Abstract
This randomized trial examined the relative effectiveness of primarily compensatory and primarily restorative cognitive interventions in an early psychosis population. A total of 56 patients were randomized to one of two treatments which were applied for four months with a five month follow up assessment. Comparisons were between (1) Cognitive Adaptation Training (CAT) – a treatment that uses environmental supports and weekly home visits to compensate for cognitive challenges and improve community functioning and (2) Action Based Cognitive Remediation (ABCR) – a treatment involving computerized cognitive drill and practice exercises, simulations, goal setting, and behavioral activation. Linear mixed effects models demonstrated significant effects on community functioning for both CAT and ABCR without a difference between conditions (n = 39), with an indication of greater gains at follow up in the ABCR group (n = 31). Improvements in symptomatology were less robust with mixed findings across neurocognition metrics. This study concluded that both CAT and ABCR hold promise as interventions for early intervention psychosis populations but more work is needed to identify illness severity, subtype and contextual considerations that might indicate an emphasis on more compensatory versus more restorative cognitive interventions.
Keywords: Psychosis, Schizophrenia, Cognitive remediation, Cognitive adaptation training, Randomized trial
1. Introduction
The past two decades have seen a rapid uptake in interventions designed to address the persistent and treatment-resistant cognitive challenges that attend schizophrenia (Green, 2006). Cognitive deficits are observed in clinically high-risk, first episode (Bora, 2014), and chronic phases of psychosis (Kurtz, 2005). Two commonly applied models of non-pharmacological intervention to address these challenges are compensatory and restorative approaches. A prominent compensatory intervention is cognitive adaptation training (CAT). CAT addresses the functional impacts of cognitive impairments through the use of home-based environmental supports such as signs, alarm reminders, checklists, and behavioral cueing to bypass cognitive and motivational challenges (Maples and Velligan, 2008). CAT has demonstrated medium to large effects on community functioning and treatment adherence in schizophrenia populations (Velligan et al., 2000, Velligan et al., 2002, Velligan et al., 2008). Improvements in neurocognition have been observed in CAT trials. However, neurocognition has not been found to be a significant mediator of the effects of CAT on community functioning (Fredrick et al., 2015).
The most studied restorative intervention to address cognitive deficits is cognitive remediation (CR). CR seeks to improve cognitive and community functioning through a range of repeated cognitive task practice and strategy acquisition activities (Wykes et al., 2011). Despite the wide range of CR approaches, meta-analyses reveal consistent and durable effects in the medium range on cognitive functioning (McGurk et al., 2007a, McGurk et al., 2007b; Wykes et al., 2011). The effects of CR on community functioning are variable. It has been observed that unless CR is integrated into broader psychiatric rehabilitation interventions such as supported employment (McGurk et al., 2007a, McGurk et al., 2007b) and education (Kidd et al., 2014a), or closely paired with strategy monitoring and real-world task simulation (Bowie et al., 2017), functional impacts are less robust.
Investigation into CAT and CR targeting early illness phase populations are less developed literatures. A small feasibility study found good engagement and acceptance of CAT interventions in an early psychosis population (Allott et al., 2016). Among early intervention populations CR has been found feasible (Breitborde et al., 2017; Cellard et al., 2016) and effective in cognitive and social domains (Fisher et al., 2014; Mendella et al., 2015). Some studies comparing outcomes as a function of age have also indicated greater effects of CR on cognitive functioning for younger participants (Bowie et al., 2014; Kontis et al., 2013), though this finding is not consistent across all such analyses (McGurk et al., 2007a, McGurk et al., 2007b; Wykes et al., 2011).
The present study was designed to contribute to the evidence base examining cognitive interventions in early psychosis treatment and explore the relative effects of primarily compensatory and restorative interventions. The study design was a randomized trial comparing the effects of CAT and Action Based Cognitive Remediation (ABCR) with community functioning as the primary outcome. Previous evidence was not available to inform hypotheses as to one intervention being more effective than the other, so the question of relative benefit was exploratory. Secondary outcomes included goal attainment, hospitalization, medication adherence, symptomatology and neurocognition.
2. Methods
2.1. Study design
Participants with psychosis were recruited through both inpatient and outpatient services at a tertiary psychiatric facility and other local outpatient community mental health services in a large urban center in Canada. They were block randomized into CAT and ABCR in stage one and, in a second stage in which a combined version of ABCR+CAT was piloted, were randomize into the combined intervention (data from the combined intervention is reported elsewhere due to the small sample size). The treatment period was 4 months with one follow up assessment at 5 months post-treatment. The assessor was blinded to treatment condition and treatment providers only provided a single type of treatment. Two assessors were used over the course of the trial who cross-trained to >80% reliability on scale delivery. The study was initiated in 2015 with the last follow up data collected in March, 2018.
2.2. Participants
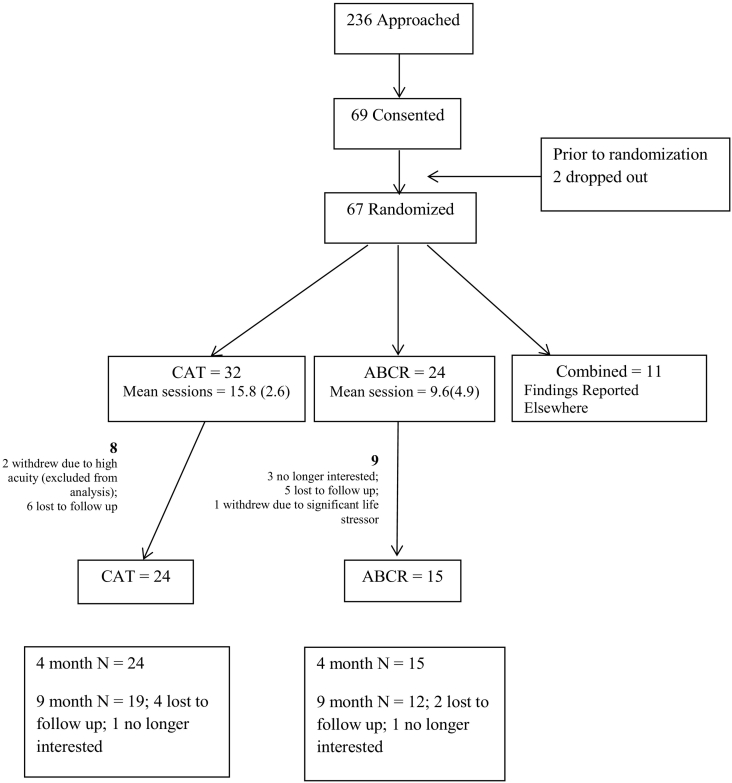
Participants were identified through a centralized recruitment process in inpatient and outpatient early psychosis services at the primary site and through clinician referral from other local sites. All participants provided written consent with forms with processes approved by an Institutional Review Board and with procedures in line with internationally recognized ethical standards. The study was registered through clinicaltrials.gov (#NCT02430935). Of the 69 participants who signed consent, 56 were randomized into CAT and ABCR arms of whom, in turn, follow up data was obtained for 39 (consort diagram – Fig. 1). The Mini International Neuropsychiatric Interview was used to confirm diagnosis. Participants were between 17 and 34 years of age, were fluent English speakers, and most were prescribed oral or depot anti-psychotic medication (96%). Prospective participants were excluded if a clinician diagnosis of intellectual disability was identified. To control for variability in support level in the follow up period, all participants were required to have an outpatient case manager in addition to physician support. In the case of multiple diagnoses, Psychosis NOS, and Bipolar Disorder with Psychotic features (noting shifting diagnoses are common in early intervention), participants were included if structured interview, chart and clinician aligned in identifying psychosis as the primary clinical concern. Of the 56 participants from whom baseline data was obtained, the mean age was 27 years (SD = 4.5), 36 were male, 30 were White, 8 were of African Descent, 6 were East Asian, 5 South Asian, 2 Latin American and 5 indicated other ethnicity. With respect to education, attainment groupings included grades 7–12 without graduation (27%), high school graduation (32%), and some community college or university (41%) (see Table 1).
Fig. 1.
Consort diagram.
Table 1.
Participant demographics at baseline.
| Variable | ABCR |
CAT |
p-Value⁎ |
||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Categorical variables | |||||
| Gender | 0.47 | ||||
| Male | 14 | 58 | 22 | 69 | |
| Female | 10 | 42 | 9 | 28 | |
| Transsexual | 0 | 0 | 1 | 3 | |
| Ethnicity | 0.40 | ||||
| Aboriginal | 0 | 0 | 0 | 0 | |
| African Descent | 4 | 17 | 4 | 13 | |
| Caucasian | 13 | 54 | 17 | 53 | |
| East Asian | 2 | 8 | 4 | 13 | |
| Latin-American | 0 | 0 | 2 | 6 | |
| South Asian | 1 | 4 | 4 | 13 | |
| Other | 4 | 17 | 1 | 3 | |
| Living situation | 0.40 | ||||
| lives alone in a private dwelling | 8 | 33 | 11 | 35 | |
| lives with spouse and children in private dwelling | 1 | 4 | 0 | 0 | |
| lives with parents in private dwelling | 14 | 58 | 14 | 45 | |
| Rooming/boarding home | 0 | 0 | 3 | 10 | |
| Supportive housing | 1 | 4 | 3 | 10 | |
| Education | 0.38 | ||||
| Grade 6 or less | 0 | 0 | 0 | 0 | |
| Grade 7 to 12 (without graduating high school) | 5 | 21 | 10 | 31 | |
| Graduated high school or high school equivalent | 9 | 38 | 9 | 28 | |
| Part college/university | 5 | 21 | 9 | 28 | |
| Graduated 2-year college | 4 | 17 | 1 | 3 | |
| Graduated 4-year undergraduate | 1 | 4 | 3 | 9 | |
| Part graduate/professional school | 0 | 0 | 0 | 0 | |
| Completed graduate or professional school | 0 | 0 | 0 | 0 | |
| Employment status | 0.21 | ||||
| Employed full time | 2 | 8 | 2 | 6 | |
| Employed part time | 0 | 0 | 5 | 16 | |
| Employed casually | 1 | 4 | 2 | 6 | |
| Unemployed | 21 | 88 | 23 | 72 | |
| Time last hospitalized | 0.29 | ||||
| 0 | 1 | 4 | 0 | 0 | |
| <1 week | 0 | 0 | 3 | 10 | |
| 1–4 weeks | 1 | 4 | 1 | 3 | |
| > 4 weeks | 21 | 91 | 26 | 87 | |
| Diagnosis | 1.00 | ||||
| Schizophrenia | 19 | 83 | 23 | 74 | |
| Schizoaffective | 4 | 17 | 5 | 16 | |
| PD-NOS | 0 | 0 | 1 | 3 | |
| Bipolar II - psychotic | 0 | 0 | 1 | 3 | |
| Psychosis | 0 | 0 | 1 | 3 | |
| Comorbidity | 0.69 | ||||
| Schizophrenia | 0 | 0 | 1 | 9 | |
| Schizoaffective | 0 | 0 | 0 | 0 | |
| Depression | 1 | 17 | 4 | 36 | |
| Dyslexia | 0 | 0 | 0 | 0 | |
| Bipolar disorder | 2 | 33 | 1 | 9 | |
| Psychosis | 0 | 0 | 2 | 18 | |
| OCD | 1 | 17 | 1 | 9 | |
| PTSD | 2 | 33 | 1 | 9 | |
| Anxiety | 0 | 0 | 1 | 9 | |
| Continuous variables | Mean (SE) | Mean (SE) | p-Value⁎⁎ |
|---|---|---|---|
| Age | 26.1(0.9) | 27.8(0.8) | 0.22 |
| Age 1st hospitalized | 20.5(1.0) | 22.4(0.7) | 0.04 |
| Total number of hospitalizations in past year | 0.5(0.2) | 1.0(0.2) | 0.29 |
| Total number of days in hospital in past year | 1.6(0.7) | 22.6(10.5) | 0.04 |
| Total number of ER visits | 0.7(0.2) | 0.9(0.2) | 0.52 |
| Medication adherence | 93.9(2.5) | 94.1(3.6) | 0.15 |
| Wide range achievement test-III | 59.3(1.3) | 59.3(1.8) | 0.365 |
Categorical variables compared using Fisher Test.
Continuous variables compared using Mann-Whitney U Test.
2.3. Treatment groups
All treatments were manualized and designed to be delivered weekly for up to 4 months/16 visits. Sessions lasted from 1 to 2 h with missed sessions rescheduled within the same week when possible.
2.3.1. CAT
CAT is a manualized, home-based intervention emphasizing environmental supports (checklists, signs, alarms) and compensatory strategies that are delivered on a weekly basis by the CAT specialist (Maples and Velligan, 2008; Velligan et al., 2002, Velligan et al., 2008). Building from initial behavioral, environmental, and cognitive assessments, individuals with poorer executive functioning require greater structure, more finely articulated steps, and more obvious cues and vice versa. Greater apathy requires more cueing and sequencing and more disinhibition requires the removal of distractions and better organization. Mixed profiles indicate combinations of the above approaches. Environmental supports are tailored to the individual's environment, needs, and recovery goals. Supports are established and maintained over the course of weekly home visits.
2.3.2. ABCR
ABCR is a manualized, group-based intervention (Bowie et al., 2017) that is provided weekly for 16 weeks in 1–2 h sessions. Scientific Brain Training Pro is used for computerized cognitive drill and practice exercises. This is an online program delivered by tablet in group sessions with participants in the present study provided with tablets for the duration of the intervention for home-based practice to ensure technology access. A total of 15 gamified exercises were used for training in attention, processing speed, visual, verbal and working memory, and executive functioning domains. In-group practice was augmented by homework practice, with participants advised to complete 20 min per day. In groups, computer exercises were complemented by strategy monitoring. Transferring to real world tasks is the second element of ABCR and involves the practice of simulated work, social, and recreational tasks and role-plays.
2.4. Assessments
2.4.1. Primary outcomes
The primary measure of functional outcome was the Multnomah Community Ability Scale (MCAS; Barker et al., 1994). Here we report both MCAS scores derived from participant interview as well as clinician (primary case manager) rated MCAS scores (cMCAS). Global level of social and occupational functioning was obtained using the Social and Occupational Functioning Scale (SOFAS; APA, 1994) and engagement in functional activities was assess with the Social Functioning Scale (SFS; Birchwood et al., 1990).
2.4.2. Secondary outcomes
To track progress on personal community recovery goals, Goal Attainment Scaling (GAS; Hurn et al., 2006) was used. GAS identifies up to 5 operationalized goals anchored of a five point scale representing less attainment (−2, −1), achieved (0), and over-attained (+1, +2). Symptoms over the past week were assessed using the expanded version of the Brief Psychiatric Rating Scale (BPRS-E; Ventura et al., 1993). The total score was used as a measure of global symptomatology and the psychosis symptom subscale was examined specifically. Negative symptoms were assessed using the Negative Symptom Assessment (NSA; Alphs et al., 1989). The Wide Range Achievement Test (WRAT-III; Wilkinson, 1993) reading subtest was used to evaluate pre-morbid intelligence. The Trail Making Test Part A (Radford et al., 1978) was used to assess scanning ability and psychomotor speed. Short term memory was evaluated with the digit span subtest of the Weschler Adult Intelligence Scale (The Psychological Corporation, 1997). Verbal learning and memory was assessed with the California Verbal Learning Test (Delis et al., 1987). Executive functioning was assessed with the Trail Making Test, Part B (Radford et al., 1978), and the Wisconsin Card Sorting Test (WCST; Berg, 1948). Sustained attention/vigilance was examined using Digit Vigilance Test (DVT; Kelland and Lewis, 1996). Global cognition was the focus of analyses with the metric calculated as the mean of neurocognition assessment z scores. Key demographics were collected along with hospitalization history with medication adherence as assessed with the Brief Adherence Rating Scale (Byerly et al., 2008).
2.5. Data analysis
All subjects that were initially randomized and had baseline data were analyzed (Intention to Treat Analysis). Initial descriptive analyses were conducted comparing baseline demographics and primary clinical measures across groups. Fisher's Exact test was used for categorical variables and the non-parametric Kruskal-Wallis H test was used for continuous variables. Means (Standard Errors) are reported and a confidence level of 0.05 was adopted along with two tailed tests. The primary analysis was conducted through Linear Mixed Effect Models, where treatment (ABCR, CAT), time (Baseline, 4 Months, 9 Months) and the treatment by time interaction was considered the fixed effect and individual subjects as random effects. Time was considered a categorical predictor. Mixed Effect Models addressed missing values in the outcome (mostly caused by attrition) through Full Maximum Likelihood estimation, which uses all available information in the data and is unbiased under Missing At Random (MAR) assumption. When reported, effects in terms of difference of means are model adjusted effects. Number Needed to Treat (NNT) was reported for binary and continuous data (Kraemer and Kupfer, 2006). Cohen's d is reported for outcome analyses except when all three time points are analyzed in which case Cohen's f is reported (Cohen, 1988). In a secondary analysis the 9 month follow up data was included and models were rerun. All analyses were conducted with R version 3.4.4., with Linear Mixed Effect Models adjusted with the R package and lme4 and p-values used with the Satterthwaite method for calculating the denominator degree of freedom using lmerTest (Bates et al., 2015; Kuznetsova et al., 2017; R Core Team, 2018).
3. Results
There were no statistically significant treatment group differences on primary demographics nor baseline WRAT (Table 1). Age of First Hospitalization was significantly older for the CAT treatment group (p = 0.04) and the number of days hospitalized in the past year was higher for the CAT group (p = 0.04). No primary outcomes presented significant differences at baseline. Of the secondary outcomes a difference was observed on baseline GAS ratings (p = 0.01) with the CAT group (1.88(0.08)) having higher GAS mean rankings than ABCR (1.88 vs. 1.47). None of these significant differences would survive a Bonferroni adjustment for all comparisons done at baseline and it was concluded that randomization was successful. Total participant numbers were: baseline n = 56; 4 months n = 39; 9 month follow up n = 31 (Fig. 1).
3.1. Primary outcomes
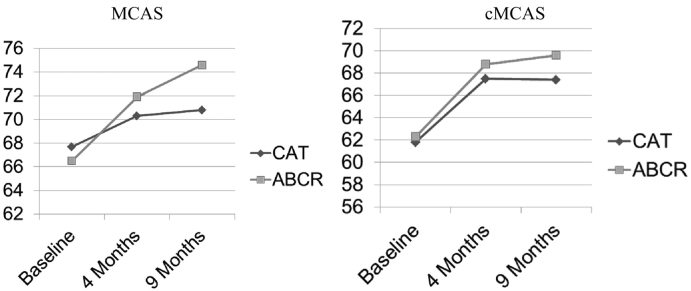
Mixed effects analysis was used to test effects on functional outcomes measured by the MCAS, cMCAS, SOFAS and SFS. While medium-large effects of time were observed at outcome on the MCAS and cMCAS respectively (Fig. 2; MCAS (F(1,90) = 20.45, p < 0.001, NNT = 2.6, d = 0.49); cMCAS F(1,65) = 21.59, p < 0.001, NNT = 1.5, d = 0.75) with increases of 5.22(1.12) points in cMCAS and 4.52(1.00) points in MCAS mean scores, there were no significant between treatment group differences. Similarly, there was a time effect in the medium range for the SOFAS (F(1,89) = 9.18, p = 0.00, NNT = 2.6, d = 0.56) with an increase of 4.6(1.5) points, but no treatment group difference. No significant findings were observed for most SFS subscales. However, a treatment group difference approached significance in the medium effect range for the prosocial activities subscale (F(1,87) = 3.90, p = 0.05, NNT = 2.8, d = 0.44), suggesting a decrease in pro-social activity in the ABCR group compared an increase in the CAT group.
Fig. 2.
Community functioning outcomes.
Incorporating 9-month follow-up data (Table 2), a significant, moderate time by treatment effect was observed on the MCAS (F(2,118) = 4.55, p = 0.01, eta2 = 0.03, Cohen's f = 0.28). This effect reflected a sustained increase in MCAS adjusted mean score in the ABCR group (from baseline to 4 months: 6.14(1.56), t(118) = 3.93, p = 0.00) and from baseline to 9 months: 8.24(1.79), t(118) = 4.61, p < 0.00) contrasted with a smaller increase from Baseline to 4 months in the CAT group (2.82(1.28), t(118) = 2.20, p = 0.033) with no significant change from 4 months to follow up. No such effect was evident for the cMCAS. At 9 months, a large time effect was observed on the SOFAS (F(2,90) = 9.36, p = 0.0002, eta2 = 0.06, Cohen's f = 0.44) without a significant treatment group difference. Considering SFS subscales at follow up, differences between treatment groups were not evident, though moderate time effects were observed for prosocial activities (F(2,82) = 5.35, p = 0.01, eta2 = 0.03, Cohen's f = 0.31), interpersonal behavior (F(2,87) = 4.19, p = 0.02, eta2 = 0.08, Cohen's f = 0.30), independence-performance (F(2,90) = 7.29, p = 0.00, eta2 = 0.05, Cohen's f = 0.36) and independence-competence (F(2,116) = 4.20, p = 0.02, eta2 = 0.03, Cohen's f = 0.27).
Table 2.
Goal Attainment and Symptomology from Baseline, Post to Follow Up by Group.
| ABCR |
CAT |
Group × time mixed model |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Post |
Follow-up |
Baseline |
Post |
Follow-up |
||||
| Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | df | F.value | Pr(>F) | |
| Functioning | |||||||||
| MCAS total | 66.5(1.32) | 71.87(2.00) | 74.6(1.93) | 67.69(1.39) | 70.29(1.20) | 70.75(1.08) | 2/118 | 4.55 | 0.01 |
| Clinician MCAS total | 62.25(1.67) | 68.77(2.26) | 69.6(2.52) | 61.83(1.57) | 67.5(2.10) | 67.36(2.79) | 2/60 | 0.92 | 0.40 |
| Social functioning scale | |||||||||
| Employment/occupation | 4.04 (0.51) | 3.92 (0.75) | 5.00 (1.07) | 4.68 (0.53) | 5.13 (0.68) | 4.79 (0.84) | 2/89 | 0.15 | 0.86 |
| Independence/competence | 33.13 (0.89) | 33.57 (1.73) | 36.40 (0.81) | 33.06 (0.98) | 35.61 (0.84) | 34.85 (1.23) | 2/116 | 1.40 | 0.25 |
| Independence/performance | 25.75 (2.06) | 26.36 (3.11) | 31.80 (3.22) | 28.28 (1.22) | 31.13 (1.19) | 32.30 (1.35) | 2/90 | 1.09 | 0.34 |
| Interpersonal behaviour | 7.08 (0.29) | 7.71 (0.38) | 8.00 (0.45) | 6.90 (0.37) | 6.92 (0.42) | 7.47 (0.34) | 2/87 | 1.39 | 0.25 |
| Prosocial activities | 24.29 (2.50) | 22.50 (3.11) | 19.30 (2.31) | 19.65 (1.58) | 20.25 (2.51) | 17.45 (1.89) | 2/82 | 1.73 | 0.18 |
| Recreation | 19.29 (1.76) | 22.57 (2.63) | 20.50 (2.18) | 17.97 (1.09) | 18.21 (2.02) | 18.65 (1.38) | 2/88 | 0.32 | 0.73 |
| Social engagement/withdrawal | 9.92 (0.48) | 10.14 (0.58) | 11.30 (0.67) | 10.10 (0.36) | 10.17 (0.39) | 10.35 (0.41) | 2/89 | 1.06 | 0.35 |
| Social & occupational functioning scale | 52.75 (2.53) | 57.70 (4.33) | 63.30 (3.59) | 50.22 (2.39) | 56.63 (2.81) | 59.80 (2.43) | 2/90 | 0.79 | 0.46 |
| Goal attainment | |||||||||
| GAS | 1.47(0.10) | 3.55(0.27) | 3.55(0.73) | 1.88(0.08) | 3.57(0.10) | 3.48(0.29) | 2/72 | 0.87 | 0.42 |
| Symptomology | |||||||||
| BPRS total | 36.52(1.75) | 35.93(1.94) | 35.78(2.03) | 36.9(1.26) | 36.92(1.55) | 39.79(1.92) | 2/79 | 1.16 | 0.32 |
| NSA global | 2.58(0.17) | 2.64(0.32) | 3(0.26) | 2.94(0.16) | 3(0.19) | 3.42(0.26) | 2/87 | 0.28 | 0.76 |
3.2. Secondary outcomes
The GAS evidenced a large time effect (F(1,44) = 241.50, p < 0.00) with an increase in adjusted mean of 1.80(0.12) points from baseline to 4 months that remained significant at follow up (F(2,72) = 99.77, p < 0.00, eta2 = 0.64, Cohen's f = 1.57). No difference between treatment groups was observed in goal attainment. No significant change was observed for Total BPRS nor for the psychoticism subscale of the BPRS at outcome and follow up. While outcome effects on negative symptoms were not observed on the NSA, a moderate time effect was evident at follow up (F(2,87) = 5.12, p = 0.01, eta2 = 0.05, Cohen's f = 0.32) again without a treatment group difference. There were no significant effects on days in hospital at outcome and follow up. However, a significant time effect in the moderate range was observed for ER visits at follow up (F(2, 106) = 4.32, p = 0.16, eta2 = 0.05, Cohen's f = 0.29) which reflected an overall decrease in ER visits for both treatment groups. No effects were observed for medication adherence, likely due to a ceiling effect. A composite score was created to represent overall neurocognition by averaging the z-score versions of each neurocognition scale (r = 0.75). No effects were observed at outcome nor follow up for this composite score. Changes were observed in some individual tests are reported in Supplemental file 1.
4. Discussion
With respect to the comparison of CAT to ABCR on change in functional outcome in an early psychosis population, we found medium-large effect size improvements in individuals randomized to either intervention. Considering follow up data, functioning in the CAT group stabilized as previously observed in an older psychosis population (Kidd et al., 2014b), while functioning in the ABCR group continued to improve in the 5-month post treatment period. Changes in symptomatology proved modest which is consistent with previous CAT and CR trials (Velligan et al., 2002; McGurk et al., 2007a, McGurk et al., 2007b). As observed previously for both CAT and ABCR (Fredrick et al., 2015; Bowie et al., 2017), global cognition did not change significantly. Taken as a whole, these findings speak to the relevance of both CAT and ABCR for early psychosis populations, wherein they had to date not been substantively studied for effects and only preliminary evidence existed (Bowie et al., 2017; Allott et al., 2016). The continued improvement in ABCR at follow up, or ‘sleeper effect’, is not unusual in restorative training programs and may speak to a degree of relative superiority of cognitive remediation in early psychosis populations. This hypothesis would require further testing to be confirmed, however, and attention would also need to be given to the influence of process variables of these quite different approaches (e.g., in home, outreach intervention with CAT and within clinic, group-based intervention with ABCR).
Implementation is also a key practice consideration for these cognitive interventions (Wykes and Spaulding, 2011). Both are resource intensive, which affects both feasibility and viability in under-resourced mental health systems. Efforts to optimize access and cost-effectiveness will be essential to bringing compensatory and restorative cognitive interventions to scale. Examples include remotely-delivered CR (Donohoe et al., 2018), CAT implementation by family supports (Kidd et al., 2018) and, more broadly, efforts to generate evidence to inform the targeting of treatment for subpopulations likely to receive the most benefit (Wykes and Spaulding, 2011),
These findings are limited due to power concerns. The lack of treatment group effect difference at outcome and the treatment group difference at follow up need to be interpreted cautiously and will require replication with a larger sample in future work. Generalizability outside of the context of a large urban tertiary care facility in Canada is also a consideration. The lack of treatment as usual and placebo control conditions also limit the degree to which this analysis can provide a clear articulation of the relative effects of each intervention. Finally, it would benefit the field to continue this type of comparative method in different contexts and to use multi-site and pooled data approaches to parse out for whom benefit is optimized as a function of treatment type – be its' emphasis compensatory, restorative or a combination of both.
The following is the supplementary data related to this article.
Changes in Cognitive Measures from Baseline, Post to Follow Up by Group.
Funding
This study was funded by the Slaight Family Centre for Youth in Transition and the Centre for Addiction and Mental Health. There is no funding body agreement in relation to the funds granted and the funders had no role in study design, operations, data analysis nor dissemination.
Declaration of Competing Interest
No author of this paper has any financial and personal relationships with other people or organizations that could inappropriately influence the research described in this paper.
References
- Allott K., Killackey E., Sun P., Brewer W., Velligan D. Cognitive adaptation training for first-episode psychosis: a feasibility study. Early. Interv. Psychiatry. 2016;10:476–484. doi: 10.1111/eip.12207. [DOI] [PubMed] [Google Scholar]
- Alphs L., Summerfelt A., Lann H., Muller R.J. The negative symptom assessment: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol. Bull. 1989;25:159–163. [PubMed] [Google Scholar]
- American Psychiatric Association . Fourth edition. APA; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Barker S., Barron N., McFarland B.H., Bigelow D.A., Carnahan T. A community ability scale for chronically mentally ill consumers: part II. Applications. Community. Ment. Hlt. J. 1994;30:459–472. doi: 10.1007/BF02189063. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. [Google Scholar]
- Berg E. A simple objective test for measuring flexibility in thinking. J. Gen. Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Birchwood M., Smith J., Cochrane R., Wetton S., Copestake S. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br. J. Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bora E. Developmental lag and course of cognitive deficits from the premorbid to post onset period in schizophrenia. Am. J. Psychiatry. 2014;171:369. doi: 10.1176/appi.ajp.2013.13091283. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Grossman M., Gupta M., Kola Oyewumi L., Harvey P.D. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early. Interv. Psychiatry. 2014;8:32–38. doi: 10.1111/eip.12029. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Grossman M., Gupta M., Holshausen K., Best M.W. Action-based cognitive remediation for individuals with serious mental illnesses: effects of real-world simulations and goal setting on functional and vocational outcomes. Psychiatr. Rehabil. J. 2017;40:53–60. doi: 10.1037/prj0000189. [DOI] [PubMed] [Google Scholar]
- Breitborde N.J., Woolverton C., Dawson S.C. Meta-cognitive skills training enhances computerized cognitive remediation outcomes among individuals with first-episode psychosis. Early. Interv. Psychiatry. 2017;11:244–249. doi: 10.1111/eip.12289. [DOI] [PubMed] [Google Scholar]
- Byerly M.J., Nakonezny P.A., Rush A.J. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2008;100:60–69. doi: 10.1016/j.schres.2007.12.470. [DOI] [PubMed] [Google Scholar]
- Cellard C., Reeder C., Paradis-Giroux A.A. A feasibility study of a new computerised cognitive remediation for young adults with schizophrenia. Neuropsychol. Rehabil. 2016;26:321–344. doi: 10.1080/09602011.2015.1019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Delis D., Kramer J., Kaplan E., Ober B. Psychological Corporation; San Antonio, TX: 1987. California Verbal Learning and Memory Test (Manual) [Google Scholar]
- Donohoe G., Dillon R., Hargreaves A. Effectiveness of a low support, remotely accessible, cognitive remediation training programme for chronic psychosis: cognitive, functional and cortical outcomes from a single blind randomised controlled trial. Psychol. Med. 2018;48:751–764. doi: 10.1017/S0033291717001982. [DOI] [PubMed] [Google Scholar]
- Fisher M., Loewy R., Carter C. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr. Bull. 2014;41:250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrick M.M., Mintz J., Roberts D.L. Is cognitive adaptation training (CAT) compensatory, restorative, or both? Schizophr. Res. 2015;166:290–296. doi: 10.1016/j.schres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Green M.F. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. Psychiat. 2006;67:3–8. [PubMed] [Google Scholar]
- Hurn J., Kneebone I., Cropley M. Goal setting as an outcome measure: a systematic review. Clin. Rehabil. 2006;20:756–772. doi: 10.1177/0269215506070793. [DOI] [PubMed] [Google Scholar]
- Kelland D., Lewis R. The digit vigilance test: reliability, validity, and sensitivity to diazepam. Arch. Neuropsychol. 1996;11 (339-334) [PubMed] [Google Scholar]
- Kidd S.A., Herman Y., Barbic S. Testing a modification of cognitive adaptation training: streamlining the model for broader implementation. Schizophr. Res. 2014;156:46–50. doi: 10.1016/j.schres.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Kidd S.A., Kaur J., Virdee G., George T.P., McKenzie K., Herman Y. Cognitive remediation for individuals with psychosis in a supported education setting: a randomized controlled trial. Schizophr. Res. 2014;157:90–98. doi: 10.1016/j.schres.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Kidd S.A., Kerman N., Ernest D. A pilot study of a family cognitive adaptation training guide for individuals with schizophrenia. Psychiatr. Rehabil. J. 2018;41:109–117. doi: 10.1037/prj0000204. [DOI] [PubMed] [Google Scholar]
- Kontis D., Huddy V., Reeder C., Landau S., Wykes T. Effects of age and cognitive reserve on cognitive remediation therapy outcome in patients with schizophrenia. Am. J. Geriat. Psychiat. 2013;21:218–230. doi: 10.1016/j.jagp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Kraemer H.C., Kupfer D.J. Size of treatment effects and their importance to clinical research and practice. Biol. Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kurtz M.M. Neurocognitive impairment across the lifespan: an update. Schizophr. Res. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P., Christensen R. lmerTest package: tests in linear mixed effects models. J Stat Software. 2017;82:1–26. [Google Scholar]
- Maples N.J., Velligan D.I. Cognitive adaptation training: establishing environmental supports to bypass cognitive deficits and improve functional outcomes. Am. J. Psychiatr. Rehabil. 2008;11(2):164–180. [Google Scholar]
- McGurk S.R., Mueser K.T., Feldman K., Wolfe R., Pascaris A. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am. J. Psychiatry. 2007;164:437–441. doi: 10.1176/ajp.2007.164.3.437. [DOI] [PubMed] [Google Scholar]
- McGurk S.R., Twamley E.W., Sitzer D.I., McHugo G.J., Mueser K.T. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendella P.D., Burton C.Z., Tasca G.A., Roy P., Louis L.S., Twamley E.W. Compensatory cognitive training for people with first-episode schizophrenia: results from a pilot randomized controlled trial. Schizoph. Res. 2015;162:108–111. doi: 10.1016/j.schres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Radford L., Chaney E., O’Leary M. Screening for cognitive impairment among inpatients. J Clin. Psychiat. 1978;39:712–715. [PubMed] [Google Scholar]
- The Psychological Corporation . Psychological Corporation; San Antonio, TX: 1997. WAIS-III Administration and Scoring Manual. [Google Scholar]
- Velligan D.I., Bow-Thomas C.C., Huntzinger C. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am. J. Psychiatry. 2000;157:1317–1323. doi: 10.1176/appi.ajp.157.8.1317. [DOI] [PubMed] [Google Scholar]
- Velligan D.I., Prihoda T.J., Ritch J.L. A randomized single-blind pilot study of compensatory strategies in schizophrenia outpatients. Schizophr. Bull. 2002;28:283–292. doi: 10.1093/oxfordjournals.schbul.a006938. [DOI] [PubMed] [Google Scholar]
- Velligan D.I., Diamond P.M., Maples N.J. Comparing the efficacy of interventions that use environmental supports to improve outcomes in patients with schizophrenia. Schizophr. Res. 2008;102:312–319. doi: 10.1016/j.schres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J., Green M.F., Shaner A., Liberman P. Training and quality assurance with the Brief Psychiatric Rating Scale: “the drift busters”. Int. J. Meth. Psych. Res. 1993;3:221–224. [Google Scholar]
- Wilkinson G. Wide Range Inc; Wilmington, DE: 1993. Wide Range Achievement Test 3 (Manual) [Google Scholar]
- Wykes T., Spaulding W.D. Thinking about the future cognitive remediation therapy—what works and could we do better? Schiz. Bull. 2011;37:S80–S90. doi: 10.1093/schbul/sbr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T., Huddy V., Cellard C. A meta-analysis of cognitive remediation for schizophrenia. Am. J. Psychiatry. 2011;23:41–46. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in Cognitive Measures from Baseline, Post to Follow Up by Group.