevens DA. RNA-Seq profile reveals Th-1 and Th-17-type of immune responses in mice infected systemically with Aspergillus fumigatus. Mycopathologia. 2018;183:645–658. doi: 10.1007/s11046-018-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung M, et al. Targeted enrichment outperforms other enrichment techniques and enables more multi-species RNA-Seq analyses. Sci Rep. 2018;8:13377. doi: 10.1038/s41598-018-31420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TB, et al. Cryptococcus inositol utilization modulates the host protective immune response during brain infection. Cell Commun Signal. 2014;12:51. doi: 10.1186/s12964-014-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, et al. Cryptococcus neoformans infection and immune cell regulation in human monocytes. Cell Physiol Biochem. 2015;37:537–547. doi: 10.1159/000430375. [DOI] [PubMed] [Google Scholar]
- 19.Linde J, et al. Defining the transcriptomic landscape of Candida glabrata by RNA-Seq. Nucleic Acids Res. 2015;43:1392–1406. doi: 10.1093/nar/gku1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang C, et al. Significance of hyphae formation in virulence of Candida tropicalis and transcriptomic analysis of hyphal cells. Microbiol Res. 2016;192:65–72. doi: 10.1016/j.micres.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Amorim-Vaz, S. et al. RNA enrichment method for quantitative transcriptional analysis of pathogens in vivo applied to the fungus Candida albicans. MBio. 6, e00942–15 (2015). [DOI] [PMC free article] [PubMed]
- 22.Andes D, Lepak A, Pitula A, Marchillo K, Clark J. A simple approach for estimating gene expression in Candida albicans directly from a systemic infection site. J Infect Dis. 2005;192:893–900. doi: 10.1086/432104. [DOI] [PubMed] [Google Scholar]
- 23.Hebecker B, et al. Dual-species transcriptional profiling during systemic candidiasis reveals organ-specific host-pathogen interactions. Sci Rep. 2016;6:36055. doi: 10.1038/srep36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdary D, et al. Prognostic gene expression signatures can be measured in tissues collected in RNAlater preservative. J Mol Diagn. 2006;8:31–39. doi: 10.2353/jmoldx.2006.050056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preté PS, et al. Multiple stages of detergent-erythrocyte membrane interaction–a spin label study. Biochim Biophys Acta. 2011;1808:164–170. doi: 10.1016/j.bbamem.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Bohnert M, et al. Melleolides induce rapid cell death in human primary monocytes and cancer cells. Bioorg Med Chem. 2014;22:3856–3861. doi: 10.1016/j.bmc.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Optimization of human dendritic cell sample preparation for mass spectrometry-based proteomic studies. Anal Biochem. 2015;484:40–50. doi: 10.1016/j.ab.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Bruin OM, Birnboim HC. A method for assessing efficiency of bacterial cell disruption and DNA release. BMC Microbiol. 2016;16:197. doi: 10.1186/s12866-016-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill C, van de Wijgert JH, Blow F, Darby AC. Evaluation of lysis methods for the extraction of bacterial DNA for analysis of the vaginal microbiota. PLoS One. 2016;11:e0163148. doi: 10.1371/journal.pone.0163148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasidharan K, Amariei C, Tomita M, Murray DB, Rapid DNA. RNA and protein extraction protocols optimized for slow continuously growing yeast cultures. Yeast. 2012;29:311–322. doi: 10.1002/yea.2911. [DOI] [PubMed] [Google Scholar]
- 31.Sasidharan K, Soga T, Tomita M, Murray DB. A yeast metabolite extraction protocol optimised for time-series analyses. PLoS One. 2012;7:e44283. doi: 10.1371/journal.pone.0044283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers JN, Rekhadevi PV, Ramesh A. Comparative evaluation of different cell lysis and extraction methods for studying benzo(a)pyrene metabolism in HT-29 colon cancer cell cultures. Cell Physiol Biochem. 2011;28:209–218. doi: 10.1159/000331732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasahara T, et al. Evaluation of methods for duration of preservation of RNA quality in rat liver used for transcriptome analysis. J Toxicol Sci. 2006;31:509–519. doi: 10.2131/jts.31.509. [DOI] [PubMed] [Google Scholar]
- 34.Mack E, Neubauer A, Brendel C. Comparison of RNA yield from small cell populations sorted by flow cytometry applying different isolation procedures. Cytometry A. 2007;71:404–409. doi: 10.1002/cyto.a.20391. [DOI] [PubMed] [Google Scholar]
- 35.Van der Poel-van de Luytgaarde SC, et al. Addition of β-mercaptoethanol is a prerequisite for high-quality RNA isolation using QIAsymphony technology as demonstrated by detection of molecular aberrations in hematologic malignancies. Genet Test Mol Biomarkers. 2013;17:475–480. doi: 10.1089/gtmb.2012.0448. [DOI] [PubMed] [Google Scholar]
- 36.Beauvais A, Latgé JP. Special issue: Fungal cell wall. J Fungi. 2018;4:91. doi: 10.3390/jof4030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez A, Vaneechoutte M. Comparison of the efficiency of different cell lysis methods and different commercial methods for RNA extraction from Candida albicans stored in RNAlater. BMC Microbiol. 2019;19:94. doi: 10.1186/s12866-019-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moyes DL, et al. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One. 2011;6:e26580. doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, et al. Different host immunological response to C. albicans by human oral and vaginal epithelial cells. Mycopathologia. 2019;184:1–12. doi: 10.1007/s11046-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 40.Ho J, et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10:2297. doi: 10.1038/s41467-019-09915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvatori O, Pathirana RU, Kay JG, Edgerton M. Candida albicans Ras1 inactivation increases resistance to phagosomal killing by human neutrophils. Infect Immun. 2018;86:1–14. doi: 10.1128/IAI.00685-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liew KL, et al. In vitro analysis of metabolites secreted during infection of lung epithelial cells by Cryptococcus neoformans. PLoS One. 2016;7:e0153356. doi: 10.1371/journal.pone.0153356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernández-Chávez MJ, et al. Role of protein phosphomannosylation in the Candida tropicalis-macrophage interaction. FEMS Yeast Res. 2018;18:1–14. doi: 10.1093/femsyr/foy053. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, He D, Gao S, Wei Y, Wang L. Aspergillus fumigatus enhances human NK cell activity by regulating M1 macrophage polarization. Mol Med Rep. 2019;20:1241–1249. doi: 10.3892/mmr.2019.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X, et al. Microfluidics-based enrichment and whole-genome amplification enable strain-level resolution for airway metagenomics. mSystems. 2019;4:1–14. doi: 10.1128/mSystems.00198-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lüttich A, Brunke S, Hube B. Isolation and amplification of fungal RNA for microarray analysis from host samples. Methods Mol Biol. 2012;845:411–421. doi: 10.1007/978-1-61779-539-8_28. [DOI] [PubMed] [Google Scholar]
- 47.Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014;4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep. 2017;7:8004. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guiver M, Levi K, Oppenheim BA. Rapid identification of Candida species by TaqMan PCR. J Clin Pathol. 2001;54:362–366. doi: 10.1136/jcp.54.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.