Abstract
Background
The present study was designed to determine whether zinc supplementation would increase the effects of restricted calorie diet (RCD) on obesity.
Methods and materials
A randomized, double-blind clinical trial was performed on 40 obese subjects who were randomly assigned to receive zinc supplements (30 mg/day) or placebo for a period of 15-weeks. Both groups were under a restricted calorie diet (~ 300 kcal lower than the estimated energy requirement). Anthropometric measurements, biochemical markers, appetite, and dietary intakes were determined during the study period.
Results
The reductions of body weight, body mass index, waist circumference, and hip circumference were significantly higher in the zinc group compared to the placebo group (P = 0.032, 0.025, 0.003, and 0.0001, respectively). Lower levels of high sensitivity C-reactive protein, apelin, homeostatic model assessment of insulin resistance (HOMA-IR), and appetite score were observed in the zinc group in comparison with the placebo group (P = 0.0001, 0.001, 0.031 and 0.001 respectively).
Conclusion
This study indicates that Zn supplementation with a restricted calorie diet has favorable effects in reducing anthropometric measurements, inflammatory markers, insulin resistance and appetite in individuals with obesity, and may play an effective role in the treatment of obesity.
Trial registration This clinical trial was registered at clinicaltrials.gov at the U.S. National Library of Medicine (NCT02516475).
Keywords: Zinc supplement, Obesity, Anthropometric measurements, hs-CRP, Insulin resistance
Background
As the etiology of obesity is complex [1], current interventions for weight management are only modestly successful [2]. Restricted calorie diets (RCD) are playing a fundamental role in prevention and treatment of obesity [3, 4]; but these diets often result in micronutrient deficiencies [5]. Furthermore, obesity and obesity-related inflammation are related to abnormal micronutrient status [5–7]. Among these micronutrients, zinc (Zn) deficiency is a common problem in obese individuals [8–10]. Furthermore, Zn has been reported as limiting nutrients in RDCs [11]. Previous studies have also demonstrated that plasma Zn level and dietary intake of Zn are insufficient in obese individuals [12–14]. So, it seems that further weight gain or development of obesity-related disorders may occur if the Zn deficiency is not corrected [15]. Payahoo et al. [16] also showed that daily intake of 30 mg Zn gluconate for 1 month decreased significantly body weight and body mass index (BMI). Two key assumptions about the possible mechanisms for the effects of Zn supplementation on weight loss are including appetite regulation [17] and improving insulin resistance (IR) [18, 19]. Another important aspect which worth to notice is the beneficial effects of dietary intake of Zn and plasma Zn level on inflammatory status [20, 21]. Zn has shown possible anti-inflammatory effects through cytokine signaling pathways [22] and the attenuation of plasma levels of Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) [23, 24]. Moreover, a growing body of literature has demonstrated that these inflammatory markers are directly or indirectly correlated with obesity-related IR through blocking the insulin signaling receptors activation in pancreatic β-cells [25]. More recently, apelin is also proposed as an adipokine mediator which might have an adaptive response to prevent chronic inflammation associated with obesity [26, 27]. Previous reports also imply that higher apelin levels are associated with both insulin resistance and chronic inflammation in individuals with obesity [28]. So, based on previous studies, low Zn concentration and high level of inflammatory markers maybe correlated to high BMI [29, 30], and it seems reasonable to assume that Zn supplementation may have favorable effects on weight loss or reversing obesity-related comorbidities such as IR. Therefore, this study was designed to evaluate the effects of daily intake of 30 mg Zn supplement along with RCD on anthropometric measurements, appetite, IR, and serum levels of inflammatory markers, apelin, and neuropeptide Y (NPY), in obese individuals.
Materials and methods
Study design and participants
This double-blind randomized clinical trial was conducted from December 2015 to April 2016. In order to detect a difference of 4.5 kg/m2 in the BMI and with respect to a pooled standard deviation of 26.21 kg/m2, obtaining from the study by Payahoo et al. [16], the sample size was calculated 20 subjects for each group. In this two-arm parallel study with two-tailed testing, a power (1–β) of 80% and α = 0.05 was used. Fifty healthy adults (men and women) with obesity and BMI more than 30 kg/m2 in the age range of 18–45 years were selected using convenience sampling from the Specialized Clinic of Nutrition & Diet Therapy located at the Faculty of Nutrition Sciences and Food Technology of Shahid Beheshti University of Medical Sciences in Tehran, Iran. In our study, exclusion criteria were the presence of pregnancy or lactation, chronic kidney or hepatic disease, autoimmune and infectious disease, chronic inflammatory diseases, recent surgery, smoking, having weight loss diets in the last 2 months, the use of Zn, calcium, or iron supplements in the last 2 months, and taking anticoagulant drugs, lipid-lowering or beta-blocker drugs. The primary outcomes were anthropometric measurements, and secondary outcome were appetite score, serum levels of inflammatory markers, apelin, NPY, glucose, Zn and insulin, and IR. The study protocol was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Iran (IR.SBMU.nntri.Rec.1394.407). The study was in adherence with the Declaration of Helsinki. Written informed consent was obtained from all subjects before initiating the study. This clinical trial was registered at clinicaltrials.gov at the U.S. National Library of Medicine (NCT02516475).
Randomization
The subjects were randomly allocated to either a Zn or placebo group by block randomization. A trained dietitian completed the block randomization with a block size of 4 and possible balanced combinations with 2 P (placebo) and 2 Z (Zn supplement) subjects, calculated as 6 blocks (ZZPP, PZPZ, PZZP, ZPZP, PPZZ, ZPPZ). Then, blocks were randomly chosen, using a simple random sampling method to determine the assignment of all the participants into the groups.
Intervention
During this study, subjects in the Zn group received 30 mg zinc sulfate as 1 capsule (between meals) while those in the placebo group received corresponding placebo capsules containing starch (also between meals). All capsules were produced by Dineh Iran Company, Tehran, Iran. According to the literature, zinc supplement is safe at a dose of 30 mg/day [31, 32]. Blinding was performed by a trained dietician, and the patients and researchers were kept blinded to the allocation. In addition, subjects in both Zn and placebo groups received a restricted calorie diet (RCD) with ~ 300 kcal lower than the estimated energy requirement based on the Mifflin-St Jeor equation in order to reduce their weight about 1 kg per month, and this RCD contained ~ 55% carbohydrate, ~ 15% protein and ~ 30% fat [33]. Adherence to the diet was monthly assessed by a registered dietitian. Participants were followed twice a month via telephone calls in order to ensure their compliance and were asked to maintain their usual physical activity level. They were also asked to return the remaining capsules, and based on the number of returned capsules by each subject and adherence to the diet, their degree of compliance was determined and the data of individuals with the degree of compliance more than 90% were analyzed at the end of the study.
Dietary intakes and appetite assessments
Dietary intakes of participations were assessed using a 3-day dietary recall (2 weekdays and
1 weekend day) at baseline and at the end of week 15. Individuals’ diets were analyzed by Nutritionist IV software (N Squared Computing, San Bruno, CA, USA). Basal metabolic rate (BMR) was calculated based on Mifflin and St Jeor et al. [34]. Underreporting was defined as a ratio reported energy intake by 3-day dietary recall/BMR < 1.1 [35]. Simplified nutritional appetite questionnaire (SNAQ), a valid 4-item questionnaire recommended for clinical purposes [36], were used to assess the appetite at baseline and week 15. The SNAQ items were as follows: #1, Appetite; #2, Feeling full; #3, Food tastes; #4, Feeling hunger, and the sum of the 4 items scores constitutes the total SNAQ score which ranges from 4 to 20. The total score of 4 to 14 and 15 to 20 indicates low and normal appetite, respectively [36].
Anthropometric assessments
Weight was measured with minimum clothes and without shoes using a calibrated scale (Seca, CA, USA) and precision of 100 g. Height was measured using a wall-mounted stadiometer with the precision of 0.5 cm. Hip and waist circumference were also measured using an inflexible tapeline with the precision of 0.5 cm, in the narrowest circumference below the rib cage and above the umbilicus and the largest circumference between the waist and knees, respectively [37]. BMI was calculated as the ratio of weight (kg)/height2 (m2). Anthropometric parameters were measured at baseline and at the end of weeks 7 and 15.
Physical activity assessment
Physical activity level was estimated using a valid and reliable physical activity questionnaire [38] and calculating metabolic equivalent (MET) at baseline and the end of the study.
Blood samples and biochemical assessments
A sample of 5 ml blood was collected from all participants after a 12 to 14 h fast, at baseline and at the end of week 15. These samples were centrifuged at 4000 rpm for 15 min. The samples of serum were separated into small aliquots and were frozen at − 80 °C. For Zn analysis, all tubes were washed by acid and rinsed with distilled water, then atomic absorption spectrometry (variant Chemthech Analytical 2000) was used to determine serum Zn concentration [39, 40]. Serum concentration of high-sensitivity C-reactive protein (hs-CRP) was determined by enzyme-linked immunosorbent assay (ELISA) kits (Diagnostics Biochem Canada, Ontario, Canada) with an intra-assay coefficient of variation (CV) of 7.2%. Serum TNF-α was measured by ELISA kits (Diaclone, Besancon, France). Intra-assay CV for serum TNF-αwas 6.5%. Serum apelin concentration was assessed by ELISA kits (ZellBio GmbH, Ulm, Germany), with an intra-assay CV of 7.2%. Serum insulin was determined by ELISA kits (Monobind, USA), with an intra-assay CV of 7.4%. Serum glucosewas measured by commercial kits (Pars Azemoon, Tehran, Iran) with the aid of a Selectra 2 Autoanalyzer (Vital Scientific, Spankeren, The Netherlands). Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was determined using the following equation:
Statistical analysis
Intention-to-treat principle was applied for anthropometric and dietary intake variables. Per-protocol analysis (PPA) was performed for analyzing the biochemical data. Data analysis was performed using SPSS version 20. The results are presented as mean (± SD) and frequency (percent) for quantitative and qualitative variables, respectively. The Kolmogorov–Smirnov test was used to assess normal distribution of data. None normal data distribution has been presented as 25/75 IQR. Natural log transformations on plasma Zn, insulin, TNF-α, NPY, apelin and HOMA-IR were transformed through Box-Cox transformation. To compare qualitative variables between the two groups, the Chi square test was used. We used a t test and paired t-test to compare quantitative parameters between and within groups, respectively. In addition, because anthropometric parameters were measured 3 times during the study, analysis of variance for repeated measurements was used to compare data between various times. Analysis of covariance was performed in order to remove the effect of confounding factors. In this study, P values of less than 0.05 were considered statistically significant.
Results
Of the 50 subjects initially enrolled, 10 subjects were eliminated because of non- compliance and medical treatment (Fig. 1). The baseline characteristics of the subjects did not differ significantly between the two groups (Table 1).
Fig. 1.
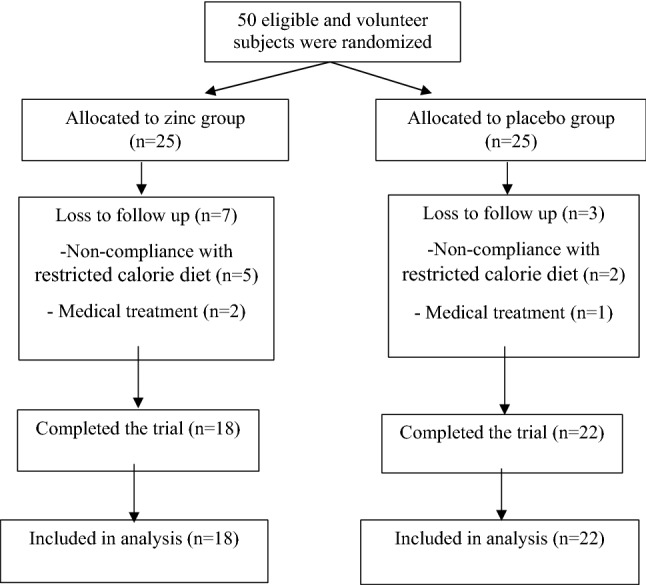
Flow diagram of the study
Table 1.
Baseline characteristics of subjects in the Zinc and Placebo groups
| Characteristics | Zinc (n = 18) | Placebo (n = 22) |
|---|---|---|
| Age (years)a | 35.63 ± 3.2 | 32.95 ± 1.7 |
| Sex (n/%) | ||
| Male | 6 (24%) | 8 (32%) |
| Female | 19 (76%) | 17 (68%) |
| Past experiences with weight-reducing treatment (n/%) | ||
| Yes | 15 (60%) | 13 (52%) |
| No | 10 (40%) | 12 (48%) |
| Marital status (n/%) | ||
| Single | 8 (32%) | 9 (36%) |
| Married | 17 (68%) | 16 (64%) |
aValues are mean ± SD
Dietary intakes and physical activity
As is shown in Table 2, dietary intakes of energy, protein, carbohydrate, fat, saturated fatty acids (SAFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), cholesterol, and Zn were not significantly different between the groups at baseline and the end of week 15. No significant changes were observed in physical activity levels between the two groups during the study.
Table 2.
Dietary intakes and physical activity in the Zinc and Placebo groups
| Variables | Zinc group | Placebo group | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 15 | Mean change | Baseline | Week 15 | Mean change | P1 | P2 | P3 | |
| Energy (kcal/d) | 1727.12 ± 378.76 | 1463.49 ± 424.07 | − 263.62 ± 514.36 | 1643.97 ± 476.19 | 1542.96 ± 500.29 | − 101.01 ± 481. 08 | 0.498 | 0.547 | 0.254 |
| P4 | 0.017 | 0.304 | |||||||
| Protein (g/d) | 64.34 ± 11.42 | 60.64 ± 7.16 | − 9.63 ± 6.98 | 69.73 ± 11.78 | 61.44 ± 19.38 | − 7.52 ± 18.99 | 0.156 | 0.688 | 0.453 |
| P4 | 0.209 | 0.057 | |||||||
| Carbohydrate (g/d) | 237.99 ± 60.18 | 201.79 ± 65.89 | − 36.27 ± 82.69 | 223.19 ± 55.26 | 208.57 ± 69.33 | − 14.62 ± 57.37 | 0.370 | 0.722 | 0.288 |
| P4 | 0.038 | 0.215 | |||||||
| Fat (g/d) | 58.83 ± 15.43 | 50.64 ± 15.36 | − 8.18 ± 22.28 | 55.31 ± 19.76 | 49.23 ± 15.93 | − 6.07 ± 20.93 | 0.486 | 0.752 | 0.731 |
| P4 | 0.079 | 0.160 | |||||||
| SFA (g/d) | 14.01 ± 4.55 | 12.64 ± 3.91 | − 1.37 ± 5.25 | 16.34 ± 15.06 | 14.84 ± 4.95 | − 1.50 ± 14.45 | 0.483 | 0.089 | 0.965 |
| P4 | 0.205 | 0.607 | |||||||
| MUFA (g/d) | 18.41 ± 5.78 | 17.69 ± 5.48 | − .71 ± 4.10 | 17.79 ± 6.52 | 18.98 ± 5.25 | 0.53 ± 8.00 | 0.722 | 0.683 | 0.491 |
| P4 | 0.391 | 0.742 | |||||||
| PUFA (g/d) | 23.70 ± 8.84 | 18.95 ± 8.68 | − 4.75 ± 10.68 | 20.57 ± 8.92 | 16.23 ± 8.58 | − 4.34 ± 10.04 | 0.219 | 0.272 | 0.88 |
| P4 | 0.036 | 0.041 | |||||||
| Cholesterol (mg/d) | 179.86 ± 87.60 | 184.76 ± 92.00 | 4.89 ± 110.06 | 189.21 ± 67.11 | 1999.92 ± 86.21 | 10.71 ± 77.50 | 0.674 | 0.550 | 0.830 |
| P4 | 0.826 | 0.496 | |||||||
| Zinc (mg/d) | 14.01 ± 1.14 | 14.02 ± 0.74 | 0.19 ± 0.94 | 13.5 ± 1.18 | 13.80 ± 1.34 | 0.30 ± 0.70 | 0.127 | 0.204 | 0.624 |
| P4 | 0.317 | 0.04 | |||||||
| Physical activity (MET/day) | 32.87 ± 3.40 | 32.77 ± 3.62 | − 0.09 ± 0.67 | 32.23 ± 4.38 | 32.44 ± 4.33 | 0.21 ± 0.65 | 0.567 | 0.770 | 0.107 |
| P4 | 0.481 | 0.119 | |||||||
All values are mean ± SD
SFA saturated fatty acids, MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid
P1: p-values for comparison of variables between two group by independent T-test at baseline
P2: p-values for comparison of variables between two group by independent T-test at week 15
P3: p-values for comparison of mean change of variables between two group by independent T-test
P4: p-values for comparison of variables within groups by Paired T-test
Effects on anthropometric measurements
Weight, BMI, waist circumference and hip circumference decreased in both groups compared to baseline. However, the reductions of weight (P = 0.032), BMI (P = 0.025), waist circumference (P = 0.003) and hip circumference (P = 0.0001) were significantly higher in the Zn group than in the placebo group (Table 3). No significant change was observed in WHR within each group during the study (Table 3).
Table 3.
Anthropometric parameters in Zinc and Control groups
| Variable | Time | Zinc group | Placebo group | P* |
|---|---|---|---|---|
| Body weight (kg) | Baseline | 89.59 ± 17.10 | 88.41 ± 12.46 | 0.781 |
| Week 7 | 83.16 ± 14.52 | 87.09 ± 12.53 | 0.312 | |
| Week 15 | 84.99 ± 13.41 | 86.93 ± 12.34 | 0.597 | |
| P** | 0.020 | 0.007 | ||
| Mean changea | − 4.60 ± 8.80 | − 1.48 ± 2.37 | 0.093 | |
| P*** | 0.032 | |||
| BMI (kg/m2) | Baseline | 33.17 ± 6.34 | 32.64 ± 2.37 | 0.701 |
| Week 7 | 30.66 ± 4.10 | 32.16 ± 2.63 | 0.129 | |
| Week 15 | 31.50 ± 5.08 | 32.09 ± 2.31 | 0.599 | |
| P** | 0.024 | 0.007 | ||
| Mean change | − 1.66 ± 3.33 | − 0.55 ± .89 | 0.113 | |
| P*** | 0.025 | |||
| Waist circumference (cm) | Baseline | 99.48 ± 10.19 | 99.32 ± 9.42 | 0.954 |
| Week 7 | 96.80 ± 10.07 | 98.10 ± 9.44 | 0.639 | |
| Week 15 | 94.36 ± 10.31 | 97.82 ± 9.90 | 0.231 | |
| P*** | 0.105 | 0.023 | ||
| Mean change | − 5.12 ± 6.67 | − 1.49 ± 3.52 | 0.020 | |
| P*** | 0.003 | |||
| Hip circumference (cm) | Baseline | 114.72 ± 8.77 | 115.16 ± 5.49 | 0.833 |
| Week 7 | 111.83 ± 8.17 | 114.68 ± 5.64 | 0.159 | |
| Week 15 | 109.84 ± 7.53 | 114.68 ± 5.68 | 0.013 | |
| P** | 0.0001 | 0.063 | ||
| Mean change | − 4.88 ± 3.58 | − 0.48 ± 1.04 | 0.0001 | |
| P*** | 0.0001 | |||
| WHR | Baseline | 0.87 ± 0.09 | 0.86 ± 0.06 | 0.734 |
| Week 7 | 0.86 ± 0.07 | 0.85 ± 0.06 | 0.597 | |
| Week 15 | 0.86 ± 0.11 | 0.85 ± 0.07 | 0.710 | |
| P** | 0.864 | 0.149 | 0.880 | |
| Mean change | − 0.07 ± 0.053 | − 0.009 ± 0.028 | ||
| P*** | 0.682 |
All values are mean ± SD
aMean change for the 15-week period
P*: p-values for comparison of variables between two group by independent T-test
P**: p-values for comparison of variables within groups by analysis of variance for repeated measurement
P***: p-values for comparison between mean changes of variables by Analysis of covariance (adjusted for age, mean change of calorie intake, mean change of zinc intake)
Effects on biochemical markers and appetite
Serum zinc concentration increased significantly in the Zn group at the end of week 15 compared with baseline (P = 0.0001), whereas no significant change was observed in the placebo group. The increment of serum zinc concentration in the Zn group was significant in comparison with the placebo group (P = 0.002; Table 4). Serum hs-CRP reduced significantly in the Zn group at the end of week 15 in comparison with baseline (P = 0.0001), whereas no significant change was observed in the placebo group. The reduction of serum hs-CRP in the Zn group was significant in comparison with the placebo group (P = .0001; Table 4). Serum TNF-α concentration did not significantly change within each group during the study (Table 4). Serum apelin reduced significantly in the Zn group at the end of week 15 in comparison with baseline (P = 0.042), whereas it increased significantly in the placebo group (P = 0.001). The reduction of serum apelin in the Zn group was significant in comparison with the placebo group (P = 0.001; Table 4). Serum glucose (P = 0.046) and insulin (P = 0.002) reduced significantly in the Zn group at the end of week 15 in comparison with baseline. However, these reductions in the Zn group were not significant in comparison with the placebo. In addition, HOMA-IR decreased significantly in the Zn group at the end of week 15 in comparison with baseline (P = 0.0001), whereas no significant change was observed in the placebo group. The reduction of HOMA-IR in the Zn group was significant in comparison with the placebo group (P = .031; Table 4). Serum NPY decreased in the Zn group and this reduction was significant in comparison with the placebo group (Table 4; P = 0.048); however, after statistical adjustment for age and calorie intake, the reduction of NPY in the Zn group was not significant in comparison with the placebo group. Appetite score decreased significantly in the Zn group at the end of week 15 in comparison with baseline (P = 0.004), whereas no significant change was observed in the placebo group. The reduction of appetite score in the Zn group was significant in comparison with the placebo group (P = .001; Table 4).
Table 4.
Biochemical markers and appetite in the Zinc and Placebo groups
| Variables | Zinc group | Placebo group | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 15 | Mean change | Baseline | Week 15 | Mean change | P2 | P3 | P4 | P5 | |
| Zinc (µg/dL)1 | 65.2 ± 5.9 | 75.4 ± 8.2 | 10.2 ± 6.8 | 71.15 ± 13.2 | 68.15 ± 10 | − 3 ± 13.1 | 0.086 | 0.018 | 0.0001 | 0.002 |
| P1 | 0.0001 | 0.296 | ||||||||
| hs-CRP (mg/L)1 | 5.27 ± 2.93b | 3.37 ± 2.24 | − 1.89 ± 1.60 | 4.75 ± 2.28 | 3.98 ± 2.04 | − 0.07 ± 1.8 | 0.124 | 0.064 | 0.0001 | 0.0001 |
| P1 | 0.0001 | 0.60 | ||||||||
| TNF-α (pg/ml) 2 | 32.41 (10.92, 708.48) a | 30.43 (11.79, 546.19) | − 1.98 | 26.07 (13.19, 67.47) | 25.07 (9.62, 94.65) | − 1.0 | 0.170 | 0.238 | 0.293 | 0.723 |
| P1 | 0.473 | 0.451 | ||||||||
| Apelin (pg/ml)2 | 1568.20 (1119, 3282) | 1245.13 (482, 2087) | − 323.07 | 1493.45 (505, 4467) | 1683.32 (963, 3804) | 189.87 | 0.805 | 0.017 | 0.002 | 0.001 |
| P1 | 0.042 | 0.001 | ||||||||
| FBS (mg/dL)1 | 86.83 ± 11.94 | 83.50 ± 7.36 | − 3.33 ± 6.56 | 86.90 ± 9.93 | 86.90 ± 13.42 | 0.00 ± 6.00 | 0.983 | 0.343 | 0.102 | 0.088 |
| P1 | 0.046 | 1.00 | ||||||||
| Insulin (microU/L)2 | 5.91 (1.7,18.60) | 4.05 (1,9.1) | − 1.86 | 5.07 (1.8, 22.6) | 5.08 (1.7, 27.2) | 0.01 | 0.135 | 0.33 | 0.019 | 0.073 |
| P1 | 0.002 | 0.735 | ||||||||
| HOMA-IR2 | 1.35 (0.37, 2.81) | 0.83 (0.21, 2.27) | − 0.52 | 1.02 (0.41, 5.88) | 1.08 (0.33, 9.27) | 0.06 | 0.160 | 0.294 | 0.011 | 0.031 |
| P1 | 0.0001 | 0.757 | ||||||||
| NPY (ng/l)2 | 306.1 (178.8, 1540.5) | 273.8 (170.1, 1022.8) | − 32.27 | 400.9 (198.9, 2239.2) | 411.6 (180.6, 1900.9) | 10.72 | 0.147 | 0.037 | 0.048 | 0.151 |
| P1 | 0.114 | 0.243 | ||||||||
| Appetite1 | 16.00 ± 2.11 | 13.77 ± 2.36 | − 2.22 ± 2.81 | 15.50 ± 1.80 | 15.45 ± 1.59 | − 0.04 ± 1.59 | 0.426 | 0.011 | 0.007 | 0.001 |
| P1 | 0.004 | 0.892 | ||||||||
aValues are geometric mean (minimum, maximum)
bValues are mean ± SD
P1: p-values for comparison of variables within groups by Paired T-test
P2: p-values for comparison of variables between two groups by independent T-test at baseline
P3: p-values for comparison of variables between two group by independent T-test at week15
P4: p-values for comparison of mean change of variables between two group by independent T-test
P5: p-values for comparison between mean changes of variables by Analysis of covariance (adjusted for age, and mean change of calorie intake)
Discussion
In our study, mean serum zinc in the Zn group (65.2 ± 5.9 µg/dL) was lower than normal range (70–120 µg/dL) at baseline [41]. At the end of week 15, mean serum zinc increased significantly in the Zn group (75.4 ± 8.2 µg/dL), whereas no significant change was observed in the placebo group. In the present study, weight, BMI, waist circumference and hip circumference decreased in both groups compared to baseline. However, the reductions of these anthropometric parameters were significantly higher in the Zn group than in the placebo group. To our knowledge, this is the first study to evaluate the co-administration of Zn supplement and RCD in individuals with obesity. In agreement with the present study, Payahoo et al. [16] showed that daily administration of 30 mg zinc gluconate for 1 month reduced body weight, BMI and waist circumferences in the healthy obese adults. It is documented that body weight management requires restricting energy intake, and increasing energy expenditure [42]. No significant changes were observed in physical activity levels between the two groups. In our study, although the difference in energy intake between the two groups was not statistically significant, the reduction of energy intake was higher in the Zn group than in the placebo group. Based on previous studies, it seems that improvement in Zn status could have beneficial effects on food intake regulation [43]. One of the suggested mechanisms may be related to the favorable effect of improvement in Zn status on leptin regulation for inhibiting eating behaviors through reduction in neuropeptide Y mRNA level [44]. Zn deficiency and obesity can lead to leptin resistance which may increase NPY levels in the hypothalamus of rodents and men [45]. Previous findings also report that Zn deficiency can cause a 50% increase in NPY levels [46], but despite the higher level of NPY in Zn deficient rats, their food intake is reduced because of NPY resistance [46, 47]. On the other hand, previous reports imply that Zn has an essential role in serotonin synthesis which stimulates satiety and reduce food intake [48]. Based on our knowledge, the functional role of zinc status in weight or appetite management of individuals with obesity has not been revealed. However, the role of lower plasma zinc level in inhibiting TSH secretion [49] and the involvement of zinc in the production, storage and release of insulin were also previously showed [50]. So it seems reasonable that zinc level may have an essential role in weight or appetite management of individuals with obesity. In our study, the baseline Zn level was below than the normal range (70–120 µg/dL) [51] in the Zn group; however, Zn levels turn to a normal status after the supplementation. In agreement with previous studies, serum NPY decreased in the Zn group and this reduction was significant in comparison with the placebo group. In addition, appetite score decreased significantly in the Zn group at the end of week 15 in comparison with baseline, and this reduction was significant in comparison with the placebo group. Welch et al. [52] also documented that NPY not only effect on food intake but also seems to be associated with macronutrient selection, such a way that increase carbohydrate intake. In agreement with Welch et al. study, carbohydrate and fat intakes were significantly reduced in the Zn group as compared to the placebo group in our study. In the present study, serum hs-CRP, an inflammatory marker, reduced significantly in the Zn group at the end of week 15 in comparison with baseline, and this reduction was significant in comparison with the placebo group. Inflammation is one of the main complications of obesity [53] and weight loss through dietary restriction may have a favorable effect on obesity-related inflammatory status [54]. Selvin et al. [55] suggested that a 1 kg weight loss through changes in diet and lifestyle will lead to a 0.13 mg/L reduction in serum CRP level. In our study, Serum TNF-α concentration did not significantly change in the Zn group. In agreement with this study, Kim et al. [30] did not find any significant reduction in serum TNF-α after a 8-week supplementation with Zn. In addition, serum apelin, an adipose tissue inflammatory biomarker [28], reduced significantly in the Zn group at the end of week 15 in comparison with baseline, and this reduction was significant in comparison with the placebo group. To our knowledge, no studies to date have evaluated the effects of Zn supplementation on apelin levels; however, some studies revealed that weight loss with RCD can cause a significant reduction in apelin level [28, 56] which seems this reduction has been largely attributed to decreased inflammation or increased insulin sensitivity [28, 55–62]. Serum glucose and HOMA-IR reduced significantly in the Zn group at the end of week 15 in comparison with baseline. Insulin sensitivity improvement is documented in previous weight loss interventions using calorie restriction [57–59]. It has been shown that a 5–10% weight loss increases insulin sensitivity [60, 61]. However, the effectiveness of the Zn supplementation on IR is controversial [18, 62, 63]. It seems that zinc supplementation with longer duration has more favorable effects on IR or glucose tolerance [64, 65]. One of the probable mechanisms for the beneficial effects of Zn on IR may be related to decreased inflammation [66]. Few studies have proposed that higher levels of hs-CRP are associated with insulin resistance and hyperinsulinemia [67–69]. Furthermore, the role of apelin in the development of insulin resistance has also attracted a lot of attention in the recent years [70, 71]. It has been shown that apelin level is higher in insulin-resistant individuals and it has also been suggested that apelin can inhibit the insulin secretion [70, 72, 73]. The proposed mechanisms for the role of apelin in insulin sensitivity include direct effects on glucose uptake or insulin signaling pathways and indirect effects on energy metabolism [28]. A limitation of our study was the small sample size.
Conclusion
This study indicates that Zn supplementation with a restricted calorie diet has favorable effects in reducing anthropometric measurements, inflammatory markers, insulin resistance and appetite in individuals with obesity, and may play an effective role in the treatment of obesity.
Acknowledgements
The authors thank the staff of Specialized Clinic of Nutrition & Diet Therapy located at the Faculty of Nutrition Sciences and Food Technology of Shahid Beheshti University of Medical Sciences in Tehran, Iran, for their invaluable assistance, and the staff of the research laboratory of Research Institute for Endocrine Sciences and the Nutrition research laboratory of the Faculty of Nutrition and Food Technology for their technical assistance.
Abbreviations
- Zn
zinc
- RCD
restricted calorie diet
- HOMA-IR
homeostatic model assessment of insulin resistance
- hs-CRP
high-sensitivity C-reactive protein
- BMI
body mass index
- IR
insulin resistance
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor-α
- NPY
neuropeptide Y
- SNAQ
Simplified Nutritional Appetite Questionnaire
- MET
metabolic equivalent
- PPA
per-protocol analysis
- SAFA
saturated fatty acids
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
Authors’ contributions
HKH and AS had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of data analysis. NH, RY and AS conceived and designed the study and provided administrative support. HKH, RY, NH, MP, and AS conducted the study. AS, ON wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the National Nutrition and Food Technology Research Institute of the Shahid Beheshti University of Medical Sciences in Tehran, Iran (Grant Number: IR.SBMU.nntri.Rec.1394.407)
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Awritten informed consent was obtained from all subjects before initiation of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nima Hosseinzadeh, Email: nima.hosseinzadeh@sbmu.ac.ir.
Atoosa Saidpour, Email: atoosa.saidpour@gmail.com, Email: a.saidpour@sbmu.ac.ir.
References
- 1.Gamboa-Gomez CI, Rocha-Guzman NE, Gallegos-Infante JA, Moreno-Jimenez MR, Vazquez-Cabral BD, Gonzalez-Laredo RF. Plants with potential use on obesity and its complications. EXCLI J. 2015;14:809–831. doi: 10.17179/excli2015-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 3.Piehowski KE, Preston AG, Miller DL, Nickols-Richardson SM. A reduced-calorie dietary pattern including a daily sweet snack promotes body weight reduction and body composition improvements in premenopausal women who are overweight and obese: a pilot study. J Am Diet Assoc. 2011;111:1198–1203. doi: 10.1016/j.jada.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng HL, Griffin HJ, Bryant CE, Rooney KB, Steinbeck KS, O’Connor HT. Impact of diet and weight loss on iron and zinc status in overweight and obese young women. Asia Pac J Clin Nutr. 2013;22:574–582. doi: 10.6133/apjcn.2013.22.4.08. [DOI] [PubMed] [Google Scholar]
- 6.Cheng HL, Bryant C, Cook R, O’Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13:150–161. doi: 10.1111/j.1467-789X.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 7.García OP, Ronquillo D, del Carmen Caamaño M, Martínez G, Camacho M, López V, et al. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients. 2013;5:5012–5030. doi: 10.3390/nu5125012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farsani GM, Targhi FZ, Pishgahroudsari M, Mokhber S, Pazouki A. High prevalence of zinc deficiency in Iranian morbid obese patients undergoing bariatric surgery. J Minim Invasive Surg Sci. 2015;4:e33347. [Google Scholar]
- 9.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa Y, Kudo H, Kagawa Y, Sakamoto S. Increased plasma levels of zinc in obese adult females on a weight-loss program based on a hypocaloric balanced diet. In Vivo. 2005;19:1035–1037. [PubMed] [Google Scholar]
- 11.Helen O, Munas Z, Griffin H, Rooney K, Cheng HL, Steinbeck K. Nutritional adequacy of energy restricted diets for young obese women. Asia Pac J Clin Nutr. 2011;20:206–211. [PubMed] [Google Scholar]
- 12.Damms-Machado A, Weser G, Bischoff SC. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr J. 2012;11:34. doi: 10.1186/1475-2891-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Med Gen Med. 2006;8:59. [PMC free article] [PubMed] [Google Scholar]
- 14.Marreiro DDN, Fisberg M, Cozzolino SMF. Zinc nutritional status in obese children and adolescents. Biol Trace Elem Res. 2002;86:107–122. doi: 10.1385/BTER:86:2:107. [DOI] [PubMed] [Google Scholar]
- 15.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Obes Surg. 2008;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 16.Payahoo L, Ostadrahimi A, Mobasseri M, Bishak YK, Farrin N, Jafarabadi MA, et al. Effects of zinc supplementation on the anthropometric measurements, lipid profiles and fasting blood glucose in the healthy obese adults. Adv Pharm Bull. 2013;3:161. doi: 10.5681/apb.2013.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.do Nascimento Marreiro D, Geloneze B, Tambascia MA, Lerário AC, Halpern A, Cozzolino SMF. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res. 2006;112:109–118. doi: 10.1385/BTER:112:2:109. [DOI] [PubMed] [Google Scholar]
- 18.Cruz KJC, de Oliveira ARS, Morais JBS, Severo JS, Mendes PMV, de Sousa Melo SR, et al. Zinc and insulin resistance: biochemical and molecular aspects. Biol Trace Elem Res. 2018;186:407–412. doi: 10.1007/s12011-018-1308-z. [DOI] [PubMed] [Google Scholar]
- 19.Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals. 2005;18:333–338. doi: 10.1007/s10534-005-3707-9. [DOI] [PubMed] [Google Scholar]
- 20.Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 2017;68:19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantzoros CS, Prasad AS, Beck FW, Grabowski S, Kaplan J, Adair C, et al. Zinc may regulate serum leptin concentrations in humans. J Am Coll Nutr. 1998;17:270–275. doi: 10.1080/07315724.1998.10718758. [DOI] [PubMed] [Google Scholar]
- 22.Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 24.Foster M, Petocz P, Samman S. Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J Nutr Biochem. 2013;24:1655–1661. doi: 10.1016/j.jnutbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23:87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 27.Antushevich H, Wójcik M. Apelin in disease. Clin Chim Acta. 2018;483:241–248. doi: 10.1016/j.cca.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Krist J, Wieder K, Klöting N, Oberbach A, Kralisch S, Wiesner T, et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes Facts. 2013;6:57–69. doi: 10.1159/000348667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67:559–572. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Ahn J. Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res. 2014;157:101–106. doi: 10.1007/s12011-013-9885-3. [DOI] [PubMed] [Google Scholar]
- 31.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009;28:257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- 33.Mahan LK, Raymond JL, Escott-Stump S. Krause’s food & the nutrition care process-E-Book. St. Louis: Elsevier Health Sciences; 2013. [Google Scholar]
- 34.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg G, Black A, Jebb S, Cole T, Murgatroyd P, Coward W, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45:569–581. [PubMed] [Google Scholar]
- 36.Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82:1074–1081. doi: 10.1093/ajcn/82.5.1074. [DOI] [PubMed] [Google Scholar]
- 37.Mahan LK, EHammond K. Dietary and clinical assessment. Krause’s food and nutrition therapy; 2000, p. 372.
- 38.Aadahl M, JØrgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35:1196–1202. doi: 10.1249/01.MSS.0000074446.02192.14. [DOI] [PubMed] [Google Scholar]
- 39.Chou D. Clinical guide to laboratory tests. JAMA. 1984;251:2587–2588. doi: 10.1001/jama.1984.03340430079046. [DOI] [Google Scholar]
- 40.Bishop ML, Fody EP, Schoeff LE. Clinical chemistry: principles, techniques, and correlations. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 41.Smith J, Butrimovitz G, Purdy W. Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem. 1979;25:1487–1491. [PubMed] [Google Scholar]
- 42.Chong PW, Beah ZM, Grube B, Riede L. IQP-GC-101 reduces body weight and body fat mass: a randomized, double-blind, placebo-controlled study. Phytother Res. 2014;28:1520–1526. doi: 10.1002/ptr.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing M-Y, Sun J-Y, Weng X-Y. Insights on zinc regulation of food intake and macronutrient selection. Biol Trace Elem Res. 2007;115:187–194. doi: 10.1007/BF02686029. [DOI] [PubMed] [Google Scholar]
- 44.Costarelli L, Muti E, Malavolta M, Cipriano C, Giacconi R, Tesei S, et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J Nutr Biochem. 2010;21:432–437. doi: 10.1016/j.jnutbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Baltaci AK, Mogulkoc R. Leptin and zinc relation: in regulation of food intake and immunity. Indian J Endocrinol Metab. 2012;16:S611. doi: 10.4103/2230-8210.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee RG, Rains TM, Tovar-Palacio C, Beverly JL, Shay NF. Zinc deficiency increases hypothalamic neuropeptide Y and neuropeptide Y mRNA levels and does not block neuropeptide Y-induced feeding in rats. J Nutr. 1998;128:1218–1223. doi: 10.1093/jn/128.7.1218. [DOI] [PubMed] [Google Scholar]
- 47.Selvais PL, Labuche C, Ninh NX, Ketelslegers JM, Denef JF, Maiter DM. Cyclic feeding behaviour and changes in hypothalamic galanin and neuropeptide Y gene expression induced by zinc deficiency in the rat. J Neuroendocrinol. 1997;9:55–62. doi: 10.1046/j.1365-2826.1997.00566.x. [DOI] [PubMed] [Google Scholar]
- 48.Johnson S. Micronutrient accumulation and depletion in schizophrenia, epilepsy, autism and Parkinson’s disease? Med Hypotheses. 2001;56:641–645. doi: 10.1054/mehy.2000.1302. [DOI] [PubMed] [Google Scholar]
- 49.Baltaci AK, Mogulkoc R, Kul A, Bediz CS, Ugur A. Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology. 2004;195:69–75. doi: 10.1016/j.tox.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Robertson R, Zhou H, Slucca M. A role for zinc in pancreatic islet β-cell cross-talk with the α-cell during hypoglycaemia. Diabetes Obes Metab. 2011;13:106–111. doi: 10.1111/j.1463-1326.2011.01448.x. [DOI] [PubMed] [Google Scholar]
- 51.Mashhadi MA, Bakhshipour A, Zakeri Z, Ansari-Moghadam A. Reference range for zinc level in young healthy population in southeast of Iran. Health Scope. 2017;6:e18181. [Google Scholar]
- 52.Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol. 1994;266:R426–R433. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- 53.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Rev Rheumatol. 2007;3:716. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013 doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 56.Heinonen M, Laaksonen D, Karhu T, Karhunen L, Laitinen T, Kainulainen S, et al. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:626–633. doi: 10.1016/j.numecd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Weickert MO. Nutritional modulation of insulin resistance. Scientifica. 2012;2012:424780. doi: 10.6064/2012/424780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Straznicky NE, Lambert EA, Grima MT, Eikelis N, Richards K, Nestel PJ, et al. The effects of dietary weight loss on indices of norepinephrine turnover: modulatory influence of hyperinsulinemia. Obesity (Silver Spring, Md). 2014;22:652–662. doi: 10.1002/oby.20614. [DOI] [PubMed] [Google Scholar]
- 59.Weiss EP, Albert SG, Reeds DN, Kress KS, Ezekiel UR, McDaniel JL, et al. Calorie Restriction and Matched Weight Loss From Exercise: independent and Additive Effects on Glucoregulation and the Incretin System in Overweight Women and Men. Diabetes Care. 2015;38:1253–1262. doi: 10.2337/dc14-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 62.Kim J, Lee S. Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutr Res Pract. 2012;6:221–225. doi: 10.4162/nrp.2012.6.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:505–510. doi: 10.1089/met.2010.0020. [DOI] [PubMed] [Google Scholar]
- 64.Ranasinghe P, Jayawardena R, Pigera A, Katulanda P, Constantine GR, Galappaththy P. Zinc supplementation in pre-diabetes: study protocol for a randomized controlled trial. Trials. 2013;14:52. doi: 10.1186/1745-6215-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Islam MR, Attia J, Ali L, McEvoy M, Selim S, Sibbritt D, et al. Zinc supplementation for improving glucose handling in pre-diabetes: a double blind randomized placebo controlled pilot study. Diabetes Res Clin Pract. 2016;115:39–46. doi: 10.1016/j.diabres.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:9. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gelaye B, Revilla L, Lopez T, Suarez L, Sanchez SE, Hevner K, et al. Association between insulin resistance and c-reactive protein among Peruvian adults. Diabetol Metab Syndr. 2010;2:30. doi: 10.1186/1758-5996-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preethi B, Kumar KP, Jaisri G, Suresh K. High-sensitivity C-reactive protein a surrogate marker of insulin resistance. J Physiol Pathophysiol. 2013;4:29–36. [Google Scholar]
- 69.Yang JS, Gerber JN, You HJ. Association between fasting insulin and high-sensitivity C reactive protein in Korean adults. BMJ Open Sport ExerMed. 2017;3:e000236-e. doi: 10.1136/bmjsem-2017-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu S, Tsao PS, Yue P. Apelin and insulin resistance: another arrow for the quiver? J Diabetes. 2011;3:225–231. doi: 10.1111/j.1753-0407.2011.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 72.Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59–E67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Assaad SN, El-Aghoury AA, El-Sharkawy EM, Azzam EZ, Salah MA. Study of serum apelin and its relation to obesity-associated hypertension. Egypt J Obes Diabetes Endocrinol. 2015;1:28. doi: 10.4103/2356-8062.159990. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


