Abstract
Exosomes, a type of extracellular vesicles, can be collected from the conditioned medium of cultured cells, and are expected to be used in disease therapy and drug delivery systems. However, since the yield of exosomes from conditioned medium is generally low, investigations to develop new methods to increase exosome secretion and to elucidate the secretion mechanism have been performed. Our previous studies demonstrated that activation of intracellular signaling including Rho GTPase and subsequent endocytosis of extraneous molecules in cells could be induced by low level electricity (0.3–0.5 mA/cm2). Since exosomes are produced in the process of endocytosis and secreted by exocytosis via certain signaling pathways, we hypothesized that low level electric treatment (ET) would increase exosome secretion from cultured cells via intracellular signaling activation. In the present study, the influence of ET (0.34 mA/cm2) on extracellular vesicle (EV) secretion from cultured cells was examined by using murine melanoma and murine fibroblast cells. The results showed that the number of EV particles collected by ultracentrifugation was remarkably increased by ET in both cell lines without cellular toxicity or changes in the particle distribution. Also, protein amounts of the collected EVs were significantly increased in both cells by ET without alteration of expression of representative exosome marker proteins. Moreover, in both cells, the ratio of particle numbers to protein amount was not significantly changed by ET. Rho GTPase inhibition significantly suppressed ET-mediated increase of EV secretion in murine melanoma, indicating that Rho GTPase activation could be involved in ET-mediated EV secretion in the cell. Additionally, there were almost no differences in uptake of each EV into each donor cell regardless of whether the cells had been exposed to ET for EV collection. Taken together, these results suggest that ET could increase EV secretion from both cancer and normal cells without apparent changes in EV quality.
Keywords: Exosomes, Extracellular vesicles, Low level electric treatment, Intracellular signaling, Exosome secretion
Highlights
-
•
Low level electric treatment (ET; 0.34 mA/cm2) increased exosome yield from cells.
-
•
The number of exosome particles was increased by ET without distribution changes.
-
•
ET increased protein amounts of the collected exosomes from conditioned media.
-
•
ET did not induce cellular toxicity and change in exosomal marker protein expression.
-
•
ET-mediated increase of exosome secretion occurred in both cancer and normal cells.
1. Introduction
Exosomes, a type of extracellular vesicle (EV), are composed of lipid bilayers, characteristically with diameters of 40–150 nm; they are secreted by a variety of cell types [1]. Exosomes are involved in intercellular communication via delivery of proteins and nucleic acids, including microRNA (miRNA) and mRNA [2]. Since exosomes can transport endogenous cargo (proteins and nucleic acids) for cell-to-cell cross-talk by their inherent potencies, they have been expected as bio-shuttles for efficient delivery of biomolecules inside the cells, especially in the field of gene therapy [3]. Indeed, it was previously reported that small interfering RNA (siRNA) was incorporated into exosomes by electroporation and the siRNA tagged-exosomes were employed as a delivery vector to decrease the target protein expression [4]. Exosomes are also involved in the pathology of various diseases such as cancer, cardiovascular diseases, and Alzheimer's diseases [[5], [6], [7]]. In particular, in the field of cancer, cancer cell-derived exosomes have been mentioned to not only participate in the formation of the tumor microenvironment and metastasis [8], but also possess tumor antigens that are expected to be used in cancer immunotherapy [9]. Thus, exosomes are expected as tools for treating diseases, and mechanisms of their production and secretion are recognized as targets for development of new therapies.
In order to obtain exosomes for research, laboratories generally use conditioned medium from cultivated cells, relying on stepwise ultracentrifugation procedures. However, low yield and purity of exosomes have been frequently observed [10]. The functions, properties, and production amounts of EVs including exosomes have been reported to be different between donor cells of EVs. For instance, mesenchymal stem cells (MSCs) were reported to secrete more than 100-1000 fold EVs compared with other cell lines such as myoblasts [11]. Other researcher also reported that protein amount and EV particle number are different between certain cell lines [12]. Thus, for elucidation of specific functions of EVs and use of their functions, low yield of EVs from donor cells can be an obstacle. In fact, it was previously reported that the dose of injected EVs into mice varied from 1 to 150 μg in the case of EVs been used as vehicles for drug delivery in some reports [[13], [14], [15]]. To collect 150 μg of EVs from cultured cells, several number of dishes or flasks are required. Therefore, obtaining higher yield of EVs from various cells can be advantage for progression of EV research. To increase their yield and purity, polymeric precipitation and affinity-based purification methods have been reported [10,16]. In addition to these methods, development of methods to increase exosome secretion from cells would be a useful approach for increasing exosome yield. Several mechanisms regarding the production and secretion of exosomes were previously reported, including plasma membrane depolarization of neurons [17], increases of intracellular Ca2+ concentrations being trigger in certain cells [18], involvement of the Rab GTPase family [19], and so on. Based on these findings, it is expected that activation of intracellular signaling via stimulation of cultured cells may increase exosome yield.
Our previous studies reported that low level electric treatment (ET; 0.3–0.5 mA/cm2), which is employed for iontophoresis, a transdermal drug delivery technology, of cultured cells induce cellular uptake of macromolecules such as small interfering RNA (siRNA) and dextran (molecular weight: 10,000 and 70,000) via induction of endocytosis [20,21]. With regard to the mechanisms of ET-mediated endocytosis, we also reported that activation of Rho GTPase via heat shock protein 90 (HSP90) and protein kinase C (PKC) is involved in endocytosis induction [22]. On the other hand, it was previously reported that early endosomes imported by endocytosis are sort to late endosomes and then form multivesicular bodies (MVB), and exosomes are produced in MVB [23]. Then, exosomes are released after exocytic fusion of the MVB with the plasma membrane via several signaling pathways [24]. Based on these findings, we hypothesized that since ET of cultured cells activate intracellular signaling and induce endocytosis, secretion of EVs including exosomes from cultured cells would also be induced via ET. In addition, if ET can increase EV secretion from cultivated cells, elucidation of underlying mechanisms of ET-mediated increase of EV secretion may lead to explore new therapeutic targets for certain diseases by regulating EV secretion as well as development of a mean to increase EV yield from the cells.
Therefore, in the present study, we investigated the effect of ET (0.34 mA/cm2) on EV secretion, including exosomes, from cultured cells by evaluating the number of particles and the amount of protein in EVs harvested from conditioned medium. For this purpose, we used B16F1 murine melanoma and 3T3 Swiss Albino cells as representative cancer and normal cell line, respectively. The expression of representative exosome markers was also examined to confirm the quality of the EVs collected following ET.
2. Materials and methods
2.1. Cell cultures
A murine melanoma cell line, B16F1, was purchased from DS Pharma Biomedical Co., Ltd (Osaka, Japan). A murine fibroblast cell line, 3T3 Swiss Albino, was obtained from RIKEN Cell Bank (Tsukuba, Japan). B16F1 and 3T3 Swiss Albino cells were cultured in Dulbecco's modified Eagle medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin (Gibco, MA, USA), and 100 μg/mL streptomycin (Gibco). The cells were cultured at 37 °C in a 5% CO2 incubator.
2.2. Collection of extracellular vesicles (EVs)
B16F1 or 3T3 Swiss Albino cells were seeded onto 150-mm dishes at a density of 4 or 6 × 106 cells/dish, respectively, to reach approximately 80% confluency after overnight incubation. Then, the medium was removed, and the cells were washed with phosphate-buffered saline (PBS). Cell culture medium was replaced with Advanced DMEM (Gibco), a FBS and exosome-free medium, supplemented with 2 mM l-glutamine (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin. When performing ET, Ag–AgCl electrodes with 2.5 cm2 surface areas (3 M Health Care, Minneapolis, MN, USA) were placed in the culture dish, and the cells were treated with a constant current of 0.34 mA/cm2 for 60 min. After 24 h of additional incubation, the conditioned medium was collected, and EVs in the medium were purified as described previously [25]. In brief, the medium was subjected to sequential centrifugation at 300×g for 10 min, 2000×g for 20 min, and 10,000×g for 30 min at 4 °C, followed by filtration with 0.22-μm syringe filters (Merck Millipore, MA, USA). Then, the samples were ultracentrifuged at 100,000×g for 70 min at 4 °C (Optima L-90K; Beckman Coulter, Tokyo, Japan) to pellet the EVs. The EVs were then resuspended in PBS and subjected to ultracentrifugation (100,000×g, 70 min, 4 °C) for washing. The resultant EVs were resuspended in PBS and used in the following experiments. Also, after harvesting the conditioned medium, the cells were trypsinized and the number of the cells was determined by trypan blue staining.
2.3. Measurement of EV particle size distribution, particle number and ζ-potential
The particle size distribution and the number of particles in the collected EV suspension were measured with a nanoparticle multi-analyzer (qNano; Meiwafosis Co., Ltd., Tokyo, Japan). To determine the ζ-potential of the EVs, a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) was employed.
2.4. Transmission electron microscopy
To observe the morphology of the collected EVs by transmission electron microscopy (TEM), the samples were absorbed to formvar film coated copper grids and were negatively stained with 2% phospho tungstic acid solution (pH 7.0) for 60 s. Then, the grids were observed by a transmission electron microscope (JEM-1400Plus; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 100 kV. Digital images were taken with a CCD camera (EM-14830RUBY2; JEOL Ltd.).
2.5. Quantification of protein amount of EVs
The protein concentration of the collected EVs was determined by using a Micro bicinchoninic acid (BCA) Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's instructions. The absorbance of each sample at 562 nm was measured with a Tecan Infinite M200 microplate reader (Salzburg, Austria).
2.6. Western blotting
Anti-CD9 rabbit monoclonal antibody (ab92726; Abcam, Cambridge, UK), anti-HSP70 mouse monoclonal antibody (ab5439; Abcam), anti-CD81 mouse monoclonal antibody (sc-166029; Santa Cruz Biotechnology, CA, USA), horse-radish peroxidase (HRP)-conjugated anti-rabbit IgG polyclonal antibody (A24531; Thermo Fisher Scientific, Waltham, MA, USA), and HRP-conjugated anti-mouse IgG rabbit polyclonal antibody (ab97046; Abcam) were purchased as indicated. To observe the expression of CD9, HSP70, and CD81 in the collected EVs, the EVs (1 μg protein) were subjected to 10% (for CD9 and HSP70) or 12.5% (for CD81) SDS-PAGE, and the proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The PVDF membrane was incubated with 3% bovine serum albumin (BSA) dissolved in Tris-HCl-buffered saline containing 0.1% Tween20 (pH 7.4) for 1 h at 37 °C, then with anti-CD9 antibody (1:2000), anti-HSP70 antibody (1:2000), or anti-CD81 antibody (1:100) for 24 h at 4 °C, respectively. Thereafter, the membrane was incubated with each secondary antibody at a dilution of 1:2000 for rabbit IgG or 1:5000 for mouse IgG for 1 h at 37 °C. After incubation of the membrane with a chemiluminescent substrate reagent (ECL prime; GE Healthcare, Little Chalfont, UK), the bands of each protein were detected with a LAS-4000 mini system (Fuji Film, Tokyo, Japan).
2.7. Rho GTPase inhibition experiment with pharmacological inhibitor
For mechanistic study, B16F1 or 3T3 Swiss Albino cells were seeded onto 150-mm dishes at a density of 4 or 6 × 106 cells/dish, respectively. After washing the cells with PBS followed by replacing with Advanced DMEM supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, a Rho GTPase inhibitor Rhosin hydrochloride (Tocris Bioscience, Bristol, UK) dissolved in dimethyl sulfoxide (DMSO; Wako Pure Chemical, Osaka, Japan) was added into the culture media to become a concentration of 10 or 30 μM. The final concentration of DMSO in the media was 0.1% in each group. Then, the cells were treated with a constant current of 0.34 mA/cm2 for 60 min in the presence or absence of the Rho GTPase inhibitor. After 24 h of additional incubation, the conditioned medium was collected, and EVs in the medium were purified as mentioned above. Thereafter, the number of particles in the collected EV suspension was measured with the nanoparticle multi-analyzer qNano.
2.8. Evaluation of EV uptake
For uptake study, the EVs collected from B16F1 and 3T3 Swiss Albino cells were collected with or without ET as described above, followed by fluorescence-labeling with PKH67 (Sigma-Aldrich, MO, USA) according to the manufacturer's protocol. The PKH67-labeled EVs were washed twice with PBS by using Amicon Ultra 10K (Merck Millipore). B16F1 and 3T3 Swiss Albino cells were seeded onto a 35-mm glass bottom dish at a density of 1 × 105 cells/dish. After 24-h incubation, PKH67-labeled EVs derived from B16F1 or 3T3 Swiss Albino were added to B16F1 or 3T3 Swiss Albino cells, respectively, to a final concentration of 5 μg protein/mL in serum free DMEM. After 6-h incubation, the media were removed and the cells were washed twice with PBS. Then, the cells were fixed with 4% paraformaldehyde for 20 min at 37 °C. After washing the cells with PBS three times, the cells were incubated with 1 μg/mL 4′, 6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific) in PBS for 15 min at 37 °C to stain nuclei. After washing with PBS, the fluorescence was observed using a confocal laser scanning microscope (LSM700, Carl Zeiss). To evaluate relative uptake amount of each PKH-labeled EVs, fluorescence intensities of PKH67 were analyzed for 12 images per each group for one experiment using an image-analysis system Image J. The experiments were performed 3 times independently.
2.9. Statistical analysis
The statistical differences were evaluated by one-way analysis of variance with the Tukey post-hoc test. Those in 2 groups were determined by using Student's t-test. Data were presented as the mean ± S.D.
3. Results
3.1. Physicochemical properties of collected EVs
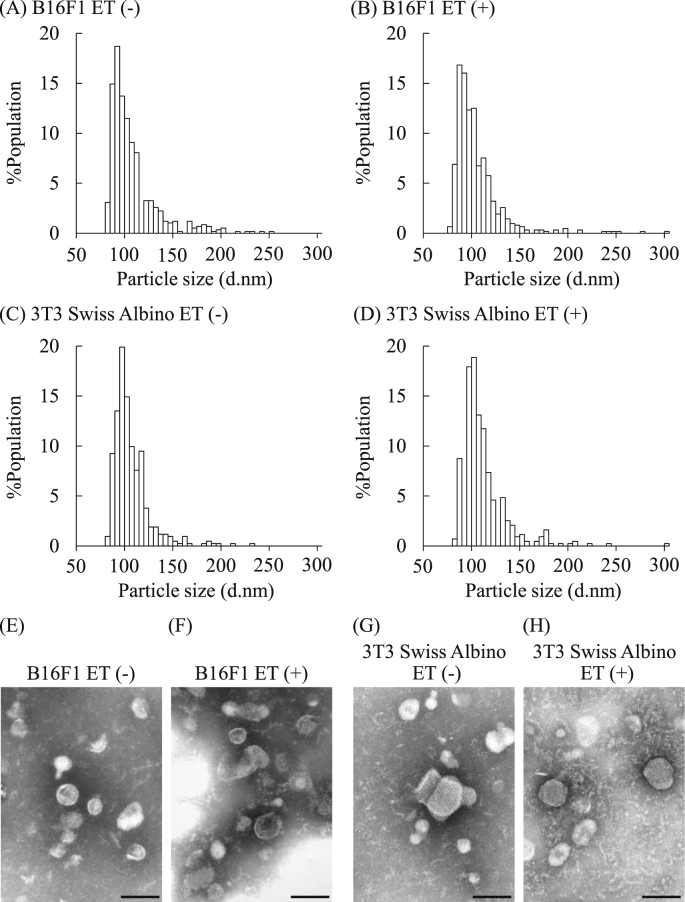
By using B16F1 and 3T3 Swiss Albino as representative cancer and normal cell lines, we examined the influence of ET (0.34 mA/cm2) on EV secretion. ET onto the cultured cells was performed as shown in Fig. 1. First, we examined physicochemical properties of EVs collected by ultracentrifugation from the culture supernatants. As shown in Table 1, the average particle sizes of the collected EVs ranged from approximately 100 to 120 nm in diameter, and their ζ-potentials were approximately −20 to −25 mV. The statistically significant differences in the particle size and the ζ-potential were not found between EVs collected from each cell exposed or not exposed to ET. Histograms of the particle size distribution obtained with a nanoparticle multi-analyzer indicated that the collected EVs from both cell lines showed similar distribution patterns regardless of the treatment with low level electricity (Figs. 2A–D). We also performed TEM observations to visualize the isolated EVs. The TEM images showed globular vesicles having approximately 100 nm in diameter in each EV sample (Figs. 2E–H). Moreover, the particle size of the EVs from B16F1 tended to be smaller than those from 3T3 Swiss Albino in agreement with the results of Table 1.
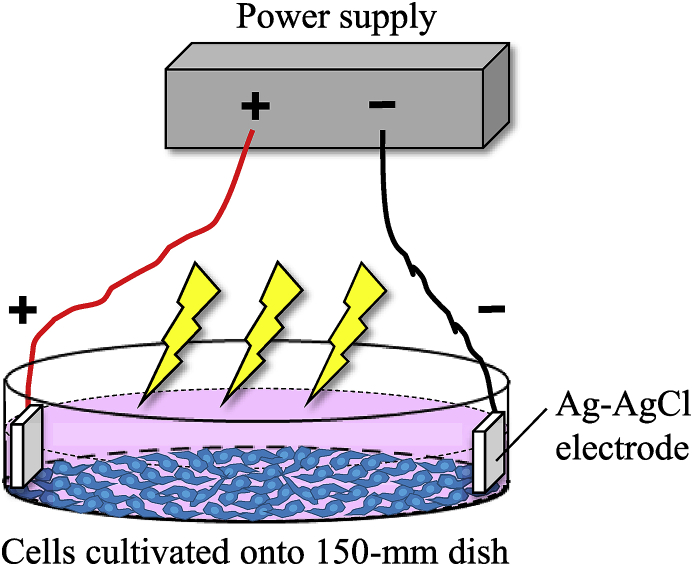
Fig. 1.
Graphic illustration of ET onto the cultured cells. For the treatment of the cultured cells with low level electricity, two Ag–AgCl electrodes with 2.5 cm2 surface areas were placed in the culture dish. Then, the cells were treated with a constant current of 0.34 mA/cm2 for 60 min. Twenty-four hours after ET, the conditioned medium was collected, and extracellular vesicle (EV) isolation was performed by the ultracentrifugation procedures.
Table 1.
Particle sizes and ζ-potentials of the EVs harvested from each cell line.
| Exosome-producing cells | Particle size (d.nm) | ζ-Potential (mV) |
|---|---|---|
| B16F1 (ET (−)) | 105.5 ± 8.5 | −26.1 ± 2.7 |
| B16F1 (ET (+)) | 108.0 ± 9.8 | −26.2 ± 8.4 |
| 3T3 Swiss Albino (ET (−)) | 111.3 ± 8.2 | −18.6 ± 7.2 |
| 3T3 Swiss Albino (ET (+)) | 118.2 ± 12.4 | −20.0 ± 5.0 |
The data indicate the mean ± S.D. (n = 5).
Fig. 2.
Particle distribution and morphology of the EVs collected from culture cells. B16F1 and 3T3 Swiss Albino cells were treated by low level electricity (ET; 0.34 mA/cm2) for 1 h. After 24 h of incubation, EVs were collected from the conditioned medium of each cell line by ultracentrifugation. Then, the particle distributions of the EVs collected from B16F1 ((A): ET (−), (B): ET (+)) and 3T3 Swiss Albino ((C): ET (−), (D) ET (+)) were analyzed with a qNano. The morphologies of the EVs collected from B16F1 ((E): ET (−), (F): ET (+)) and 3T3 Swiss Albino ((G): ET (−), (H): ET (+)) were observed by TEM. Scale bars = 100 nm.
3.2. ET increased EV secretion from cultured cells
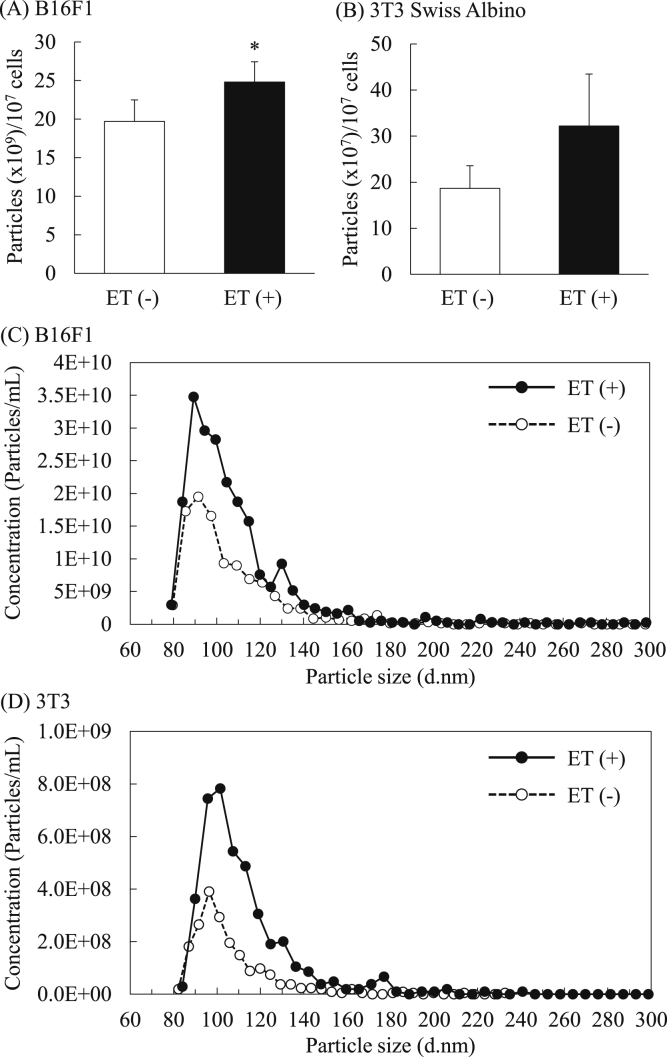
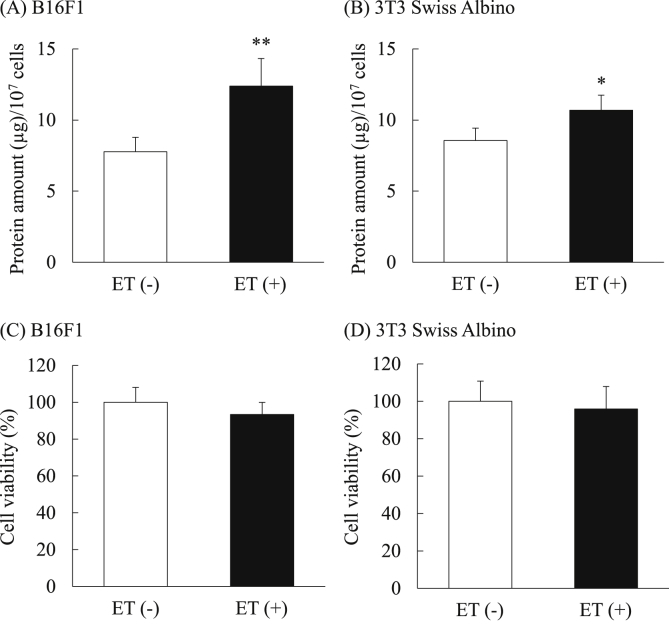
Next, we investigated the effect of ET on EV secretion from cultured cells by determining particle number and protein amount. The results showed that ET increased the particle number of EVs from both B16F1 (1.26-fold) and 3T3 Swiss albino (1.7-fold) cells (Figs. 3A and B). In particular, the number of EVs in the size (diameter) range 90–130 nm was markedly increased as shown in the particle distribution data (Figs. 3C and D). We also analyzed the particle distribution of EVs collected without the procedure of filtration using 0.22-μm syringe filter. The results showed that ET-mediated increase of the number of EVs having over 150 nm was not remarkable compared with that of less than 150 nm in both cell lines (Supplementary Fig. 1). The amount of protein in the collected EVs was significantly higher in the group treated with low level electricity compared with the non-treated group, and this tendency was observed with both B16F1 (1.6-fold) and 3T3 Swiss albino (1.25-fold) cell lines (Figs. 4A and B). On the other hand, the protein contents of the cells were comparable between non-treated cells and low level electricity-treated cells at 24 h after ET (Supplementary Fig. 2). The number of viable cells 24 h after 1 h of ET was also evaluated for each cell line. There was almost no difference in cell viability between the groups treated with low level electricity and the non-treated group in both cell lines (Figs. 4C and D). By changing the current strength and time of ET, we evaluated the increase of EV secretion in B16F1 cells by BCA assay. When ET (0.34 or 0.5 mA/cm2) was performed for 15 min, the protein amount of EVs tended to increase compared with non-treated group (Supplementary Fig. 3A). Treatment-time dependent increase in the protein amounts of the collected EVs was observed, and the amounts were significantly increased in the groups of ET with a constant current of 0.34 or 0.5 mA/cm2 for 60 min. On the other hand, significant decrease in cell viability determined based on the cell number after 24 h of ET was observed in the cells exposed to ET (0.5 mA/cm2) for 60 min (Supplementary Fig. 3B). Considering the balance between increase of protein amounts and cell viability, ET (0.34 mA/cm2) for 60 min was suggested to be best among the examined conditions, and the condition was used in the following experiments.
Fig. 3.
Enhancement of EV secretion from cultivated cells by ET. The particle number of the collected EVs from B16F1 (A) and 3T3 Swiss Albino (B) cells treated with or without low level electricity (0.34 mA/cm2, 1 h) was determined. The data show the mean ± S.D. (n = 5). *P < 0.05 vs. ET (−). The representative graphs of the particle concentration curve were obtained from B16F1- (C) and Swiss 3T3 Albino-derived EVs (D).
Fig. 4.
ET increased the amount of protein in the collected EVs. The quantity of protein in the EVs harvested from the conditioned medium of B16F1 (A) and 3T3 Swiss Albino (B) was measured by BCA assay. The number of cells was counted by trypan blue staining after collecting their conditioned media. The relative percentage of viability to that of non-treated cells is indicated (C: B16F1, D: 3T3 Swiss Albino). The data show the mean ± S.D. (n = 5). *P < 0.05, **P < 0.01 vs. ET (−).
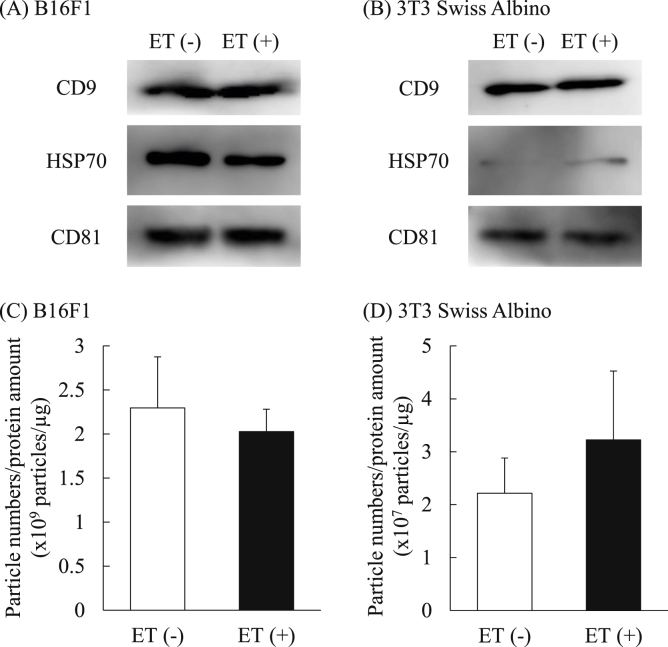
3.3. Influence of ET on the expression of representative exosomal marker proteins
To assess the influence of ET on the quality of the collected EVs, expression of representative exosomal markers, namely CD9, HSP70, and CD81, in the EVs was confirmed by Western blotting. Western blotting showed that each exosome marker was present in the EVs derived from each cell line regardless of the treatment with low level electricity, although the expression of HSP70 was quite low in 3T3 Swiss Albino-derived EVs (Figs. 5A and B), as reported previously [12]. These results suggest that ET could increase the secretion of EVs from both cancer and normal cells without changes in expression of representative exosomal markers. In addition, the ratios of particle numbers to the amounts of protein were calculated based on the results of Fig. 3, Fig. 4B. Although particle number/protein quantity tended to increase by ET in 3T3 Swiss Albino cells, significant differences were not observed in either cell line regardless of the treatment by low level electricity (Figs. 5C and D), suggesting that the increased EV protein amount is due to the increased number of secreted EVs. Based on these results, it was suggested that ET did not change the quality of the secreted EVs from either cancer or normal cells.
Fig. 5.
Western blot analysis of the expression of the exosomal marker proteins. EVs derived from B16F1 (A) and 3T3 Swiss Albino (B) were subjected to SDS-PAGE (1 μg/lane). Western blotting was performed to observe the expression of exosome markers CD9, HSP70, and CD81. Per the product datasheet, the detected molecular weights of CD9, HSP70, and CD81 are approximately 25, 70–78, and 22–26 kDa, respectively. The experiments were performed 3 times independently. The ratios of particle number per protein amount of the EVs collected from B16F1 (C) and 3T3 Swiss Albino (D) were calculated based on the results of Fig. 2, Fig. 3.
3.4. Influence of Rho GTPase inhibition on ET-mediated increase in EV secretion
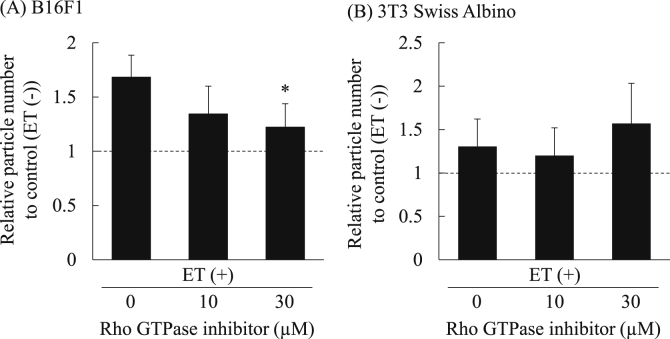
Since our previous study demonstrated that ET of cultured cells induces endocytosis of macromolecules via activation of Rho GTPase [22], we evaluated the influence of Rho GTPase inhibition on ET-mediated increase in EV secretion by using a Rho GTPase inhibitor, Rhosin hydrochloride [26]. The results showed that increase in the particle number of the collected EVs from 3T3 Swiss Albino cells treated by low level electricity was hardly inhibited by the Rho GTPase inhibitor (Fig. 6B). On the other hand, the number of particles was decreased in the Rho GTPase inhibitor dose-dependent manner in the EVs collected from B16F1 exposed to ET (Fig. 6A). Notably, significant decrease in the particle number was observed in B16F1 cells treated with 30 μM of the inhibitor. These results suggest that Rho GTPase activation could be involved in ET-mediated increase in EV secretion in a cancer cell B16F1.
Fig. 6.
Effect of Rho GTPase inhibition on increase in EV secretion by ET. B16F1 and 3T3 Swiss Albino cells were exposed to ET (0.34 mA/cm2) for 1 h in the presence of Rho GTPase inhibitor at the indicated concentration. After 24 h of additional incubation, EVs were collected, and the particle number of the collected EVs from B16F1 (A) and 3T3 Swiss Albino (B) cells was then measured with a qNano. The relative particle number of each group to that in control (ET (−)) are presented. The data show the mean ± S.D. (n = 4). Significant difference; *P < 0.05.
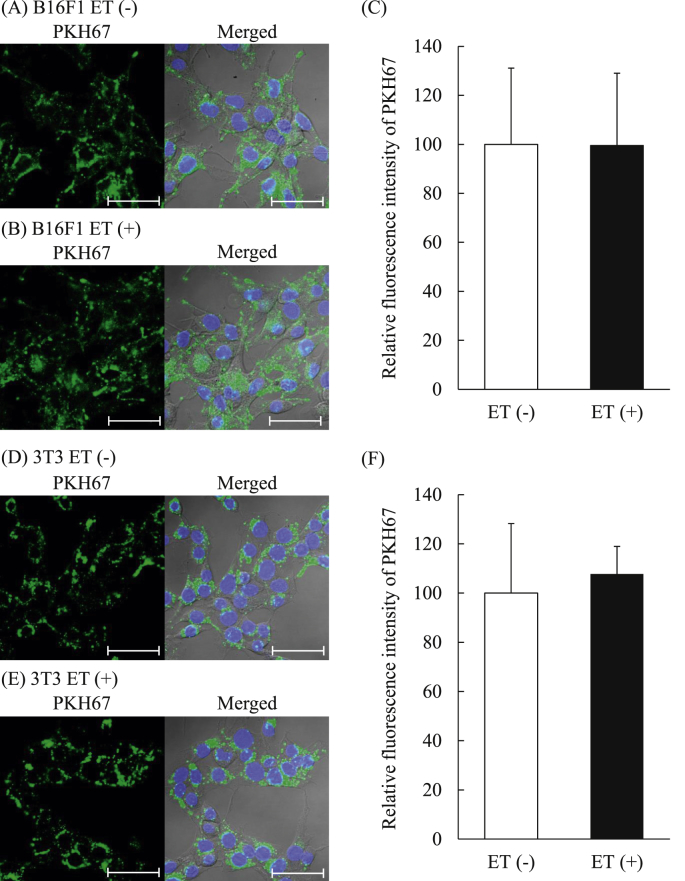
3.5. Cellular uptake of EVs collected from each cell exposed to ET
To evaluate whether ET affects functionalities of the collected EVs, cellular uptake of the EVs collected with or without ET into each donor cell was observed by fluorescence labeling of EVs with PKH67. Confocal images showed that PKH-labeled EVs derived from B16F1 cells tended to similarly be uptaken into B16F1 (donor) cells regardless of whether the EVs had been collected after ET of the cells (Figs. 7A and B). The quantitative data of relative fluorescence intensity indicated that uptake amounts of EVs into B16F1 cells were comparable between EVs collected with or without ET (Fig. 7C). The EVs derived from 3T3 Swiss Albino cells exposed to ET were similarly uptaken into 3T3 Swiss Albino cells in comparison to those derived from the cells without ET (Fig. 7D and E). Their uptake amounts into 3T3 Swiss Albino cells were also comparable between EVs harvested with or without ET (Fig. 7F). Based on the results of EV uptake study, it was suggested that ET would not affect the apparent functionality of the secreted EVs.
Fig. 7.
Cellular uptake of EVs collected with or without exposure of the cells to ET. B16F1 and 3T3 Swiss Albino cells (1 × 105 cells/35-mm dish) were incubated with PKH67-labeled EVs (5 μg protein/mL) derived from B16F1 and 3T3 Swiss Albino cells, respectively, for 6 h at 37 °C. Then, the cells were fixed, and their nuclei were counterstained with DAPI. Fluorescence images of B16F1 (A and B) and 3T3 Swiss Albino (D and E) were obtained by confocal laser scanning microscopy. Blue and green colors show the fluorescence of DAPI (nuclei) and PKH67 (EVs), respectively. The images of right columns are merged images of those of DAPI, PKH67, and phase contrast. Scale bars = 50 μm. The relative fluorescence intensities of EVs (ET (+)) to that of EV (ET (−)) was calculated from 12 images per each group of B16F1 (C) and 3T3 Swiss Albino cells (F) for one experiment by using Image J. The experiments were performed three times independently. The data show the mean ± S.D. (n = 3).
4. Discussion
It was previously reported that exosomes produced in MVB were released from cells after MVB fusion with the cellular membrane, a process depending upon several intracellular signaling pathways [23]. Thus, external stimuli that activate intracellular signaling would likely increase exosome secretion from the cells. Our previous reports demonstrated that treatment of cultivated cells with ET (0.34 mA/cm2) activates intracellular signaling and induces endocytosis of extraneous macromolecules such as siRNA and FITC-conjugated dextran (molecular weight: 10,000 and 70,000) [[20], [21], [22]]. Hence, in this study, we investigated whether ET-mediated intracellular signaling activation leads to increased EV secretion from cultured cells. Towards that end, we used murine melanoma B16F1 and murine fibroblast 3T3 Swiss Albino cells as a representative cancer cell line and a normal cell line, respectively.
The analysis of the physicochemical properties showed that the average particle size, ζ-potential, particle distribution, and morphology of EVs collected from cells exposed to ET were comparable with those collected from non-treated cells (Fig. 2 and Table 1). On the other hand, the number of secreted EVs was increased in the group treated by low level electricity (Figs. 3A and B). Notably, the particle distribution analysis showed that EVs approximately 90–130 nm in diameter prominently increased by ET (Figs. 3C and D). As shown in Supplementary Fig. 1, in case of the particle distribution of EVs collected without the procedure of filtration using 0.22-μm syringe filter been analyzed, ET-mediated increase of the number of EVs having over 150 nm was not remarkable compared with that of less than 150 nm. Regarding the definition of EVs, it was previously reported that particles with diameters in the 40–150 nm range were defined as exosomes and those with diameters of 100-1000 nm were microvesicles [1,27]. Based on our results and previous reports, it is suggested that secretion of exosomes would be increased by ET, although microvesicles having approximately 100 nm could also be contained. In addition to particle number, ET significantly increased the protein amounts of harvested EVs from B16F1 and 3T3 Swiss Albino cells without remarkable cellular toxicity (Fig. 4). The results also suggest that EV secretion from cultured cells was promoted by ET. A slight decrease of cell viability was observed in the cells exposed to ET as shown in Figs. 4C and D. Our previous study demonstrated that ET induces the dissociation of intracellular junctions via cell signaling activation accompanying with filamentous-actin (F-actin) depolymerization [28]. After ET of the cells, their slight detachment from the dish was seen, whereas the growth rate of the cells was not changed even at 48 h after ET between the cells exposed to or not exposed to ET (data not shown). It is speculated that the ET-mediated F-actin depolymerization may cause slight detachment of the cells, resulting in slight decrease in cell viability determined based on the cell number at 24 h after ET. The results of Western blotting showed that the expression of representative exosomal marker proteins, namely CD9, HSP70, and CD81, was hardly changed in comparisons of cells exposed to ET vs. non-treated cells (Figs. 5A and B). Moreover, the ratios of particle numbers to protein amounts were not significantly changed by ET in either cell line (Figs. 5C and D), indicating that the quality of the collected EVs seems to be unaltered by ET.
Since the expression of representative exosomal marker proteins was almost not changed by ET, we next investigated the functionality of EVs collected from the cells exposed to ET by evaluating uptake of the EVs derived from B16F1 and 3T3 Swiss Albino cells into each donor cell, respectively. As shown in Fig. 7, there were almost no differences in uptake of each EV into each donor cell regardless of whether the cells had been exposed to ET for EV collection. In addition, the ζ-potential of each EV was not changed by ET as shown in Table 1. These results indicate that ET could not influence at least surface properties of the lipid membranes of the secreted EVs, which led to almost no change in the uptake of each EV into the donor cells. However, since certain conditions (e.g. hypoxia) and stimuli were reported to alter components of lipids in EV membranes and nucleic acids [[29], [30], [31]], there is a possibility that ET also changes the components of lipid membranes and nucleic acid contents in the secreted EVs. Although the uptake of the EVs derived from the cells exposed to ET did not apparently change in comparison with the EVs derived from non-treated cells, further detailed studies to reveal changes in the components of lipid membranes and nucleic acid contents in EVs are needed in future.
Other researchers previously reported that several stimuli, such as acidic pH [32,33], hypoxic condition [29], exposure to anticancer drugs [34], and treatment with liposomes of certain lipid compositions [35], can increase EV secretion from cancer cells. However, acidic pH, hypoxic conditions and anticancer drug treatment alter the lipid composition of EV membranes and the contents in the exosomes, resulting in a change of the quality of the EVs compared with those collected under normal conditions. Regarding the method by using liposomes, there is a need to exhaustively separate EVs and liposomes to achieve high purity. As other modality to increase EV secretion, low-level laser irradiation (LLLI) was also previously reported [36]. LLLI at a power intensity of 80 J/cm2 could increase EV biogenesis via induction of autophagy and transcription factors which promote Wnt signaling pathways and GTPase activity in human endothelial cells, although the power intensity was cytotoxic dose. Based upon the expression of exosomal marker proteins and the ratio of particle number to protein amount, our current results demonstrated that ET can easily increase EV secretion from cultured cells without apparent cellular toxicity or changes in exosome quality. In addition, ET could increase EV secretion from not only a cancer cell (B16F1) but also a normal cell (3T3 Swiss Albino), implying that the method using ET may be applied for various types of cells, whereas other stimuli reported previously were investigated only in cancer cell lines except that the method using LLLI was performed in human ECs. Although the detailed mechanisms of ET-mediated EV secretion need to be elucidated, our previous studies reported that ET induces Rho GTPase activation via HSP90 and PKC by Ca2+ influx, and also phosphorylates Ras-related protein Rab-10, a protein associated with vesicle-mediated transport processes [22]. Since cellular signalings including Rho GTPase [37], the Rab family [19], and increase of intracellular Ca2+ were reportedly involved in the production and secretion of exosomes [18], ET-mediated activation of those signaling pathways could increase EV secretion. In fact, in the present study, Rho GTPase inhibition significantly suppressed the ET-mediated increase of EV secretion in a dose-dependent manner in B16F1 (Fig. 6), implying that Rho GTPase activation could be involved in ET-mediated EV secretion in a cancer cell B16F1. On the other hand, the ET-mediated increase in the particle number of the EVs was hardly inhibited in the presence of the Rho GTPase inhibitor in 3T3 Swiss Albino cells. Regarding the reason why the effect of Rho GTPase inhibition was different between B16F1 and 3T3 Swiss Albino cells, it is speculated that sensitivity to the Rho GTPase inhibitor might be different between the cell species. To clarify the mechanism of ET-mediated increase in EV secretion, further detailed studies are needed in future. The pathways of exosome production and secretion have recently been analyzed as novel therapeutic targets for various diseases [[5], [6], [7],38,39]. For instance, it was previously reported that sera from type 2 diabetic patients disturb the normal exosome signaling in human mesenchymal stem cells (hMSCs), and that the diabetic condition could decrease angiogenic potential and restorative effects of hMSCs [40,41]. Based on these findings, elucidation of the central role of the activated exosome secretion pathway has been needed to predict the behavior of hMSCs under diabetic condition. Thus, to clarify the mechanisms of EV secretion by ET may lead to explore new therapeutic targets as well as development of methods to increase EV yield from cultured cells. In addition, there is a possibility that ET can be applied for a tool to elucidate mechanisms involved in EV secretion, including Rho GTPase focused in the present study, in certain types of the cells.
In clinical area, transcranial direct current stimulation (tDCS), a non-invasive brain stimulation procedure by the treatment of brain through the skull by low level electricity, has been shown effective for alleviation of neuropsychiatric and neurological conditions such as major depression, and also effective for enhancement of learning and memory formation in humans [42]. However, detailed cellular mechanisms for positive effects by ET onto brain remain largely unclear. Our previous and present studies demonstrated that ET induces activation of intercellular signaling such as Rho GTPase via HSP90 and PKC [22], and also increases the secretion of EV from cultured cells. Although the effect of ET on brain-derived cells are needed to be examined in future, ET-mediated intracellular signaling activation and increase of EV secretion may have the potential to be involved in beneficial effects of tDCS in the brain.
In conclusion, the present study demonstrated that low level ET of cultured cells markedly increased both particle number and protein amount of the resultant EVs. This was observed in murine melanoma B16F1 and murine fibroblast 3T3 Swiss Albino cells without obvious cellular toxicity or a change in the ratio of particle number to protein quantity. Moreover, the expression of representative exosome markers, namely CD9, HSP70, and CD81, was comparable between the cells regardless of ET. As a mechanism, Rho GTPase inhibition significantly suppressed ET-mediated increase of EV secretion in B16F1, suggesting that Rho GTPase activation could be involved in ET-mediated EV secretion in the cell. Additionally, there were almost no differences in uptake of each EV into donor cells used in this study regardless of whether the cells had been exposed to ET for EV collection. Thus, ET could be used as a method to increase the yield of EVs from both cancer and normal cells.
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS, No. 17H06906 and No. 19K16336). The authors are also thankful for support from the Research Program for the Development of Intelligent Tokushima Artificial Exosome (iTEX) from Tokushima University, and from Takeda Science Foundation, Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100713.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Tkach M., Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Rezaie J., Ajezi S., Avci C.B., Karimipour M., Geranmayeh M.H., Nourazarian A., Sokullu E., Rezabakhsh A., Rahbarghazi R. Exosomes and their application in biomedical field: difficulties and advantages. Mol. Neurobiol. 2018;55:3372–3393. doi: 10.1007/s12035-017-0582-7. [DOI] [PubMed] [Google Scholar]
- 4.Shokrollahi E., Nourazarian A., Rahbarghazi R., Salimi L., Karbasforush S., Khaksar M., Salarinasab S., Abhari A., Heidarzadeh M. Treatment of human neuroblastoma cell line SH-SY5Y with HSP27 siRNA tagged-exosomes decreased differentiation rate into mature neurons. J. Cell. Physiol. 2019;234:21005–21013. doi: 10.1002/jcp.28704. [DOI] [PubMed] [Google Scholar]
- 5.Naito Y., Yoshioka Y., Yamamoto Y., Ochiya T. How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell. Mol. Life Sci. 2017;74:697–713. doi: 10.1007/s00018-016-2346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z.G., Chopp M. Exosomes in stroke pathogenesis and therapy. J. Clin. Investig. 2016;126:1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kugler S., Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo N., Akiyoshi K., Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109:2998–3004. doi: 10.1111/cas.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J., Lim S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Charoenviriyakul C., Takahashi Y., Morishita M., Matsumoto A., Nishikawa M., Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. 2017;96:316–322. doi: 10.1016/j.ejps.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C., Huang N.P., Xiao Z.D., Lu Z.H., Tannous B.A., Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., Gotoh N., Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 16.Nakai W., Yoshida T., Diez D., Miyatake Y., Nishibu T., Imawaka N., Naruse K., Sadamura Y., Hanayama R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016;6:33935. doi: 10.1038/srep33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachenal G., Pernet-Gallay K., Chivet M., Hemming F.J., Belly A., Bodon G., Blot B., Haase G., Goldberg Y., Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., Goud B., Benaroch P., Hacohen N., Fukuda M., Desnos C., Seabra M.C., Darchen F., Amigorena S., Moita L.F., Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- 20.Hasan M., Nishimoto A., Ohgita T., Hama S., Kashida H., Asanuma H., Kogure K. Faint electric treatment-induced rapid and efficient delivery of extraneous hydrophilic molecules into the cytoplasm. J. Control. Release. 2016;228:20–25. doi: 10.1016/j.jconrel.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Hasan M., Tarashima N., Fujikawa K., Ohgita T., Hama S., Tanaka T., Saito H., Minakawa N., Kogure K. The novel functional nucleic acid iRed effectively regulates target genes following cytoplasmic delivery by faint electric treatment. Sci. Technol. Adv. Mater. 2016;17:554–562. doi: 10.1080/14686996.2016.1221726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan M., Hama S., Kogure K. Low electric treatment activates Rho GTPase via heat shock protein 90 and protein kinase C for intracellular delivery of siRNA. Sci. Rep. 2019;9:4114. doi: 10.1038/s41598-019-40904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakkaraju A., Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T., Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Shang X., Marchioni F., Sipes N., Evelyn C.R., Jerabek-Willemsen M., Duhr S., Seibel W., Wortman M., Zheng Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hama S., Kimura Y., Mikami A., Shiota K., Toyoda M., Tamura A., Nagasaki Y., Kanamura K., Kajimoto K., Kogure K. Electric stimulus opens intercellular spaces in skin. J. Biol. Chem. 2014;289:2450–2456. doi: 10.1074/jbc.M113.514414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Canc. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong O.G., Verhaar M.C., Chen Y., Vader P., Gremmels H., Posthuma G., Schiffelers R.M., Gucek M., van Balkom B.W. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phuyal S., Skotland T., Hessvik N.P., Simolin H., Overbye A., Brech A., Parton R.G., Ekroos K., Sandvig K., Llorente A. The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC-3 cells. J. Biol. Chem. 2015;290:4225–4237. doi: 10.1074/jbc.M114.593962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logozzi M., Spugnini E., Mizzoni D., Di Raimo R., Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;38:93–101. doi: 10.1007/s10555-019-09783-8. [DOI] [PubMed] [Google Scholar]
- 33.Ban J.J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015;461:76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 34.Lv L.H., Wan Y.L., Lin Y., Zhang W., Yang M., Li G.L., Lin H.M., Shang C.Z., Chen Y.J., Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emam S.E., Ando H., Lila A.S.A., Shimizu T., Ukawa M., Okuhira K., Ishima Y., Mahdy M.A., Ishida T. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol. Pharm. Bull. 2018;41:733–742. doi: 10.1248/bpb.b17-00919. [DOI] [PubMed] [Google Scholar]
- 36.Bagheri H.S., Mousavi M., Rezabakhsh A., Rezaie J., Rasta S.H., Nourazarian A., Avci C.B., Tajalli H., Talebi M., Oryan A., Khaksar M., Kazemi M., Nassiri S.M., Ghaderi S., Bagca B.G., Rahbarghazi R., Sokullu E. Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med. Sci. 2018;33:1131–1145. doi: 10.1007/s10103-018-2495-8. [DOI] [PubMed] [Google Scholar]
- 37.Liang B., Peng P., Chen S., Li L., Zhang M., Cao D., Yang J., Li H., Gui T., Li X., Shen K. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteom. 2013;80:171–182. doi: 10.1016/j.jprot.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Rashed M.H., Bayraktar E., Helal G.K., Abd-Ellah M.F., Amero P., Chavez-Reyes A., Rodriguez-Aguayo C. Exosomes: from garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quek C., Hill A.F. The role of extracellular vesicles in neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2017;483:1178–1186. doi: 10.1016/j.bbrc.2016.09.090. [DOI] [PubMed] [Google Scholar]
- 40.Rezaie J., Nejati V., Khaksar M., Oryan A., Aghamohamadzadeh N., Shariatzadeh M.A., Rahbarghazi R., Mehranjani M.S. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res. 2018;374:555–565. doi: 10.1007/s00441-018-2895-x. [DOI] [PubMed] [Google Scholar]
- 41.Rezaie J., Mehranjani M.S., Rahbarghazi R., Shariatzadeh M.A. Angiogenic and restorative abilities of human mesenchymal stem cells were reduced following treatment with serum from Diabetes Mellitus type 2 patients. J. Cell. Biochem. 2018;119:524–535. doi: 10.1002/jcb.26211. [DOI] [PubMed] [Google Scholar]
- 42.Monai H., Ohkura M., Tanaka M., Oe Y., Konno A., Hirai H., Mikoshiba K., Itohara S., Nakai J., Iwai Y., Hirase H. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 2016;7:11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.