Abstract
Niemann-Pick disease type C (NPC) is an autosomal recessive disorder characterized by progressive nervous degeneration. Because of the diversity of clinical symptoms and onset age, the diagnosis of this disease is difficult. Therefore, biomarker tests have attracted significant attention for earlier diagnostics. In this study, we developed a simultaneous analysis method for five urinary conjugated cholesterol metabolites, which are potential diagnostic biomarkers for a rapid, convenient, and noninvasive chemical diagnosis, using LC/MS/MS. By the method, their urinary concentrations were quantified and the NPC diagnostic performances were evaluated. The developed LC/MS/MS method showed high accuracy and satisfied all analytical method validation criteria. When the urine of healthy controls and patients with NPC was analyzed, three of five urinary conjugated cholesterol metabolite concentrations corrected by urinary creatinine were significantly higher in the patients with NPC. As a result of receiver operating characteristics analysis, these urinary metabolites might have excellent diagnostic marker performance. 3β-Sulfooxy-7β-hydroxy-5-cholenoic acid showed particularly excellent diagnostic performance with both 100% clinical sensitivity and specificity, suggesting that it is a useful NPC diagnostic marker. The urinary conjugated cholesterol metabolites exhibited high NPC diagnostic marker performance and could be used for NPC diagnosis.
Keywords: Niemann-Pick disease type C, urinary biomarkers, liquid chromatography/tandem mass spectrometry
Niemann-Pick disease type C (NPC) is a progressive and life-limiting autosomal recessive inherited disorder (1). The prevalence of this disease is approximately 1/100,000, and it is classified as a lysosomal disease. It is caused by mutations in the NPC intracellular cholesterol transporter 1 (NPC1) gene coding for membrane proteins or NPC intracellular cholesterol transporter 2 (NPC2) coding for secreted proteins (2, 3). Lack of these functional proteins, which work cooperatively with lysosomal free cholesterol efflux, causes excessive accumulation of free cholesterol and sphingolipids (4). However, the relationship between the characteristic lipid abnormalities and pathology of the disease remains unclear, as patients with NPC present a wide variety of clinical symptoms (5). The onset age of NPC ranges from neonatal to adult, and the symptoms are diverse and include systemic, visceral, nervous, and psychiatric abnormalities. Because the prognosis of patients with this disease is poor, it is important to diagnose NPC early and apply the treatment to maintain the quality of life of the patient (5). However, few trained specialists are available and the process leading to the discovery and diagnosis of NPC is complex. As conventional laboratory tests, the filipin test and genetic examination are considered to be the gold standards (5). However, both of these tests are complicated, so biomarker tests have attracted significant attention as a rapid screening method for NPC. Oxysterols are generated from the accumulated cholesterol in NPC cells and are present in higher concentrations in the plasma of the affected patients (6). The concentration of lysosphingomyelin, which is metabolized from sphingomyelin, is also elevated in the plasma of patients with NPC (7). Lysosphingomyelin-509 is a blood biomarker that has recently been used, but its precise structure remains unknown (8).
Following the previous report regarding urinary metabolites in patients with NPC by Alvelius et al. (9), we developed a noninvasive diagnostic method using urine analysis. First, we developed an analytical method for three multi-conjugated cholesterol metabolites, nonamidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid (SNAG-Δ5-CA) as well as its glycine and taurine conjugates [glycine-amidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid (SNAG-Δ5-CG) and taurine-amidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid (SNAG-Δ5-CT)], using LC/MS/MS (10). It was observed that the metabolites in the urine of two patients with NPC were much higher than those of the controls without NPC. Subsequently, we collected over 20 urine samples and preliminarily investigated their diagnostic performance, assuming that they might be useful for NPC screening (11). However, several patients with NPC had extremely low concentrations of the relevant metabolites and false-negatives. Thus, a comprehensive analysis method was used to search for other biomarker candidates (12), which yielded two strongly detected metabolite peaks in urine of patients with NPC, 3β-sulfooxy-7β-hydroxy-5-cholenoic acid (S7B-Δ5-CA) and 3β-sulfooxy-7-oxo-5-cholenoic acid (S7O-Δ5-CA) (13). In this study, we evaluated the NPC diagnostic marker performance of five urinary conjugated cholesterol metabolites. Therefore, we developed an LC/MS/MS method that could accurately and simultaneously analyze the urinary concentrations of the five conjugated cholesterol metabolites for each sample. The urinary conjugated cholesterol metabolites in all samples were quantified by the developed method, and their utility as NPC diagnostic markers was evaluated.
MATERIALS AND METHODS
Chemicals and reagents
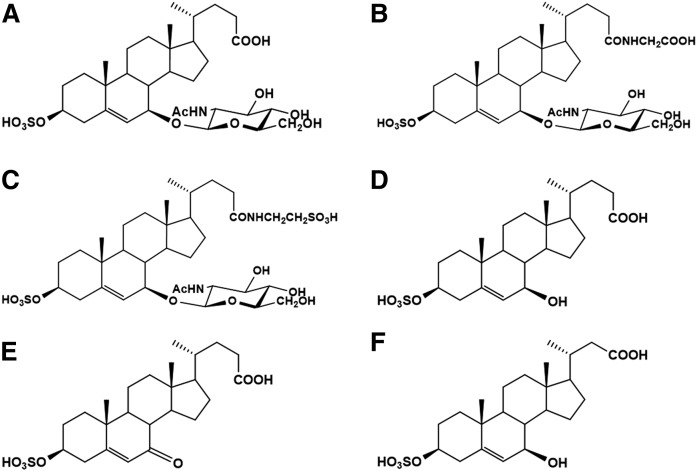
SNAG-Δ5-CA, SNAG-Δ5-CG, SNAG-Δ5-CT, S7B-Δ5-CA, S7O-Δ5-CA, and 3β-sulfooxy-7β-hydroxy-23-nor-5-cholenoic acid [as an internal standard (IS)] were synthesized as described in previous reports (the structures are shown in Fig. 1) (13–15). Ultrapure water was prepared with a PURELAB ultra apparatus (Organo Co. Ltd., Tokyo, Japan). All reagents (HPLC grade) were purchased from FUJIFILM Wako Pure Chemical Co. Ltd. (Osaka, Japan). The urine samples were collected in the morning, stored at −80°C, and analyzed within 1 month.
Fig. 1.
Chemical structures of analytes and IS. SNAG-Δ5-CA (A), SNAG-Δ5-CG (B), SNAG-Δ5-CT (C), S7B-Δ5-CA (D), S7O-Δ5-CA (E), and IS (F).
LC/MS/MS analysis
A Prominence model high-performance liquid chromatograph system (Shimadzu Co., Kyoto, Japan) was connected to a triple quadrupole tandem mass spectrometer (API 5000) equipped with an ESI probe (SCIEX, Framingham, MA). MS/MS was acquired in selected reaction monitoring (SRM) mode with negative ion detection. Ion spray voltage, turbo spray temperature, curtain gas, nebulizer gas, turbo gas, and collision gas were set at −4,500 V, 700°C, 20 psi, 50 psi, 50 psi, and 6 units, respectively. SRM conditions were set as listed in supplemental Table S1. The dwell and pause times were set to 160 and 5 ms. Data acquisition was performed using Analyst version 1.5.0 (SCIEX) and SCIEX OS-Q software (SCIEX) for data integration. With respect to the LC, a column-switching system was used (10–13, 16). After injection of the sample aliquot, a 20 mM ammonium acetate buffer (pH 5.5)/methanol (9:1, v/v) mixture was loaded on an OASIS HLB column (2.1 mm i.d. × 20 mm, 5 μm; Waters, Milford, MA). Pretreatment of the sample was performed at a flow rate of 1.0 ml/min for 3 min. After washing and concentrating the analytes, the sample eluent was loaded on a Capcell pak C18 BB-H column (2.1 mm i.d. × 150 mm, 3 μm; Osaka Soda, Osaka) by switching the valve used for changing the flow path. Mobile phase A [20 mM ammonium acetate buffer (pH 5.5)] and mobile phase B (methanol) were gradually changed from A:B = 65:35 to A:B = 45:55 over 50 min.
Preparation of the stock and working solutions
The analytes and IS were adjusted to a concentration of 100 μg/ml using water/ethanol (1:1, v/v, as stock solution). The IS was diluted with water/ethanol (1:1, v/v) to 33 ng/ml and used as the IS solution. The analytes were mixed and diluted with water/ethanol (1:1, v/v) to 0.3, 1, 3, 10, 30, 100, 300, and 1,000 ng/ml (working solutions for the calibration curve). For quality control (QC), mixed solutions of 2, 50, and 800 ng/ml were set as the low QC (LQC), middle QC (MQC), and high QC (HQC) (working solution for QC), respectively.
Calibration curve
A total of 50 μl of water was used as a surrogate matrix, and 50 μl of IS solution, 50 μl of working solution for the calibration curve, and 350 μl of water were added and mixed. The mixture was then centrifuged at 15,000 g at 4°C for 3 min, and 200 μl of the supernatant were injected for LC/MS/MS analysis. The peak area ratio of each analyte to IS was plotted against the standard concentration and the calibration curves were prepared using the least squares method with 1/x2 weighting.
Matrix effects
To determine matrix effects, 50 μl of the IS solution, 50 μl of water/ethanol (1:1, v/v) or QCM solution, and 350 μl of water were added to 50 μl of urine from a healthy control or water. After mixing and centrifugation, the supernatant was injected into the LC/MS/MS system. The matrix factor (MF) for each analyte was calculated using the following formula, and the ratio considering the MF of the IS was calculated as the IS-normalized MF (7).
Intra-assay and inter-assay reproducibility
To determine intra- and inter-assay reproducibility, 50 μl of QC solution (Blank, LQC, MQC, HQC), 50 μl of IS solution, and 350 μl of water were added to 50 μl of urine from a healthy control, and the specimens were analyzed using the procedure described above. Every 3 days, urine samples were prepared and analyzed for every Blank, LQC, MQC, and HQC (N = 6). Generally, the recovery (percent) was calculated by relative error [R.E. (%)]. However, because the analytes in this study were endogenous, it was calculated by adding the concentration contained in the healthy control urine (Blank).
Precision (percent) was calculated by relative standard deviation [R.S.D. (%)].
Stability test
For the stability test, 50 μl of QC solution (Blank, LQC, HQC) was dried under a nitrogen gas stream, and the urine of a healthy control was added and stored under various conditions including: 6 months at −80°C, 24 h at 4°C, 12 h at 25°C as room temperature, freeze-thaw cycles (repeated three times), and 48 h in an autosampler. Afterwards, analysis was performed using the same pretreatment as described above, and the ratio between the data immediately after preparation and the quantitative value was calculated as recovery (percent).
Dilution test
A mixture of standard solutions was added to 1.5 ml of healthy human urine to a final standard solution concentration of 645 ng/ml (dilute 1). Dilute 1 was further diluted 20-fold with water (dilute 2) and dilutes 1 and 2 were analyzed as described above. Dilution factor (percent) was calculated as follows.
Urine analysis
For analysis of the urine samples, 50 μl of urine from healthy subjects (N = 38) and patients with NPC (N = 28) were subjected to analysis. The data were processed using JMP Pro version 13.2.1 software (SAS Institute Inc.). Wilcoxon’s t-test and receiver operating characteristic (ROC) analysis were used for intergroup analysis and diagnostic performance tests. Urinary creatinine was analyzed with an enzymatic creatinine analysis kit (Serotec, Sapporo, Japan). The urinary concentrations of five metabolites were corrected with the urinary creatinine concentration.Urine samples were collected after obtaining informed consent from untreated patients diagnosed with NPC and healthy volunteers. This study abided by the Declaration of Helsinki principles and was performed according to the protocol approved by the Ethics Committee of the Graduate School of Medicine in Tohoku University (Approval number, 2013-1-293).
RESULTS AND DISCUSSION
Detection and separation of analytes with column-switching LC/ESI-MS/MS
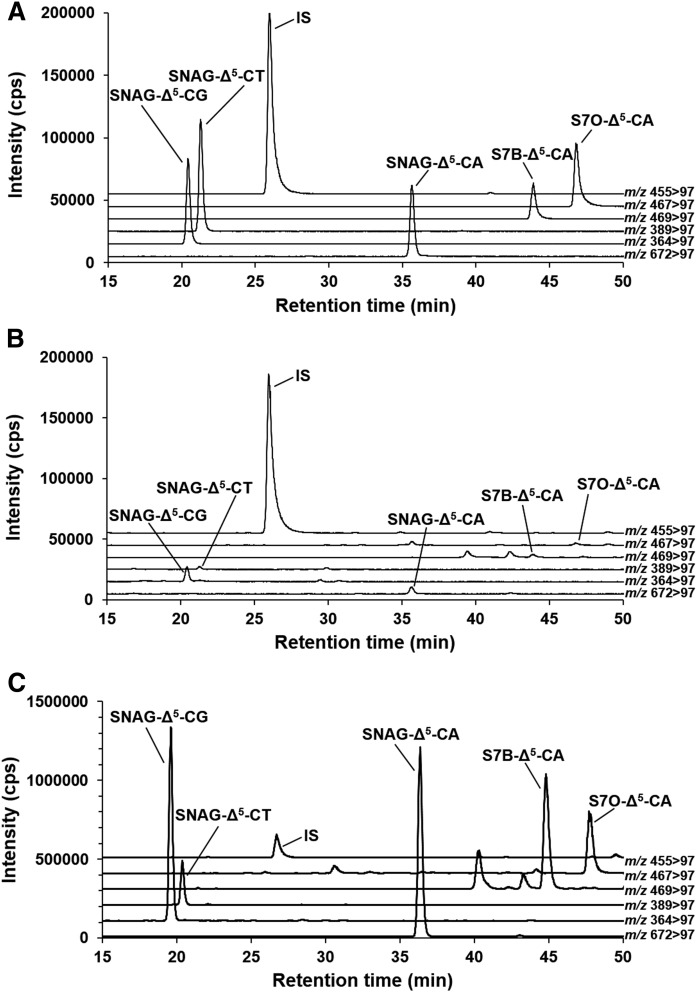
The analytes and IS, which are sulfate conjugates (Fig. 1), were detected with high sensitivity in negative ion mode (10–13). As a result of optimization, the SRM condition was set as listed in supplemental Table S1. A column-switching LC system, which was capable of large volume injection and online solid phase extraction, was used for the analysis (10–13, 16). Under this LC condition, the separation of all analytes and IS was achieved with sharp peak shapes (Fig. 2A). In addition, the peaks were separated from urinary contaminant peaks, which were detected constantly at the SRM transitions of m/z 469 > 97 and m/z 467 > 97 (Fig. 2B).
Fig. 2.
SRM chromatograms of analytes and IS. Standard mixture (30 ng/ml) (A), urine of a healthy control (B), and urine of a patient with NPC (C). All of the analytes and IS were separated from each other and completely separated from the contaminant peaks.
Calibration curves and matrix effects
In general bioanalysis, working solution-spiked sample matrices are used for preparing calibration curves. Because the analytes in this study were endogenous in urine, it was necessary to use a surrogate matrix. Therefore, we investigated the matrix effects for quantification of analytes. Procedures of sample preparation for calibration curves, QC samples, and urine samples are summarized in supplemental Table S2. We prepared calibration curves using water as a surrogate matrix, and all the calibration curves showed high linearity over a wide range from 0.3 to 1,000 ng/ml (supplemental Table S3A). Next, the matrix effects were investigated. The matrix effect is usually calculated by the ratio of peak intensity of the standard solution spiked in a pretreated matrix to that of the neat standard solution (17). However, the analytical system used herein features an online solid phase extraction, so we could not evaluate the typical method (17). Therefore, it was evaluated using MF, which is the parameter combining the pretreatment extraction efficiency and matrix effects from biological contaminants (7). As a result, the MFs of all analytes and IS were 101–105% (supplemental Table S3B). The IS-normalized MFs of all analytes were nearly 100%, and it was found that the analytes could be quantified without considering the matrix effect.
Reproducibility test
The method reproducibility was investigated using QC samples. Accuracy was evaluated by subtracting the concentration in the healthy control urine as Blank. The accuracy of the inter- and intra-day assays was within 100 ± 10% for all QC samples and their precision (percent) was within 10% (Table 1).
TABLE 1.
Analytical validation data, intra-day and inter-day assay
| Number | Compound | Recovery (%) | Accuracy (%) | |||||
| Blank | LQC | MQC | HQC | LQC | MQC | HQC | ||
| Intra-day assay (N = 6) | ||||||||
| 1 | SNAG-Δ5-CA | 4.69 | 2.53 | 2.59 | 2.20 | 3.64 | −6.01 | −6.73 |
| 2 | SNAG-Δ5-CG | 3.07 | 4.36 | 3.19 | 2.87 | 2.56 | −6.63 | −7.99 |
| 3 | SNAG-Δ5-CT | 3.07 | 6.68 | 2.39 | 2.54 | 4.21 | −4.06 | −3.40 |
| 4 | S7B-Δ5-CA | 3.69 | 2.12 | 1.86 | 3.96 | −4.94 | −6.49 | −10.23 |
| 5 | S7O-Δ5-CA | 7.48 | 1.54 | 2.13 | 4.28 | 5.73 | 6.10 | 3.17 |
| Inter-day assay (N = 6) | ||||||||
| 1 | SNAG-Δ5-CA | 4.27 | 5.50 | 2.95 | 2.25 | −0.94 | −3.60 | −4.64 |
| 2 | SNAG-Δ5-CG | 2.43 | 4.29 | 3.50 | 2.03 | −0.81 | −4.25 | −7.35 |
| 3 | SNAG-Δ5-CT | 3.11 | 6.15 | 1.69 | 1.82 | 0.100 | −3.92 | −4.26 |
| 4 | S7B-Δ5-CA | 8.31 | 6.79 | 4.57 | 3.00 | −5.79 | −2.46 | −8.75 |
| 5 | S7O-Δ5-CA | 5.76 | 4.11 | 3.55 | 4.25 | 3.06 | 9.76 | 7.67 |
LQC, 2 ng/ml; MQC, 50 ng/ml; and HQC, 800 ng/ml.
Stability test
The QC solution-spiked urine samples were stored under various conditions and the analytes were subsequently quantified. All analytes could be stably stored under all conditions tested and could be quantified even for the long-term-preserved specimens (Table 2).
TABLE 2.
Analytical validation data, stability and dilution test
| Number | Compound | Freeze and Thaw | −80°C for 6 Months | 4°C for 24 h | 24°C for 12 h | Autosampler for 48 h | Dilution (10 μg/ml) | |||||
| LQC | HQC | LQC | HQC | LQC | HQC | LQC | HQC | LQC | HQC | |||
| 1 | SNAG-Δ5-CA | 99.9 ± 3.75 | 104 ± 0.687 | 95.3 ± 4.58 | 95.0 ± 2.64 | 99.1 ± 3.19 | 97.1 ± 0.227 | 95.5 ± 3.23 | 95.8 ± 2.16 | 92.2 ± 2.80 | 93.7 ± 0.797 | 109 ± 0.759 |
| 2 | SNAG-Δ5-CG | 97.9 ± 1.17 | 99.1 ± 0.642 | 110 ± 3.76 | 97.6 ± 1.88 | 101 ± 3.84 | 97.2 ± 0.931 | 98.4 ± 2.36 | 95.9 ± 1.15 | 99.7 ± 2.21 | 94.4 ± 0.844 | 109 ± 0.976 |
| 3 | SNAG-Δ5-CT | 98.1 ± 2.34 | 101 ± 1.08 | 96.8 ± 2.13 | 98.5 ± 1.66 | 97.7 ± 4.48 | 97.2 ± 1.97 | 95.6 ± 2.17 | 94.6 ± 0.399 | 94.7 ± 2.38 | 96.3 ± 2.18 | 107 ± 1.81 |
| 4 | S7B-Δ5-CA | 98.6 ± 2.47 | 97.4 ± 1.18 | 98.9 ± 5.89 | 103 ± 1.46 | 96.8 ± 1.34 | 92.1 ± 0.347 | 94.4 ± 7.45 | 93.8 ± 2.30 | 101 ± 3.09 | 106 ± 1.09 | 104 ± 1.11 |
| 5 | S7O-Δ5-CA | 96.8 ± 4.99 | 104 ± 1.64 | 99.9 ± 3.05 | 104 ± 0.168 | 102 ± 6.30 | 93.4 ± 1.65 | 95.3 ± 2.92 | 96.9 ± 3.36 | 103 ± 1.43 | 109 ± 1.40 | 102 ± 1.76 |
Recovery (%, mean ± SD). LQC, 2 ng/ml; MQC, 50 ng/ml; HQC, 800 ng/ml.
Dilution test
When the upper limit of the calibration curve was exceeded, it became necessary to dilute with the matrix and remeasure the sample using general bioanalytical techniques. Because endogenous analytes of this study are included in urine, water was used as a surrogate matrix. The influence on the quantitative value was investigated and it was found that 20-fold dilution of the urine sample by water did not affect the quantitative results (Table 2).
Analysis of five urinary cholesterol metabolites in healthy controls and patients with NPC
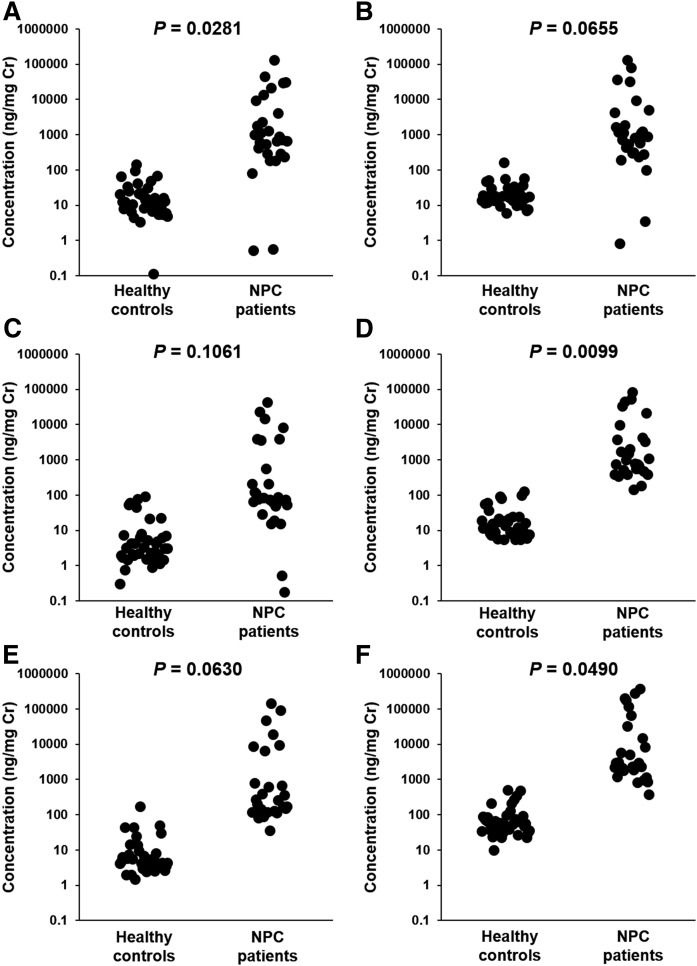
Subsequently, all urine samples from the healthy controls and patients with NPC were analyzed. A total of 66 specimens were collected from patients with NPC and healthy controls, and their demographics are listed in supplemental Table S4. The age of the groups did not differ between healthy controls (0.33–47 years) and patients with NPC (0.0274–48 years; P = 0.1739), but a larger proportion of females were recruited in the NPC patient group (P = 0.0179). A typical SRM chromatogram of patients with NPC is shown in Fig. 2C. The data are summarized in both creatinine-corrected concentrations, which are often used for biochemical examinations (Fig. 3, supplemental Table S5), and uncorrected concentrations (supplemental Fig. S1, supplemental Table S6). All metabolites were significantly higher in patients with NPC in terms of creatinine-corrected concentrations and uncorrected concentrations other than SNAG-Δ5-CT (Fig. 3, supplemental Fig. S1). The correlations between each of the metabolites were investigated and observed to generally correlate (supplemental Fig. S2). On the other hand, the correlation for S7B-Δ5-CA and other metabolites was slightly lower than other combinations. Similar to the reports of Mazzacuva et al. (18) and Jiang et al. (19), we speculate that the analytes in this study were produced via oxysterols. It was also assumed that S7O-Δ5-CA is metabolized from 7-ketocholesterol, and SNAG-Δ5-CA, SNAG-Δ5-CG, SNAG-Δ5-CT, and S7B-Δ5-CA are produced from 7β-hydroxycholesterol. The sequence of cleavage of the side chain, conjugation with sulfuric acid, amino acid, and N-acetylglucosamine (GlcNAc) remains unknown. Because SNAG-Δ5-CA, SNAG-Δ5-CG, and SNAG-Δ5-CT showed high correlations, it is expected that they are produced via similar metabolic pathways. In contrast, S7B-Δ5-CA and S7O-Δ5-CA may pass through a slightly different route. In addition, S7B-Δ5-CA did not overlap at all between the samples from the patients with NPC and healthy controls in any cases tested. In our previous studies (11) and the report by Mazzacuva et al. (18), several cases where metabolites bearing the 7β-GlcNAc group were present in extremely low concentration were observed due to mutation of the UGT3A1 gene, which codes for UDP glucosyltransferase 3A1 as a GlcNAc conjugation enzyme (20). In this study, the concentrations of the metabolites of SNAG-Δ5-CA, SNAG-Δ5-CG, and SNAG-Δ5-CT were very low in the urine of patients with NPC numbers 10 and 17. Conversely, the concentration of S7B-Δ5-CA, which does not contain a GlcNAc group, in NPC samples was higher than those of healthy controls, and it is likely that the discrimination between patients with NPC from other subjects by urinary S7B-Δ5-CA concentration may be possible. Similarly, S7O-Δ5-CA does not contain a GlcNAc group, but some overlap was observed between the concentrations present in the urine samples of the patients with NPC and healthy subjects. The results suggested that analysis of urinary S7B-Δ5-CA may prevent overlooking of patients with NPC with false negative results based on abnormally low concentrations due to the UGT3A1 mutation (18, 20). Because the concentrations of urinary cholesterol metabolites were generally higher than plasma oxysterols (Fig. 3, supplemental Table S7, Refs. 6, 21), these metabolites act as an excretion pathway of excessive accumulated cholesterol due to metabolic abnormalities similar to other cholesterol metabolic disorder diseases (22–26).
Fig. 3.
The creatinine-corrected concentrations of SNAG-Δ5-CA (A), SNAG-Δ5-CG (B), SNAG-Δ5-CT (C), S7B-Δ5-CA (D), S7O-Δ5-CA (E), and their total concentration (F) in the urine of healthy controls and patients with NPC. SNAG-Δ5-CA, S7B-Δ5-CA, and their total concentration in the urine of patients with NPC were significantly higher than those observed in healthy controls.
Diagnostic performance of the urinary NPC biomarker candidates.
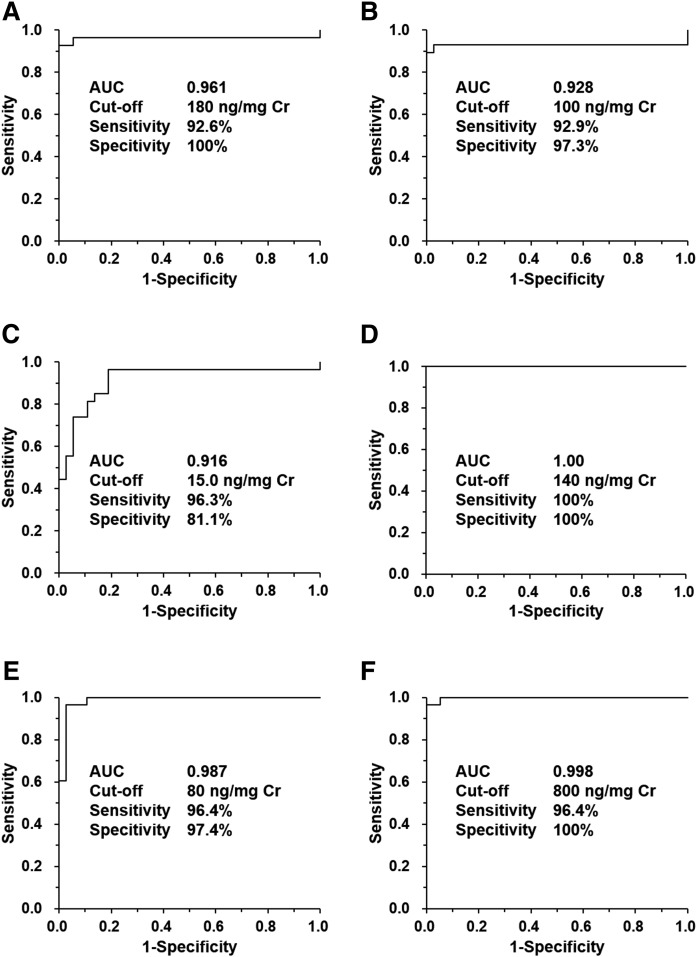
Finally, the NPC diagnostic performance of each urinary cholesterol metabolite was evaluated using ROC analysis (Fig. 4). This study investigated the biomarkers for a rare lysosomal disease, NPC, and we experienced difficulty collecting urine specimens and collected a total of 66 specimens. This limited sample size is not ideal, but the sample number in this study exceeded the threshold that could yield significant differences as a result of power analysis (data not shown). Accordingly, the analytical results were subjected to statistical analysis and the area under the curve (AUC) value exceeded 0.92 for each metabolite. In particular, because S7B-Δ5-CA exhibited no overlap between NPC and control patients, the AUC value of the metabolite was 1.0. The cut-off concentration was set to the concentration with the highest value of sensitivity-(1-specificity), which is representative of the highest true positive rate and lowest false negative rate. The sensitivity was 92.6–100% and specificity was 81.1–100%, but S7B-Δ5-CA showed 100% for both parameters. These results were nearly equivalent to the plasma oxysterols (6) and their metabolites (18, 19). Therefore, the metabolites investigated herein represent a series of metabolites produced from cholesterol accumulated by NPC pathology (18, 19). These results also suggest that urinary metabolites are a series of metabolites generated from cholesterol accumulation in an NPC-dependent manner (6, 18, 19). In addition, some patients with other lysosomal diseases and cholesterol metabolic disorders provided almost low concentrations (supplemental Tables S5, S6). Thus, it is suggested that these urinary metabolites can serve as useful NPC diagnostic biomarkers, reflecting the pathology of NPC.
Fig. 4.
ROC analysis results of the urinary concentration of SNAG-Δ5-CA (A), SNAG-Δ5-CG (B), SNAG-Δ5-CT (C), S7B-Δ5-CA (D), S7O-Δ5-CA (E), and their total concentration (F). AUC, cut-off concentration, sensitivity, and specificity are also shown. The AUC values ranged between 0.916 and 1.0. The sensitivities were 92.6–100% and the specificities were 81.1–100%. The cut-off concentrations ranged from 15 to 800 ng/mg creatinine (Cr) and S7B-Δ5-CA showed clear-cut diagnostic performance.
CONCLUSIONS
A simultaneous analytical method for five urinary conjugated cholesterol metabolites identified from the urine of patients with NPC was developed using LC/MS/MS. The performance of the five metabolites as NPC diagnostic biomarkers was also evaluated. First, we developed a reliable analytical method using column-switching LC/MS/MS, and then five NPC diagnostic biomarker candidates in urine were quantified. All five metabolites were generally present in higher concentrations in the urine of patients with NPC compared with that of healthy controls and showed excellent diagnostic marker performance. It was observed that the conjugated cholesterol metabolites are useful as diagnostic markers of NPC. In particular, S7B-Δ5-CA is a valuable biomarker, exhibiting both 100% sensitivity and specificity. In the future, it is expected that these five urinary cholesterol metabolites (S7B-Δ5-CA in particular) will be used for a noninvasive diagnostic screening method for NPC.
Supplementary Material
Acknowledgments
The authors are grateful to all donors who provided their valuable urine samples. The authors thank Editage by Cactus Commununiations Co., Ltd. (Tokyo) for English language editing.
Footnotes
Abbreviations:
- AUC
- area under the curve
- GlcNAc
- N-acetylglucosamine
- HQC
- high quality control
- IS
- internal standard (3β-sulfooxy-7β-hydroxy-23-nor-5-cholenoic acid)
- LQC
- low quality control
- MF
- matrix factor
- MQC
- middle quality control
- NPC
- Niemann-Pick disease type C
- QC
- quality control
- ROC
- receiver operating characteristic
- S7B-Δ5-CA
- 3β-sulfooxy-7β-hydroxy-5-cholenoic acid
- SNAG-Δ5-CA
- nonamidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid
- SNAG-Δ5-CG
- glycine-amidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid
- SNAG-Δ5-CT
- taurine-amidated 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholenoic acid
- S7O-Δ5-CA
- 3β-sulfooxy-7-oxo-5-cholenoic acid
- SRM
- selected reaction monitoring
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grants 16K20900 and 18K15699.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Vanier M. T. 2010. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carstea E. D., Polymeropoulos M. H., Parker C. C., Detera-Wadleigh S. D., O’Neill R. R., Patterson M. C., Goldin E., Xiao H., Straub R. E., Vanier M. T., et al. 1993. Linkage of Niemann-Pick disease type C to human chromosome 18. Proc. Natl. Acad. Sci. USA. 90: 2002–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg S. J., Ward C. P., and Fensom A. H.. 1994. Complementation studies in Niemann-Pick disease type C indicate the existence of a second group. J. Med. Genet. 31: 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon H. J., Abi-Mosleh L., Wang M. L., Deisenhofer J., Goldstein J. L., Brown M. S., and Infante R. E.. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geberhiwot T., Moro A., Dardis A., Ramaswami U., Sirrs S., Marfa M. P., Vanier M. T., Walterfang M., Bolton S., Dawson C., et al. 2018. Consensus clinical management guidelines for Niemann-Pick disease type C. Orphanet J. Rare Dis. 13: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welford R. W., Garzotti M., Marques L. C., Mengel E., Marquardt T., Reunert J., Amraoui Y., Kolb S. A., Morand O., and Groenen P.. 2014. Plasma lysosphingomyelin demonstrates great potential as a diagnostic biomarker for Niemann-Pick disease type C in a retrospective study. PLoS One. 9: e114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giese A. K., Mascher H., Grittner U., Eichler S., Kramp G., Lukas J., te Vruchte D., Eisa N. A., Cortina-Borja M., Porter F. D., et al. 2015. A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet J. Rare Dis. 10: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvelius G., Hjalmarson O., Griffiths W. J., Björkhem I., and Sjövall J.. 2001. Identification of unusual 7-oxygenated bile acid sulfates in a patient with Niemann-Pick disease, type C. J. Lipid Res. 42: 1571–1577. [PubMed] [Google Scholar]
- 10.Maekawa M., Misawa Y., Sotoura A., Yamaguchi H., Togawa M., Ohno K., Nittono H., Kakiyama G., Iida T., Hofmann A. F., et al. 2013. LC/ESI-MS/MS analysis of urinary 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholen-24-oic acid and its amides: new biomarkers for the detection of Niemann-Pick type C disease. Steroids. 78: 967–972. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa M., Narita A., Jinnoh I., Iida T., Marquardt T., Mengel E., Eto Y., Clayton P. T., Yamaguchi H., and Mano N.. 2019. Diagnostic performance evaluation of sulfate-conjugated cholesterol metabolites as urinary biomarkers of Niemann-Pick disease type C. Clin. Chim. Acta. 494: 58–63. [DOI] [PubMed] [Google Scholar]
- 12.Maekawa M., Shimada M., Ohno K., Togawa M., Nittono H., Iida T., Hofmann A. F., Goto J., Yamaguchi H., and Mano N.. 2015. Focused metabolomics using liquid chromatography/electrospray ionization tandem mass spectrometry for analysis of urinary conjugated cholesterol metabolites from patients with Niemann-Pick disease type C and 3β-hydroxysteroid dehydrogenase deficiency. Ann. Clin. Biochem. 52: 576–587. [DOI] [PubMed] [Google Scholar]
- 13.Maekawa M., Omura K., Sekiguchi S., Iida T., Saigusa D., Yamaguchi H., and Mano N.. 2016. Identification of two sulfated cholesterol metabolites found in the urine of a patient with Niemann-Pick disease type C as novel candidate diagnostic markers. Mass Spectrom. (Tokyo). 5: S0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iida T., Kakiyama G., Hibiya Y., Miyata S., Inoue T., Ohno K., Goto T., Mano N., Goto J., Nambara T., et al. 2006. Chemical synthesis of the 3-sulfooxy-7-N-acetylglucosaminyl-24-amidated conjugates of 3β,7β-dihydroxy-5-cholen-24-oic acid, and related compounds: unusual, major metabolites of bile acid in a patient with Niemann-Pick disease type C1. Steroids. 71: 18–29. [DOI] [PubMed] [Google Scholar]
- 15.Kakiyama G., Muto A., Shimada M., Mano N., Goto J., Hofmann A. F., and Iida T.. 2009. Chemical synthesis of 3β-sulfooxy-7β-hydroxy-24-nor-5-cholenoic acid: an internal standard for mass spectrometric analysis of the abnormal Δ5-bile acids occurring in Niemann-Pick disease. Steroids. 74: 766–772. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa M., Mori M., Fujiyoshi M., Suzuki H., Yanai K., Noda A., Tanaka M., Takasaki S., Kikuchi M., Akasaka K., et al. 2018. A direct injection LC/ESI-MS/MS analysis of urinary cyclophosphamide as an anticancer drug for monitoring occupational exposure. Chromatography (Basel). 39: 41–47. [Google Scholar]
- 17.Matuszewski B. K., Constanzer M. L., and Chavez-Eng C. M.. 2003. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 75: 3019–3030. [DOI] [PubMed] [Google Scholar]
- 18.Mazzacuva F., Mills P., Mills K., Camuzeaux S., Gissen P., Nicoli E. R., Wassif C., te Vruchte D., Porter F. D., Maekawa M., et al. 2016. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 590: 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X., Sidhu R., Mydock-McGrane L., Hsu F. F., Covey D. F., Scherrer D. E., Earley B., Gale S. E., Farhat N. Y., Porter F. D., et al. 2016. Development of a bile acid-based newborn screen for Niemann-Pick disease type C. Sci. Transl. Med. 8: 337ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie P. I., Rogers A., Treloar J., Jorgensen B. R., Miners J. O., and Meech R.. 2008. Identification of UDP glycosyltransferase 3A1 as a UDP N-acetylglucosaminyltransferase. J. Biol. Chem. 283: 36205–36210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X., Sidhu R., Porter F. D., Yanjanin N. M., Speak A. O., te Vruchte D. T., Platt F. M., Fujiwara H., Scherrer D. E., Zhang J., et al. 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res. 52: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton P. T., Leonard J. V., Lawson A. M., Setchell K. D. R., Andersson S., Egestad B., and Sjövall J.. 1987. Familial giant cell hepatitis associated with synthesis of 3β, 7α-dihydroxy-and 3β, 7α, 12α-trihydroxy-5-cholenoic acids. J. Clin. Invest. 79: 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setchell K. D. R., Suchy F. J., Welsh M. B., Zimmer-Nechemias L., Heubi J., and Balistreri W. F.. 1988. Δ4-3-oxosteroid-5β-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J. Clin. Invest. 82: 2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton P. T., Casteels M., Mieli-Vergani G., and Lawson A. M.. 1995. Familial giant cell hepatitis with low bile acid concentrations and increased urinary excretion of specific bile alcohols: a new inborn error of bile acid synthesis? Pediatr. Res. 37: 424–431. [DOI] [PubMed] [Google Scholar]
- 25.Setchell K. D., Schwarz M., O’Connell N. C., Lund E. G., Davis D. L., Lathe R., Thompson H. R., Tyson R. W., Sokol R. J., and Russell D. W.. 1998. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Invest. 102: 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton P. T. 2011. Disorders of bile acid synthesis. J. Inherit. Metab. Dis. 34: 593–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.