Abstract
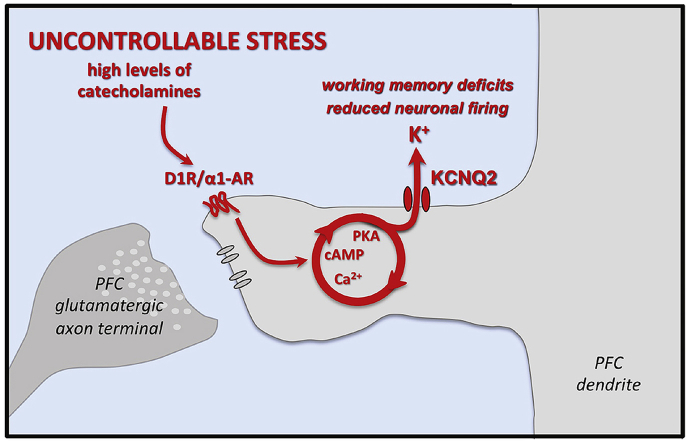
The prefrontal cortex (PFC) mediates higher cognition but is impaired by stress exposure when high levels of catecholamines activate calcium-cAMP-protein kinase A (PKA) signaling. The current study examined whether stress and increased cAMP-PKA signaling in rat medial PFC (mPFC) reduce pyramidal cell firing and impair working memory by activating KCNQ potassium channels. KCNQ2 channels were found in mPFC layers II/III and V pyramidal cells, and patch-clamp recordings demonstrated KCNQ currents that were increased by forskolin or by chronic stress exposure, and which were associated with reduced neuronal firing. Low dose of KCNQ blockers infused into rat mPFC improved cognitive performance and prevented acute pharmacological stress-induced deficits. Systemic administration of low doses of KCNQ blocker also improved performance in young and aged rats, but higher doses impaired performance and occasionally induced seizures. Taken together, these data demonstrate that KCNQ channels have powerful influences on mPFC neuronal firing and cognitive function, contributing to stress-induced PFC dysfunction.
Keywords: cAMP-PKA, KCNQ, Pyramidal neurons, Working memory, Stress, Prefrontal cortex
Graphical abstract
1. Introduction
The prefrontal cortex (PFC) guides behavior by representational knowledge, a process often probed using working memory (WM) tasks in rodents, monkeys and humans. Lesions to the medial PFC (mPFC) in rats impair spatial WM performance (Larsen and Divac, 1978), while recordings from this region show neurons with persistent firing during the delay period, which are thought to mediate WM (Yang et al., 2014). Exposure to either acute or chronic uncontrollable stress impairs PFC WM functions, a finding of great relevance to the etiology of mental disorders (A. F. Arnsten, 2015). For example, both schizophrenia and bipolar disorder are characterized by PFC dysfunction (Blumberg et al., 2003; Hoftman et al., 2017; Weinberger et al., 1986), and are exacerbated by stress exposure (Docherty et al., 1994; Leverich et al., 1990). Furthermore, recent evidence suggests that the degenerative process in Alzheimer's disease may also be exacerbated by stress exposure during aging (Johansson et al., 2013). Thus, understanding the molecular mechanisms by which stress causes PFC dysfunction has a direct clinical relevance.
Research to date has found that stress weakens PFC network connections, reduces PFC neuronal firing, and impairs working memory performance through high levels of catecholamine release in the PFC, which in turn activate feedforward calcium-cAMP signaling to open HCN channels (A. F. Arnsten, 2015). Acute mild stress exposure can impair PFC cognitive functions in rodents (Murphy et al., 1996; Shansky et al., 2006), monkeys (A. F. T. Arnsten and Goldman-Rakic, 1998; Murphy et al., 1996), and humans (Hartley and Adams, 1974; Qin et al., 2009). These studies have used a variety of environmental stressors (e.g. restraint stress in rats, noise stress in monkeys and humans). They have also used the benzodiazepine inverse agonist FG7142 to induce a stress response, as it activates anxiety circuits, mimics the effects of environmental stressors, and induces glucocorticoid release in blood and catecholamine release in the PFC. For example, FG7142 has been shown to induce glucocorticoid release in rodents (Leidenheimer and Schechter, 1988; Patki et al., 2015), monkeys (Ninan et al., 1982) and humans (Dorow et al., 1983), and has been shown to activate a fear/anxiety circuit in rodent brain (Singewald et al., 2003). Thus, FG7142 can be a useful experimental tool. FG7142 also has the advantage of being active during cognitive testing, in contrast to stressors such as restraint stress where the animal can only be tested after release from the stressor. There is extensive evidence that stress-induced working memory deficits arise from excessive catecholamine actions in the PFC, engaging feedforward cAMP-calcium signaling to open HCN channels and reduced neuronal firing (A. F. Arnsten, 2015). For example, the cognitive deficits induced by environmental stressors or FG7142 can be reversed by dopamine D1R (A. F. T. Arnsten and Goldman-Rakic, 1998; Murphy et al., 1996) or norepinephrine alpha-1R (S. G. Birnbaum et al., 1999) blockade, or by blocking HCN channels in PFC (N.J. Gamo et al., 2015). Conversely, stress-induced working memory deficits can be mimicked by increasing D1R (A. F. T. Arnsten et al., 2015; N.J. Gamo et al., 2015; Vijayraghavan et al., 2007; J. Zahrt et al., 1996) or alpha-1R (S. B. Birnbaum et al., 2004; Mao et al., 1999) stimulation in the PFC, or by activating cAMP-PKA signaling (J. R. Taylor et al., 1999; Wang et al., 2007). For example, high levels of dopamine D1R stimulation increase cAMP signaling, which opens nearby HCN channels, reduce PFC neuronal firing, and impair WM in rats and monkeys (N. J. Gamo et al., 2015).
Similar mechanisms contribute to age-related WM deficits, where cAMP signaling is disinhibited in the aged rodent and primate PFC due to loss of phosphodiesterase PDE4A (Carlyle et al., 2014; Ramos et al., 2003). For example, age-related WM deficits correlate with increased protein kinase A (PKA) signaling in mPFC (Ramos et al., 2003). Recordings from the dorsolateral PFC (dlPFC) in monkeys have found an age-related decline in persistent firing that could be normalized by inhibiting cAMP opening of HCN channels (Wang et al., 2011). Intriguingly, neuronal firing in aged dlPFC could also be restored through blockade of KCNQ channels (Wang et al., 2011), potassium channels whose open state is increased by cAMP activation of PKA. These data suggest that KCNQ channels may also play a key role in WM impairment with age or stress.
KCNQ channels are of great interest, as they have powerful influences on firing patterns (Greene and Hoshi, 2017; Gu et al., 2005; Guan et al., 2011; Peng et al., 2017; Santini and Porter, 2010; Yue and Yaari, 2004), and because their open state is regulated by many neuromodulators and second messenger actions, and thus can serve as the intermediary for altering neuronal excitability based on arousal state (Delmas and Brown, 2005; Greene and Hoshi, 2017). KCNQ channels are also called Kv7 channels or “M” channels (mediating the M-current, IM), as they are closed by muscarinic receptor stimulation (Brown and Adams, 1980; Delmas and Brown, 2005). The 4 isoforms of KCNQ channels: KCNQ2, KCNQ3, KCNQ4 and KCNQ5 (Delmas and Brown, 2005; Jentsch, 2000) can form homomers or heteromers, e.g. KCNQ2/KCNQ3 or KCNQ3/KCNQ5. Importantly, the open state of the KCNQ2 isoform, and its heteromer KCNQ2/KCNQ3, are increased by PKA phosphorylation. This mechanism likely plays a negative feedback role to prevent seizures in a recurrent network, as mutations that prevent PKA phosphorylation of KCNQ2 are associated with seizures (Delmas and Brown, 2005; Schroeder et al., 1998). KCNQ2 mRNA is expressed in neurons in both the superficial and deep layers of rat medial PFC (Saganich et al., 2001), indicating that these receptors may influence neuronal firing in PFC.
We hypothesize that large increases in KCNQ2 opening may also contribute to reduced PFC neuronal firing and induce cognitive deficits during acute or chronic stress exposure (A. F. Arnsten, 2015), and with advanced age (Carlyle et al., 2014; Ramos et al., 2003) when cAMP-PKA signaling is increased, and WM is impaired. The role of KCNQ channels in PFC physiology and working memory function was tested in rats, in which intracellular recordings of KCNQ currents were performed from the prelimbic mPFC neurons. We utilized bath application of forskolin to mimic the acute stress response in vitro, and administrated FG7142 systemically to test for effects on stress-induced working memory deficits. The findings show that cAMP-PKA signaling or stress exposure increases KCNQ currents, reduces mPFC pyramidal cell firing, and impairs WM performance.
2. Materials and methods
All procedures described followed the National Institutes of Health guidelines outlined in “Preparation and Maintenance of Higher Animals During Neuroscience Experiments” (publication 91–3207) and were approved by the Yale University Institutional Animal Care and Use Committee.
2.1. Immunohistochemistry
For KCNQ2 channel immunolabeling, 3 male, 4 to 6-week-old Sprague-Dawley rats were anesthetized with Nembutal (50 mg/mL, i.p.) and perfused transcardially with 4% paraformaldehyde, 0.05% glutaraldehyde and 0.2% picric acid in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed and sectioned coronally at 60 μm. Sections of the mPFC were transferred for 1 h to Tris-buffered saline (TBS) containing 5% bovine serum albumin, plus 0.05% Triton X-100 to block non-specific reactivity, and incubated in mouse KCNQ2 channel antibody (clone S26-A-23; Abcam, Cambridge, MA; labels a protein of appropriate molecular weight: https://www.abcam.com/kcnq2-antibody-n26a23-ab84812.html#description_images_3) at 1:100 in TBS for 48 h at 4 °C. Next, sections were incubated in goat anti-mouse biotinylated antibody (Vector Laboratories, Burlingame, CA) at 1:300 in TBS for 2 h, and developed using the Elite ABC kit (Vector Laboratories) and diaminobenzidine (DAB) as a chromogen. In a parallel set of experiments, 200 μm-thick sections from the series prepared for electrophysiological recordings were immersion-fixed in 4% paraformaldehyde in PB overnight, and immunolabeled for KCNQ2 channels as described above. Omission of the primary antibody eliminated all labeling (see Fig. 1d). Sections were mounted on microscope slides and examined and photographed under an Axiophot microscope equipped with Axiocam camera (Zeiss, Jena, Germany).
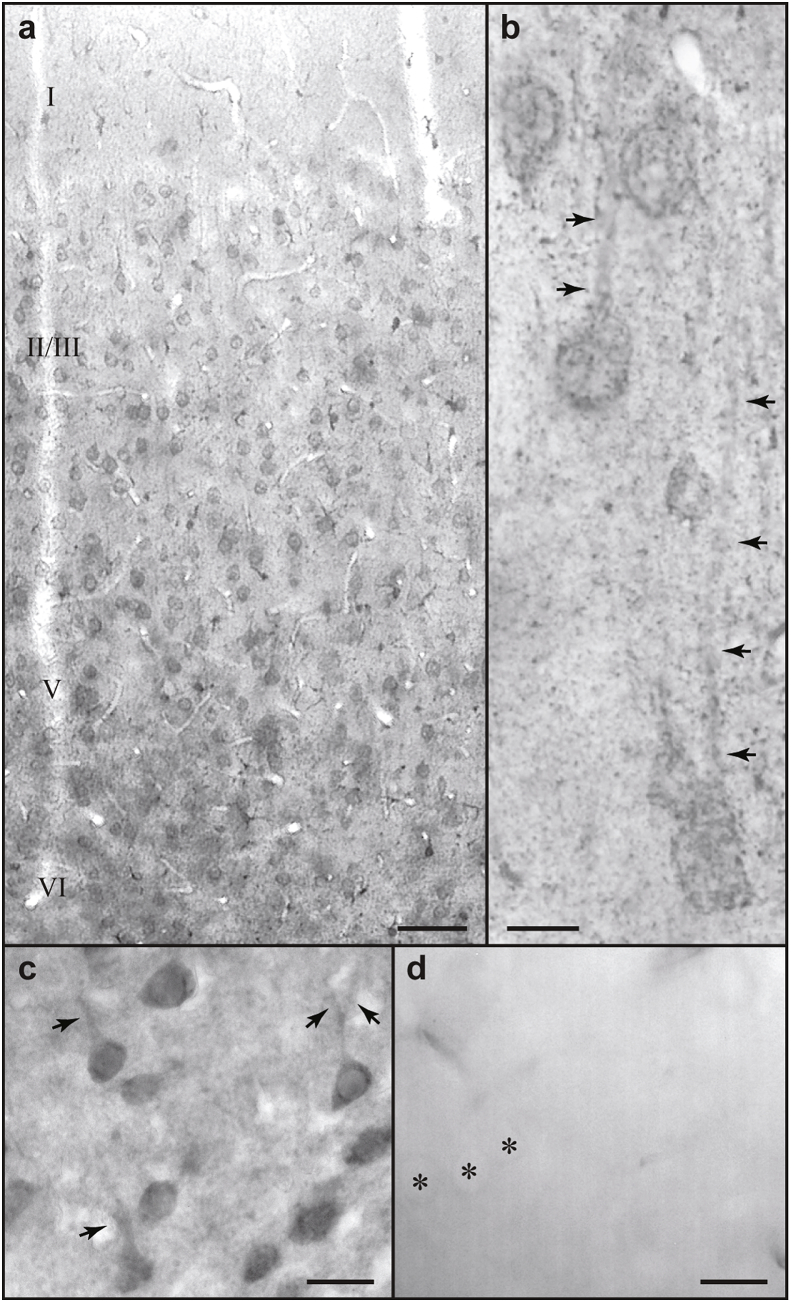
Fig. 1.
Expression of KCNQ2 channels in rat mPFC. A. Pyramidal neurons in layers II-VI are immunolabeled against KCNQ2. B and C Note the reactive pyramidal somata and principal apical dendrites (arrows) in layer V (B) and in layers II/III (C). D. Omission of the anti-KCNQ2 primary antibody eliminated all labeling; asterisks mark non-reactive pyramidal neurons. Scale bars, 50 μm (a); 10 μm (b); 20 μm (c and d).
2.2. Slice preparation and electrophysiology
Acute mPFC slices (200–350 μm) including the prelimbic, infralimbic, and cingulate cortices anterior to the corpus callosum, were extracted from adult 6-15 week-old male Sprague-Dawley rats (Hagenston et al., 2008). Recordings were made from pyramidal cells in the prelimbic region of the mPFC from both superficial (II/III) and deep (V) layers and were performed in a chamber continuously perfused (1–2 mL/min) with oxygenated and warmed (31–33 °C) artificial cerebrospinal fluid containing (in mM): 124 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, 10 dextrose. Recordings were made from visualized mPFC pyramidal neurons using an upright Zeiss Axioskop 2 FS or Olympus BX51WI with DIC optics. Recording electrodes (3–5 MΩ) were filled with (in mM): 134 KMeSO4, 3.0 KCl, 10 HEPES, 1.0 MgCl2, 4.0 Mg-ATP, 0.5 Na-GTP, 5 K2-phosphocreatine, 5.0 Na2-phosphocreatine (pH 7.5, ~288 mOsm). The inert fluorescent dye Alexa 488 was also included in some recordings for better visualization of neuronal processes (5–15 μM). Whole-cell patch-clamp recordings of mPFC pyramidal neurons were performed using either SEC 05L (NPI, Electronic, Tamm, Germany) or Multiclamp 700B (Molecular Device) amplifiers. Neurons > −50 mV were discarded, regardless of the treatment. Whole-cell series resistance was between 10 and 20 MΩ for all cells; more than 20% variation led to rejection of the cell. Voltage-clamp recordings of KCNQ currents were performed using the same amplifier. Access and holding current were continuously monitored for stability, and a 20% increase led to rejection of the recorded cell from analysis. Averaged resting membrane potential was −60.53 ± 0.47 mV for control and −59.83 ± 0.73 mV for the stressed group. All data collection and data analysis software were custom written in the IGOR Pro programming environment (WaveMetrics, Portland, OR).
2.2.1. KCNQ current recordings
We used the voltage-clamp protocol first described by Constanti A. and Brown D.A. in 1981, which involves depolarizing the cell membrane from its resting membrane potential of ~ −63 mV to holding potentials of – 40 mV for 1 s duration, followed by hyperpolarizing steps of 20 mV over 1 s duration and a step back to – 40 mV (for 2 more sweeps) before returning to holding potential of ~ −63 mV (at the end of the 3rd sweep). KCNQ currents were averaged from 3 sweeps per set of recordings. The cell was held near resting membrane potential between periods of KCNQ current recording. Recordings of KCNQ currents in mPFC pyramidal neurons were performed in the presence of Na+, Ca2+ and HCN channels blockers (TTX: 1 μM; CdCl2: 200 μM; ZD7288: 10 μM). These blockers were not used during current-clamp experiments. To quantify the magnitude of the KCNQ current, we analyzed the amplitude of the outward current (Iout or holding current) induced by depolarizing the membrane from its resting membrane potential of (~ −63 mV) to ~ −40 mV for 1 s duration before and after drugs. We also measured the relaxation of KCNQ currents (slow inward current following the instantaneous or ohmic inward current decline) by calculating the difference between the instantaneous peak current at the command onset and the average of the steady state current within the last 100 ms just before the command offset of the hyperpolarization step. In most cases, when characterizing KCNQ currents, only one hyperpolarizing step of 20 mV was used. Additional hyperpolarizing steps of 40 and 50 mV were added to better investigate changes in the magnitude of KCNQ currents occurring in the stressed group of animals (Fig. 5).
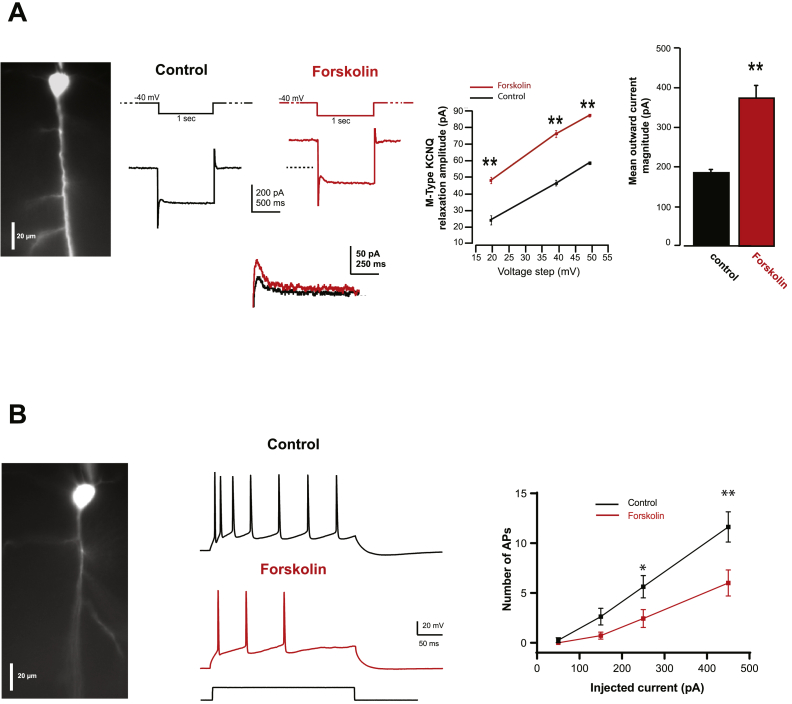
Fig. 5.
cAMP-PKA signaling increases KCNQ currents and reduces the firing of mPFC pyramidal cells.
Voltage-clamp recordings of IM-KCNQ currents (A) and current-clamp recordings (B) of mPFC pyramidal neurons, filled with an inert fluorescent dye (Alexa 488) during whole-cell recordings, before and after bath application of the cAMP-PKA signaling activator, forskolin (10 μM). Raising cAMP with forskolin increased KCNQ currents and decreased firing in mPFC pyramidal cells. A. Representative trace of KCNQ currents in response to a depolarizing voltage step from cell's resting membrane potential (~−63 mV) to a holding potential of ~−40 mV for 1 s followed by a hyperpolarizing step of 20 mV for 1 s. Note the increase in IOut amplitude under forskolin quantified in the summary graph. The relaxation amplitude of KCNQ current was also quantified in this set of experiment in response to 3 different hyperpolarizing voltage steps (20, 40 and 50 mV). Note the significant increase in the KCNQ relaxation magnitude at all hyperpolarizing voltage steps tested (**p < 0.01, paired Student's t-test, n = 3) B. Current-clamp recordings of APs in response to intracellular depolarizing current (250 pA, 300 ms) in a regular firing type of mPFC pyramidal neurons showing a significant decrease of the number of APs in presence of forskolin (*p < 0.05, **p < 0.01, paired Student's t-test, n = 7).
2.2.2. Firing pattern of mPFC pyramidal neurons
Current-clamp recordings of mPFC pyramidal were performed in both superficial (II/III) and deep (V) layers and revealed that 77% of neurons had a regular firing pattern profile and 23% of neurons had a bursting firing pattern profile in response to a depolarizing injected current pulses. Some of these recordings were performed in the presence of biocytin (0.5%) added into the intracellular recording solution to further confirm the localization of recorded neurons within cell layers of the mPFC.
2.2.3. Pharmacological agents
TTX, CdCl2, ZD7288, Retigabine, Linopirdine and XE991 were obtained from Tocris Bioscience. Biocytin was obtained from Thermo Scientific. Forskolin was obtained from Sigma-Aldrich. Alexa 488 was obtained from Molecular Probes.
2.3. Chronic stress paradigm
Chronic, daily treatment with FG7142 can induce seizures (S. C. Taylor et al., 1988), and thus the effects of chronic stress on KCNQ currents utilized repeated restraint stress, which also impairs working memory function in rats (Hains et al., 2009; Shansky et al., 2006). Sprague Dawley rats (6–12 old weeks, male) were restrained in plastic restraint tubes for 6 h/day for 21 consecutive days. Animals did not have access to food or water during the restraint period but had access to their regulated diet and unlimited water following stress exposure. Animals were monitored for signs of pain or distress during the stress procedure and immediately afterwards in their home cage. If they exhibited signs of acute pain or impaired health, rats were removed from the study.
2.4. Statistical analyses of the ex-vivo study
All data are presented as mean ± SEM. Unless otherwise stated, statistical significance (*p < 0.05; **p < 0.01 and ***p < 0.001) was tested using paired or unpaired Student's t tests (t-test) assuming unequal variance.
2.5. Behavioral analyses
Subjects: The behavioral experiments utilized young (2–3 months old) or aged (22 months old at purchase) male adult Sprague-Dawley rats (Harlan, now Envigo, East Millstone, NJ).
2.6. Performance of the delayed alternation task
Rats were trained in a delayed alternation task in a T-shaped maze (90 cm × 65 cm), as described in Murphy et al., 1996. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 10 trials per session, rats were rewarded only if they entered the maze arm which was not chosen previously. Between trials, the choice point was wiped with alcohol to remove potential olfactory cues. The intertrial delay was adjusted until a rat's performance showed stable baseline performance of ~70–80% correct responses. Rats had to perform at stable baseline for at least two consecutive test sessions prior to subsequent drug treatment. Delays continued to be adjusted for each animal across the course of the study to maintain a baseline of ~70–80%; delays ranged from “0” sec (~2sec from arm to start box), to 60sec across animals. Maintaining a consistent level of baseline performance at ~75% correct allowed for detection of either improvement or impairment during drug administration.
2.7. Intra-PFC infusions
The effects of the KCNQ blocker, linopirdine, or the PKA inhibitor, Rp-cAMPS, on delayed alternation performance were tested through direct drug infusions into the mPFC, Young male Sprague-Dawley rats were implanted with chronic infusion cannulae directed above the prelimbic mPFC (anterior-posterior [AP] +3.2 mm; medial-lateral [ML] ±75 mm; dorsal-ventral [DV] −4.2 mm). In the first experiment (n = 9), linopirdine (0.001 μg) was infused into both sides of PFC (0.5 μL/side) 15 min before testing and compared with vehicle. In studies investigating mechanisms underlying the FG7142 stress response, we tested whether infusion of linopirdine (0.0001–0.01 μg/0.5 μL/side; n = 6) or Rp-cAMPS (21 nmol/0.5 μL/side; n = 5) would prevent working memory deficits. As additive drug effects would interfere with interpretation of the data, it was important to find a dose of linopirdine or Rp-cAMPS that did not improve performance on its own. Thus, pilot experiments were conducted to identify the highest dose that had no effect on performance for each individual animal. This dose was then co-administered with FG7142. Linopirdine and Rp-cAMPS were purchased from Tocris Bioscience, Ellisville, MO, and dissolved and diluted in sterile saline. Animals were required to return to stable baseline testing before subsequent infusions; there was at least a one-week washout between drug doses.
2.8. Systemic injections
The first set of experiments determined whether the detrimental effects of the pharmacological stressor, FG7142, could be reversed by intra-PFC infusion of Rp-cAMPS or linopirdine as described above. A dose of FG7142 (2.5–10 mg/kg, i.p., 30 min before testing) was identified in each rat that produced reliable impairment in delayed alternation performance but still allowed completion of all trials. The FG7142 response was then challenged with intra-PFC infusions drug or vehicle as described above. FG7142 (Tocris, Ellisville, MO) was finely ground and suspended in a saline vehicle containing Tween 80, hydroxybetacylodextrin and ethanol.
In a second set of experiments, the effects of systemic administration of the KCNQ blocker, XE991 (Tocris, Ellisville, MO), were examined on delayed alternation performance (i.p., 15 min prior to testing). The first experiment examined XE991 effects (0.001–2.5 mg/kg) in young adult rats (n = 11). A second experiment evaluated the effects of XE991 (0.001–1.0 mg/kg) in aged rats (n = 10).
Statistical analyses of behavioral studies. Analyses were performed using SPSS. Within-subjects one-way or two-way ANOVA and paired-samples t tests were used to test drug effects on % correct compared to vehicle or FG7142 conditions.
3. Results
3.1. Anatomy
3.1.1. KCNQ2 immunostaining in mPFC
KCNQ2 subunits were localized using a monoclonal antibody against the intracellular N-terminus of the KCNQ2 protein. Immunoreactivity was widespread in all layers of mPFC (Fig. 1A). Directly relevant to our physiological recordings, KCNQ2 channels were found in the soma and along the principal apical dendrites of layer V (Fig. 1B) and layers II/III (Fig. 1C) pyramidal neurons. The punctate neuropil labeling likely reflects KCNQ2 channel expression in fine processes such as in dendritic spines, as omission of the primary antibody eliminated all signal (Fig. 1D).
3.2. Physiology
3.2.1. Overview
KCNQ currents were recorded in pyramidal cells from ex-vivo mPFC slices, using the deactivation protocol described by Adams et al. (1982) (Adams et al., 1982). Briefly, KCNQ current was activated by depolarizing the cell membrane from its resting potential of ~ −63 mV to a holding potential of −40 mV for 1 s in the presence of Na+, Ca2+ and HCN channel blockers. This elicits an outward steady state current termed IOut (indicated by the broken line in Fig. 2) driven by outward potassium currents, including KCNQ channel opening. When this current was deactivated by a hyperpolarizing step (20 mV for 1 s), it revealed the typical slow relaxation of KCNQ currents at the onset of the hyperpolarizing pulse, as shown on Fig. 2A. This protocol was repeated three times (3 sweeps) before returning the cell to its resting membrane potential. For the sake of clarity, only one sweep is illustrated in the figures.
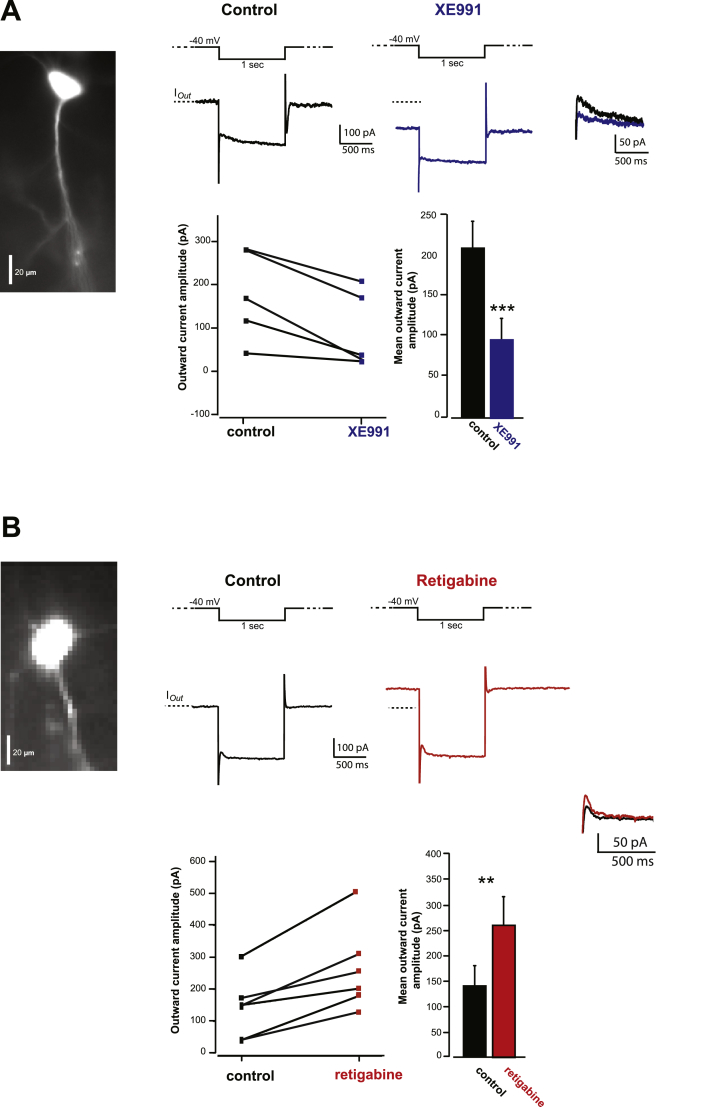
Fig. 2.
Identification of KCNQ currents in rat mPFC pyramidal cells.
A and B. Voltage-clamp recordings of IM-KCNQ currents in mPFC pyramidal neurons before bath application of the specific KCNQ channel blocker (XE991, 10 μM, Fig. 2A) and of the specific KCNQ channel opener (Retigabine, 10 μM, Fig. 2B). Each panel shows an example of a mPFC pyramidal neuron filled with an inert fluorescent dye (Alexa 488) during whole-cell recordings and a representative trace of IM-KCNQ currents in response to a depolarizing voltage step from cell's resting membrane potential (~−63 mV) to a holding potential of −40 mV for 1 s followed by a hyperpolarizing step of 20 mV for 1 s. This protocol elicits a steady state or outward current (IOut) driven by potassium channels opening, including KCNQ channels (indicated by the broken line) followed by the typical relaxation of KCNQ currents at the onset of the hyperpolarization pulse. These recordings were performed in the presence of Na+, Ca2+ and HCN blockers. Note the decrease (A) and the increase (B) of KCNQ currents (represented by IOut) after bath application of XE991 and Retigabine, respectively. Graphs showing the individual and mean values of IOut (**p < 0.01, ***< 0.001, paired Student's t-test).
3.2.2. Characterization of KCNQ currents in mPFC pyramidal neurons
The outward potassium current (Iout) reached at the depolarizing membrane potential was significantly reduced by 46% after addition of the KCNQ-selective channel blocker XE991 (10 μM). Iout decreased from 208 ± 39 pA to 95 ± 27 pA; n = 5, p < 0.001, Student's paired t-test; Fig. 2A). The KCNQ-selective channel blocker XE991 (10 μM) also decreased the slow relaxation amplitude of KCNQ channels (measured by the difference of the peak amplitude between the hyperpolarizing pulse and the steady state current) from 24.6 ± 4.83 pA to 8.68 ± 1.28 pA (p = 0.01, Unpaired Student t-test). Following recording, mPFC slices were fixed and saved for post-hoc analysis. We found that 3/5 cells were recorded from layers II/III, 1/5 was from layer V. These results indicate that KCNQ currents can be recorded from pyramidal cells in layers II/III and V of the mPFC.
To further confirm the presence of KCNQ currents in mPFC pyramidal cells, we investigated the effects of retigabine (10 μM), which increases the open state of KCNQ channels by shifting their activation curve to negative potentials (Tatulian et al., 2001; Wickenden et al., 2000). As shown in Fig. 2B, retigabine increased the Iout by 180% (control: Iout = 141 ± 40 vs. retigabine: Iout = 262 ± 54 pA, n = 6 p < 0.01). Retigabine also increased the slow relaxation amplitude of KCNQ channels from 24.7 ± 1.9 pA to 41 ± 2.2 pA, n = 6 (p < 0.01 Student paired t-test).
In summary, the expression of KCNQ2 subunits in mPFC layers II/III and V, as well as the efficacy of KCNQ-specific compounds on Iout currents, indicate the presence of functional KCNQ channels in mPFC pyramidal neurons.
3.2.3. KCNQ potassium currents regulate the firing rate of mPFC pyramidal neurons
One of the major roles of KCNQ channels is to regulate the intrinsic excitability of neurons (Greene and Hoshi, 2017), limiting the generation of action potentials and therefore controlling neuronal firing patterns (Gu et al., 2005; Guan et al., 2011; Yue and Yaari, 2004). Thus, we examined how manipulations of KCNQ channels would change the firing patterns of mPFC pyramidal neurons.
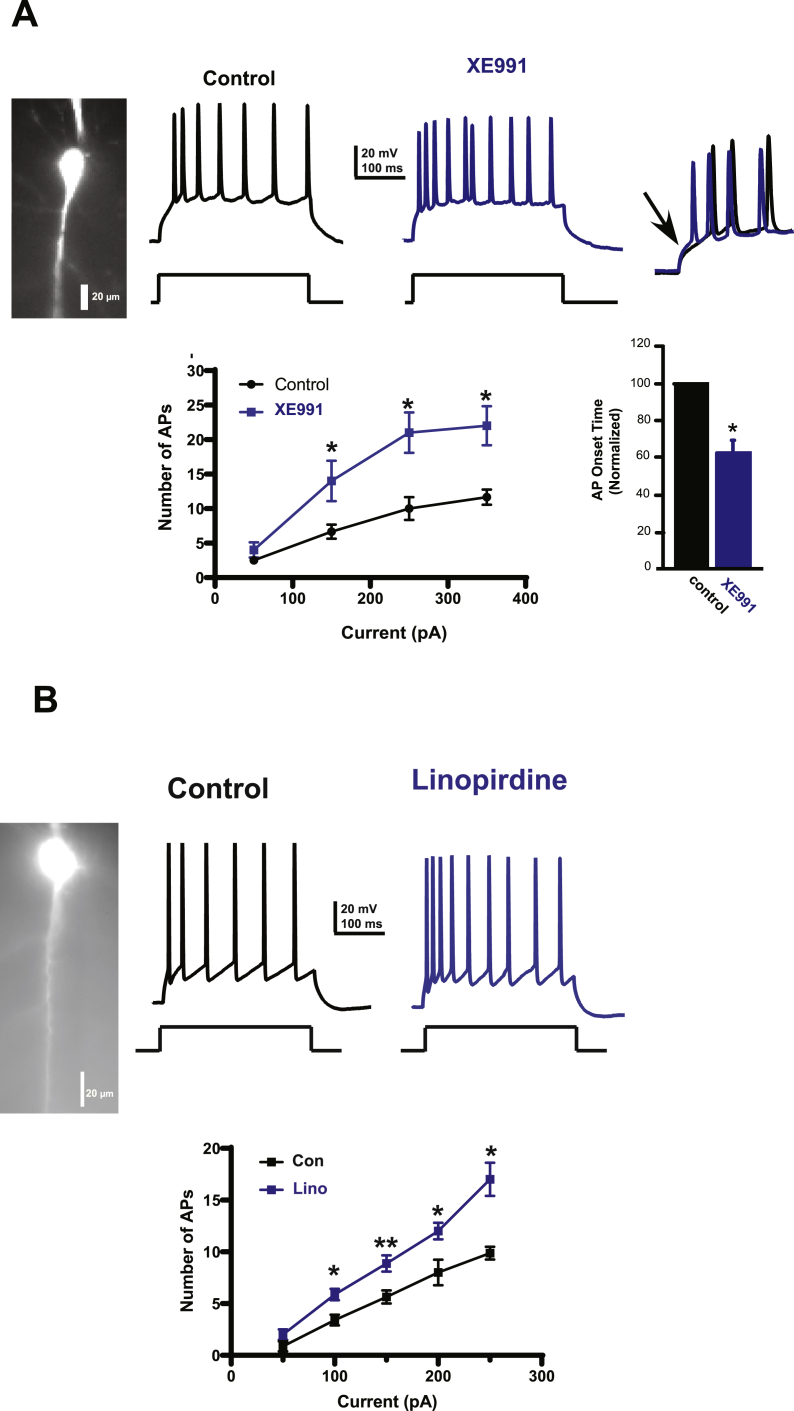
We first tested the effects of the KCNQ-selective channel blocker XE991 (10 μM) on mPFC pyramidal neuronal firing. We found that the KCNQ-specific channel blocker, XE991 (10 μM), significantly increased firing of mPFC pyramidal cells. The number of action potentials (APs) increased from 10 ± 1.65 to 21 ± 3 (250 pA, 300 ms, p < 0.05, paired Student t-test, n = 6, Fig. 3A) with a significant change in the input resistance (Rin), which increased from 121.4 ± 12.5 MΩ in control to 191 ± 32 MΩ under XE991, p < 0.05). We also found that KCNQ channel blockers significantly reduced the spike onset time by ~38%, (Control: 13.64 ± 3.11 ms to 8.35 ± 2.2 ms under XE991, p < 0.05 Student t-test, n = 6, Fig. 3A). Because 10 μM of XE991 has been reported to block additional, non KCNQ channels (Elmedyb et al., 2007; Wladyka and Kunze, 2006), we tested a lower concentration and found that 3 μM of XE991 also produced a significant increase to 220 ± 34% in the number of action potentials (p < 0.05 Student t-test, n = 4, data not shown).
Fig. 3.
Pharmacological inhibition of KCNQ channels increases the firing rate of mPFC pyramidal cells.
Current-clamp recordings of action potentials (APs) in mPFC pyramidal neurons before and after specific KCNQ channel blockers, XE911 (A) and linopirdine (B). A. mPFC pyramidal neuron filled with an inert fluorescent dye (Alexa 488) during whole-cell recordings with a representative trace of APs in response to intracellular depolarizing current (150 pA, 300 ms) before and after XE911 (10 μM). B. Another specific KCNQ channels blocker, linopirdine also increased the number of APs evoked by a depolarizing square pulse (150 pA, 300 ms) Note the significant increase in the APs number and the earlier onset of APs under two different KCNQ blockers. *p < 0.05, **p < 0.01, paired Student's t-test.
Similar effects were found with the KCNQ channel blocker, linopirdine (10 μM), which also increased the number of action potentials from 9.87 ± 0.61 to 17 ± 1.6 in response to injected depolarizing current (250 pA, 300 ms, p < 0.05, paired Student t-test, n = 10, Fig. 3B). Because all cells recorded in this set of experiments showed a linear relation when the change in firing was plotted against the increased amplitude of depolarizing current, we concluded that they might all belong to the regular profile type of firing (van Aerde and Feldmeyer, 2015).
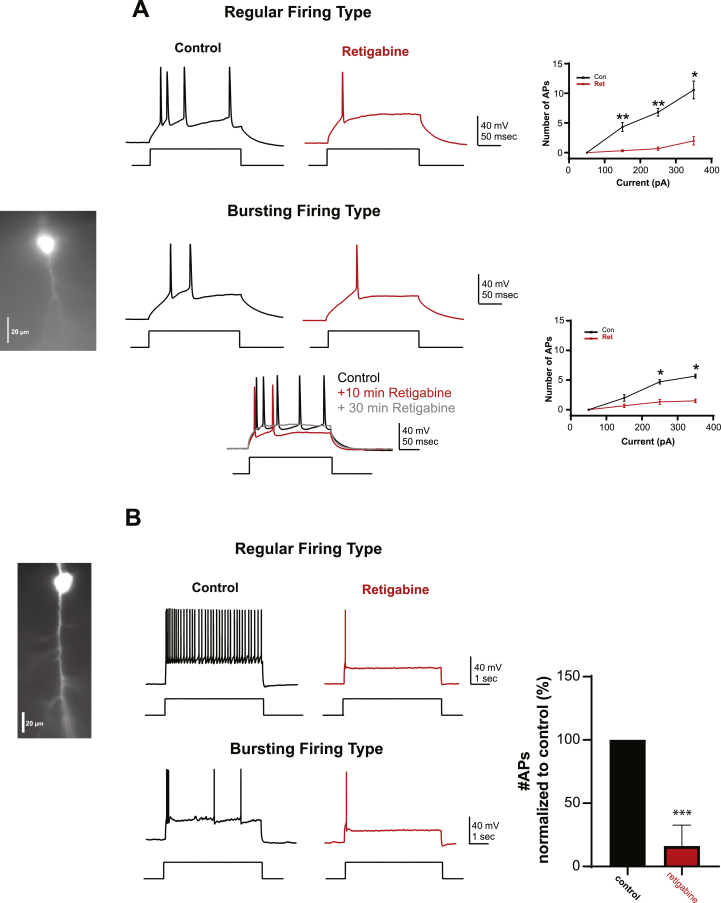
Conversely, increasing the open state of KCNQ channels with retigabine decreased the firing of mPFC pyramidal cells (Fig. 4). In this set of experience, recorded cells (n = 15), exhibited two different types of firing patterns. One group, had a regular firing profile (10/15), as shown by the linear input-output relationship in response to increasing depolarizing current (Fig. 4A, upper right graph). In contrast, the bursting profile neurons (5/15) typically had a non-linear input-output relationship, showing a modest rising phase in the firing frequency in response to increasing depolarizing current, Fig. 4 A, lower right graph).
Fig. 4.
Pharmacological opening of KCNQ decreased firing rate of mPFC pyramidal cells.
A, Representative traces of regular and bursting firing type of mPFC pyramidal neuron before and after bath application of retigabine (10 μM) in response to a short pulse (300 ms) of intracellular depolarizing current. Both traces top (regular) and bottom (bursting) were evoked by same level of stimulation (150 pA). The cell filled with an inert fluorescent dye (Alexa 488) during whole-cell recording corresponds to the bursting type of neuron. Note the progressive decrease in the number of APs following retigabine application over time in a regular firing type of neuron B, Representative trace of a regular and bursting firing type of mPFC pyramidal neuron in response to a longer pulse of stimulation (5 s, 250 pA) with an example of the regular firing neuron type filled with an inert fluorescent dye (Alexa 488) during whole-cell recordings. Longer pulse of stimulation confirmed that retigabine-decreasef the firing frequency in both type of neurons, as shown in the bar graph where the number of APs has been normalized to control (pre-drug treatment). “*”p < 0.05, **p < 0.001 and *** < 0.0001, paired Studen's t-test.
In the regular-firing type of neurons, bath application of retigabine (10 μM) decreased the number of APs from 6.8 ± 0.65 to 0.66 ± 0.3 (p < 0.01, n = 10) in response to depolarizing current (250 pA, 300 ms) and from 10.6 ± 1.5 to 2 ± 0.7 (p < 0.05, n = 10) in response to higher level of depolarizing current (350 pA, 300 ms). In the bursting-firing type group of neurons, the number of APs decreased from 4.71 ± 0.36 to 1.33 ± 0.42 (n = 5, p < 0.05) in response to depolarizing current (250 pA, 300 ms) and from (5.66 ± 0.33 to 1.5 ± 0.3, p < 0.01) in response to higher level of depolarizing current (350 pA, 300 ms). Using a longer pulse of depolarizing current (5 s, 250 pA), we further confirmed the effect of retigabine on the two different group of firing profile. In the regular neurons, retigabine decreased the number of action potentials from 36 ± 6.93 to 0.66 ± 0.33 (p < 0.05, n = 5, Fig. 4B) and in bursting type of neurons, retigabine decreased the number of APs from 6.43 ± 1.2 to 1.8 ± 0.2 (p < 0.05, n = 5, Fig. 4B). Regardless of the firing profile, we found that retigabine significantly decreased firing in both (regular and bursting) type of neurons, as shown in the summary graph of Fig. 4B, in which the number of APs has been normalized to the control condition (pre-retigabine treatment), the percentage of APs is decreased to 16.16 ± 6% after retigabine (10 μM) treatment (p < 0.001, t-test paired, n = 15).
Following application of retigabine, Rin decreased from 136 ± 15 MΩ to 95 ± 15 MΩ (p < 0.05, n = 15), suggesting that retigabine decreased mPFC cell firing through opening of KCNQ channels.
3.2.4. Driving cAMP-PKA signaling increases KCNQ currents in mPFC pyramidal cells
As PKA signaling increases the open probability of KCNQ2 channels, we next tested whether activating cAMP-PKA signaling with forskolin would increase KCNQ currents. Using the same protocol as described in Fig. 2, we found that forskolin (10 μM) significantly increased the Iout current, as well as the relaxation magnitude of KCNQ currents. Depolarizing mPFC pyramidal neurons under forskolin increased Iout current amplitude from 185 ± 13 to 372 ± 68 pA (p < 0.01, Student paired t-test, Fig. 5A). Forskolin also increased the KCNQ relaxation at all voltage steps tested (20, 40 and 50 mV). For example, at 20 mV hyperpolarizing step, the KCNQ relaxation magnitude increased from 23.84 ± 2.72 pA to 47.57 ± 2.02 pA (p < 0.01, Student paired t-test, n = 3, Fig. 5A).
The effects of forskolin were also tested on pyramidal cell firing in response to depolarizing current steps. Under forskolin (10 μM), the number of APs significantly decreased from 5.63 ± 1.11 to 2.43 ± 0.9 in response to depolarizing current (250 pA, 300 ms) and from 11.62 ± 1.52 to 6 ± 1.3 in response to higher level of depolarizing current (450 pA, 300 ms, p < 0.05 and p < 0.01, respectively, n = 7, Student paired t-test, Fig. 5B). The forskolin treated neurons presented all a regular spiking profile of neurons, as indicated by the linear relation of the firing frequency with increasing amplitude of injected current.
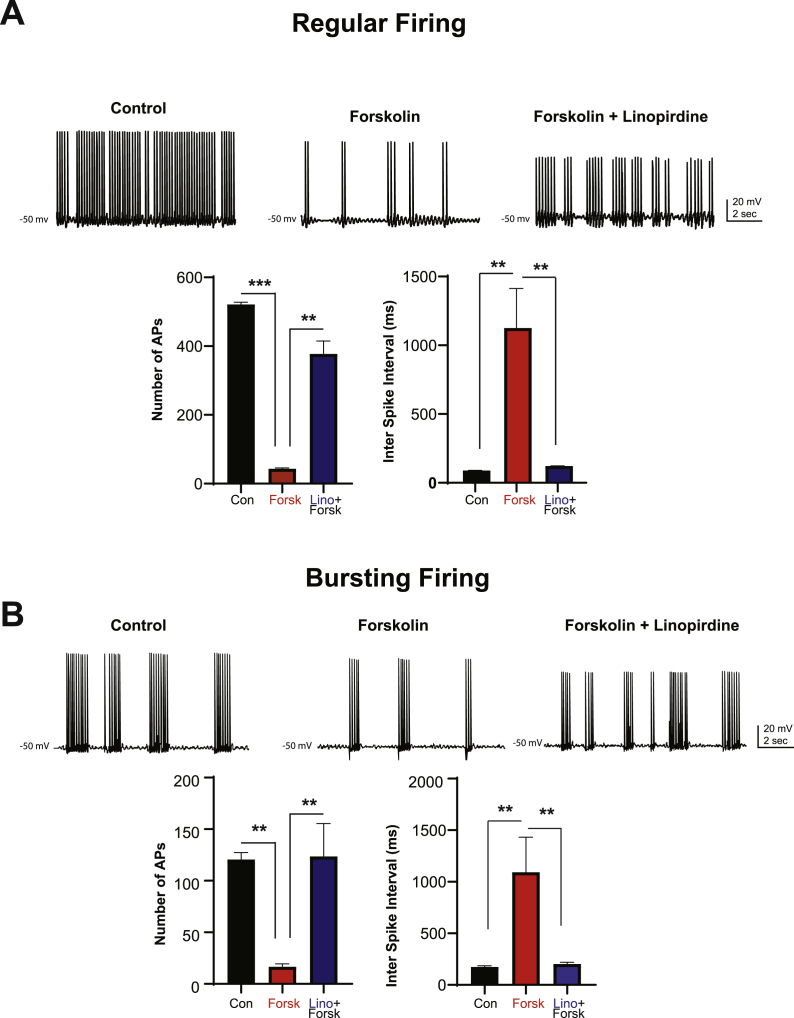
To further prove that stimulation of cAMP signaling, as it may occur during stress decreases mPFC cell firing through opening of KCNQ channels, we next mimicked stress condition by raising levels of cAMP with forskolin and recorded mPFC pyramidal neurons in gap-free current-clamp mode at a holding potentials slightly subthreshold for spiking (~−50 mV) to mimic the active “recurrent” network that occurs during WM task (Goldman-Rakic, 1995). Forskolin (10 μM) was first applied alone (for 10 min) and then co-applied with 10 μM of linopirdine. As shown in Fig. 6, we found that pyramidal neurons fired either with a regular pattern or with a burst pattern of action potentials when held at subthreshold membrane potentials. Bath application of forskolin reduced the number of APs from 521 ± 6.64 to 43 ± 2.91 (p < 0.001, paired student's t-test, n = 3) in regular type of neurons; further addition of linopirdine rescued this decrease in firing, as the number of APs increased from 43 ± 2.91 to 377 ± 37.6 (p < 0.01 paired Student t-test, n = 3, Fig. 6A). This result is consistent with the significant increase in the inter-spike interval from 89.2 ± 1.47 to 1126 ± 287 ms (p < 0.01) under forskolin and with further decrease in the inter-spike interval to 121.3 ± 1.4 ms after addition of linopirdine (p < 0.01 paired Student t-test, n = 3, Fig. 6A). In the bursting type of neurons, bath application of forskolin reduced the number of APs from 121 ± 6.65 to 16.5 ± 2.91 (p < 0.01, paired student's t-test, n = 3); further addition of linopirdine rescued this decrease in firing, as the number of APs increased from 16.5 ± 2.91 to 124 ± 32 (p < 0.01 paired Student t-test, n = 3, Fig. 6B). As in the regular type of firing, bath application of forskolin increased the inter spike interval from 174 ± 11.63 to 1092 ± 340 ms (p < 0.01 paired Student t-test, n = 3, Fig. 6B) and further addition of linopirdine reduced the inter spike interval to 202 ± 17 ms p < 0.01 paired Student t-test, n = 3, Fig. 6B). These data indicate that forskolin-induced rise in cAMP-PKA signaling decreased mPFC cell firing by increasing the activity of KCNQ channels.
Fig. 6.
Blocking KCNQ channels with Linopirdine reversed the Forskolin-induced decrease in mPFC pyramidal neuronal firing.
Representative current-clamp recordings of regular type (A) and bursting (B) type of firing recorded in gap free mode before and after bath application of forskolin alone and then followed by co-application of forskolin with linopirdine (10 μM each). Note that Forskolin decreases firing in both (regular and bursting) type of mPFC pyramidal neurons. This decrease was reversed by addition of KCNQ channels blocker linopirdine. Histograms showing the number of actions potentials (APs) and the number of inter-spike interval (within 500 ms bin period) before and after bath application of forskolin alone and co-application of forskolin with linopirdine. (***p < 0.001 and **p < 0.01, paired Student t-test).
3.2.5. Chronic stress exposure induces an increase in KCNQ currents
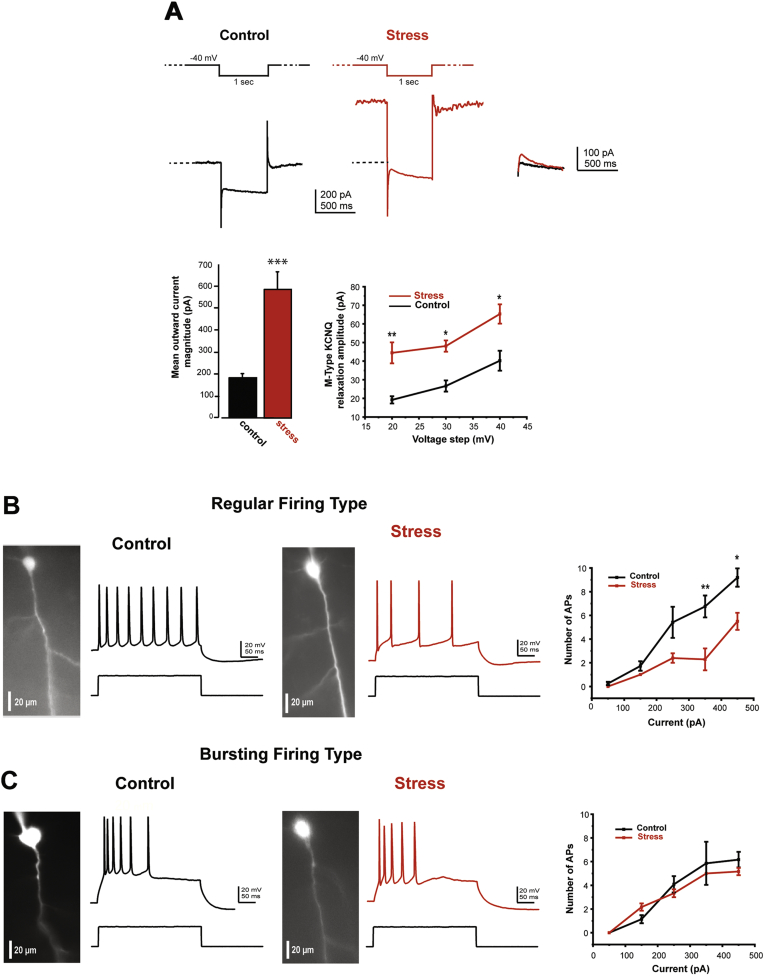
Stress exposure, like forskolin, increases cAMP-PKA signaling in the rat mPFC (A. F. Arnsten, 2015). Thus, we tested whether KCNQ currents were increased in pyramidal cells from rats that had been exposed to chronic restraint stress. We found that chronic stress exposure significantly increased the magnitude of KCNQ currents (Iout in stressed animals was 587 ± 82 pA, n = 7 vs Iout in controls: 182 ± 23 pA, n = 11, p < 0.001, Student unpaired t-test, Fig. 7A). The relaxation amplitude of KCNQ currents in response to hyperpolarizing steps was also increased by 230%. For example, at 20 mV hyperpolarizing step, the relaxation amplitude increased from 19.19 ± 6.62 pA, (n = 11) to 44.44 ± 5.62 pA (n = 7, p < 0.001, Student unpaired t-test, Fig. 7A). Post-hoc analysis of recorded cells revealed that 6/7 neurons were located in layer II/III mPFC (cell filling was unsuccessful for the 7th neuron).
Fig. 7.
Chronic stress exposure increases KCNQ currents in mPFC pyramidal cells
A. Representative trace of KCNQ currents in response to a depolarizing voltage step from cell's resting membrane potential (~−63 mV) to a holding potential of ~−40 mV for 1 s followed by a hyperpolarizing step of 20 mV for 1 s in control and in stressed group of animals. Note that chronic stress exposure significantly increased IOut amplitude (**p < 0.01, unpaired Student's t-test) and the relaxation amplitude of KCNQ currents induced by 20 mV hyperpolarizing step (**p < 0.01, unpaired Student's t-test), and by 30 and 40 mV hyperpolarizing steps (*p < 0.05, unpaired Student's t-test). B and C Current-clamp recordings of APs in response to intracellular depolarizing current (450 pA, 300 ms) in regular-firing (B) and in bursting firing (C) type of mPFC pyramidal neurons in control and stressed group of animals. Graphs showing the effect of stress on the number of action potentials with increasing depolarizing currents. Note that chronic stress exposure reduced firing in the regular-type (**p < 0.01 and *p < 0.05, unpaired Student's t-test) but not in the bursting-type of mPFC pyramidal cells. All recorded cells presented in this figure were filled with an inert fluorescent dye (Alexa 488). Interestingly, the regular firing-type of cells that were affected by chronic stress exposure were located in both (II/III) and (V) cell layers.
A second experiment examined the effects of chronic restraint stress on mPFC neuronal firing rates. We found that chronic stress exposure significantly reduced the firing rate of regular type of neurons without changing the firing rate of bursting type. APs of regular firing decreased from 6.75 ± 0.92 to 2.28 ± 0.3 (p < 0.01) and from 9.2 ± 0.77 to 5.5 ± 0.71 (p < 0.05) in chronically-stressed animals in response to depolarizing current (350 and 450 pA, respectively, n = 30 (control) and n = 6 (stress), Student unpaired t-test, Fig. 7B). However, APs of burst-firing neurons was unchanged by stress exposure (control: 6.16 ± 2.23, n = 11 vs. stress: 5.16 ± 0.3, n = 6, p = 0.3, 450 pA, 300 ms, Student unpaired t-test, Fig. 7C). Post-hoc analysis of the regular firing neurons from stressed rats revealed that 3 neurons were located in layers II/III, 2 neurons was located in layer V, and 1 neuron were unidentified because of poor cell filling.
3.3. Behavior
3.3.1. Blocking KCNQ channels in rat mPFC improves spatial working memory performance
To test the functional significance of these physiological data, we first examined the effects of blocking KCNQ channels in mPFC on spatial working memory (WM) performance. Rats were tested on the spatial delayed alternation task in a T maze, a task that requires WM, decision-making, behavioral inhibition, and depends upon the integrity of the mPFC (Larsen and Divac, 1978). This is the same task used to document WM deficits with stress (Murphy et al., 1996; Shansky et al., 2006), increased catecholamine, calcium-cAMP-PKA signaling (J. R. Taylor et al., 1999; J. Zahrt et al., 1997), or advanced age (Ramos et al., 2003).
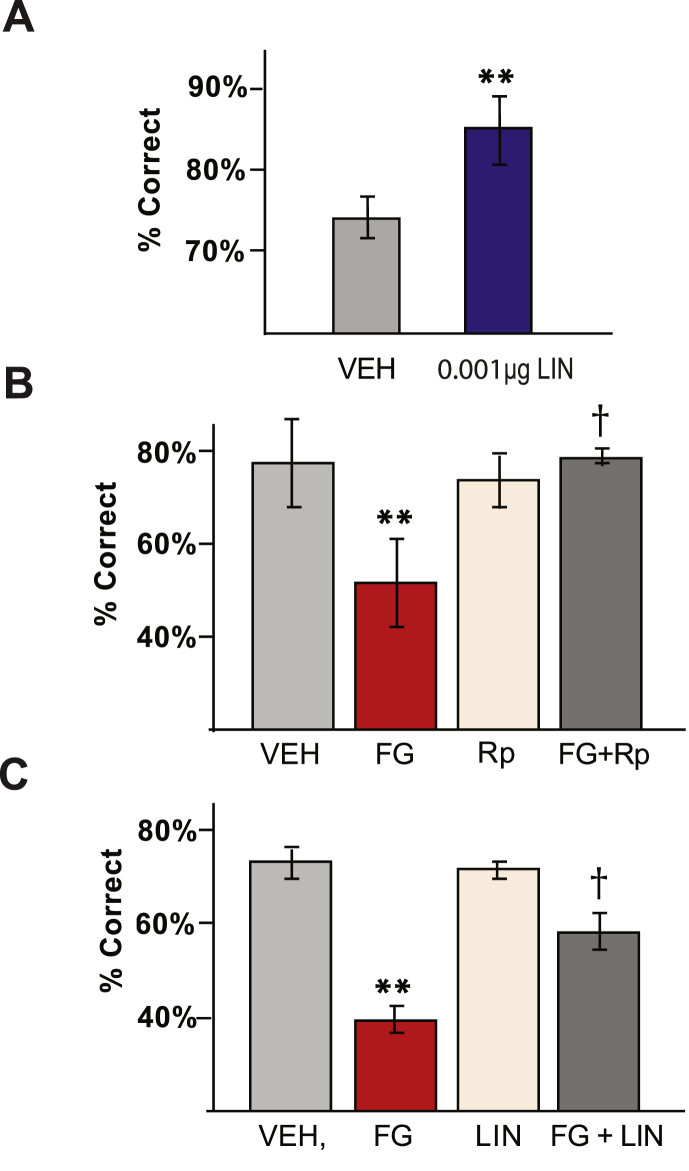
The KCNQ channel blocker, linopirdine, was used in the current study to facilitate translational significance, as we have previous experience with this compound in patients with Alzheimer's Disease (van Dyck et al., 1996). We tested the effects of linopirdine infusions aimed just above the prelimbic mPFC, the same site where infusions of D1 receptor agonists or cAMP-PKA activators have been shown to impair task performance (J. R. Taylor et al., 1999; J. Zahrt et al., 1997), and where KCNQ currents were recorded in ex-vivo mPFC slices in the current study. Bilateral intra-PFC infusion of a low dose of linopirdine, significantly improved performance in the delayed alternation task in young adult rats. Delays were adjusted such that the rats performed at about 75% correct (average of 74.4 ± 3.1% correct) following vehicle infusions, thus leaving room for either impairment or improvement in task performance with drug treatment. Infusion of a low dose of linopirdine (0.001 μg/0.5 μL) significantly improved performance (84.4 ± 4.0% correct, p = 0.017, n = 9, paired T test) compared to vehicle control (Fig. 8A).
Fig. 8.
Blockade of KCNQ channels within mPFC improves delayed alternation performance and prevents stress-induced working memory deficits
A. Infusion of the KCNQ blocker, linopirdine (0.001 μg/0.5 μL) into the rat mPFC improves working memory performance in young adult rats (**p < 0.02; n = 6). B. Infusion of the PKA inhibitor, Rp-cAMPS, into the rat mPFC blocked the delayed alternation deficits induced by the pharmacological stressor, FG7142, in young adult rats. A dose of Rp-cAMPS was chosen for each rat that had no effect on its own to prevent additive effects (**p < 0.001 compared to vehicle; †p < 0.05 compared to FG7142; n = 5). C. Infusion of the KCNQ blocker, linopirdine, into the rat mPFC blocked the delayed alternation deficits induced by the pharmacological stressor, FG7142, in young adult rats. A dose of linopirdine was chosen for each rat that had no effect on its own to prevent additive effects (**p < 0.001 compared to vehicle; †p < 0.02 compared to FG7142; n = 6).
3.3.2. KCNQ potassium channels in mPFC contribute to FG7142-induced cognitive deficits
Given that stress activates cAMP signaling in the mPFC, we tested whether cAMP-PKA opening of KCNQ channels contributes to stress-induced WM deficits. The anxiogenic pharmacological stressor, FG7142, was utilized, as this agent produces a stable stress response, i.e. animals do not habituate or sensitize with repeated weekly exposure needed for within-subjects comparisons (N. J. Gamo et al., 2015). FG7142 induces a stress response similar to classic stressors (Leidenheimer and Schechter, 1988; Major et al., 2009), produces changes in WM performance similar to those seen with restraint stress (Shansky et al., 2006), and has been the focus of previous research on the molecular mechanisms underlying stress-induced PFC dysfunction as summarized above.
A dose of FG7142 (between 2.5 and 10 mg/kg, i.p.) was identified for each rat that produced reliable impairment in delayed alternation performance, but still allowed completion of all trials. FG7142 impaired WM performance compared to non-stress control rats administered vehicle into the mPFC (p < 0.001, n = 5, Fig. 8B–C). Intra-mPFC infusion of the cAMP-PKA signaling inhibitor, Rp-cAMPS (21 nmol/0.5 μL), at a dose that has no effect on performance in non-stressed rats, significantly reduced stress-induced WM deficits (p < 0.05 compared to FG7142 + vehicle, n = 5, Fig. 8B). Rp-cAMPS + FG7142 was indistinguishable from vehicle control (p > 0.9).
A parallel experiment examined the effects of KCNQ channel blockade in mPFC on the stress response. A dose of the KCNQ channel blocker, linopirdine, (0.0001–0.01 μg/0.5 μL) was identified that had no effect on its own, to prevent additive effects of the drug treatments (Fig. 8C). Intra-mPFC infusion of linopirdine significantly ameliorated the detrimental effects of FG7142 exposure on WM performance (p < 0.02 compared to FG7142 + vehicle infusion, n = 6, Fig. 8C). Performance following FG + linopirdine was still significantly lower than vehicle control (p < 0.02). It is possible that a cognitive-enhancing dose of linopirdine may have been more effective, but this would have confounded interpretation of the data. It is also possible that HCN channels must also be blocked to fully ameliorate cognitive deficits. In contrast to infusions in the mPFC, control infusions of linopirdine into the premotor cortex dorsal to the PFC did not rescue performance in stressed rats (average of 50% correct; p > 0.8, n = 5). As the Rp-cAMPS or linopirdine treatment had no effect when given alone, additive drug effects could not explain the blockade of the FG7142 response. Instead, these results are consistent with PKA opening of KCNQ channels in mPFC contributing to stress-induced cognitive impairment.
3.3.3. Systemic administration of low doses of KCNQ blockers improves working memory performance in young adult and aged rats in-vivo
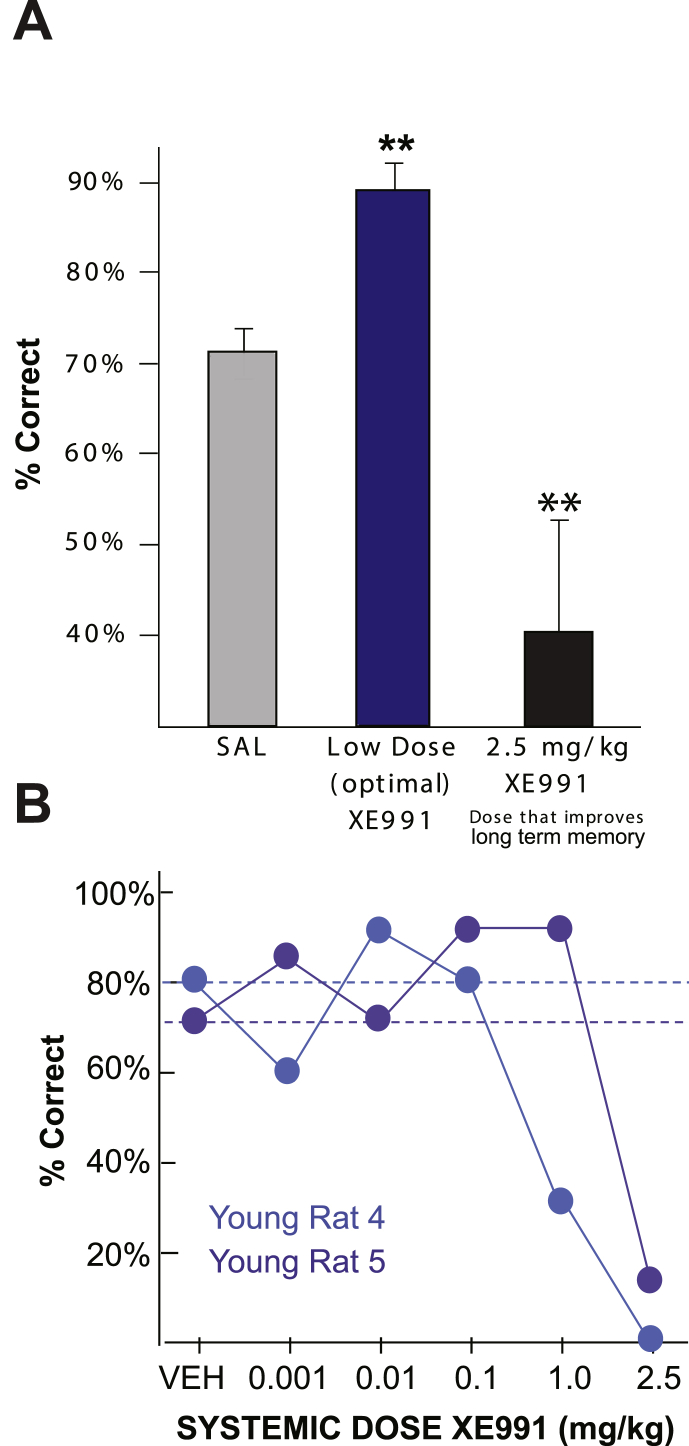
Given the enhancing effects of KCNQ blockade in mPFC, we further examined whether systemic administration of XE991 would improve WM in young adult or aged rats in order to assess potential therapeutic use. The first experiment examined the effects of XE991 in young adult rats, including a high dose (2.5 mg/kg) that was known to improve long-term memory consolidation and hippocampal plasticity in mice (Fontán-Lozano et al., 2011). XE991 produced an inverted U dose response curve in young adult rats (Fig. 9A). Although there was individual variation in sensitivity to the drug (e.g. Fig. 9B), a lower dose (0.001–1.0 mg/kg) could be found in 10/11 rats that improved performance compared to vehicle control (p < 0.001, n = 8, Fig. 9A). In contrast, the higher 2.5 mg/kg dose that improved long-term memory consolidation in previous studies significantly impaired WM performance in this study (p < 0.02, n = 12, Fig. 8B). Five rats were unable to test following this dose and thus were given scores of “0”; this may have reflected covert side effects such as subthreshold seizures.
Fig. 9.
Systemic administration of the KCNQ blocker, XE991, has an inverted U dose-response on working memory performance in young adult rats
A. Systemic injection of XE991 (0.001–2.5 mg/kg, i.p.) produced an inverted U dose-response curve on performance of the delayed alternation task in young adult rats (n = 12). A low dose was identified for each animal that improved performance. In contrast, the 2.5 mg/kg dose impaired performance. This high dose was chosen due to its enhancing effects on long term memory consolidation in mice (*p < 0.05; **p < 0.001 compared to saline control). B. Examples of XE991 dose-response curves in two young adult rats. Note that the drug appears to be more potent in rat 4 than in rat 5. Both are improved by lower doses and markedly impaired by the 2.5 mg/kg dose. The dashed lines represent the vehicle baselines for each individual rat.
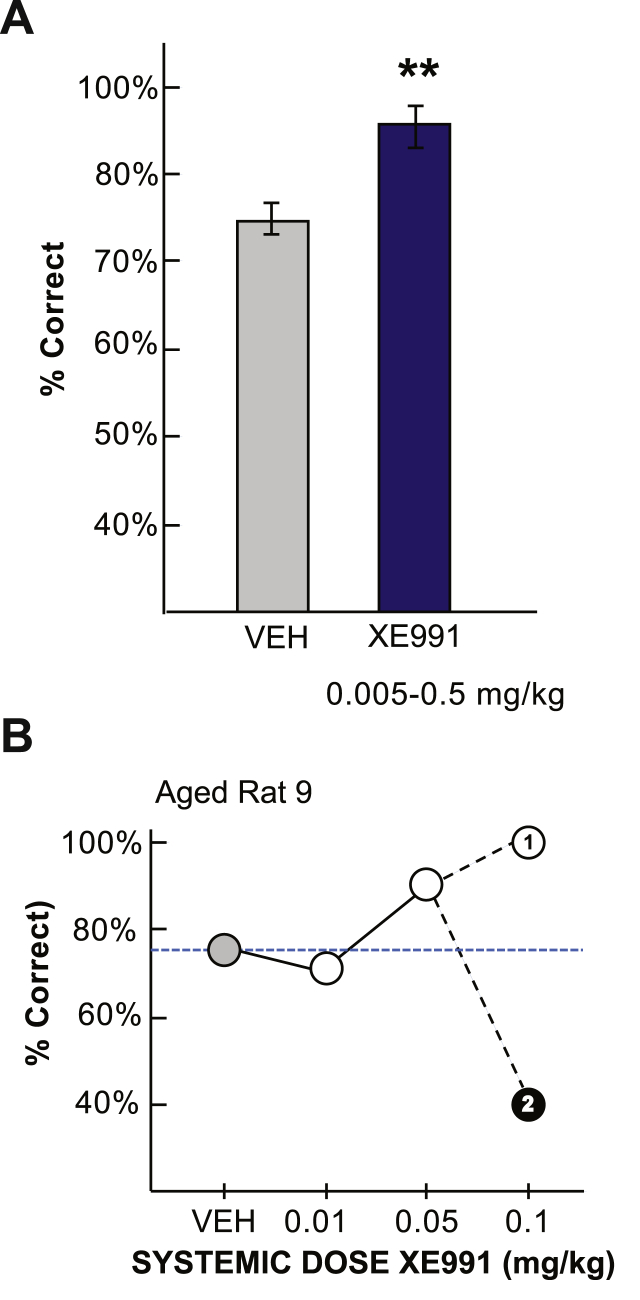
Aged rats have naturally occurring increases in PKA activity in mPFC that impair WM function (Ramos et al., 2003). Thus, we tested whether lower dose XE991 treatment (<1.0 mg/kg) might improve WM performance in aged rats (24 months). A lower dose of XE991 improved performance above vehicle control in 7/8 aged rats (p < 0.01, n = 8, Fig. 10A). However, the inverted U dose-response observed in aged rats was less clear, and the drug had more variable effects, e.g. the same dose could improve performance at the initial administration (100% correct) but impair performance upon repetition (40% correct) within the same animal (Fig. 10B), perhaps due to drug sensitization inducing subthreshold seizures. Importantly, we observed behavioral evidence of seizures in 2 aged rats following the 0.05 and 0.1 mg/kg doses, where animals briefly lay on their sides exhibiting repetitive, involuntary-like movements. These data caution that KCNQ blockers may be problematic as therapeutic treatments for cognitive impairment.
Fig. 10.
Low dose KCNQ blockade improves working memory performance in aged rats
A. Systemic injection of an optimal low dose of XE991 (0.005–0.5 mg/kg, i.p.; n = 8) significantly improved performance of the delayed alternation task in aged rats (**p < 0.01). B. Example of an XE991 dose-response curve in aged rat 9. Note that the first time 0.1 mg/kg dosage was administered it markedly improved performance (noted by the “1”), but the second time this dose was given, it impaired performance (noted by the “2”).
Altogether, these results suggest that increased levels of cAMP signaling with forskolin, stress or aging, decrease mPFC pyramidal cell firing and impair spatial WM performance through opening of KCNQ channels.
4. Discussion
This study found that rat mPFC pyramidal neurons express KCNQ2 channels, and that excessive opening of KCNQ channels reduces neuronal firing contributing to WM impairment. These data have particular clinical relevance, as the open state of KCNQ2 is increased by PKA signaling (Schroeder et al., 1998), and both stress exposure and advanced age increase PKA activity and cause PFC dysfunction (A. F. Arnsten, 2015; Carlyle et al., 2014; Ramos et al., 2003).
KCNQ currents in mPFC pyramidal cells exhibited properties qualitatively similar to those recorded in other neurons, e.g. retigabine increased KCNQ currents from layer V somatosensory cortical neurons (Battefeld et al., 2014), or cell lines expressing KCNQ2/3 heteromers (Tatulian et al., 2001). Importantly, driving PKA activity increased KCNQ currents and reduced neuronal firing, consistent with the behavioral data showing that KCNQ blockade in mPFC improved performance of a WM task, and that cognitive deficits induced by stress or aging could be ameliorated by KCNQ blockade. Altogether, the data suggest that KCNQ channels have powerful influences on mPFC cellular and cognitive function, and may contribute to the reduction in PFC activity with stress (Johansson et al., 2013) and advanced age (Wang et al., 2011). However, the data also caution that systemic administration of KCNQ blockers may be problematic, as there was evidence of seizures following higher doses, particularly in aged animals.
4.1. Role in stress response
This study provides evidence that KCNQ channel opening contributes to stress-induced WM deficits. The behavioral data showed that impairments in delayed alternation performance arising from acute stress could be reversed by either PKA inhibition or KCNQ blockade in the mPFC, supporting this hypothesis. In addition, the physiological data showed that chronic stress increased KCNQ currents in the layers II/III mPFC pyramidal neurons known to be especially vulnerable to stress exposure (Radley et al., 2006). The chronic restraint paradigm used here has been previously shown to impair WM (Hains et al., 2009) and attention-set-shifting performance (Liston et al., 2006), both cognitive abilities dependent on mPFC. Interestingly, stress effects were limited to regular-firing neurons, which were located in both layers II/III and V. It is possible that some of these neurons may correspond to the corticopontine neurons, which may contribute to the delay period of WM function (Dembrow et al., 2010), and/or most likely to cortico-cortical neurons, which have a regular firing phenotype (Anastasiades et al., 2018), and are especially sensitive to chronic stress exposure (Shansky et al., 2009). The specific function of mPFC neurons that are stress-sensitive would be an important area for future research. The data suggest that greater KCNQ currents in chronically stressed rats would result in reduced PFC neuronal firing needed for higher cognitive function.
4.2. KCNQ actions in PFC vs. hippocampus
This study of prelimbic mPFC WM function parallels previous studies demonstrating KCNQ channel contributions to the ventral PFC regulation of emotion. For example, increased KCNQ actions can reduce infralimbic mPFC neuronal firing and weaken extinction of the conditioned fear response (Santini and Porter, 2010). KCNQ actions in mPFC circuits have also been linked to alcohol consumption (Rinker et al., 2017) and sucrose palatability (Parent et al., 2015). Here we expand on this body of work, demonstrating that KCNQ actions in mPFC contribute to cognitive deficits induced by stress or advanced age. Sustained hyperpolarization resulting from increased KCNQ may contribute to dendritic atrophy and WM deficit with chronic stress (Radley et al., 2006), which increases vulnerability to mental illness. Previous research has established that increased cAMP signaling with stress and advancing age contributes to PFC cognitive deficits by increasing the open state of HCN channels (Abel et al., 1997; A. F. Arnsten, 2015; Carlyle et al., 2014; N.J. Gamo et al., 2015; Li et al., 2014; Paspalas et al., 2013). The current study builds on this foundation, revealing an additional role of KCNQ channels in PFC-related dysfunction.
cAMP-PKA signaling effects are distinct in PFC vs. hippocampal circuits, and these distinctions may extend to KCNQ actions as well. In contrast to cAMP weakening the WM functions of the PFC via opening of HCN and KCNQ channels, cAMP signaling strengthens the long-term memory functions of hippocampus through classic neuroplastic signaling events (Abel et al., 1997). These differences are also seen in KCNQ channel actions in hippocampus, where stress exposure decreases the expression of KCNQ2/3 channels (Li et al., 2014), in contrast to the increase in KCNQ currents we found in stressed mPFC. Additionally, a high dose of the KCNQ channel inhibitor, XE991 (2.5 mg/kg), that impaired WM in this study, improved long-term memory consolidation (Fontán-Lozano et al., 2011). It is possible that the hyperexcitability induced by KCNQ channel blockade in mPFC pyramidal cells is sufficient to obscure the pattern of information held in WM, whereas the same degree of excitability in hippocampus may facilitate increased neuronal activity needed for long term memory consolidation.
4.3. Role in aging
cAMP-PKA signaling becomes dysregulated in the aging rat and monkey PFC, at least in part due to loss of the phosphodiesterase, PDE4A (Carlyle et al., 2014; Ramos et al., 2003). Loss of WM-related neuronal firing with advancing age in monkey dlPFC is improved KCNQ blockers (Wang et al., 2011), suggesting that elevated PKA signaling erodes WM by increasing the activity of KCNQ channels in the aged PFC. Forskolin induced elevated cAMP-PKA can also affect the activity of other ion channels but its effect on KCNQ current and on mPFC cell firing is consistent with what we obeserve after modulating KCNQ channels with retigabine.
These data provide the first direct evidence that elevated cAMP-PKA signaling in PFC, as occurs with advanced age and stress, reduces neuronal firing and cognitive function by increasing the open state of KCNQ channels. Given the powerful effect of these channels on PFC pyramidal cell excitability, this is a significant mechanism for regulating the strength of top-down PFC function.
5. Clinical relevance
In addition to stress and aging, KCNQ actions likely contribute to impaired PFC function in mental illness. Altered splice variants of KCNQ2 are linked to bipolar disorder (Borsotto et al., 2007), where manic episodes are driven by stress exposure (Hammen and Gitlin, 1997) and are associated with hypoactivity of PFC (Blumberg et al., 2003). Lithium treatment of bipolar disorder is thought to involve inhibition of GSK3ß signaling, and there is recent evidence that GSK3ß increases the open state of KCNQ2 channels (Kapfhamer et al., 2010). Thus, the loss of top-down PFC regulation of behavior during bipolar mania may involve increased KCNQ2 channel actions.
KCNQ-targeting compounds are already in widespread clinical use. Retigabine (marketed as Ezogabine in the USA), which increases the open state of KCNQ channels, has been prescribed as an anticonvulsant (Amabile and Vasudevan, 2013). Interestingly, common side effects of this medication include cognitive deficits such as confusion, speech disorder, amnesia and abnormal thinking (Porter et al., 2007), as well as aggression, difficulties with concentration and even psychosis (FDA.gov), all signs consistent with PFC dysfunction. The current results suggest that some of these side effects may arise from excessive reduction in PFC neuronal firing.
Conversely, KCNQ blockers have been considered as potential treatments for cognitive impairment (Gribkoff, 2003). Although these data indicate that systemic treatment with a KCNQ blocker can improve WM at low doses, the data also caution that risk of seizures, e.g. due to neuronal hyperexcitability, could be problematic. Seizures were observed in two aged rats, and subthreshold seizures might have contributed to marked cognitive impairment following high doses in both young and aged animals. These data suggest that other, more indirect modulation of KCNQ channel open state, e.g. through inhibition of cAMP-PKA signaling, may be more appropriate for treatment of cognitive impairment.
Financial disclosures
A.F.T.A. and Yale University receive royalties from the USA sales of Intuniv, but not from generic or nonUSA sales. All other authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
This work was supported by NIH grants DC01919 (L.K.K.), DP1AG047744-01 (A.F.T.A.), 1RL1AA017536-01 (A.F.T.A.), R01NS060803 (N.S.B.), and U54RR024350-01 (A.F.T.A.)
Contributor Information
Amy F.T. Arnsten, Email: amy.arnsten@yale.edu.
Lynda El-Hassar, Email: Lynda.El-Hassar@unice.fr.
References
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Adams P.R., Brown D.A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J. Physiol. 1982;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile C.M., Vasudevan A. Ezogabine: a novel antiepileptic for adjunctive treatment of partial-onset seizures. Pharmacotherapy. 2013;33:187–194. doi: 10.1002/phar.1185. [DOI] [PubMed] [Google Scholar]
- Anastasiades P.G., Marlin J.J., Carter A.G. Cell-type specificity of callosally evoked excitation and feedforward inhibition in the prefrontal cortex. Cell Rep. 2018;22:679–692. doi: 10.1016/j.celrep.2017.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T., Goldman-Rakic P.S. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatr. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T., Wang M., Paspalas C.D. Dopamine's actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol. Rev. 2015;67:681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A., Tran B.T., Gavrilis J., Cooper E., Kole M.H. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J. Neurosci. 2014;34:3719–3732. doi: 10.1523/JNEUROSCI.4206-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum S.B., Yuan P., Wang M., Vijayraghavan S., Bloom A., Davis D. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Birnbaum S.G., Gobeske K.T., Auerbach J., Taylor J.R., Arnsten A.F.T. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Blumberg H.P., Leung H.C., Skudlarski P., Lacadie C.M., Fredericks C.A., Harris B.C. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch. Gen. Psychiatr. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Borsotto M., Cavarec L., Bouillot M., Romey G., Macciardi F., Delaye A. PP2A-Bgamma subunit and KCNQ2 K+ channels in bipolar disorder. Pharmacogenomics J. 2007;7:123–132. doi: 10.1038/sj.tpj.6500400. [DOI] [PubMed] [Google Scholar]
- Brown D.A., Adams P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Carlyle B.C., Nairn A.C., Wang M., Yang Y., Jin L.E., Simen A.A. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5036–5041. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P., Brown D.A. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Dembrow N.C., Chitwood R.A., Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J. Neurosci. 2010;30(50):16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty N.M., Evans I.M., Sledge W.H., Seibyl J.P., Krystal J.H. Affective reactivity of language in schizophrenia. J. Nerv. Ment. Dis. 1994;182:98–102. doi: 10.1097/00005053-199402000-00006. [DOI] [PubMed] [Google Scholar]
- Dorow R., Horowski R., Pashelke G., Amin M., Braestrup C. Severe anxiety induced by FG7142, a B-carboline ligand for benzodiazepine receptors. The Lancet. 1983:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Elmedyb P., Calloe K., Schmitt N., Hansen R.S., Grunnet M., Olesen S.P. Modulation of ERG channels by XE991. Basic Clin. Pharmacol. Toxicol. 2007;100(5):316–322. doi: 10.1111/j.1742-7843.2007.00048.x. [DOI] [PubMed] [Google Scholar]
- Fontán-Lozano A., Suárez-Pereira I., Delgado-García J.M., Carrión A.M. The M-current inhibitor XE991 decreases the stimulation threshold for long-term synaptic plasticity in healthy mice and in models of cognitive disease. Hippocampus. 2011;21:22–32. doi: 10.1002/hipo.20717. [DOI] [PubMed] [Google Scholar]
- Gamo N.J., Lur G., Higley M.J., Wang M., Paspalas C.D., Vijayraghavan S. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with hyperpolarization-activated cyclic nucleotide-gated channels. Biol. Psychiatry. 2015;78(12):860–870. doi: 10.1016/j.biopsych.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Greene D.L., Hoshi N. Modulation of Kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2017;74:495–508. doi: 10.1007/s00018-016-2359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff V.K. The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin. Ther. Targets. 2003;7:737–748. doi: 10.1517/14728222.7.6.737. [DOI] [PubMed] [Google Scholar]
- Gu N., Vervaeke K., Hu H., Storm J.F. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J. Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D., Higgs M.H., Horton L.R., Spain W.J., Foehring R.C. Contributions of Kv7-mediated potassium current to sub- and suprathreshold responses of rat layer II/III neocortical pyramidal neurons. J. Neurophysiol. 2011;106:1722–1733. doi: 10.1152/jn.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenston A.M., Fitzpatrick J.S., Yeckel M.F. mGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cerebr. Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains A.B., Vu M.A., Maciejewski P.K., van Dyck C.H., Gottron M., Arnsten A.F. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc. Natl. Acad. Sci. U. S. A. 2009;106(42):17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C., Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. Am. J. Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Hartley L.R., Adams R.G. Effect of noise on the Stroop test. J. Exp. Psychol. 1974;102:62–66. doi: 10.1037/h0035695. [DOI] [PubMed] [Google Scholar]
- Hoftman G.D., Datta D., Lewis D.A. Layer 3 excitatory and inhibitory circuitry in the prefrontal cortex: developmental trajectories and alterations in schizophrenia. Biol. Psychiatry. 2017;81:862–873. doi: 10.1016/j.biopsych.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Johansson L., Guo X., Hällström T., Norton M.C., Waern M., Ostling S., Skoog I. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhamer D., Berger K.H., Hopf F.W., Seif T., Kharazia V., Bonci A., Heberlein U. Protein Phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-Type potassium channel activity. J. Neurosci. 2010;30:8830–8840. doi: 10.1523/JNEUROSCI.1292-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J.K., Divac I. Selective ablations within the prefrontal cortex of the rat and performance of delayed alternation. Physiol. Psychol. 1978;6:15–17. [Google Scholar]
- Leidenheimer N.J., Schechter M.D. Discriminative stimulus control by the anxiogenic b-carboline FG7142: generalization to a physiological stressor. Pharmacol. Biochem. Behav. 1988;30:351–355. doi: 10.1016/0091-3057(88)90467-4. [DOI] [PubMed] [Google Scholar]
- Leverich G.S., Post R.M., Rosoff A.S. Factors associated with relapse during maintenance treatment of affective disorders. Int. Clin. Psychopharmacol. 1990;5:135–156. doi: 10.1097/00004850-199004000-00007. [DOI] [PubMed] [Google Scholar]
- Li C., Huang P., Lu Q., Zhou M., Guo L., Xu X. KCNQ/Kv7 channel activator flupirtine protects against acute stress-induced impairments of spatial memory retrieval and hippocampal LTP in rats. Neuroscience. 2014;280:19–30. doi: 10.1016/j.neuroscience.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Liston C., Miller M.M., Goldwater D.S., Radley J.J., Rocher A.B., Hof P.R. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major C.A., Kelly B.J., Novak M.A., Davenport M.D., Stonemetz K.M., Meyer J.S. The anxiogenic drug FG7142 increases self-injurious behavior in male rhesus monkeys (Macaca mulatta) Life Sci. 2009;85:753–758. doi: 10.1016/j.lfs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z.-M., Arnsten A.F.T., Li B.-M. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Murphy B.L., Arnsten A.F.T., Goldman-Rakic P.S., Roth R.H. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan P.T., Insel T.M., Cohen R.M., Cook J.M., Skolnick P., Paul S.M. Benzodiazepine receptor-mediated experimental anxiety in primates. Science. 1982;218(4579):1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- Parent M.A., Amarante L.M., Swanson K., Laubach M. Cholinergic and ghrelinergic receptors and KCNQ channels in the medial PFC regulate the expression of palatability. Front. Behav. Neurosci. 2015;9:284. doi: 10.3389/fnbeh.2015.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas C.D., Min Wang M., Arnsten A.F.T. Constellation of HCN Channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex ─ Potential substrate for working memory deficits in schizophrenia. Cerebr. Cortex. 2013;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G., Salvi A., Liu H., Atrooz F., Alkadhi I., Kelly M., Salim S. Tempol treatment reduces anxiety-like behaviors induced by multiple anxiogenic drugs in rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117498. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Peng H., Bian X.L., Ma F.C., Wang K.W. Pharmacological modulation of the voltage-gated neuronal Kv7/KCNQ/M-channel alters the intrinsic excitability and synaptic responses of pyramidal neurons in rat prefrontal cortex slices. Acta Pharmacol. Sin. 2017;38(9):1248–1256. doi: 10.1038/aps.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.J.,., Partiot A., Sachdeo R., Nohria V., Alves W.M., Group S. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- Qin S., Hermans E.J., van Marle H.J.F., Lou J., Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G., Liston C., Hof P.R. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr. Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Ramos B., Birnbaum S.B., Lindenmayer I., Newton S.S., Duman R., Arnsten A.F.T. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Rinker J.A., Fulmer D.B., Trantham-Davidson H., Smith M.L., Williams R.W., Lopez M.F. Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol Alcohol. 2017;58:33–45. doi: 10.1016/j.alcohol.2016.05.007. Jun 27, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saganich M.J., Machado E., Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J. Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E., Porter J.T. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J. Neurosci. 2010;30(37):12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.C., Kubisch C., Stein V., Jentsch T.J. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Rubinow K., Brennan A., Arnsten A.F. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav. Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Hamo, Hof P.R., McEwen B.S., Morrison J.H. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebr. Cortex. 2009;19:2479. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N., Salchner P., Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Tatulian L., Delmas P., Abogadie F.C., Brown D.A. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R., Birnbaum S.G., Ubriani R., Arnsten A.F.T. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Johnston A.L., Wilks L.J., Nicholass J.M., File S.E., Little H.J. Kindling with the b-carboline FG7142 suggests separation between changes in seizure threshold and anxiety-related behaviour. Neuropsychobiology. 1988;19:195–201. doi: 10.1159/000118460. [DOI] [PubMed] [Google Scholar]
- van Aerde K.I., Feldmeyer D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cerebr. Cortex. 2015;25(3):788–805. doi: 10.1093/cercor/bht278. [DOI] [PubMed] [Google Scholar]
- van Dyck C.H., Lin C.H., Robinson R., Cellar J., Arnsten A.F.T., Hoffer P.B. Effects of the acetylcholine-releaser linopirdine on SPECT regional cerebral blood flow and cognitive function in Alzheimer's disease. Am. J. Geriatr. Psychiatry. 1996;40(4):339–340. [Google Scholar]
- Vijayraghavan S., Wang M., Birnbaum S.G., Bruce C.J., Williams G.V., Arnsten A.F.T. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M., Gamo N.J., Yang Y., Jin L.E., Wang X.J., Laubach M. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Ramos B., Paspalas C., Shu Y., Simen A., Duque A. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Weinberger D.R., Berman K.F., Zec R.F. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatr. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wickenden A.D., Yu W., Zou A., Jegla T., Wagoner P.K. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol. Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- Wladyka C.L., Kunze D.L. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J. Physiol. 2006;575(Pt 1):175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.T., Shi Y., Wang Q., Peng J.Y., Li B.M. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol. Brain. 2014;7:61. doi: 10.1186/s13041-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C., Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J. Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J., Taylor J.R., Arnsten A.F.T. Supranormal stimulation of dopamine D1 receptors in the prefrontal cortex impairs spatial working memory in rats. Soc. Neurosci. Abstr. 1996;22:1128. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J., Taylor J.R., Mathew R.G., Arnsten A.F.T. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]