Abstract
Objective
Although disseminated nontuberculous mycobacterial infection is attributed to defects in the interleukin (IL)-12/interferon-γ circuit, the immunophenotype of idiopathic pulmonary nontuberculous mycobacterial (PNTM) disease is not well defined.
Method
We phenotyped Th1, Th2, Th17, and Treg cytokines and colony-stimulating factor production from patients with idiopathic PNTM disease. Data were compared with healthy donors, cystic fibrosis (CF), and primary ciliary dyskinesia (PCD) patients with PNTM disease. Both supernatant cytokine production and intracellular cytokines expressed by various leukocyte subpopulations following mitogen and antigen stimulation were assayed by electrochemiluminescence–based multiplex immunoassay and flow cytometry, respectively.
Results
Regardless of antigen or mitogen stimulation, neither intracellular nor extracellular Th1, Th2, and Treg cytokine levels differed between patients and controls. Th17 cells and IL-17A levels were lower in idiopathic PNTM patients, whereas monocyte granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in response to NTM stimulation was higher compared with healthy donors. Besides, distinct cytokine responses following stimulation by Mycobacterium abscessus and Mycobacterium avium were observed consistently within each group.
Conclusions
The IL-12/IFN-γ circuit appeared intact in patients with idiopathic PNTM disease. However, idiopathic PNTM patients had reduced Th17 response and higher mycobacteria-induced monocyte GM-CSF expression.
Keywords: cystic fibrosis, cytokines, nontuberculous mycobacteria, PNTM, primary ciliary dyskinesia, Th17
This study found no defects in the interleukin-12/interferon-γ circuit among idiopathic pulmonary nontuberculous mycobacterial patients. Interleukin-17─producing T cells were low, but also similarly low in cystic fibrosis and primary ciliary dyskinesia patients, suggesting that the Th17 defect is an infection-associated and not a primary one.
INTRODUCTION
Although the incidence of tuberculosis is declining gradually in many countries, the prevalence of pulmonary nontuberculous mycobacterial (PNTM) disease is increasing worldwide [1–5]. It often is seen in the setting of structural lung abnormalities, including bronchiectasis, pneumoconiosis, chronic obstructive lung disease (COPD), immunologic or genetic disorders (eg, cystic fibrosis [CF], primary ciliary dyskinesia [PCD], α-1-antitrypsin deficiency), and in patients receiving tumor necrosis factor-α (TNF-α) antagonists. However, it has become increasingly recognized that PNTM disease occurs in persons without any know underlying abnormalities. The majority of this population are nonsmoking, postmenopausal women, who have increased rates of distinctive morphologic features, including slender body habitus, increased height, scoliosis, pectus excavatum, and mitral valve prolapse.
Difficult to treat, PNTM disease requires multiple antibiotics and an extended treatment course with a high recurrence rate [6]. There is an unmet need to clarify the host susceptibility factors to “idiopathic” PNTM disease so that we can explore alternative therapeutic options other than antimicrobial treatment alone. However, the critical question of where vulnerability to PNTM disease lies has been perplexing, though preexisting somatic features and family clusters suggest critical genetic underpinnings [7, 8]. Much effort has been placed on examining the integrity of the interleukin (IL)-12 dependent interferon-gamma (IFN-γ) pathway, the defects of which are well-established to cause disseminated mycobacterial infections [9]. Yet, previous studies that attempted to evaluate the integrity of this pathway in PNTM patients have yielded widely contradictory results [7, 10–14]. Apart from the lack of consistent immunologic abnormalities, the late age of disease onset and the lack of disseminated disease in patients with extensive and fatal pulmonary disease (and vice versa) suggest distinctive immunologic defects between patients with idiopathic PNTM disease and disseminated NTM infection.
In light of the conflicting data surrounding PNTM susceptibility, we undertook a comprehensive study to examine not only the IL-12/IFN-γ axis, but also Th17/Treg cytokine levels in patients with idiopathic PNTM disease through comparisons with CF and PCD patients with the same infection. Mitogens, lipopolysaccharide, as well as rapid- and slow-growing NTM strains were used as agonists with the aim to evaluate the integrity of different pathways.
METHODS
Patient Recruitment
Patients were recruited from established cohorts for the study of PNTM disease, PCD, and bronchiectasis at the National Institutes of Health (NIH). Whole blood was obtained from 30 idiopathic PNTM patients, 7 PCD patients, 10 CF patients, and 40 healthy controls. All subjects provided consent for the National Institute of Allergy and Infectious Diseases’ Institutional Review Board–approved protocol (01-I-0202). All patients had microbiologic and radiographic evidence of PNTM infection and fulfilled the American Thoracic Society criteria for PNTM disease [6]. Patients with idiopathic PNTM disease did not meet diagnostic criteria for CF or PCD, whereas both CF and PCD patients fulfilled published criteria, respectively [15–17]. All patients tested negative for antibodies to HIV. The patients’ demographics, most recent sputum microbiology results, medical comorbidities, antibiotic use, and clinical laboratory data performed on the same date as their research blood were recorded. A CT scan of the chest was reviewed for parenchymal abnormalities by the same experienced pulmonologist (K.N.O.).
Isolation of Fresh PBMC, Stimulation, and Cell Culture
Fresh peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by density-gradient centrifugation using lymphocyte separation medium (Lonza, Walkersville, MD) and resuspended in RPMI 1640 (Gibco BRL, Grand Island, NY) containing penicillin and streptomycin (Life Technologies, Carlsbad, CA), 10% fetal bovine serum, and 20 mM HEPES. For each stimulation, 1 × 106 cells in 100 μl media were cultured in sterile snap-cap 5 ml polypropylene tubes (Becton Dickinson Labware, Franklin Lakes, NJ) containing (1) media alone; (2) 50 ng/ml phorbol 12-myristate 13-acetate (PMA; Calbiochem, San Diego, CA) plus 1 μM ionomycin (Sigma-Aldrich, St Louis, MO) in the presence of 10 μg/mL brefeldin A (Sigma-Aldrich); (3) 1% phytohemagglutinin (PHA; Gibco) plus 50 ng/ml IL-12 (R&D Systems, Minneapolis, MN); (4) 5 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich); and (5) live mycobacteria (live Mycobacterium abscessus [multiplicity of infection {MOI}, 10:1]; live Mycobacterium avium [MOI, 10:1]; or live Mycobacterium intracellulare [MOI, 0.2:1]). Cells were incubated separately with different mitogens and mycobacteria at 37°C in an atmosphere containing 5% CO2 for 6 hours with PMA plus ionomycin, or 18 hours for the other stimulation conditions with brefeldin A (10 μg/mL) added to all tubes for the last 15 hours of culture. Brefeldin A was not added to cultures set up for supernatant collection. Methods of mycobacteria preparation can be found in the Supplementary Data online.
Flow Cytometry and Cytokine Detection
Cells were harvested and washed with phosphate buffered saline (PBS) and stained with the LIVE/DEAD Near-IR dead cell stain kit (Invitrogen, Carlsbad, CA) to exclude dead cells. For cell surface staining, PBMCs were incubated with antibodies at 4°C in dark for 20 minutes. Cells then were washed with flow cytometry staining buffer (PBS containing 2% bovine serum albumin), fixed in 4% paraformaldehyde, and then permeabilized in 0.1% saponin and nonfat milk solution. After incubation with antibodies for intracellular staining at room temperature for 45 min, cells were washed in 0.1% saponin solution and analyzed by flow cytometry on an LSR-Fortessa (BD Biosciences, San José, CA). Details of fluorochrome-conjugated monoclonal antibodies used (Table S1) and flow cytometric data analysis (Fig S1) can be found in the Supplementary Data online.
Measurement of Supernatant Cytokines
Supernatants of cultured PBMC were collected after incubation for 24 hours at 37°C in a 5% CO2-humidified cell culture incubator and stored at -20°C until analysis using an electrochemiluminescence (ECL)–based multiplex immunoassay on an MSD technology platform (Meso Scale Discovery, Gaithersburg, MD). According to the manufacturer’s instructions, GM-CSF and IL-17A were measured with the Cytokine Panel 1 V-plex kit (Meso Scale Discovery), TGF-β1 with the Human TGF- β1 kit (Meso Scale Discovery), and IL-2, IL-4, IL-10, IL-12p70, IL-13, IFN-γ, and TNF-α with the TH1/TH2 10-plex Ultrasensitive kit (Meso Scale Discovery).
Statistics
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA). Continuous variables were expressed as means ± standard errors. One-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc tests were used to compare data between healthy volunteers, idiopathic PNTM patients, and disease controls. Two-way ANOVA was used to compare cytokine responses between M. abscessus and M. avium stimulation in different groups of subjects. Categorical variables were compared using χ 2or Fisher exact tests, as appropriate; P values less than .05 were considered to be statistically significant.
RESULTS
Subjects
Demographics and clinical characteristics of the patients and healthy controls enrolled in this study are illustrated in Table 1. The absolute lymphocyte and monocyte counts, percentages of T cell subsets, NK cells, and NK T cells did not differ significantly among the groups (Table 1).
Table 1.
Demographic Features of Patients With idiopathic PNTM Disease, PCD, CF and Healthy Controls a
| Characteristics | Healthy controls (n = 40) | PNTM (n = 30) | PCD (n = 7) | CF (n = 10) |
|---|---|---|---|---|
| Age, mean ± SD years | 48.8 ± 11.7 | 66.0 ± 8.3 b,c,d | 40.3 ± 17.0 | 44 ± 17.8 |
| Gender (% female) | 12 (30) | 27 (90)b | 7 (100) | 6 (60) |
| Ethnicity | ||||
| Caucasian | 28 (70) | 27 (96.6) | 4 (57.1) | 10 (100) |
| African-American | 6 (15) | 0 | 0 | 0 |
| Asian | 3(8) | 1 (3.4) | 2 (28.6) | 0 |
| Hispanic | 2 (5) | 2 (6.7) | 1 (14.3) | 0 |
| Native American | 1 (3) | |||
| BMI (Kg/m2) | n.a. | 21.6 ± 3.1 | 23.3 ± 7.0 | 22.7 ± 5.7 |
| Current smoker | n.a. | 1 (3.3) | 0 | 0 |
| Ex-smoker | n.a. | 6 (20) | 0 | 2 (20) |
| NTM treatment status | ||||
| Treatment-naive | n.a. | 2 (6.7) | 1 (14.3) | 0 |
| On therapy | n.a. | 19 (63.3) | 3 (42.9) | 8 (80) |
| Off therapy | n.a. | 9 (30) | 3 (42.9) | 2 (20) |
| Respiratory pathogens | ||||
| M. abscessus | n.a. | 7 (23.3) | 3 (42.9) | 6 (60) |
| M. intracellulare | n.a. | 9 (30) | 1 (14.3) | 1 (10) |
| M. avium | n.a. | 8 (26.7) | 1 (14.3) | 2 (20) |
| MAC (unspeciated) | n.a. | 4 (13.3) | 1 (14.3) | 0 |
| Mixed NTM | n.a. | 2 (6.7) | 0 | 1 (10) |
| Radiologic features | ||||
| Nodular/bronchiectatic | n.a. | 25 (83.3) | 7 (100) | 9 (90) |
| Cavitary | n.a. | 5 (16.7) | 0 | 1 (10) |
| Severity | n.a. | |||
| Mild | n.a. | 8 (26.7) | 3 (42.9) | 2 (20) |
| Moderate | n.a. | 20 (66.7) | 3 (42.9) | 6 (60) |
| Severe | n.a. | 2 (6.7) | 1 (14.3) | 2 (20) |
| WBC (k/μl, mean ± SD) | n.a. | 7.0 ± 2.7 | 7.3 ± 2.6 | 7.7 ± 2.8 |
| Lymphocytes (k/μl) | n.a. | 1.6 ± 0.6 | 2.0 ± 1.5 | 1.8 ± 0.6 |
| Monocytes (k/μl) | n.a. | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.3 |
| CD3+ % (out of all PBMCs) | 59.8 ± 13.3 | 58.2 ± 8.4 | 63.0 ± 8.3 | 57.3 ± 7.1 |
| CD4+/CD3+ % | 65.6 ± 13.1 | 72.3 ± 12.1 | 63.0 ± 9.2 | 59.9 ± 10.2 |
| CD8+/CD3+ % | 26.1 ± 11.5 | 22.2 ± 10.83 | 29.1 ± 7.0 | 30.6 ± 7.8 |
| CD56+/CD3+ % | 3.6 ± 3.6 | 3.0 ± 2.0 | 5.8 ± 5.2 | 4.4 ± 2.5 |
| CD3-CD56+ (out of all PBMCs) | 9.9 ± 4.7 | 11.6 ± 4.3 | 9.2 ± 6.0 | 11.8 ± 4.4 |
| CRP (mg/L) | n.a. | 8.3 ± 24.8 | 4.1 ± 4.6 | 16.8 ± 26.1 |
Abbreviations: BMI, body mass index; CF, cystic fibrosis; CRP, C-reactive protein; MAC, Mycobacterium avium complex; n.a., not available; PCD, primary ciliary dyskinesia; PNTM, idiopathic pulmonary nontuberculous mycobacterial disease; SD, standard deviation; WBC, white blood cells.
a Data are expressed as n (%) unless otherwise specified.
b P value < .001 using one-way analysis of variance (ANOVA) test with Bonferroni adjustment for comparisons between healthy volunteers and PNTM patients.
c P value < .001 using one-way ANOVA test with Bonferroni adjustment for comparisons between PNTM patients and PCD patients.
d P value < .001 using one-way ANOVA test with Bonferroni adjustment for comparisons between PNTM patients and CF patients.
Th1, Th2, and Treg Cytokine Production Did Not Differ Between Idiopathic PNTM Patients and Controls
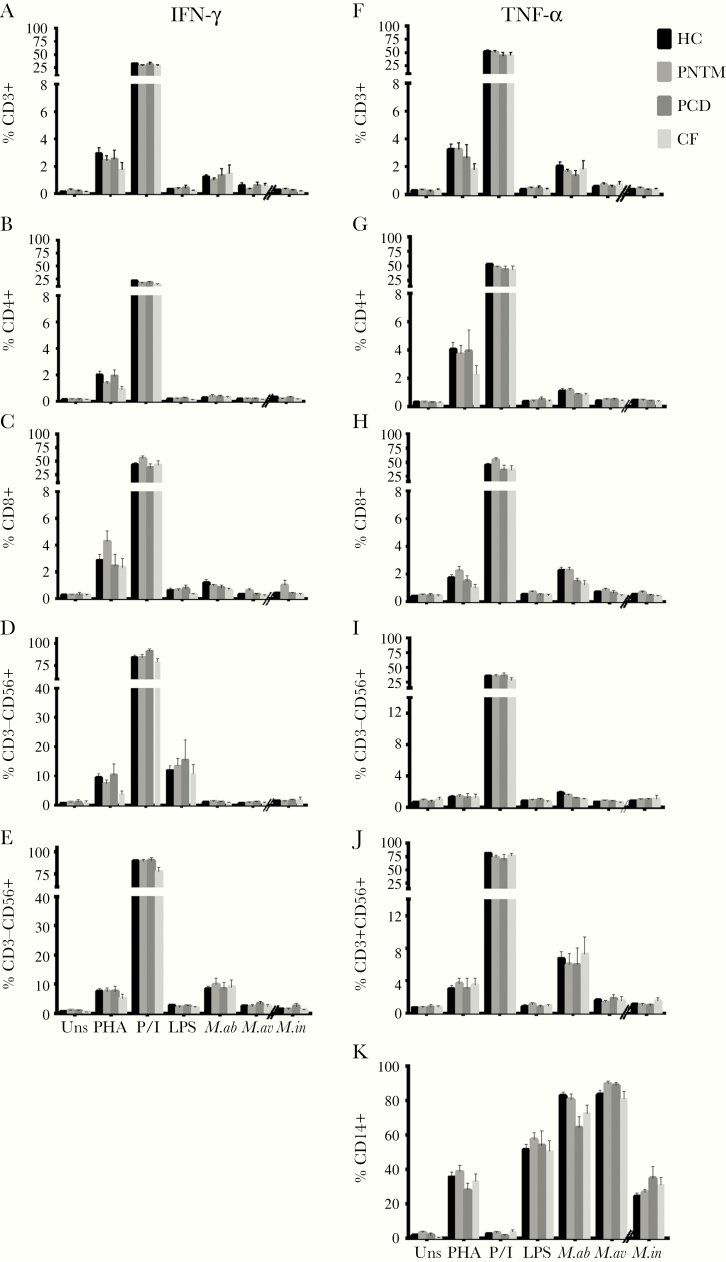
We detected no significant differences in the frequencies of IFN-γ and TNF-α produced by various T cells subsets, NK cells or monocytes between idiopathic PNTM patients and disease or healthy controls (Fig 1). The percentages of cell subsets that produced IL-2, IL-4, IL-10, IL-12, IL-13, and TGF-β1 following mitogen and antigen stimulation also were comparable (Fig S2). Likewise, there were no significant differences of these cytokines in the supernatant levels except a modest decrease of TNF-α in idiopathic PNTM patients with regard only to PMA plus ionomycin but not mycobacterial stimulation (Fig S3).
Fig 1.
Flow cytometric analyses showing the mean percentages of IFN-γ (A-F) and TNF-α (G-L) expressed by different leukocyte subpopulations after stimulation with various mitogens and agonists, as indicated. No significant differences were found in the percentages of CD3+ T cells, CD4+ T cells, CD8+ T cells, CD3-CD56+ NK cells, and CD3+CD56+ NK T cells that expressed IFN-γ and TNF-α (A–E, F–J), and in the percentage of TNF-α –expressing CD14+ cells (K) between healthy controls and patients with idiopathic pulmonary nontuberculous mycobacterial (PNTM) disease, primary ciliary dyskinesia (PCD), and cystic fibrosis (CF). HC indicates healthy controls; uns, unstimulated; PHA, phytohemagglutinin + IL-12; P/I, phorbol 12-myristate 13-acetate + ionomycin; M.ab, Mycobacterium abscessus (MOI: 10:1); M.av, Mycobacterium avium (MOI: 10:1); M.in; Mycobacterium intracellulare (MOI: 0.2:1). Data are presented as means; error bars show standard error.
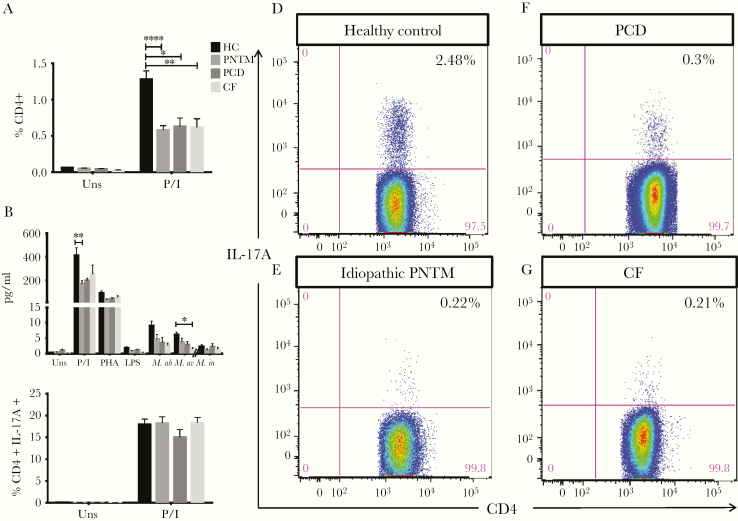
Patients With Idiopathic PNTM Disease and PCD Have Diminished Th17 Cytokine Responses
The proportion of CD4+ T cells producing IL-17A was significantly lower in idiopathic PNTM patients (0.6 ± 0.1 %, P < .001) than in healthy controls (1.3 ± 0.7%) after 6h stimulation with PMA plus ionomycin (Fig 2A). However, to understand whether this was associated with idiopathic PNTM disease per se or with pulmonary mycobacterial disease in other contexts, we also examined the frequencies of Th17 cells in PCD patients (0.6 ± 0.1 %, P = .03) and CF patients (0.6 ± 0.1 %, P = .006) and found them similarly decreased (Fig 2A). Levels of IL-17A assayed in culture supernatant also were significantly reduced in idiopathic PNTM patients following PMA plus ionomycin stimulation (169.6 ± 29.1 pg/ml vs 411.5 ± 64.7 pg/ml, P = .009), and in CF patients following M. avium stimulation (1.5 ± 0.4 pg/ml vs 6.1 ± 4.9 pg/ml, P = .04) compared with healthy controls (Fig 2B). Given that human Th17 cells comprise heterogeneous subsets and IL-17A+ IFN-γ + double-positive Th17 cells have been described as more pathogenic [18, 19], we next compared the frequency of IFN-γ-expressing CD4+ IL-17A+ Th17 cells and observed similar distribution among the groups (Fig 2C).
Fig 2.
Th17 responses were decreased in patients with PNTM disease. The percentages of IL-17A-expressing CD4+ cells were significantly lower in patients with idiopathic PNTM disease, PCD, and CF compared with healthy controls in response to PMA + ionomycin stimulation (A). Supernatant IL-17A level also was significantly reduced in idiopathic PNTM patients in response to PMA + ionomycin stimulation, and in CF patients in response to M. avium stimulation as compared with healthy controls (B). The frequencies of IFN-γ-expressing CD4+ IL-17A+ cells were comparable among the groups (C). Representative plots show expression of IL-17A in CD4+ T cells in a healthy volunteer (D), an idiopathic PNTM patient (E), a PCD patient (F) and a CF patient (G). Both (D) and (E) were performed in the same experiment, while (F) and (G) were performed simultaneously in another experiment. HC indicates healthy controls; uns, unstimulated; PHA, phytohemagglutinin + IL-12; P/I, phorbol 12-myristate 13-acetate + ionomycin; M.ab, Mycobacterium abscessus (MOI: 10); M.av, Mycobacterium avium (MOI: 10); M.in; Mycobacterium intracellulare (MOI: 0.2). Data are presented as means; error bars show standard error. *P < .05, **P < .01, ***P < .001.
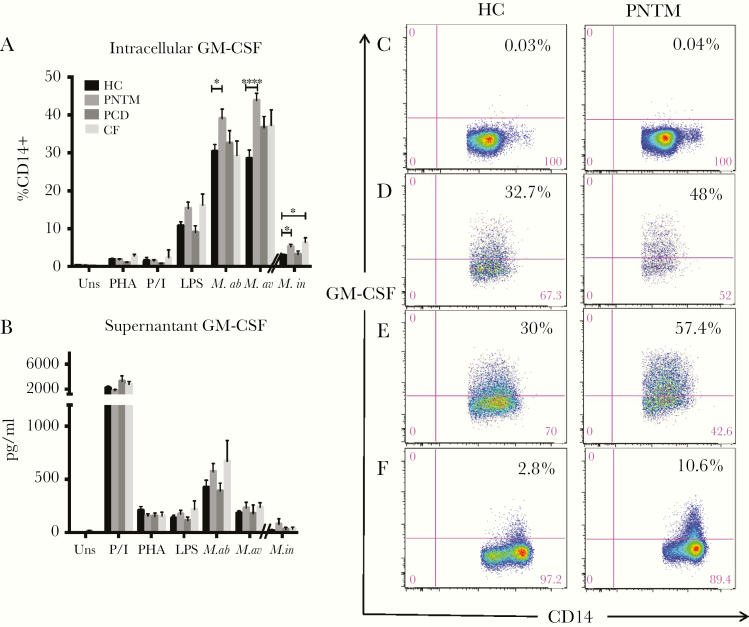
PNTM Patients Have More GM-CSF Expressing Monocytes in Response to Mycobacterial Stimulation
Granulocyte-macrophage colony-stimulating factor expressing CD14+ cells were significantly increased in idiopathic PNTM patients following M. abscessus (39.0 ± 2.5% vs 30.3 ± 1.8%, P = .03), M. avium (43.8 ± 1.9% vs 28.5 ± 2.2%, P < .0001) and M. intracellulare (5.0 ± 3.7% vs 2.8 ± 2.4%, P = .02) stimulation compared to healthy controls (Fig 3A). The number of GM-CSF expressing CD14+ cells also was greater in CF patients following M. intracellulare stimulation (6.3 ± 1.3 pg/ml vs 2.9 ± 0.4 pg/ml, P = .02). Supernatant GM-CSF levels produced by PBMCs from idiopathic PNTM patients and CF patients following mycobacterial infection were insignificantly elevated (Fig 3B).
Fig 3.
Intracellular (A) and extracellular (B) GM-CSF levels among patients and healthy controls. Idiopathic PNTM patients had a higher percentage of GM-CSF expressing CD14+ cells than healthy controls in response to M. abscessus, M. avium and M. intracellulare stimulation. CF patients also had a higher percentage of GM-CSF expressing CD14+ cells than normal controls in response to M. intracellulare stimulation. However, no significant differences in supernatant GM-CSF levels were found between patients and normal controls. Representative dot plots show GM-CSF expression in CD14+ cells from unstimulated (C), M. abscessus (D), M. avium (E), and M. intracellulare-stimulated conditions (F) performed in the same experiment. HC indicates healthy controls; uns, unstimulated; PHA, phytohemagglutinin + IL-12; P/I, phorbol 12-myristate 13-acetate + ionomycin; M.ab, Mycobacterium abscessus (MOI: 10); M.av, Mycobacterium avium (MOI: 10); M.in; Mycobacterium intracellulare (MOI: 0.2). Data are presented as means; error bars show standard error. *P < .05, **P < .01, ***P < .001.
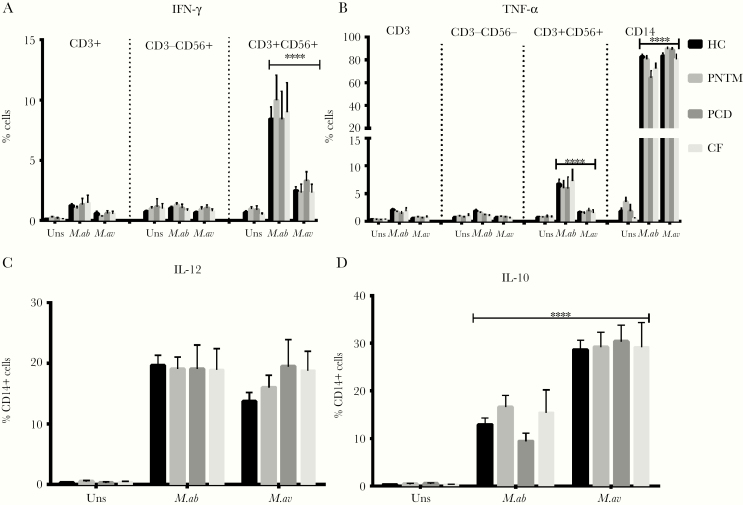
M. abscessus and M. avium Triggered Distinct Cytokine Responses
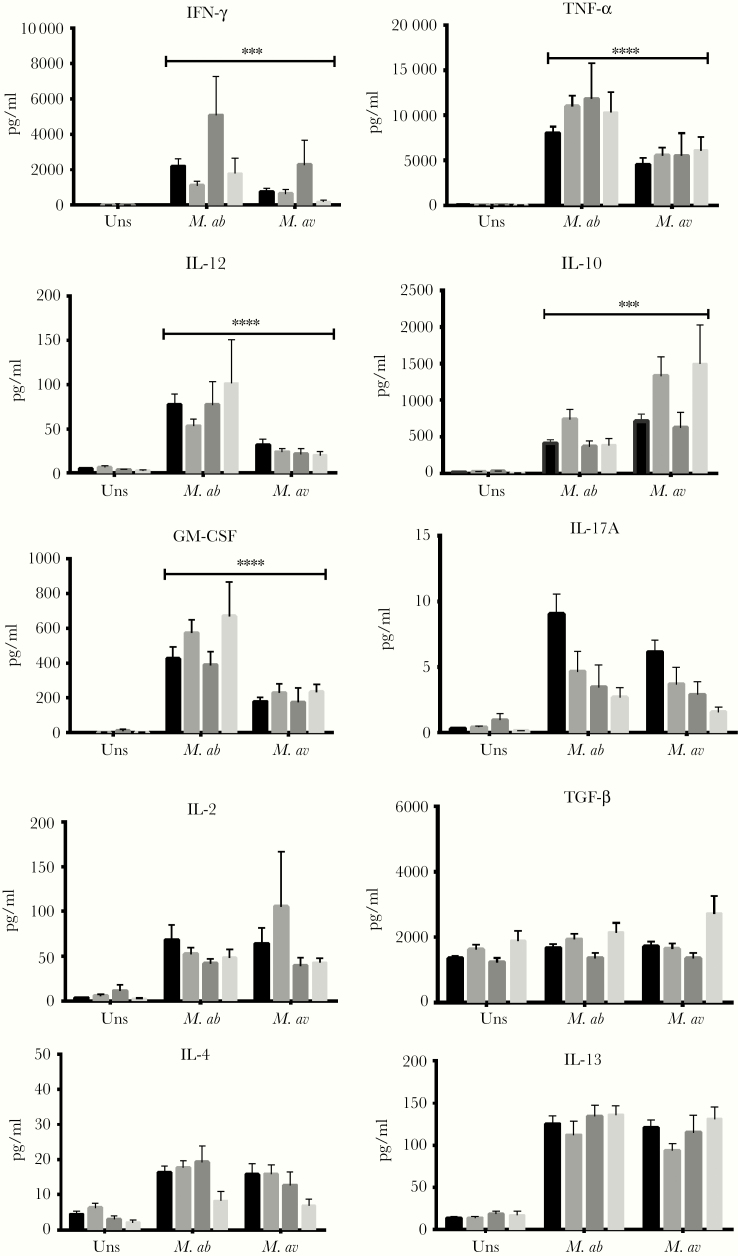
After in vitro exposure with M. abscessus and M. avium, IFN-γ primarily was expressed by CD3+CD56+ NK T cells rather than other T cell subpopulations or NK cells (Fig 4A), whereas CD14+ cells were the major source of TNF-α (Fig 4B), IL-10, IL-12 (Fig 4C-D), and GM-CSF (Fig 3A). The mean frequencies of CD3+ CD56+ NK T cell subsets expressing IFN-γ (P < .0001) and TNF-α (P < .0001) were higher following M. abscessus than M. avium stimulation (Fig 4A-B). However, TNF-α− (P < .0001) and IL-10−producing CD14+ cells (P < .001) were more upregulated in response to M. avium than M. abscessus (Fig 4B&D). In supernatants, M. abscessus induced higher levels of IFN-γ (P = .0005), TNF-α (P < .0001), IL-12 (P < .0001), and GM-CSF (P < .0001) secretion than M. avium, whereas the latter induced a higher IL-10 (P = .0002) level (Fig 5).
Fig 4.
Comparisons of intracellular cytokine expression by various leukocyte subsets following stimulation of peripheral blood mononuclear cells with M. abscessus versus M. avium. HC, healthy controls; uns, unstimulated; M.ab, Mycobacterium abscessus (MOI: 10:1); M.av, Mycobacterium avium (MOI: 10:1). Data are presented as means; error bars show standard error. *P < .05, **P < .01, ***P < .001.
Fig 5.
Comparisons of supernatant cytokine concentrations following stimulation of peripheral blood mononuclear cells with M. abscessus versus M. avium. HC indicates healthy controls; uns, unstimulated; M.ab, Mycobacterium abscessus (MOI: 10:1); M.av, Mycobacterium avium (MOI: 10:1). Data are presented as means; error bars show standard error. *P < .05, **P < .01, ***P < .001.
DISCUSSION
Understanding the origins and complications of idiopathic PNTM disease has been complicated by its late onset and the difficultly of identifying informative family clusters. Although there have been extensive advances made in genetic and functional susceptibility to disseminated NTM infection, disease limited to the lung only has been clearly associated with respiratory epithelial disorders like CF and PCD. In the present study, we found similar intracellular and extracellular Th1/Th2/Treg cytokine profiles in idiopathic PNTM patients, CF patients, PCD patients, and healthy controls in response to mitogen and antigen stimulations. Indeed, in a recent large-scale study that examined the genetic variants in PNTM patients, none of the previously recognized mutations of genes involved in the synthesis of and response to IFN-γ were identified [20].
Our findings are in contrast to previous studies that reported various defects in the IL-12/IFN-γ pathway, ranging from impaired intracellular IFN-γ production to impaired IFN-γ secretion [10–12, 14, 21], but agree with other reports that have failed to identify significant systemic immune defects in these patients [7, 13, 22, 23]. The overall discrepancies between previous reports could have been due to methodological variations (eg, enzyme-linked immunosorbent assay vs flow cytometry; PBMCs vs whole blood; mitogen vs antigen stimulation); to heterogeneity of the subjects (eg, disease severity, control groups); or to limited sample sizes. As such, we sought to conduct a comprehensive study that focused on patients with confirmed “idiopathic” PNTM disease who have been regularly followed up at NIH to avoid subject heterogeneity. Given that patients with PCD and CF are known to be predisposed to PNTM disease through mechanisms that are presumably due to their underlying structural pulmonary disease (and not immune-based), we included them along with healthy blood donors in the analysis. With both healthy and disease controls, we sought to distinguish differences in cytokine production specific to idiopathic PNTM disease per se from those that may be related to infection itself. Previous studies have relied on supernatant cytokine levels, which cumulate cellular responses but do not reflect the functional competence of individual subpopulations of leukocytes [24]. We thus assess both intracellular and secreted cytokines to anticipate the possibility of CD4+ T-lymphocytopenia in the context of chronic illness and to identify possible cellular subsets associated with the disease. We used freshly isolated PBMCs instead of whole blood in order to avoid any possible interference by humoral factors in the serum and to reduce the variability in input cell numbers. The use of early time points allowed us to focus more on innate immune functions than acquired ones, because antigen-specific T cell defects are hard to characterize without well-defined antigens and responses. In addition, isolated pulmonary disease is not a major feature of primary T cell defects [25, 26].
Our findings that PNTM patients have a lower level of IL-17A production is noteworthy [23, 27]. It has been well described that inborn errors of IL-17 immunity contribute to chronic mucocutaneous candidiasis (CMC) [28], which was absent in our patients. Interestingly, there is accumulating evidence for the role of Th17 cells in response to human mycobacterial infection. In vivo experiments showed Th17 cells confer partial protection against M. tuberculosis (Mtb) infection in the absence of IFN-γ [29]. Interleukin-17 also was reported to inhibit apoptosis of M. bovis BCG- or Mtb-infected macrophages, thus hampering their ability to control bacterial growth [30]. In patients with active TB, a deficient Th17 response also was demonstrated, especially when compared to healthy controls [31]. Recently, patients with bi-allelic mutations of RORC, the master gene controlling Th17 differentiation, were reported to display severe mycobacterial infections in addition to CMC, highlighting the essential role of Th17 immunity in protection against mycobacterial disease [32]. In the present study, however, the percentage of IL-17A–producing CD4+ T cells was diminished not only in idiopathic PNTM patients but also in CF and PCD patients with NTM infections, indicating that this phenomenon is not specific to idiopathic PNTM disease. Further investigation is required to explore the potential clinical applications Th17 responses as adjunct biomarkers in improving diagnosis or monitoring therapeutic outcomes.
Granulocyte-macrophage colony-stimulating factor plays a crucial role in the differentiation, activation, and survival of various hematopoietic cell lineages, particularly monocytes and dendritic cells, as well as induction of Th1 responses and host defense [33, 34]. It is known to be involved in immune response against Mtb in mouse models [35]. Few case series showed GM-CSF augments macrophage phagocytosis and impairs NTM growth [36]. Our findings are consistent with previous studies that showed that monocytes can produce GM-CSF more readily than T cells when directly stimulated with NTM, which might in turn augment the antimycobacterial capabilities of phagocytes [37]. The marked increase of GM-CSF production by CD14+ cells obtained from our idiopathic PNTM patients might simply reflect the preexisting priming effect of peripheral CD14+ cells due to PNTM infections. However, overexpression of GM-CSF can prevent proper granuloma formation in the lung by depressing type 1 chemokine production [38]. Intriguingly, hyper immunoglobulin E syndrome (HIES), which shares several distinctive characteristics with idiopathic PNTM disease, including recurrent pulmonary infections, skeletal, and connective tissue abnormalities, also was reported to have increased GM-CSF and decreased IL-17 production [39, 40]. About one-third of HIES patients have NTM isolated in at least 1 sputum culture, and 16% of patients met ATS criteria of pulmonary NTM infection [41]. Longitudinal studies are required to differentiate whether the cytokine imbalance is a cause or just a consequence of PNTM disease.
This study also allowed, at the same time, comparisons of human cytokine responses between a slow-grower and a rapid-grower in healthy people and different subpopulations of NTM-infected patients. Our results showed distinct cytokine profiles elicited by M. abscessus versus Mycobacterium avium-intracellulare complex (MAC), in that the former were more capable to induce production of proinflammatory cytokines, whereas the latter elicited a high production of anti-inflammatory cytokines. Because IL-12 contribute to production of IFN-γ by T cells and NK cells, the enhanced IFN-γ production following M. abscessus stimulation can be secondary to enhanced IL-12 production, or vice versa.
In addition, NKT cells, a T cell subset known to mediate protection against Mtb infection via a CD1d-dependent lipid recognition, appeared to be more activated by M. abscessus than MAC in terms of cytokine production. Although these data reflect only 1 representative organism per species and may not represent all members, they do demonstrate an important organism- or species-specific variation in cellular responses, as has been shown previously [42]. Indeed, the intriguing question of why MAC is the most predominant NTM in patients with advanced HIV infection [43], whereas M. abscessus complex is isolated more frequently in CF patients in some centers [44] and in nosocomial outbreaks [45], has never been addressed. The interplay between different strains of NTM and specific hosts warrant further elucidation.
Our cross-sectional study design does not allow us to distinguish whether the very few abnormal cytokine levels found are due to the primary pathogenesis of the disease or are a consequence of it. However, the inclusion of PCD and CF patients as disease controls enabled us to differentiate cytokine changes due to idiopathic PNTM disease from those likely secondary to PNTM infection. We were unable to select age- or sex-matched controls for each case; nevertheless, we did not observe marked differences in the cytokine levels with regard to age or sex (data not shown). Our testing only explored cells in the systemic circulation and not at the sites of infection, so finding normal number and function of cytokine-producing cells ex vivo does not exclude a possible impairment of the development, homing, or functions of these cells in the airways. Levels of cytokines in the circulation likely reflect local immune responses at disease sites [46, 47]. Recently, impairments in nasal nitric oxide, ciliary beat frequency, and toll-like receptor responses in the respiratory epithelial cells have been identified in PNTM patients [48]. Studies are underway to evaluate possible immune defects localized to the respiratory compartment in this population.
Distinctive from disseminated NTM infections, patients with idiopathic PNTM disease had no identifiable defects in the Th1/Th2/Treg paradigm. Our study provides insights into revisiting alternative immune defects underlying the pathogenesis of PNTM disease. Whether dysregulation of Th17 and GM-CSF response in idiopathic PNTM patients can be used as an indicator for disease severity or the normalization of these cytokine imbalances can improve clinical outcomes is worth further exploration in longitudinal studies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Kevin L. Holmes and David A. Stephany from Flow Cytometry Section, Research Technologies Branch, National Institute of Allergy and Infectious Disease for their excellent technical assistance.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NCT00018044).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions: U.I.W. performed cell cultures, flow cytometric analysis, and wrote the manuscript; K.N.O. enrolled subjects, analyzed clinical data, and interpreted radiographic studies; D.B.K. and D.L.F. conducted supernatant cytokine analysis; E.P.S. assisted in data interpretation; A.M.Z., M.S.L., and S.J.S. prepared mycobacteria for cell culture; B.E.M. procured clinical data; S.M.H. conceived and directed these studies. S.M.H. is also the guarantor of the study. All authors participated in writing, proof reading, and/or editing the manuscript.
REFERENCES
- 1. Andréjak C, Thomsen VØ, Johansen IS, et al. . Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010; 181:514–21. [DOI] [PubMed] [Google Scholar]
- 2. Lai CC, Tan CK, Chou CH, et al. . Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis 2010; 16:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I.. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health 2010; 10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Ingen J, Bendien SA, de Lange WC, et al. . Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax 2009; 64:502–6. [DOI] [PubMed] [Google Scholar]
- 5. Prevots DR, Shaw PA, Strickland D, et al. . Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 7. Kim RD, Greenberg DE, Ehrmantraut ME, et al. . Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178:1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colombo RE, Hill SC, Claypool RJ, et al. . Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest 2010; 137:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis 2015; 15:968–80. [DOI] [PubMed] [Google Scholar]
- 10. Greinert U, Schlaak M, Rüsch-Gerdes S, et al. . Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol 2000; 20:445–52. [DOI] [PubMed] [Google Scholar]
- 11. Hallstrand TS, Ochs HD, Zhu Q, Liles WC. Inhaled IFN-gamma for persistent nontuberculous mycobacterial pulmonary disease due to functional IFN-gamma deficiency. Eur Respir J 2004; 24:367–70. [DOI] [PubMed] [Google Scholar]
- 12. Kwon YS, Kim EJ, Lee SH, et al. . Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung 2007; 185:337–41. [DOI] [PubMed] [Google Scholar]
- 13. Safdar A, Armstrong D, Murray HW. A novel defect in interferon-gamma secretion in patients with refractory nontuberculous pulmonary mycobacteriosis. Ann Intern Med 2003; 138:521. [DOI] [PubMed] [Google Scholar]
- 14. Kartalija M, Ovrutsky AR, Bryan CL, et al. . Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013; 187:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noone PG, Leigh MW, Sannuti A, et al. . Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004; 169:459–67. [DOI] [PubMed] [Google Scholar]
- 16. Barbato A, Frischer T, Kuehni CE, et al. . Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J 2009; 34:1264–76. [DOI] [PubMed] [Google Scholar]
- 17. Farrell PM, Rosenstein BJ, White TB, et al. ; Cystic Fibrosis Foundation Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008; 153:S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boniface K, Blumenschein WM, Brovont-Porth K, et al. . Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol 2010; 185:679–87. [DOI] [PubMed] [Google Scholar]
- 19. Lee YK, Turner H, Maynard CL, et al. . Late developmental plasticity in the T helper 17 lineage. Immunity 2009; 30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szymanski EP, Leung JM, Fowler CJ, et al. . Pulmonary nontuberculous mycobacterial infection. a multisystem, multigenic disease. Am J Respir Crit Care Med 2015; 192:618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safdar A, White DA, Stover D, et al. . Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am J Med 2002; 113:756–9. [DOI] [PubMed] [Google Scholar]
- 22. Vankayalapati R, Wizel B, Samten B, et al. . Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis 2001; 183:478–84. [DOI] [PubMed] [Google Scholar]
- 23. Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol 2010; 137:296–302. [DOI] [PubMed] [Google Scholar]
- 24. Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res 2007; 38:342–6. [DOI] [PubMed] [Google Scholar]
- 25. Buckley RH. Primary cellular immunodeficiencies. J Allergy Clin Immunol 2002; 109:747–57. [DOI] [PubMed] [Google Scholar]
- 26. Pozniak A. Mycobacterial diseases and HIV. J HIV Ther 2002; 7:13–6. [PubMed] [Google Scholar]
- 27. Kim SY, Koh WJ, Park HY, et al. . Changes in serum immunomolecules during antibiotic therapy for Mycobacterium avium complex lung disease. Clin Exp Immunol 2014; 176:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puel A, Cypowyj S, Bustamante J, et al. . Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun 2010; 78:4187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cruz A, Ludovico P, Torrado E, et al. . IL-17A promotes intracellular growth of Mycobacterium by inhibiting apoptosis of infected macrophages. Front Immunol 2015; 6:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scriba TJ, Kalsdorf B, Abrahams DA, et al. . Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 2008; 180:1962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okada S, Markle JG, Deenick EK, et al. . Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015; 349:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dranoff G, Crawford AD, Sadelain M, et al. . Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994; 264:713–6. [DOI] [PubMed] [Google Scholar]
- 34. Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol 2005; 25:405–28. [DOI] [PubMed] [Google Scholar]
- 35. Szeliga J, Daniel DS, Yang CH, Sever-Chroneos Z, Jagannath C, Chroneos ZC. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinborough) 2008; 88:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Silva TI, Cope A, Goepel J, Greig JM. The use of adjuvant granulocyte-macrophage colony-stimulating factor in HIV-related disseminated atypical mycobacterial infection. J Infect 2007; 54:e207–10. [DOI] [PubMed] [Google Scholar]
- 37. Blanchard DK, Michelini-Norris MB, Pearson CA, McMillen S, Djeu JY. Production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by monocytes and large granular lymphocytes stimulated with Mycobacterium avium-M. intracellulare: activation of bactericidal activity by GM-CSF. Infect Immun 1991; 59:2396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez-Juarrero M, Hattle JM, Izzo A, et al. . Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol 2005; 77:914–22. [DOI] [PubMed] [Google Scholar]
- 39. Milner JD, Brenchley JM, Laurence A, et al. . Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008; 452:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vargas L, Patiño PJ, Rodríguez MF, et al. . Increase in granulocyte-macrophage-colony-stimulating factor secretion and the respiratory burst with decreased L-selectin expression in hyper-IgE syndrome patients. Ann Allergy Asthma Immunol 1999; 83:245–51. [DOI] [PubMed] [Google Scholar]
- 41. Melia E, Freeman AF, Shea YR, et al. . Pulmonary nontuberculous mycobacterial infections in hyper-IgE syndrome. J Allergy Clin Immunol 2009; 124:617–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sampaio EP, Elloumi HZ, Zelazny A, et al. . Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am J Respir Cell Mol Biol 2008; 39:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benator DA, Gordin FM. Nontuberculous mycobacteria in patients with human immunodeficiency virus infection. Semin Respir Infect 1996; 11:285–300. [PubMed] [Google Scholar]
- 44. Leung JM, Olivier KN. Nontuberculous mycobacteria: the changing epidemiology and treatment challenges in cystic fibrosis. Curr Opin Pulm Med 2013; 19:662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atkins BL, Gottlieb T. Skin and soft tissue infections caused by nontuberculous mycobacteria. Curr Opin Infect Dis 2014; 27:137–45. [DOI] [PubMed] [Google Scholar]
- 46. Belli F, Capra A, Moraiti A, et al. . Cytokines assay in peripheral blood and bronchoalveolar lavage in the diagnosis and staging of pulmonary granulomatous diseases. Int J Immunopathol Pharmacol 2000; 13:61–7. [PubMed] [Google Scholar]
- 47. Wakeham J, Wang J, Magram J, et al. . Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guérin in IL-12-deficient mice. J Immunol 1998; 160:6101–11. [PubMed] [Google Scholar]
- 48. Fowler CJ, Olivier KN, Leung JM, et al. . Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med 2013; 187:1374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.