Abstract
The role of genetics in cognitive remediation therapies in schizophrenia has not been completely understood yet. Different genes involved in neurotrophic, dopaminergic and serotonin systems have reported to influence cognitive functioning in schizophrenia. These genetic factors could also be contributing to the variability in responsiveness to cognitive treatments. No comprehensive synthesis of the literature of the role of genetics in the context of cognitive remediation has been conducted until now. We aimed to systematically review the published works through three electronic database searches: PubMed, Scopus, and the Cochrane Library. Eligible studies revealed a rising interest in the field although the number of published studies was rather small (n = 10). Eventually, promising results showing a relationship between some phenotypic variations based on different polymorphisms and different levels of responsivity to cognitive remediation therapies have been described although results are still inconclusive. In case those findings will be replicated, they could be guiding future research and informing clinical decision-making in the next future.
Keywords: Cognitive remediation, Genetics, COMT, BDNF, EAAT2, 5-HT1A-R
1. Introduction
Cognitive remediation in schizophrenia has proved to be an evidence-based approach as confirmed in different meta-analytic studies (McGurk et al., 2007; Grynszpan et al., 2011; Wykes et al., 2011; Revell et al., 2015). However, effect sizes over cognition and functioning have been reported to be only modest. Besides, in some studies showing positive results it was found a percentage of participants who do not benefit significantly from the treatment (Medalia and Richardson, 2005; Penadés et al., 2006; Bryce et al., 2018). Thus, the analysis of putative moderators or mediators that could influence individual response seems pertinent.
Searching for biomarkers in schizophrenia is an important line of research to detect putative moderators or mediators in cognitive response (Penadés et al., 2015). Recently, a growing number of studies have pointed to a number of candidate genes and proteins that could become excellent biomarkers (Chana et al., 2013). Unfortunately, biomarkers are still waiting for validation but research on genetic candidates is increasingly growing and they have a potentially big value to define endophenotypes in schizophrenia. Recent meta-analyses based on Genome-Wide Association Studies (GWAS) data for schizophrenia demonstrate that polygenic risk scores are associated with decreased cognitive abilities even in nonclinical cohorts (Smeland et al., 2017; Ohi et al., 2018). Moreover, most of the loci found to be shared between schizophrenia and cognitive traits confirmed a negative correlation between risk of schizophrenia and cognitive performance (Davies et al., 2018). Specifically, some genes have been identified with some influence on cognitive functioning in schizophrenia (Zai et al., 2017).
These genes are involved in different systems, particularly in the neurotrophic, dopaminergic, glutamatergic and serotoninergic systems. Genetic factors could influence not only cognition but they could also contribute to the response variability to cognitive treatments (O'Tuathaigh et al., 2017).
However, to date no comprehensive synthesis of the literature concerning the role of genetics in the field of cognitive remediation has been conducted yet. We aim to make available a review that could be used to guide future research and to eventually inform clinical decision-making. The main target of the current paper is to systematically review the published works testing genetic variables in the context of cognitive remediation in schizophrenia.
2. Methods
Studies were identified through three electronic database searches: PubMed, Scopus, and the Cochrane Library. Search terms were “cognitive training” OR “cognitive remediation” OR “cognitive rehabilitation” OR “cognitive enhancement” AND “schizophrenia” AND “genetics” OR “gene” OR “CNV” OR “COMT” OR “BDNF” OR “GWAS”. Eligibility was implemented independently in blinded conditions by two of the authors: RP and RC. Screening was initially focused on abstracts and later full articles were revised. Results from the search were organized in a table (Table 1). Disagreements were resolved by discussion with a third party, the author CG.
Table 1.
Studies on genetics in the context of cognitive remediation therapies in schizophrenia.
| Sample | Intervention/approach | Gene | Variants | Comparisons | Results | Effect size | CTAM index | |
|---|---|---|---|---|---|---|---|---|
| Bosia et al. (2007) | SCH = 50 (CRT: 27/SRT: 23) |
Cogpack Software Drill-and-practice |
COMT | rs4680 | Baseline/post-treatment differences in cognition between Val/Val and Met-carriers | Greater improvement of executive functions and quality of life in Met carriers | Met carriers EF = 0.7 QoL = 0.50 |
57 |
| Greenwood et al. (2011) | SCH = 87 | Cognitive Remediation Therapy Strategy-based |
COMT | rs4680 | Baseline/post-treatment differences in cognition between the three genotype groups (Met/Met, Val/Met, Val/Val) | No effect of COMT genotype on CRT improvement | No effect | 77 |
| Panizzutti et al. (2013) | SCH = 48 | Computerized cognitive training exercises Drill and practice |
COMT | rs165599, rs9265, rs5993891, rs758373, rs2239395, rs2240713, rs739368, rs1544325 | Association between variation in the COMT gene and global cognition improvement | Cumulative effect of COMT SNPs on cognitive improvement | N.A. | 77 |
| Mak et al. (2013) | SCH = 41 HC = 40 |
RehaCom software Drill and practice |
COMT BDNF |
rs4680 rs6265 |
Baseline/post-treatment differences in cognition between Val/Val and Met-carriers | No significant effects | No effect | 40 |
| Bosia et al., 2014a, Bosia et al., 2014b | SCH = 86 |
Cogpack Software Drill-and-practice |
COMT 5-HT1A-R |
rs4680 rs6295 |
A general linear model analysis, with COMT and 5-HT1A-R genotypes as categorical predictors | COMT Val/Val plus 5-HT1A G/G: lower improvement of EF. | COMT Met Carriers plus 5-HT1A G/G group EF = 0.9 |
48 |
| Bosia et al., 2014a, Bosia et al., 2014b | SCH = 98 |
Cogpack Software Drill-and-practice |
COMT | rs4680 | Baseline/post-treatment differences in cognition between Val/Val and Met-carriers | Met carriers: greater improvement in processing speed in patients treated with D2 blockers | Met Carriers PS = 0.44 |
48 |
| Burton et al. (2015) | SCH = 41 | Compensatory Cognitive Training Strategy-based |
COMT | rs4680 | COMT genotype as a predictor of response | No effect of COMT genotype on cognitive improvement | No effect | 40 |
| Lindenmayer et al. (2015) | SCH = 145 |
Cogpack Software Drill and practice |
COMT | rs4680 | Baseline/post-treatment differences in cognition between the three genotype groups (Met/Met, Val/Met, Val/Val) | Met carriers: greater improvements in multiple cognitive domains | Met/Met PS = 0.310 A/V = 0.021 WM = 0.028 VL = 0.183 VIL = 0.314 R-PS = 0.029 GCI = 0.024 |
56 |
| Penadés et al. (2017) | SCH = 70 (CRT: 35/SST: 35) HC: 15 |
Cognitive Remediation Therapy Strategy-based |
BDNF | rs6265 | Differences in BDNF-serum levels between Val/Val and Met-carriers after CRT | Val/Val: higher BDNF serum levels after cognitive treatment Met-carriers: no changes |
Week-4 = 0.54 After CRT = 0.64 |
70 |
| Spangaro et al. (2018) | SCH = 88 |
Cogpack Software Drill-and-practice |
SLC1A2 | (EAAT2) rs4354668 | Differences between T/T homozygote and carriers of the G allele | T/T: greater Working Memory improvements after cognitive treatment Interaction between genotype and therapy on EF outcome |
T/T WM = 0.69 (3 months); 0.64 (6 months) T/T not treated with clozapine EF = 0.68 |
49 |
CTAM: Clinical Trials Assessment Measure; SCH: Schizophrenia; HC: Healthy controls; CRT: Cognitive Remediation Therapy; SST: Social Skills Training; SRT: Standard Rehabilitation Treatment; COMT: Catechol-O-methyltransferase; BDNF: Brain derived neurotrophic factor, SLC1A2: Excitatory amino acid transporter 2; 5-HT1A-R: 5-hydroxytryptamine receptor. EATT2: Excitatory amino acid transporter 2; PS: Psychomotor Speed; A/V: Attention/vigilance; VL: Verbal Learning; VIL: Visual Learning; R-PS: Reasoning and Problem Solving; EF: Executive functions; GCI: Global Composite Index; QoL: Quality of Life.
As the study of genetic variables in the context of cognitive remediation is nearly always exploratory and does not appear directly in the titles and abstracts of the articles, we also hand searched for articles included in the recent meta-analyses (McGurk et al., 2007; Grynszpan et al., 2011; Wykes et al., 2011; Revell et al., 2015). Additionally, authors manually search articles in all the aforementioned databases following the search terms “cognitive training” OR “cognitive remediation” OR “cognitive rehabilitation” OR “cognitive enhancement” AND “schizophrenia” until the date of 29/06/2018.
Inclusion criteria were: a) Participants with a diagnosis of schizophrenia or schizoaffective disorder; b) Use of a cognitive remediation approach as defined in Wykes et al. (2011); c) Analysis of at least one gene as a predictor or mediator of outcome; d) Peer reviewed work being a randomized controlled trial, case-referent study or a single arm trial.
3. Results
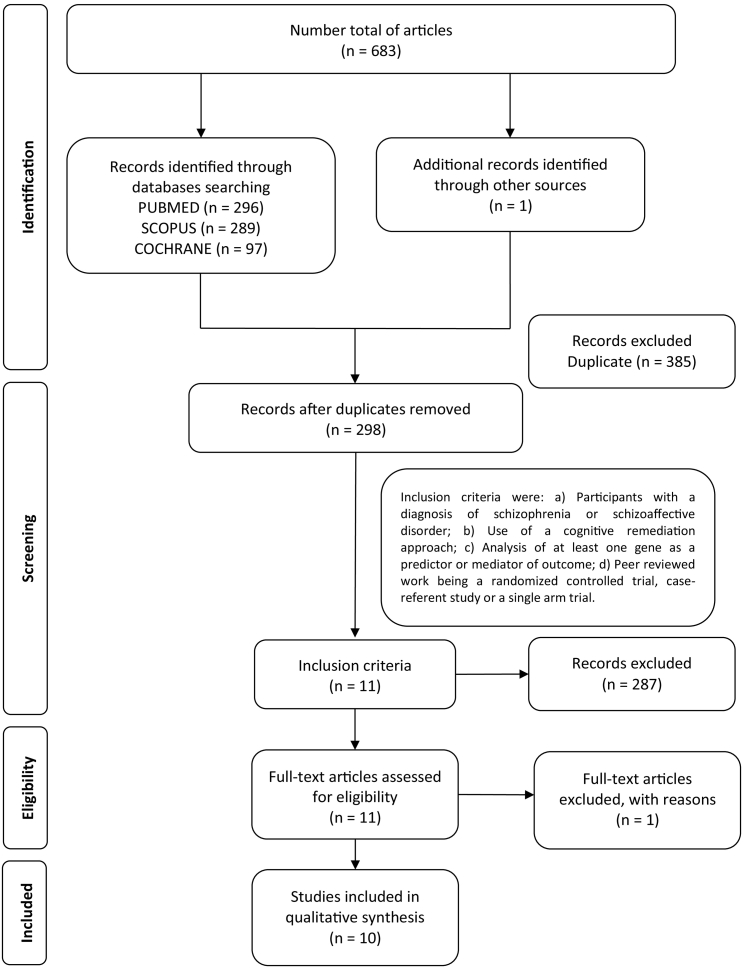
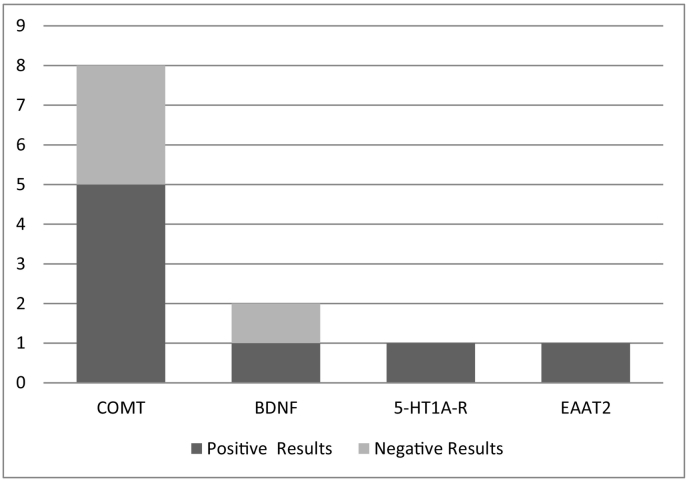
Ten articles, considering 809 participants were finally included in the review. Fig. 1 presents the flow of studies through the selection process. Seven studies showed positive association between different genetic polymorphisms and responsiveness to cognitive remediation whereas three studies (Greenwood et al., 2011; Mak et al., 2013; Burton et al., 2015) did not find any relationship (Fig. 2). Single nucleotide polymorphisms (SNPs) of the following genes were investigated by the ten studies included in this review: COMT (catechol-O-methyltransferase), 8 studies; HTR1A (hydroxytryptamine receptor 1A) encoding 5-HTA1-R, 1 study; BDNF (brain derived neurotrophic factor), 2 studies; SLC1A2 (solute carrier family 1 member 2) encoding EAAT2 (excitatory amino acid transporter 2), 1 study.
Fig. 1.
Flow diagram of the databases research.
Fig. 2.
Frequency that different genotypes have been tested in the context of cognitive remediation.
3.1. COMT
The COMT gene is one of the main research targets in schizophrenia because of its major role in the degradation of dopamine (DA). COMT is widely expressed throughout the brain and strongly modulates DA levels in the prefrontal cortex (PFC), due to the very low expression of DA Transporter in this area. A functional SNP (rs4680, Val108/158Met) modulates COMT expression, with the Val form associated to higher enzymatic activity and therefore to lower prefrontal DA levels. Several studies reported an association between COMT genotype and cognitive functioning among both healthy subjects and patients with schizophrenia (Egan et al., 2001). Specifically, subjects carrying the COMT Met allele show optimal prefrontal dopamine availability, thus determining an advantageous effect in both neurocognitive and neurophysiological terms, compared to Val/Val homozygotes (Witte and Floel, 2012). COMT genotype was also found to predict antipsychotic response among subjects with schizophrenia (Bertolino et al., 2004), and to influence brain structure, being associated with variability of cortical and subcortical volumes (Bollettini et al., 2018; Ira et al., 2013). Moreover, Val allele is associated with higher expression D1 receptor, which is inversely related to cognitive improvement after training (Slifstein et al., 2008). Given this strong evidence, different studies examined the COMT genotype as predictor of cognitive remediation, with the hypothesis that COMT polymorphism could influence the degree of cognitive change achievable with cognitive remediation. The first study, conducted by Bosia et al., 2007, analyzed the possible additive effect of cognitive remediation and COMT genotype in a sample of 50 patients with schizophrenia, through a randomized controlled trial comparing 3 months cognitive remediation plus standard rehabilitation treatment (SRT) versus non-specific computerized exercise plus SRT. Results indicated that subjects carrying the COMT Met allele treated with cognitive remediation reached a significantly greater cognitive improvement, especially in cognitive flexibility, as well as a significantly higher change in quality of life, compared to Val/Val homozygotes treated with placebo (Bosia et al., 2007). Following these results, a second study analyzed the possible influence of COMT genotype on cognitive remediation outcome in a sample of 87 patients. In contrast with previous evidence, results showed no significant effects of COMT polymorphism on cognitive improvement after treatment, nor at follow-up (Greenwood et al., 2011), suggesting that cognitive change relies on a complex medley of factors. In line with these results, a negative findings were also reported by Burton et al. (2015) and Mak et al. (2013), observing no effect of COMT genotype on response to cognitive training. An additional study by Panizzutti et al. (2013), further investigated the effect of dopamine availability on the dynamic changes in cognition. In details, 48 patients with schizophrenia treated with a computerized cognitive training were genotyped for 42 common single nucleotide polymorphisms (SNP) within the COMT gene. Gene-based analyses showed a significant aggregate effect of variation in the COMT gene on the cognitive improvement observed after training. Moreover, 8 SNP were nominally associated with cognitive changes, including rs165599, previously reported to interact with rs4680 on prefrontal activation during working memory tasks (Meyer-Lindenberg et al., 2006), supporting the hypothesis that COMT genotype may influence cognitive remediation outcome in schizophrenia. Based on these contrasting results, two subsequent studies reanalyzed the possible COMT effect on cognitive remediation. The first focused on the interaction between COMT and serotonin 1A receptor (5-HT1A-R) genotypes. 5-HT1A-R is able to modulate DA release in the PFC in dose dependent manner and the functional polymorphism, rs6295, consisting of a C to G substitution at position −1019, regulates transcription, with the G allele leading to higher levels. The analysis confirmed a main effect of COMT rs4680 and revealed a significant interaction with 5-HT1A-R rs6295 on dynamic modulation of executive functions after cognitive remediation (Bosia et al., 2014a). The second study investigated the interaction between COMT and antipsychotic treatment in 98 patients with schizophrenia, showing a significantly greater improvement in processing speed among patients carrying the Met allele treated with antipsychotics other than clozapine, compared to Val/Val treated with the same antipsychotics (Bosia et al., 2014b). Finally, a larger study on 145 subjects with diagnosis of schizophrenia or schizoaffective disorder, confirmed an effect of COMT genotype on cognitive remediation outcome. In details, the results showed a significant association between COMT Met allele and greater in cognitive domains of verbal and visual learning, as well as attention vigilance after cognitive remediation (Lindenmayer et al., 2015).
Although further evidence in larger samples is needed to establish the role genetic predictors of response to cognitive remediation, these data overall suggest that COMT genotype may at least partially influence the degree of cognitive and functional improvement after cognitive remediation and, consequently that cognitive treatment could be personalized in order to optimize outcomes.
3.2. BDNF gene
The BDNF gene is located at chromosome 11p13–14 and it encodes a precursor peptide, the so-called proBDNF, which is proteolytically cleaved to form the BDNF protein. BDNF is a neurotrophin belonging to the family of growth factors that can be found not only in the brain but also in the periphery. BDNF gene contains a functional polymorphism, Val66Met, which has been tested in different psychiatric conditions. It is a frequent single nucleotide polymorphism (SNP) and it is found in the position 196 of the gene (rs6265). It produces a nucleotide change (G → A), which results in a substitution of amino acids at position 66, a valine (Val) is replaced by a methionine (Met) in the amino acid 66 of the protein.
Some preliminary evidence indicates the importance of this functional polymorphism. Thus, the Val variant is related with higher levels of BDNF activity in neurons (Chen et al., 2004). On the other hand, carriers of the Met allele in the BDNF Val66Met polymorphism present characteristics of worse overall prognosis with worse cognitive functioning (Kambeitz et al., 2012), more hippocampus abnormalities (Molendijk et al., 2012) and less cortical plasticity capacity (Strube et al., 2014). In addition, the co-expression of Val and Met alleles is associated with decreased BDNF secretion in heterozygotes resulting in a less efficient trafficking and processing in cells (Egan et al., 2001).
Moreover, BDNF seems to be related with conditions like schizophrenia although genetic studies are still not able to conclusively establish that association. Thus, one meta-analytic study (Gratacós et al., 2007) suggested that the polymorphism can increase the risk of schizophrenia and other psychiatric disorders. Consequently, persons with the Met/Met homozygous allele appeared to have 19% higher risk of developing schizophrenia than those with the Val/Met alleles. On the other hand, it was not possible to find that association of the Val66Met polymorphism and the risk of schizophrenia on other meta-analytic study (Kawashima et al., 2009). Furthermore, recent studies are providing growing evidence about positive associations between the BDNF Val66Met polymorphism and some crucial aspects of schizophrenia condition ranging from clinical symptoms and brain morphology to cognitive function (Hong et al., 2011).
Not only the BDNF gene but also the BDNF protein itself, as determined in plasma or serum, has been proposed as a marker of cognitive recovery (Penadés et al., 2013). So far, the role of BDNF as a response marker to cognitive remediation has been directly tested only in two studies. Vinogradov et al. (2009) reported a significant increase in serum BDNF levels in patients who followed cognitive training when compared to the control group. The same authors published the study with the complete sample confirming the preliminary results (Fisher et al., 2016). Surprisingly, it was not possible to find a significant correlation between increased BDNF levels and cognitive improvement, suggesting that the relationship between BDNF levels and cognition is not direct and linear. In a recent study, Penadés et al. (2017) tried to replicate the study but it was not possible to reproduce the previous results in terms of increased serum levels after cognitive remediation. However, when the influence of genetic variability related to the BDNF gene was taken into account, some interesting data were found suggesting the potential value of genetic variability. Indeed, when the sample was divided according to the resulting polymorphisms, the carriers of the Met allele behaved totally differently from the non-carriers as no increase in serum BDNF levels was observed whatsoever for Met carriers. On the other hand, Val/Val carriers did experience a significant increase in BDNF serum levels that was similar to the increment found in the previous studies.
Those findings could be reflecting the importance of genetic variability as a mediator factor in the determination of responsiveness to cognitive remediation in persons with schizophrenia. Particularly, regarding the Val66Met polymorphism, the presence of the Met allele could be considered like a putative marker of negative response to cognitive remediation. However, in other study (Mak et al., 2013) no association was found with the same polymorphism and the cognitive improvement after cognitive remediation. Therefore, further data obtained in prospective and more accurate trials are needed to specifically test if the genetic variability related to the BDNF gene is playing or not a mediator role in the responsiveness to cognitive remediation.
3.3. EAAT2
EAAT2 is encoded by the SLC1A2 gene and plays a key role in glutamatergic neurotransmission, being responsible for more than 90% of cerebral glutamate uptake. Its activity is crucial in order to prevent neuronal excitotoxicity, to maintain an efficient energy metabolism, and to limit glutamate spillover between synapses, thus ensuring signal input specificity (Kim et al., 2011; Robinson and Jackson, 2016). EAAT2 expression is modulated by a functional SNP (rs4354668, T −181 G), located in the promoter region (Mallolas et al., 2006). Previous studies showed that the G allele, linked to lower EAAT2 expression, is associated with worse working memory and executive functions abilities among both healthy subjects and patients with schizophrenia (Spangaro et al., 2012; Zhang et al., 2015). Moreover, Poletti and colleagues showed that poor working memory performance was associated with reduced frontal cortical volumes among patients carrying the G allele (Poletti et al., 2014). Based on these evidences, Spangaro et al. recently investigated possible effects of rs4354668 on cognitive remediation outcome. The study involved 88 patients with schizophrenia, included in a cognitive remediation + SRT protocol. Authors reported greater working memory improvements among subjects homozygous for the T allele, also showing a significant interaction between pharmacological treatment and rs4354668 on executive functions outcome (Spangaro et al., 2018). In details, a greater improvement in executive functions was observed among T/T patients treated with antipsychotic other than clozapine. Consistently with previous literature, results of this study further evidenced the influence of EAAT2 genotype on cognitive functions, suggesting an effect of the transporter on both basal cognition and the degree of improvement and a possible interaction with antipsychotic treatment.
4. Discussion
Literature analyzing the role of genetics in cognitive remediation in schizophrenia is very limited and results are still inconclusive. In spite of the scarce and controversial results (Fig. 2), some relationships may be proposed between some phenotypic variations based on different polymorphisms and different levels of responsivity to cognitive remediation therapies. However, it is not possible for this review to provide any guidance on clinical decision-making.
COMT genotype seems to be a good candidate for a marker of response owing to the association of cognitive remediation outcomes with different genetic variants. The majority of studies showed that COMT genotype influences the degree of cognitive and functional improvement after cognitive remediation. Particularly, COMT Met allele has been associated with greater response in cognitive flexibility, verbal and visual learning, and attention vigilance. At different level, BDNF Val66Met polymorphism could be playing a moderating effect on responsiveness. Thus, the presence of the BDNF Met allele could be considered like a putative marker of negative response to cognitive remediation in terms of inhibiting the positive changes in BDNF serum levels after cognitive remediation. Finally, EAAT2 genotype has been suggested to influence CRT outcome as well. Particularly, higher transporter expression (T/T genotype) was associated to greater improvement of working memory, and of executive functions among T/T patients treated with antipsychotic other than clozapine. Thus, an effect of the transporter on the degree of cognitive improvement and a possible interaction with antipsychotic treatment has been proposed.
Despite this evidence concerning the influence of genetic variability on cognitive remediation outcome, specific biological pathways underlying cognitive remediation are still unclear and in order to confirm these preliminary results, future studies will have to include larger samples. This would also allow gaining a better understanding of biological correlates of cognitive remediation therapies. Moreover, evaluating cost-effectiveness seems to be unavoidable. In addition, to establish causal associations between genetic variations and treatment response, prospective studies might be preferred.
The possibility that most or even all the reported positive findings represent type I errors cannot be discarded, owing to the chosen error rate (typically α = 0.05), the proportion of tested hypotheses, and the low statistical power. Unfortunately, calculation of the false discovery rate has not been normally performed. Besides, although GWAS instead of candidate gene approach seems to be more appropriate to minimize false discoveries, only studies with candidate-gene approach have been published. In addition, cognition appears as unifactorial in larger-scale studies (Keefe et al., 2006; Harvey et al., 2016) but studies in the current revision are based on specific cognitive domains. Some authors (Wilkening et al., 2009) still stand for the need of candidate gene studies and the choice of separate cognitive domains (Thomas et al., 2017), especially for polymorphisms whose allele frequencies are low or effect sizes are small, but only replication studies will reveal the true meaning of that findings. Another caveat in this review is the lack of control for the publication bias toward positive findings. The only way to facilitate the necessary evidence for the decisive goal of offering personalized remediation therapies to targeted participants with appropriate genotypes is to generate more accurate research and trying to overcome the current barriers.
Acknowledgements
This work was supported by: the NARSAD Independent investigator grant (Project 24618) and Fondo de Investigación Sanitaria (FIS), Ministerio de Economía y Competitividad, Instituto de Salud Carlos III: FIS (PI 17/00872) Fondo Europeo de Desarrollo Regional, Unión Europea, “Un manera de hacer Europa” to RP.
References
- Bertolino A., Caforio G., Blasi G., De Candia M., Latorre V., Petruzzella V., Altamura M., Nappi G., Papa S., Callicott J.H., Mattay V.S., Bellomo A., Scarabino T., Weinberger D.R., Nardini M. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am. J. Psychiatry. 2004;161(10):1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bollettini I., Spangaro M., Poletti S., Lorenzi C., Pirovano A., Vai B., Smeraldi E., Cavallaro R., Benedetti F. Sexually divergent effect of COMT Val/met genotype on subcortical volumes in schizophrenia. Brain Imaging Behav. 2018;12(3):829–836. doi: 10.1007/s11682-017-9748-1. [DOI] [PubMed] [Google Scholar]
- Bosia M., Bechi M., Marino E., Anselmetti S., Poletti S., Cocchi F., Smeraldi E., Cavallaro R. Influence of catechol-O-methyltransferase Val158Met polymorphism on neuropsychological and functional outcomes of classical rehabilitation and cognitive remediation in schizophrenia. Neurosci. Lett. 2007;417(3):271–274. doi: 10.1016/j.neulet.2007.02.076. [DOI] [PubMed] [Google Scholar]
- Bosia M., Bechi M., Pirovano A., Buonocore M., Lorenzi C., Cocchi F., Cavallaro R. COMT and 5-HT1A-receptor genotypes potentially affect executive functions improvement after cognitive remediation in schizophrenia. Health Psychol. Behav. Med. 2014;2(1):509–516. doi: 10.1080/21642850.2014.905206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosia M., Zanoletti A., Spangaro M., Buonocore M., Bechi M., Cocchi F., Pirovano A., Lorenzi C., Bramanti P., Smeraldi E., Cavallaro R. Factors affecting cognitive remediation response in schizophrenia: the role of COMT gene and antipsychotic treatment. Psychiatry Res. 2014;217(1–2):9–14. doi: 10.1016/j.psychres.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Bryce S.D., Rossell S.L., Lee S.J., Lawrence R.J., Tan E.J., Carruthers S.P., Ponsford J.L. Neurocognitive and self-efficacy benefits of cognitive remediation in schizophrenia: a randomized controlled trial. J. Int. Neuropsychol. Soc. 2018:1–14. doi: 10.1017/S1355617717001369. [DOI] [PubMed] [Google Scholar]
- Burton C.Z., Vella L., Kelsoe J.R., Bilder R.M., Twamley E.W. Catechol-O-methyltransferase genotype and response to Compensatory Cognitive Training in outpatients with schizophrenia. Psychiatr. Genet. 2015;25(3):131–134. doi: 10.1097/YPG.0000000000000085. [DOI] [PubMed] [Google Scholar]
- Chana G., Bousman C.A., Money T.T., Gibbons A., Gillett P., Dean B., Everall I.P. Biomarker investigations related to pathophysiological pathways in schizophrenia and psychosis. Front. Cell. Neurosci. 2013;7:95. doi: 10.3389/fncel.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.Y., Patel P.D., Sant G., Meng C.X., Teng K.K., Hempstead B.L., Lee F.S. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild type BDNF in neurosecretory cells and cortical neurons. J. Neurosci. 2004;24(18):4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G., Lam M., Harris S.E. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 2018;9(1):2098. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M.F., Goldberg T.E., Kolachana B.S., Callicott J.H., Mazzanti C.M., Straub R.E., Goldman D., Weinberger D.R. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Mellon S.H., Wolkowitz O., Vinogradov S. Neuroscience-informed auditory training in schizophrenia: a final report of the effects on cognition and serum brain-derived neurotrophic factor. Schizophr. Res. Cogn. 2016;3:1–7. doi: 10.1016/j.scog.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacós M., González J.R., Mercader J.M., de Cid R., Urretavizcaya M., Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of casecontrol studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol. Psychiatry. 2007;61(7):911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Greenwood K., Hung C.F., Tropeano M., McGuffin P., Wykes T. No association between the Catechol-O-Methyltransferase (COMT) val158met polymorphism and cognitive improvement following cognitive remediation therapy (CRT) in schizophrenia. Neurosci. Lett. 2011;496(2):65–69. doi: 10.1016/j.neulet.2011.03.075. [DOI] [PubMed] [Google Scholar]
- Grynszpan O., Perbal S., Pelissolo A., Fossati P., Jouvent R., Dubal S., Perez-Diaz F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol. Med. 2011;41(1):163–173. doi: 10.1017/S0033291710000607. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Aslan M., Du M., Zhao H., Siever L.J., Pulver A., Gaziano J.M., Concato J. Factor structure of cognition and functional capacity in two studies of schizophrenia and bipolar disorder: implications for genomic studies. Neuropsychology. 2016;30(1):28–39. doi: 10.1037/neu0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C.J., Liou Y.J., Tsai S.J. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 2011;86(5–6):287–297. doi: 10.1016/j.brainresbull.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Ira E., Zanoni M., Ruggeri M., Dazzan P., Tosato S. COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J. Psychiatry Neurosci. 2013;38(6):366–380. doi: 10.1503/jpn.120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J.P., Bhattacharyya S., Kambeitz-Ilankovic L.M., Valli I., Collier D.A., McGuire P. Effect of BDNF val (66) met polymorphism on declarative memory and its neural substrate: a meta-analysis. Neurosci. Biobehav. Rev. 2012;36(9):2165–2177. doi: 10.1016/j.neubiorev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Ikeda M., Kishi T., Kitajima T., Yamanouchi Y., Kinoshita Y., Okochi T., Aleksic B., Tomita M., Okada T., Kunugi H., Inada T., Ozaki N., Iwata N. BDNF is not associated with schizophrenia: data from a Japanese population study and meta-analysis. Schizophr. Res. 2009;112(1–3):72–79. doi: 10.1016/j.schres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Bilder R.M., Harvey P.D. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Kim K., Lee S.G., Kegelman T.P., Su Z.Z., Das S.K., Dash R., Dasgupta S., Barral P.M., Hedvat M., Diaz P., Reed J.C., Stebbins J.L., Pellecchia M., Sarkar D., Fisher P.B. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J. Cell. Physiol. 2011;226(10):2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer J.P., Khan A., Lachman H., McGurk S.R., Goldring A., Thanju A., Kaushik S. COMT genotype and response to cognitive remediation in schizophrenia. Schizophr. Res. 2015;168(1–2):279–284. doi: 10.1016/j.schres.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak M., Samochowiec J., Tybura P., Bieńkowski P., Karakiewicz B., Zaremba-Pechmann L., Mroczek B. The efficacy of cognitive rehabilitation with RehaCom programme in schizophrenia patients. The role of selected genetic polymorphisms in successful cognitive rehabilitation. Ann Agric Environ Med. 2013;20(1):77–81. [PubMed] [Google Scholar]
- Mallolas J., Hurtado O., Castellanos M., Blanco M., Sobrino T., Serena J., Vivancos J., Castillo J., Lizasoain I., Moro M.A., Davalos A. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J. Exp. Med. 2006;203(3):711–717. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk S.R., Twamley E.W., Sitzer D.I., McHugo G.J., Mueser K.T. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A., Richardson R. What predicts a good response to cognitive remediation interventions? Schizophr. Bull. 2005;31(4):942–953. doi: 10.1093/schbul/sbi045. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Nichols T., Callicott J.H., Ding J., Kolachana B., Buckholtz J., Mattay V.S., Egan M., Weinberger D.R. Impact of complex genetic variation in COMT on human brain function. Mol. Psychiatry. 2006;11(9):867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., van Tol M.J., Penninx B.W., van der Wee N.J., Aleman A., Veltman D.J., Spinhoven P., Elzinga B.M. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Transl. Psychiatry. 2012;2:e74. doi: 10.1038/tp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K., Sumiyoshi C., Fujino H. Genetic overlap between general cognitive function and schizophrenia: a review of cognitive GWASs. Int. J. Mol. Sci. 2018;19(12):3822. doi: 10.3390/ijms19123822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh C.M., Moran P.M., Zhen X.C., Waddington J.L. Translating advances in the molecular basis of schizophrenia into novel cognitive treatment strategies. Br. J. Pharmacol. 2017;174(19):3173–3190. doi: 10.1111/bph.13938. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzutti R., Hamilton S.P., Vinogradov S. Genetic correlate of cognitive training response in schizophrenia. Neuropharmacology. 2013;64:264–267. doi: 10.1016/j.neuropharm.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penadés R., Catalán R., Salamero M., Boget T., Puig O., Guarch J., Gastó C. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr. Res. 2006;87:323–331. doi: 10.1016/j.schres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Penadés R., Catalán R., López-Vílchez I., Arias B., González-Rodríguez A., Galán A.M., Gastó C. Brain-derived neurotrophic factor as a potential biomarker of cognitive recovery in schizophrenia. World J. Psychiatry. 2013;3(4):93–102. [Google Scholar]
- Penadés R., García-Rizo C., Bioque M., González-Rodríguez A., Cabrera B., Mezquida G., Bernardo M. The search for new biomarkers for cognition in schizophrenia. Schizophr. Res. Cogn. 2015;2(4):172–178. doi: 10.1016/j.scog.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penadés R., López-Vílchez I., Catalán R., Arias B., González-Rodríguez A., García-Rizo C., Masana G., Ruíz V., Mezquida G., Bernardo M. BDNF as a marker of response to cognitive remediation in patients with schizophrenia: a randomized and controlled trial. Schizophr. Res. 2017;197:458–464. doi: 10.1016/j.schres.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Poletti S., Radaelli D., Bosia M., Buonocore M., Pirovano A., Lorenzi C., Cavallaro R., Smeraldi E., Benedetti F. Effect of glutamate transporter EAAT2 gene variants and gray matter deficits on working memory in schizophrenia. Eur. Psychiatry. 2014;29(4):219–225. doi: 10.1016/j.eurpsy.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Revell E.R., Neill J.C., Harte M., Khan Z., Drake R.J. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr. Res. 2015;168(1–2):213–222. doi: 10.1016/j.schres.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Robinson M.B., Jackson J.G. Astroglial glutamate transporters coordinate excitatory signaling and brain energetics. Neurochem. Int. 2016;98:56–71. doi: 10.1016/j.neuint.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M., Kolachana B., Simpson E.H., Tabares P., Cheng B., Duvall M., Frankle W.G., Weinberger D.R., Laruelle M., Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol. Psychiatry. 2008;13(8):821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Smeland O.B., Frei O., Kauppi K. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74(10):1065–1075. doi: 10.1001/jamapsychiatry.2017.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangaro M., Bosia M., Zanoletti A., Bechi M., Cocchi F., Pirovano A., Lorenzi C., Bramanti P., Benedetti F., Smeraldi E., Cavallaro R. Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neurosci. Lett. 2012;522(2):151–155. doi: 10.1016/j.neulet.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Spangaro M., Bosia M., Bechi M., Buonocore M., Cocchi F., Guglielmino C., Bianchi L., Mastromatteo A., Lorenzi C., Cavallaro R. Neurobiology of cognitive remediation in schizophrenia: effects of EAAT2 polymorphism. Schizophr. Res. 2018 doi: 10.1016/j.schres.2018.06.059. (in press) [DOI] [PubMed] [Google Scholar]
- Strube W., Nitsche M.A., Wobrock T., Bunse T., Rein B., Herrmann M., Schmitt A., Nieratschker V., Witt S.H., Rietschel M., Falkai P., Hasan A. BDNFVal66Met polymorphism impact on cortical plasticity in schizophrenia patients: a proof-of-concept study. Int. J. Neuropharmacol. 2014;18(4) doi: 10.1093/ijnp/pyu040. (pii: pyu040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E.H.X., Bozaoglu K., Rossell S.L., Gurvich C. The influence of the glutamatergic system on cognition in schizophrenia: a systematic review. Neurosci. Biobehav. Rev. 2017;77:369–387. doi: 10.1016/j.neubiorev.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Vinogradov S., Fisher M., Holland C., Shelly W., Wolkowitz O., Mellon S.H. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol. Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S., Chen B., Bermejo J.L., Canzian F. Is there still a need for candidate gene approaches in the era of genome-wide association studies? Genomics. 2009;93:415–419. doi: 10.1016/j.ygeno.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Witte A.V., Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 2012;88(5):418–428. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Wykes T., Huddy V., Cellard C., McGurk S.R., Czbor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zai G., Robbins T.W., Sahakian B.J., Kennedy J.L. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci. Biobehav. Rev. 2017;72:50–67. doi: 10.1016/j.neubiorev.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Zhang B., Guan F., Chen G., Lin H., Zhang T., Feng J., Li L., Fu D. Common variants in SLC1A2 and schizophrenia: association and cognitive function in patients with schizophrenia and healthy individuals. Schizophr. Res. 2015;169(1–3):128–134. doi: 10.1016/j.schres.2015.10.012. [DOI] [PubMed] [Google Scholar]