Summary
The invasion of a suitable host hepatocyte by Plasmodium sporozoites is an essential step in malaria infection. We demonstrate that in infected hepatocytes, lysosomes are redistributed away from the nucleus, and surface exposure of lysosome-associated membrane protein 1 (LAMP1) is increased. Lysosome exocytosis in infected cells occurs independently of sporozoite traversal. Instead, a sporozoite-secreted factor is sufficient for the process. Knockdown of SNARE proteins involved in lysosome-plasma membrane fusion reduces lysosome exocytosis and Plasmodium infection. In contrast, promoting fusion between the lysosome and plasma membrane dramatically increases infection. Our work demonstrates parallels between Plasmodium sporozoite entry of hepatocytes and infection by the excavate pathogen Trypanosoma cruzi and raises the question of whether convergent evolution has shaped host cell invasion by divergent pathogens.
Subject Areas: Biological Sciences, Molecular Biology, Parasitology, Microbiology Parasite, Molecular Microbiology, Cell Biology
Graphical Abstract
Highlights
-
•
Plasmodium sporozoites induce host lysosome exocytosis during invasion
-
•
Hepatocyte lysosome exocytosis occurs in a SPECT2-independent manner
-
•
Inhibition of lysosome-plasma membrane fusion inhibits sporozoite invasion
-
•
Secreted parasite factors are sufficient to induce lysosome exocytosis
Biological Sciences; Molecular Biology; Parasitology; Microbiology Parasite; Molecular Microbiology; Cell Biology
Introduction
Plasmodium parasites, the causative agents of malaria, are transmitted to humans by the bite of infected female Anopheles mosquitoes. The sporozoite form of the parasite is deposited into human skin during a blood meal. Sporozoites are motile and rapidly migrate through the skin to enter a capillary, which allows the parasite to travel to the liver. Plasmodium sporozoites have the capacity to transmigrate through cells using a process termed cell traversal (CT) (Mota and Rodriguez, 2001). Recent studies have demonstrated that sporozoites can also enter hepatocytes within a transient vacuole, independently of CT, and that parasites that are CT deficient within a transient vacuole associate with lysosomes and are eliminated (Risco-Castillo et al., 2015). In contrast, productive invasion occurs when sporozoites invade a hepatocyte, form a parasitophorous vacuole (PV), and develop into a liver stage (LS) schizont, from which merozoites are released into the bloodstream and invade erythrocytes. The secretion of a multitude of sporozoite factors are released during motility, CT, and invasion, yet a precise role for most secreted factors remains undefined.
Membrane vesicle trafficking is fundamental to eukaryotic life and plays a regulatory role in nearly all cellular activities. Many intracellular pathogens target and subvert these trafficking events for their own benefit (Alix et al., 2011, Asrat et al., 2014). Previous work has demonstrated that the Plasmodium liver stage PV membrane co-localizes with late endosomes (Petersen et al., 2017), lysosomes (Lopes da Silva et al., 2012, Niklaus et al., 2019, Prado et al., 2015, Risco-Castillo et al., 2015), and autophagic vesicles (Prado et al., 2015, Real et al., 2018, Wacker et al., 2017). Although these studies have suggested that the vesicle association is gradually lost over the course of liver stage infection (Niklaus et al., 2019, Prado et al., 2015, Risco-Castillo et al., 2015), when this association is initiated during Plasmodium life cycle progression remains unknown, and none of these studies have evaluated infection prior to 3 h post-entry. Moreover, the role of host vesicular trafficking processes in sporozoite entry of hepatocytes has not been explored.
Results and Discussion
Plasmodium Sporozoites Co-localize with LAMP1-Positive Vesicles
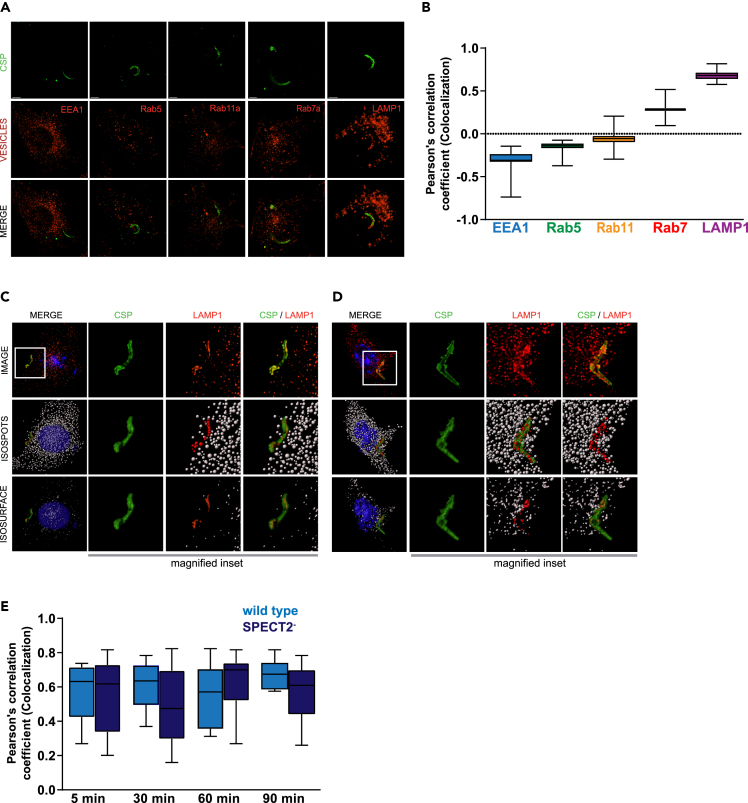
We quantitatively surveyed the extent of co-localization between the parasite and five markers of endocytic compartments. Freshly isolated Plasmodium yoelii sporozoites were added to Hepa1-6 cells. After 90 min, cells were fixed, stained, and visualized by 3D fluorescence deconvolution microscopy. We used antibodies against early endosome antigen 1 (EEA1) and Ras-related protein 5 (Rab5) to mark early endosomes, Rab7a to mark late endosomes (LE), Rab11a to mark recycling endosomes, and lysosome-associated membrane protein 1 (LAMP1) to mark LE/lysosomes. Sporozoites were labeled with an antibody against circumsporozoite protein (CSP) (Figure 1A). Intensity-based co-localization was used (Bolte and Cordelieres, 2006) to evaluate the extent of overlap between CSP and staining for each vesicular compartment (Figures 1A and 1B). The Pearson's correlation coefficient between CSP and LAMP1 was ∼0.6, but staining did not significantly overlap between CSP and EEA1, Rab5, Rab7a, or Rab11a (Figures 1A and 1B). These data are consistent with earlier observations (Lopes da Silva et al., 2012, Petersen et al., 2017).
Figure 1.
Plasmodium Sporozoites Co-localize with LAMP1-Positive Vesicles
(A) Hepa1-6 cells were infected with P. yoelii sporozoites for 90 min and processed for fluorescence microscopy using antibodies to EEA1, Rab5a, Rab11, Rab7a, and LAMP1 (red) and PyCSP (green). Scale bar represents 5 μm.
(B) Pearson's correlation coefficients were calculated for each endocytic vesicle channel with the sporozoite CSP channel of 25 different microscopic fields from three independent experiments. Hepa1-6 cells were infected with P. yoelii sporozoites for 5 min (C) and 30 min (D) and processed for fluorescence microscopy using DAPI (blue) for DNA, phalloidin (white) for actin visualization, antibodies to LAMP1 (red) for LE/lysosomes, and CSP (green) for parasites. Isospot rendering for LE/lysosomes and isosurface rendering for LE/lysosomes, parasites, host cell nucleus, and plasma membrane are shown. Red isospots represent LAMP1-positive structures co-localized with CSP. Magnified inset is 15 μm × 15 μm.
(E) Hepa1-6 cells were infected with wild type or SPECT2-P. yoelii sporozoites and fixed after 5, 30, 60, and 90 min. Intensity-based colocalization was performed on at least 25 parasites per time point and Pearson's correlation coefficients were calculated. Box and whiskers plot depict mean ± SD of three independent experiments.
We next assessed the kinetics of co-localization between sporozoites and LAMP1. Hepa1-6 cells were infected with P. yoelii sporozoites and fixed after 5 (Figure 1C), 30 (1D), 60, or 90 min (1E). LAMP1 structures that co-localized with CSP were observed as early as 5 min and were elongated in the infected cells (Figure 1C). These elongated LAMP1 structures were not observed in bystander or unexposed cells. Thus, the association between LAMP1-postive LE/lysosomes and sporozoites occurs during or very soon after infection and is maintained. We observed a very similar association between LAMP1 and CSP in the CT-deficient parasite, PySPECT2−(Ishino et al., 2004) (Figures 1E, S1A, and S1B). Our data are consistent with the hypothesis that lysosomes interact with the sporozoite during or very soon after entry, independently of CT. Since PySPECT2- parasites cannot egress from the transient vacuole, these data do not distinguish between productive and non-productive entry.
Sporozoite Entry Is Associated with LE/Lysosome Redistribution
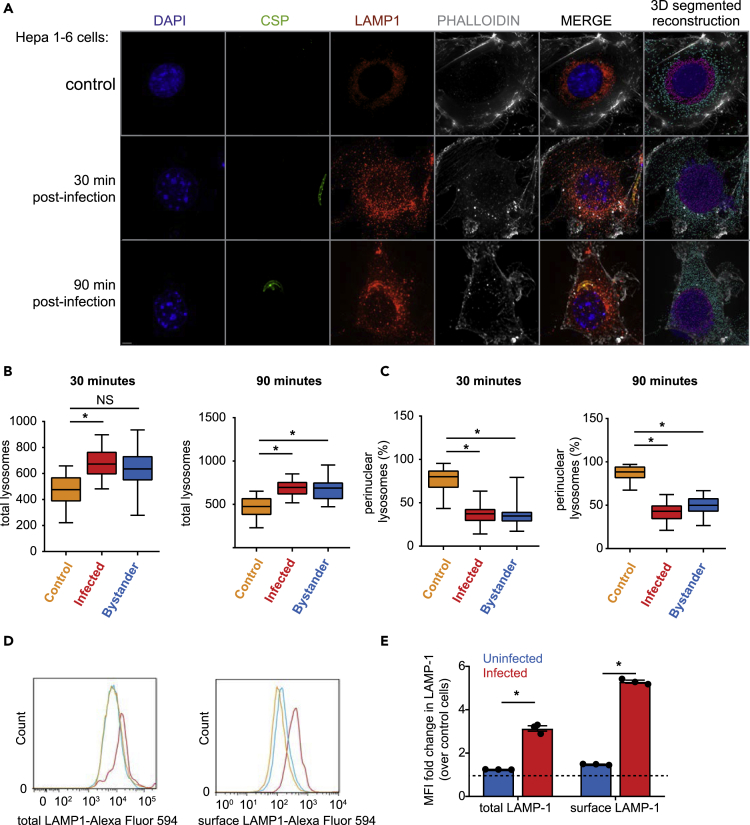
Lysosomes are typically located in juxtanuclear regions of the cell under basal conditions but can be redistributed under times of stress or during infection (Yokota et al., 1989). To evaluate lysosome localization during Plasmodium infection, we infected Hepa1-6 cells with P. yoelii sporozoites and fixed after 30 or 90 min (Figure 2A). To assess the quantity and localization of lysosomes within infected and uninfected cells we defined LE/lysosomes as LAMP1-positive structures between 0.25 and 1 μm in diameter, corresponding to the typical size of LE/lysosomes within the mammalian cell. Hepa1-6 cells contained an average of ∼450 LAMP1-positive structures, similar to measurements obtained by other groups (Cortez et al., 2016). We defined perinuclear lysosomes as LAMP1-positive structures that were within a region surrounding the nucleus, delineated by extrapolating the DAPI signal (Cortez et al., 2016). In unexposed or mock-treated samples containing material from the salivary glands of uninfected mosquitoes, ∼85% of LE/lysosomes were perinuclear (Figure 2A). In infected cells, lysosomes were slightly higher in number (Figure 2B) and significantly less perinuclear (Figure 2C). Interestingly, bystander cells, which were defined as being immediately proximate to the infected cell, also exhibited an increase in lysosome numbers and redistribution (Figures 2B and 2C).
Figure 2.
Sporozoite Entry Is Associated with LE/lysosome Redistribution
(A) Hepa1-6 cells were infected with P. yoelii sporozoites and fixed after 30 or 90 min. DNA was visualized with DAPI (blue), actin with phalloidin (white), LE/Lysosomes with anti-LAMP1 (red), and parasites with anti-CSP (green). Images are maximum intensity projections of the 3D dataset. Scale bar represents 5 μm. The isospots corresponding to lysosomes away from the nucleus are cyan while perinuclear lysosomes are shown in magenta.
(B and C) Values represented in box and whiskers plots correspond to lysosomes from total and perinuclear area represented as mean ± SD of 25 different microscopic fields from three independent experiments. *p < 0.001.
(D) Hepa1-6 cells were infected with P. yoelii sporozoites for 90 min and analyzed by flow cytometry using antibodies specific to LAMP1 and CSP. The histogram depicts the distribution of total and surface LAMP1 from infected, uninfected, and unexposed control cells from one representative experiment.
(E) Total and surface LAMP1 levels were compared between uninfected and infected cells as a fold change over control cells. The bar graph depicts the mean ± the SD of three independent experiments. *p < 0.001.
To assess the fate of redistributed lysosomes, we asked if there was evidence of LAMP1 positive-vesicle fusion with the hepatocyte plasma membrane in infected cells. We infected Hepa1-6 cells with P. yoelii sporozoites and evaluated total and surface-exposed LAMP1 (sLAMP1) by flow cytometry (Figure 2D) and immunofluorescence microscopy (Figure S2A). Both LAMP1 and sLAMP1 were elevated in infected cells compared with exposed uninfected and unexposed control cells (Figures 2D and 2E). Interestingly, little impact on sLAMP1, if any, was observed in uninfected cells, despite our earlier observation that lysosomes redistribute in these cells. These data suggest that lysosomes traffic away from the nucleus in infected and neighboring cells but undergo exocytosis only in infected cells. Although we observed an increase in the total number of lysosomes in bystander cells using microscopy-based methodology, we did not observe an increase in total LAMP1 when it was evaluated by flow cytometry on uninfected cells within infected cultures. This difference might originate from the lack of spatial information that can be resolved in flow cytometry experiments. Alternatively, the ability to quantify LAMP1-positive vesicles may be greater using microscopy-based methodology, which could result in our inability to distinguish the modest difference in total LAMP1 levels between bystander and uninfected cells. We observed a similar pattern of lysosome redistribution (Figures S2B–S2D) and elevated levels of sLAMP1 (Figures S3A and S3B) when we infected with PySPECT2-. Together, these data suggest that lysosome trafficking and exocytosis are altered in infected hepatocytes, independently of CT.
The PV membrane (PVM) is critical for liver-stage development. Soon after productive infection, parasite factors, including upregulated in infectious sporozoites 4 (UIS4), are translated and trafficked to the PVM (Matuschewski et al., 2002). We infected Hepa1-6 cells with wild-type P. yoelii sporozoites and fixed samples 3 h after infection. LS parasites with an intact PVM were distinguished by positive CSP and UIS4 staining, and LS parasites positive for CSP but negative for UIS4 were defined as unsuccessful invasion events. We observed co-localization of LAMP1 with parasite markers in both cases (Figure S3C), suggesting that lysosomal contents are associated with intracellular parasites, independently of the status of their PVM.
The Role of Lysosome Exocytosis Varies across Species of Intracellular Parasites
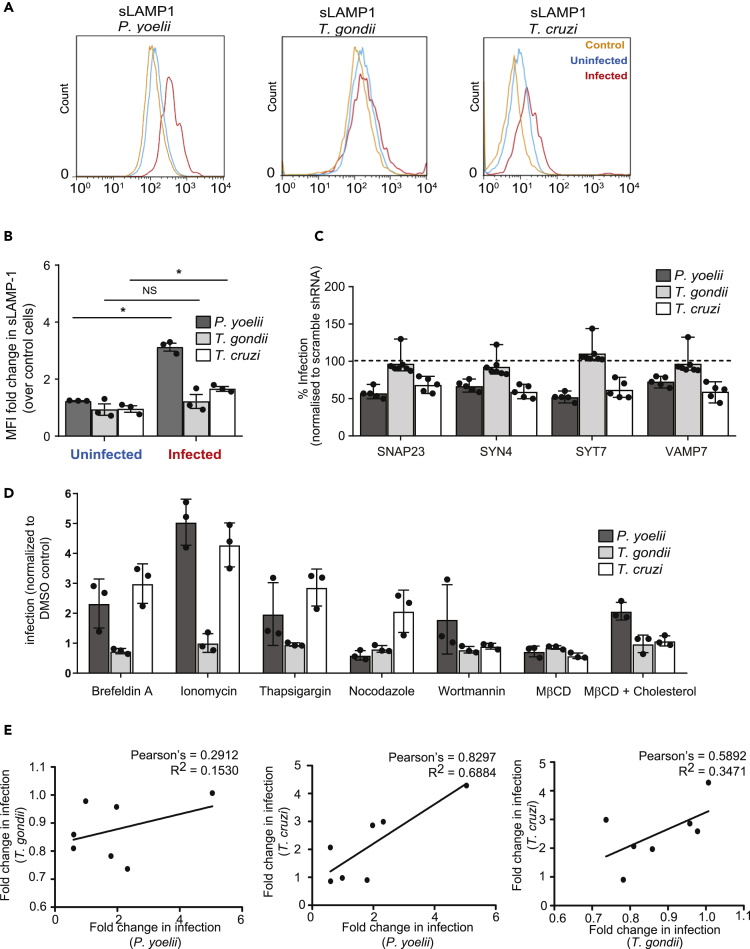
Lysosomes have been previously demonstrated to play a role in Trypanosoma cruzi host cell entry (Tardieux et al., 1992). Specifically, a portion of T. cruzi parasites utilize a lysosome-mediated event to enter the host cell (Hissa et al., 2012, Tardieux et al., 1992). In contrast, Toxoplasma gondii, an apicomplexan parasite closely related to Plasmodium, sequesters host lysosomes to the vacuolar space (Coppens et al., 2006) but is not thought to use lysosomes to facilitate host cell entry. To elucidate how the invasion of Plasmodium parasites is compared with these two disparate systems, we infected Hepa1-6 cells with P. yoelii sporozoites, T. gondii tachyzoites, or T. cruzi trypomastigotes and assessed infection and sLAMP1 by flow cytometry after 90 min. Cells infected with P. yoelii or T. cruzi, but not T. gondii, exhibited increased sLAMP1 (Figures 3A and 3B).
Figure 3.
The Role of Lysosome Exocytosis Varies across Species Of Intracellular Parasites
(A and B) Hepa1-6 cells were infected with P. yoelii sporozoites, T. gondii tachyzoites or T. cruzi trypomastigotes for 90 min and analyzed by flow cytometry. The histogram shows surface LAMP1 from infected, uninfected, and unexposed control cells. Surface LAMP1 is expressed as fold change over uninfected cells. The bar graph displays the mean ± SD of three independent experiments. *p < 0.001.
(C) Hepa1-6 cells were transduced with shRNA lentiviruses against SNAP23, SYN4, SYT7, VAMP7, or a scrambled control and challenged with P. yoelii sporozoites, T. gondii tachyzoites, or T. cruzi trypomastigotes for 90 min. The bar graph displays the infection rate after knockdown of each transcript of interest normalized to scramble shRNA cells, indicated by dashed line (n = 5 for Plasmodium and Trypanosoma; n = 7 for Toxoplasma infection; mean ± SD).
(D) Hepa1-6 cells were incubated with or without the indicated compound for 15 min, washed, and then infected with the indicated parasite for 90 min. The bar graph represents mean ± SD of three independent experiments.
(E) Pearson's correlation coefficients were calculated from the data in (D) for each pairwise combination of infections.
Fusion between lysosomes and the plasma membrane is mediated by the SNARE complex, which includes synaptotagmin VII (SYT7), syntaxin 4 (SYN4), vesicle associate membrane protein 7 (VAMP7), and synaptosomal-associated protein 23 (SNAP23) (Rao et al., 2004). We knocked down each factor in Hepa1-6 cells using lentivirus-encoded shRNAs and observed decreased levels of transcript (Figure S4A) and reduced sLAMP1 (Figure S4B) (Rao et al., 2004). We then infected each knockdown line with T. gondii, P. yoelii, or T. cruzi parasites. Knockdown of each member of the SNARE complex significantly reduced P. yoelii and T. cruzi infections, but not T. gondii infection (Figure 3C), and did not reduce the viability of infected cells (Figure S4C).
Genetic knockdowns can sometimes lead to compensatory changes that produce off-target effects. To partially circumvent this, we evaluated the impact of a range of small molecules that modulate lysosome exocytosis (Table S1). We treated Hepa1-6 cells with each compound for 15 min, washed the cells, and then infected with P. yoelii sporozoites, T. gondii tachyzoites, or T. cruzi trypomastigotes for 90 min. Molecules that increase lysosome exocytosis or redistribution (ionomycin, thapsigargin, brefeldin A; Table S1 and Figure S4C) significantly increased P. yoelii and T. cruzi infection (Figure 3D). In contrast, pretreatment with MβCD, which has been previously shown to reduce surface LAMP1 levels by disrupting lipid rafts, reduced our readout of sLAMP1 (Figure S4D) and diminished P. yoelii and T. cruzi infections (Figure 3D). Levels of sLAMP1 and infection returned to baseline when cholesterol was added, restoring lipid raft formation. No inhibitors substantially altered T. gondii infection (Figure 3D). Overall, changes in P. yoelii and T. cruzi infections were tightly correlated (Pearson Correlation Coefficient, 0.8297) (Figure 3E), whereas other pairwise comparisons were less correlated. Our data suggest that T. cruzi and P. yoelii, but not T. gondii, rely on a lysosome-mediated mechanism to enter the host cell. The extent of these parallels, and ways in which the entry strategies diverge, remains an important area for further investigation.
Secreted Sporozoite Factor(s) Contribute to Lysosome Redistribution during Invasion
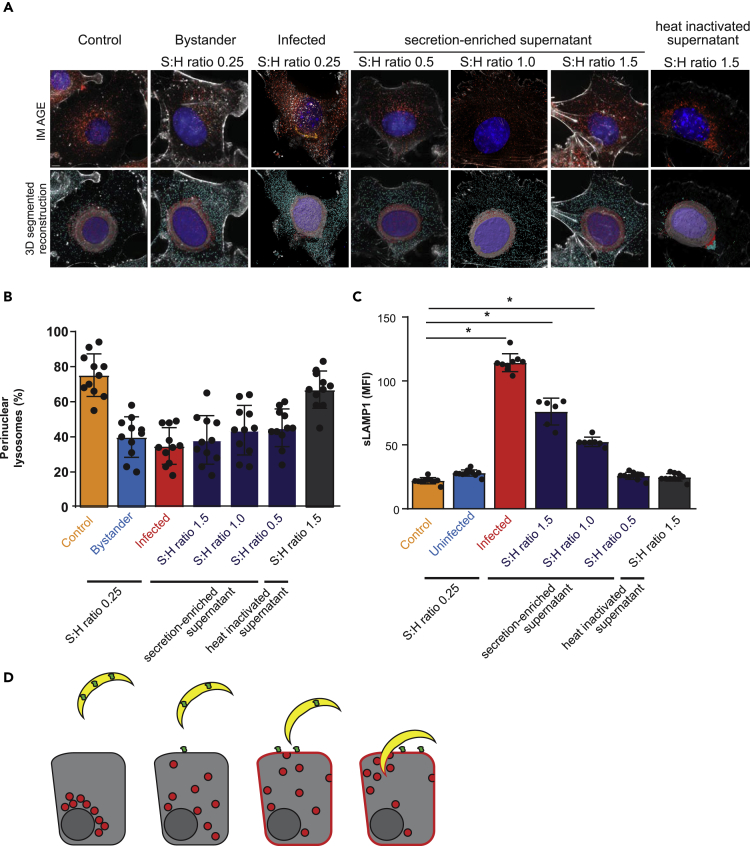
Lysosome exocytosis is induced by the soluble T. cruzi factor Tcgp82 (Cortez et al., 2016). To assess if a parallel process occurs during Plasmodium entry, we treated sporozoites with FBS to induce secretion and then collected supernatants. We exposed Hepa1-6 cells to this sporozoite-derived, secretion-enriched supernatant at different sporozoite:hepatocyte ratios for 90 min. Cells were monitored for lysosome redistribution by 3D fluorescence microscopy (Figures 4A, 4B, S5A) and sLAMP1 by flow cytometry (Figure 4C). Treating cells with even low quantities of secretion-enriched supernatant, but not heat-inactivated supernatant, promoted lysosome redistribution (Figure 4B), and sLAMP1 was induced in a dose-dependent manner (Figures 4C and S5B). Therefore, sporozoite-induced lysosome redistribution is impacted by different factors or the same factors at different levels than lysosome exocytosis. These results are consistent with a model where two separate secretion-mediated events induce hepatocyte lysosome redistribution and lysosome exocytosis (Figure 4D). Taken together, our data suggest a role for lysosome exocytosis in hepatocyte entry of sporozoites, independently of CT or the presence of the PVM.
Figure 4.
Secreted Sporozoite Factor(s) Contribute to Lysosome Redistribution during Invasion
(A) Hepa1-6 cells were infected with P. yoelii sporozoites or treated with sporozoite secretion-enriched supernatants at different sporozoite:hepatocyte (S:H) ratios. After 90 min, cells were processed using DAPI (blue) for DNA, phalloidin (white) for actin visualization, antibodies to LAMP1 (red) for LE/lysosomes, and CSP (green) for parasites and displayed as maximum intensity projections. Scale bar represents 5 μm. The isospots corresponding to lysosomes away from the nucleus and perinuclear lysosomes were depicted in cyan and magenta, respectively.
(B) Values represented in bar graphs correspond to percentage of perinuclear lysosomes identified in (A). Data represent the mean ± SD of at least 10 different microscopic fields per condition from three independent experiments.
(C) Hepa1-6 cells were infected with P. yoelii sporozoites or exposed to sporozoite secretion-enriched supernatants. Surface LAMP1 was analyzed by flow cytometry. Values represent the mean ± the SD of three independent experiments. *p < 0.001.
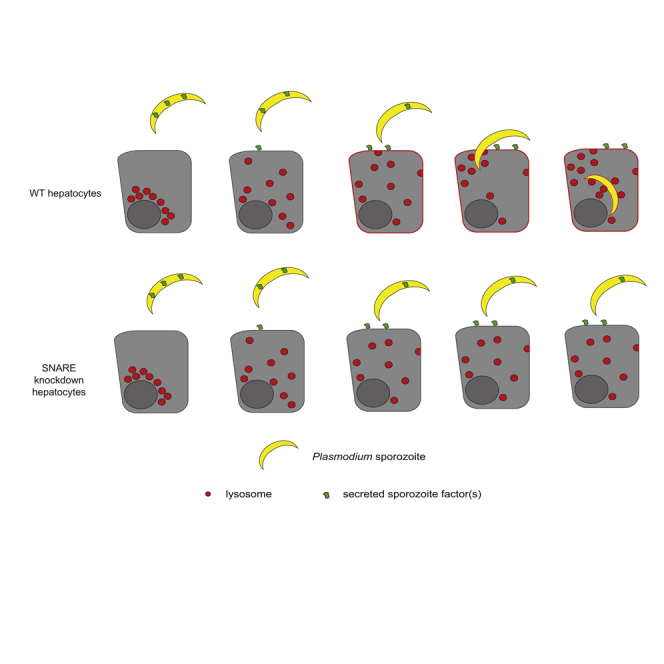
(D) Model of Plasmodium sporozoites promoting lysosome exocytosis. Sporozoites are depicted in yellow, hepatocytes in gray, lysosomes in red, and a Plasmodium-derived secreted factor in green.
A growing collection of evidence suggests that parasites that differ only slightly in genetic makeup can exhibit drastically altered host cell tropism. For example, Plasmodium species rely differentially on host proteins CD81 and SRB1 for entry (Manzoni et al., 2017, Risco-Castillo et al., 2014, Silvie et al., 2003), and this relationship cannot be predicted by evolutionary similarity alone (Frech and Chen, 2011). Here, we demonstrate that lysosome-related alterations impact P. yoelii and T. cruzi infections similarly but have no effect on the apicomplexan parasite, T. gondii. These observations raise the question of how quickly pathogens can evolve host cell tropism and whether the similarities we observe are sculpted by convergent evolution. Systematic investigation into mechanisms of host cell invasion across many pathogens with well-defined evolutionary relationships, will allow us to obtain a better understanding of the major influences that shape host cell engagement over evolutionary time.
Limitations of the Study
In this study, we addressed the role of lysosome exocytosis during Plasmodium liver stage infection. Although we systematically explored this process using temporally resolved immunofluorescence imaging, our work does not definitively demonstrate that lysosome exocytosis occurs at the moment of infection. Subsequent work that utilizes live cell imaging could add temporal detail to our findings.
The functional studies contained within this manuscript are performed using pharmacological inhibitors and shRNA-mediated knockdown, each which, has off-target effects. Moreover, we have performed these experiments using cell lines in vitro. These technical limitations represent potential caveats of our study.
Finally, we demonstrate that secreted parasite factor(s) are sufficient to induce hepatocyte lysosome exocytosis. Subsequent work will elucidate the specific effectors of this process.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Marilyn Parsons for the RHΔHXGPRT T. gondii strain. We thank the Center for Infectious Disease Research/Seattle Children's Research Institute vivarium staff for their work with mice. All work was done according to IACUC procedures and protocols. This work was supported by National Institutes of Health grants R01GM101183 (AK), 1K99/R00AI111785 (AK), R01AI014102 (KS, IC and SM), P41 GM109824. (JDA), T32 Post-Doctoral Fellowship AI07509 (EKKG), a W.M. Keck Foundation award (AK), Science and Engineering Research Board, and INDO-US Science and Technology Forum - Overseas Post-Doctoral Fellowship (KV). FDM is a postdoctoral fellow with the Canadian Institutes of Health Research.
Author Contributions
K.V., E.K.K.G., I.C., S.M., H.S.K., and A.M.B. performed experiments. K.V., I.C., and F.D.M. analyzed data. J.D.A., K.S., and A.K. supervised the research. K.V. and A.K. wrote the paper with input from all other authors.
Declaration of Interests
The authors declare no competing interests.
Published: November 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.054.
Data and Code Availability
The data supporting the findings of this study are available within the paper and its Supplemental Information.
Supplemental Information
References
- Alix E., Mukherjee S., Roy C.R. Subversion of membrane transport pathways by vacuolar pathogens. J. Cell Biol. 2011;195:943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrat S., de Jesus D.A., Hempstead A.D., Ramabhadran V., Isberg R.R. Bacterial pathogen manipulation of host membrane trafficking. Annu. Rev. Cell Dev. Biol. 2014;30:79–109. doi: 10.1146/annurev-cellbio-100913-013439. [DOI] [PubMed] [Google Scholar]
- Bolte S., Cordelieres F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Coppens I., Dunn J.D., Romano J.D., Pypaert M., Zhang H., Boothroyd J.C., Joiner K.A. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Cortez C., Real F., Yoshida N. Lysosome biogenesis/scattering increases host cell susceptibility to invasion by Trypanosoma cruzi metacyclic forms and resistance to tissue culture trypomastigotes. Cell Microbiol. 2016;18:748–760. doi: 10.1111/cmi.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech C., Chen N. Genome comparison of human and non-human malaria parasites reveals species subset-specific genes potentially linked to human disease. PLoS Comput. Biol. 2011;7:e1002320. doi: 10.1371/journal.pcbi.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissa B., Duarte J.G., Kelles L.F., Santos F.P., del Puerto H.L., Gazzinelli-Guimaraes P.H., de Paula A.M., Agero U., Mesquita O.N., Guatimosim C. Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells. PLoS Negl. Trop. Dis. 2012;6:e1583. doi: 10.1371/journal.pntd.0001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T., Yano K., Chinzei Y., Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva M., Thieleke-Matos C., Cabrita-Santos L., Ramalho J.S., Wavre-Shapton S.T., Futter C.E., Barral D.C., Seabra M.C. The host endocytic pathway is essential for Plasmodium berghei late liver stage development. Traffic. 2012;13:1351–1363. doi: 10.1111/j.1600-0854.2012.01398.x. [DOI] [PubMed] [Google Scholar]
- Manzoni G., Marinach C., Topcu S., Briquet S., Grand M., Tolle M., Gransagne M., Lescar J., Andolina C., Franetich J.F. Plasmodium P36 determines host cell receptor usage during sporozoite invasion. Elife. 2017;6:e25903. doi: 10.7554/eLife.25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K., Ross J., Brown S.M., Kaiser K., Nussenzweig V., Kappe S.H. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. 2002;277:41948–41953. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- Mota M.M., Rodriguez A. Migration through host cells by apicomplexan parasites. Microbes Infect. 2001;3:1123–1128. doi: 10.1016/s1286-4579(01)01473-3. [DOI] [PubMed] [Google Scholar]
- Niklaus L., Agop-Nersesian C., Schmuckli-Maurer J., Wacker R., Grunig V., Heussler V.T. Deciphering host lysosome-mediated elimination of Plasmodium berghei liver stage parasites. Sci. Rep. 2019;9:7967. doi: 10.1038/s41598-019-44449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen W., Stenzel W., Silvie O., Blanz J., Saftig P., Matuschewski K., Ingmundson A. Sequestration of cholesterol within the host late endocytic pathway restricts liver-stage Plasmodium development. Mol. Biol. Cell. 2017;28:726–735. doi: 10.1091/mbc.E16-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado M., Eickel N., De Niz M., Heitmann A., Agop-Nersesian C., Wacker R., Schmuckli-Maurer J., Caldelari R., Janse C.J., Khan S.M. Long-term live imaging reveals cytosolic immune responses of host hepatocytes against Plasmodium infection and parasite escape mechanisms. Autophagy. 2015;11:1561–1579. doi: 10.1080/15548627.2015.1067361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.K., Huynh C., Proux-Gillardeaux V., Galli T., Andrews N.W. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- Real E., Rodrigues L., Cabal G.G., Enguita F.J., Mancio-Silva L., Mello-Vieira J., Beatty W., Vera I.M., Zuzarte-Luis V., Figueira T.N. Plasmodium UIS3 sequesters host LC3 to avoid elimination by autophagy in hepatocytes. Nat. Microbiol. 2018;3:17–25. doi: 10.1038/s41564-017-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco-Castillo V., Topcu S., Marinach C., Manzoni G., Bigorgne A.E., Briquet S., Baudin X., Lebrun M., Dubremetz J.F., Silvie O. Malaria sporozoites traverse host cells within transient vacuoles. Cell Host Microbe. 2015;18:593–603. doi: 10.1016/j.chom.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Risco-Castillo V., Topcu S., Son O., Briquet S., Manzoni G., Silvie O. CD81 is required for rhoptry discharge during host cell invasion by Plasmodium yoelii sporozoites. Cell Microbiol. 2014;16:1533–1548. doi: 10.1111/cmi.12309. [DOI] [PubMed] [Google Scholar]
- Silvie O., Rubinstein E., Franetich J.F., Prenant M., Belnoue E., Renia L., Hannoun L., Eling W., Levy S., Boucheix C. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat. Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- Tardieux I., Webster P., Ravesloot J., Boron W., Lunn J.A., Heuser J.E., Andrews N.W. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Wacker R., Eickel N., Schmuckli-Maurer J., Annoura T., Niklaus L., Khan S.M., Guan J.L., Heussler V.T. LC3-association with the parasitophorous vacuole membrane of Plasmodium berghei liver stages follows a noncanonical autophagy pathway. Cell. Microbiol. 2017;19:12754. doi: 10.1111/cmi.12754. [DOI] [PubMed] [Google Scholar]
- Yokota S., Himeno M., Kato K. Immunocytochemical localization of acid phosphatase in rat liver. Cell Struct Funct. 1989;14:163–171. doi: 10.1247/csf.14.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper and its Supplemental Information.