Abstract
CXC chemokine ligand 12 (CXCL12) is a member of the CXC chemokine family and mainly acts on cell chemotaxis. CXCL12 also elicits a proatherogenic role, but the molecular mechanisms have not been fully defined yet. We aimed to reveal if and how CXCL12 promoted atherosclerosis via regulating lipid metabolism. In vitro, our data showed that CXCL12 could reduce ABCA1 expression, and it mediated cholesterol efflux from THP-1-derived macrophages to apoA-I. Data from the luciferase reporter gene and chromatin immunoprecipitation assays revealed that transcription factor 21 (TCF21) stimulated the transcription of ABCA1 via binding to its promoter region, which was repressed by CXCL12. We found that CXCL12 increased the levels of phosphorylated glycogen synthase kinase 3β (GSK3β) and the phosphorylation of β-catenin at the Thr120 position. Inactivation of GSK3β or β-catenin increased the expression of TCF21 and ABCA1. Further, knockdown or inhibition of CXC chemokine receptor 4 (CXCR4) blocked the effects of CXCL12 on TCF21 and ABCA1 expression and the phosphorylation of GSK3β and β-catenin. In vivo, the overexpression of CXCL12 in Apoe−/− mice via lentivirus enlarged the atherosclerotic lesion area and increased macrophage infiltration in atherosclerotic plaques. We further found that the overexpression of CXCL12 reduced the efficiency of reverse cholesterol transport and plasma HDL-C levels, decreased ABCA1 expression in the aorta and mouse peritoneal macrophages (MPMs), and suppressed cholesterol efflux from MPMs to apoA-I in Apoe−/− mice. Collectively, these findings suggest that CXCL12 interacts with CXCR4 and then activates the GSK-3β/β-cateninT120/TCF21 signaling pathway to inhibit ABCA1-dependent cholesterol efflux from macrophages and aggravate atherosclerosis. Targeting CXCL12 may be a novel and promising strategy for the prevention and treatment of atherosclerotic cardiovascular diseases.
Keywords: CXC chemokine ligand 12, CXC chemokine receptor 4, ATP binding cassette transporter A1, cholesterol efflux, glycogen synthase kinase 3β, transcription factor 21
Atherosclerosis is a chronic vascular disease that has been identified as one of the pathogenesis of cardiovascular disease. It is well known that atherosclerosis is driven by the dysregulation of cholesterol metabolism, leading to the formation of foam cells, a hallmark of atherosclerosis (1). Reverse cholesterol transport (RCT) is an important approach in preventing lipid accumulation during atherogenesis (2, 3). ABCA1 locates in the plasma membrane, serving as a cholesterol transporter to mediate excessive cholesterol efflux from macrophages to apoA-I. This is believed to be the first and most important step of RCT. Indeed, several lines of evidence have demonstrated that ABCA1 expression and cholesterol efflux are inversely associated with the development and progression of atherosclerosis (4–7). Thus, understanding how ABCA1 expression is regulated has always been an important research topic.
Chemokines are a group of molecules that mainly function on cell chemotaxis and are extensively expressed in vascular cells, such as macrophages, endothelial cells (ECs), and smooth muscle cells (SMCs). CXC chemokine ligand 12 (CXCL12) belongs to the CXC subclass of the chemokine family. Several clinical reports have shown that higher serum levels of CXCL12 are closely related to an increase in atherosclerotic risk (8–11). The overexpression of CXCL12 can aggravate atherosclerosis progression in Apoe−/− mice (12, 13). CXC chemokine receptor 4 (CXCR4), a specific receptor for CXCL12, is strongly correlated with plaque stability, lesion area, and risk for cardiovascular disease (14–16). Merckelbach et al. (17) reported that CXCL12 and CXCR4 were abundantly expressed in macrophages within human carotid artery atherosclerosis. It has been shown that CXCL12 stimulates the formation of macrophage-derived foam cells via CXCR4 (18). However, the role of CXCL12 in cholesterol efflux is unclear.
Glycogen synthase kinase 3β (GSK3β) is a multifunctional serine/threonine kinase that is primarily involved in cellular glucose metabolism (19). The activation and knockdown of GSK3β accelerate and inhibit atherosclerosis, respectively (20, 21). Although GSK3β has been implicated in dyslipidemia during atherogenesis, the role of GSK3β in ABCA1-mediated cholesterol efflux has yet to be determined. β-catenin belongs to the catenin family and commonly acts as a nuclear transcriptional activator to activate the transcription of target genes when Wnt/β-catenin is activated (22). GSK3β-mediated change in protein phosphorylation regulates the translocation of β-catenin from the cytoplasm to the nucleus and the subsequent GSK3β/β-catenin pathway. This has been implicated in the development of cardiovascular disease (23–26). Transcription factor 21 (TCF21) is a member of the basic helix-loop-helix transcription factor (TCF) family and affects the development of coronary vasculature (27). Lu et al. (28) found that TCF21 was associated with the risk of coronary artery disease (CAD) in a Chinese Han population. The inhibition of TCF21 expression dramatically increases CAD risk (29). However, the underlying mechanisms remain to be addressed.
In this study, we found that the binding of CXCL12 to CXCR4 activated the GSK3β/β-cateninT120/TCF21pathway. This downregulated ABCA1 expression and reduced cholesterol efflux. Further, our data confirmed the proatherogenic role of CXCL12 in Apoe−/− mice.
MATERIALS AND METHODS
Animals
Apoe−/− male mice from a C57BL/6 background (8 weeks old) were purchased from Cavens Animal Research Laboratories, Changzhou, China. Apoe−/− mice were injected with 1 × 1011 viral particles of lentiviral vector (LV)-CXCL12 or LV via the tail vein and then fed the Western-type diet for 12 weeks. Afterward, mice were euthanized using pentobarbital sodium. Blood samples and tissues were collected. Prior to euthanasia, mice were intraperitoneally injected with 4% thioglycollate broth, followed by an injection of 5 ml PBS for the collection of peritoneal macrophages. All procedures were conducted in accordance with the Institutional Animal Ethics Committee and the University of South China Animal Care Guidelines for the Use of Experimental Animals.
Atherosclerosis analysis
Aortas were dissected from mice with all adventitia removed. The quantification of lesion size and composition is in agreement with Arteriosclerosis, Thrombosis, and Vascular Biology guidelines and previous studies (30, 31). Aortas were then unfolded along the longitudinal axis, stained with Oil Red O, and photographed with a CASIO EX-ZR3700 digital camera en face to measure the percentage of total atherosclerotic lesions on the aortic surface. Mouse hearts were sectioned perpendicular to the axis of the aorta once the aortic root was identified by the appearance of aortic valve leaflets. Eight serial sections (10 μm intervals) of aortic sinus were obtained per mouse. We randomly selected three sections from each mouse per group for Oil Red O, H&E, and Masson staining. The lesion areas were quantified with Oil Red O staining, and the collagen contents were quantified with Masson staining. All analyses were quantified using Image J software.
Plasma lipid profiles
Plasma lipid levels were analyzed as previously described (7). Blood samples were collected from the retro-orbital plexus of Apoe−/− mice injected with or without LV-CXCL12 or saline and fed the Western-type diet for 12 weeks. Plasma levels of total cholesterol (TC), LDL-C, HDL-C, and triglyceride (TG) levels were analyzed by enzymatic methods using their specific kits (BioSino Bio-Technology and Science Inc.).
Immunofluorescence assay
The sections of aortic roots were washed with 1× PBS three times and blocked with 5% goat serum for 30 min at room temperature. THP-1-derived macrophages were incubated with or without CXCL12, washed with PBS, and then fixed with methanol. Afterward, the sections and cells were incubated with anti-ABCA1 antibody (mouse monoclonal antibody; 1:200; Abcam), anti-CD68 antibody (mouse monoclonal antibody; 1:200; Abcam), or anti-CXCR4 antibody (mouse monoclonal antibody; 1:200; Abcam) overnight at 4°C, followed by Cy3-labeled goat anti-mouse IgG (1:200; Beyotime) for 1 h at room temperature in a dark place. Nuclei were counterstained with DAPI (Thermo Fisher Scientific). Fluorescence microscopy of ABCA1 and CD68 in the sections and ABCA1 and CXCR4 in cells were performed using an EVOS FL AUTO 2 (Thermo Fisher Scientific). The mean fluorescence intensity on the stained sections of aortic roots and cells were quantified using Image J software.
Immunohistochemistry
Mice aorta root sections were incubated with UV block (Thermo Fisher Scientific) containing 10% goat serum (Abcam) for 30 min and then with mouse monoclonal anti-CD68 antibody (1:1000; Abcam) overnight in a humid chamber at 4°C. Afterward, sections were incubated with a biotin-conjugated secondary antibody for 1 h at 37°C, followed by streptavidin-HRP for 30 min. Sections were then counterstained with hematoxylin for 15 s, differentiated with a hydrochloric acid/alcohol mixture, and then stained with 3,3′-diaminobenzidine for 30 s. After washing with water for 15 min, sections were imaged using an EVOS FL AUTO 2 (Thermo Fisher Scientific).
Measurement of RCT in vivo
The efficiency of RCT was determined as previously described (7). J774.1 macrophages were loaded with 50 μg/ml ac-LDL and 5 μCi/ml [3H]cholesterol for 24 h in DMEM. The labeled J774 cells were injected into the abdominal cavity of individual mice. Plasma was collected at 6, 24, and 48 h after injection. The feces were continuously collected from 0 to 48 h until the endpoint of the experiment. The feces were weighed and dissolved in 50% ethanol. Aliquots (20 μl) were used for scintillation counting after shaking overnight. The liver was collected from euthanized mice, washed in ice-cold PBS, blotted up with filter papers, weighed, and stored at −20°C. Frozen liver tissue (80 mg) was added into n-hexane and isopropanol at a proportion of 3:2 with shaking for 10 min. Afterward, the samples were vacuum-dried for the extraction of liver lipids, and radioactivity was counted with a liquid scintillation counter. The results were calculated as the percentage of injected = cpm (plasma, liver, or feces)/total cpm (5 μCi/ml [3H]cholesterol).
Cell culture and transfection
Human THP-1 monocytes were purchased from the Chinese Academy of Sciences cell bank and cultured in RPMI-1640 supplemented with 0.1% nonessential amino acids, penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% FBS. HEK293T cells were purchased from the Chinese Academy of Sciences cell bank and cultured in DMEM containing 10% FBS. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. The differentiation of THP-1 monocytes into macrophages was induced by 160 nM phorbol-12-myristate acetate for 24 h.
THP-1-derived macrophages and HEK293T cells were cultured in 12-well plates for different treatments. β-catenin agonist SKL2001 (MedChemExpress), GSK3β inhibitor TWS119 (MedChemExpress), CXCR4 inhibitor LY2510924 (MedChemExpress), or recombinant human CXCL12 protein (Abcam) was incubated with cells. CXCR4 siRNA (Abcam) and TCF21 (Santa Cruz Biotechnology) were introduced to cells by transfection and LV, respectively. Approximately 6 to 12 h later, the medium was changed to DMEM containing antibiotics and 10% FBS.
Western blot analysis
Tissues and cell-derived total proteins were extracted by the regular method. Nuclear and cytoplasmic proteins were extracted with a nuclear and cytoplasmic protein extraction kit (Sangon Biotech). β-actin was used as the loading control for total proteins derived from tissues and cells and cytoplasmic proteins. H3 was used as the loading control for nuclear fractions (32). Total proteins were quantified and subjected to SDS-PAGE, followed by immunoblotting. Primary antibodies included anti-GSK3β (1:1000; Santa Cruz Biotechnology), anti-phospho-GSK3β (1:1000; Santa Cruz Biotechnology), anti-β-catenin (1:1000; Santa Cruz Biotechnology), anti-phospho-β-cateninY654 (1:1000; Abcam), anti-phospho-β-cateninT120 (1:2000; Santa Cruz Biotechnology), anti-CXCR4 (1:200; Santa Cruz Biotechnology), anti-ABCA1 (1:200; Santa Cruz Biotechnology), anti-TCF21 (1:10000; Santa Cruz Biotechnology), anti-histone H3 (1:500; Santa Cruz Biotechnology), and anti-β-actin (1:2000; Santa Cruz Biotechnology). Secondary antibodies were HRP-labeled goat anti-mouse IgG (1:1000; Santa Cruz Biotechnology). Antibody binding was visualized with a Tanon 5500 and BeyoECL Plus (Beyotime).
Coimmunoprecipitation
THP-1-derived macrophages were incubated with CXCL12 and then homogenized and lysed in RIPA buffer. Protein A plus-agarose (Thermo Fisher Scientific) was washed in PBS three times. Lysates were incubated with antibodies overnight at 4°C, followed by an incubation with prewashed protein A plus-agarose overnight at 4°C. Beads were then washed three times in PBS. Immunoprecipitated proteins were eluted from the bead with 60 μl 2× SDS sample buffer (Beyotime) containing 2-mercaptoethanol at 60°C for 5 min and then subjected to immunoblotting.
Cholesterol efflux assay
Cholesterol efflux was performed as described previously (4). THP-1-derived macrophages were incubated with or without CXCL12 for 12 h and then cultured in 0.1% BSA and RPMI 1640 medium for 24 h. Peritoneal macrophages were isolated from Apoe−/− mice and cultured in RPMI 1640 medium containing 0.1% BSA for 24 h. Mouse peritoneal macrophages (MPMs) and THP-1-derived macrophages were incubated with 50 μg/ml ox-LDL and then labeled with 0.5 μCi/ml [3H]cholesterol for 24 h in serum-free medium. Afterward, the cells were washed in PBS and then cultured in RPMI 1640 medium containing 0.1% BSA and 25 μg/ml apoA-I or 50 μg/ml HDL (Sigma-Aldrich) for 24 h. Radioactivity in culture medium and cells was counted in a liquid scintillation counter separately. Cholesterol efflux was calculated as the ratio of [3H]cholesterol in medium to total [3H]cholesterol in cells and medium.
HPLC analysis of lipids
The lipid content in THP-1-derived macrophages was examined as previously described (4). THP-1-derived macrophages were washed three times with PBS. The cells were homogenized in 1 ml 0.9% NaCl on ice using the ultrasonic processor (Cole-Parmer). Protein concentrations were measured using the BCA protein assay kit (Abcam). Isopropanol (1 mg cholesterol/ml) was added to extract cholesterol and then stored at 20°C. The stock solution was diluted as cholesterol standard liquid and then supplied with 10 μl reaction mixture containing 500 mM MgCl2, 500 mM Tris-HCl (pH 7.4), 10 mM dithiothreitol, and 5% NaCl to 0.1 ml cholesterol standard liquid. Afterward, 0.4 U cholesterol oxidase in 10 μl 0.5% NaCl was added into each sample to examine free cholesterol or 0.4 U cholesterol oxidase together with 0.4 U cholesterol esterase for the measurement of TC. Samples were incubated at 37°C for 30 min. The reaction was then terminated by adding 100 ml methanol-ethanol (1:1). After cooling for 30 min, samples were centrifuged at 1,500 rpm for 10 min at 15°C. Ten microliters of supernatant was applied to a 2790 chromatographer (Waters Corporation) for chromatographic analysis. Absorbance at 216 nm was monitored, with cholesterol content indicated by the peak area.
Quantitative RT-PCR
THP-1-derived macrophages, MPMs, and tissues were lysed for RNA extraction using TRIzol. The sequences of the RT-PCR primers were as follows: human ABCA1, 5′-GTCCTCTTTCCCGATTATCTGG-3′ and 5′-CACTCACTCTCGCTCGCAAT-3′; human TCF21, 5′-GGTTAGTTAGGAGGGGAAGTA-3′ and 5′-ACACCCAAAACAAAATAATCTTA-3′; and mouse ABCA1, 5′-GGGTGGTGTTCTTCCTCATTAC-3′ and 5′-GAATGACGAGGATGAGGATGTG-3′. mRNA levels were analyzed with an ABI PRISM 7900 sequence detection system (Applied Biosystems).
Luciferase reporter gene
The TCF21 expression vector was acquired from Genechem. The luciferase vectors with the promoter region of 3,000 bp of the human ABCA1 were prepared by Genechem. HEK293T cells were treated with CXCL12 or transfected with 0.5 μg TCF21 overexpression vector using Lipofectamine 2000 (Invitrogen). The cells were then cotransfected with 0.5 μg of the ABCA1 promoter reporter construct and renilla luciferase control reporter vector. After 12 h, cells were washed in PBS and incubated in medium containing 0.1% BSA and RPMI 1640. The luciferase activity was detected using the Dual-Glo Luciferase Assay System (Promega). The results were standardized in the corresponding luciferase activity and plotted as a percentage of the control.
Chromatin immunoprecipitation assay
THP-1-derived macrophages transfected with CXCL12 were cross-linked in 1% formaldehyde for 15 min at 37°C. The reaction was stopped by adding glycine solution. SDS lysis buffer (Beyotime) and PMSF (Beyotime) were then added to the cells before sonication with an ultrasonic processor (Sonics) for 14 bursts of 4.5 s with 9 s intervals under 60 W on the ice. Cell lysates were centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant containing sheared chromatin was kept on ice. DNA fragment sizes were measured using agarose gel electrophoresis. Afterward, the samples were subjected to immunoprecipitation using a chromatin immunoprecipitation assay kit (Abcam) and antibody against TCF21 (Abcam) or antibodies against IgG (Abcam). The DNA was eluted and collected for analysis of quantitative RT-PCR using human ABCA1 primers (forward: 5′-CTCGGTGCAGCCGAATCTAT-3′; reverse: 5′-CACTCACTCTCGCTCGCAAT-3′).
Statistical analysis
All data are presented as means ± SDs. The distribution of all data was analyzed by an F-test and showed no significant difference (P > 0.05). Statistical significance was evaluated by either one-way ANOVA or Student’s t-test. Student’s t-test was used for assessing the differences between two groups, while one-way ANOVA was used for analyzing the difference between multiple groups. Scheffe’s test was used for specific comparisons. P < 0.05 was considered statistically significant.
RESULTS
CXCL12 downregulates ABCA1 expression and inhibits cholesterol efflux in THP-1-derived macrophages
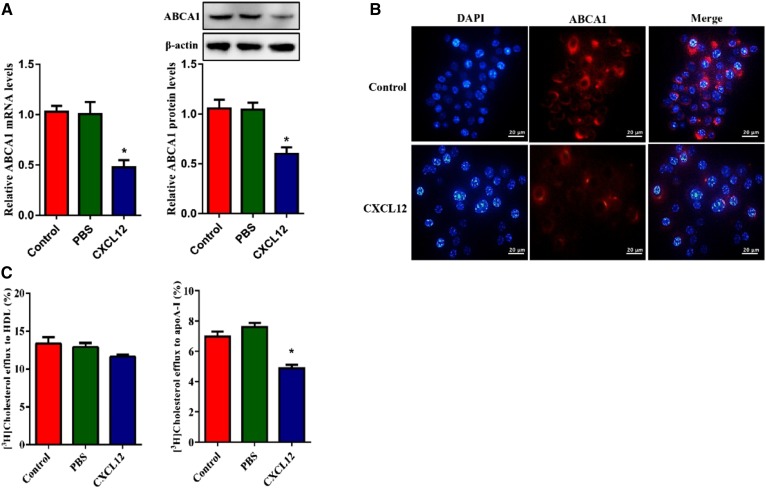
To investigate the role of CXCL12 in lipid metabolism, we treated THP-1-derived macrophages with CXCL12. As shown in Fig. 1A, CXCL12 reduced the mRNA and protein levels of ABCA1. To confirm this finding, we detected ABCA1 in THP-1-derived macrophages using immunofluorescence microscopy. Consistently, cellular ABCA1 signal was markedly reduced in CXCL12-treated cells (Fig. 1B). Given the important role of ABCA1 in the maintenance of cellular cholesterol homeostasis, we measured cholesterol efflux onto apoA-I and HDLs and found that CXCL12 reduced cholesterol efflux to apoA-I but not HDLs (Fig. 1C). Furthermore, CXCL12 significantly increased the cellular content of TC, TGs, and cholesteryl ester (CE) in THP-1-derived macrophages (Table 1). Together, these findings indicate that CXCL12 exerts an inhibitory effect on cholesterol efflux from THP-1-derived macrophages via the reduction of ABCA1 expression.
Fig. 1.
CXCL12 reduced ABCA1 expression for the inhibition of cholesterol efflux in THP-1-derived macrophages. The effect of CXCL12 on ABCA1 expression and cholesterol efflux. THP-1-derived macrophages were incubated with 80 ng/ml CXCL12. A: Analysis of mRNA and protein levels of ABCA1 using quantitative RT-PCR and Western blot, respectively. B: Analysis of ABCA1 by immunofluorescent staining. Scale bar = 20 μm. C: Analysis of cholesterol efflux to apoA-I and HDL using liquid scintillation counting assays. All data are shown as means ± SDs from three independent experiments, each experiment performed in triplicate. *P < 0.05 versus the control group.
TABLE 1.
The effect of CXCL12 on the lipid contents of THP-1-derived macrophages
| TC | CE | FC | |
| mg/g | |||
| Control | 489.73 ± 28.47 | 312.63 ± 18.69 | 177.10 ± 18.67 |
| PBS | 492.56 ± 30.35 | 308.28 ± 29.56 | 184.28 ± 22.49 |
| CXCL12 | 597.48 ± 23.46* | 359.32 ± 26.37* | 238.16 ± 23.41* |
Analysis of the levels of cellular TC, FC, and CE using high-performance liquid chromatography. All data are shown as means ± SDs from three independent experiments, each performed in triplicate. *P < 0.05 versus the control group. FC, free cholesterol.
TCF21 plays an important role in CXCL12-induced reduction in ABCA1 expression
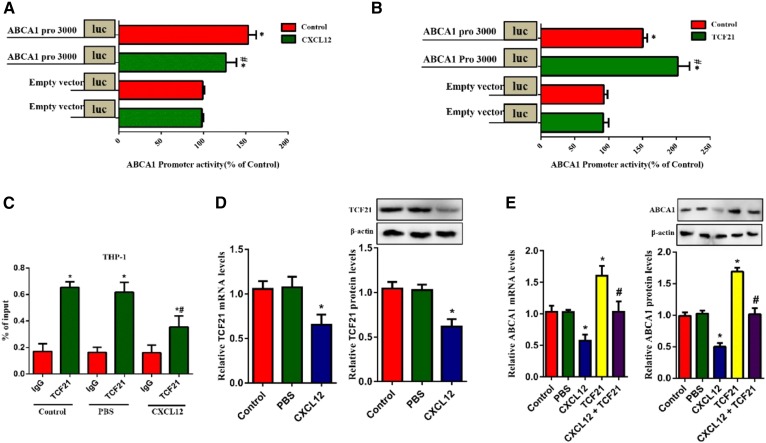
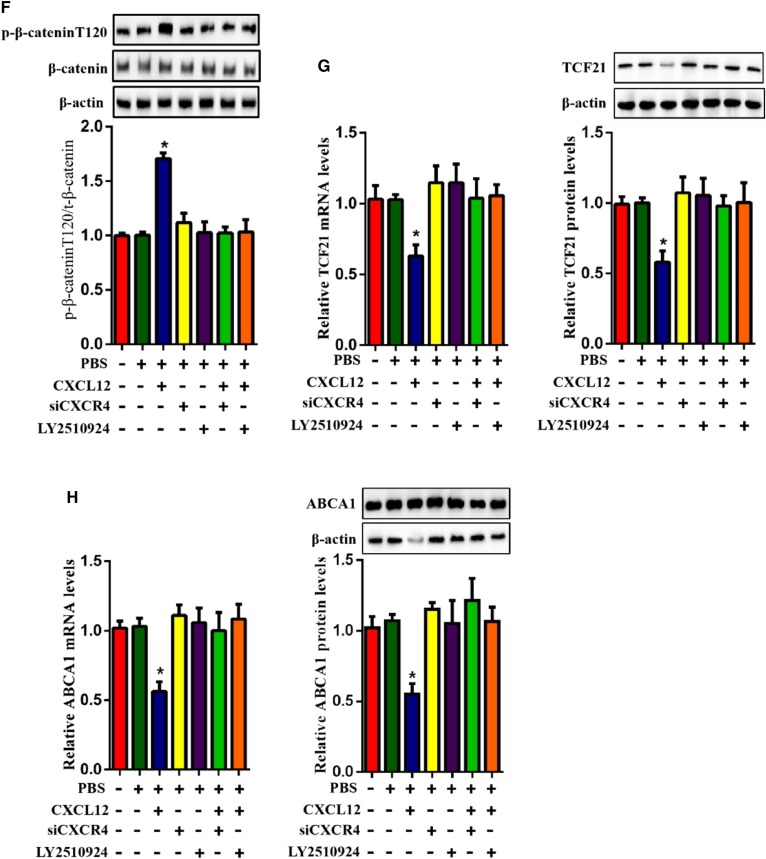
TCF21 binds to the promoter region of its target and regulates its expression. We explored the promoter activity of ABCA1 in response to TCF21 and CXCL12 in HEK293T cells using the luciferase assay and found that TCF21 enhanced while CXCL12 decreased ABCA1 promoter activity (Fig. 2A, B). Consistently, results obtained from the chromatin immunoprecipitation assay indicated that CXCL12 markedly inhibited the binding of TCF21 to ABCA1 in THP-1-derived macrophages (Fig. 2C). Next, we assessed the effect of CXCL12 on TCF21 and ABCA1 expression in THP-1-derived macrophages. As shown in Fig. 2D and E, CXCL12 significantly decreased the mRNA and protein levels of TCF21 and inhibited the TCF21-induced upregulation of ABCA1 expression. These results suggest that CXCL12 inhibits TCF21 expression, decreasing ABCA1 transcription and consequently downregulating its expression.
Fig. 2.
CXCL12 downregulated TCF21 expression and inhibited the binding of TCF21 to the ABCA1 promoter. The role of TCF21 in CXCL12-induced reduction ABCA1 expression. A, B: HEK293T cells were transfected with TCF21 and ABCA1 promoter 3000 or incubated with 80 ng/ml CXCL12 and transfected with ABCA1 promoter 3000. ABCA1 promoter activity was analyzed using the luciferase reporter gene assay. C: THP-1-derived macrophages were transfected with ABCA1 promoter 3000 and TCF21 and then incubated with or without 80 ng/ml CXCL12. Analysis of the action of CXCL12 on the binding of TCF21 to the ABCA1 promoter region using the chromatin immunoprecipitation assay. D, E: THP-1-derived macrophages were transfected with or without TCF21 and then incubated with 80 ng/ml CXCL12. mRNA and protein levels of TCF21 (D) or ABCA1 (E) were measured using quantitative RT-PCR and Western blot, respectively. All data are shown as means ± SDs from three independent experiments, each performed in triplicate. *P < 0.05 versus the control group or IgG group. *P < 0.05 versus the ABCA1 promoter 3000 group or TCF21 of the control group or TCF21 group.
CXCL12-induced reduction in ABCA1 expression is mediated by GSK3β/β-cateninT120
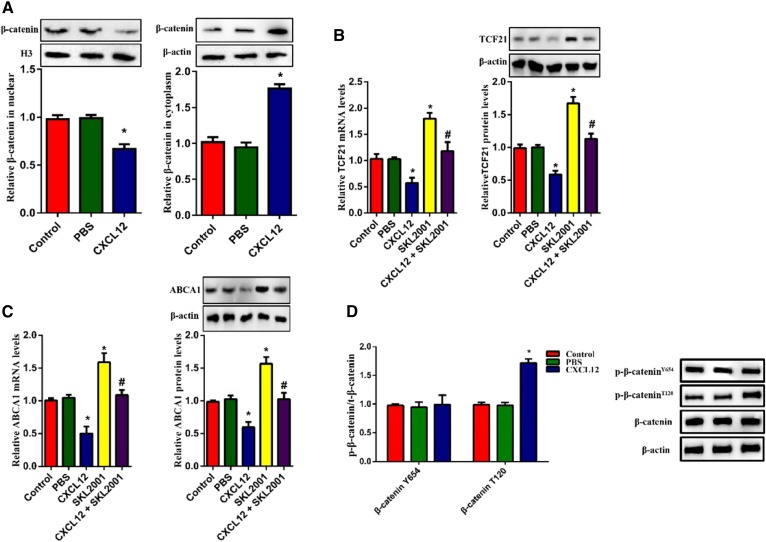
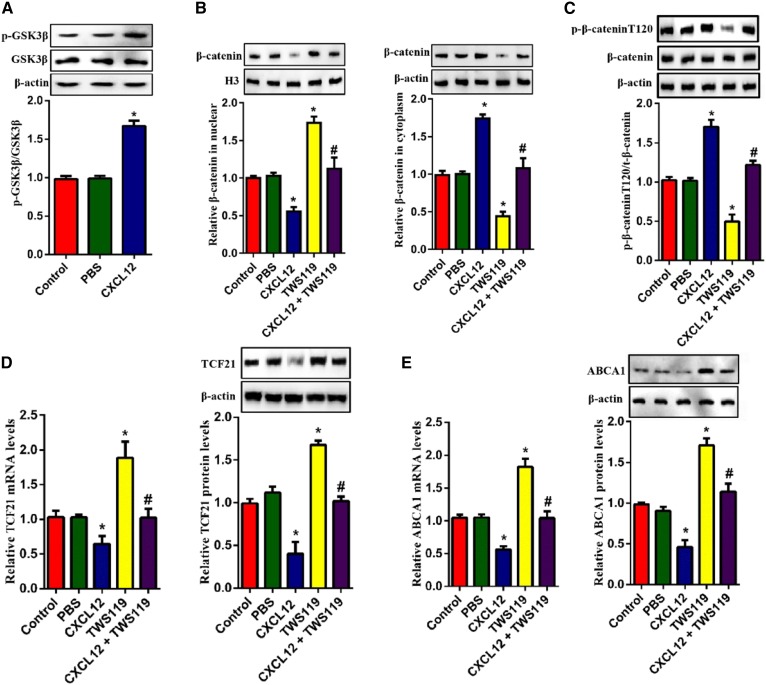
Given that β-catenin nuclear translocation regulates the transcriptional activation of TCF family members, we hypothesized that β-catenin might act as an upstream molecule for the CXCL12-regualted expression of ABCA1. To test this hypothesis, we evaluated the protein levels of β-catenin in THP-1-derived macrophages. β-actin and H3 were used as the loading control for cytoplasmic and nuclear fractions, respectively. Our results indicated that the treatment of CXCL12 strikingly decreased the amount of nuclear β-catenin but increased cytoplasmic β-catenin (Fig. 3A). Additionally, the expression of TCF21 and ABCA1 were significantly upregulated by the β-catenin agonist SKL2001 (Fig. 3B, C). Because the cellular localization of β-catenin is regulated by its phosphorylation, we examined phosphorylated β-catenin using two antibodies that can specifically recognize phosphorylation at positions Thr120 and Tyr654. As shown in Fig. 3D, CXCL12 increased levels of p-β-cateninT120 but not p-β-cateninY654. Considering that the phosphorylation of GSK3β reduced nuclear β-catenin, we assessed the contribution of GSK3β to CXCL12-reduced ABCA1 expression. Indeed, CXCL12 increased the level of p-GSK3β (Fig. 4A). Furthermore, the GSK3β inhibitor TWS119 partially blocked CXCL12-induced cytoplasmic retention of β-catenin and the levels of phosphorylated β-cateninT120 (Fig. 4B, C). In addition. TWS199 markedly upregulated the expression of TCF21 and ABCA1 (Fig. 4D, E). Taken together, these data suggest that the GSK3β/β-catenin T120 pathway acts as the upstream signal for the inhibitory effect of CXCL12 on the expression of TCF21 and ABCA1.
Fig. 3.
CXCL12-inhibited translocation of β-catenin was responsible for the downregulation of ABCA1. The role of β-catenin in CXCL12-reduced ABCA1 expression. THP-1-derived macrophages were incubated with 80 ng/ml CXCL12 and/or SKL2001. A: Analysis of protein levels of β-catenin in the nucleus and cytoplasm using Western blot. B, C: The measurement of mRNA and protein levels of TCF21 (B) or ABCA1 (C) using quantitative RT-PCR and Western blot, respectively. D: Analysis of the levels of phosphorylated β-catenin at Thr120 and Tyr654 using Western blot. E, F: THP-1-derived macrophages were incubated with 80 ng/ml CXCL12 and/or transfected with β-catenin mutant T120I. mRNA and protein levels of TCF21 (E) or ABCA1 (F) were measured using quantitative RT-PCR and Western blot, respectively.*P < 0.05 versus the control group. #P < 0.05 versus the SKL2001 or T120I group.
Fig. 4.
GSK3β was essential for the phosphorylation of β-catenin induced by CXCL12. THP-1-derived macrophages were incubated with 80 ng/ml CXCL12 and/or TWS119. A: Analysis of phosphorylation levels of GSK3β using Western blot. B: Measurement of nuclear and cytoplasmic β-catenin using Western blot. C: Detection of the levels of phosphorylated β-cateninY654 and β-cateninT120 using Western blot. D, E: The measurement of mRNA and protein levels of TCF21 (D) or ABCA1 (E) using quantitative RT-PCR and Western blot, respectively. All data are shown as means ± SDs from three independent experiments, each performed in triplicate. *P < 0.05 versus the control group. #P < 0.05 versus the TWS119 group.
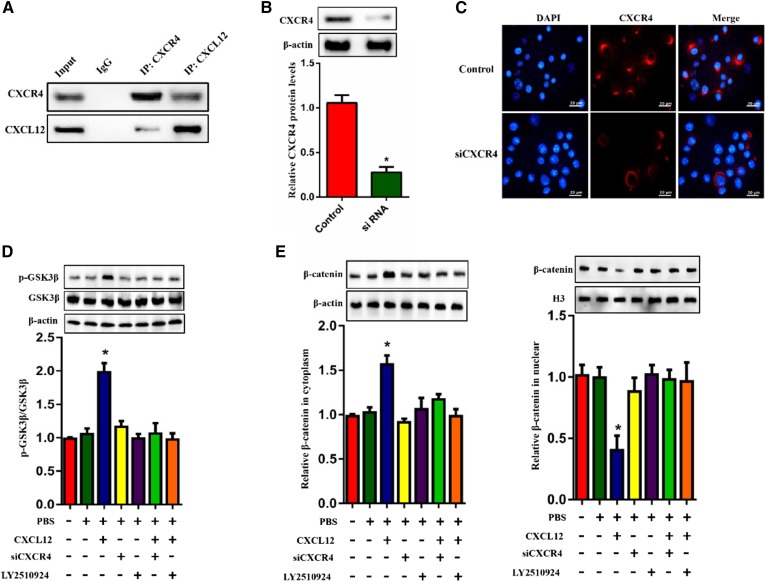
CXCR4 is essential for the effect of CXCL12 on ABCA1 expression
CXCR4 is a specific receptor for CXCL12 and involves multiple CXCL12-induced signal transductions. We performed coimmunoprecipitation and observed that CXCL12 indeed interacted with CXCR4 in THP-1 macrophages (Fig. 5A). To analyze the knockdown efficiency of CXCR4 siRNA, we examined the protein levels and fluorescence intensity of CXCR4 in THP-1-derived macrophages using Western blot and immunofluorescence assay, respectively. As shown in Fig. 5B and C, the expression and fluorescence intensity of CXCR4 were efficiently reduced in THP-1-derived macrophages transfected with CXCR4 siRNA. In addition, we found that the effect of CXCL12 on the phosphorylation of GSK3β was blocked by the knockdown of CXCR4 and the CXCR4 inhibitor LY2510924 (Fig. 5D). We then isolated nuclear and cytoplasmic fractions. H3 and β-actin were used as a control protein for the nucleus and cytoplasm, respectively, according to a previous study (32). We observed that the inhibition of CXCR4 significantly suppressed the CXCL12-induced increase in the cytoplasmic retention of β-catenin (Fig. 5E) and the levels of p-β-cateninT120 (Fig. 5F). Moreover, the inhibition of CXCR4 also suppressed the CXCL12-induced reduction in the mRNA and protein levels of TCF21 and ABCA1 (Fig. 5G, H). Together, these findings indicate that the binding of CXCL12 to CXCR4 is required for the activation of the GSK3β/β-cateninT120/TCF21 pathway, leading to the downregulation of ABCA1 expression.
Fig. 5.
Binding of CXCL12 to CXCR4 was required for the reduction in ABCA1. The role of CXCR4 in CXCL12-induced reduction in ABCA1 expression. A: THP-1-derived macrophages were incubated with 80 ng/ml CXCL12. The interaction between CXCL12 and CXCR4 was examined using coimmunoprecipitation. B, C: Detection of the silent efficiency of CXCR4 siRNA using Western blot and an immunofluorescence assay. Scale bar = 20 μm. D, E: THP-1-derived macrophages were incubated with 80 ng/ml CXCL12 and/or treated with CXCR4 siRNA or LY2510924. Analysis of the levels of phosphorylated GSKβ (D) and protein levels of β-catenin (F) and levels of phosphorylated β-cateninY654 and β-cateninT120 (E) using Western blot. G, H: The measurement of mRNA and protein levels of TCF21 (F) and ABCA1 (G) using quantitative RT-PCR and Western blot, respectively. All data are shown as means ± SDs from three independent experiments, each performed in triplicate. *P < 0.05 versus the control group.
CXCL12 promotes atherosclerosis in Apoe−/− mice
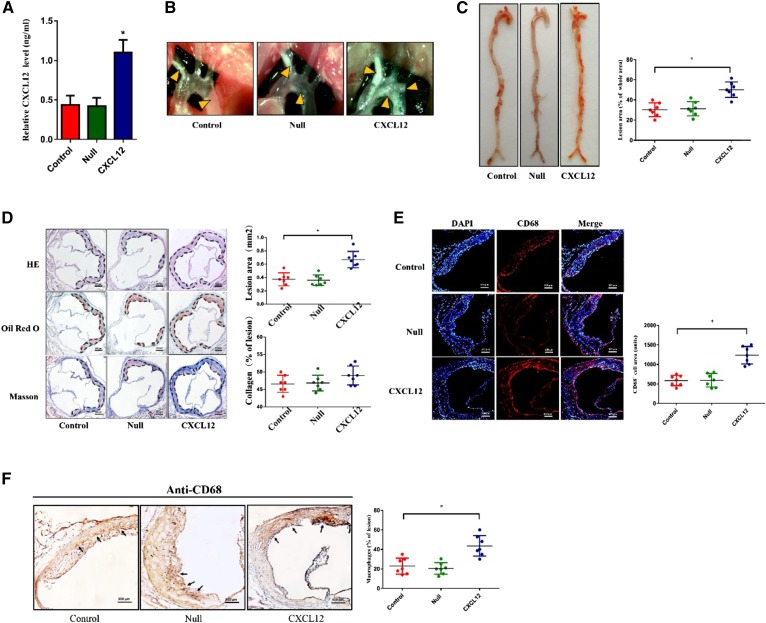
To determine the role of CXCL12 in atherosclerosis, we analyzed atherosclerotic lesions in en face aortas and the aortic sinuses of Apoe−/− mice. We first measured plasma levels of CXCL12 in Apoe−/− mice and found that CXCL12 was significantly increased in Apoe−/− mice transduced with LV-CXCL12 (Fig. 6A). As shown in Fig. 6B and C, CXCL12 profoundly increased the size of en face and aortic sinus lesions. The section staining also revealed that a lipid-positive area in the aortic root was remarkably increased in CXCL12-overexpressing mice, while the collagen content in a lesion area of LV-CXCL12 mice did not show any statistically difference compared with the control and null groups (Fig. 6D). Because CXCL12 is a potent stimulator of cell chemotaxis, we analyzed its effect on macrophage infiltration within atherosclerotic plaques using immunohistochemistry and immunofluorescence. As expected, CXCL12 markedly increased the numbers of macrophages in atherosclerotic plaques compared with the control and null groups (Fig. 6E, F). To assess the effect of CXCL12 on lipid metabolism, we examined the plasma lipid profile of Apoe−/− mice and found that HDL-C was reduced in the overexpressing CXCL12 group, while there was no significant difference in LDL-C, TGs, and TC (Table 2). Collectively, our data indicate that CXCL12 lowers plasma HDL levels and promotes the formation of atherosclerotic plaques in Apoe−/− mice.
Fig. 6.
CXCL12 promoted atherosclerotic plaque formation in Apoe−/− mice. The role of CXCL12 in the atherogenesis. Male Apoe−/− mice were treated with saline alone (control), lentivirus, or lentivirus-CXCL12 via a tail-vein injection and then fed the Western diet for 12 weeks. A: Analysis of the transduction efficiency of CXCL12 using ELISA. B: The lesion (yellow arrow) in aortic arches of Apoe−/− mice that were viewed by a stereoscopic microscope. C: Atherosclerotic lesion areas. All aortas were measured by Oil Red O staining and quantified by analyzing the positive staining regions using Image J software. D: Section of the aorta stained with Oil Red O, H&E, and Masson staining. The percentage of lesion areas detected by Oil Red O staining and collagen content examined by Masson staining were quantified by analyzing the positive staining regions of aortic root sections with Oil Red O and Masson staining, respectively, using Image J software. Scale bar = 300 μm. E, F: Immunofluorescence and immunohistochemistry analysis of CD68-postive macrophages in the aortic sections. Scale bar = 100 μm. All data are shown as means ± SDs and were collected from seven mice for each group. *P < 0.05 versus the control group.
TABLE 2.
The role of CXCL12 in the regulation of plasma lipid levels in Apoe−/−mice fed a Western diet
| TC | TGs | LDL-C | HDL-C | |
| mmol/l | ||||
| Control | 27.18 ± 3.81 | 1.43 ± 0.35 | 25.62 ± 2.47 | 1.86 ± 0.76 |
| Null | 29.68 ± 4.23 | 1.56 ± 0.47 | 27.36 ± 2.21 | 1.73 ± 0.37 |
| CXCL12 | 30.28 ± 3.01 | 1.63 ± 0.56 | 28.59 ± 1.38 | 1.68 ± 0.28* |
The assessment of TC, TGs, HDL-C, and LDL-C in Apoe−/− mice by enzymatic methods. All data are shown as means ± SDs from three independent experiments, each performed in triplicate. *P < 0.05 versus the control group.
CXCL12 downregulates ABCA1 expression and reduces RCT in Apoe−/− mice
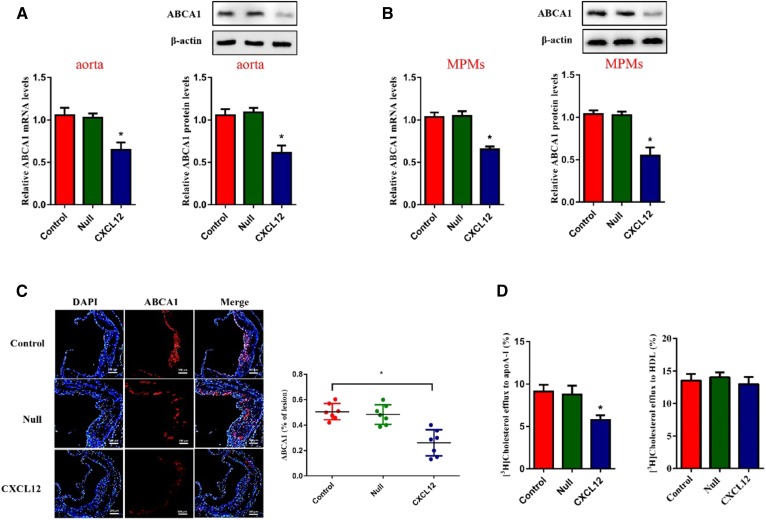
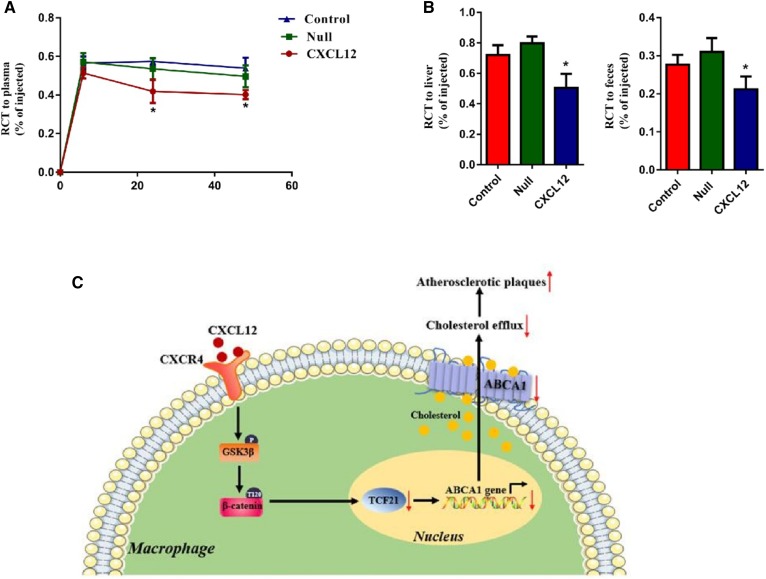
Next, we analyzed the levels of ABCA1 in the aortas and MPMs of Apoe−/− mice and found an inhibitory effect of CXCL12 on ABCA1 expression (Fig. 7A, B). Results from immunofluorescence staining further revealed that the positive areas of ABCA1 were reduced in the aortic sinuses of Apoe−/− mice overexpressing CXCL12 (Fig. 7C). Consistently, we found that CXCL12 reduced cholesterol efflux from MPMs to apoA-I (Fig. 7D) and exerted an inhibitory effect on the efficiency of RCT to plasma, the liver, and feces in Apoe−/− mice (Fig. 8A–C). These data suggest that CXCL12 downregulates ABCA1 expression and then inhibits RCT, enhancing the development of atherosclerosis.
Fig. 7.
CXCL12-reduced ABCA1 expression and cholesterol efflux in Apoe−/− mice. Male Apoe−/− mice were treated with saline alone (control), lentivirus, or lentivirus-CXCL12 via tail-vein injection and then fed the Western diet for 12 weeks. A, B: Analysis of mRNA and protein levels of ABCA1 in aortas (A) and MPMs (B) isolated from Apoe−/− mice using quantitative RT-PCR and Western blot, respectively. C: The evaluation of ABCA1-mediated cholesterol efflux to apoA-I and HDL using liquid scintillation counting assays. D: Immunofluorescence analysis of the levels of ABCA1 in the lesion of Apoe−/− mouse aortas. Scale bar = 100 μm. All data are shown as means ± SDs and were collected from seven mice for each group. *P < 0.05 versus the control group.
Fig. 8.
CXCL12 inhibited RCT efficiency. [3H]cholesterol and ox-LDL-loading J774 macrophages were injected into the abdominal cavity of Apoe−/− mice transfected with lentivirus or lentivirus-CXCL12 and fed the Western diet. A–C: The examination of the concentration of 3H tracer in serum (A), the liver (B), and feces (C) using radioactive counting. All data are shown as means ± SDs (n = 7 mice per group). *P < 0.05 versus the control group. D: A proposed model for CXCL12-regulated cholesterol efflux in macrophages. CXCL12 promotes phosphorylation of GSK3β and reduces nuclear β-catenin content. This downregulates the expression of TCF21 and ABCA1, suppressing macrophage cholesterol efflux. Thus, CXCL12 exerts a stimulating effect on atherosclerosis.
DISCUSSION
We found for the first time that CXCL12 reduced the expression of ABCA1 and subsequent cholesterol efflux from macrophages to apoA-I. Mechanistically, CXCL12 bound CXCR4 and downregulated the expression of TCF21, thereby decreasing the promoter activity of ABCA1. In addition, our findings revealed that the activation of the GSK3β/β-catenin pathway served as the upstream signal for the CXCL12-induced reduction in TCF21 expression and verified that CXCL12 promoted the development of atherosclerotic lesions in Apoe−/− mice.
Increasing evidence suggests that CXCL12 involves various cardiovascular diseases, including atherosclerosis (10, 13, 33, 34). Most previous studies in animal models focused on revealing the proatherogenic role of CXCL12 by examining the sizes, counts, and stability of atherosclerotic plaques (12). These studies rarely defined the underlying mechanisms of CXCL12 in vitro. Dyslipidemia is the leading cause of atherosclerosis. Chatterjee et al. (18) reported that CXCL12 induced the differentiation of macrophages into foam cells; we therefore proposed that CXCL12 might regulate lipid metabolism in macrophages. For the first time, we showed that CXCL12 reduced ABCA1 expression and subsequent cholesterol efflux from macrophages to apoA-I. ABCA1 mainly mediates the efflux of intracellular cholesterol onto lipid-free apoA-I, while ABCG1 mainly facilitates cholesterol efflux onto HDL (35–38). Therefore, the CXCL12-induced decrease in cholesterol efflux to apoA-I but not to HDL implies that CXCL12 reduces cholesterol efflux by downregulating ABCA1 expression. The retention of TC in THP-1-derived macrophages also provides evidence to support CXCL12-induced cellular lipid accumulation. CXCR4, the receptor of CXCL12, is abundantly expressed in atherosclerotic plaques and acts as an important mediator of macrophage migration toward the regions of atherosclerotic lesions (14, 39). We expounded a novel mechanism of CXCR4-accelerated atherosclerosis, impairing ABCA1-mediated cholesterol efflux from macrophages via binding to CXCL12. Consistently, the overexpression of CXCL12 in Apoe−/− mice led to a significant reduction in plasma HDL-C levels, which is attributed to the downregulation of ABCA1 expression. The inhibition of ABCA1 expression and cholesterol efflux from MPMs in Apoe−/− mice were among the primary reasons for the reduction in plasma HDL-C levels. HDL plays a crucial role in RCT that promotes the transport of excess cholesterol from peripheral cells to the liver for excretion (40). To evaluate the effect of CXCL12 on lipid metabolism, we measured RCT efficiency in Apoe−/− mice and found that the overexpression of CXCL12 significantly reduced RCT. Together, our data suggest that CXCL12 adversely affects lipid metabolism by impairing cholesterol efflux, therefore promoting atherosclerosis.
Döring et al. (41) recently reported that EC-derived CXCL12 contributes to plasma CXCL12 levels and is an exciter for atherogenesis. Our data consistently indicate that the overexpression of CXCL12 in Apoe−/− mice enhanced the development of atherosclerosis. We also found that the incubation of macrophages with CXCL12 led to a reduction in cholesterol efflux and a promotion of intracellular lipid accumulation. However, whether specific deletion of CXCL12 in macrophages affects plasma CXCL12 levels and the occurrence and progression of atherosclerosis needs to be investigated in the future. It has been reported that CXCL12 antibody treatment has no effect on neointimal macrophage content in Apoe−/− mice. Cell-specific deletion of CXCL12 in ECs or SMCs also has no significant effect on macrophage content in atherosclerotic plaques (41, 42). However, our study revealed that the global overexpression of CXCL12 significantly increased macrophage infiltration within atherosclerotic plaques of Apoe−/− mice. This may indicate that macrophage-derived CXCL12 could serve as an underlying driver for macrophage infiltration. Previous studies showed that neutralization of CXCL12 in Apoe−/− mice led to a reduction in the neointimal lesion area and SMC content, suggesting the implication of CXCL12 in the phenotype switching of SMCs (42). Given the fact that SMC phenotypes are involved in the regulation of atherosclerotic plaque stability and atherosclerosis progression (42), the effect of CXCL12 on SMC phenotypes will be explored in our future studies.
TCF21 has been implicated in CAD and hypertension in different populations worldwide (43–45). Nurnberg et al. (46) reported that TCF21 was involved in the progression of atherosclerotic lesions. Conversely, Lyer et al. (47) identified TCF21 as an atheroprotective gene. However, little is known about the underlying mechanism by which TCF21 regulates atherogenesis. In this study, our findings suggest that TCF21 exerts an antiatherogenic action by increasing ABCA1 expression, which may indicate a novel role of TCF21 in cardiovascular disease. In addition, we found that TCF21 increased ABCA1 promoter activity, but CXCL12 elicited an inverse action. This suggests an underlying association of CXCL12 with TCF21. Further studies showed that CXCL12 inhibited the binding of TCF21 to the ABCA1 promoter region by downregulating TCF21 expression. In addition, the inhibition of CXCR4 blocks the effect of CXCL12 on the expression of TCF21 and ABCA1. Therefore, the effect of the CXCL12/CXCR4 axis on ABCA1-mediated cholesterol efflux may depend on TCF21.
β-catenin, a member of the Wnt signaling pathway, is translocated into the nucleus followed by the activation of TCF family members (48). The canonical Wnt/β-catenin pathway is triggered by hyperlipidemia as a protective mechanism (49). Chen et al. (50, 51) reported that β-catenin affected the uptake of lipids by macrophages. We found for the first time that the CXCL12/CXCR4 axis has an inhibitory effect on β-catenin translocation, leading to the downregulation of TCF21 and ABCA1 expression in THP-1-derived macrophages. GSK3β elicits a common inhibitory effect on the Wnt/β-catenin pathway and is closely related to atherosclerosis. McAlpine and Werstuck (52) found that GSK3β expedites atherosclerotic lesions and foam-cell formation in Apoe−/− mice. Treatment of a GSK3β inhibitor can alleviate intracellular lipid accumulation (53). We consistently found that GSK3β impaired ABCA1-mediated cholesterol efflux and then promoted atherosclerosis, which depends on β-catenin. It has been reported that the GSK3β-dependent phosphorylation of β-catenin regulates the distribution of β-catenin between the nucleus and cytoplasm (23–26). Here, we further explored the phosphorylation sites of β-catenin and found that phosphorylation at Thr120 in β-catenin by GSK3β was essential for CXCL12-regulated TCF21 and ABCA1 expression. Moreover, Du et al. (54) reported that cytoplasmic β-catenin retention was influenced by phosphorylation at Thr120, consistent with our finding that CXCL12 increased cytoplasmic β-catenin accumulation.
Taken together, our findings reveal for the first time that CXCL12 accelerates atherosclerosis via the downregulation of ABCA1 expression and then the reduction of cholesterol efflux from macrophages. In this process, the binding of CXCL12 to CXCR4 is required for the activation of the GSK3β/β-cateninT120 signaling pathway and downregulation of TCF21 expression (Fig. 8D). Therefore, CXCL12 might be a promising target for protecting against atherosclerosis.
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CE
- cholesteryl ester
- CXCL12
- CXC chemokine ligand 12
- CXCR4
- CXC chemokine receptor 4
- EC
- endothelial cell
- GSK3β
- glycogen synthase kinase 3β
- LV
- lentiviral vector
- MPM
- mouse peritoneal macrophage
- RCT
- reverse cholesterol transport
- SMC
- vascular smooth muscle cell
- TC
- total cholesterol
- TCF
- transcription factor
- TCF21
- transcription factor 21
- TG
- triglyceride
This work was supported by Hunan Provincial Innovation Foundation for Postgraduate Grant CX20190750 and National Natural Science Foundation of China Grant 81770461. The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Yu X. H., Zhang D. W., Zheng X. L., and Tang C. K.. 2018. C1q tumor necrosis factor-related protein 9 in atherosclerosis: mechanistic insights and therapeutic potential. Atherosclerosis. 276: 109–116. [DOI] [PubMed] [Google Scholar]
- 2.Marques L. R., Diniz T. A., Antunes B. M., Rossi F. E., Caperuto E. C., Lira F. S., and Gonçalves D. C.. 2018. Reverse cholesterol transport: molecular mechanisms and the non-medical approach to enhance HDL cholesterol. Front. Physiol. 9: 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu X. H., Zhang D. W., Zheng X. L., and Tang C. K.. 2019. Cholesterol transport system: an integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 73: 65–91. [DOI] [PubMed] [Google Scholar]
- 4.Gao J. H., Zeng M. Y., Yu X. H., Zeng G. F., He L. H., Zheng X. L., Zhang D. W., Ouyang X. P., and Tang C. K.. 2018. Visceral adipose tissue-derived serine protease inhibitor accelerates cholesterol efflux by up-regulating ABCA1 expression via the NF-κB/miR-33a pathway in THP-1 macrophage-derived foam cells. Biochem. Biophys. Res. Commun. 500: 318–324. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M., Li L., Xie W., Wu J. F., Yao F., Tan Y. L., Xia X. D., Liu X. Y., Liu D., and Lan G.. 2016. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis. 248: 149–159. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M., Zhao G. J., Yin K., Xia X. D., Gong D., Zhao Z. W., Chen L. Y., Zheng X. L., Tang X. E., and Tang C. K.. 2018. Apolipoprotein A-1 binding protein inhibits inflammatory signaling pathways by binding to apolipoprotein A-1 in THP-1 macrophages. Circ. J. 82: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z. W., Zhang M., Chen L. Y., Gong D., Xia X. D., Yu X. H., Wang S. Q., Ou X., Dai X. Y., and Zheng X. L.. 2018. Heat shock protein 70 accelerates atherosclerosis by downregulating the expression of ABCA1 and ABCG1 through the JNK/Elk-1 pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 1863: 806–822. [DOI] [PubMed] [Google Scholar]
- 8.Mehta N. N., Li M., William D., Khera A. V., DerOhannessian S., Qu L., Ferguson J. F., McLaughlin C., Shaikh L. H., Shah R., et al. 2011. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur. Heart J. 32: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camnitz W., Burdick M. D., Strieter R. M., Mehrad B., and Keeley E. C.. 2012. Dose-dependent effect of statin therapy on circulating CXCL12 levels in patients with hyperlipidemia. Clin. Transl. Med. 1: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farouk S. S., Rader D. J., Reilly M. P., and Mehta N. N.. 2010. CXCL12: a new player in coronary disease identified through human genetics. Trends Cardiovasc. Med. 20: 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavakolian Ferdousie V., Mohammadi M., Hassanshahi G., Khorramdelazad H., Falahati-Pour S. K., Mirzaei M., Tavakoli M. A., Kamiab Z., Ahmadi Z., and Vazirinejad R.. 2017. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int. J. Cardiol. 233: 23–28. [DOI] [PubMed] [Google Scholar]
- 12.Schober A., Knarren S., Lietz M., Lin E. A., and Weber C.. 2003. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 108: 2491–2497. [DOI] [PubMed] [Google Scholar]
- 13.Gao J-H., Yu X-H., and Tang C-K.. 2019. CXC chemokine ligand 12 (CXCL12) in atherosclerosis: an underlying therapeutic target. Clin. Chim. Acta. 495: 538–544. [DOI] [PubMed] [Google Scholar]
- 14.Weiberg D., Thackeray J. T., Daum G., Sohns J. M., Kropf S., Wester H. J., Ross T. L., Bengel F. M., and Derlin T.. 2018. Clinical molecular imaging of chemokine receptor CXCR4 expression in atherosclerotic plaque using (68)Ga-pentixafor PET: correlation with cardiovascular risk factors and calcified plaque burden. J. Nucl. Med. 59: 266–272. [DOI] [PubMed] [Google Scholar]
- 15.Zernecke A., Schober A., Bot I., von Hundelshausen P., Liehn E. A., Mopps B., Mericskay M., Gierschik P., Biessen E. A., and Weber C.. 2005. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 96: 784–791. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z., Han Y., Li L., Lu H., Meng G., Li X., Shirhan M., Peh M. T., Xie L., Zhou S., et al. 2013. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(−/−) mice. Br. J. Pharmacol. 169: 1795–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merckelbach S., Epc V. D. V., Kallmayer M., Rischpler C., Burgkart R., Döring Y., J. G., Schwaiger M., Eckstein H. H., and Weber C.. 2018. Expression and cellular localization of CXCR4 and CXCL12 in human carotid atherosclerotic plaques. Thromb. Haemost. 118: 195–206. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee M., von Ungern-Sternberg S. N., Seizer P., Schlegel F., Buttcher M., Sindhu N. A., Muller S., Mack A., and Gawaz M.. 2015. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis. 6: e1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eldar-Finkelman H. 2002. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol. Med. 8: 126–132. [DOI] [PubMed] [Google Scholar]
- 20.McAlpine C. S., Bowes A. J., Khan M. I., Shi Y., and Werstuck G. H.. 2012. Endoplasmic reticulum stress and glycogen synthase kinase-3beta activation in apolipoprotein E-deficient mouse models of accelerated atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32: 82–91. [DOI] [PubMed] [Google Scholar]
- 21.Choi S. E., Jang H. J., Kang Y., Jung J. G., Han S. J., Kim H. J., Kim D. J., and Lee K. W.. 2010. Atherosclerosis induced by a high-fat diet is alleviated by lithium chloride via reduction of VCAM expression in Apoe-deficient mice. Vascul. Pharmacol. 53: 264–272. [DOI] [PubMed] [Google Scholar]
- 22.Valenta T., Hausmann G., and Basler K.. 2012. The many faces and functions of beta-catenin. EMBO J. 31: 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H., Li Y., Zhu H., Wang Q., Chen Z., Chen L., Zhu Y., Zheng C., Wang Y., Liao W., et al. Lansoprazole alleviates pressure overload-induced cardiac hypertrophy and heart failure in mice by blocking the activation of beta-catenin. Cardiovasc. Res. Epub ahead of print. January 24, 2019; doi:10.1093/cvr/cvz016. [DOI] [PubMed] [Google Scholar]
- 24.Xu B., Wang T., Xiao J., Dong W., Wen H. Z., Wang X., Qin Y., Cai N., Zhou Z., Xu J., et al. 2019. FCPR03, a novel phosphodiesterase 4 inhibitor, alleviates cerebral ischemia/reperfusion injury through activation of the AKT/GSK3beta/ beta-catenin signaling pathway. Biochem. Pharmacol. 163: 234–249. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Lin F., Fu Y., Chen W., Liu W., Chi J., Zhang X., and Yin X.. 2018. Cortistatin inhibits arterial calcification in rats via GSK3beta/beta-catenin and protein kinase C signalling but not c-Jun N-terminal kinase signalling. Acta Physiol. (Oxf.). 223: e13055. [DOI] [PubMed] [Google Scholar]
- 26.Sharma M., Chuang W. W., and Zijie S.. 2002. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 277: 30935–30941. [DOI] [PubMed] [Google Scholar]
- 27.Hidai H., Bardales R., Goodwin R., Quertermous T., and Quertermous E. E.. 1998. Cloning of capsulin, a basic helix-loop-helix factor expressed in progenitor cells of the pericardium and the coronary arteries. Mech. Dev. 73: 33–43. [DOI] [PubMed] [Google Scholar]
- 28.Lu X., Laiyuan W., Shufeng C., Lin H., Xueli Y., Yongyong S., Jing C., Liang Z., Charles G. C., and Jianfeng H.. 2012. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat. Genet. 44: 890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller C. L., Ulrike H., Roxanne D., Leeper N. J., Kundu R. K., Bhagat P., Assimes T. L., Kaiser F. J., Ljubica P., and Ulf H.. 2014. Coronary heart disease-associated variation in TCF21 disrupts a miR-224 binding site and miRNA-mediated regulation. PLoS Genet. 10: e1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty A., Tall A. R., Mjap D., Falk E., Fisher E. A., Garcíacardeña G., Lusis A. J., Rd O. A., Rosenfeld M. E., and Virmani R.. 2017. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 37: e131–e157. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M., Zhao G. J., Yao F., Xia X. D., Gong D., Zhao Z. W., Chen L. Y., Zheng X. L., Tang X. E., and Tang C. K.. 2018. AIBP reduces atherosclerosis by promoting reverse cholesterol transport and ameliorating inflammation in Apoe −/− mice. Atherosclerosis. 273: 122–130. [DOI] [PubMed] [Google Scholar]
- 32.Li B., He J., Lv H., Liu Y., Lv X., Zhang C., Zhu Y., and Ai D.. 2019. c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J. Clin. Invest. 129: 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta N. N., Matthews G. J., Krishnamoorthy P., Shah R., Mclaughlin C., Patel P., Budoff M., Chen J., Wolman M., and Go A.. 2014. Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur. Heart J. 35: 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghasemzadeh N., Hritani A. W., De S. C., Eapen D. J., Veledar E., Al K. H., Khayata M., Zafari A. M., Sperling L., and Hooper C.. 2015. Plasma stromal cell-derived factor 1伪/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis. 238: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M., Mei X., Herscovitz H., and Atkinson D.. 2019. N-terminal mutation of apoA-I and interaction with ABCA1 reveal mechanisms of nascent HDL biogenesis. J. Lipid Res. 60: 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Xu Y., Jadhav K., Zhu Y., Yin L., and Zhang Y.. 2019. Hepatic forkhead box protein A3 regulates ApoA-I (aolipoprotein A-I) expression, cholesterol efflux, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 39: 1574–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai C., Zhu H., Ning X., Li L., Yang B., Chen S., Wang L., Lu X., and Gu D.. 2019. LncRNA ENST00000602558.1 regulates ABCG1 expression and cholesterol efflux from vascular smooth muscle cells through a p65-dependent pathway. Atherosclerosis. 285: 31–39. [DOI] [PubMed] [Google Scholar]
- 38.Takata K., Honda S., Sidharta S. L., Duong M., Shishikura D., Kim S. W., Andrews J., Di Bartolo B. A., Psaltis P. J., Bursill C. A., et al. 2019. Associations of ABCG1-mediated cholesterol efflux capacity with coronary artery lipid content assessed by near-infrared spectroscopy. Cardiovasc. Diagn. Ther. 9: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamesch K., Subramanian P., Li X., Dembowsky K., Chevalier E., Weber C., and Schober A.. 2012. The CXCR4 antagonist POL5551 is equally effective as sirolimus in reducing neointima formation without impairing re-endothelialisation. Thromb. Haemost. 107: 356–368. [DOI] [PubMed] [Google Scholar]
- 40.Lewis G. F., and Rader D. J.. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 41.Döring Y., van der Vorst E. P. C., Duchene J., Jansen Y., Gencer S., Bidzhekov K., Atzler D., Santovito D., Rader D. J., Saleheen D., et al. 2019. CXCL12 derived from endothelial cells promotes atherosclerosis to drive coronary artery disease. Circulation. 139: 1338–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryma C. S., Ortega C., Dubland J. A., and Francis G. A.. 2019. Pathways of smooth muscle foam cell formation in atherosclerosis. Curr. Opin. Lipidol. 30: 117–124. [DOI] [PubMed] [Google Scholar]
- 43.Fujimaki T., Oguri M., Horibe H., Kato K., Matsuoka R., Abe S., Tokoro F., Arai M., Noda T., Watanabe S., et al. 2015. Association of a transcription factor 21 gene polymorphism with hypertension. Biomed. Rep. 3: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastami M., Ghaderian S. M., Omrani M. D., Mirfakhraie R., Vakili H., Parsa S. A., Nariman-Saleh-Fam Z., and Masotti A.. 2016. MiRNA-related polymorphisms in miR-146a and TCF21 are associated with increased susceptibility to coronary artery disease in an Iranian population. Genet. Test. Mol. Biomarkers. 20: 241–248. [DOI] [PubMed] [Google Scholar]
- 45.Miller C. L., Anderson D. R., Kundu R. K., Raiesdana A., Nurnberg S. T., Diaz R., Cheng K., Leeper N. J., Chen C. H., Chang I. S., et al. 2013. Disease-related growth factor and embryonic signaling pathways modulate an enhancer of TCF21 expression at the 6q23.2 coronary heart disease locus. PLoS Genet. 9: e1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nurnberg S. T., Cheng K., Raiesdana A., Kundu R., Miller C. L., Kim J. B., Arora K., Carcamo-Oribe I., Xiong Y., Tellakula N., et al. 2015. Coronary artery disease associated transcription factor TCF21 regulates smooth muscle precursor cells that contribute to the fibrous cap. Genom. Data. 5: 36–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer D., Zhao Q., Wirka R., Naravane A., Nguyen T., Liu B., Nagao M., Cheng P., Miller C. L., Kim J. B., et al. 2018. Coronary artery disease genes SMAD3 and TCF21 promote opposing interactive genetic programs that regulate smooth muscle cell differentiation and disease risk. PLoS Genet. 14: e1007681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Xiao Y., Mou Y., Zhao Y., Blankesteijn W. M., and Hall J. L.. 2002. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ. Res. 90: 340–347. [DOI] [PubMed] [Google Scholar]
- 49.Borrell-Pagès B. P., July Carolina R., and Lina B.. 2015. LRP5 deficiency down-regulates Wnt signalling and promotes aortic lipid infiltration in hypercholesterolaemic mice. J. Cell. Mol. Med. 19: 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huangfu N., Xu Z., Zheng W., Wang Y., Cheng J., and Chen X.. 2018. LncRNA MALAT1 regulates oxLDL-induced CD36 expression via activating beta-catenin. Biochem. Biophys. Res. Commun. 495: 2111–2117. [DOI] [PubMed] [Google Scholar]
- 51.Cheng S. L., Shao J. S., Halstead L. R., Distelhorst K., Sierra O., and Towler D. A.. 2010. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 107: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAlpine C. S., and Werstuck G. H.. 2014. Protein kinase R-like endoplasmic reticulum kinase and glycogen synthase kinase-3alpha/beta regulate foam cell formation. J. Lipid Res. 55: 2320–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang Y. S., Tsai C. T., Huangfu C. A., Huang W. Y., Lei H. Y., Lin C. F., Su I. J., Chang W. T., Wu P. H., Chen Y. T., et al. 2011. ACSL3 and GSK-3beta are essential for lipid upregulation induced by endoplasmic reticulum stress in liver cells. J. Cell. Biochem. 112: 881–893. [DOI] [PubMed] [Google Scholar]
- 54.Du C., Zhang C., Li Z., Biswas M. H., and Balaji K. C.. 2012. Beta-catenin phosphorylated at threonine 120 antagonizes generation of active beta-catenin by spatial localization in trans-Golgi network. PLoS One. 7: e33830. [DOI] [PMC free article] [PubMed] [Google Scholar]