Abstract
Background
The medicinal mushroom Trametes versicolor (Tv, Turkey Tail) is often prepared for consumption as a powder from the fungal mycelium and the fermented substrate on which it grew. The goal for this study was to evaluate the immune-modulating properties of the mycelium versus the fermented substrate, to document whether an important part of the immune-activating effects resides in the metabolically fermented substrate.
Methods
Tv mycelium was cultured on rice flour. The mycelium and the fermented substrate were mechanically separated, dried, and milled. The initial substrate served as a control. Aqueous fractions were extracted and passed through 0.22-μm filters. The remaining solids were passed through homogenization spin columns without filtration. The aqueous and solid fractions of the initial substrate (IS), the fermented substrate (FS), and the Trametes versicolor mycelium (TvM) were tested for immune-activating and modulating activities on human peripheral blood mononuclear cell cultures, to examine expression of the CD69 activation marker on lymphocytes versus monocytes, and on the T, NKT, and NK lymphocyte subsets. Culture supernatants were tested for cytokines using Luminex arrays.
Results
Both aqueous and solid fractions of TvM triggered robust induction of CD69 on lymphocytes and monocytes, whereas FS only triggered minor induction of CD69, and IS had no activating effect. The aqueous extract of TvM had stronger activating effects than the solid fraction. In contrast, the solid fraction of IS triggered a reduction in CD69, below levels on untreated cells.
Both aqueous and solid fractions of FS triggered large and dose-dependent increases in immune-activating pro-inflammatory cytokines (IL-2, IL-6), anti-inflammatory cytokines Interleukin-1 receptor antagonist (IL-1ra) and Interleukin-10 (IL-10), anti-viral cytokines interferon-gamma (IFN-γ) and Macrophage Inflammatory Protein-alpha (MIP-1α), as well as Granulocyte-Colony Stimulating Factor (G-CSF) and Interleukin-8 (IL-8). TvM triggered more modest cytokine increases. The aqueous extract of IS showed no effects, whereas the solid fraction showed modest effects on induction of cytokines and growth factors.
Conclusion
The results demonstrated that the immune-activating bioactivity of a mycelial-based medicinal mushroom preparation is a combination of the mycelium itself (including insoluble beta-glucans, and also water-soluble components), and the highly bioactive, metabolically fermented substrate, not present in the initial substrate.
Keywords: Anti-inflammatory, Immune modulation, CD69, Cytokines
Background
Medicinal mushrooms describe a category of edible members of the kingdom Fungi, traditionally associated with health-supporting properties. They have been used for centuries to treat an array of ailments, particularly in traditional Asian medicine and Eastern European traditions. They are well regarded for supporting longevity, treating infectious disease and cancer, and promoting overall well-being [1, 2]. Contemporary research has mainly focused on the broad immune activity of mushrooms. Several preclinical findings suggest that mushrooms may specifically support NK cell upregulation, [2–5] enhancement of T-cell and NK cell cytotoxicity [6], and the induction of immune-regulating cytokines such as TNF-α, IL-2, IFN-y, and IL-10 [7–10]. As a category, medicinal mushrooms stimulate host defense and immunity due to the complex and varying polysaccharides; some well-studied examples include (1,3;1,6)-β-glucans, proteoglycans, heteroglucans, that comprise the chitin-based fungal cell wall [11–13]. Mushrooms are also the source of other pharmacologically relevant compounds, such as proteins like Ling zhi-8 in Ganoderma lucidum [14] and lectins in several species [15], triterpenes [16, 17], phenols [18], and sterols [16]. While medicinal mushrooms generally confer broad immune activity, individual species often possess unique immunological properties. Trametes versicolor (Tv), commonly known as Turkey tail and previously named Coriolus versicolor, is known to enhance innate and adaptive immune responses [19, 20]. Recent clinical research involving consumption of Tv mycelium on rice substrate by Standish and colleagues [21] suggests NK cell induction in women with breast cancer. Other researchers cite antitumor effects [4, 22, 23], but this is generally considered to be a result of its underlying immunologic activity [24]. Tv contains precursors to the proteoglycans polysaccharide peptide (PSP) and polysaccharide-K (Krestin, PSK), the latter of which is frequently prescribed to gastric cancer patients in Japan [25]. The constituents responsible for these immunological effects are believed to be the polysaccharides. However, recent research suggests that the lipid fraction of PSK isolated from Tv is instrumental to its TLR-2 induction activity [26].
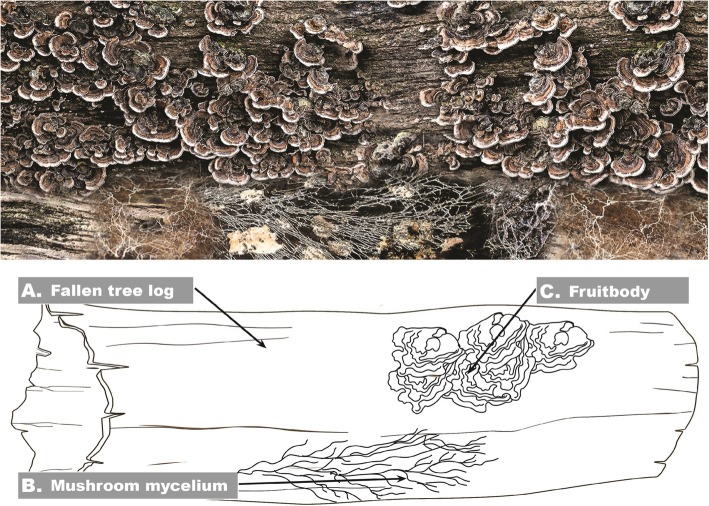
The natural ecological role of mushrooms is to assist the breakdown of dead plant matter, and therefore they engage in a highly dynamic interaction with the environment in which they grow. The fungal organism contains a vegetative state consisting of progressive extensions of tissue into a substrate, as well as a reproductive state for spore dispersal (Fig. 1). Mycelium is an aggregation of multinucleate hyphae that typically appear as strands or thin filaments. As a mycelium grows throughout its environment, it secretes an array of compounds into its substrate, altering the chemical nature of the substrate. This enzyme-rich exudate helps catalyze the breakdown of macromolecules for absorption—an example of which are the extracellular lignin-modifying enzymes laccase, lignin peroxidase, and manganese peroxidase [27]. Fungi that engage in this type of enzymatic lignin biodegradation and decompose wood are known as white-rot fungi, named for their white appearance. As a white rot fungus with notable laccase production, Tv has the capacity to enzymatically affect its environment, primarily by degradation of its substrate [28–30]. This enzymatic activity is not limited to lignin-containing woody tissues; rice bran can also function as an efficient substrate for laccase production [31].
Fig. 1.
Decomposition of a fallen tree log by Trametes versicolor (Tv). a Fallen tree log, presenting fresh organic plant matter. b Tv mycelium is growing inside the log, decomposing the plant biomass by fermentation, in a highly dynamic exchange of solubilized nutrients from the tree log, resulting from secreted fungal enzymes, combined with anti-microbial defense compounds to protect the mycelial territory. c Fruitbodies serve to spread the spores of the Tv mushrooms, and have a narrower chemical composition, focused on beta-glucans, spores, attractants to animals that may eat and transport the spores, and protectants to protect the fruitbodies from bacteria and other fungi
Of importance for the understanding of medicinal mushrooms for human consumption, fungal hyphae also secrete a wide variety of defense compounds to deter predators and pathogens [32]. Secreted defense compounds allow the fungi to maintain their territory and evade invasion by bacteria and molds. These compounds may be evolutionarily conserved and offer biological effects for other species such as humans. The medical significance of this is apparent in the famous example of the first antibiotic—penicillin, isolated from the Penicillium chrysogenum mold [33].
As structurally different as the mycelium is from the fruitbody, so too are their biological functions. Whereas the mycelium is the major biomass of a fungus and serves to gather nutrients and interact with the substrate during decomposition, the fruitbodies (the most commonly known form of edible mushrooms) are the instruments of spore dispersal in higher fungi (Basidiomycota). They commonly appear as a cap on top of a stem or stalk, with either gills or pore structures underneath the cap. Mycelium and fruiting bodies share similar cell wall structures and contain the polysaccharide complexes that enhance the innate and adaptive immune response [12, 34, 35]. However, concentrations vary, and β-glucans are considered to be present in higher concentrations in the fruiting body compared to the mycelium [36], whereas the mycelial tissue may contain a broader profile of bioactive compounds. Other metabolic variances may exist: recent proteomic research suggests that 40% more protein-coding genes in G. lucidum are expressed in the mycelial state, compared to the fruiting body [37].
The production of medicinal mushroom products utilizes a wide spectrum of substrates, including sawdust to mimic the natural habitat, as well as various grains. It is well known that the biological properties of raw grain are altered by fungal fermentation, likely due to the secreted enzymes. A simple fungal organism, namely yeast, grown on red rice, is considered a dietary supplement, and documented to reduce LDL in preclinical and clinical settings [38], properties not associated with consumption of plain unfermented rice. Another example is the Saccharomyces yeast-based fermentate EpiCor®, which is composed of the fungal cell walls as well as secreted metabolites produced during the fermentation. The aqueous extract of the dried fermentate has well-documented immune activating, anti-inflammatory, and antioxidant properties [39–41]. Consuming the whole dried fermentate is associated with clinical benefits including improved mucosal immune health [42], and reduced incident and duration of colds [43] and allergies [44]. Research using the SHIME model for digestive health has shown beneficial effects on the gut microbiome [45].
Many medicinal mushroom products are sold as a crude powder consisting of mycelium and its fermented substrate. While pre-clinical and clinical studies have been performed on these products [19, 21], the immunological contributions of the fermented substrate have not been examined. The purpose of this study was to characterize the immunological activity of each of the components, namely the mycelia and the fermented substrate, using the initial substrate as a control. Tv was selected as a model organism for this effort because the physical structure of the mycelium is well defined and allows for harvest of both the mycelium and the fermented substrate (including secreted fungal metabolites) when cultivated in specially designed solid substrate fermentation (SSF) systems. The test model involved evaluation of the early activation marker CD69 on different subsets of immune cells and the induction of production of cytokines and growth factors. The choice of CD69 is based on its role in natural killer (NK) cell function, where CD69 is rapidly induced in NK cells shortly after activation [46] and has a direct role in NK cytotoxicity (killing of target cells) [47].
Methods
Reagents
Roswell Park Memorial Institute 1640 medium, penicillin–streptomycin 100×, interleukin-2 (IL-2), phosphate-buffered saline, and lipopolysaccharide (LPS) from Salmonella enterica were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). CD69 fluorescein isothiocyanate, CD56 phycoerythrin, CD3 peridinin chlorophyll protein, and heparin Vacutainer tubes were purchased from Becton-Dickinson (Franklin Lakes, NJ, USA). Customized Bio-Plex Pro™ human cytokine arrays were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
Trametes versicolor (Turkey tail) culture and separation of mycelial and fermented substrate
The mycelial culture work and sample processing was performed at Fungi Perfecti LLC, following a three-step process of substrate preparation, mycelial culturing, and sample separation (Fig. 2). Certified organic rice flour (Azure Farms, Dufur, Oregon, USA) mixed with water to form a paste and sterilized by autoclaving at 1 bar for 60 min. This resulted in a solid biscuit-like disc of rice grain media (0.4–0.45 g/g water content; aw 0.99). This material constituted the initial substrate (IS). A Petri dish containing 60 g (dry mass) of the milled and sterilized rice flour was inoculated with 50 mg of Trametes versicolor agar media spawn. The resulting inoculated media disc solid substrate fermentation microcosm was stored at 20-24 °C for 42 days in a class 1000 clean room. The Trametes versicolor mycelium spread radially over the growth substrate, preferentially developing biomass on the surface of the substrate where gas exchange was highest. Mycelium was separated mechanically by removing the surface mycelium from the underlying substrate with a scalpel.
Fig. 2.
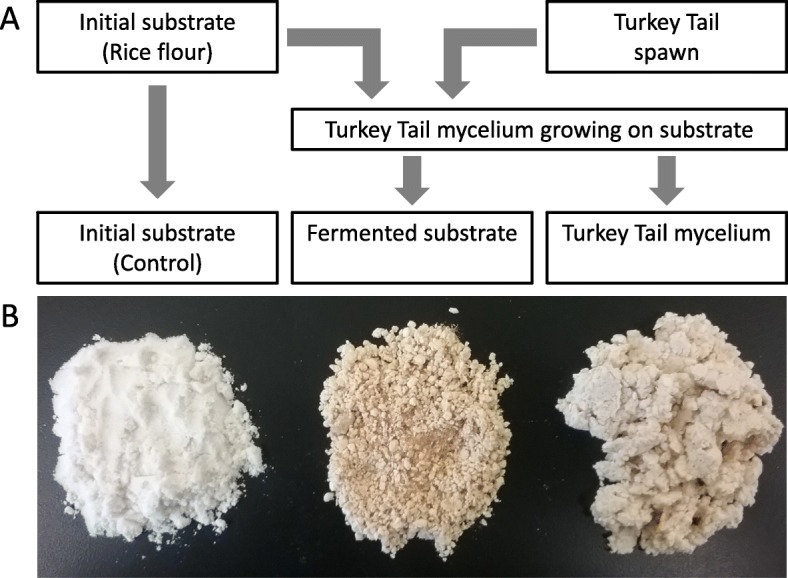
Trametes versicolor (Tv) was used as an experimental model to isolate and compare the mycelium and its fermented substrate. a Diagram showing the origin of the three test products compared: Initial substrate (rice flour), fermented substrate, and Tv mycelium. b Photo of the three powders: Initial substrate (left) is plain rice flour prior to use as a substrate for growing the Tv mycelium. The fermented substrate (center) is the dried residual powder where the mycelium has been removed. The mycelium (right) is the collection of fungal hyphae, removed from the fermented substrate on which it was grown
Preparation of mycelium and substrate for in vitro testing
The three powders were handled in the following manner: 1) Liquid extraction using phosphate-buffered saline (PBS) and referred to as the aqueous fraction; 2) Harvesting the non-aqueous, solid fractions left after aqueous extractions were completed, and passing them through homogenization spin columns (QIAshredder, Qiagen, Hercules, CA). The aqueous fractions were filtered through a 0.22-μm filter before adding to cell cultures. The solid fractions were not filtered through a 0.22-μm filter. This provided two “test products/fractions” from each product, namely the aqueous fraction and the solid fraction. From each fraction, serial dilutions were made in phosphate-buffered saline.
Dry weight determinations of aqueous extracts
The data graphs show the biological activities per gram starting material. However, in order to understand the relative contributions from aqueous constituents, dry weight assessments were performed for the aqueous fractions. From each of the three powders, a 100 g/L suspension was prepared in distilled H2O. The powder was allowed to hydrate and water-soluble compounds were extracted for 1 h under gentle agitation. Solids were precipitated by centrifugation in conical polypropylene vials for 10 min at 400 g. The liquid fraction was harvested, passed through a 0.22-μm cellulose acetate filter, and dried at 100 °C. The weights of the filtrates were 45 mg/g (4.5%w/w) for the initial substrate, 110 mg/g (11% w/w) for the fermented substrate, and 120 mg/g (12% w/w) for the mycelium.
Immune cell activation
Peripheral venous blood was drawn from three healthy human donors upon written informed consent, as approval by the Sky Lakes Medical Center Institutional Review Board, Federalwide Assurance 2603. The blood was drawn into heparin vacutainer vials, and the peripheral blood mononuclear cells (PBMC) isolated using Lympholyte Poly (Cedarlane Labs, Burlington, Ontario, CA) by centrifugation for 35 min at 450 g. The PBMC were washed twice in PBS, counted, and the density adjusted to establish cultures with a cell density at 106/mL, using Roswell Park Memorial Institute 1640 medium containing penicillin–streptomycin and 10% heat-inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific, Asheville, NC).
Serial dilutions of products or LPS were added to cultures at a volume of 20 μL, and cultures were then incubated at 37 °C, 5% CO2 for 24 h. The highly inflammatory LPS from Salmonella enterica was used as a positive control for immune-cell activation at a dose of 10 ng/mL. In parallel, IL-2 was used as a positive control for natural killer (NK)-cell activation, at a concentration of 100 IU/mL. Untreated negative control cultures consisted of PBMC exposed to phosphate-buffered saline in the absence of test products. All treatments, including each dose of test product and each positive and negative control, were tested in triplicate. After 24 h, blood cells were isolated from each culture well and stained for 10 min with fluorochrome-labeled antibodies at the recommended concentration. PBMC were then fixed using 0.5% formalin. The fluorescence intensities for CD3, CD56, and CD69 were measured by flow cytometry, using an Attune acoustic-focusing flow cytometer (Thermo Fisher Scientific).
During data analysis, gating on forward and side scatter facilitated evaluation of the levels of CD69 expression on lymphocyte and monocyte subsets. The lymphocyte subpopulation was further analyzed for CD69 expression on CD3+ T lymphocytes, CD3+ CD56+ NKT lymphocytes, and CD3- CD56+ NK cells.
Production of cytokines, chemokines, and growth factors
After 24 h of incubation, the supernatants were harvested from the PBMC cultures described above. Levels of 10 cytokines and chemokines were quantified (IL-1ra, IL-2, IL-4, IL-6, IL-8 (CXCL8), IL-10, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), MIP-1α (CCL3), and G-CSF) using Bio-Plex Pro™ multiplex immunoassays (Bio-Rad Laboratories, Hercules, CA) and utilizing xMAP technology (Luminex, Austin, TX, USA).
Statistical analysis
Average and standard deviation for each data set was calculated using Microsoft Excel. Statistical analysis of in vitro data was performed using the 2-tailed, independent t-test. Statistical significance was set at P < 0.05, and a high level of significance at P < 0.01.
Results
Induction of the CD69 activation marker on immune cell subsets
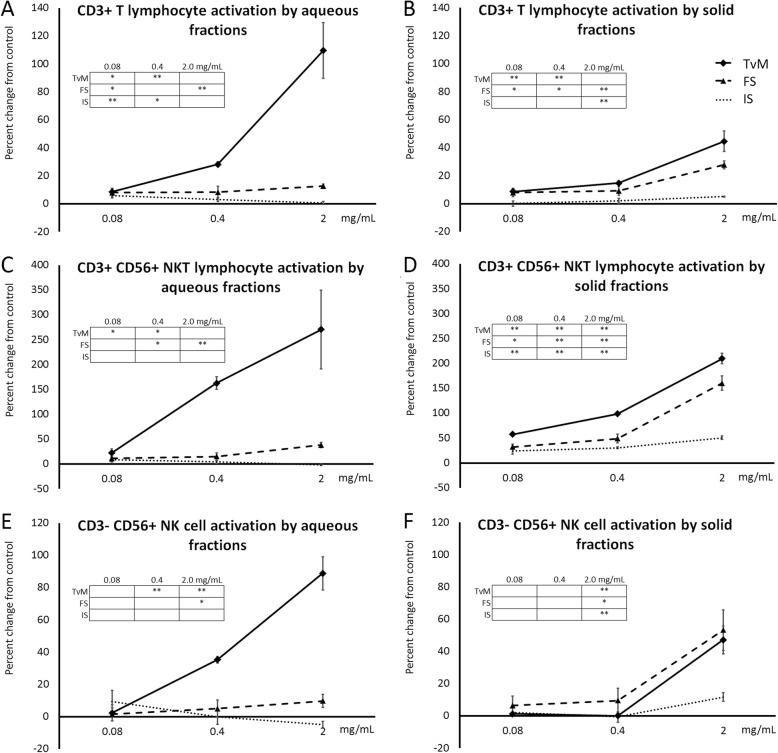
The cell surface expression of the early activation marker CD69 was measured on peripheral blood mononuclear cells (PBMC), after 24 h incubation in the absence versus presence of initial substrate (IS), fermented substrate (FS), and Trametes versicolor mycelium (TvM). Representative results from one blood donor are shown in Figure 3 and 4, and results from the 2 other blood donors are available in Additional file 1. During flow cytometric data analysis, the gating on the physical characteristics of the cell subsets, allowed analysis on lymphocytes versus monocytes (Fig. 3).
Fig. 3.
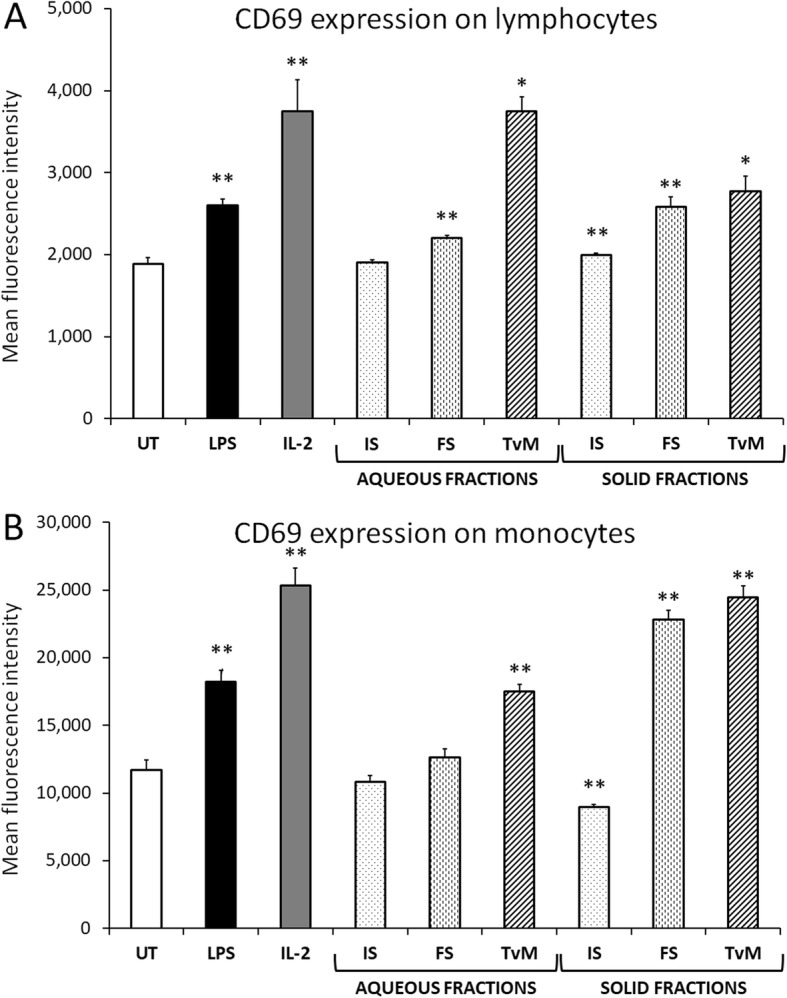
Induction of the CD69 cellular activation marker on lymphocyte (a) and monocyte (b) subsets in human PBMC cultures. The PBMC cultures were treated for 24 h in the presence of the aqueous versus solid fractions of initial substrate (IS), fermented substrate (FS), and Trametes versicolor mycelium (TvM). Data are shown for the highest dose tested (2 mg/mL), where the dose represents the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the mean fluorescence intensities in triplicate cultures, and represents one of three separate experiments using PBMC cells from three different healthy human donors. Positive controls included lipopolysaccharide (LPS, 10 ng/mL) and Interleukin-2 (IL-2, 100 IU/mL). Statistical significance is indicated as * for P < 0.05 and **for P < 0.01
The aqueous fraction of IS showed no effect on CD69 expression (Fig. 3a). The solid fraction showed a very minor increase in CD69 expression on lymphocytes, and a highly significant suppression of CD69 expression on monocytes (P < 0.001) (Fig. 3b).
The induction of CD69 on human lymphocytes by the aqueous and solid fractions of FS showed higher CD69 induction by the solid fraction than the induction seen by the aqueous fraction. The difference in CD69 induction by the aqueous and solid fractions of FS was statistically significant (P < 0.03).
The treatment of human lymphocytes with both the aqueous and the solid fractions of TvM resulted in a robust and statistically significant increase of the CD69 marker, indicating immune cell activation (Fig. 3a). The induction of CD69 on human lymphocytes by the TvM aqueous fraction was more robust than the induction seen by the TvM solid fraction (Fig. 3a), where the difference between the TvM aqueous and solid fractions was statistically significant (P < 0.02).
In contrast, the induction of CD69 on human monocytes by the TvM solid fraction was more robust than the induction seen by the TvM aqueous fraction (Fig. 3b), where the difference between the TvM aqueous and solid fractions was highly significant (P < 0.001).
The lymphocyte subset was further analyzed for expression of the CD69 activation marker of CD3+ T cells, CD3+ CD56+ NKT lymphocytes, and CD3- CD56+ Natural Killer (NK) cells (Fig. 4). It was found that the aqueous extract from TvM triggered a very potent activation of NKT cells (Fig. 4c), and a more moderate activation of T cells and NK cells (Fig. 4a and e). The aqueous extract of the fermented substrate only induced minor increases in CD69 on all three cell types, and the aqueous extract of the initial substrate did not induce CD69 on any of the three cell types (Fig. 4a, c, e).
Fig. 4.
Induction of the CD69 cellular activation marker on immune cell subsets in human PBMC cultures. The PBMC cultures were treated for 24 h in the presence of serial dilutions of Trametes versicolor mycelium (TvM), fermented substrate (FS), or initial substrate (IS). The percent change when compared to untreated control cultures is shown for T lymphocytes (a-b), NKT cells (c-d), and NK cells (e-f). The effects of aqueous extracts are shown in a, c, and e, and the effects of the solid fractions are shown in B, d, and f. Data are shown for three doses tested (0.08, 0.4, and 2 mg/mL), where the doses represent the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the percent change seen in triplicate cultures, and represents one of three separate experiments using PBMC cells from three different healthy human donors. Positive controls included LPS and IL-2. The mean ± standard deviation percent change induced by LPS were 19 ± 2.1% for T lymphocytes, 54 ± 8.3% for NKT cells, and 114 ± 10% for NK cells. The mean ± standard deviation percent change induced by IL-2 were 39 ± 0.9% for T lymphocytes, 150 ± 25% for NKT cells, and 446 ± 60.0% for NK cells. Inserted tables: Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01
In contrast, the solid fractions of TvM and FS induced comparable levels of cellular activation, as measured by increased CD69 expression (Fig. 4b, d, f). The activation was not as strong as what was seen for the aqueous extract of the mycelium but was stronger than the cell activation by the aqueous extract of the fermented substrate. The solid fraction from the initial substrate showed minor activation of NK cells and NKT cells (Fig. 4d, f).
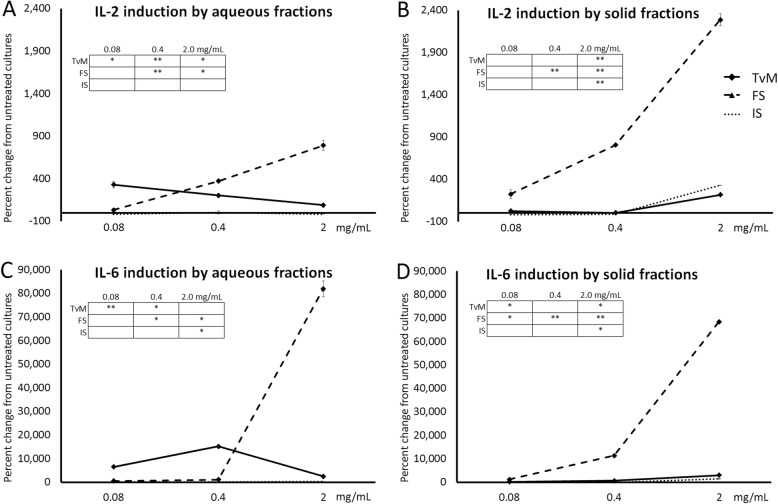
Increased production of pro-inflammatory, immune-activating cytokines
The culture supernatants from the PBMC cultures were tested for the levels of two cytokines involved in immune cell activation, Interleukin-2 (IL-2) and Interleukin-6 (IL-6). Representative results from one blood donor are shown in Figure 5, and results from the 2 other blood donors are available in Additional file 1. Both the aqueous and the solid fractions of the fermented substrate (FS) induced robust increases in IL-2 and IL-6 levels (Fig. 5). The aqueous fraction of Trametes versicolor mycelium (TvM) also induced IL-2 and IL-6, but was more potent at doing so at lower doses (Fig. 5a,c). The solid fraction of TvM had mild effects on IL-2 and IL-6 production in the cultures, and the induction was comparable to the solid fraction of the initial substrate (Fig. 5b,d). The aqueous fraction of the initial substrate did not have any effect on IL-2 or IL-6 induction (Fig. 5a, c).
Fig. 5.
Changes in levels of the cytokines Interleukin-2 (IL-2) and Interleukin-6 (IL-6) in supernatants from human PBMC cultures. The PBMC were cultured for 24 h in the presence of serial dilutions on Trametes versicolor mycelium (TvM), fermented substrate (FS), or initial substrate (IS). The effects on IL-2 and IL-6, cytokines involved in immune activation, of aqueous extracts shown in a and c, and of the solid fractions are shown in b and d. Data are shown for three doses (0.08, 0.4, and 2 mg/mL), where the doses represent the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the percent change seen in triplicate cultures, and represents one of three separate experiments using PBMC cells from three different healthy human donors. Inserted tables: Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01
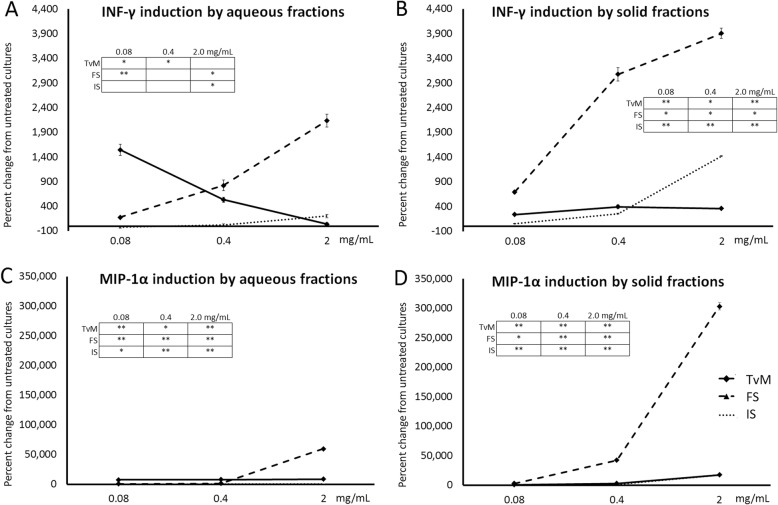
Increased anti-viral cytokine production
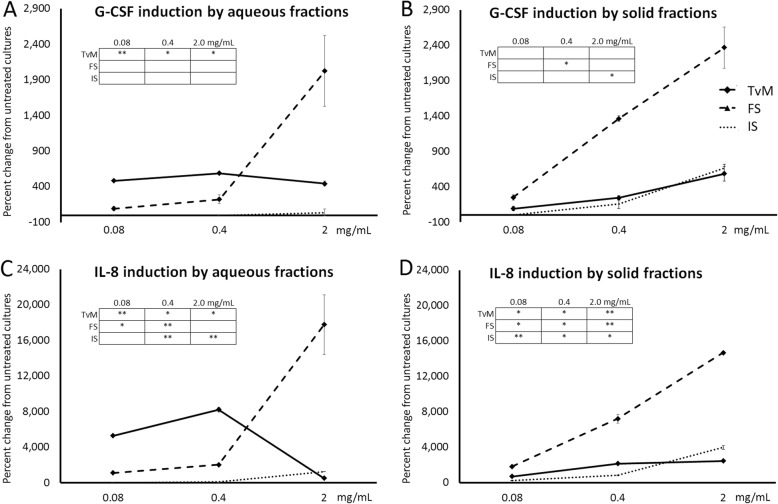
The treatment of human PBMC with the fungal extracts triggered increased production of two anti-viral cytokines, namely Interferon-gamma (IFN-γ) and MIP-1α (Fig. 6). Representative results from one blood donor are shown in Figure 6, and results from the 2 other blood donors are available in Additional file 1. Both the aqueous and solid fractions from the fermented substrate triggered robust increases in these two cytokines, whereas treatment of cultures with the Trametes versicolor mycelium (TvM) led to modest increases in these cytokines. The aqueous fraction of TvM showed more potent effect on IFN-γ at lower doses than at higher doses (Fig. 6a). Interestingly, treatment of human PBMC with the solid fraction of the initial substrate showed a minor increase in IFN-γ and MIP-1α production. The induction of IFN-γ exceeded that induced by the solid fraction of TvM (Fig. 6b). The MIP-1α induced by both aqueous and solid fractions of TvM and the initial substrate were similar in magnitude (Fig. 6c, d).
Fig. 6.
Changes in levels of the cytokines Interferon-gamma (IFN-γ) and Macrophage Inflammatory Protein-1-alpha (MIP-1α) in supernatants from human PBMC cultures. The PBMC were cultured for 24 h in the presence of serial dilutions of Trametes versicolor mycelium (TvM), fermented substrate (FS), or initial substrate (IS). The effects on IFN-γ and MIP-1α, cytokines involved in anti-viral immune defense activity, of aqueous extracts shown in A and C, and of the solid fractions are shown in B and D. Data are shown for three doses (0.08, 0.4, and 2 mg/mL), where the doses represent the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the percent change seen in triplicate cultures, and represents one of three separate experiments using PBMC cells from three different healthy human donors. Inserted tables: Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01
Increased anti-inflammatory cytokine production
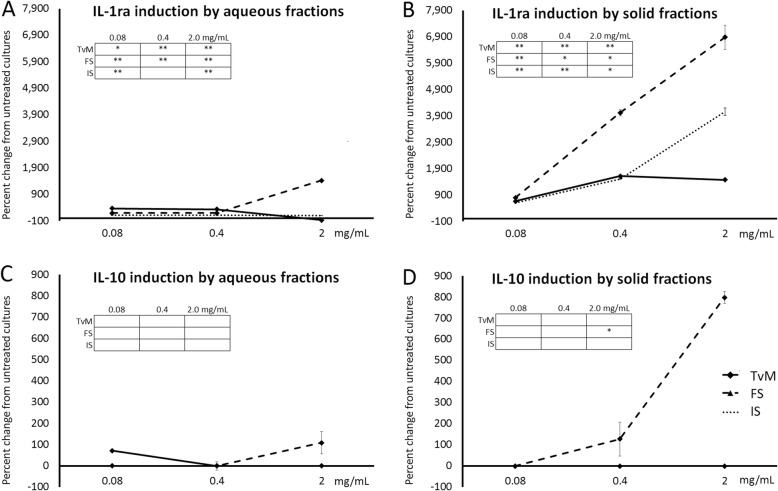
The treatment of human PBMC with the fungal extracts triggered increased production of two anti-inflammatory cytokines, namely Interleukin-1-Receptor Antagonist (IL-1ra) and Interleukin-10 (IL-10) (Fig. 7). Representative results from one blood donor are shown in Figure 7, and results from the 2 other blood donors are available in Additional file 1. Both the aqueous and solid fractions from the fermented substrate triggered increases in both these two cytokines, with the most robust induction being associated with the solid fraction. The aqueous fraction of Trametes versicolor mycelium (TvM) showed more potent effect on both IL-1ra and IL-10 at lower doses than at higher doses (Fig. 7a, c). Interestingly, treatment of human PBMC with the solid fraction of the initial substrate showed a moderate increase in IL-1ra production, exceeding that induced by the solid fraction of TvM (Fig. 7b).
Fig. 7.
Changes in levels of the cytokines Interleukin-1 receptor antagonist (IL-1ra) and Interleukin-10 in supernatants from human PBMC cultures. The PBMC were cultured for 24 h in the presence of serial dilutions of Trametes versicolor mycelium (TvM), fermented substrate (FS), or initial substrate (IS). The effects on IL-1ra and IL-10, both involved in anti-inflammatory processes as part of the resolution of inflammatory processes, of aqueous extracts are shown in a and c, and of the solid fractions are shown in b and d. Data are shown for three doses (0.08, 0.4, and 2 mg/mL), where the doses represent the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the percent change seen in triplicate cultures, and represents one of three separate experiments using PBMC cells from three different healthy human donors. Inserted tables: Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01
Increased production of markers involved in regenerative processes
The treatment of human PBMC with the fungal extracts triggered increased production of two biomarkers involved in regenerative processes involving stem cells, Granulocyte Colony-Stimulating Factor (G-CSF) and Interleukin-8 (IL-8) (Fig. 8). Representative results from one blood donor are shown in Figure 8, and results from the 2 other blood donors are available in Additional file 1. For the fermented substrate, both the aqueous and solid fractions triggered increases in both these two markers. The aqueous fraction of Trametes versicolor mycelium (TvM) showed effects at a broad dose range (Fig. 8a, c), whereas the effects of the solid fraction from the TvM showed similar effects as the solid fraction from the initial substrate (Fig. 8b, d).
Fig. 8.
Changes in levels of the growth factor Granulocyte-Colony Stimulating Factor (G-CSF) and the cytokine Interleukin-8 in supernatants from human PBMC cultures. The PBMC were cultured for 24 h in the presence of serial dilutions of Trametes versicolor mycelium (TvM), fermented substrate (FS), or initial substrate (IS). The effects on the stem cell mobilizing growth factor G-CSF and Interleukin-8 (IL-8) of aqueous extracts are shown in a and c, and of the solid fractions are shown in b and d. Data are shown for three doses (0.08, 0.4, and 2 mg/mL), where the doses represent the amount of starting material used to produce a given fraction. Data are presented as mean ± standard deviation of the percent change seen in triplicate cultures and represents one of three separate experiments using PBMC cells from three different healthy human donors. Inserted tables: Statistical significance is indicated as * for P < 0.05 and ** for P < 0.01
Discussion
The principal finding of the work reported here was a highly differentiated immune activating effect by the Trametes versicolor mycelium (TvM) when compared to its fermented substrate (FM). It was noteworthy that both aqueous and solid fractions of both materials had potent immune modulating activities.
TvM triggered robust increases in the CD69 activation marker on lymphocytes and monocytes, unlike FM which did not induce CD69 on immune cells. The cell surface marker CD69 is rapidly upregulated on many immune cell types after activation, and correlations have been made between Natural Killer (NK) cell CD69 expression and NK cell-mediated tumor-killing activity in the classical target cell-based assay, by a number of research teams over the past 25 years. We have found the induction of the CD69 activation marker a helpful tool for natural products research, both in vitro [48–54] and in clinical studies [55, 56]. When human NK cells are co-cultured with K562 target cells, CD69 expression is upregulated, and the increase significantly correlated with NK cell activity, as measured by today’s gold-standard CD107 mobilization assay [57]. CD69 has the capacity to activate the NK cytolytic machinery in the absence of other NK–target cell adhesion molecule interactions [58]. A direct and highly significant correlation between CD69 levels and NK cell activity was demonstrated by Clausen et al 2003 [59], in a study involving 14 breast cancer patients tested repeatedly during chemotherapy.
NK cells do not function in a vacuum; they are regulatory cells engaged in crosstalk with other cell types [60]. Therefore, the work reported here focused not only on NK cells, but also on NKT cells, T cells, and monocytes. The TvM-mediated induction of CD69 expression on lymphocytes and monocytes was triggered by both aqueous and solid fractions, however, the CD69 expression on lymphocytes was more robust when cells were treated with the aqueous fraction than the solid fraction, with the aqueous fraction comparable to the induction caused by LPS. Given that the aqueous fraction contained almost 10 times less material than the solid fraction, this further demonstrates the potency of the aqueous compounds in the mycelium. For monocytes, this was reversed, where the solid fraction triggered a stronger CD69 expression than the aqueous fraction of the TvM. This is expected, since monocytes are known to be robustly activated by insoluble fungal beta-glucans through Toll-Like receptors (TLR), specifically TLR-2 and TLR-4 [61].
The cytokine induction by aqueous and insoluble compounds in the fermented substrate was seen in the absence of CD69 up-regulation, suggesting activation via alternate pathways, such as has been demonstrated for NK cell activation, which may involve CD69 expression or as an alternative mode of activation involve upregulation of the Interleukin-2 receptor CD25. This was demonstrated by Clausen’s team to be associated with distinct functional differences, where CD69 expression is associated with cytotoxicity as described above, whereas the CD25 expression is associated with increased cell proliferation [59].
Interestingly, the initial un-fermented substrate was not very bioactive, devoid of aqueous bioactive compounds, and only minor effects on cytokine induction by the solid fraction. This further helps demonstrate the uniqueness of the fermented substrate, in terms of fermentation of the rice along with fungal exudates. This is important for the many consumable products that are produced from mycelia along with their fermented substrates.
The aqueous extracts were produced by cold-water extraction, and not using heat or pressure. This is in contrast to other types of fungal extracts and teas, where heat and sometimes pressure is applied to produce the extract. Examples include the use of batch reactors and subcritical water extraction, with heat up to 300 °C, to produce extracts from golden oyster mushrooms (Pleurotus citrinopileatus) [62] and Chaga mushrooms (Inonotus obliquus) [63].
The results clearly demonstrate that most of the bioactivity for cytokine induction lies in the fermented substrate. Based on the known enzyme secretions by mycelium during active growth, the fermented substrate likely represents a broad array of fungal products, in conjunction with breakdown products from the substrate.
Tv has proved to be an effective model for demonstrating the bioactivities of mycelium versus its fermented substrate. This model will be useful for further evaluation of Tv and other medicinal mushrooms, and this model can help mycology research in the isolation and identification of bioactive compounds. There may be potentially pharmaceutical uses of isolated novel compounds form both mycelium and fermentate, however, one of the more unique and interesting challenges will be to understand their synergistic relationships in traditional medicinal use of the whole crude fermentate. Further work from our team is ongoing and includes this evaluation of synergy, by pretreating isolated NK cells and monocytes with TvM, FM, and a blend thereof, followed by the classical NK activity assay in co-culture with tumor cells, and by co-cultures of Tv-primed monocytes with lymphocytes to evaluate the role of NK cells and monocytes in the overall immunological cross-talk between cell types. In addition, a clinical trial will compare the TvM, FM, and the blend thereof. This will help further document the importance of the multi-faceted and complex actions of the natural mixture of TvM and its fermented substrate, traditionally used for immune support in the integrative medicine setting.
Conclusions
The work reported here has helped demonstrate that the mycelial fermentation of its substrate dramatically alters the biological effects of the fermented substrate. Furthermore, the mushroom mycelium has distinctly different biological and immune-modulating properties than its fermented substrate. The mycelium was very potent in terms of triggering immune cell activation, whereas the fermented substrate was very active in terms of cytokine induction. Complex immune-activating bioactivity of mycelial-based medicinal mushrooms go beyond effects of insoluble beta-glucans, as potent effects were also seen in the aqueous fraction. The results suggest that overall medicinal effects are associated both with the mycelium itself (including insoluble beta-glucans, but also water-soluble components), and the highly bioactive fermented substrate. Novel applications for animal and human immune health may be identified in the future for components isolated from fermented substrates, independent of mushroom mycelium.
Supplementary information
Additional file 1. The graphs in the paper shows representative data on immune effects on cells from one of three healthy donors. The full sets of data from all 3 blood donors are shown in the supplementary information.
Abbreviations
- FS
Fermented substrate
- G-CSF
Granulocyte-Colony Stimulating Factor
- IFN-γ
Interferon-gamma
- IL-10
Interleukin-10
- IL-1ra
Interleukin-1 Receptor Antagonist
- IL-2
Interleukin-2
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IS
Initial substrate
- LPS
lipopolysaccharide
- MIP-1α
Macrophage Inflammatory Protein 1-alpha
- PBMC
Peripheral Blood Mononuclear Cells
- TNF-α
Tumor Necrosis Factor-alpha
- Tv
Trametes versicolor (Turkey Tail mushroom)
- TvM
Trametes versicolor mycelium
Authors’ contributions
PS and AT conceived of the questions to be tested. GSJ and KFB wrote the research protocol and designed the study. KFB, SS, and AT conducted the work presented here. KFB and GSJ performed the data analysis. GSJ, RD, RN, and KFB wrote the manuscript. All co-authors participated in the writing and final edit of the manuscript.
Authors’ information
KFB is a molecular geneticist with a specific interest in the human microbiome, including the normal and diseased microbial composition in gut, skin, and blood.
PS is a mycologist with a broad spectrum of interests, and active as advisor on NIH panels for complementary and alternative medicine. PS is also deeply engaged in ecological research, particularly in successful anti-viral protection of endangered honey bees by mushroom extracts. PS served as a consultant to Star Trek Discovery, pertaining to contemporary and futuristic visions for astrobiology/astromycology.
RD is a writer, herbalist, and researcher with an interest in botanical and mycological medicine to improve clinical treatment strategies and health outcomes. She has been involved in health policy research and consultations in indigenous tribal communities, and research in botanical medicine around women’s health and herb-drug interactions.
RN is an organic chemist whose academic research focused on the synthesis of supramolecular systems capable of altering their physical properties based upon environmental stimuli. She has a profound interest applying scientific tools to the development of the natural products industry.
AT is a biological systems engineer specializing in medicinal mushroom cultivation and fungal biotechnology. He has 8 yrs of academic and industry experience spanning diverse topics in fungal biotechnology including development of quantitative methods for assessing biomass production, evaluation of field-scale fungal deployment for environmental restoration, and development of mushroom-derived antiviral formulations. His research interests include microbial growth dynamics, biofiltration, biological biomass conversion, mathematical modeling and industrial fermentation.
SS is a research scientist with over 18 yrs of experience working in molecular biology and immunology in Seattle area biotech companies and the University of Washington with a focus on B cell directed drug therapies: Cloning of recombinant DNA molecules, subsequent protein expression in mammalian cell lines, protein purification and characterization, immunological assays to test the efficacy of the protein against B cell tumors and in autoimmune diseases.
GSJ has an academic background in cancer research, with a special focus on immune surveillance and systems biology. This is combined with a strong interest in natural products, nutrition, herbal and complementary medicine. She has applied her research to serving traditional integrative medicine as well as novel developments in the natural products industry with mainstream research tools.
Funding
The study was sponsored by Fungi Perfecti, LLC, a grower and producer of commercially available mushroom mycelia and finished consumable products. Authors RD, RN, PS, AT, and SS are scientists from the funding body and their roles in the design, execution, and analysis is described below.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Written informed consent, as approved by the Sky Lakes Medical Center Institutional Review Board (Federalwide Assurance 2603), was obtained from the healthy blood donors from which venous blood samples were obtained for this work.
Consent for publication
Not applicable.
Competing interests
GSJ and KFB declare that they have no competing interests. RD, RN, AT, and SS are employed by the sponsor of the study. PS holds several patents on topics related to the presented work, and is the founder and owner of the sponsoring company.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kathleen F. Benson, Email: kathy@nislabs.com
Paul Stamets, Email: paul.s@fungi.com.
Renee Davis, Email: renee.d@fungi.com.
Regan Nally, Email: regan.n@fungi.com.
Alex Taylor, Email: alex.t@fungi.com.
Sonya Slater, Email: sonya.s@fungi.com.
Gitte S. Jensen, Phone: (541) 884-0112, Email: gitte@nislabs.com
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12906-019-2681-7.
References
- 1.Aung SK: The Clinical Use of Mushrooms from a Traditional Chinese Medical Perspective. 2005, 7(3):375–376.
- 2.Jin X, Ruiz Beguerie J, Sze DM-Y, Chan GCF. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2012;6:CD007731. doi: 10.1002/14651858.CD007731.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Yim M-H, Shin J-W, Son J-Y, Oh S-M, Han S-H, Cho J-H, Cho C-K, Yoo H-S, Lee Y-W, Son C-G. Soluble components of Hericium erinaceum induce NK cell activation via production of interleukin-12 in mice splenocytes. Acta Pharmacol Sin. 2007;28(6):901–907. doi: 10.1111/j.1745-7254.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Yang Y, Gad E, Inatsuka C, Wenner CA, Disis ML, Standish LJ. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin Cancer Res. 2011;17(21):6742–6753. doi: 10.1158/1078-0432.CCR-11-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Zhao J, Liu J, Huang Y, Zhong J-J, Tang W. Enhancement of IL-2 and IFN-gamma expression and NK cells activity involved in the anti-tumor effect of ganoderic acid me in vivo. Int Immunopharmacol. 2007;7(6):864–870. doi: 10.1016/j.intimp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Chien CM, Cheng JL, Chang WT, Tien MH, Tsao CM, Chang YH, Chang HY, Hsieh JF, Wong CH, Chen ST. Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56+ NK-cell cytotoxicity in cord blood. Bioorg Med Chem. 2004;12(21):5603–5609. doi: 10.1016/j.bmc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Yan ZF, Liu NX. Activation effects of polysaccharides of Flammulina velutipes mycorrhizae on the T lymphocyte immune function 2014, 285421. [DOI] [PMC free article] [PubMed]
- 8.Xu X, Li J, Hu Y. Polysaccharides from Inonotus obliquus sclerotia and cultured mycelia stimulate cytokine production of human peripheral blood mononuclear cells in vitro and their chemical characterization. Int Immunopharmacol. 2014;21(2):269–278. doi: 10.1016/j.intimp.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 9.De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4(3):239–248. doi: 10.1016/1043-4666(92)90062-V. [DOI] [PubMed] [Google Scholar]
- 10.Dai X, Stanilka JM, Rowe CA, Esteves EA, Nieves C, Spaiser SJ, et al. Consuming Lentinula edodes (shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J Am Coll Nutr. 2015;34(6):478–487. doi: 10.1080/07315724.2014.950391. [DOI] [PubMed] [Google Scholar]
- 11.Wasser SP. Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biom J. 2014;37(6):345–356. doi: 10.4103/2319-4170.138318. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Liang H, Luo L. Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr Res. 2016;424:30–41. doi: 10.1016/j.carres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Kodama N, Komuta K, Sakai N, Nanba H. Effects of D-fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull. 1647;2002:25(12). doi: 10.1248/bpb.25.1647. [DOI] [PubMed] [Google Scholar]
- 14.Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, et al. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidium. J Biol Chem. 1989;264(1):472–478. [PubMed] [Google Scholar]
- 15.Singh SS, Wang H, Chan YS, Pan W, Dan X, Yin CM, et al. Lectins from edible mushrooms. Molecules. 2014;20(1):446–469. doi: 10.3390/molecules20010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akihisa T, Nakamura Y, Tagata M, Tokuda H, Yasukaw, K, Uchiyama E, Kimura Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. 2007, Chem Biodivers, 4(2), 224–231. [DOI] [PubMed]
- 17.Xue Z, Li J, Cheng A, Yu W, Zhang Z, Kou X, Zhou F. Structure identification of Triterpene from the mushroom Pleurotus eryngii with inhibitory effects against breast Cancer. Plant Foods Hum Nutr. 2015;70(3):291–296. doi: 10.1007/s11130-015-0492-7. [DOI] [PubMed] [Google Scholar]
- 18.Durgo K, Koncar M, Komes D, Belscak-Cvitanovic A, Franekic J, Jakopovich I, et al. Cytotoxicity of blended versus single medicinal mushroom extracts on human cancer cell lines: contribution of polyphenol and polysaccharide content. Int J Med Mushrooms. 2013;15(5):435–448. doi: 10.1615/IntJMedMushr.v15.i5.20. [DOI] [PubMed] [Google Scholar]
- 19.Standish LJ, Wenner CA, Sweet ES, Bridge C, Nelson A, Martzen M, et al. Trametes versicolor mushroom immune therapy in breast cancer. J Soc Integr Oncol. 2008;6(3):122–128. [PMC free article] [PubMed] [Google Scholar]
- 20.Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J. 2010;9:54. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torkelson CJ, Sweet E, Martzen MR, Sasagawa M, Wenner CA, Gay J, Putiri A, Standish LJ. Phase 1 clinical trial of Trametes versicolor in women with breast Cancer. ISRN Oncol. 2012;2012:251632. doi: 10.5402/2012/251632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DC, Reet J. Single agent polysaccharopeptide delays metastases and improves survival in naturally occurring hemangiosarcoma. Evid Based Complement Alternat Med. 2012;384301. [DOI] [PMC free article] [PubMed]
- 23.Rosendahl AH, Sun C, Wu D, Andersson R. Polysaccharide-K (PSK) increases p21(WAF/Cip1) and promotes apoptosis in pancreatic cancer cells. Pancreatology. 2012;12(6):467–474. doi: 10.1016/j.pan.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Konagai A, Yoshimura K, Hazama S, Yamamoto N, Aoki K, Ueno T, et al. Correlation between NKG2DL expression and antitumor effect of protein-bound polysaccharide-K in tumor-bearing mouse models. Anticancer Res. 2017;37(8):4093–4101. doi: 10.21873/anticanres.11796. [DOI] [PubMed] [Google Scholar]
- 25.Fritz H, Kennedy DA, Ishii M, Fergusson D, Fernandes R, Cooley K, Seely D. Polysaccharide K and Coriolus versicolor extracts for lung cancer: a systematic review. Integr Cancer Ther. 2015;14(3):201–211. doi: 10.1177/1534735415572883. [DOI] [PubMed] [Google Scholar]
- 26.Coy Catherine, Standish Leanna J., Bender Geoff, Lu Hailing. Significant Correlation between TLR2 Agonist Activity and TNF-α Induction in J774.A1 Macrophage Cells by Different Medicinal Mushroom Products. International Journal of Medicinal Mushrooms. 2015;17(8):713–722. doi: 10.1615/IntJMedMushrooms.v17.i8.20. [DOI] [PubMed] [Google Scholar]
- 27.Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and role from in lignin degradation. FEMS Microbiol Rev. 1994;13(2):125–135. doi: 10.1111/j.1574-6976.1994.tb00039.x. [DOI] [Google Scholar]
- 28.Collins PJ, Dobson A. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol. 1997;63(9):3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand T, Jolivalt C, Caminade E, Joly N, Mougin C, Briozzo P. Purification and preliminary crystallographic study of Trametes versicolor laccase in its native form. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 2):319–321. doi: 10.1107/S0907444901019898. [DOI] [PubMed] [Google Scholar]
- 30.Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551–565. doi: 10.1016/S0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- 31.Chawachart N, Khanongnuch C, Watanabe T, Lumyong S. Rice bran as an efficient substrate for laccase production from thermotolerant basidiomycete Coriolus versicolor strain RC3. Fungal Divers. 2004;15:23–32. [Google Scholar]
- 32.Gloer JB. The chemistry of fungal antagonism and defense. Can J Bot. 1995;73(S1):1265–1274. doi: 10.1139/b95-387. [DOI] [Google Scholar]
- 33.Elder AL. (1970). History of penicillin production. Chem. Eng. Prog. Symp. Series, no 100. New York: American Institute of Chemical Engineers; 1970. [Google Scholar]
- 34.Su C-H, Lai M-N, Lin C-C, Ng L-T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl Microbiol Biotechnol. 2016;100(10):4385–4393. doi: 10.1007/s00253-015-7260-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee JS, Min KM, Cho JY, Hong EK. Study of macrophage activation and structural characteristics of purified polysaccharides from the fruiting body of Hericium erinaceus. J Microbiol Biotechnol. 2009;19(9):951–959. doi: 10.4014/jmb.0901.013. [DOI] [PubMed] [Google Scholar]
- 36.Bak WC, Park JH, Park YA, Ka KH. Determination of Glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology. 2014;42(3):301–304. doi: 10.5941/MYCO.2014.42.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Zhang J, Chen H, Chen X, Lan J, Liu C. Complete mitochondrial genome of the medicinal mushroom Ganoderma lucidum. PLoS One. 2013;8(8):e72038. doi: 10.1371/journal.pone.0072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasliwal RR, Bansal M, Gupta R, Shah S, Dani S, Oomman A, Pai V, Prasad GM, Singhvi S, Patel J, et al. ESSENS dyslipidemia: a placebo-controlled, randomized study of a nutritional supplement containing red yeast rice in subjects with newly diagnosed dyslipidemia. Nutrition. 2016;32(7–8):767–776. doi: 10.1016/j.nut.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Jensen GS, Hart AN, Schauss AG. An antiinflammatory immunogen from yeast culture induces activation and alters chemokine receptor expression on human natural killer cells and B lymphocytes in vitro. Nutr Res. 2007;27:327–335. doi: 10.1016/j.nutres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Jensen GS, Carter SG, Reeves SG, Robinson LE, Benson KF. Anti-inflammatory properties of a dried fermentate in vitro and in vivo. J Med Food. 2015;18(3):378–384. doi: 10.1089/jmf.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen GS, Redman KA, Benson KF, Carter SG, Mitzner MA, Reeves S, Robinson L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: results of a placebo-controlled double-blinded crossover pilot study. J Med Food. 2011;14(9):1002–1010. doi: 10.1089/jmf.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen GS, Patterson KM, Barnes J, Schauss AG, Beaman R, Reeves SG, Robinson LE. A double-blind placebo-controlled, randomized pilot study: consumption of a high-metabolite Immunogen from yeast culture has beneficial effects on erythrocyte health and mucosal immune protection in healthy subjects. The Open Nutrition Journal. 2008;2:68–75. doi: 10.2174/1874288200802010068. [DOI] [Google Scholar]
- 43.Moyad MA, Robinson LE, Zawada ET, Jr Kittelsrud JM. Chen DG. Reeves SG. Weaver SE. Effects of a modified yeast supplement on cold/flu symptoms. Urol Nurs. 2008;28(1):50–55. [PubMed] [Google Scholar]
- 44.Moyad MA, Robinson LE, Kittelsrud JM, Reeves SG, Weaver SE, Guzman AI, Bubak ME. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo-controlled trial. Adv Ther. 2009;26(8):795–804. doi: 10.1007/s12325-009-0057-y. [DOI] [PubMed] [Google Scholar]
- 45.Possemiers S, Pinheiro I, Verhelst A, Van den Abbeele P, Maignien L, Laukens D, Reeves SG, Robinson LE, Raas T, Schneider YJ, Van de Wiele T, Marzorati M. A dried yeast fermentate selectively modulates both the luminal and mucosal gut microbiota and protects against inflammation, as studied in an integrated in vitro approach. J Agric Food Chem. 2013;61(39):9380–9392. doi: 10.1021/jf402137r. [DOI] [PubMed] [Google Scholar]
- 46.Borrego F, Peña J, Solana R. Regulation of CD69 expression on human natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol. 1993;23(5):1039–1043. doi: 10.1002/eji.1830230509. [DOI] [PubMed] [Google Scholar]
- 47.Moretta A, Poggi A, Pende D, Tripodi G, Orengo AM, Pella N, Augugliaro R, Bottino C, Ciccone E, Moretta L. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174(6):1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen GS, Hart AN. Immunomodulation by SanPharma fungal metabolic products. J Altern Complement Med. 2006;12(4):409–416. doi: 10.1089/acm.2006.12.409. [DOI] [PubMed] [Google Scholar]
- 49.Hart AN, Zaske LA, Patterson KM, Drapeau C, Jensen GS. Natural killer cell activation and modulation of chemokine receptor profile in vitro by an extract from the cyanophyta Aphanizomenon flos-aquae. J Med Food. 2007;10(3):435–441. doi: 10.1089/jmf.2007.401. [DOI] [PubMed] [Google Scholar]
- 50.Jensen GS, Patterson KM, Yoon I. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp Immunol Microbiol Infect Dis. 2008;31(6):487–500. doi: 10.1016/j.cimid.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Benson Kathleen F., Carter Steve G., Patterson Kelly M., Patel Dilip, Jensen Gitte S. A novel extract from bovine colostrum whey supports anti-bacterial and anti-viral innate immune functions in vitro and in vivo. Preventive Medicine. 2012;54:S116–S123. doi: 10.1016/j.ypmed.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Benson KF, Beaman JL, Ou B, Okubena A, Okubena O, Jensen GS. West African Sorghum bicolor leaf sheaths have anti-inflammatory and immune-modulating properties in vitro. J Med Food. 2013;16(3):230–238. doi: 10.1089/jmf.2012.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benson KF, Newman RA, Jensen GS. Antioxidant, anti-inflammatory, anti-apoptotic, and skin regenerative properties of an Aloe vera-based extract of Nerium oleander leaves (nae-8(®)) Clin Cosmet Investig Dermatol. 2015;8:239–248. doi: 10.2147/CCID.S79871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benson KF, Newman RA, Jensen GS. Water-soluble egg membrane enhances the immunoactivating properties of an Aloe vera-based extract of Nerium oleander leaves. Clin Cosmet Investig Dermatol. 2016;9:393–403. doi: 10.2147/CCID.S114471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen GS, Redman KA, Benson KF, Carter SG, Mitzner MA, Reeves S, Robinson L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: results of a placebo-controlled double-blinded crossover pilot study. J Med Food. 2011;14(9):1002–1010. doi: 10.1089/jmf.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen Gitte S., Patel Dilip, Benson Kathleen F. A novel extract from bovine colostrum whey supports innate immune functions. II. Rapid changes in cellular immune function in humans. Preventive Medicine. 2012;54:S124–S129. doi: 10.1016/j.ypmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Dons'koi BV, Chernyshov VP, Osypchuk DV. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J Immunol Methods. 2011;372(1–2):187–195. doi: 10.1016/j.jim.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Borrego F, Robertson MJ, Ritz J, Peña J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97(1):159–165. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207(2):85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 60.Benlahrech A, Donaghy H, Rozis G, Goodier M, Klavinskis L, Gotch F, Patterson S. Human NK cell up-regulation of CD69, HLA-DR, interferon γ secretion and cytotoxic activity by Plasmacytoid dendritic cells is regulated through overlapping but different pathways. Sensors (Basel) 2009;9(1):386–403. doi: 10.3390/s90100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batbayar S, Kim MJ, Kim HW. Medicinal mushroom Lingzhi or Reishi, Ganoderma lucidum (W.Curt.:Fr.) P. karst., beta-glucan induces toll-like receptors and fails to induce inflammatory cytokines in NF-kappaB inhibitor-treated macrophages. Int J Med Mushrooms. 2011;13(3):213–225. doi: 10.1615/IntJMedMushr.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- 62.Jo E-K, Heo D-J, Kim J-H, Lee Y-H, Ju Y-C, Lee S-C. The effects of subcritical water treatment on antioxidant activity of Golden oyster mushroom. Food Bioprocess Technol. 2013;6:2555–2561. doi: 10.1007/s11947-012-0793-x. [DOI] [Google Scholar]
- 63.Seo H-K, Lee S-C. Antioxidant activity of subcritical water extracts from Chaga mushroom (Inonotus obliquus) Sep Sci Technol. 2009;45(2):198–203. doi: 10.1080/01496390903423899. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The graphs in the paper shows representative data on immune effects on cells from one of three healthy donors. The full sets of data from all 3 blood donors are shown in the supplementary information.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.