Abstract
Background
Lung (LC), prostate (PCa) and colorectal (CRC) cancers are the most incident in males worldwide. Despite recent advances, optimal population-based cancer screening methods remain an unmet need. Due to its early onset, cancer specificity and accessibility in body fluids, aberrant DNA promoter methylation might be a valuable minimally invasive tool for early cancer detection. Herein, we aimed to develop a minimally invasive methylation-based test for simultaneous early detection of LC, PCa and CRC in males, using liquid biopsies.
Results
Circulating cell-free DNA was extracted from 102 LC, 121 PCa and 100 CRC patients and 136 asymptomatic donors’ plasma samples. Sodium-bisulfite modification and whole-genome amplification was performed. Promoter methylation levels of APCme, FOXA1me, GSTP1me, HOXD3me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me were assessed by multiplex quantitative methylation-specific PCR.
SEPT9me and SOX17me were the only biomarkers shared by all three cancer types, although they detected CRC with limited sensitivity. A “PanCancer” panel (FOXA1me, RARβ2me and RASSF1Ame) detected LC and PCa with 64% sensitivity and 70% specificity, complemented with “CancerType” panel (GSTP1me and SOX17me) which discriminated between LC and PCa with 93% specificity, but with modest sensitivity. Moreover, a HOXD3me and RASSF1Ame panel discriminated small cell lung carcinoma from non-small cell lung carcinoma with 75% sensitivity, 88% specificity, 6.5 LR+ and 0.28 LR–. An APCme and RASSF1Ame panel independently predicted disease-specific mortality in LC patients.
Conclusions
We concluded that a DNA methylation-based test in liquid biopsies might enable minimally invasive screening of LC and PCa, improving patient compliance and reducing healthcare costs. Moreover, it might assist in LC subtyping and prognostication.
Keywords: Lung cancer, Prostate cancer, Colorectal cancer, Epigenetic biomarkers, DNA methylation, Circulating cell free DNA, Liquid biopsy
Background
According to GLOBOCAN, cancers of the lung (LC), prostate (PCa), and colorectal (CRC) were estimated to account, globally, for 39% of all cancers diagnosed in males worldwide in 2018, or 46% if only Europe was considered [1], representing major public health issues. Despite advances in therapeutic strategies over the years, mortality rates remain high, mainly due to late diagnosis. Hence, the development and implementation of effective screening methods that might allow for easy detection of these cancers at early stages, when treatment is most likely to be curative, is crucial. Although no LC screening method is currently implemented, low-dose computed tomography (LD-CT) has been suggested owing to a reduction in LC-related mortality in screening trials targeting high-risk smokers [2–4], although at the expense of a high rate of false-positive results [2, 5]. The widespread adoption of serum prostate-specific antigen (PSA) and digital rectal examination (DRE) based PCa screening has increased the detection of early stage disease [6]. However, serum PSA-based screening is associated with significant overdiagnosis and overtreatment of non-life threatening PCa [7], whereas DRE results are dependent on clinicians’ expertise and mostly detects advanced-stage disease [6]. For CRC screening, both faecal occult blood test (FOBT) and coloscopy-based screening are available options [8]. Nonetheless, the former has limited sensitivity to detect precancerous lesions, and although colonoscopy is very precise and enables resection of precancerous polyps during examination, it is an invasive and expensive procedure with low patient compliance [9]. Although the aforementioned strategies might help decrease cancer-related mortality among those three cancer types, considering the limitations, potential harms for men’s quality of life and the negative economic impact in healthcare systems, more effective and minimally invasive methods are required.
Aberrant DNA promoter methylation, which is closely associated with inappropriate gene transcription and is thought to precede the emergence of the malignant phenotype, can be easily detected with minimal-invasiveness in circulating cell-free DNA (ccfDNA) from body fluids, such as serum/plasma [10–13], representing a valuable tool for early cancer detection. Several studies have assessed the feasibility of ccfDNA methylation for specific cancer type detection [13, 14], including “Epi proColon” (SEPT9me) and “Epi proLung” (PTGER4me and SHOX2me), two commercially available tests for CRC and LC detection, respectively [15, 16]. Nonetheless, to the best of our knowledge, only few research teams have investigated the feasibility of this approach for simultaneous detection of several types of cancer [17–20]. Herein, we sought to determine the feasibility of using a minimally invasive methylation-based test (comprising eight candidate genes selected based on a literature review) in liquid biopsies for simultaneous detection of LC, PCa and CRC in males. Additionally, the prognostic value of the selected genes was also tested.
Results
Clinical and pathological data
Plasma samples were obtained from 323 male patients diagnosed with LC (n = 102), PCa (n = 121) and CRC (n = 100), and 136 male AC (Table 1). Overall, cancer patients’ median age was significantly higher than that of controls (p < 0.0001). Nonetheless, except for SOX17me levels that correlated with controls’ age (R = 0.179; p = 0.037), and FOXA1me levels which correlated with cancer patients’ age (R = 0.144; p = 0.010), no other correlations were disclosed.
Table 1.
Clinical and pathological features of LC, PCa and CRC patient and ACs included in this study
| Clinicopathological features | AC | Cancer patients |
|---|---|---|
| Number | 136 | 323 |
| Age median (range) | 57 (48–66) | 68 (27–93) |
| Lung cancer | ||
| Number | 102 | |
| Age median (range) | 66 (45–89) | |
| Histological type | n.a. | |
| Non-small cell lung carcinoma (NSCLC) | ||
| Adenocarcinoma | 42 | |
| Squamous cell carcinoma | 43 | |
| Large cell carcinoma | 1 | |
| Small cell lung carcinoma (SCLC) | 16 | |
| Primary tumour (T)a | n.a. | |
| T1 | 12 | |
| T2/T3/T4 | 82 | |
| Regional lymph node (N)b | n.a. | |
| N0 | 25 | |
| N+ | 72 | |
| Distant metastasis (M) | n.a. | |
| M0 | 47 | |
| M+ | 55 | |
| Clinical stage | n.a. | |
| I/II | 17 | |
| III/IV | 85 | |
| Prostate cancer | ||
| Number | 121 | |
| Age median (range) | 71 (52–88) | |
| Histological type | n.a. | |
| Adenocarcinoma | 121 | |
| Primary tumour (T)c | n.a. | |
| T1/T2 | 104 | |
| T3 | 16 | |
| Regional lymph node (N) | n.a. | |
| N0 | 119 | |
| N+ | 2 | |
| Distant metastasis (M) | n.a. | |
| M0 | 116 | |
| M+ | 5 | |
| Grade group | n.a. | |
| 1 | 59 | |
| 2 | 38 | |
| 3/4/5 | 24 | |
| Serum PSA levels (ng/mL) | n.a. | |
| < 10 | 71 | |
| 10–20 | 27 | |
| > 20 | 23 | |
| Clinical stage | n.a. | |
| I | 31 | |
| II | 55 | |
| III/IV | 35 | |
| Colorectal cancer | ||
| Number | 100 | |
| Age median (range) | 66 (27–93) | |
| Histological Type | n.a. | |
| Adenocarcinoma (all subtypes) | 99 | |
| Squamous cell carcinoma | 1 | |
| Tumour location | n.a. | |
| Proximal colon | 23 | |
| Distal colon | 36 | |
| Rectum | 41 | |
| Primary tumour (T)d | n.a. | |
| T1/T2 | 26 | |
| T3/T4 | 72 | |
| Regional lymph node (N)e | n.a. | |
| N0 | 40 | |
| N+ | 57 | |
| Distant metastasis (M) | n.a. | |
| M0 | 82 | |
| M+ | 18 | |
| Clinical stage | n.a. | |
| I/II | 39 | |
| III/IV | 61 | |
aNo information available in 8 cases
bNo information available in 5 cases
cNo information available in 1 case
dNo information available in 2 cases
eNo information available in 3 cases
n.a. not applicable
Gene promoter methylation levels in ccfDNA
CcfDNA’s APCme, FOXA1me, GSTP1me, HOXD3me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me levels were compared between each cancer type and controls. APCme (p = 0.033), FOXA1me (p = 0.024), RARβ2me, RASSF1Ame, SEPT9me and SOX17me (p < 0.0001) levels were significantly higher in LC patients than in AC, whereas no differences were disclosed for GSTP1me and HOXD3me (p = 0.718 and p = 0.174, respectively) (Fig. 1).
Fig. 1.
Distribution of a APC, b FOXA1, c GSTP1, d HOXD3, e RARβ2, f RASSF1A, g SEPT9 and h SOX17 relative methylation levels of asymptomatic controls (AC) (n = 136), lung cancer (LC) (n = 102), prostate cancer (PCa) (n = 121) and colorectal cancer (CRC) (n = 100) samples. Mann-Whitney U Test between AC and each cancer type, n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Red horizontal lines represent median methylation levels
In PCa patients, significantly higher levels were observed for FOXA1me, GSTP1me, HOXD3me, RARβ2me, SOX17me (p < 0.0001), RASSF1Ame and SEPT9me (p = 0.014 and p = 0.0001, respectively), although no differences were apparent for APCme (p = 0.443) (Fig. 1).
Concerning CRC patients, only SEPT9me and SOX17me (p = 0.012 and p = 0.014, respectively) displayed significantly higher levels in patients comparing with controls. Moreover, HOXD3me levels (p = 0.009) were significantly lower in CRC patients than in controls (Fig. 1).
Biomarker performance of ccfDNA
Since our main goal was to test biomarker performance for LC, PCa and CRC detection of a methylation-based panel in ccfDNA, genes displaying significantly higher methylation levels in cancer patients compared to controls were selected for further analysis.
SEPT9me and SOX17me were the only two biomarkers shared by all three cancer types, displaying specificity between 93 and 100% (Table 2). Nonetheless, these two biomarkers detected CRC with limited sensitivity (8 and 11%, respectively) (Table 2), thus, no further analyses were performed for this tumour.
Table 2.
Biomarker performance of each gene promoter methylation for LC, PCa and CRC detection in ccfDNA
| Genes | AUC (95 % CI) | p-value | Cut-off value | Sensitivity % (95% CI) | Specificity % (95% CI) | Accuracy % (95% CI) | Positive likelihood ratio (LR+) (95% CI) | Negative likelihood ratio (LR-) (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Lung Cancer | ||||||||
| APCme | 0.556 (0.482-0.631) | 0.136 | 7.2629 | 26 (18-36) | 85 (77-90) | 60 (53-66) | 1.7 (1.0-2.9) | 0.87 (0.76-1.00) |
| FOXA1me | 0.579 (0.505-0.653) | 0.037 | 301.1682 | 38 (29-48) | 77 (69-84) | 61 (54-67) | 1.7 (1.1-2.5) | 0.80 (0.67-0.96) |
| RARβ2me | 0.599 (0.525-0.674) | 0.009 | 0.3548 | 24 (16-33) | 96 (92-99) | 65 (59-71) | 6.4 (2.5-16.2) | 0.79 (0.71-0.89) |
| RASSF1Ame | 0.602 (0.528-0.677) | 0.007 | 14.5588 | 25 (17-34) | 96 (91-98) | 65 (59-71) | 5.6 (2.4-13.0) | 0.79 (0.70-0.89) |
| SEPT9me | 0.596 (0.522-0.671) | 0.011 | 9.1907 | 21 (13-30) | 99 (95-100) | 65 (59-71) | 14.0 (3.4-58.4) | 0.81 (0.73-0.89) |
| SOX17me | 0.619 (0.546-0.693) | 0.002 | 70.0725 | 29 (21-39) | 94 (89-97) | 66 (60-72) | 5.0 (2.4-10.4) | 0.75 (0.66-0.86) |
| LC panel | - | - | - | 66 (56-75) | 70 (61-77) | 68 (62-74) | 2.2 (1.6-2.9) | 0.49 (0.37-0.66) |
| Prostate Cancer | ||||||||
| FOXA1me | 0.719 (0.656-0.782) | <0.001 | 295.8133 | 61 (52-70) | 77 (69-84) | 70 (64-75) | 2.7 (1.9-3.8) | 0.50 (0.40-0.64) |
| GSTP1me | 0.564 (0.493-0.634) | 0.078 | 36.7086 | 15 (9-22) | 98 (94-100) | 59 (52-65) | 6.7 (2.0-22.3) | 0.87 (0.80-0.94) |
| HOXD3me | 0.650 (0.583-0.717) | <0.001 | 320.9365 | 80 (72-87) | 43 (34-51) | 60 (54-66) | 1.4 (1.2-1.7) | 0.47 (0.31-0.70) |
| RARβ2me | 0.594 (0.524-0.664) | 0.010 | 5.2251 | 22 (15-31) | 96 (92-99) | 61 (55-67) | 6.1 (2.4-15.3) | 0.81 (0.73-0.89) |
| RASSF1Ame | 0.543 (0.472-0.614) | 0.232 | 0.5627 | 13 (8-21) | 96 (91-98) | 57 (51-64) | 3.0 (1.2-7.4) | 0.91 (0.84-0.98) |
| SEPT9me | 0.550 (0.479-0.621) | 0.164 | 7.7096 | 12 (6-19) | 99 (95-100) | 58 (51-64) | 7.9 (1.8-33.9) | 0.90 (0.84-0.96) |
| SOX17me | 0.611 (0.542- 0.681) | 0.002 | 16.153 | 29 (21-38) | 93 (88-97) | 63 (57-69) | 4.4 (2.2-8.7) | 0.76 (0.67-0.86) |
| PCa panel | - | - | - | 72 (63-80) | 72 (64-79) | 72 (66-77) | 2.6 (1.9-3.5) | 0.39 (0.29-0.53) |
| Colorectal cancer | ||||||||
| SEPT9me | 0.533 (0.458 - 0.608) | 0.383 | 265.2606 | 8 (4-15) | 100 (97-100) | 61 (54-67) | - | 0.92 (0.87-0.97) |
| SOX17me | 0.550 (0.475 - 0.625) | 0.189 | 732.3866 | 11 (6-19) | 100 (97-100) | 62 (56-68) | - | 0.89 (0.83-0.95) |
| CRC panel | - | - | --- | 12 (6-20) | 100 (97-100) | 63 (56-69) | - | 0.88 (0.82-0.95) |
LC panel—FOXA1me, RARβ2me, RASSF1Ame and SOX17me; PCa panel—FOXA1me, RARβ2me, RASSF1Ame and GSTP1me; CRC panel—SEPT9me and SOX17me
RARβ2 me, RASSF1A me, SEPT9 me and SOX17 me were able to detect both LC and PCa with over 93% specificity (Table 2). Conversely, FOXA1me disclosed the highest sensitivity for detecting LC and the second highest for detecting PCa (38% and 61%, respectively), with 77% specificity for both. SOX17me detected both LC and PCa, individually, with 29% sensitivity, and RARβ2me identified both cancers with 22–24% sensitivity (Table 2).
Gene panels were further constructed to increase detection sensitivity. Hence, the best LC panel (FOXA1me, RARβ2me, RASSF1Ame and SOX17me) achieved 66% sensitivity and 70% specificity, whereas for PCa, the panel (FOXA1me, RARβ2me, RASSF1Ame and GSTP1me) depicted 72% sensitivity and specificity (Table 2).
Aiming to obtain a gene panel for simultaneous LC and PCa detection (designated as “PanCancer” panel), all genes (FOXA1me, RARβ2me, RASSF1Ame and SEPT9me) that were shared [except for SOX17me that displayed a relatively different cut-off between these two cancer types (70.0725 for LC and 16.153 for PCa)) (Table 2), were further tested as panel. Additionally, APCme, GSTP1me, HOXD3me and SOX17me were tested as a suitable panel for tumour’s primary location discrimination (“CancerType” panel). Remarkably, the “PanCancer” panel (FOXA1me, RARβ2me and RASSF1Ame) identified LC and PCa with 64% sensitivity, 70% specificity, 66% accuracy, positive likelihood ratio (LR+) of 2.3 and a negative likelihood ratio (LR–) of 0.53 (Table 3 and Fig. 2).
Table 3.
Biomarker performance detection of “PanCancer” panel (FOXA1me, RARβ2me and RASSF1Ame) in ccfDNA
| PanCancer | |
|---|---|
| Sensitivity % | 64.3 |
| Specificity % | 69.8 |
| Accuracy % | 66.4 |
| Positive likelihood ratio (LR+) | 2.3 |
| Negative likelihood ratio (LR-) | 0.52 |
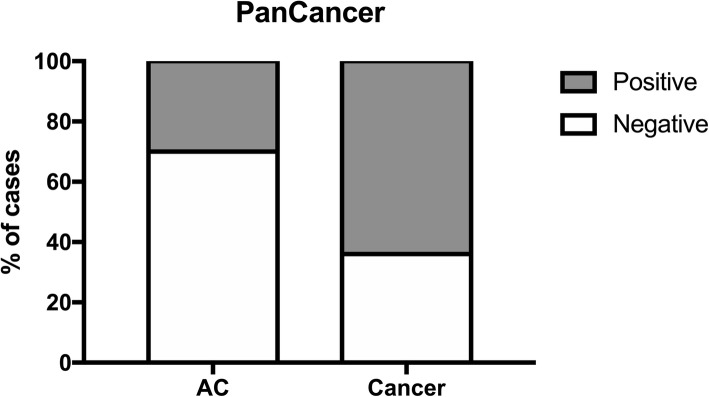
Fig. 2.
Percentage of cases identified by “PanCancer” panel in cancer samples (64% positive, 36% negative) and in asymptomatic controls (ACs) (30% positive, 70% negative)
As early diagnosis is imperative and specifically for PCa it is also relevant to detect clinically significant disease, we further tested this panel for those purposes. “PanCancer” panel detected LC stages I and II with 95% (95% CI 90–98%) specificity, however with modest 35% (95% CI 14–62%) sensitivity, displaying 88% (95% CI 81–93%) accuracy, LR+ of 7.1 (95% CI 2.6–19.6) and LR– of 0.68 (95% CI 0.48–0.97). Importantly, this panel was able to discriminate intermediate and high-risk PCa patients (stage II and III-IV) from controls and low-risk (stage I) PCa patients with 71% (95% CI 61–80%) sensitivity, 65% (95% CI 57–72%) specificity, 67% (61–73%) accuracy, LR+ of 2.0 (1.6–2.6) and LR– of 0.45 (0.32–0.63).
Among the four genes tested for “CancerType” panel, SOX17me and GSTP1me demonstrated the best performance for discriminating LC from PCa. Indeed, SOX17me discriminated LC from PCa with 93% specificity, whereas GSTP1me detected PCa with 97% specificity, although with limited sensitivity (Table 4).
Table 4.
Biomarker performance of “CancerType” for discrimination among LC and PCa
| Gene | Sensitivity % | Specificity % | Accuracy % | Positive likelihood ratio (LR+) | Negative likelihood ratio (LR–) |
|---|---|---|---|---|---|
| Lung cancer | |||||
| SOX17me | 15.2 | 93.4 | 57.7 | 3.0 | 0.91 |
| GSTP1me | --- | --- | --- | --- | --- |
| Prostate cancer | |||||
| SOX17me | --- | --- | --- | --- | --- |
| GSTP1me | 13.6 | 97.0 | 51.8 | 3.6 | 0.89 |
Association between promoters’ methylation levels and clinicopathological features
Concerning associations between promoters’ methylation levels and clinicopathological features (Additional file 1: Tables S1–S3), in LC patients, higher circulating FOXA1me and RARβ2me levels associated with advanced primary tumour stage (T) (p = 0.014 and p = 0.044, respectively) (Fig. 3), whereas higher levels of those two genes and APCme and HOXD3me were also depicted in LC patients with regional lymph node involvement (p = 0.021, p = 0.015, p = 0.044 and p = 0.022, respectively) (Fig. 4).
Fig. 3.
a FOXA1 and b RARβ2 promoter’s methylation levels according with T stage (T1 (n = 12) and T2-4 (n = 82)) in LC patients. Mann-Whitney U Test, *p < 0.05. Red horizontal lines represent median methylation levels
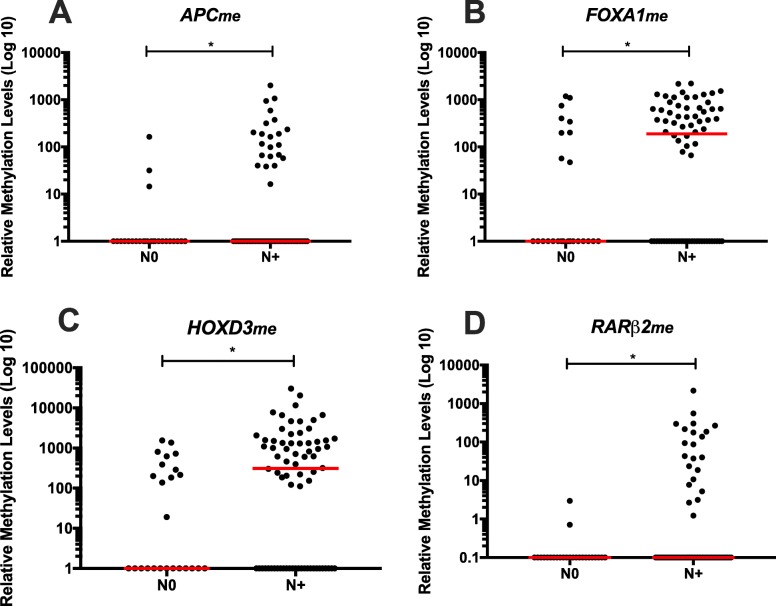
Fig. 4.
a APC, b FOXA1, c HOXD3 and d RARβ2 promoter’s methylation levels according with node status, node-negative (N0) (n = 25) and node-positive (N+) (n = 72) in LC patients. Mann-Whitney U Test, *p < 0.05. Red horizontal lines represent median methylation levels
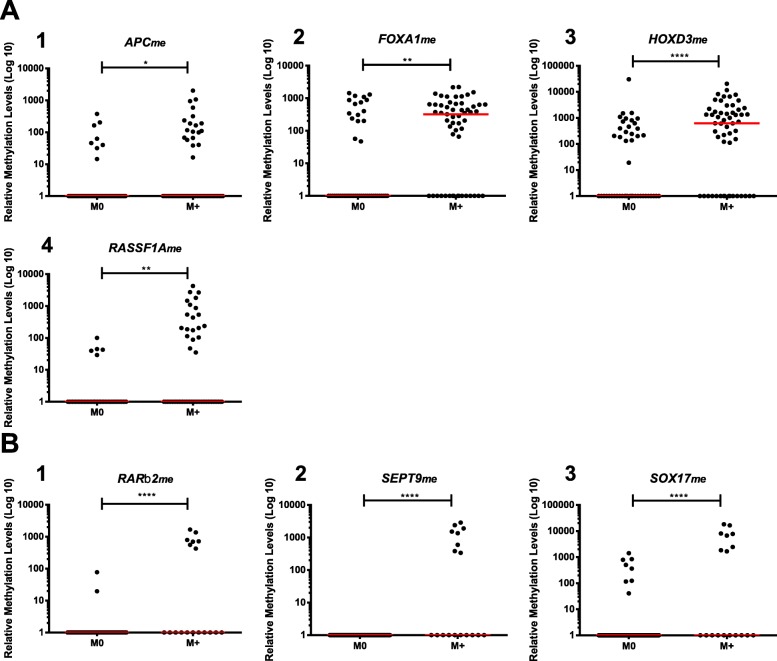
Furthermore, circulating APCme, FOXA1me, HOXD3me and RASSF1Ame levels were significantly higher in LC patients with distant metastatic disease (p = 0.028, p = 0.001, p < 0.0001 and p = 0.001, respectively) (Fig. 5a), whereas RARβ2me, SEPT9me and SOX17me levels were higher in CRC metastatic patients (p < 0.0001, in all comparisons) (Fig. 5b). Similarly, significantly higher APCme, GSTP1me, HOXD3me, RARβ2me, RASSF1Ame and SEPT9me levels were observed in the five PCa patients with metastatic disease at diagnosis (p < 0.0001, for all comparisons, except for HOXD3me, p = 0.003) (Additional file 2: Figure S1).
Fig. 5.
Distribution of methylation levels in LC a and in CRC patients according with metastatic dissemination b. a (1) APC, (2) FOXA1, (3) HOXD3 and (4) RASSF1A promoter’s methylation levels in non-metastatic (M0) (n = 47) and metastatic (M+) (n = 55) LC patients. b (1) RARβ2, (2) SEPT9 and (3) SOX17 promoter’s methylation levels in non-metastatic (M0) (n = 82) and metastatic (M+) (n = 18) CRC patients. Mann-Whitney U Test, *p < 0.05, **p < 0.01, ****p < 0.0001. Red horizontal lines represent median methylation levels
Significantly higher circulating RASSF1Ame levels were also apparent in LC patients with advanced clinical stage (III and IV) (p = 0.043) (Fig. 6a), whereas higher GSTP1me levels were observed in PCa patients with advanced clinical stage (III and IV) (p = 0.039) comparing to patients with clinical stage I (Fig. 6b). Similar results were found for RARβ2me and SEPT9me in CRC patients (p = 0.012 and p = 0.019, respectively) (Fig. 6c).
Fig. 6.
Scatter plot of a RASSF1A promoter methylation levels between clinical stage I & II (n = 17) and III & IV (n = 85) LC patients. b GSTP1 promoter methylation levels between clinical stage I (n = 31), II (n = 55) and III & IV (n = 35) PCa patients. c (1) RARβ2 and (2) SEPT9 promoters’ methylation levels between clinical stage I & II (n = 39) and III & IV (n = 61) CRC patients. Mann-Whitney U Test for a and c and Mann-Whitney U test with Bonferroni’s correction for b, n.s. p < 0.05, *p < 0.05. Red horizontal lines represent median methylation
Concerning LC patients, significantly higher circulating HOXD3me, RASSF1Ame and SOX17me levels were observed in small cell lung cancer (SCLC) vs non-small cell lung cancer (NSCLC) patients (p = 0.001, p < 0.001 and p = 0.013, respectively) (Fig. 7). Therefore, we further assessed biomarker’s performance of these three genes to discriminate between these two major histological subtypes (Table 5). Individually, all three genes identified SCLC with specificities above 75%. RASSF1Ame disclosed the highest specificity (98% (95% CI 92–100%)), correctly identifying 10 out of the 16 SCLC patients, misclassifying only 2 out of 86 NSCLC patients. Importantly, the panel including the two genes with the highest specificity (HOXD3me and RASSF1Ame) discriminated between LC subtypes with 75% (95% CI 48–93%) sensitivity, 88% (95% CI 80–94%) specificity, 86% (95% CI 78–92%) accuracy, LR+ of 6.5 (95% CI 3.4–12.3) and LR– of 0.28 (95% CI of 0.12–0.66) (Table 5).
Fig. 7.
Scatter plot of a HOXD3, b RASSF1A, and c SOX17 promoter’s methylation levels according with histological subtype (Non-small cell lung carcinoma (NSCLC) (n = 86) and small cell lung carcinoma (SCLC) (n = 16)). Mann-Whitney U Test, *p < 0.05, **p < 0.01, ****p < 0.0001. Red horizontal lines represent median methylation levels
Table 5.
Biomarker performance of HOXD3, RASSF1A and SOX17 promoters’ methylation levels for discrimination among small cell lung carcinoma (SCLC) and non-small cell Lung carcinoma (NSCLC)
| Genes | AUC (95 % CI) | p-value | Cut-off value | Sensitivity % (95% CI) | Specificity % (95% CI) | Accuracy % (95% CI) | Positive likelihood ratio (LR+) (95% CI) | Negative likelihood ratio (LR–) (95% CI) |
|---|---|---|---|---|---|---|---|---|
| SCLC vs NSCLC | ||||||||
| HOXD3me | 0.742 (0.577–0.907) | 0.002 | 1401.1301 | 69 (41–89) | 88 (80–94) | 85 (77–92) | 5.9 (3.0–11.6) | 0.35 (0.17–0.73) |
| RASSF1Ame | 0.775 (0.617–0.934) | < 0.001 | 204.8474 | 63 (35–85) | 98 (92–100) | 92 (85–97) | 26.9 (6.5–113.3) | 0.38 (0.20–0.72) |
| SOX17me | 0.657 (0.504–0.811) | 0.046 | 30.1876 | 56 (30–80) | 76 (65–84) | 73 (63–81) | 2.3 (1.3–4.1) | 0.58 (0.33–1.02) |
| HOXD3me/RASSF1Ame | - | - | - | 75 (48–93) | 88 (80–94) | 86 (78–92) | 6.5 (3.4–12.3) | 0.28 (0.12–0.66) |
Prognostic biomarker performance of ccfDNA
The median patient follow-up time for LC patients’ cohort was 9 months (range from 0 to 40 months), during which 62 patients have deceased due to cancer. Since only 15 out of 102 LC patients were submitted to surgery, disease-free survival (DFS) was not assessed. Moreover, no significant associations were disclosed between circulating methylation levels of the selected genes and progression-free survival (PFS) (data not shown).
Cumulative incidence of disease-specific mortality (DSM) was significantly increased in LC patients with lymph node involvement or distant metastasis (both p < 0.001), advanced clinical stage (p < 0.001) and SCLC subtype (p = 0.004) (Additional file 1: Table S4; Additional file 2: Figure S2), as expected. Interestingly, methylation of APC (p < 0.001), FOXA1 (p = 0.033), HOXD3 (p = 0.047), RASSF1A (p < 0.001), SEPT9 (p = 0.009) and SOX17 (p = 0.037) also significantly associated with increased cumulative incidence of DSM (Fig. 8 and Additional file 1: Table S5).
Fig. 8.
Cumulative incidence function plots according to a APC, b FOXA1, c RASSF1A, d SEPT9 and e SOX17 promoter methylation status in LC patients. Dashed red line and full black line represent positive and negative for promoter methylation status, respectively. p values obtained by Gray’s test for disease-specific mortality
As both APC and RASSF1A methylation positivity were strongly associated with increased cumulative incidence of DSM, the value as a prognostic panel to better stratify outcome in LC patients was assessed for the combined genes. Indeed, this prognostic panel was also significantly associated with cumulative incidence of DSM (p < 0.001). LC patients classified as negative for methylation for the both genes presented an estimated 32% of cumulative incidence of DSM at 12 months, whereas the patients with only one methylated gene promoter presented a DSM cumulative incidence of 73%. Remarkably, the estimated DSM cumulative increased to 82% in patients classified as positive for both genes (Additional file 1: Table S4; Fig. 9).
Fig. 9.

Cumulative incidence function plots according to panel (APCme and RASSF1Ame) promoter methylation status in LC patients. p values obtained by Gray’s test for disease-specific mortality
On univariable Cox-regression analysis, circulating APCme (p < 0.0001), FOXA1me (p = 0.039), RASSF1Ame (p = 0.001), SEPT9me (p = 0.018) and prognostic panel (APCme and RASSF1Ame) (both negative vs one positive and both negative vs both positive) (p < 0.0001) were DSM predictors in LC patients, along with nodal involvement (p = 0.001) and distant metastasis (p < 0.0001) at diagnosis, clinical stage (p = 0.001) and histological subtype (p = 0.015) (Table 6). Remarkably, on multivariable Cox-regression analysis comprising the significant variables in univariable analysis (clinical stage, histological subtype, FOXA1me, SEPT9me and prognostic panel (APCme and RASSF1Ame)), both clinical stage (p = 0.002) and prognostic methylation panel (APCme and RASSF1Ame) (p < 0.0001) were found to be independent disease-specific mortality predictors (Table 6). Indeed, LC patients with detectable circulating APCme and RASSF1Ame displayed a 3.918-fold risk (95%CI for HR 1.935–7.933, p < 0.0001) of dying from LC comparing to those lacking methylation (Table 6).
Table 6.
Cox-regression models assessing the potential of clinical variables and detection of circulating APCme, FOXA1me, HOXD3me, RASSF1Ame, SEPT9me, SOX17me and prognostic panel (APCme, RASSF1Ame) in the prediction of disease-specific mortalityin LC patients
| Disease-Specific Mortality | Variable | HR | 95% CI for HR | P value |
|---|---|---|---|---|
| Univariable | Regional node (N) | |||
| N0 vs N+ | 4.067 | 1.827-9.051 | 0.001 | |
| Distant Metastasis (M) | ||||
| M0 vs M+ | 3.320 | 1.897-5.809 | <0.0001 | |
| Clinical Stage | ||||
| I&II vs III&IV | 5.594 | 2.012-15.549 | 0.001 | |
| Histological Subtype | ||||
| NSCLC vs SCLC | 2.098 | 1.153-3.819 | 0.015 | |
| APCme | ||||
| Negative vs positive | 2.800 | 1.634-4.800 | <0.0001 | |
| FOXA1me | ||||
| Negative vs positive | 1.716 | 1.027-2.867 | 0.039 | |
| HOXD3me | ||||
| Negative vs positive | 1.666 | 0.982-2.828 | 0.058 | |
| RASSF1Ame | ||||
| Negative vs positive | 2.669 | 1.529-4.660 | 0.001 | |
| SEPT9me | ||||
| Negative vs positive | 2.011 | 1.130-3.579 | 0.018 | |
| SOX17me | ||||
| Negative vs positive | 1.696 | 0.991-2.902 | 0.054 | |
| APCme / RASSF1Ame | <0.0001 | |||
| Both negative vs One positive | 2.582 | 1.428-4.671 | 0.002 | |
| Both negative vs Both Positive | 3.958 | 1.967-7.965 | <0.0001 | |
| Multivariable | Clinical Stage | |||
| I&II vs III&IV | 5.014 | 1.781-14.114 | 0.002 | |
| APCme / RASSF1Ame | <0.0001 | |||
| Both negative vs One positive | 1.992 | 1.100-3.608 | 0.023 | |
| Both negative vs Both positive | 3.918 | 1.935-7.933 | <0.0001 |
PCa patient’s cohort displayed the longer follow-up time, with a median of 85 months (range, 3 to 155 months). From the 85 patients treated with curative intent (radical prostatectomy, external beam radiotherapy or brachytherapy), 24 developed biochemical relapse and 12 endured disease progression. Moreover, nine out of the remainder 36 patients (subjected to androgen deprivation therapy or active surveillance) presented disease progression. Nonetheless, no relevant significant associations were disclosed between circulating methylation positivity and PFS or DFS (data not shown). Although 38 patients deceased during follow-up, only 11 deaths were attributable to PCa. Cumulative incidence of DSM was significantly increased in PCa patients with higher serum PSA levels at diagnosis (p < 0.001), higher group grade (p = 0.007) and advanced clinical stage (p = 0.003). Interestingly, patients with detectable circulating APCme (p < 0.001), GSTP1me (p = 0.003), RARβ2me (p = 0.001), RASSF1Ame, SEPT9me (both p < 0.001) or SOX17me (p = 0.014) also disclosed increased cumulative incidence of DSM (Additional file 1: Table S5; Additional file 2: Figure S3). Nevertheless, Cox-regression analysis was not performed due to the reduced number of events in each group.
Regarding CRC patients, a median follow-up time of 13 months (range from 0 to 44 months) was achieved. From the 90 CRC patients submitted to surgery, 10 developed recurrence/progression. Given the difficulty to ascertain whether patients submitted to surgery were indeed free of the disease, DFS was not performed. CRC was the cause of death of nine of the 11 patients that deceased during follow-up. The presence of distant metastasis (p < 0.001) and detection of circulating RARβ2me (p = 0.002), SEPT9me (p < 0.001) and SOX17me (p = 0.031) significantly associated with increased cumulative incidence of DSM (Additional file 1: Table S6; Additional file 2: Figure S4), although due to lack of events, no Cox-regression analysis was performed.
Discussion
LC, PCa and CRC are the three most incident and prevalent cancers in males worldwide, and the most deadly in Europe and North America [1]. Despite efforts to develop more effective screening methods, due to either low sensitivity, high false-positive rate leading to overdiagnosis [7, 21, 22] or high invasiveness and cost [9], currently available strategies are suboptimal for population-based screening. Thus, development of new minimally invasive and effective pre-screening methods to triage patients for subsequent screening/detection tests are urgently needed. Aberrant DNA methylation of cancer-related genes occurs at very early stages of tumorigenesis, is cancer-specific, and amenable to be assessed in ccfDNA [13, 23], representing a valuable candidate tool for minimally invasive early cancer detection. Thus, we assessed the clinical utility of a liquid biopsy-based strategy for simultaneous detection of LC, PCa and CRC in males using multiplex qMSP in ccfDNA.
The eight candidate genes tested were selected based on a critical analysis of published data available in PubMed, including previously published data by our research team [14, 20, 24]. Firstly, the performance of candidate genes was individually analysed for each cancer type. In accordance with previously published studies [20, 25–33], significantly higher circulating APCme, FOXA1me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me levels were observed in LC patients. Although the best panel for LC detection—FOXA1me, RARβ2me, RASSF1Ame, SOX17me—disclosed similar specificity to “Epi proLung”, it showed modest sensitivity [16]. Concerning PCa, except for APCme that was only reported in tissue and urine of cancer patients [34, 35], our results are in line with previously published studies showing GSTP1me, RARβ2me and RASSF1Ame hypermethylation in ccfDNA of PCa patients [36–39]. Furthermore, to the best of our knowledge, we are the first research team reporting the biomarker potential of FOXA1me, HOXD3me, SOX17me and SEPT9me levels for PCa detection in blood-based liquid biopsies. Interestingly, the combination of FOXA1me, RARβ2me, RASSF1Ame and GSTP1m identified PCa with 72% sensitivity and specificity. Although a panel comprising RARβ2me, RASSF1Ame and GSTP1me disclosing 29% sensitivity and 100% specificity to detect PCa was previously shown [38], the addition of FOXA1 to this panel in our study increased sensitivity although at expense of lower specificity. Nonetheless, the former panel was assessed using MSP [38], whereas we performed multiplex qMSP, which requires less amounts of DNA, is less time-consuming and more sensitive. We also confirmed SEPT9 and SOX17 hypermethylation in ccfDNA of CRC patients, although displaying modest sensitivity (12%), in contrast with previous reports [20, 40]. Moreover, the disparate results observed for APCme FOXA1me, RARβ2me and RASSF1Ame [20, 41–45] might be due to differences in sample collection procedures, methodology or population studied, since we have only evaluated these genes in males. Interestingly, HOXD3 was significantly hypomethylated in CRC samples.
Although the initial goal was to identify a panel suitable for simultaneous detection of LC, PCa and CRC in males, due to the results observed in CRC, we decided to move forward with simultaneous detection of LC and PCa, only, which constitute the two most incident cancers in males. The best combination to this end—“PanCancer” panel (FOXA1me, RARβ2me and RASSF1Ame)—disclosed 70% specificity and 64% sensitivity, which is slightly lower than we have reported for the simultaneous detection of breast cancer (BrC), CRC and LC in women [20]. Nonetheless, compared to LD-CT LC screening [2], our test displayed considerable less false-positive results. Although asymptomatic donors included in our study do not represent a high-risk population, they might better reproduce the “real world” setting, nonetheless. Furthermore, the panel performance for detecting PCa, outperformed serum PSA specificity (which is around 20–45%) [46], although with limited sensitivity. Interestingly, our results are within the range reported for urinary long-non coding RNA Prostate Cancer Antigen 3 (PCA3), which is analysed in urine collected after prostatic massage [46, 47], a more invasive PCa screening approach. Thus, we are tempted to speculate whether our panel might add clinical value to serum PSA, increasing specificity and potentially reducing the number of unnecessary prostate biopsies, a hypothesis that deserves further studies.
Despite the modest sensitivity to detect early stage (I and II) LC, the high specificity of “PanCancer” panel to detect those patients suggests a potential usefulness for early detection of LC, reducing false-positive rates of some previously reported screening strategies. Moreover, our panel also discriminated intermediate and high-risk PCa (stage II-IV), according to the EAU risk groups [48], with 71% sensitivity and 65% specificity, thus increasing the ability to identify clinically significant PCa. Therefore, it has the potential to prevent the excess morbidity associated with unnecessary biopsies and reduce overdiagnosis and consequent overtreatment. Hence, the minimally invasive “PanCancer” panel might be advantageous for population-based screening, limiting drawbacks of currently available methods, with the advantage of potentially detecting two cancer types in the same test.
Following a positive result in “PanCancer” panel, identifying the primary location (i.e. LC or PCa) is of foremost importance. We, thus, propose a second panel (“CancerType”) comprising SOX17me and GSTP1me, due to their remarkable specificity to discriminate between LC and PCa. Nonetheless, given the limited sensitivity of these two genes, this strategy must be complemented with other ancillary tests, including imaging. Notwithstanding, a positive “PanCancer” test should always trigger a search for LC or PCa and if any of these is identified, then, closer follow-up and repeat testing must be performed.
Correlations between clinicopathological features and circulating methylation levels of candidate genes were also assessed. Interestingly, higher FOXA1me and RARβ2me associated with advanced LC tumour stage. Moreover, methylation of those two genes, as well as of APCme and HOXD3me, was also increased in node-positive disease. Furthermore, higher APCme, FOXA1me, HOXD3me and RASSF1Ame associated with metastatic dissemination. To the best of our knowledge, only RASSF1Ame and SOX17me have been previously associated with nodal and distant metastasis and advanced stage in ccfDNA of LC patients [20, 49]. Increased HOXD3me levels were similarly observed in all five PCa patients with distant metastasis at diagnosis, along with higher APCme, GSTP1me, RARβ2me, RASSF1Ame and SEPT9me in four out of the same five patients. Likewise, higher RARβ2me, SEPT9me and SOX17me levels were disclosed in metastatic CRC patients, paralleling recent reports which indicate the same trend for SEPT9me and SOX17me [20, 50, 51]. Thus, despite the early onset of epigenetic alterations in tumorigenesis, higher circulating methylation levels are detectable in cancer progression probably owing to increased tumour burden and metastatic spread.
Remarkably, a panel constituted by HOXD3me and RASSF1Ame was able to discriminate SCLC from NSCLC with 75% sensitivity and 88% specificity. Interestingly, RASSF1Ame potential for SCLC discrimination from NSCLC in plasma samples was previously observed in our group [52]. This distinction is critical as SCLC and NSCLC require quite different therapeutic strategies and display different prognosis. Although, an IVD-approved miRNA-based test (miRview) identifies the four main LC subtypes, in fine-needle aspirates and cytological samples, with 90% sensitivity and specificity [53], our panel can discriminated two major LC subtypes in liquid biopsies, which has a far less invasive collection method. Moreover, our panel displays similar performance to a three miRNAs panel assessed in plasma [54].
We further evaluated whether gene methylation might convey independent prognostic information. Indeed, detection of circulating APCme, FOXA1me, HOXD3me, RASSF1Ame, SEPT9me and SOX17me associated with increased cumulative incidence of DSM in LC patients, alongside with all clinicopathological variables except tumour stage (T). Remarkably, the prognostic panel (APCme and RASSF1Ame) is an independent predictor of DSM in LC patients. These results are in line with the prognostic value reported for circulating APCme and RASSF1Ame by other research teams, namely as biomarkers for evaluation of treatment efficacy and disease monitoring [26, 55, 56]. Moreover, detectable circulating APCme, GSTP1me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me methylation, in PCa patients, and RARβ2me, SEPT9me and SOX17me, in CRC patients, associated with increased cumulative incidence of DSM, suggesting that methylation of selected genes in plasma might convey prognostic information already at the time of diagnosis. Our observations are limited owing to the short follow-up time and the favourable prognosis of most PCa and CRC patients, and, thus, additional validation in larger studies with longer follow-up is warranted.
Overall and notwithstanding the promising results of “PanCancer” panel for simultaneous detection of LC and PCa in males, it should be acknowledged that the lack of clinical information and long-term follow-up of asymptomatic controls of our exploratory study constitutes a limitation. Therefore, the screening utility of “PanCancer” panel in general population, as well as, in high-risk populations, such as high-risk smokers and family history of PCa, remains to be ascertained. Hence, our results should be validated in future large multicentre prospective studies. Nonetheless, one may envisage that high-risk populations could benefit from the implementation of this minimally invasive screening test given the lack of widely implemented options able to detect LC and PCa at early stages in these population subsets. Moreover, further studies should also be considered to find the best DNA methylation-based test to detect CRC, and ultimately, to be able to detect the four major cancers in both genders (LC, BrC, CRC and PCa).
Conclusions
In conclusion, our findings corroborate the hypothesis that a single minimally invasive test can be devised to simultaneously detect multiple malignancies, which might improve patient compliance and, thereby, increase tumours’ detection rate at earlier stages, reducing cancer morbidity and mortality. Moreover, we also provide a novel tool to aid discrimination between the major LC subtypes and a prognostic panel that carries independent information for those patients.
Material and methods
Patients and sample collection
A case control retrospective cohort of LC, PCa and CRC male patients retrieved from consecutive series was included in this study. A total of 323 male patients diagnosed with LC (n = 102) or CRC (n = 100) between 2015 and 2019, and PCa (n = 121) between 2006 and 2010, at Portuguese Oncology Institute of Porto, Portugal, without previous oncological treatment were selected. For control purposes, blood samples were donated from 2016 to 2018 by 136 male asymptomatic blood donors (AC), without any known malignancy at the same institution.
After collection of peripheral blood into EDTA-containing tubes, plasma was separated by centrifuging at 2000 rpm for 10 min at 4 °C, and subsequently, stored at − 80 °C in the institutional tumour bank until further use. Relevant clinical and pathological data was retrieved from clinical charts and an anonymized database was constructed for analysis purposes.
CcfDNA extraction, sodium-bisulfite modification and whole genome amplification
CcfDNA was extracted from 2 to 3 mL of plasma using QIAmp MinElute ccfDNA (Qiagen, Hilden, Germany), according to manufacturer’s instructions, and subsequently eluted in 20 μL of sterile distilled water and stored at − 20 °C until further use. Then, 20 μL of each extracted ccfDNA sample and 1 μg of CpGenome™ Universal Methylated DNA (Merck Milipore, Burlington, MA, USA) were sodium-bisulfite-modified using EZ DNA Methylation-GoldTM Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s recommendations. Afterwards, the bisulfite-modified DNA was eluted in 10 μL of sterile distilled water and stored at − 80 °C until further use. EpiTect Whole Bisulfitome Kit (Qiagen, Hilden, Germany) was used to perform Whole Genome Amplification (WGA) of 10 μL sodium-bisulfite-modified DNA following manufacturer’s recommendations. Lastly, amplified DNA samples were diluted in 25 μL of sterile distilled water, in a final volume of 65 μL, and stored at − 20 °C until further use. Extracted, sodium-bisulfite converted and amplified DNA were quantified using Qubit 2 Fluorometer (Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions.
Multiplex qMSP
Promoter methylation levels of eight genes (APCme, FOXA1me, GSTP1me, HOXD3me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me) were assessed by multiplex quantitative methylation-specific PCR (qMSP), using WGA-amplified DNA as template and Xpert Fast Probe (GRiSP, Porto, Portugal). Primers and TaqMan probes designed specifically for the modified gene sequence plus fluorochromes and quenchers selected for each probe are listed in Additional file 1: Table S7. The housekeeping gene β-Actin was used an internal reference gene to normalize the assay. Multiplex qMSP assay was carried out in triplicate using a 7500 Sequence Detector (Applied Biosystems, Perkin Elmer, CA, USA). Sterile distilled water was used as negative control in all plates. WGA-amplified CpGenome™ Universal Methylated DNA subjected to six serial dilutions (5x factor dilution) was used to generate a standard curve in each plate, allowing for relative quantification and PCR efficiency evaluation. All plates displayed efficiency values above 90%. Relative methylation levels were calculated as the ratio between the mean methylation levels of each gene and the respective value for β-Actin, multiplied by 1000, for easier tabulation.
Statistical analysis
Non-parametric tests were performed to compare methylation levels of each gene promoter and to evaluate associations with clinicopathological features. Mann-Whitney U test was used for comparisons between two groups, while Kruskal-Wallis test was used for multiple groups, followed by Mann-Whitney U test with Bonferroni’s correction for pairwise comparisons. Scatter dot plots were constructed using a log10 scale in y axis. Spearman non-parametric test was performed to assess correlations between methylation levels and patients’ age. A result was considered statistically significant when p value < 0.05.
For each gene’s promoter, samples were categorized as methylated or non-methylated based on the cut-off values established using Youden’s J index (value combining highest sensitivity and specificity), through receiver operator characteristic (ROC) curve analysis [57], and areas under the curve (AUC) were calculated. When more than one value fulfilled this condition, the cut-off value allowing for higher sensitivity was chosen. Validity estimates (sensitivity, specificity, accuracy and positive (LR+) and negative (LR–) likelihood ratios) with 95% confidence intervals (CI) were determined to assess detection biomarker performance.
To improve detection performance of the selected genes, panels were constructed considering a positive result whenever at least one gene promoter was plotted as methylated in individual analysis, favouring gene combinations allowing maximum sensitivity. For “PanCancer” panel, validity estimates were calculated by joining LC and PCa (n = 223) vs AC samples (n = 136), whereas for “CancerType” panel, these were calculated by comparing one tumour type to the other. Validity estimates for the two panels were assessed by constructing multiple ROC curves via resampling analysis [58]. In short, samples were randomly divided into training (70%) and validation (30%) sets. Then, the cut-off value obtained combining the highest sensitivity and specificity in the training set, was used to calculate validity estimates in the validation set. This procedure was repeated 1000 times, and the mean value of sensitivity, specificity, accuracy, LR+ and LR– was computed.
Using a competing risk approach, cumulative incidence function (CIF) plots were constructed, and Gray’s test was used to test differences in disease-specific mortality (DSM) between groups considering clinicopathological variables and categorized gene promoter methylation status (positive: relative methylation levels > 0, and negative: relative methylation levels = 0). DSM was calculated as the time between the date of diagnosis and the date of cancer-related death. Kaplan-Meier curves were constructed, and log-rank test was used to compare progression-free survival (PFS) and disease-free survival (DFS) between groups, considering clinicopathological variables and categorized gene promoter methylation. PFS was calculated as the time between the date of diagnosis and the date of the first imaging exam showing disease progression, whereas DFS as the time between the date of curative intent treatment and the date of the first imaging exam showing disease progression or biochemical recurrence (for PCa). Cox proportional hazards regression was employed to calculate hazard ratios (HR) and 95% CI. Backwards conditional multivariable Cox-regression model comprising all significant variables on univariable analysis was computed to determine whether genes’ promoter methylation status was independently associated with DSM.
Two-tailed p values calculation, ROC curve analysis and PFS and DFS survival analysis and cox-regression analysis were performed using SPSS 25.0 for MacOS software (IBM-SPSS Inc., Chicago, IL, USA). Multiple ROC curves via resampling analysis and CIF analysis were performed using R v.3.4.4 (Vienna, Austria). Scatter dot plots were assembled using GraphPad Prism 7.0a for MacOS Software (GraphPad Software Inc., LA Jolla, CA, USA).
Supplementary information
Additional file 1: Table S1. Associations between lung cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test. Table S2. Associations between prostate cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test for Primary Tumour (T) and Distant Metastasis (M), and by Kruskal-Wallis Test for Grade Group (GG), serum PSA levels and Clinical Stage. Table S3. Associations between colorectal cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test for Primary Tumour (T), Regional Node (N), Distant Metastasis (M) and Clinical Stage, and by Kruskal-Wallis Test for Tumour Location. Table S4. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in lung cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S5. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in prostate cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S6. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in colorectal cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S7. Primers and probes sequences with respective fluorochrome and quencher.
Additional file 2: Figure S1. Distribution of methylation levels in PCa patients according with metastatic dissemination. (A) APC, (B) GSTP1, (C) HOXD3, (D) RARβ2, (E) RASSF1A and (F) SEPT9 promoter’s methylation levels between non-metastatic (M0) (n=116) and metastatic (M+) (n=5) PCa patients. Mann-Whitney U Test, ***p<0.001, ****p<0.0001. Red horizontal lines represent median methylation levels. Figure S2. Cumulative incidence function plots according to clinicopathological variables (A) regional node, (B) distant metastasis, (C) clinical stage and (D) histological subtype in LC patients. p-values obtained by Gray’s test for disease-specific mortality. Figure S3. Cumulative incidence function plots according to clinicopathological variables (A) ISUP Grade Group, (B) serum PSA levels, (C) clinical stage, and (D) APC, (E) GSTP1, (F) RARβ2, (G) RASSF1A, (H) SEPT9, (I) SOX17 promoter methylation levels in PCa patients. p-values obtained by Gray’s test for disease-specific mortality. Figure S4. Cumulative incidence function plots according to clinicopathological variable (A) distant metastasis, and (B) RARβ2, (C) SEPT9 and (D) SOX17 promoter methylation levels in CRC patients. p-values obtained by Gray’s test for disease-specific mortality.
Acknowledgments
The authors are grateful to the Lung, Urological and Digestive Tract Cancer Clinics, as well as to the Department of Laboratory Medicine, namely to Berta Reis, BSN for their collaboration in sample collection. The authors would like to extend their appreciation to all the patients and control subjects that kindly provided the biological samples for this study.
Abbreviations
- AC
Asymptomatic controls
- APCme
Methylated APC regulator of WNT signalling pathway
- CcfDNA
Circulating cell-free DNA
- CI
Confidence interval
- CIF
Cumulative incidence function
- CRC
Colorectal cancer
- DFS
Disease-free survival
- DRE
Digital rectal examination
- DSM
Disease-specific mortality
- FOBT
Faecal occult blood test
- FOXA1me
Methylated Fork-head box A1
- GSTP1me
Methylated Glutathione S-transferase pi 1
- HOXD3me
Methylated Homeobox D3
- HR
Hazard ratio
- LC
Lung cancer
- LD-CT
Low-dose computed tomography
- NSCLC
Non-small cell lung carcinoma
- PCa
Prostate cancer
- PCA3
Prostate Cancer antigen 3
- PFS
Progression-free survival
- PSA
Prostate-specific antigen
- qMSP
Quantitative methylation-specific PCR
- RARβ2me
Methylated Retinoic acid receptor beta 2
- RASSF1Ame
Methylated Ras association domain family 1 isoform A
- SCLC
Small cell lung carcinoma
- SEPT9me
Methylated Septin 9
- SOX17me
Methylated SRY-box transcription factor 17
- WGA
Whole genome amplification
Authors’ contributions
CJ and RH conceived and designed the experiments. VC and SPN performed the experiments. VC, SPN, CM-B, LA, RH and CJ analysed the data. RF, JO, IP, JO, MS, CGD and TD assisted in patients’ enrolment for plasma samples’ collection and provided the clinical data from clinical charts. VC drafted the manuscript. CJ and RH designed and supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by Research Center—Portuguese Oncology Institute of Porto (grant PI 74-CI-IPOP-19-2015). S.P.N. was supported by a fellowship from Liga Portuguesa contra o Cancro/Pfizer.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
This study was approved by the institutional review board (Comissão de Ética para a Saúde) of Portuguese Oncology Institute of Porto, Portugal (CES-IPOFG-EPE 120/015). Written informed consent, in accordance with the Declaration of Helsinki ethical principles, was provided by all patients and asymptomatic donors enrolled in this study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vera Constâncio, Email: vera.salvado.constancio@ipoporto.min-saude.pt.
Sandra P. Nunes, Email: sandra.pinto.nunes@ipoporto.min-saude.pt
Catarina Moreira-Barbosa, Email: catarina.moreira.barbosa@gmail.com.
Rui Freitas, Email: rui.azevedo.freitas@gmail.com.
Jorge Oliveira, Email: jorge.oliveira@ipoporto.min-saude.pt.
Inês Pousa, Email: ines.ruiz@ipoporto.min-saude.pt.
Júlio Oliveira, Email: julio.oliveira@ipoporto.min-saude.pt.
Marta Soares, Email: martasoares@ipoporto.min-saude.pt.
Carlos Gonçalves Dias, Email: carlosgdias@ipoporto.min-saude.pt.
Teresa Dias, Email: teresa.dias@ipoporto.min-saude.pt.
Luís Antunes, Email: luis.antunes@ipoporto.min-saude.pt.
Rui Henrique, Email: rmhenrique@icbas.up.pt.
Carmen Jerónimo, Email: carmenjeronimo@ipoporto.min-saude.pt, Email: cljeronimo@icbas.up.pt.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13148-019-0779-x.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed]
- 2.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM et al: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011, 365(5):395-409. [DOI] [PMC free article] [PubMed]
- 3.De Koning H. VDAC, Ten Haaf K., Oudkerk M.: Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial. In: World Conference on Lung Cancer 2018. Toronto, Canada: Journal of Thoracic Oncology; 2018: S185.
- 4.Balata H, Evison M, Sharman A, Crosbie P, Booton R. CT screening for lung cancer: are we ready to implement in Europe? Lung Cancer. 2019;134:25–33. doi: 10.1016/j.lungcan.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Wu GX, Raz DJ. Lung Cancer Screening. Cancer Treat Res. 2016;170:1–23. doi: 10.1007/978-3-319-40389-2_1. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman RM. Clinical practice. Screening for prostate cancer. N Engl J Med. 2011;365(21):2013–2019. doi: 10.1056/NEJMcp1103642. [DOI] [PubMed] [Google Scholar]
- 7.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, Feuer EJ. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 8.Rawson JB, Bapat B. Epigenetic biomarkers in colorectal cancer diagnostics. Expert Rev Mol Diagn. 2012;12(5):499–509. doi: 10.1586/erm.12.39. [DOI] [PubMed] [Google Scholar]
- 9.Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–976. doi: 10.2147/CIA.S109285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 11.Jeronimo C, Henrique R. Epigenetic biomarkers in urological tumors: a systematic review. Cancer Lett. 2014;342(2):264–274. doi: 10.1016/j.canlet.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Ellinger J, Muller SC, Dietrich D. Epigenetic biomarkers in the blood of patients with urological malignancies. Expert Rev Mol Diagn. 2015;15(4):505–516. doi: 10.1586/14737159.2015.1019477. [DOI] [PubMed] [Google Scholar]
- 13.Costa-Pinheiro P, Montezuma D, Henrique R, Jeronimo C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics. 2015;7(6):1003–1015. doi: 10.2217/epi.15.56. [DOI] [PubMed] [Google Scholar]
- 14.Constancio V, Barros-Silva D, Jeronimo C, Henrique R. Known epigenetic biomarkers for prostate cancer detection and management: exploring the potential of blood-based liquid biopsies. Expert Rev Mol Diagn. 2019:1–9. [DOI] [PubMed]
- 15.Payne SR. From discovery to the clinic: the novel DNA methylation biomarker (m)SEPT9 for the detection of colorectal cancer in blood. Epigenomics. 2010;2(4):575–585. doi: 10.2217/epi.10.35. [DOI] [PubMed] [Google Scholar]
- 16.Weiss G, Schlegel A, Kottwitz D, Konig T, Tetzner R. Validation of the SHOX2/PTGER4 DNA methylation marker panel for plasma-based discrimination between patients with malignant and nonmalignant lung disease. J Thorac Oncol. 2017;12(1):77–84. doi: 10.1016/j.jtho.2016.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Toung JM, Jassowicz AF, Vijayaraghavan R, Kang H, Zhang R, Kruglyak KM, Huang HJ, Hinoue T, Shen H, et al. Targeted methylation sequencing of plasma cell-free DNA for cancer detection and classification. Ann Oncol. 2018;29(6):1445–1453. doi: 10.1093/annonc/mdy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Li Q, Kang S, Same M, Zhou Y, Sun C, Liu CC, Matsuoka L, Sher L, Wong WH, et al. CancerDetector: ultrasensitive and non-invasive cancer detection at the resolution of individual reads using cell-free DNA methylation sequencing data. Nucleic Acids Res. 2018;46(15):e89. doi: 10.1093/nar/gky423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Li Q, Chen Q, Zhou Y, Park S, Lee G, Grimes B, Krysan K, Yu M, Wang W, et al. CancerLocator: non-invasive cancer diagnosis and tissue-of-origin prediction using methylation profiles of cell-free DNA. Genome Biol. 2017;18(1):53. doi: 10.1186/s13059-017-1191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes Sandra, Moreira-Barbosa Catarina, Salta Sofia, Palma de Sousa Susana, Pousa Inês, Oliveira Júlio, Soares Marta, Rego Licínio, Dias Teresa, Rodrigues Jéssica, Antunes Luís, Henrique Rui, Jerónimo Carmen. Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women. Cancers. 2018;10(10):357. doi: 10.3390/cancers10100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer Control. 2014;21(1):9–14. doi: 10.1177/107327481402100102. [DOI] [PubMed] [Google Scholar]
- 22.Plumb AA, Halligan S. Colorectal cancer screening. Semin Roentgenol. 2015;50(2):101–110. doi: 10.1053/j.ro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 24.Freitas M, Ferreira F, Carvalho S, Silva F, Lopes P, Antunes L, Salta S, Diniz F, Santos LL, Videira JF, et al. A novel DNA methylation panel accurately detects colorectal cancer independently of molecular pathway. J Transl Med. 2018;16(1):45. doi: 10.1186/s12967-018-1415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usadel H, Brabender J, Danenberg KD, Jeronimo C, Harden S, Engles J, Danenberg PV, Yang S, Sidransky D. Quantitative adenomatous polyposis coli promoter methylation analysis in tumor tissue, serum, and plasma DNA of patients with lung cancer. Cancer Res. 2002;62(2):371–375. [PubMed] [Google Scholar]
- 26.Ponomaryova AA, Rykova EY, Cherdyntseva NV, Skvortsova TE, Dobrodeev AY, Zav'yalov AA, Bryzgalov LO, Tuzikov SA, Vlassov VV, Laktionov PP. Potentialities of aberrantly methylated circulating DNA for diagnostics and post-treatment follow-up of lung cancer patients. Lung Cancer. 2013;81(3):397–403. doi: 10.1016/j.lungcan.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara K, Fujimoto N, Tabata M, Nishii K, Matsuo K, Hotta K, Kozuki T, Aoe M, Kiura K, Ueoka H, et al. Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin Cancer Res. 2005;11(3):1219–1225. [PubMed] [Google Scholar]
- 28.Powrozek T, Krawczyk P, Kucharczyk T, Milanowski J. Septin 9 promoter region methylation in free circulating DNA-potential role in noninvasive diagnosis of lung cancer: preliminary report. Med Oncol. 2014;31(4):917. doi: 10.1007/s12032-014-0917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Qi W, Sun L, Zhou H, Zhou B, Hu Y. DNA methylation analysis of selected genes for the detection of early-stage lung cancer using circulating cell-free DNA. Adv Clin Exp Med. 2019;28(3):361–366. doi: 10.17219/acem/87114. [DOI] [PubMed] [Google Scholar]
- 30.Balgkouranidou I, Chimonidou M, Milaki G, Tsaroucha E, Kakolyris S, Georgoulias V, Lianidou E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin Chem Lab Med. 2016;54(8):1385–1393. doi: 10.1515/cclm-2015-0776. [DOI] [PubMed] [Google Scholar]
- 31.Hsu HS, Chen TP, Hung CH, Wen CK, Lin RK, Lee HC, Wang YC. Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer. 2007;110(9):2019–2026. doi: 10.1002/cncr.23001. [DOI] [PubMed] [Google Scholar]
- 32.Begum S, Brait M, Dasgupta S, Ostrow KL, Zahurak M, Carvalho AL, Califano JA, Goodman SN, Westra WH, Hoque MO, et al. An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin Cancer Res. 2011;17(13):4494–4503. doi: 10.1158/1078-0432.CCR-10-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulbert A, Jusue-Torres I, Stark A, Chen C, Rodgers K, Lee B, Griffin C, Yang A, Huang P, Wrangle J, et al. Early detection of lung cancer using DNA promoter hypermethylation in plasma and sputum. Clin Cancer Res. 2017;23(8):1998–2005. doi: 10.1158/1078-0432.CCR-16-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira-Barbosa C, Barros-Silva D, Costa-Pinheiro P, Torres-Ferreira J, Constancio V, Freitas R, Oliveira J, Antunes L, Henrique R, Jeronimo C. Comparing diagnostic and prognostic performance of two-gene promoter methylation panels in tissue biopsies and urines of prostate cancer patients. Clin Epigenetics. 2018;10(1):132. doi: 10.1186/s13148-018-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao F, Olkhov-Mitsel E, Kamdar S, Jeyapala R, Garcia J, Hurst R, Hanna MY, Mills R, Tuzova AV, O'Reilly E, et al. A urine-based DNA methylation assay, ProCUrE, to identify clinically significant prostate cancer. Clin Epigenetics. 2018;10(1):147. doi: 10.1186/s13148-018-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu T, Giovannucci E, Welge J, Mallick P, Tang WY, Ho SM. Measurement of GSTP1 promoter methylation in body fluids may complement PSA screening: a meta-analysis. Br J Cancer. 2011;105(1):65–73. doi: 10.1038/bjc.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumache R, Puiu M, Motoc M, Vernic C, Dumitrascu V. Prostate cancer molecular detection in plasma samples by glutathione S-transferase P1 (GSTP1) methylation analysis. Clin Lab. 2014;60(5):847–852. doi: 10.7754/clin.lab.2013.130701. [DOI] [PubMed] [Google Scholar]
- 38.Sunami E, Shinozaki M, Higano CS, Wollman R, Dorff TB, Tucker SJ, Martinez SR, Mizuno R, Singer FR, Hoon DS. Multimarker circulating DNA assay for assessing blood of prostate cancer patients. Clin Chem. 2009;55(3):559–567. doi: 10.1373/clinchem.2008.108498. [DOI] [PubMed] [Google Scholar]
- 39.Ellinger J, Haan K, Heukamp LC, Kahl P, Buttner R, Muller SC, von Ruecker A, Bastian PJ. CpG island hypermethylation in cell-free serum DNA identifies patients with localized prostate cancer. Prostate. 2008;68(1):42–49. doi: 10.1002/pros.20651. [DOI] [PubMed] [Google Scholar]
- 40.Song L, Jia J, Peng X, Xiao W, Li Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: a meta-analysis. Sci Rep. 2017;7(1):3032. doi: 10.1038/s41598-017-03321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassinotti E, Melson J, Liggett T, Melnikov A, Yi Q, Replogle C, Mobarhan S, Boni L, Segato S, Levenson V. DNA methylation patterns in blood of patients with colorectal cancer and adenomatous colorectal polyps. Int J Cancer. 2012;131(5):1153–1157. doi: 10.1002/ijc.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YC, Yu ZH, Liu C, Xu LZ, Yu W, Lu J, Zhu RM, Li GL, Xia XY, Wei XW, et al. Detection of RASSF1A promoter hypermethylation in serum from gastric and colorectal adenocarcinoma patients. World J Gastroenterol. 2008;14(19):3074–3080. doi: 10.3748/wjg.14.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roperch JP, Incitti R, Forbin S, Bard F, Mansour H, Mesli F, Baumgaertner I, Brunetti F, Sobhani I. Aberrant methylation of NPY, PENK, and WIF1 as a promising marker for blood-based diagnosis of colorectal cancer. BMC Cancer. 2013;13:566. doi: 10.1186/1471-2407-13-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pack SC, Kim HR, Lim SW, Kim HY, Ko JY, Lee KS, Hwang D, Park SI, Kang H, Park SW, et al. Usefulness of plasma epigenetic changes of five major genes involved in the pathogenesis of colorectal cancer. Int J Color Dis. 2013;28(1):139–147. doi: 10.1007/s00384-012-1566-8. [DOI] [PubMed] [Google Scholar]
- 45.Lee BB, Lee EJ, Jung EH, Chun HK, Chang DK, Song SY, Park J, Kim DH. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009;15(19):6185–6191. doi: 10.1158/1078-0432.CCR-09-0111. [DOI] [PubMed] [Google Scholar]
- 46.Kearns JT, Lin DW. Improving the specificity of PSA screening with serum and urine markers. Curr Urol Rep. 2018;19(10):80. doi: 10.1007/s11934-018-0828-6. [DOI] [PubMed] [Google Scholar]
- 47.Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, Xu J, Shao Q. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:25776. doi: 10.1038/srep25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Yu Z, Wang T, Zhang J, Hong L, Chen L. Identification of epigenetic aberrant promoter methylation of RASSF1A in serum DNA and its clinicopathological significance in lung cancer. Lung Cancer. 2007;56(2):289–294. doi: 10.1016/j.lungcan.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Fu B, Yan P, Zhang S, Lu Y, Pan L, Tang W, Chen S, Chen S, Zhang A, Liu W. Cell-Free Circulating Methylated SEPT9 for Noninvasive Diagnosis and Monitoring of Colorectal Cancer. Dis Markers. 2018;2018:6437104. doi: 10.1155/2018/6437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergheim J, Semaan A, Gevensleben H, Groening S, Knoblich A, Dietrich J, Weber J, Kalff JC, Bootz F, Kristiansen G, et al. Potential of quantitative SEPT9 and SHOX2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: a prospective observational cohort study. Br J Cancer. 2018;118(9):1217–1228. doi: 10.1038/s41416-018-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunes SP, Diniz F, Moreira-Barbosa C, Constancio V, Silva AV, Oliveira J, Soares M, Paulino S, Cunha AL, Rodrigues J, et al. Subtyping Lung Cancer Using DNA Methylation in Liquid Biopsies. J Clin Med. 2019;8:9. doi: 10.3390/jcm8091500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilad S, Lithwick-Yanai G, Barshack I, Benjamin S, Krivitsky I, Edmonston TB, Bibbo M, Thurm C, Horowitz L, Huang Y, et al. Classification of the four main types of lung cancer using a microRNA-based diagnostic assay. J Mol Diagn. 2012;14(5):510–517. doi: 10.1016/j.jmoldx.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Lu S, Kong H, Hou Y, Ge D, Huang W, Ou J, Yang D, Zhang L, Wu G, Song Y, et al. Two plasma microRNA panels for diagnosis and subtype discrimination of lung cancer. Lung Cancer. 2018;123:44–51. doi: 10.1016/j.lungcan.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Zhai X, Li SJ. Methylation of RASSF1A and CDH13 genes in individualized chemotherapy for patients with non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(12):4925–4928. doi: 10.7314/APJCP.2014.15.12.4925. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Zhang B, Chen D, Xia W, Zhang J, Wang F, Xu J, Zhang Y, Zhang M, Zhang L, et al. Real-time monitoring efficiency and toxicity of chemotherapy in patients with advanced lung cancer. Clin Epigenetics. 2015;7:119. doi: 10.1186/s13148-015-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 58.Baker SG, Kramer BS. Identifying genes that contribute most to good classification in microarrays. BMC Bioinformatics. 2006;7:407. doi: 10.1186/1471-2105-7-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Associations between lung cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test. Table S2. Associations between prostate cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test for Primary Tumour (T) and Distant Metastasis (M), and by Kruskal-Wallis Test for Grade Group (GG), serum PSA levels and Clinical Stage. Table S3. Associations between colorectal cancer patients’ clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels. p-values obtained by Mann-Whitney U Test for Primary Tumour (T), Regional Node (N), Distant Metastasis (M) and Clinical Stage, and by Kruskal-Wallis Test for Tumour Location. Table S4. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in lung cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S5. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in prostate cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S6. Demographics of the clinicopathological features and APC, FOXA1, GSTP1, HOXD3, RARβ2, RASSF1A, SEPT9 and SOX17 promoters’ methylation levels in colorectal cancer patients, and their association with disease-specific mortality. p-values obtained by Gray’s test. Table S7. Primers and probes sequences with respective fluorochrome and quencher.
Additional file 2: Figure S1. Distribution of methylation levels in PCa patients according with metastatic dissemination. (A) APC, (B) GSTP1, (C) HOXD3, (D) RARβ2, (E) RASSF1A and (F) SEPT9 promoter’s methylation levels between non-metastatic (M0) (n=116) and metastatic (M+) (n=5) PCa patients. Mann-Whitney U Test, ***p<0.001, ****p<0.0001. Red horizontal lines represent median methylation levels. Figure S2. Cumulative incidence function plots according to clinicopathological variables (A) regional node, (B) distant metastasis, (C) clinical stage and (D) histological subtype in LC patients. p-values obtained by Gray’s test for disease-specific mortality. Figure S3. Cumulative incidence function plots according to clinicopathological variables (A) ISUP Grade Group, (B) serum PSA levels, (C) clinical stage, and (D) APC, (E) GSTP1, (F) RARβ2, (G) RASSF1A, (H) SEPT9, (I) SOX17 promoter methylation levels in PCa patients. p-values obtained by Gray’s test for disease-specific mortality. Figure S4. Cumulative incidence function plots according to clinicopathological variable (A) distant metastasis, and (B) RARβ2, (C) SEPT9 and (D) SOX17 promoter methylation levels in CRC patients. p-values obtained by Gray’s test for disease-specific mortality.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.